Abstract
Anastomotic bleeding in vascular surgery can be difficult to control. Patients, in particular those undergoing carotid surgery, have often been started on treatment with dual antiplatelet agents and receive systemic heparinization intraoperatively. The use of local hemostatic agents as an adjunct to conventional methods is widely reported. 3-D Matrix’s absorbable hemostatic material RADA16 (PuraStat®), is a fully synthetic resorbable hemostatic agent. The aim of this study is to confirm the safety and performance of this agent when used to control intraoperative anastomotic bleeding during carotid endarterectomy (CEA). A prospective, single-arm, multicenter study involving 65 patients, undergoing CEA, in whom the hemostatic agent was applied to the suture line after removal of arterial clamps. Patients were followed up at 24 h, discharge, and one month after surgery. Time to hemostasis was measured as the primary endpoint. Secondary endpoints included hemostasis efficacy and safety outcomes, blood loss, intraoperative and postoperative administration of blood products, and incidence of reoperation for bleeding. A total of 65 cases (51 male and 14 female) undergoing CEA, utilizing patch reconstruction (90. 8%), eversion technique (6.1%), and direct closure (3.1%) were analyzed. All patients received dual antiplatelet therapy preoperatively and were administered systemic intravenous heparin intraoperatively, as per local protocol. The mean time to hemostasis was 83 s ± 105 s (95% CI: 55-110 s). Primary hemostatic efficacy was 90.8%. The mean volume of product used was 1.7 mL ± 1.1 mL. Hemostasis was achieved with a single application of the product in 49 patients (75.3%). Two patients required a transfusion of blood products intraoperatively. There were no blood product transfusions during the postoperative period. The intraoperative mean blood loss was 127 mL ± 111.4 mL and postoperatively, the total mean drainage volume was 49.0 mL ± 51.2 mL. The mean duration of surgery was 119 ± 35 min, and the mean clamp time was 35 min 12 s ± 19 min 59 s. In 90.8% of patients, there was no presence of hematoma at 24 h postoperatively. Three returned to theatre due to bleeding (2 in the first 24 h), however, none of these cases were considered product related. Overall, there were no device-related serious adverse events (SAE) or unanticipated device-related SAEs reported. Use of the hemostatic agent PuraStat® is associated with a high rate of hemostatic efficacy (90.8%) and a short time to hemostasis. The safety of the product for use on vascular anastomoses has been demonstrated.
Keywords: hemostasis, self-assembling peptides, RADA16, vascular surgery, carotid endarterectomy, case series
Introduction
Carotid endarterectomy (CEA) is the definitive treatment for long-term stroke prevention in patients who have suffered a recent cerebral ischemic event with moderate to severe internal carotid artery stenosis.1,2 Patients with symptomatic carotid stenosis of 50% to 99%, according to the North American Symptomatic Carotid Endarterectomy Trial (NASCET) criteria, or more than 70% according to the European Carotid Surgery Trial criteria, should be assessed and referred urgently for CEA.3–5 CEA is a procedure whereby the atherosclerotic plaque is removed from the carotid artery via an arteriotomy. Despite it demonstrating significant long-term benefits with regard to primary and secondary stroke prevention and mortality reduction, complications of the operation can provide significant perioperative challenges.1,6
Perioperative bleeding is common in patients undergoing CEA and other major vascular reconstructions. It is well-recognized that treatment before surgery with dual antiplatelet therapy reduces the risk of perioperative stroke. The patient often also receive intraoperative anticoagulation to further reduce thromboembolic risk, however, these contribute to a significant risk of anastomotic bleeding.7,8 These patients often have friable vessels with extensive atherosclerotic disease and calcification, further contributing to anastomotic bleeding risk.3,9
Anastomotic bleeding itself can be unpredictable, persistent, and life-threatening. Postoperative hematoma after CEA has the potential to cause rapid onset of airway obstruction, respiratory failure, and emergency reintervention.10,11 In the NASCET study postoperative neck hematomas occurred in 7.1% of patients after CEA, which was subsequently identified as a statistically significant risk factor for perioperative stroke and death (14.9% in patients with wound hematoma, compared with 5.9% in patients without hematoma).12 More recently, Baracchini et al8 reported neck bleeding in 8.2% of 1458 cases undergoing eversion CEA.
Hemostasis may be challenging in peripheral vascular surgery, due to the requirement of direct arterial and arterial graft suturing. The risk of anastomotic bleeding in vascular surgery may be reduced by meticulous technique, using fine needles, suture material, and surgical loupes. The use of antiplatelet agents and systemic anticoagulants in the prevention of thrombosis during periods of operative vessel occlusion will increase the risk despite good surgical technique. Increasingly, hemostatic agents are being used as an adjunct to promote hemostasis.13
Rapid hemostasis during CEA results in shorter operative times, decreased transfusion requirement, improved patient recovery time, and decreased wound healing time.13 Adjuvant hemostatic methods alongside accurate methods of primary hemostasis may aid in achieving these outcomes.
