Abstract
The outer membrane (OM) of the intracellular parasite Brucella abortus is permeable to hydrophobic probes and resistant to destabilization by polycationic peptides and EDTA. The significance of these unusual properties was investigated in a comparative study with the opportunistic pathogens of the genus Ochrobactrum, the closest known Brucella relative. Ochrobactrum spp. OMs were impermeable to hydrophobic probes and sensitive to polymyxin B but resistant to EDTA. These properties were traced to lipopolysaccharide (LPS) because (i) insertion of B. abortus LPS, but not of Escherichia coli LPS, into Ochrobactrum OM increased its permeability; (ii) permeability and polymyxin B binding measured with LPS aggregates paralleled the results with live bacteria; and (iii) the predicted intermediate results were obtained with B. abortus-Ochrobactrum anthropi and E. coli-O. anthropi LPS hybrid aggregates. Although Ochrobactrum was sensitive to polymyxin, self-promoted uptake and bacterial lysis occurred without OM morphological changes, suggesting an unusual OM structural rigidity. Ochrobactrum and B. abortus LPSs showed no differences in phosphate, qualitative fatty acid composition, or acyl chain fluidity. However, Ochrobactrum LPS, but not B. abortus LPS, contained galacturonic acid. B. abortus and Ochrobactrum smooth LPS aggregates had similar size and zeta potential (−12 to −15 mV). Upon saturation with polymyxin, zeta potential became positive (1 mV) for Ochrobactrum smooth LPS while remaining negative (−5 mV) for B. abortus smooth LPS, suggesting hindered access to inner targets. These results show that although Ochrobactrum and Brucella share a basic OM pattern, subtle modifications in LPS core cause markedly different OM properties, possibly reflecting the adaptive evolution of B. abortus to pathogenicity.
Brucella spp. are gram-negative bacteria of the α-2 subgroup of the domain Proteobacteria characteristically able to multiply intracellularly in a variety of cells (35). The pathogenicity of Brucella is not related to production of classical toxin, antiphagocytic capsules, proteinaceous adhesins, or plasmid-encoded virulence factors. On the other hand, the low endotoxicity of the lipopolysaccharide (LPS) (49), the resistance to a wide range of bactericidal cationic peptides (13, 32), and the ability to prevent lysosome fusion and to locate in autophagosome-like compartments (46, 47) are characteristics of Brucella in all likelihood related to pathogenicity. Reduced endotoxicity and polycation resistance reflect properties of the LPS, and this molecule is also linked to additional peculiarities of the Brucella outer membrane (OM), such as its resistance to EDTA (13, 31, 38) and permeability to hydrophobic compounds (31). Because the OM plays a critical role in interaction of the bacteria with the environment, we have proposed (38, 39, 49) that the unusual set of properties of the Brucella OM is linked to its intracellular parasitism.
A first approach to test this possibility is to obtain mutants altered in OM properties. We have recently produced polycation-sensitive mutants of B. abortus which, in support of our hypothesis, also show increased OM permeability to hydrophobic dyes, cannot penetrate into HeLa cells, and are avirulent in the mouse model (55). These mutants are disrupted in either of the two genes of a sensory/regulatory system (BvrR/BvrS) highly homologous to sensory/regulatory systems essential for virulence and endosymbiosis in Agrobacterium and Rhizobium, two plant-associated bacteria also belonging to the α-2 Proteobacteria (55). Moreover, we found that the divergence in BvrS is concentrated in the periplasmic sensory section (55); this shows that in the α-2 Proteobacteria, slight modifications of some basic systems reflect the adaptation to seemingly very different hosts and environments. Thus, a complementary approach to assess the relevance of the OM properties in the biology of Brucella is to perform comparative studies with phylogenetically related bacteria differing in pathogenicity. For this purpose, the members of the genus Ochrobactrum are particularly suitable. These bacteria are very close to Brucella by DNA, rRNA, and protein analysis (12, 23, 59, 62, 65), but they are primarily soil dwellers known to be pathogenic only in critically ill or immunocompromised patients or in patients with indwelling catheters (10, 11, 25, 26, 34, 52, 66). Although in such situations Ochrobactrum can cause meningitis, osteomyelitis, bacteremia, and septicemia, these bacteria are unable to establish chronic infections by themselves and are cleared from normal hosts after catheter removal (16). Infections by Ochrobactrum are, therefore, in sharp contrast with the highly contagious, tenacious, and harmful disease caused by their Brucella relatives. Since both bacteria have a common evolutionary stem, appropriate comparative studies should help to clarify whether the above-described set of properties represent the result of an adaptive evolution of the Brucella OM to thwart host defensive systems, and hence a feature relevant in pathogenicity, or whether they merely represent an earlier OM pattern shared with other α-2 Proteobacteria. The results of a structural and functional study is presented here. We found that despite their close phylogenetic relatedness, B. abortus and Ochrobactrum differ markedly in OM properties and that these wide differences are caused at least in part by little changes in the LPS.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
B. abortus 2308 (smooth [S], virulent, biotype 1), O. anthropi LMG3331T (S), and O. intermedium LMG3301T (rough [R]), Escherichia coli O:8 K27 (S), and E. coli O111 K58H2 (S) have been used in previous work (1, 13, 32, 62). When used in cell permeability studies, they were grown in tryptic soy broth in sidearm flasks on an orbital shaker at 250 rpm and 37°C. For LPS extraction, bacteria were propagated in 1.7% Casitone (E. Merck, Darmstadt, Germany)–0.3% Soytone (Difco Laboratories, Detroit, Mich.)–0.5% yeast extract (Merck)–0.25% K2HPO4–2% glucose–0.5% NaCl–0.01% antifoam A-butyl acetate (1:3) (Sigma Chemical Co., St. Louis, Mo.) in a 15-liter Biostat fermentor (B. Braun Melsungen AG, Leinfelden, Germany) (1). Bacteria were harvested in the stationary phase by tangenital flow filtration, washed, and dried (60).
LPS.
S-LPSs were obtained by the water-phenol method, from either the water (E. coli O111 K58H2, E. coli O:8 K27, and O. anthropi LMG3331) or phenol (B. abortus) phase (29). The R-LPS of O. intermedium LMG3301 was extracted with phenol-chloroform-light petroleum (15). Crude Brucella and Ochrobactrum S-LPS extracts (10 mg/ml in 5 mM MgCl2–0.1 M Tris-HCl [pH 7.0]) were digested first with nucleases (50 μg each of DNase II type V and RNase A [Sigma] per ml, 30 min at 37°C) and then three times with proteinase K (50 μg/ml, 3 h at 55°C), sedimented by ultracentrifugation (6 h, 100,000 × g), and freeze-dried. Ornithine lipids and phospholipids were removed by a fourfold extraction with chloroform-methanol (2:1 [vol/vol]), as demonstrated by lipid and amino sugar analyses (see below). Contents in 3-deoxy-d-manno-octulosonic acid (Kdo) (determined by the thiobarbituric acid method) were as follows: B. abortus S-LPS, 1.0%; E. coli S-LPS, 1.5%; O. anthropi S-LPS, 0.52%; and O. intermedium R-LPS, 1.38%. Protein content (30) was less than 0.2% for all preparations. The natural salt form of the LPS was used in the physicochemical analyses described below.
