Abstract
Introduction
Atrial Fibrillation (AF) is the most common cardiac arrythmia in the world. Structural remodeling and fatty acid metabolism dysregulation are believed to play a role in the development of AF. This study explored different biomarkers in the blood of AF patients and a control population to determine if there was a significant difference between the two groups.
Material and Methods
Plasma samples were collected from 73 patients with confirmed diagnosis of AF from Loyola University Clinic. Control group represented commercially available plasma (n = 50). Sandwich ELISA kits were used to quantify the collagen remodeling proteins and liver type fatty acid binding protein (L-FABP) in the AF population and the control population. Non-esterified fatty acids (NEFAs) were measured using an enzymatic colorimetric kit from Wako Diagnostics. Statistical analyses were performed using GraphPad Prism.
Results
All the collagen remodeling biomarkers were significantly higher in AF patients compared to the control group. The fatty acid dysregulation biomarkers were elevated in the AF patients. Spearman correlation analyses yielded significant correlations between L-FABP and TIMP-1 (r = 0.47, P < 0.001), NEFA and TIMP-2 (r = 0.41, P = 0.002), NEFA and ICTP (r = 0.41, P =0 .002), and NEFA and PIIINP (r = 0.61, P < 0.0001).
Summary and Conclusions
The elevation of collagen remodeling biomarkers suggests an upregulation of these biomarkers and their potential role in AF, which may contribute to atrial fibrosis. L-FABP and NEFAs were elevated in AF patients. The correlations between the collagen remodeling and fatty acid dysregulation biomarkers may be due to their involvement in structural remodeling of the atria.
Keywords: atrial fibrillation, collagen turnover peptides, fatty acid binding protein, non-esterified fatty acids, metalloproteases
Introduction
Atrial fibrillation (AF) is now the most common cardiac arrythmia around the world and has developed into a cardiovascular epidemic over the past few decades.1,2 The United States spends around $26.1 billion each year towards the clinical management of AF.3 Many complications, such as heart failure, strokes, and even death, are commonly seen in AF. Patients with AF suffer from a two-times increased risk of mortality secondary to thrombotic complications induced by the disease.4,5 It is believed that up to 15% of all strokes occurring worldwide are associated with AF, due to the hypercoagulable nature of the disease.6 Since the number of cases of AF are quickly rising, it is vital to develop more useful ways to prevent, diagnose and manage the disorder. Despite the burden that AF places on the medical system, the complete molecular pathophysiology has never been fully elucidated. A multifactorial pathogenesis is likely involved in the development of AF. Inflammation and structural remodeling are some factors believed to be important contributors towards the emergence of AF.7 Another factor that may play a role in the development and progression of AF is cardiac fatty acid metabolism dysregulation.8
Atrial structural remodeling, in the form of fibrosis, is one of the main contributors towards the development of AF.9 Fibrosis is a multifactorial process that is still not clearly understood. Collagen and other extracellular matrix (ECM) proteins deposit into the atrial walls leading to impaired conduction and contraction.10 Along with proteins, long-chain fatty acids may also be involved in cardiac structural remodeling and fibrosis.11 Currently, noninvasive imaging modalities are used to measure the extent of atrial fibrosis, including echocardiography and delayed contrast enhancement cardiac magnetic resonance imaging (DCE-CMRI). However, both techniques have their limitations. Cost, interobserver variability, differences in software manufacturers, and presence of implanted medical devices in patients are some of the limitations.12,13 Quantifying plasma collagen remodeling and fatty acid metabolism biomarker levels may provide an alternative way to detect atrial fibrosis with less limitations and at a cheaper cost.
Fibrosis is one of the heart's main adaptive mechanisms to injury. Collagen is the most well-known ECM protein in fibrotic tissue. Collagen plays a pivotal role in providing structure and strength to tissue. Therefore, even minor changes in myocardial collagen levels can have a significant impact on myocardial stiffness and contraction.14 The most abundant collagens found in the heart are types I and III.15 Collagen is produced by fibroblasts in the procollagen form. Post-translational modifications then take place. The N-terminal (amino) and C-terminal (carboxyl) ends are proteolytically cleaved off the procollagen molecules. This leads to the release of procollagen I carboxyterminal propeptide (PICP), procollagen I aminoterminal propeptide (PINP), procollagen III carboxyterminal propeptide (PIIICP), procollagen III aminoterminal propeptide (PIIINP), and type I collagen degradation product (ICTP) into the blood.16 If these collagen remodeling biomarkers are elevated in patient plasma, that could indicate that there is increased collagen turnover. Multiple previous studies show that the levels of collagen remodeling biomarkers are elevated in AF patients, compared to control populations.17–19 Another class of biomarkers that may be useful in AF are the matrix metalloproteinases (MMPs) and their inhibitors, tissue inhibitors of matrix metalloproteinases (TIMPs). MMPs play a critical role in regulating ECM turnover, including collagen remodeling. The balance between MMP and TIMP activity in the body is important because it decides the extent of ECM turnover, including in the atria.20 Upregulation of MMP activity and/or downregulation of TIMP activity may lead to increased atrial fibrosis. A prior experiment showed that MMP-2 activity was increased, and TIMP-2 activity was decreased in explanted atrial tissue from AF patients compared to a control population.21 The importance of collagen turnover in the development of AF led to our investigation on the potential usefulness of PICP, PINP, PIIICP, PIIINP, ICTP, MMP-1, MMP-2, TIMP-1, and TIMP-2 in noninvasively detecting the extent of atrial fibrosis.