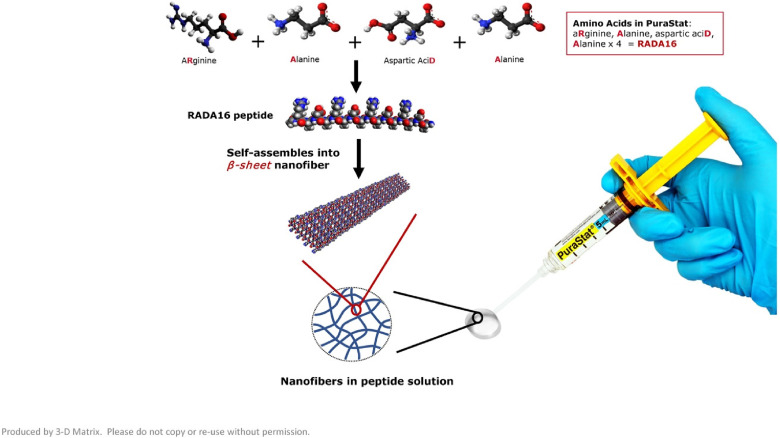
Multiple hemostatic agents are available to the surgeon, with the aim of controlling and reducing anastomotic bleeding but, to date, a reliable option that is easy to use and shows good risk and cost–benefit potential is not widely available. Those hemostatic agents can be divided into 3 categories: hemostats, sealants, and adhesives.13 Five classes of these agents have been historically available: fibrin sealants, bovine collagen and thrombin, cyanoacrylate, albumin cross-linked with glutaraldehyde, and polyethylene glycol polymer.9 The self-assembling peptide, RADA16, an absorbable hemostatic material, was developed for use as a hemostat (PuraStat®, 3-D Matrix Europe, Caluire and Cuire, France) for controlling intra- and postoperative bleeding in diverse surgical procedures (eg, bleeding from vascular anastomosis, small vessel hemorrhage of the gastrointestinal tract and oozing from capillaries of the parenchyma and surrounding tissues of solid organs). It is made from a chain of 3 naturally occurring amino acids; arginine, alanine, and aspartic acid bonded together and repeated 4 times to form a 16-amino acid oligo-peptide chain, RADA16, as shown in Figure 1. It is a transparent, amphiphilic self-assembling peptide (SAP), available in a prefilled syringe as a 2.5% aqueous solution. Contact with the fluid of physiological pH, such as blood causes the acidic peptide solution to be neutralized and, as a result, the peptide molecule, which has a β structure, quickly forms a peptide hydrogel. This hydrogel coats and adheres to the point of vessel bleeding, forming a mechanical barrier that effectively seals the damaged part of the vessel. This facilitates coagulation deep into the gel, thus aiding hemostasis. The gel remains in situ after surgery and is gradually absorbed. The majority will have been absorbed within 30 days, but some may remain in place for longer.14
Figure 1.
Constituents of PuraStat®. In contact with the fluid of physiological pH, the β sheet nanofiber network rapidly forms a hydrogel creating a mechanical barrier to aid in hemostasis.14
RADA16 was commercialized under the name of PuraStat® following Masuhara et al15 first demonstrating its safety and efficacy as a hemostat in cardiac surgery. Since 2014, both pro-active and reactive postmarket surveillance data have been collected to monitor and record serious adverse events causally related to PuraStat®, of which none have been declared as of July 2022.
Subsequent clinical trials have been carried out since 2014 to assess and demonstrate the safety and efficacy of PuraStat® in multiple surgical fields, including cardiology, hepatology, gastrointestinal, otolaryngology, and endocrine surgery.16–24
Aim/Hypotheses
The objective of this postmarket clinical follow-up study is to report safety and performance data from patients undergoing elective CEA where the SAP, PuraStat®, was used for intraoperative hemostasis.
The primary endpoint was defined as total time-to-hemostasis (TTH).
Secondary endpoints evaluated were blood loss, drainage volume, ease of use of PuraStat®, and rate and quantity of transfusion of blood products. Safety data were also collected including reintervention for bleeding, adverse events, postoperative complications, and length of hospital stay.
Methods
Study Design
This was a single-arm, prospective, multicenter study involving consecutive patients undergoing CEA in whom RADA16, the hemostatic agent was applied to the suture line after the removal of arterial clamps. Ethics approval was obtained from all centers. The study was registered at HRA under number 17/LO/0082 and at ClinicalTrials.gov under the Identifier: NCT03103282.
Patient Selection
Patients over the age of 18 years, undergoing elective CEA either by direct closure (without the use of patch), patch reconstruction, or eversion technique were screened for eligibility. Those, undergoing elective CEA, requiring the use of RADA16 were eligible for inclusion. Those with a known coagulation disorder or hypersensitivity to any components of RADA16 were excluded. No formal statistical hypothesis was conducted to derive the sample size.
Patient Enrollment
Routine standard of care regarding the informed consent process for CEA was followed independently of any information or discussion relating to this study. Study inclusion was first discussed at the preoperative visit. Informed consent forms related to the study were provided to each site and were signed by the patients. Once deemed eligible, patients were assigned a unique identification number for the duration of the study.
Patient demographics, medical history, and their use of antiplatelet or anticoagulant drugs were recorded prospectively, alongside standard local site preoperative examinations and blood tests (full blood count, pregnancy test, prothrombin time, fibrinogen or partial thromboplastin time).
Procedure
CEA was undertaken by 7 senior surgeons with previous experience with different hemostatic agents. CEA was carried out to the standard of care of the local site by either patch reconstruction, direct closure, or eversion technique, subject to the lead surgeon's discretion. CEA involves the removal of atherosclerotic plaque via an arteriotomy in the internal carotid artery (ICA). Direct closure involves closing the arteriotomy with direct suturing of the ICA, whereas patch reconstruction involves the incorporation of a synthetic, bovine pericardial, or venous patch as the method of closure. The eversion technique involves the disconnection of the ICA from the carotid bulb via an arteriotomy. The ICA is then everted to allow for the atherosclerotic plaque to be removed before the ICA is re-anastomosed to the carotid bulb.25 All patients were on dual antiplatelet therapy preoperatively and were administered systemic intravenous heparin intraoperatively, as per local site protocol.
Procedural instructions for PuraStat® use were provided to the study sites. PuraStat® was supplied in prefilled syringes, of either 1, 3, or 5 mL. Primary hemostasis was achieved through techniques at the lead surgeon's discretion, including single pressure, and further monofilament sutures, with or without pledgets, for bleeding in between existing sutures. Cautery was not used on the suture line itself. RADA16 was applied if oozing bleeding from suture lines was present after the clamps on the vessel were removed. As much blood as possible was removed from the bleeding site following initial hemostasis before the application of RADA16. The syringe nozzle was held as close to the tissue as possible to allow for adequate application in a volume sufficient to fully cover the bleeding site. The gel was left undisturbed until complete hemostasis occurred. In some cases, more than one application or syringe was required to achieve this and there was no maximum number of applications defined.