Lipid A and polysaccharide preparations.
S-LPS was hydrolyzed (2% acetic acid, 100°C, 3 h), and the polysaccharide and lipid A fractions were separated by centrifugation. The soluble polysaccharide fraction was freeze-dried and purified by chromatography on Sephadex G-50 with water-pyridine-acetate (14). The crude lipid A was dissolved in chloroform, applied to a Silica Gel 60 column, and eluted stepwise with chloroform and chloroform-methanol mixtures of increasing polarity (95:5 to 50:50). As judged by thin-layer chromatography (TLC), pure lipid A eluted in the 95:5 fraction. This fraction was further purified by high-pressure liquid chromatography (HPLC) on Silica Gel 60 and shown to be free of phospholipids and ornithine lipids by TLC, gas-liquid chromatography (GLC)-mass spectrometry (MS), and amino acid analysis (see below).
Permeability to NPN. (i) Normal cells.
Exponentially growing cells were harvested (5,000 × g, 20 min, 5°C) and resuspended immediately in 2 mM HEPES (pH 7.2) at an optical density at 600 nm (OD600) of 0.5. Partition of the fluorescent hydrophobic probe N-phenyl-1-naphthylamine (NPN; 10 μM) into the cell envelope was monitored with an LS-50 fluorimeter (excitation, 350 nm; emission, 420 nm; slit width for both windows, 2.5 nm; Perkin-Elmer Ltd., Beaconsfield, England); OM disturbances caused by cationic peptides or EDTA were assessed by measuring changes in NPN partition (32). The agents tested and their final concentrations in the cuvettes were as follows: polymyxin B, 12.5 U/ml; poly-l-lysine, 20 μg/ml; poly-l-ornithine, 20 μg/ml; and EDTA, 10 mM. Polymyxin B sulfate (8,000 U/mg), poly-l-lysine hydrobromide (molecular weight, 7,000 to 10,000), and poly-l-ornithine hydrobromide (12,000 to 22,000) were purchased from Sigma.
(ii) O. intermedium-B. abortus S-LPS and O. anthropi-E. coli S-LPS chimeras.
Viable O. intermedium chimeras carrying B. abortus or E. coli S-LPS were obtained as described previously for Brucella-Salmonella enterica serovar Minnesota S-LPS chimeras (13), with modifications. Stock suspensions of B. abortus or E. coli S-LPS (20 mg/ml in 0.01 M phosphate buffer [pH 7.4]) were prepared by ultrasonic treatment (see below) and sterilized by heating at 100°C for 1 h. Exponentially growing cells of O. intermedium were harvested and resuspended in 1% soybean peptone (Soytone; Merck)–0.01 M phosphate buffer (pH 7.4) to an OD600 of 0.30. Six hundred microliters of this suspension was mixed with 200 μl of the LPS stocks in sterile Eppendorf-type tubes, and the mixtures were sonicated for 1 to 2 s. After incubation for 18 h at 37°C, growing chimeras were sedimented (12,000 × g, 15 min), washed twice with sterile 0.01 M phosphate buffer (pH 7.4), and resuspended in 1 mM KCN–2 mM HEPES (pH 7.2) at an OD600 of 0.30. Coating was shown by coagglutination (28) with immune sera of the appropriate specificity. NPN partition was assessed as described for normal bacteria, but because lower numbers of cells were available, a slit width of 4 for both windows was used. As controls, measurements with the same settings were performed with normal bacteria grown under the same conditions but for only 7 (B. abortus) or 4 (O. intermedium) h to correct for the longer doubling time of chimeras.
(iii) LPS and LPS hybrids.
One milligram of B. abortus S-LPS was mixed with 1 mg of O. anthropi S-LPS in 2 ml of double-distilled water, and the mixture was sonicated for 30 s at 10 W in a Branson Sonifier 450 (Branson Ultrasonics Corp., Danbury, Conn.) equipped with a microtip probe. Two milliliters of 2% sodium deoxycholate–0.1 M Tris-HCl (pH 8.5) was added to the suspension, and the mixture was incubated for 15 min at 20°C. LPS hybrids were then precipitated with 6 volumes of ethanol (18 h, −20°C), sedimented (10,000 × g, 15 min), washed with 6 volumes of cold ethanol, resuspended in distilled water (1 ml per mg of LPS), and dialyzed extensively (51). To measure NPN partition, a volume of the LPS or hybrid LPS was mixed with the appropriate volume of a stock HEPES solution to give final suspensions containing 400 μg of LPS per ml in 2 mM HEPES (pH 7.1). NPN fluorescence was determined before and after polymyxin B (70 U/ml, final concentration) or EDTA (2.5 mM) addition as described for normal cells. Plain O. anthropi and B. abortus S-LPSs treated as the hybrid mixtures were used as controls.
Interaction of polymyxin B with LPS. (i) HPLC.
Stock LPS suspensions were prepared in 0.133 M NaCl–0.1 M NaH2PO4 (pH 4.6) by ultrasonic treatment (see above) and clarified by brief centrifugation, and final LPS concentration was determined by measuring the Kdo content. Aliquots of these suspensions were incubated (10 min, 37°C) with increasing amounts of polymyxin B, and the bound and free forms of polymyxin B were separated and measured by HPLC (32).
(ii) Dansyl-cadaverine displacement.
Appropriate amounts of stock LPS suspensions (3 mg/ml; prepared as described above) were diluted in 1 ml of 2 mM HEPES (pH 7.5) directly in the fluorimetric cuvettes to obtain concentrations equivalent to 4 nmol of Kdo per ml (B. abortus S-LPS, 73 μg/ml; O. anthropi S-LPS, 102 μg/ml; O. anthropi R-LPS, 56 μg/ml). Approximately 4 mg (equivalent to 12 nmol/ml) of dansyl-cadaverine (Sigma) was added to the cuvettes (90% maximum fluorescence) (4). The decrease in fluorescence upon addition of polymyxin B was recorded, and the occupancy was calculated as (F − F0)/(Fmax − F0), where F0 is the fluorescence intensity of dansyl-cadaverine alone, Fmax is the intensity in the presence of LPS alone, and F is the intensity of the dansyl-cadaverine-LPS mixtures at different polymyxin B concentrations. Fifty percent occupancy values (concentration [micromolar] of polymyxin B necessary to displace 50% of dansyl-cadaverine) were calculated graphically from the occupancy-versus-polymyxin B concentration plots. Measurements were performed with the following settings: excitation, 340 nm; emission scan from 400 to 600 nm; slit width for both windows, 1.5 nm.