Along with collagen and other ECM proteins, non-esterified fatty acids (NEFAs) may also deposit in the walls of the atria. Higher serum NEFA levels lead to epicardial adipose tissue formation, which may increase the risk for cardiovascular disorders such as AF.11 A prior study showed a positive association between plasma NEFA levels and risk of AF development among a group of 4000 patients in a Cardiovascular Health Study.22 Therefore, plasma NEFA levels may be a useful noninvasive way to measure risk for epicardial adipose tissue formation. NEFAs, also known as long-chain fatty acids, are the predominant metabolic energy source used by cardiac tissue. To avoid the accumulation of NEFAs in cardiomyocytes, cytosolic fatty acid binding proteins (FABPs) bind to free NEFAs and transport them to different intracellular locations. Different isoforms of FABPs exist in the body, including heart-FABP (H-FABP or FABP3). If there is damage to cardiac tissue, such as in AF, H-FABPs are released into the circulation. H-FABPs are currently used as a marker for early myocardial infarction detection.23 Therefore, H-FABP may serve as a biomarker for the early detection of AF as well. Along with H-FABP, liver FABP (L-FABP or FABP1) and adipocyte (A-FABP or FABP4) have also been implicated in cardiovascular disorders.11,24
Materials and Methods
Blood samples from 73 AF patients at the Loyola University Medical Center were collected under an approved IRB protocol (Dr Mushabbar Syed, IRB #205530). The demographic details of the AF cohort are shown in Table 1. The blood samples were collected in tubes containing 3.2% sodium citrate and the tubes were then centrifuged at 3000 × g for 15 min. The plasma supernatant was then separated, aliquoted, and frozen at −80°C until the samples were analyzed. All the samples were identified by the patient's study number under an IRB-approved protocol, with no identifying information labeled on the vials. The samples were then blindly analyzed in the Hemostasis and Thrombosis Research Laboratories at the Loyola University Medical Center. Controls were comprised of 50 normal human plasma (NHP) samples obtained from a commercial vendor, George King Biomedical (Overland Park, Kansas).
Table 1.
Summary of the Atrial Fibrillation Patients’ Demographic Information.
| Demographic categories | N | Mean ± SD | Percentage of the population |
|---|---|---|---|
| Age | 59 | 62.8 ± 11.1 | |
| Gender | |||
| Male | 42 | 71.2% | |
| Female | 17 | 28.8% | |
| BMI | 31.97 ± 5 | ||
| Smoking status | |||
| No smoking history | 37 | 62.7% | |
| Former Smoker | 20 | 33.9% | |
| Current smoker | 1 | 1.7% | |
| Anticoagulation Status | |||
| Not on anticoagulation | 6 | 10.2% | |
| On anticoagulation | 53 | 89.8% | |
| Comorbidities | |||
| Diabetes Mellitus II | 10 | 16.9% | |
| Hypertension | 36 | 61.0% | |
| Acute Coronary Syndrome | 14 | 23.7% | |
| Stroke/TIA | 4 | 6.8% | |
| Peripheral Artery Disease | 2 | 3.4% | |
| Chronic Kidney Disease | 3 | 5.1% | |
| Heart Failure | 4 | 6.8% | |
| Dyslipidemia | 33 | 55.9% | |
N represents the number of patients. The percentage of the population column represents the percent of the population within the respective demographic category.
Abbreviations: SD, standard deviation.
Commercially available sandwich enzyme-linked immunosorbent assay (ELISA) kits were used to quantify the collagen remodeling biomarkers, including PICP, PINP, ICTP, PIIINP, PIIICP, MMP-1, MMP-2, TIMP-1, and TIMP-2 in the AF and control plasma samples. Some of the ELISA kits required different amounts of plasma. Therefore, samples that did not have enough plasma left over were excluded from that ELISA experiment. The sandwich ELISA kits were purchased from Aviva Systems Biology (San Diego, California), R&D Systems (Minneapolis, Minnesota), Neobiolabs (Cambridge, Massachusetts), and Antibodies On-line.
In regards to the fatty acids in our study, NEFAs were measured using the NEFA-HR 2 kit purchased from Fujifilm Wako Diagnostics (Mountain View, California). The NEFA-HR2 kit is an enzymatic colorimetric test. After following the manufacturer's directions, a purple-colored final product was formed. The purple chromogen was then colorimetrically measured at 550 nm to determine the amount of NEFAs present in the plasma samples. Any patients receiving heparin therapy were excluded from the analysis because the NEFA-HR2 kit cannot be used on samples containing heparin. L-FABP levels were quantified using a sandwich ELISA kit obtained from Diapharma Group (West Chester, Ohio). If there was not an adequate amount of plasma left over in an AF patient sample, that patient was excluded from the experiment.