The primary endpoint was defined as total time-to-hemostasis (TTH). TTH was measured using a stopwatch from the initial application of RADA16 to the bleeding site, after vessel clamp release, until all visible bleeding had ceased, and complete hemostasis was judged as achieved by the operator.
In the case of multiple applications of RADA16, TTH was defined as the time from the very first application. TTH is a well-accepted outcome measure for hemostasis and has been used in previous postmarket studies evaluating the safety and efficacy of PuraStat®.17,18 This should allow adequate comparability of the results across the different surgical fields.
Secondary endpoints evaluated were blood loss, drainage volume, ease of use of PuraStat®, and rate and quantity of transfusion of blood products. Safety data were also collected including reintervention for bleeding, adverse events, postoperative complications, and length of hospital stay.
Surgeon-Assessed Outcomes
Surgeons were asked to categorize the degree of bleeding following anastomosis as being “mild,” “moderate,” or “severe.” They were asked to comment on the quality of the vessel being treated as “poor,” “moderate,” or “good.” At the completion of the procedure, investigators were asked to determine hemostasis as “complete” or as “showing presence of bleeding.” The ease of use of the PuraStat® application system was assessed by the operating surgeons as “excellent,” “good,” “fair,” or “poor.”
Follow-up Protocol
Patients were followed up at 24 h, at discharge, and at one month postoperatively. One-month follow-up was completed in person or over the phone. Primary endpoint data were collected intraoperatively, whereas secondary endpoints and safety data were collected throughout the study follow-up period.
Statistical Analysis
Statistical analysis was undertaken using SAS System®, Version 9.4. No replacement of missing data has been performed. Descriptive statistics were utilized with continuous variables summarized as mean (with 95% confidence intervals [CIs]), standard deviation, median, quartiles, and range. The number of missing observations has also been summarized. Categorical variables were summarized as percentages by categories.
All statistical analysis was performed on the intention-to-treat (ITT) population of the study, defined as all the patients with signed informed consent forms that were treated with at least one application of RADA16.
Results
Patient Recruitment and Enrollment
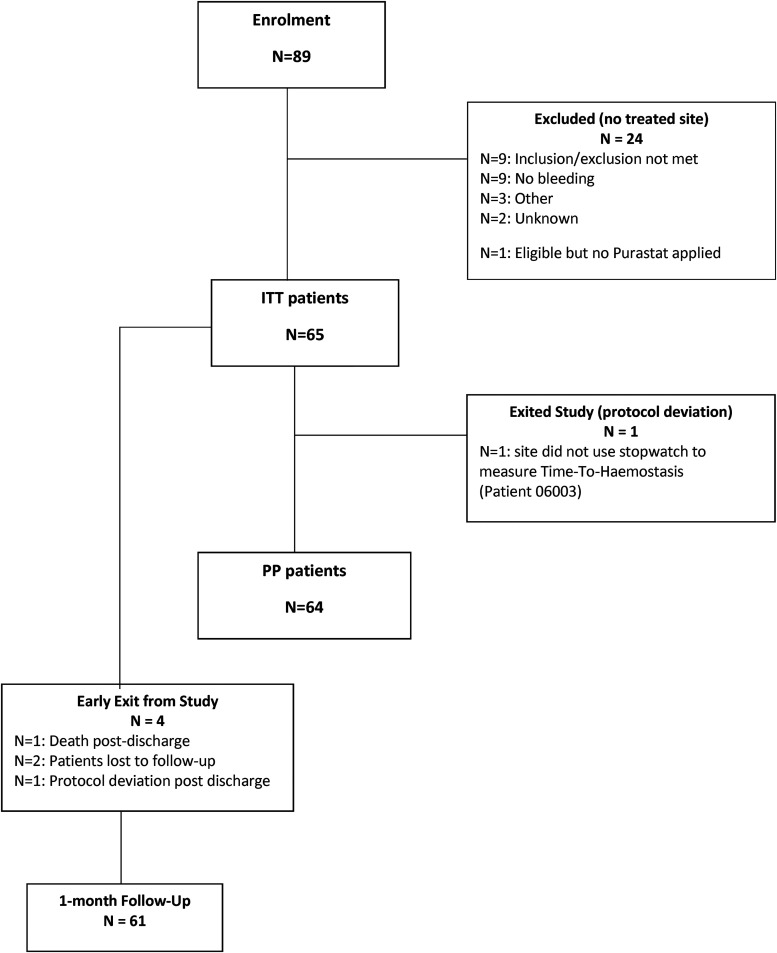
Eighty-nine patients were enrolled in the study between June 2017 and July 2019 (72 patients in 4 hospitals in the United Kingdom and 17 patients in one hospital in Belgium). Twenty-four (27%) of this cohort were excluded from the ITT population as RADA16 was not used either because patients were ineligible (9), or because there were no active bleeding sites after primary hemostasis (9) for other reasons not reported by the site (3), for unknown reasons (2), 1 patient who was eligible but did not have RADA16 applied during the procedure. Recruitment and enrolment data are summarized in Figure 2.
Figure 2.
Patient recruitment and enrolment flowchart.
Abbreviations: ITT, Intent To Treat; TTH, Time To Hemostasis; PP, Per Protocol.
In the remaining 65 cases, RAD16 was used during elective CEA, and these formed the ITT population. One patient completed the study with a major deviation, whereby the site did not use a stopwatch to measure the TTH resulting in a per-protocol population (PP) of 64 participants. 24-h follow-up and discharge data were complete for 100% of patients. 93.8% (61) completed the 1-month follow-up visit, of which 83.6% (51) were completed within the predefined study window.