Zeta potential measurements.
The surface charge density of LPS aggregates and the effect of polycations was measured as the electrophoretically effective potential (zeta potential ζsm) (7) in dependence on the concentration of polymyxin B. The LPS aggregates were prepared in 1 mM CsCl–2.5 mM Tris-HCl (pH 7.0) by sonication for 20 min at 40°C in an ultrasonic bath and allowed to equilibrate at 4°C overnight, and the suspensions were adjusted to the same Kdo concentrations (approximately 40.5 μM). Particle size and ζsm were measured in a ZetaSizer 4 apparatus (Malvern Instruments GmbH, Herrsching, Germany), which uses a 5 mW He-Ne laser and a temperature-controlled electrophoresis cell to measure the electrophoretic mobility by laser-Doppler-anemometry and the size distribution by photon correlation spectroscopy at a scatter angle of 90°. The electrokinetic mobility μcl of the aggregates was measured at 22°C in a driving electric field of 19.2 V cm−1 from which, independent of the particle size, ζsm can be calculated according to the Helmholtz-Smoluchowsky equation ζsm = (μcl · η)/(ɛr · ɛ0), where μcl is the electrokinetic mobility, η is the viscosity, ɛr is the dielectric constant of the buffer (0.97 mPa and 79, respectively), and ɛ0 is the permittivity of free space (24). The cell was frequently checked for instrumental drifts and, if necessary, cleaned and recalibrated.
Determination of the β↔α phase transitions.
The LPS gel↔liquid crystalline β↔α phase transition of the acyl chains of LPS from a well-ordered (gel) into a fluid (liquid crystalline) state at a lipid-specific temperature Tc was measured by Fourier-transformed infrared spectroscopy (FTIR). This allows the determination of the acyl chain fluidity—inversely proportional to the state of order—which is a measure of the mobility of the hydrocarbon chains at a given temperature. To ensure homogeneity, LPS dispersions were prepared in 2.5 mM HEPES (pH 7.0) at room temperature, incubated at 56°C for 15 min, stirred, and cooled to 4°C. This heating-cooling step was repeated three times, and suspensions were stored at 4°C for several hours before analysis. Measurements were performed with a Bruker IFS 55 apparatus (Bruker, Karlsruhe, Germany) as previously described (5). Briefly, LPS dispersions (water content, >90%) were analyzed in CaF2 cuvettes with 12.5-μm-thick Teflon spacers, and in each measurement 50 interferograms were accumulated, Fourier transformed, and converted to absorbance spectra. The measurements were performed in continuous heating scans (2°C/min) from 10 to 60°C. The peak position of the symmetric stretching vibration of the methylene groups νs(CH2) around 2,850 cm−1 was taken as a measure of the state of order (fluidity) of the acyl chains. The S-LPS of E. coli O:8 K27 was used as a reference.
Electron microscopy.
Bacteria were adjusted to 4 × 107 CFU/ml and incubated with polymyxin B at 20 μg/ml for 15 min before being fixed with 4% glutaraldehyde (Sigma) in 0.1 M phosphate buffer (pH 7.4) for 1 h at 4°C, preembedded in 3% low-gelling-temperature agarose (Sea Plaque; FMC Corp.), and stored for 12 h in fixative solution at 4°C. Agarose blocks were cut into 3-mm3 pieces, fixed, dehydrated, and embedded in Spurr resin (Agar Scientific Ltd.) (13). Ultrathin sections were stained with 4% uranyl acetate (Agar Scientific) for 15 min and with 2.66% lead citrate (Agar Scientific) for 15 min and then examined with a Hitachi 1100 transmission electron microscope (Hitachi Scientific Instruments, Mountain View, Calif.) operating at 100 kV.
Chemical analysis. (i) Sugar analysis.
Methylation analysis of the polysaccharide LPS fraction (see above) was done by methylation, hydrolysis (3 M trifluoroacetic acid, 100°C, 2 h), reduction (NaBH4), and acetylation. The derivatives of uronic acids were obtained by methanolysis, reduction with NaBD4 and acetylation. Alditol acetates were identified by GLC or GLC-MS (see below) (61, 62).
(ii) Fatty acid analysis.
Amide- and ester-linked fatty acids were released from lipid A by acid (4 M HCl, 100°C, 4 h) or alkaline (1 M NaOH-methanol [1:1], 65°C, 1 h) hydrolysis. Fatty acids were extracted with chloroform and derivatized with diazomethane or with diazomethane and bistrimethylsilyl trifluoroacetamide for GLC and GLC-MS (64). GLC was performed on a Varian gas chromatograph (model 3700) and a silica SPB-5 column (30 m, 0.25-μm film, 0.25-mm inside diameter; Supelco Inc., Bellefonte, Pa.) with N2 as the carrier gas. The injector and detector temperature was 290°C. The column was programmed at 150°C for 3 min, a 3°C raise per min from 150 to 320°C, and 320°C for 30 min. GLC-MS was performed on a Hewlett-Packard 5890 II chromatograph equipped with a silica HP-5 column (30 m, 0.25-μm film, 0.25-mm inside diameter; Hewlett-Packard GmbH, Waldbronn, Germany) and fitted to a mass spectrometer 5989 A (Hewlett-Packard). The column was programmed as described above for GLC. Electronic impact mass spectra were recorded at 70 eV, and chemical ionization mass spectra were obtained with ammonia as the reactant gas. The temperature of the ion source was 200°C. The mass spectra were analyzed with a HP Vectra 488/66 computer using the Hewlett-Packard MS data analysis program version C.00.07.
(iii) Amino acid and amino sugar analysis.
Lipid A samples were hydrolyzed (4 M HCl, 100°C for 16 to 24 h), and fatty acids were eliminated by repeated extraction with chloroform. Analyses were carried out with a 4151 Alpha Plus apparatus (LKB Biochrom Ltd., Cambridge, England) with the appropriate amino sugars and amino acids as standards.
(iv) Laser desorption (LD)-MS.
Dephosphorylated (48% HF at 4°C for 48 h) O. intermedium lipid A was analyzed with a laser microprobe mass analyzer apparatus (LAMMA 500 Leybold AG, Köln, Germany) as described previously (54).
(v) TLC.
Mono- and bisphosphorylated forms of lipid A were detected by TLC on Silica Gel 60 plates (Merck), using chloroform-methanol-water (100:75:15). Plates were developed with ethanol-sulfuric acid, and mono- and bisphosphorylated S. enterica serovar minnesota Re595 lipids A were used as standards. The degree of acylation was examined by the same method, using chloroform–methanol–25% ammonia (80:20:3) and the hexa-acylated lipid A of E. coli F515 as a standard.
RESULTS
Permeability to NPN.