Statistical analyses were performed using Microsoft Excel and GraphPad Prism Software. For each biomarker, we calculated the mean ± SEM for the AF samples and the NHP. We also calculated the percent change between the AF mean and the NHP mean for each biomarker using the Mann–Whitney U test. A P-value <0 .05 was considered statistically significant. A spearman correlation analysis was then performed to test for interrelationship between the biomarkers. Finally, spearman correlation plots were compiled for any biomarker pair showing a significant correlation.
Results
Collagen Metabolism Biomarkers in the Control Population versus AF Patients
A comparison of collagen turnover biomarkers in the AF cohort (n = 58–73) with normal controls (n = 50) is shown in Figure 1. This composite graph shows that the collagen turnover biomarkers, such as PICP, PIIICP, and PIIINP are significantly elevated in the AF patients. As can be seen, in comparison to NHP, all the biomarkers in the AF cohort were upregulated. The most pronounced increase was seen in PIIICP, while TIMP-2 showed the lowest percent change. The AF cohort exhibited a wide range in each of the biomarkers, in comparison to NHP.
Figure 1.
Concentrations of some of the collagen turnover biomarkers in the AF cohort compared to NHP. Results are reported as means with the errors bars representing the standard error of the mean. (A) The comparison of PICP; (B) The comparison of PIIICP; (C) The comparison of PINP; (D) The comparison of PIIINP; (E) The comparison of ICTP. Abbreviations: NHP, normal human plasma; AF, atrial fibrillation; PICP, procollagen I carboxyterminal propeptide; PIIICP, procollagen III carboxyterminal propeptide; PINP, procollagen I aminoterminal propeptide; PIIINP, procollagen III aminoterminal propeptide; ICTP, type I collagen degradation product.
For ICTP, the range in the AF group was 7.28 ng/mL and the range in the NHP was 2.37 ng/mL. In regards to PICP, the range in the AF group was 10.04 ng/mL and the range in the NHP was 2.78 ng/mL. The range for PINP was 1532.68 and 348.48 ng/mL for the AF group and NHP, respectively. For PIIICP, the range in the AF group was 13.6 ng/mL and the range in the NHP was 1.1 ng/mL. PIIINP showed a range of 207.33 and 9.1 ng/mL in the AF group and NHP, respectively. For MMP-1, the range in the AF group was 10.28 ng/mL and the range in the NHP was 2.43 ng/mL. MMP-2 showed a range of 249.72 and 160.6 ng/mL for the AF cohort and NHP, respectively. TIMP-1 showed a range of 583.08 and 149.91 ng/mL in the AF samples compared to NHP, respectively. For TIMP-2, the range in the AF group was 81.55 ng/mL and the range in the NHP was 48.96 ng/mL.
Table 2 shows a composite description of the individual biomarkers, including the matrix metalloproteases, tissue inhibitors of the matrix metalloproteases, collagen turnover peptides, fatty acid binding proteins, and non-esterified fatty acids. As tabulated, all these biomarkers were increased in the AF cohort. The most dramatic increase was noted in the collagen turnover peptides, PIIICP and PIIINP.
Table 2.
Comparison of the Collagen Remodeling Biomarkers and Fatty Acid Metabolism Dysregulation Biomarkers.
| Biomarker | AF mean ± SEM (SD) | NHP mean ± SEM (SD) | P-values | % Change |
|---|---|---|---|---|
| MMP-1 (ng/mL) | 1.347 ± 0.19 (1.519) | 0.55 ± 0.07 (0.495) | <0.0001 | 146.25% |
| MMP-2 (ng/mL) | 263.5 ± 6.41 (55.17) | 183.4 ± 5.58 (39.45) | <0.0001 | 43.70% |
| TIMP-1 (ng/mL) | 125.9 ± 9.0 (76.45) | 63.97 ± 3.99 (28.18) | <0.0001 | 96.80% |
| TIMP-2 (ng/mL) | 68.79 ± 2.1 (17.8) | 61.64 ± 1.7 (12.05) | 0.0328 | 11.60% |
| ICTP (ng/mL) | 4.0 ± 0.13 (1.083) | 1.4 ± 0.07 (0.4611) | <0.0001 | 186.20% |
| PICP (ng/mL) | 1.40 ± 0.29 (2.287) | 0.68 ± 0.08 (0.5847) | 0.0042 | 104.80% |
| PINP (ng/mL) | 422.3 ± 43.8 (350.3) | 159.9 ± 9.6 (67.94) | <0.0001 | 164.10% |
| PIIICP (pg/mL) | 1435 ± 288 (2461) | 147.8 ± 39.2 (277.1) | <0.0001 | 870.90% |
| PIIINP (ng/mL) | 26.36 ± 4.3 (36.1) | 2.82 ± 0.35 (2.484) | <0.0001 | 833.80% |
| L-FABP (ng/mL) | 9.09 ± 1.18 (9.786) | 5.12 ± 0.25 (1.768) | <0.0001 | 77.50% |
| NEFAs (mEq/L) | 0.87 ± 0.06 (0.4742) | 0.6 ± 0.06 (0.3045) | 0.0174 | 45.70% |
Fatty Acid Metabolism Biomarkers in the Control Population versus AF Patients
A comparison of L-FABP and NEFA levels in the AF cohort and NHP is depicted in Figure 2. This figure shows that both NEFA and L-FABP were significantly elevated in the AF population. The p-values for both analyses were less than 0.02. This figure clearly demonstrates that both L-FABP and NEFA are upregulated in AF.