Patient Demographics
Sixty-five patients underwent CEA with intraoperative use of RADA16 and were included in the study. Of these, 51 (78.5%) of participants were male and 14 (21.5%) females, with a mean age of 71.8 ± 8.9 years (range 48-87 years). Most of the participants, 60 (92.3%) had a normal physical examination at the preoperative consultation, with a mean body mass index of 26.3 ± 5.3 kg/m2. 44 (68.8%) had a history of smoking and 12 (18.5%) were diabetic. A total of 47 out of 63 (74.6%, data were missing for 2 patients), were deemed at high risk, having an American Society of Anesthesiologists (ASA) grade of 3 or more.26 Mean preoperative systolic blood pressure was 135.7 ± 19.9 mm Hg and diastolic blood pressure was 71.5 ± 13.8 mm Hg. Full patient characteristics are presented in Table 1.
Table 1.
Demographics of the Patient Cohort Included in the Statistical Analysis.
| Characteristic | ITT N = 65 |
|---|---|
| (n/N) %, (missing) | |
| Smoking | (44/64) 68.8%, (1) |
| Diabetes | (12/65) 18.5%, (0) |
| Type I | (1/12) 8.3%, (0) |
| Type II | (11/12) 91.7%, (0) |
| Diabetes treatment | (0) |
| Oral treatment | (8/12) 66.7%, (0) |
| Insulin-dependent | (4/12) 33.3%, (0) |
| Renal failure | (2/65) 3.1%, (0) |
| Family History (of CVD) | (19/65) 29.2%, (0) |
| ASA score | (2) |
| 1 | (2/63) 3.2% |
| 2 | (14/63) 22.2% |
| 3 | (42/63) 66.7% |
| 4 | (5/63) 7.9% |
| 5 | (0) 0% |
| Other vascular condition | (24/65) 36.9%, (0) |
| Other medical history | (24/65) 36.9%, (0) |
Abbreviations: ITT, Intent To Treat; CVD, Cardiovascular Disease; ASA score, American Society of Anesthesiology score.
Procedure
Fifty-nine (90.8%) CEAs were performed using patch reconstruction, of which most cases, 37 (56.9%), used a bovine pericardial patch. The eversion technique was used in 4 (6.1%) cases and direct closure (without a patch) was performed in 2 (3.1%) of cases. The mean duration of the procedure was 118 ± 35 min (range: 61-255 min). The mean carotid artery clamp time was 35 min 12 s ± 19 min 59 s (range: 25 s-68 min), with data reported in 59 cases.
After clamp removal, in 50 cases (76.9%) “rescue” sutures were inserted to achieve primary hemostasis before the application of RADA16. The quality of vessels at the suture or the anastomotic site was reported as “good” in 51 cases (79.7%) and “moderate” in 13 cases (20.3%). Data were missing for one patient. No patients had suture site vessels deemed as “poor” quality.
The mean total number of applications of PuraStat® was 1.3 ± 0.6, with the mean total amount of product used recorded as 1.7 ± 1.1 mL.
Bleeding and RADA16 Efficacy
The degree of bleeding prior to the application of RADA16 was reported by the operating surgeon as “mild” in 59 cases (90.8%) and “moderate” in 6 (9.2%), with no “severe” bleeding, recorded. This classification was subjective and observer-dependent. RADA16 appeared to be effective in the majority of cases.
In 59 cases (90.8%), investigators describe hemostasis as “complete” including 3 cases where it had been obtained by combining RADA16 with additional hemostatic action. In 6 cases (9.2%), the investigator reported ongoing “presence of bleeding,” of these, 4 had decreased bleeding compared with preapplication levels described by the investigator, and 2 showed no change in bleeding levels. Further action was required to achieve hemostasis using additional rescue sutures in 3 cases, compressions in 2, and the use of a fibrillar, surgical absorbable hemostat in 1 case. Of the 59 cases that achieved hemostasis the number of applications of the product was as follows: 49 requiring 1 application, 7 requiring 2 applications, and 3 required 3 applications of the product. Overall, 6 cases (9.2%) did not achieve hemostasis using RADA16. Full data on the bleeding condition after the RADA16 application are shown in Table 2.
Table 2.
Condition After Application of PuraStat®.
| Status postapplication (s) | ITT patients n (%) |
|
|---|---|---|
| N = 65 | ||
| Missing | 0 | |
| Complete hemostasis | 59 (90.8%) | |
| Persistent bleeding | 6 (9.2%) | |
| Severity of persistent bleeding compared to before application | N = 6 | |
| Identical | 2 (33.3%) | |
| Decreased | 4 (66.7%) | |
| Worse | 0 (0.0%) | |
Abbreviation: ITT, Intent To Treat.
Primary Endpoint
The mean TTH reported was in seconds 83 ± 105 (range: 4-653 s), with a median time of 52 s. TTH was predictably greater in those with moderate bleeding compared with mild, 307 ± 266 s and 66 ± 63 s, respectively, and in those requiring additional applications of RAD16. In 49 cases, 1 application of RADA16 was used, with a mean TTH of 50 ± 35 s. The mean TTH in patients receiving 2 applications was 180 ± 79 s, and in those receiving 3 applications was 399 ± 256 s. Primary endpoint results for the ITT population are presented in Table 3.
Table 3.