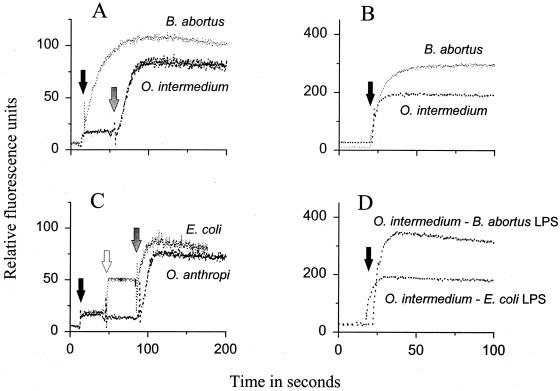
Confirming previous reports (13, 31), NPN readily partitioned into the cell envelopes of B. abortus, yielding fluorescence values of 115 or 312 relative fluorescence units (RFU) (Fig. 1A and B), depending on the experimental conditions. Under the same conditions, and in the absence of any OM-disturbing agent, partition of NPN into O. intermedium cells was stabilized at 20 or 196 RFU (Fig. 1A and B). Like O. intermedium, O. anthropi and E. coli (Fig. 1C) hardly took up NPN. To test whether these differences in permeability related to LPS differences, two sets of experiments were carried out. First, we constructed O. intermedium LPS chimeras and tested permeability to NPN. Compared to O. intermedium normal cells, O. intermedium-B. abortus S-LPS chimeras showed increased permeability to NPN, with final fluorescence levels similar to those obtained with B. abortus under the same conditions (Fig. 1B and D). On the other hand, an increase in permeability was not observed for the O. intermedium-E. coli S-LPS chimeras (Fig. 1D). Second, S-LPS aggregates were tested directly for NPN partition. The results (Table 1) show that B. abortus S-LPS took up more NPN than S-LPS aggregates of either O. anthropi or E. coli, with intermediate values for the O. anthropi-B. abortus LPS hybrid aggregates.
FIG. 1.
Permeability to NPN and effect of OM-disturbing agents on normal bacteria (A to C) and O. intermedium heterologous S-LPS chimeric bacteria (D). Arrows mark the time at which the bacteria were exposed to NPN (black arrow), EDTA (10 mM; empty arrow), or polymyxin B (12.5 units; grey arrow). Experimental conditions: (A and C) bacterial suspensions at OD600 of 0.5; slit width, 2.5 nm; (B and D) bacterial suspensions at OD600 0.3; slit width, 4 nm.
TABLE 1.
NPN permeability of S-LPS aggregates of E. coli, O. anthropi, and B. abortus, and effect of EDTA and polymyxin B
| Agent added | Fluorescence (RFU)a obtained with S-LPS aggregates from:
|
|||
|---|---|---|---|---|
| E. coli | O. anthropi | O. anthropi-B. abortus | B. abortus | |
| None | 3 ± 1 | 4 ± 1 | 5 ± 1 | 6 ± 1 |
| 10 μM NPN | 39 ± 6 | 38 ± 3 | 49 ± 2 | 54 ± 3 |
| 10 μM NPN + 2.5 μM EDTA | 104 ± 2 | 47 ± 2 | 57 ± 2 | 61 ± 2 |
| 10 μM NPN + polymyxin B (70 U/ml) | 220 ± 2 | 200 ± 5 | 177 ± 7 | 129 ± 3 |
Mean ± standard deviation of 10 measurements.
In many gram-negative bacteria, removal of the divalent cations that stabilize the OM causes an increase in permeability (41, 58). EDTA (10 mM) did not affect NPN uptake by O. anthropi (Fig. 1C) or by O. intermedium (not shown), but it increased the NPN uptake by E. coli from 25 to 50 RFU (Fig. 1C). When tested on LPS aggregates, EDTA (2.5 mM) produced only a low (less than 10 RFU) increase in NPN partition into O. anthropi and B. abortus S-LPS aggregates or into the corresponding hybrids, whereas it increased the E. coli S-LPS values by 65 RFU (Table 1).
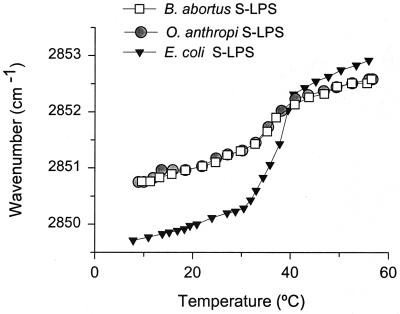
O. anthropi and B. abortus S-LPSs were examined for acyl chain fluidity differences which could relate to OM permeability and/or lipid A acyl chain differences (3, 5). Figure 2 shows that the shift in the maximum of the peak position of the symmetric stretching vibration νs(CH2) of LPS aggregates in dependence on temperature (as an estimate of the LPS acyl chain fluidity) was identical for the two S-LPSs over the whole range of temperatures tested, and that both displayed a β↔α transition in the 30 to 40°C range with almost identical Tc (35.4 and 35.3°C, respectively). Although with similar Tc, the LPS of E. coli O:8 K27 showed a different fluidity profile indicative of a very different acyl chain composition, with a more restricted and a higher degree of acyl chain mobility below and above Tc, respectively. As expected because of the dependence of Tc on the length of the sugar chain (6), the R-LPS of O. intermedium showed a broader temperature interval for the β↔phase transition, with a lower Tc (29.4°C) and a less fluid state (not shown).
FIG. 2.
Maximum of the peak position of the symmetric stretching vibration νs(CH2) versus temperature for the S-LPSs of B. abortus, O. anthropi, and E. coli O:8 K27.
Action of polycationic peptides.
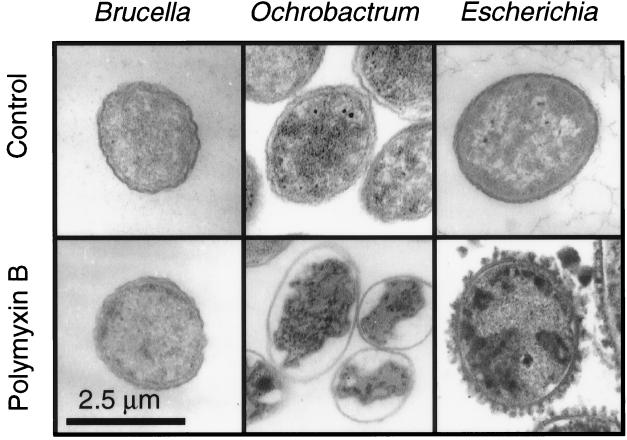
Figure 1A shows that polymyxin B disturbed the OM of O. intermedium, causing a sudden increase in permeability to NPN. Similar results were obtained for O. anthropi (not shown) and E. coli (Fig. 1C). A synergistic effect of EDTA and polymyxin B on O. anthropi (Fig. 1C) and O. intermedium was not observed. Poly-l-ornithine and poly-l-lysine also permeabilized the OM of O. anthropi, O. intermedium, and E. coli (not shown). Figure 3 shows that polymyxin B caused a characteristic (13) OM blebbing in E. coli but not in B. abortus or O. anthropi. Although the OM was morphologically unharmed, self-promoted uptake of polymyxin B was inferred in O. anthropi from the extensive damage caused on the cytoplasmic membrane and from the subsequent cytoplasm coagulation.