Figure 2.
Comparison of the fatty acid dysregulation biomarkers between the AF patients and NHP. Results are reported as means with the errors bars representing the standard error of the mean. (A) The comparison of L-FABP; (B) The comparison of NEFAs. Abbreviations: L-FABP, liver type fatty acid binding protein; NEFA, non-esterified fatty acids.
Spearman Correlation Analysis
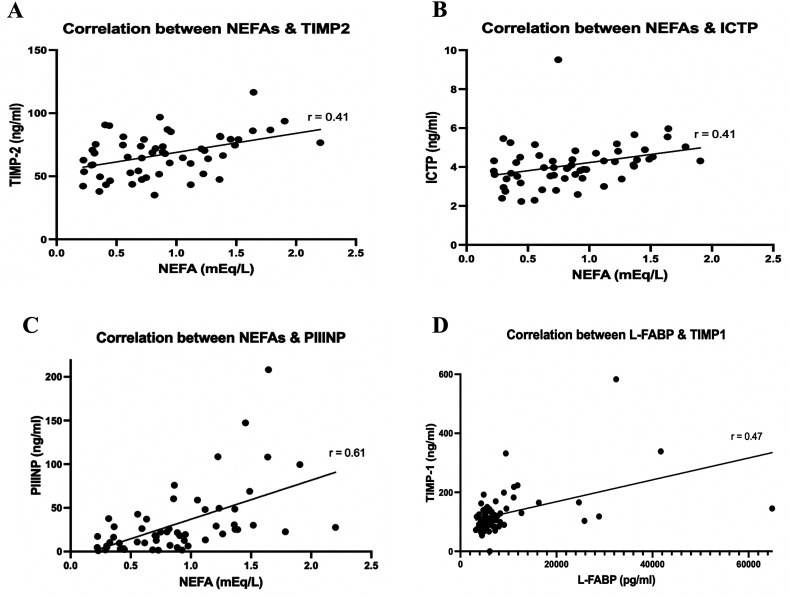
A spearman correlation matrix comparing various biomarkers in the AF cohort is shown in Figure 3. Varying degrees of correlation were noted among these biomarkers. The range in r-values was 0.05–0.61. NEFA showed the strongest correlation with PIIINP with an r-value of 0.61, followed by L-FABP and TIMP-1 with an r-value of 0.47. NEFA also showed modest correlation with TIMP-2 (r = 0.41) and ICTP (r = 0.41). The correlation plots for each biomarker pair are shown in Figure 4.
Figure 3.
Spearman correlation matrix illustrating some of the notable correlations between different biomarkers. Blue boxes indicate positive correlations, while red boxes indicate negative correlations. The numbers in the box represent the r value, otherwise known as the correlation coefficient.
Figure 4.
Spearman correlation plots for various biomarker pairs. (A) The correlation between NEFAs and TIMP-2; (B) The correlation between NEFAs and ICTP; (C) The correlation between NEFAs and PIIINP; (D) The correlation between L-FABP and TIMP-1.
Discussion
Current data regarding the clinical utility of plasma biomarkers in the diagnosis, management, and prognosis of AF is limited. Currently, clues to AF diagnosis may be seen during the physical exam, such as an irregular pulse or irregularly irregular heart rhythm on cardiac auscultation. More commonly, AF is diagnosed via an electrocardiogram demonstrating an irregularly irregular rhythm. A significant concern is that AF is commonly found incidentally. Clinical signs and symptoms may not be evident. It is important to be able to diagnose AF early due to the risk of developing other complications, such as heart failure and thrombotic events. The goal of this study was to evaluate if collagen remodeling biomarkers and fatty acid metabolism biomarkers could be useful in assessing the severity of atrial fibrosis and adipose tissue deposition, both of which are implicated in the pathogenesis of AF.11,17–19,22 We measured the levels of each biomarker in plasma obtained from AF patients and normal human plasma purchased from a commercial vendor. We hypothesized that the levels of the collagen remodeling biomarkers and fatty acid metabolism biomarkers would be elevated in the AF samples, compared to the control plasma samples.