Total Time-to-Hemostasis, Severity of Bleeding Pre-Application, and Number of Applications (ITT Population).
| Study primary endpoint ITT population N = 65 |
N | Missing | N (%) or mean ± SD [95% CI] |
Range |
|---|---|---|---|---|
| Total TTH (sec) | 59 | 0 | 83 ± 105 [55-110] | 4-653 |
| Number of patients per range of TTH | 59 | 0 | ||
| [ 4 (sec), 29 (Q25%)] | 14 (23.7%) | |||
| [ 29 (Q25%), 53 (Q50%)] | 16 (27.1%) | |||
| [ 53 (Q50%), 931 (Q75%)] | 15 (25.4%) | |||
| [ 91 (Q75%), 653 (Max)] | 14 (23.7%) | |||
| TTH per severity of bleeding (sec) | ||||
| Mild | 55 | 0 | 66 ± 63 | 5-401 |
| Moderate | 4 | 0 | 307 ± 266 | 4-63 |
| TTH per number of applications of PuraStat® (sec) | ||||
| 1 | 49 | 0 | 50 ± 35 | 4-66 |
| 2 | 7 | 0 | 180 ± 79 | 68-286 |
| 3 | 3 | 0 | 399 ± 256 | 142-653 |
Abbreviations: TTH: Time To Hemostasis; sec: seconds.
Secondary Endpoints
Secondary performance data included intraoperative blood loss, drainage volume at 24 h postprocedure and at discharge, rate, and quantity of transfusion of blood products received (intraoperatively at 24 h postoperatively and overall), and ease of use of PuraStat® during CEA.
The mean intraoperative blood loss was 127 ± 111.4 mL (95% CI: 99.2-154.8; range: 0-600 mL), with data missing for one case. The case recording 600 mL of blood loss required no specific further action and no adverse event was reported.
At 24 h, 60 patients (92.3%) had a surgical drain in situ. Five patients did not have a drain. The mean drainage volume was 49.0 ± 51.2 mL (range: 0-230 mL) for 52 patients and drainage volume data was missing in 13 patients. Two patients (3.1%) received blood products intraoperatively, with a mean volume of 580 ± 113.1 mL received. Neither of these was related to bleeding after the RADA16 application. No further blood products were received throughout the study period.
Full data for secondary performance endpoints are presented in Table 4.
Table 4.
Secondary Performance Endpoints: Blood Loss During Surgery, Drain Insertion and Volume Drained, and Transfusion Products Received.
| Secondary endpoints ITT population N = 65 |
N | Missing | (n/N)% or mean ± SD [95% CI] |
Range |
|---|---|---|---|---|
| Blood loss assessed during the surgery (mL) | 64 | 1 | 127 ± 111.4 [99.2-154.8] | 0.0-600.0 |
| Drainage volume (mL) until drain removal | ||||
| At 24-h postprocedure | 52 | 13 | 49 ± 51.2 [34.7-63.2] | 0.0-230.0 |
| At discharge | 1 | 64 | 60 | |
| Rate of transfusion of blood products | ||||
| At surgery | 65 | 0 | ||
| No | 63 (96.9%) | |||
| Yes | 2 (3.1%) [0.4%-10.7%] | |||
| At 24-h postoperation | 65 | 0 | ||
| No | 65 (100%) | |||
| Yes | 0 (0.00%) | |||
| At discharge | 65 | 0 | ||
| No | 65 (100%) | |||
| Yes | 0 (0.00%) | |||
| Overall | 65 | 0 | ||
| No | 63 (96.9%) | |||
| Yes | 2 (3.1%) [0.4%-10.7%] | |||
| Quantity of blood products and/or substitutes | ||||
| At surgery | 2 | 0 | 580 ± 113.1 | 500-660 |
| At 24-h postoperation | 0 | 0 | ||
| At discharge | 0 | 0 | ||
| Overall | 2 | 0 | 580 ± 113.1 | 500-660 |
Overall ease of use was deemed “good” or “excellent” in 93.8% of the patients (60.0% “excellent”). The ease of preparation, the application system, and the application of the gel were graded as “excellent” or “good” in 100%. The most valuable properties of PuraStat®, as recorded by surgeons, were its transparency and its overall ease of use.
Safety Outcomes
In 3 cases (4.6% [95% CI: 1.0%-12.9%] of the ITT population) there was postoperative bleeding requiring a return to the theatre; 2 within 24 h and one within 15 days. These events were resolved without sequelae and evaluated by the surgeons as related to the vascular procedure, with no causal relationship to RADA16. Adverse events (AEs) were described based on System Organ Class (SOC) in the Medical Dictionary for Regulatory Activities (MedDRA).27 After 1 month of follow-up, 24 AEs had been reported in 20 patients. Of these, there were 11 procedural complications, 6 nervous system disorders (including 3 strokes), 2 cardiac disorders, and one each of the following: ear and labyrinthine, gastrointestinal, general disorders, infection, and vascular disorders. Eleven AEs were reported at the time of the procedure, 5 within 24-h postprocedure, 3 within 15 days, and 5 at up to 1 month postprocedure. Seventeen patients (26.2%) had AEs related to their CEA, of these, 5 (7.7%) were deemed serious adverse events (SAEs). The hematoma was specifically reported in 6 cases (9.2%) at 24-h assessment, with one of these requiring surgical revisions at 24 h. Two further hematomas were recorded within 1 month of CEA but did not require additional treatment.
Overall, 7 AEs in 7 different patients (10.8%) were deemed SAEs by the investigators; of these, 3 were classed as stroke (1 hemorrhagic, 1 intraoperative, and 1 postoperative). The remaining 4 SAEs were bradycardia, a hematoma, pneumonia, and 1 death. The overall incidence of stroke was 4.6%. No SAEs were considered to be related to RADA16.
Two AEs were considered device-related. Of these, one was reported at 24-h postoperatively as a small hematoma that required no further action to resolve. The second was reported as a failure to achieve hemostasis after 3 applications of RADA16, requiring the additional hemostatic effort of compression of the carotid vessel with a surgical swab. Neither were classed as serious, and no device or product deficiencies were reported during the study.
The mean length of hospital stay for the study group was 4.3 ± 11 days (range: 0-85 days). The overall mortality at 1-month follow-up post-CEA was 1.5% [95% CI: 0.0%-8.3%].