FIG. 3.
Effect of polymyxin B (160 U, 15 min) on B. abortus, O. intermedium, and E. coli.
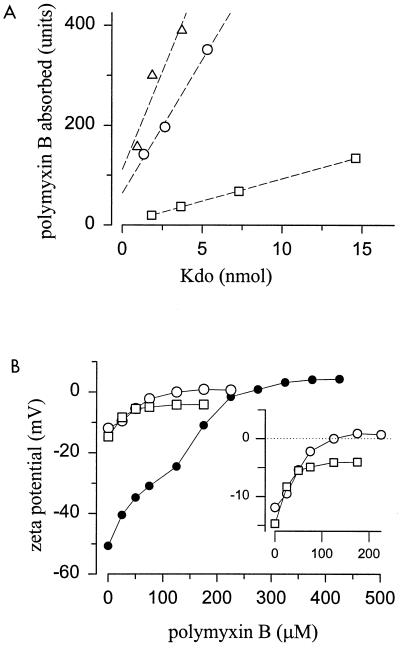
The above-described effects were due, at least in part, to the action of polymyxin B on LPSs. First, this lipopeptide also altered the permeability of the S-LPS aggregates of E. coli and O. anthropi to NPN but had a comparatively reduced effect on the S-LPS of B. abortus or on the O. anthropi-B. abortus S-LPS hybrids (Table 1). Second, either on a dry weight basis (not shown) or with respect to the amount of Kdo (Fig. 4A), the S-LPS of O. anthropi bound polymyxin B in amounts close to those bound by the E. coli S-LPS, and higher than those bound by B. abortus S-LPS. Finally, by measuring the dansyl-cadaverine displacement, the following 50% polymyxin B occupancy values were calculated: 0.66 μM for B. abortus S-LPS; 1.85 μM for O. anthropi S-LPS; and 4.15 μM for O. intermedium R-LPS. In keeping with these results, increasing blue shifts of the maximum peak emission of the dansyl-cadaverine (556 nm) were observed (dansyl-cadaverine–B. abortus S-LPS, 542 nm; dansyl-cadaverine–O. anthropi S-LPS, 524 nm; and dansyl-cadaverine–O. intermedium R-LPS, 496 nm).
FIG. 4.
Interaction of polymyxin B with O. anthropi S-LPS (○), O. intermedium R-LPS (●), B. abortus S-LPS (□), and E. coli S-LPS (▵). (A) Polymyxin B absorbed by increasing amounts of S-LPSs; (B) zeta potential (ζsm) of LPS aggregates on dependence of polymyxin B concentration (each point corresponds to six measurements, and the standard deviations are included in the size of the symbol).
Zeta potential ζsm of LPS in dependence on polymyxin B concentration.
Charged components of lipid express at the surface of aggregates an electrical potential extending into the aqueous bulk solution that modulates the binding or repulsion of polyions and that can be assessed as the zeta potential ζsm (24). The sizes of the B. abortus and O. anthropi S-LPS aggregates (134.4 nm [polydispersity, 0.175] and 102.3 nm [polydispersity, 0.223], respectively) were very close and, in the absence of polymyxin B, had very close ζsm values (−14.7 and −11.9 mV, respectively [Fig. 4B]). As expected, the absence of the O chain in the R-LPS of O. intermedium caused larger particle size (236.2 nm [polydispersity, 0.281]) of the aggregates and a more negative ζsm (−50.7 mV). Because of their negative value, the ζsm of the three LPS preparations became progressively neutralized upon addition of polymyxin B (Fig. 4B). However, whereas saturation of O. anthropi S- and R-LPS with polymyxin B produced aggregates with positive ζsm values (0.9 and 4.1 mV, respectively), saturation of the B. abortus S-LPS did not neutralize the aggregates completely, with ζsm values of −4.1 mV, suggesting that not all negatively charged groups were accessible.
Chemical analysis of LPS.
The isolated lipids A of B. abortus, O. anthropi, and O. intermedium contained 2,3-dideoxy,2,3-diaminoglucose (diaminoglucose) but no 2-deoxy,2-aminoglucose (glucosamine), ethanolamine, ornithine, heptose, or Kdo. The three lipids A showed similar qualitative fatty acid compositions (Table 2), with the presence of 27-OH-C28:0 and minor amounts of 29-OH-C30:0, 27-keto-C28:0, and 29-keto-C30:0. Lactobacillic acid (C19 cyclopropane) and C18:1 were also present. All 3-hydroxylated fatty acids were amide bound. TLC analyses revealed that the three lipids A consisted mostly of bis- and monophosphorylated forms in similar proportions. Larger amounts of purified lipid A were obtained from the O. intermedium R-LPS, and this preparation was analyzed further. It contained mostly hexa-acylated lipid A forms and by LD-MS showed major peaks at m/z 2064 and 2078 (M + Cs)+ and 1954 and 1968 (M + Na)+. These peaks correspond to C115H218N4O18 (molecular weight, 1,945) and C114H216N4O18 (molecular weight, 1,931), equivalent to a diaminoglucose disaccharide carrying amide-bound 12:O(3-OH), 14:O(3-OH), 19cyclo:O[3-O(14:O)] or 18:1[3-O(14:0)], and 28:O(27-OH)[3-O(16:0)]. Heterogeneity in other fatty acids was also observed.
TABLE 2.
Fatty acids identified in the lipids A of B. abortus 2308, O. anthropi LMG3331, and O. intermedium LMG33301
| Fatty acids | Type of linkagea | Amt (nmol/mg) in lipid A of:
|
||
|---|---|---|---|---|
| B. abortus 2308 | O. anthropi LMG3331 | Ochrobactrum intermedium LMG3301 | ||
| 12:0(3-OH) | A | 86 | 44 | 51 |
| 14:0(3-OH) | A | 126 | 208 | 362 |
| 16:0(3-OH) | A | 221 | 239 | 474 |
| 18:0(3-OH) | A | 48 | 215 | 57 |
| 12:1 | E | 48 | 76 | 79 |
| 16:0 | E | 266 | 51 | 31 |
| 18:0 | E | 71 | 244 | 76 |
| 18:1 | E | 15 | 135 | 82 |
| 19c:0 | E | 244 | 64 | 210 |
| 28:0(27-OH) | E | 118 | 185 | 359 |
| 28:0(27-keto) | E | 52 | 49 | 18 |
| 30:0(29-OH) | E | 17 | 56 | 23 |
| 30:0(29-keto) | E | 8 | 17 | 10 |
A, amide; E, ester.