The role of collagen turnover in the pathogenesis of AF has been discussed extensively in previous publications. The exact mechanism underlying atrial fibrosis is not completely understood. However, it has been shown that interstitial fibrosis, a form of structural remodeling, plays a significant role in the development of AF.9,25 Several profibrotic factors, including angiotensin II and transforming growth factor-β1, are involved in the downstream activation of molecules involved in collagen turnover, such as MMPs and TIMPs.25 Since collagen I and III are the most abundant collagens in the myocardium,15 we can potentially measure collagen levels or plasma collagen degradation products as a biomarker for increased collagen remodeling. For example, a study in China showed that patients with AF and mitral valve disease had higher levels of collagen I and III than patients with sinus rhythm and mitral valve disease.26 Another study illustrated, through the process of immunohistochemistry, that collagen I was elevated in patients with AF compared to those in normal sinus rhythm.27 Polyakova et al also showed an increase in multiple collagen remodeling biomarkers, such as PINP, PIIINP, ICTP, MMP-2, and TIMP-1 in right atrial wall samples from patients with AF compared to tissue samples isolated from patients in normal sinus rhythm.20 Multiple other studies also showed that levels of collagen remodeling biomarkers were elevated in patients with AF compared to those unaffected by AF.17–19 An interesting experiment from 2007 that contrasted the gene expression of two canine models indicated that there was a 10-fold upregulation of eight collagen-genes and a 4.5-fold increase in MMP-2 expression in left atrial tissue isolated from animals with AF.28 All these previous studies support the hypothesis that atrial collagen remodeling could be contributing to the pathogenesis of AF and that collagen remodeling biomarkers could be useful in the assessment of AF.
Along with ECM proteins, such as collagen, NEFAs may also be implicated in structural remodeling of the atria via the development of epicardial adipose tissue.11 The mechanism through which fatty acid metabolism dysregulation leads to AF remains unknown. Currently, it is known that NEFAs are the main metabolic energy source utilized by the myocardium. NEFAs are a broad classification and include many different types of fatty acids. A previous prospective evaluation by Pellegrini et al.29 measured the concentration of NEFAs in 1872 serum samples isolated from patients unaffected by AF at an annual examination. The patients were then followed to determine which of them developed AF. The researchers found that only two of the 35 NEFAs isolated via gas chromatography showed significant correlation with the development of AF. They concluded that higher amounts of nervonic acid (24:1n-9) were associated with increased incidence of AF, but higher concentration of GLA (18:3n-6) was associated with a decreased risk of AF development.29 Another prospective study referenced in my introduction showed that increased levels of plasma free fatty acids were associated with development of AF.22 As previously stated, FABPs are also involved in fatty acid metabolism. For that reason, they may also be used as biomarkers to monitor for cardiovascular disease. A previous study from 2017 used a novel custom proteomics chip to explore new biomarkers for AF.30 They discovered four proteins that could be important in the development of AF, one of them being FABP4.30 An earlier study demonstrated that increased levels of FABP4 were associated with an increased risk of AF recurrence following catheter ablation.31 The other isoforms of FABPs have also been shown to be involved in AF in some studies. Otaki et al.32 illustrated that patients with congestive heart failure and AF had higher plasma levels of FABP3 compared to patients with congestive heart failure and sinus rhythm. The previous study also showed that patients with higher levels of FABP3 had an increased rate of future cardiovascular events.32 A manuscript published by Rader et al indicated that a greater rise in FABP3 levels after cardiac surgery was associated with an increased incidence of post-operative AF.33 However, a study performed by Shingu et al.34 revealed that levels of FABP3 were decreased in patients that developed post-operative AF compared to those that did not develop AF. Overall, it seems that the data regarding the utility of NEFAs and FABPs as plasma biomarkers for AF is limited.
Some of our findings regarding the collagen remodeling biomarkers are depicted in Figure 1. Bar graphs were used to illustrate the difference in the means of some of the collagen biomarkers in the plasma of the AF group compared to NHP. The graphs show a statistically significant elevation in the collagen metabolism biomarkers, including PICP, PIIICP, PINP, PIIINP, and ICTP, in the AF population compared to the control samples. Table 2 summarizes the findings in tabular form, with the associated percent change for each biomarker studied. The results in Table 2 also include our findings for the other collagen remodeling biomarkers, MMP-1, MMP-2, TIMP-1, and TIMP-2. We yielded a statistically significant increase of all the collagen remodeling biomarkers in the AF cohort compared to the NHP. These findings indicate that there is an upregulation of collagen degradation and deposition in patients with AF. Our discovery supports the previous experiments that also demonstrated an increase in collagen remodeling biomarkers in patients diagnosed with AF.17–21,26–28 However, our study noted an increase in TIMP-2, while a previous study showed a decrease in TIMP-2 in AF patients.21 Although all the collagen remodeling biomarkers were elevated in the AF patients, some of the biomarkers were much more extensively elevated than others. For example, PIIINP and PIIICP were elevated over eight-fold in the AF group compared to the NHP, while PINP and PICP were only increased about one–two fold in the AF group. This could indicate that collagen type III may play a more significant role in the pathogenesis of AF compared to collagen type I. Overall, our findings may help support the hypothesis that collagen remodeling is involved in the pathogenesis of AF. Our results may also show that the collagen remodeling biomarkers could serve as a useful tool to determine the extent of atrial fibrosis. If these biomarkers can help detect increased atrial fibrosis early, it may give us a noninvasive way to assess for development of AF prior to symptom onset.