Discussion
CEA is associated with an increased risk of bleeding intra- and postoperatively.1,12 This risk can be mitigated using the meticulous surgical techniques. In this study, once the anastomosis was complete, primary hemostasis was undertaken using methods at the lead surgeon's discretion to ensure the integrity of the suture line. Such methods would include simple pressure or further monofilament sutures, with or without pledgets, for bleeding in between existing sutures.
Techniques such as additional “rescue” sutures or electrocoagulation may be utilized to achieve primary hemostasis. Other methods that show hemostatic benefits include intraoperative neck flexion as well as simple postoperative direct neck pressure.11,28 Despite these options, the risk of bleeding is still relatively high, reported as 8.2% by Baracchini et al8 for eversion CEA. Increasingly, vascular surgeons are looking at hemostatic agents to aid in reducing this risk.13 The mode of action of PuraStat® is different from comparable products. Self-assembling peptides in contact with the tissues rapidly form a hydrogel, which acts as a barrier and blocks the flow of blood from the wound.14 PuraStat® has been used successfully as a hemostat in cardiovascular, gastrointestinal, otolaryngology, and endocrine surgery.
This study has demonstrated RADA16 (PuraStat®) to be an effective hemostatic agent in vascular surgery, successfully controlling suture site and anastomotic bleeding in 90.8% of cases. This has been achieved without prolonging the length of the procedure, while gaining favorable assessment from the surgeons in terms of the ease of use, with 93.8% of surgeons assessing its overall ease of use as “good” or “excellent.” Each of the surgeons operated on approximately the same number of patients and therefore there is no bias in this evaluation, which despite everything remains obviously subjective. Mean TTH, reported in 59 patients, was recorded as 83 ± 1058 s and the primary hemostatic efficacy rate was 90.8%. This predictably increased with the number of applications of PuraStat® required and the amount of bleeding present prior to application. Given the very small numbers that did not use the patch technique in this study (6% eversion and 3% direct closure), it would be difficult to glean any significant differences in the incidence of bleeding when comparing the different techniques. There is little in the literature to adequately compare the incidence of bleeding complications when different techniques are used. This is largely related to the fact that bleeding is relatively uncommon and so the numbers available to compare are too small to draw meaningful conclusions from. Gisbert et al29 did not find any difference in bleeding when comparing patch closure with eversion in a series of 455 patients.
The results are comparable to a study published in 2019 by Morshuis et al,17 demonstrating the performance and safety of PuraStat® in left ventricular assist device therapy support, where complete hemostasis was achieved in 93.1% of surgical sites with a mean TTH of 19.38 ± 13.01 s. The results also concur with the 2018 study by Giritharan et al16 that assessed the safety and efficacy of PuraStat® in a range of cardiac surgical procedures, reporting that PuraStat® was solely sufficient in achieving complete hemostasis in 84% of cases. When compared to other hemostatic agents available in cardiovascular surgery, TTH achieved with PuraStat® appears favorable. Nasso et al30 reported on a gelatin-thrombin-based hemostatic agent in cardiac surgery with a mean TTH of 3.8 ± 2.4 min and successful hemostasis achieved in 91.8% of patients. Few studies on the use of hemostatic agents in CEA exist. Pellenc et al31 report preliminary results for a poly(glycerol-sebacate) acrylate-based sealant for use in vascular reconstruction, achieving complete hemostasis in 84% of cases. It is noteworthy that the comparability of previously published data using TTH as an outcome measure may be challenging since varying definitions exist. Its calculation is also subjective to the operator and study populations may differ significantly. Stangenberg et al32 reported 1.7% of patients undergoing CEA between 2012 and 2013 who received a transfusion of blood products before discharge, while 2 patients in our study were transfused during surgery and none before discharge (3.1%). This should be taken in the context of the study populations, with most patients receiving PuraStat® having an ASA grade of 3 or more.
Adverse events were reported in 30.8% of cases in the 1-month follow-up period, of which only 2 (3.1%) were related to the device and neither were serious. The rate of reoperation for bleeding was reported as 4.6% (3 cases), comparable with results published by Weinrich et al33 who reported a reoperation rate of 3.5% after CEA in 565 patients. The mortality rate during the study period was 1.5% (1 patient) and was unrelated to the device. Kashyap et al34 reported a mortality rate of 0.7% after CEA in 371 patients.
Currently available hemostatic agents have the potential to enable transmission of viral and prion diseases from human blood component-based agents, and systemic inflammatory response syndrome to animal-derived agents.35,36 The purely synthetic nature of PuraStat® negates these risks. As summarized by Sankar et al,14 the completely synthetic nature and the transparent aspect of PuraStat® provide unique advantages. The transparency of PuraStat®, reported as its best feature in 70.8% of cases, allows unique visualization of the bleeding site after application, meaning potential revisions of primary hemostasis can take place intraoperatively instead of requiring subsequent re-exploration.14 The application method as a gel in a syringe with a nozzle may also enhance precision in a congested operating field. In addition, PuraStat® is ready to use in a prefilled syringe that is stored at refrigerator temperature and is thus immediately ready for use.
Vyas and Saha13 describe the ideal characteristics of a hemostatic agent as being one that could combine biodegradability, rapid action of hemostasis, and minimal side effects while preventing thrombosis. Lumsden and Heyman9 described their perceived ideal characteristics of a hemostat as “easily applied in a controlled fashion, highly predictable in creating hemostasis, and nontoxic and must not have an adverse effect on anastomotic patency.” This study on CEA has been able to demonstrate the safety and rapid efficacy of PuraStat®, alongside its inherent ability to be naturally absorbed by the body and the favorable opinion of surgeons in terms of its application and ease of use.
Limitations of this study include patients not being stratified according to operative technique and general risk factors, but the study centers and the cohort of patients were homogenous. The proportion of patients with an ASA grade greater than 3 was significantly high and could well have impacted on the results reported. Any further analysis and comparison should take this into account. A follow-up study with risk scores stratified to evaluate their effects on PuraStat® efficacy and safety may be useful.