Results of sugar analysis of the LPSs are presented in Table 3. In addition to the O-chain sugars reported previously (N-formyl-perosamine for B. abortus and glucosamine plus rhamnose for O. anthropi LMG3331) (9, 61), O. anthropi S-LPS contained mannose, glucose, and galacturonic acid, all previously shown to be constituents of the core of the LPS of O. intermedium LMG3301 (60), plus galactose and quinovosamine (2-amino,2,6-dideoxy-d-glucose). As reported previously (9, 36, 69), mannose, glucose, glucosamine, and quinovosamine, but not galactose or galacturonic acid, were detected in B. abortus LPS.
TABLE 3.
Sugar analysis of LPSs of B. abortus 2308, O. anthropi LMG3331, and O. intermedium LMG3301
| Bacterium | Amt (nmol/mg of LPS)a
|
|||||||
|---|---|---|---|---|---|---|---|---|
| N-formyl-perosamine | Rha | Man | Glc | Gal | GlcNe | QuiN | GalAd | |
| B. abortus 2308 | +++ | 34.7 | 73.5 | — | 42.4 | + | − | |
| O. anthropi LMG3331 | − | +++ | 6.5 | 103.9 | 36.7 | +++ | + | 36.5 |
| O. intermedium LMG3301 | − | 4.2 | 19.7 | 268.7 | 4.0 | 189.4 | 0.0 | 179.5 |
+++, O-chain sugars present in large amounts but not quantified; +, present in significant amounts but not quantified; —, not detected.
DISCUSSION
The results of this and previous works (13, 31, 32) show that a high polycation resistance and a permeability to hydrophobic compounds are traits that mark a clear difference between Brucella and many gram-negative bacteria, significantly including those that are phylogenetically closest. EDTA resistance, on the other hand, was displayed by both Brucella and Ochrobactrum, although not by many other gram-negative bacteria. The significance of these findings, and their structural basis and implications in the adaptive evolution of Brucella to pathogenicity, are discussed below.
Many gram-negative bacteria are able to defend against noxious hydrophobic compounds by the combined effects of an efficient OM barrier and efflux pumps (41, 43, 44, 58). Conspicuous exceptions are Brucella and some bacteria carrying lipooligosaccharides (20). Based on the data for a phospholipid exposure in S. enterica serovar Typhimurium deep R mutants and on the model proposed to explain the effect of EDTA (see below) in enteric bacteria and pseudomonads (41), we have suggested (31) that phospholipid exposure on the outer leaflet of B. abortus OMs could account for its permeability to hydrophobic agents. Although this possibility cannot be rigorously ruled out, the results presented here demonstrate that the properties of Brucella LPS can explain such permeability. First, O. anthropi-B. abortus S-LPS chimeras, but not O. anthropi-E. coli S-LPS chimeras, were readily permeable to NPN. Second, LPS aggregates of E. coli, O. anthropi, and B. abortus showed an NPN permeability that correlated with that of the respective OMs. Although some efflux pumps are active on NPN (43), our experiments were performed in the absence of metabolic inhibitors; therefore, it is clear that Brucella lacks pumps active on this substrate and that, if present in Ochrobactrum, they could not compensate for the inward NPN flow caused by the B. abortus LPS in the OM of the chimeras. It is important to note that the results obtained with the O. intermedium-E. coli S-LPS control chimeras rule out an artifactual permeability to NPN in this kind of constructs.
Divalent cation bridging of negatively charged groups of the lipid A and core oligosaccharide is essential for the OM to act as an effective barrier, and it is thought that the partial removal of LPS caused by EDTA is compensated for by a phospholipid flip which breaks the barrier (41). Accordingly, the observation that EDTA has no effect on cells and LPS of Brucella (13, 31, 32, 38) and Ochrobactrum has functional and structural implications: in these bacteria, divalent cation stabilization of LPS neither plays a role in permeability nor is necessary for a firm anchoring of the LPS in the OM. The lipids A of both B. abortus and Ochrobactrum contained longer acyl chains than the lipids A of E. coli, Salmonella, pseudomonads, and other bacteria sensitive to EDTA; they included 28:0(27-OH) and 30:0(29-OH), two fatty acids proposed to be spanning the OM. This lipid A structure should result in a more firm anchorage and explain why further stabilization by divalent cations is not necessary. FTIR analyses also showed differences in a relevant physicochemical property between B. abortus and O. anthropi LPSs on one hand and E. coli LPS on the other. These analyses were performed to test whether differences in permeability correlated with acyl chain fluidity, as previously shown for Yersinia pseudotuberculosis and Y. pestis (3). Although this possibility was ruled out, the analyses showed that the fluidity profiles of O. anthropi and B. abortus S-LPS were identical, and this is in agreement with the similar overall lipid A composition found in chemical analyses. Interestingly, although their Tc were similar, the fluidity profiles of O. anthropi and B. abortus on the one hand and of E. coli on the other were different, particularly at temperatures below Tc. In all likelihood, this reflects the markedly different fatty acid compositions and the presence of unsaturated fatty acids in the lipids A of B. abortus and Ochrobactrum. Although such fatty acids have been detected seldom in lipids A, they have been found in the lipid A of Rhodobacter spp. (68), a phylogenetic relative of Brucella and Ochrobactrum (35), and in Legionella (33, 67).
The resistance to EDTA does not mean that differences in anionic groups of the LPS core, possibly complemented by efflux pumps in Ochrobactrum, could not contribute to differences in permeability. In fact, the following evidence supports this possibility. Brucella LPS binds lesser amounts polycationic peptides than E. coli and S. enterica serovar Montevideo S-LPS (13, 32), and this explains the comparative resistance of Brucella to the bactericidal peptides of leukocytes. Based on the factors known to modulate the self-promoted polymyxin B action (63), the described mechanisms of polymyxin B and polycation resistance (18, 19, 21, 42, 56, 58), and the phospholipid (35) and lipid A composition of Brucella and Ochrobactrum (see above), three non-mutually exclusive hypotheses could explain this reduced binding: (i) the unusual O polysaccharide (an N-formyl-perosamine homopolymer [9]) of S brucellae could efficiently block access to inner anionic groups; (ii) the Brucella LPS could contain a reduced proportion of anionic groups (Kdo and phosphate) or these groups could be replaced (17, 18, 21, 42, 56); and (iii) the sort of fatty acid lipid A could contribute to resistance to amphipathic polycations (19). By comparing S and R Brucella variants and the N-formyl-perosamine-bearing Y. enterocolitica O:9 (45), the O polysaccharide was shown previously to play only a minor role (13, 32). Hypothesis ii is in agreement with the contrasting polycationic peptide sensitivity of O. anthropi and B. abortus cells, the polymyxin binding and displacement experiments performed with the LPSs, the intermediate effect of polymyxin B on hybrid LPS aggregates, and the chemical data for the core and lipid A. In addition to the strong similarities at lipid A level (backbone, acyl chain substitution, and degree of phosphorylation [this work and references 37 and 48), the cores of the LPSs of Ochrobactrum and B. abortus (this work and references 36, 60, and 69) were similar in that both lacked heptose and phosphate and contained mannose, glucosamine, quinovosamine (in O. anthropi), and Kdo. On the other hand, O. intermedium and O. anthropi but not B. abortus (this work and references 9, 36, and 69) or the R Brucella spp. characterized (57) contain galacturonic acid. Galacturonic or glucuronic acid but not amino sugars are also present in the LPS core of the α-2 Proteobacteria characterized so far (8), and since these oligosaccharides are often conserved in phylogenetically related bacteria, their absence in Brucella LPS is conspicuous. This is likely to be a key structural difference contributing to the peculiarities of the Brucella OM.