Table 2 also shows the mean concentrations of the fatty acid metabolism biomarkers, NEFAs and FABP, in the AF cohort compared to the NHP with associated percent changes. Figure 2 shows these findings in the form of bar graphs. We found a statistically significant elevation in NEFAs and L-FABPs in the plasma of the AF population compared to the control population. These findings are consistent with prior experiments that showed elevations of NEFAs in AF patients.22,29 However, the previous experiments showed elevations in different isoforms of FABP, such as FABP3 and FABP4, than the one we measured in our experiment.31–33 Currently available data is limited on the role of L-FABP in AF. Our results may indicate that all FABPs, independent of the isoform, may play a role in AF. Regardless, our findings underscore the theory that excess fatty acids could also be contributing to AF via the formation of atrial fibrosis and epicardial adipose tissue. FABP and NEFAs may be helpful as fatty acid metabolism biomarkers that can help estimate the severity of epicardial adipose tissue formation. With this information, we can assess for possible increased risk of AF development due to the arrhythmic nature of epicardial adipose tissue. However, imaging data utilizing techniques such as MRI and echocardiography would be useful to demonstrate the potential correlation of these biomarkers with the observed remodeling of the cardiac muscle.
Figure 3 depicts a spearman correlation matrix between the different biomarkers to assess for any significant correlations. The notable correlations are summarized in the spearman correlation plots in Figure 4. We found significant correlations between the collagen remodeling biomarkers, PIIINP, ICTP, TIMP-2, and the fatty acid metabolism biomarker, NEFA. A significant association was also found between L-FABP and TIMP-1. The correlations between the collagen remodeling biomarkers and fatty acid dysregulation biomarkers could be due to both being involved in the process of structural remodeling of the atria in AF. These results could also indicate that there is a more complex interplay between collagen remodeling and fatty acid metabolism dysregulation in the development of AF.
The studies presented herein represent an integrated and comprehensive analysis of the biomarkers which are generated in response to the underlying pathophysiological processes in AF. This integrated study provided the evidence that collagen remodeling plays an important role in the progression of AF. In addition to collagen remodeling protein turnover biomarkers, this study has also reported on the role of NEFAs and L-FABP. This study provided the evidence on the relevance of NEFA to metalloproteases and their regulators and some of the collagen turnover proteins underscoring the interplay between the two pathways. Of all the biomarkers studied, the collagen turnover proteins, PIIICP and PIIINP, showed marked upregulation, whereas a modest increase was noted in PINP and PICP. Varying levels of increase were also noted in the MMPs and the TIMPs. This integrated fingerprinting of collagen remodeling proteins and the relevance to fatty acid regulatory parameters represent evidence that the two mechanisms involved in the upregulation of the collagen turnover proteins and fatty acid generated in AF may have some crosstalk. Additional studies are needed at a molecular level to establish such relevance between the collagen turnover proteins and other biomarkers studied. Overall, an integrated fingerprinting of these biomarkers provides a panel profiling that can be used for the prognostic purposes. Significant progress has been made in the understanding of the underlying mechanism in atrial remodeling. The results reported in this study have potentially important implications in not only the pathophysiology of AF, but also the development of newer therapeutic approaches.
This study has several limitations, including a small cohort size of the AF patients, lack of random sampling, and nonavailability of age-matched controls. Moreover, the population is not balanced since there are significantly higher number of males in the AF cohort. An additional limitation to our study is that an elevation of collagen biomarkers and fatty acid metabolism biomarkers in the plasma does not prove that they are necessarily involved in AF, especially since many AF patients have other significant comorbidities. For future studies, it may be useful to correlate the biomarker findings with imaging data of the AF patients to assess for the extent of atrial fibrosis and epicardial adipose tissue formation. Additionally, clinical data on atrial fibrosis and adipose tissue deposition will be helpful in validating the utility of these biomarkers in the understanding of the pathogenesis of AF. Despite these limitations, this study provides pilot data on the role of collagen remodeling biomarkers and L-FABP, along with NEFAs, in the pathogenesis of AF with reference to the dysregulation of metalloproteases.
In conclusion, our study showed that there is an elevation in collagen remodeling biomarkers and fatty acid metabolism biomarkers in plasma obtained from AF patients compared to normal human plasma. These findings support some of the previous studies that also showed elevations of these biomarkers in AF patients. Our results support the theory that collagen remodeling and fatty acid metabolism dysregulation could be implicated in the pathogenesis of AF. Profiling of these markers may provide useful information on the understanding of the pathophysiology of atrial remodeling and risk stratification of patients with this arrhythmia. The information generated in this study is also helpful in the development of newer therapeutic agents targeting various mechanisms, including collagen degradation.