TTH, although an accepted outcome measure for hemostasis, can be defined in a variety of ways and is a possible cofounder. The reporting of “complete hemostasis” is also subjective to the observer operating the stopwatch, but evaluation of complete hemostasis is common to all surgeons and TTH has been widely used to assess the hemostasis efficacy, both in the preclinical and clinical setting. However, the development of a well-structured definition for TTH would be beneficial. A systematic review of hemostasis in vascular surgery to identify key outcomes and outcome measures on this topic, along with subsequent development of a core outcome set could help to provide unified direction in this area of research and improve the comparability of studies.
Conclusion
This multicentre, postmarket clinical follow-up study was able to confirm the safety and efficacy of 3-D Matrix's PuraStat® self-assembling peptide (RADA16) in the management of bleeding during elective CEA and opens a promising new perspective to manage suture bleeding in carotid endarterectomy. PuraStat® provides a safe and effective option to aid hemostasis in surgical situations where standard means of hemostasis are insufficient or impractical. The efficacy is demonstrated through the results achieved with a mean TTH of 83 s and a complete hemostasis postapplication in 90.8% of cases. No SAEs related to the device, or its application confirm its safety, alongside low volumes of intraoperative blood loss and the subsequent need for blood transfusion. However, the efficacy of long-term hemostasis and reduction of relevant hematoma need further evaluation in a larger cohort with a prospective two-armed study design.
Acknowledgments
All of the authors would like to thank Dr Maurice Bagot d’Arc of BluePharm for his invaluable help in preparing the manuscript for publication.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval: The study was conducted in accordance with the ethical principles that have their origins in the Declaration of Helsinki and the requirements for medical device investigations as presented in EN/ISO 14155:2011, Clinical investigation of medical devices for human subjects—Good clinical practice; Annex X of the European Medical Devices Directive 93/42/EEC, as amended by Directive 2007/47/EEC, MEDDEV 2.7/4 and applicable local regulatory requirements. Ethics approval was obtained from all centers. The registration number at HRA 17/LO/0082 was granted in March 2017and the study was registered under the ClinicalTrials.gov Identifier: NCT03103282.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Katherine M. Stenson https://orcid.org/0000-0002-1795-4578
References
- 1.Saha SP, Saha S, Vyas K. Carotid endarterectomy: current concepts and practice patterns. Int J Angiology. 2015;24(3):223-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jim J, Fairman R. Complications of carotid endarterectomy [Internet]. UpToDate; 2020. [cited 2020 Dec 11]. Available from: https://www.uptodate.com/contents/complications-of-carotid-endarterectomy [Google Scholar]
- 3.North American Symptomatic Carotid Endarterectomy Trial (NASCET) Steering Committee. North American symptomatic carotid endarterectomy trial: Methods, patient characteristics, and progress. Stroke. 1991;22(6):711-720. [DOI] [PubMed] [Google Scholar]
- 4.European Carotid Surgery Trialists’ Collaborative Group. Randomised trial of endarterectomy for recently symptomatic carotid stenosis: final results of the MRC European carotid surgery trial (ECST). Lancet. 1998;351(9113):1379-1387. [PubMed] [Google Scholar]
- 5.National Institute for Health and Care Excellence. Stroke and transient ischaemic attack in over 16s: diagnosis and initial management [Internet]. NICE; 2019, 2019 May 1. (NICE guideline [NG128]). [PubMed] [Google Scholar]
- 6.Barnett H, Taylor D, Eliasziw M, et al. Benefit of carotid endarterectomy in patients with symptomatic moderate or severe stenosis. N Engl J Med. 1998;339(20):1415-1425. [DOI] [PubMed] [Google Scholar]
- 7.Jones DW, Goodney PP, Conrad MF, et al. Dual antiplatelet therapy reduces stroke but increases bleeding at the time of carotid endarterectomy. J Vasc Surg. 2016;63(5):1262-1270.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baracchini C, Gruppo M, Mazzalai F, Lorenzetti R, Meneghetti G, Ballotta E. Predictors of neck bleeding after eversion carotid endarterectomy. J Vasc Surg. 2011;54(3):699-705. [DOI] [PubMed] [Google Scholar]
- 9.Lumsden A, Heyman E. Prospective randomized study evaluating an absorbable cyanoacrylate for use in vascular reconstructions. J Vasc Surg. 2006;44(5):1002-1009. [DOI] [PubMed] [Google Scholar]
- 10.McCready R, Siderys H, Pittman J, et al. Delayed postoperative bleeding from polytetrafluoroethylene carotid artery patches. J Vasc Surg. 1992;15(4):661-663. [DOI] [PubMed] [Google Scholar]
- 11.Tamaki T, Morita A. Neck haematoma after carotid endarterectomy: risks, rescue, and prevention. Br J Neurosurg. 2019;33(2):156-160. [DOI] [PubMed] [Google Scholar]
- 12.Ferguson G, Eliasziw M, Barr H, et al. The North American symptomatic carotid endarterectomy trial: surgical results in 1415 patients. Stroke. 1999;30(9):1751-1758. [DOI] [PubMed] [Google Scholar]