In addition to the absence of galacturonic acid, other factors might be at play, including those of hypothesis iii. Electron microscopy showed that although self-promoted uptake occurred, polymyxin B did not alter the morphology of the Ochrobactrum OM. Perusal of the literature shows that such absence of morphological effects in polymyxin-sensitive bacteria has not been observed before. However, the characteristic OM blebbing that marks the self-promoted uptake has been observed with bacteria (enterics, pseudomonads, and others) that have lipids A and envelopes markedly different from those of Ochrobactrum (41, 58). Thus, because of the detergent-like action of polymyxin B (63), it might be that the longer average span of the lipid A and phospholipid acyl chains and the postulated stronger anchorage prevent disruption of the OM into small polymyxin B-LPS aggregates. In fact, an effect of the acyl chain composition of lipid A on polycation binding has also been observed in S. enterica serovar Typhimurium (19), and under certain conditions, O. intermedium is relatively resistant to polymyxin B (62). Although these factors by themselves cannot account for the high polycation resistance of the brucellae, they could reduce the disrupting action of amphipathic polycations. An additional effect was suggested by the measurements of the zeta potential ζsm of B. abortus and O. anthropi LPSs on dependence on polymyxin B concentration. Although the multimolecular nature of the LPS aggregates prevents an unequivocal determination of the binding stoichiometry, the sizes of the O. anthropi and B. abortus S-LPS were almost identical making comparisons possible. This, and the fact that the surface of the LPS aggregates mimics the exposed surface of the OM, makes this sort of analyses particularly meaningful for the purpose of the present work. Thus, it is noteworthy that an excess of polymyxin B produced a positive ζsm in the LPS of O. anthropi (and of O. intermedium) while not neutralizing completely the negative ζsm of B. abortus S-LPS. This suggests that the latter S-LPS is comparatively resistant to cationic peptides not only because of a reduction of acidic groups but also because access to them is sterically hindered.
How the above-discussed Brucella OM features are linked to pathogenicity is known only in part; the clearest evidence concerns the LPS. In contrast to the O antigen, the overall physicochemical features that depend on the core and lipid A section (i.e., a dense negative charge at core level and the strongly hydrophobic character of lipid A) are highly conserved due to functional constrains, in part related to the OM barrier function (41). Thus, they constitute a molecular pattern recognized by innate immune systems (22, 53), and its alteration should profoundly modulate the response of vertebrate and invertebrate animal cells to LPS. As such systems include bactericidal peptides and proteins that need to penetrate the OM to cause extensive damage (58), the alteration in Brucella LPS of the typical physicochemical pattern constitutes a virulence factor accounting in part for the previously shown resistance to the oxygen-independent systems of phagocytes (13, 32, 50). Moreover, the same molecular pattern is recognized by LPS cell receptors and shuttle proteins (2, 53), and on this basis an anomalous or diminished recognition of Brucella LPS can be predicted. Indeed, like Brucella LPS, Legionella LPS is resistant to polycations, carries the same type of fatty acids in the lipid A, and shows reduced endotoxicity due to poor interaction with the receptors of typical LPSs (40). Thus, such factors are likely to contribute to the reduced stimulation (low endotoxicity) of the host competent cells by Brucella LPS and cells demonstrated before (27, 49), a property useful for an intracellular parasite. Experiments are in progress to test some aspects of these hypotheses.
Finally, it is also worthwhile to comment briefly on the evolutionary meaning of the results of the present work. As pointed out before (35), the data available suggest that many members of the α-2 Proteobacteria share an OM pattern whose most relevant structural features are phosphatidylcholine as a major phospholipid, the presence of positively charged ornithine lipids, possibly shielding the negatively charged groups of LPS, and the dominance of fatty acids longer than those found in the enterics in both free lipids and lipid A. This pattern offers a starting point on which the strong hydrophobicity of the lipid A of many α-2 Proteobacteria is complemented in Brucella by modifications of the LPS core and by the acquisition of N-formyl-perosamine O-chain genes as two of the steps in the adaptive evolution of these bacteria to their present intracellular niche.
ACKNOWLEDGMENTS
This research was supported by a PIUNA grant from the University of Navarra, by the Deutsche Forschungsgemeinschaft (SFB 470 project B5 and projects ZA 149/3-2 and LU514/2-2), by CR-USA agreement 7/10-(4E)-99, and by the Agencia Española de Cooperación Internacional-Instituto de Cooperación Iberoamericana. Fellowship support for J. Velasco from the Ministerio de Educación, Ciencia y Tecnología of Spain and for J. A. Bengoechea from the Eusko Jaurlaritza is gratefully acknowledged.