Acknowledgements
We are thankful for the fellowship stipend received through the NHLBI T35 HL120835 grant provided to medical students in heart, lung, and blood related research. We also thank the Student Training in Approaches to Research (STAR) program and the STAR directors for their continuous support. This study was partially supported by the Cardiovascular Institute, Health Sciences Division of Loyola University Chicago. A special thanks to Mr Jonas Kingo of Aniara for providing the kits for this study. Thank you to Ms. Erin Healy-Erickson for her skillful assistance in the preparation of this manuscript. We are thankful to Dr Eva Wojcik, Chairperson of the Department of Pathology and Laboratory Medicine, Dr Omer Iqbal of the Departments of Pathology and Ophthalmology, Dr Colleen Fitzgerald, Director of the STAR program, Dr Meharvan Singh, Vice Provost of Research and Provost Margaret Callahan for their support and encouragement during this project. A special thanks to Dr Seth Robia and Dr Alain Heroux, Co-directors of the CVRI Cardiovascular Research Insitute and Dr Lowell Steen, Director of Cardiology Division of the Department of Medicine for their facilitation of this research program.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article
ORCID iDs: Ameer Odeh https://orcid.org/0000-0001-5848-0201
Debra Hoppensteadt https://orcid.org/0000-0001-9342-4213
Fakiha Siddiqui https://orcid.org/0000-0002-2219-7049
Bulent Kantarcioglu https://orcid.org/0000-0003-3060-721X
Jawed Fareed https://orcid.org/0000-0003-3465-2499
References
- 1.Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: The framingham study. Stroke. 1991;22(8):983-988. doi: 10.1161/01.str.22.8.983 [DOI] [PubMed] [Google Scholar]
- 2.Shah KS, Held EP. Utilizing biomarkers to refine risk prediction in atrial fibrillation: A step toward precision medicine. J Am Coll Cardiol. 2019;73(12):1411-1412. doi: 10.1016/j.jacc.2018.10.092 [DOI] [PubMed] [Google Scholar]
- 3.Rahman F, Kwan GF, Benjamin EJ. Global epidemiology of atrial fibrillation. Nat Rev Cardiol. 2014;11(11):639-654. doi: 10.1038/nrcardio.2014.118. Epub 2014 Aug 12. Erratum in: Nat Rev Cardiol. 2016 Jul 14;13(8):501. PMID: 25113750. [DOI] [PubMed] [Google Scholar]
- 4.Benjamin EJ, Wolf PA, D'Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: The framingham heart study. Circulation. 1998;98(10):946-952. doi: 10.1161/01.cir.98.10.946 [DOI] [PubMed] [Google Scholar]
- 5.Choudhury A, Lip GY. Atrial fibrillation and the hypercoagulable state: From basic science to clinical practice. Pathophysiol Haemost Thromb. 2003;33(5-6):282-289. doi: 10.1159/000083815 [DOI] [PubMed] [Google Scholar]
- 6.Hart RG, Pearce LA, Aguilar MI. Meta-analysis: Antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007;146(12):857-867. doi: 10.7326/0003-4819-146-12-200706190-00007 [DOI] [PubMed] [Google Scholar]
- 7.Iwasaki YK, Nishida K, Kato T, Nattel S. Atrial fibrillation pathophysiology: Implications for management. Circulation. 2011;124(20):2264-2274. doi: 10.1161/CIRCULATIONAHA.111.019893 [DOI] [PubMed] [Google Scholar]
- 8.Shingu Y, Takada S, Yokota Tet al. Correlation between increased atrial expression of genes related to fatty acid metabolism and autophagy in patients with chronic atrial fibrillation. PLoS One. 2020;15(4):e0224713. doi: 10.1371/journal.pone.0224713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burstein B, Nattel S. Atrial fibrosis: Mechanisms and clinical relevance in atrial fibrillation. J Am Coll Cardiol. 2008;51(8):802-809. doi: 10.1016/j.jacc.2007.09.064 [DOI] [PubMed] [Google Scholar]
- 10.Rosenberg MA, Maziarz M, Tan AYet al. Circulating fibrosis biomarkers and risk of atrial fibrillation: The cardiovascular health study (CHS). Am Heart J. 2014;167(5):723-728.e2. doi: 10.1016/j.ahj.2014.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Golaszewska K, Harasim-Symbor E, Polak-Iwaniuk A, Chabowski A. Serum fatty acid binding proteins as a potential biomarker in atrial fibrillation. J Physiol Pharmacol. 2019;70(1):25-35. doi: 10.26402/jpp.2019.1.11 [DOI] [PubMed] [Google Scholar]
- 12.Lacalzada-Almeida J, García-Niebla J. How to detect atrial fibrosis. J Geriatr Cardiol. 2017;14(3):185-194. doi: 10.11909/j.issn.1671-5411.2017.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oakes RS, Badger TJ, Kholmovski EGet al. Detection and quantification of left atrial structural remodeling with delayed-enhancement magnetic resonance imaging in patients with atrial fibrillation. Circulation. 2009;119(13):1758-1767. doi: 10.1161/CIRCULATIONAHA.108.811877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baicu CF, Stroud JD, Livesay VAet al. et al. Changes in extracellular collagen matrix alter myocardial systolic performance. Am J Physiol Heart Circ Physiol. 2003;284(1):H122-H132. doi: 10.1152/ajpheart.00233.2002 [DOI] [PubMed] [Google Scholar]
- 15.Mays PK, Bishop JE, Laurent GJ. Age-related changes in the proportion of types I and III collagen. Mech Ageing Dev. 1988;45(3):203-212. doi: 10.1016/0047-6374(88)90002-4 [DOI] [PubMed] [Google Scholar]