- 13.Vyas KS, Saha SP. Comparison of hemostatic agents used in vascular surgery. Expert Opin Biol Ther. 2013;13(12):1663-1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sankar S, O’Neill K, Bagot D’Arc M, et al. Clinical use of the self-assembling peptide RADA16: a review of current and future trends in biomedicine. Front Bioeng Biotechnol. 2021;2(9):679525. doi: 10.3389/fbioe.2021.679525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Masuhara H, Fujii T, Watanabe Y, Koyama N, Tokuhiro K. Novel infectious agent-free hemostatic material (TDM-621) in cardiovascular surgery. Ann Thorac Cardiovasc Surg. 2012;18(5):444-451. [DOI] [PubMed] [Google Scholar]
- 16.Giritharan S, Salhiyyah K, Tsang G, Ohri S. Feasibility of a novel, synthetic, self-assembling peptide for suture-line haemostasis in cardiac surgery. J Cardiothorac Surg. 2018;13(1):68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morshuis M, Schönbrodt M, Gummert J. Safety and performance of a self-assembling peptide haemostat for the management of bleeding after left ventricular assist device implantation: outcomes of a post market clinical follow-up study. J Heart Lung Transplant. 2019;38(4):S194. [Google Scholar]
- 18.Nahm C, Popescu I, Botea F, et al. A multi-center post-market clinical study to confirm safety and performance of PuraStat® in the management of bleeding during open liver resection. HPB (Oxford). 2021;24(5):700-707. doi: 10.1016/j.hpb.2021.09.020. [DOI] [PubMed] [Google Scholar]
- 19.Subramaniam S, Kandiah K, Chedgy F, et al. A novel self-assembling peptide for hemostasis during endoscopic submucosal dissection: a randomized controlled trial. Endoscopy 2020;53(1):27-35. doi: 10.1055/a-1198-0558. [DOI] [PubMed] [Google Scholar]
- 20.Uraoka T, Ochiai Y, Fujimoto A, et al. A novel fully synthetic and self-assembled peptide solution for endoscopic submucosal dissection-induced ulcer in the stomach. Gastrointest Endosc. 2016;83(6):1259-1264. [DOI] [PubMed] [Google Scholar]
- 21.de Nucci G, Reati R, Arena I, et al. Efficacy of a novel self-assembling peptide hemostatic gel as rescue therapy for refractory acute gastrointestinal bleeding. Endoscopy. 2020;52(9):773-779. [DOI] [PubMed] [Google Scholar]
- 22.Lee MF, Ma Z, Ananda A. A novel haemostatic agent based on self-assembling peptides in the setting of nasal endoscopic surgery, a case series. Int J Surg Case Rep. 2017;41:461-464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Friedland Y, Ha J, Bagot d’Arc M, Delin C. ‘The use of self-assembling peptides (PuraStat™) in functional endoscopic sinus surgery for haemostasis and reducing adhesion formation. A case series of 94 patients. Surg Technol Int. 2022;41:sti41/1594. [DOI] [PubMed] [Google Scholar]
- 24.Gangner Y, Bagot d’Arc M, Delin C. The use of self-assembling peptides (PuraStat) for hemostasis in cervical endocrine surgery. A real-life case series of 353 patients. Int J Surg Case Rep. 2022;94:107072. doi: 10.1016/j.ijscr.2022.107072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Galyfos G, Geropapas G, Kerasidis S, Kastrisios G. Carotid endarterectomy: which technique prevails? J Vasc Endovascular Surg. 2016;1(1):5. [Google Scholar]
- 26.American Society of Anesthesiologists. ASA Physical Status Classification System. [Internet]. 2020 [cited 2020 Dec 15]. Available from: https://www.asahq.org/standards-and-guidelines/asa-physical-status-classification-system
- 27.MedDRA. MedDRA Introductory Guide Version 14.0. [Internet]. 2011 [cited 2020 Dec 15] Available from: https://www.who.int/medical_devices/innovation/MedDRAintroguide_version14_0_March2011.pdf
- 28.Saghir R, Humm G, Rix T. Haematomas after carotid endarterectomy can be reduced by direct pressure to the neck postoperatively. Ann R Coll Surg Engl. 2018;100(7):580-583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gisbert SM, Sala Almonacil V, Zaragozá García JM, Genovés Gascó B, Gómez Palonés FJ, Ortiz Monzón M. Predictors of cervical bleeding after carotid endarterectomy. Ann Vasc Surg. 2014;28(2):366-374. doi: 10.1016/j.avsg.2013.04.011. Epub 2013 Sep 29. [DOI] [PubMed] [Google Scholar]
- 30.Nasso G, Piancone F, Bonifazi R, et al. Randomized clinical trial of the FloSeal matrix sealant in cardiac surgery. Ann Thorac Surg. 2009;88(5):1520-1526. [DOI] [PubMed] [Google Scholar]
- 31.Pellenc Q, Touma J, Coscas R, et al. Preclinical and clinical evaluation of a novel synthetic bioresorbable, on-demand, light-activated sealant in vascular reconstruction. J Cardiovasc Surg. 2019;60(5):599-611. [DOI] [PubMed] [Google Scholar]
- 32.Stangenberg L, Curran T, Shuja F, Rosenberg R, Mahmood F, Schermerhorn ML. Development of a risk prediction model for transfusion in carotid endarterectomy and demonstration of cost-saving potential by avoidance of “type and screen”. J Vasc Surg. 2016;64(6):1711-1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weinrich M, Schindler P, Kundt G, Klar E, Bünger CM. Influence of local hemostatic and antiplatelet agents on the incidence of bleeding complications in carotid endarterectomies. Clin Hemorheol Microcirc. 2014;58(1):71-79. [DOI] [PubMed] [Google Scholar]
- 34.Kashyap V, King AH, Foteh MI, et al. A multi-institutional analysis of transcarotid artery revascularization compared to carotid endarterectomy. J Vasc Surg. 2019;70(1):123-129. [DOI] [PubMed] [Google Scholar]
- 35.Despotis G, Avidan M, Eby C. Prediction and management of bleeding in cardiac surgery. J Thromb Haemostasis. 2009;7(s1):111-117. [DOI] [PubMed] [Google Scholar]
- 36.Taflampas P, Sanidas E, Christodoulakis M, Askoxylakis J, Melissas J, Tsiftsis DD. Sealants after axillary lymph node dissection for breast cancer: good intentions but bad results. Am J Surg. 2009;198(1):55-58. [DOI] [PubMed] [Google Scholar]