- 16.Jabati S, Fareed J, Liles Jet al. Biomarkers of inflammation, thrombogenesis, and collagen turnover in patients with atrial fibrillation. Clin Appl Thromb Hemost. 2018;24(5):718-723. doi: 10.1177/1076029618761006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duprez DA, Heckbert SR, Alonso A, et al. Jr. Collagen biomarkers and incidence of new onset of atrial fibrillation in subjects with No overt cardiovascular disease at baseline: The multi-ethnic study of atherosclerosis. Circ Arrhythm Electrophysiol. 2018;11(10):e006557. doi: 10.1161/CIRCEP.118.006557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kallergis EM, Manios EG, Kanoupakis EMet al. Extracellular matrix alterations in patients with paroxysmal and persistent atrial fibrillation: Biochemical assessment of collagen type-I turnover. J Am Coll Cardiol. 2008;52(3):211-215. doi: 10.1016/j.jacc.2008.03.045 [DOI] [PubMed] [Google Scholar]
- 19.Sonmez O, Ertem FU, Vatankulu MAet al. et al. Novel fibro-inflammation markers in assessing left atrial remodeling in non-valvular atrial fibrillation. Med Sci Monit. 2014;20:463-470. doi: 10.12659/MSM.890635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Polyakova V, Miyagawa S, Szalay Z, Risteli J, Kostin S. Atrial extracellular matrix remodelling in patients with atrial fibrillation. J Cell Mol Med. 2008;12(1):189-208. doi: 10.1111/j.1582-4934.2008.00219.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu J, Cui G, Esmailian Fet al. et al. Atrial extracellular matrix remodeling and the maintenance of atrial fibrillation. Circulation. 2004;109(3):363-368. doi: 10.1161/01.CIR.0000109495.02213.52 [DOI] [PubMed] [Google Scholar]
- 22.Khawaja O, Bartz TM, Ix JHet al. Plasma free fatty acids and risk of atrial fibrillation (from the Cardiovascular Health Study). Am J Cardiol. 2012;110(2):212-216. doi: 10.1016/j.amjcard.2012.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Otaki Y, Watanabe T, Kubota I. Heart-type fatty acid-binding protein in cardiovascular disease: A systemic review. Clin Chim Acta. 2017;474:44-53. doi: 10.1016/j.cca.2017.09.007 [DOI] [PubMed] [Google Scholar]
- 24.Furuhashi M, Saitoh S, Shimamoto K, Miura T. Fatty acid-binding protein 4 (FABP4): Pathophysiological insights and potent clinical biomarker of metabolic and cardiovascular diseases. Clin Med Insights Cardiol. 2014;8(Suppl 3):23-33. doi: 10.4137/CMC.S17067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nattel S, Burstein B, Dobrev D. Atrial remodeling and atrial fibrillation: Mechanisms and implications. Circ Arrhythm Electrophysiol. 2008;1(1):62-73. doi: 10.1161/CIRCEP.107.754564 [DOI] [PubMed] [Google Scholar]
- 26.Qian Y, Meng J, Tang Het al. et al. Different structural remodelling in atrial fibrillation with different types of mitral valvular diseases. Europace. 2010;12(3):371-377. doi: 10.1093/europace/eup438 [DOI] [PubMed] [Google Scholar]
- 27.Kostin S, Klein G, Szalay Z, Hein S, Bauer EP, Schaper J. Structural correlate of atrial fibrillation in human patients. Cardiovasc Res. 2002;54(2):361-379. doi: 10.1016/s0008-6363(02)00273-0 [DOI] [PubMed] [Google Scholar]
- 28.Cardin S, Libby E, Pelletier Pet al. Contrasting gene expression profiles in two canine models of atrial fibrillation. Circ Res. 2007;100(3):425-433. doi: 10.1161/01.RES.0000258428.09589.1a [DOI] [PubMed] [Google Scholar]
- 29.Pellegrini CN, Buzkova P, Lichtenstein AHet al. Individual non-esterified fatty acids and incident atrial fibrillation late in life. Heart. 2021;107(22):1805-1812. doi: 10.1136/heartjnl-2020-317929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lind L, Sundström J, Stenemo M, Hagström E, Ärnlöv J. Discovery of new biomarkers for atrial fibrillation using a custom-made proteomics chip. Heart. 2017;103(5):377-382. doi: 10.1136/heartjnl-2016-309764 [DOI] [PubMed] [Google Scholar]
- 31.López-Canoa JN, Couselo-Seijas M, González-Ferrero Tet al. The role of fatty acid-binding protein 4 in the characterization of atrial fibrillation and the prediction of outcomes after catheter ablation. Int J Mol Sci. 2022;23(19):11107. 10.3390/ijms231911107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Otaki Y, Arimoto T, Takahashi Het al. Prognostic value of myocardial damage markers in patients with chronic heart failure with atrial fibrillation. Intern Med. 2014;53(7):661-668. doi: 10.2169/internalmedicine.53.1293 [DOI] [PubMed] [Google Scholar]
- 33.Rader F, Pujara AC, Pattakos Get al. Perioperative heart-type fatty acid binding protein levels in atrial fibrillation after cardiac surgery. Heart Rhythm. 2013;10(2):153-157. doi: 10.1016/j.hrthm.2012.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shingu Y, Yokota T, Takada Set al. Decreased gene expression of fatty acid binding protein 3 in the atrium of patients with new onset of atrial fibrillation in cardiac perioperative phase. J Cardiol. 2018;71(1):65-70. doi: 10.1016/j.jjcc.2017.07.003 [DOI] [PubMed] [Google Scholar]