REFERENCES
- 1.Aragón V, Díaz R, Moreno E, Moriyón I. Characterization of Brucella abortus and Brucella melitensis native haptens as outer membrane O-type polysaccharides independent from the smooth lipopolysaccharide. J Bacteriol. 1996;178:1070–1079. doi: 10.1128/jb.178.4.1070-1079.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beamer L J, Carroll S F, Eisenberg D. The BPI/LBP family of proteins: a structural analysis of conserved regions. Protein Sci. 1998;7:906–914. doi: 10.1002/pro.5560070408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bengoechea J A, Brandenburg K, Seydel U, Díaz R, Moriyón I. Yersinia pseudotuberculosis and Yersinia pestis show increased outer membrane permeability to hydrophobic agents which correlates with lipopolysaccharide acyl-chain fluidity. Microbiology. 1998;144:1517–1526. doi: 10.1099/00221287-144-6-1517. [DOI] [PubMed] [Google Scholar]
- 4.Bengoechea J A, Lindner B, Seydel U, Díaz R, Moriyón I. Yersinia pseudotuberculosis and Yersinia pestis are more resistant to bactericidal cationic peptides than Yersinia enterocolitica. Microbiology. 1998;144:1509–1515. doi: 10.1099/00221287-144-6-1509. [DOI] [PubMed] [Google Scholar]
- 5.Brandenburg K, Seydel U. Investigation into the fluidity of lipopolysaccharide and free lipid A membrane systems by Fourier-transform infrared spectroscopy and differential scanning calorimetry. Eur J Biochem. 1990;191:229–236. doi: 10.1111/j.1432-1033.1990.tb19114.x. [DOI] [PubMed] [Google Scholar]
- 6.Brandenburg K, Seydel U. Fourier transformed infrared spectroscopy of cell surface polysaccharides. In: Mantsch H H, Chapman D, editors. Infrared spectroscopy of biomolecules. London, England: Wiley-Liss, Inc.; 1996. pp. 203–237. [Google Scholar]
- 7.Cafiso D, McLaughlin A, McLaughlin S, Wimshi A. Measuring electrostatic potentials adjacent of membranes. Methods Enzymol. 1983;171:342–346. doi: 10.1016/s0076-6879(89)71019-3. [DOI] [PubMed] [Google Scholar]
- 8.Carlson R W, Reuhs B, Forsberg L S, Kannenberg E L. Rhizobial cell surface carbohydrates: their structures, biosynthesis, and functions. In: Goldberg J B, editor. Genetics of bacterial polysaccharides. Boca Raton, Fla: CRC Press; 1999. pp. 53–90. [Google Scholar]
- 9.Caroff M, Bundle D R, Perry M B, Cherwonogrodzky J W, Duncan J R. Antigenic S-type lipopolysaccharide of Brucella abortus 1119-3. Infect Immun. 1984;46:384–388. doi: 10.1128/iai.46.2.384-388.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang H J, Christenson J C, Pavia A T, Bobrin B D, Bland L A, Carson L A, Arduino M J, Verma P, Aguero S M, Carroll K, Jenkins E, Daly J A, Woods M L, Jarvis W R. Ochrobactrum anthropi meningitis in pediatric pericardial allograft transplant recipients. J Infect Dis. 1996;173:656–660. doi: 10.1093/infdis/173.3.656. [DOI] [PubMed] [Google Scholar]
- 11.Cieslak T J, Drabick C J, Robb M L. Pyogenic infections due to Ochrobactrum anthropi. Clin Infect Dis. 1996;22:845–847. doi: 10.1093/clinids/22.5.845. [DOI] [PubMed] [Google Scholar]
- 12.Cloeckaert A, Tibor A, Zygmunt M S. Brucella outer membrane lipoproteins share antigenic determinants with bacteria of the family Rhizobiaceae. Clin Diagn Lab Immunol. 1999;6:627–629. doi: 10.1128/cdli.6.4.627-629.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Freer E, Moreno E, Moriyón I, Pizarro-Cerdá J, Weintraub A, Gorvel J P. Brucella-Salmonella lipopolysaccharide chimeras are less permeable to hydrophobic probes and more sensitive to cationic peptides and EDTA than are their native Brucella sp. counterparts. J Bacteriol. 1996;178:5867–5876. doi: 10.1128/jb.178.20.5867-5876.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Freer E, Rojas N, Weintraub A, Lindberg A A, Moreno E. Heterogeneity of Brucella abortus lipopolysaccharides. Res Microbiol. 1995;146:569–578. doi: 10.1016/0923-2508(96)80563-8. [DOI] [PubMed] [Google Scholar]
- 15.Galanos C, Lüderitz O, Westphal O. A new method for the extraction of R lipopolysaccharide. Eur J Biochem. 1969;9:245–249. doi: 10.1111/j.1432-1033.1969.tb00601.x. [DOI] [PubMed] [Google Scholar]
- 16.Gill M V, Ly H, Mueenuddin M, Schoch P E, Cunha B A. Intravenous line infection due to Ochrobactrum anthropi (CDC group Vd) in a normal host. Heart Lung. 1997;26:335–336. doi: 10.1016/s0147-9563(97)90092-3. [DOI] [PubMed] [Google Scholar]
- 17.Gunn J S, Lim K B, Krueger J, Kim K, Guo L, Hackett M, Miller S I. PmrA-PmrB-regulated genes necessary for 4-aminoarabinose lipid A modification and polymyxin resistance. Mol Microbiol. 1998;27:1171–1182. doi: 10.1046/j.1365-2958.1998.00757.x. [DOI] [PubMed] [Google Scholar]
- 18.Guo L, Lim K B, Gunn J S, Bainbridge B, Darveau R P, Hackett M, Miller S I. Regulation of lipid A modifications by Salmonella typhimurium virulence genes phoP-phoQ. Science. 1997;276:250–253. doi: 10.1126/science.276.5310.250. [DOI] [PubMed] [Google Scholar]
- 19.Guo L, Lim K B, Poduje C M, Daniel M, Gunn J S, Hackett M, Miller S I. Lipid A acylation and bacterial resistance against vertebrate antimicrobial peptides. Cell. 1998;95:189–198. doi: 10.1016/s0092-8674(00)81750-x. [DOI] [PubMed] [Google Scholar]
- 20.Hancock R E. Alterations in outer membrane permeability. Annu Rev Microbiol. 1984;38:237–264. doi: 10.1146/annurev.mi.38.100184.001321. [DOI] [PubMed] [Google Scholar]
- 21.Helander I M, Kato Y, Kilpelainen I, Kostiainen R, Lindner B, Nummila K, Sugiyama T, Yokochi T. Characterization of lipopolysaccharides of polymyxin-resistant and polymyxin-sensitive Klebsiella pneumoniae O3. Eur J Biochem. 1996;237:272–278. doi: 10.1111/j.1432-1033.1996.0272n.x. [DOI] [PubMed] [Google Scholar]
- 22.Hoffmann J A, Kafatos F C, Janeway C A, Ezekowitz R A. Phylogenetic perspectives in innate immunity. Science. 1999;284:1313–1318. doi: 10.1126/science.284.5418.1313. [DOI] [PubMed] [Google Scholar]
- 23.Holmes B, Popoff M, Kiredjian M, Kersters K. Ochrobactrum anthropi gen. nov., sp. nov. from human clinical specimens and previously known as group Vd. Int J Syst Bacteriol. 1988;38:406–416. [Google Scholar]
- 24.Hunter R J. Zeta potential in colloid science. London, England: Academic Press; 1981. [Google Scholar]
- 25.Jelveh N, Cunha B A. Ochrobactrum anthropi bacteremia. Heart Lung. 1999;28:145–146. doi: 10.1053/hl.1999.v28.a94602. [DOI] [PubMed] [Google Scholar]
- 26.Kern W V, Oethinger M, Kaufhold A, Rozdzinski E, Marre R. Ochrobactrum anthropi bacteremia: report of four cases and short review. Infection. 1993;21:306–310. doi: 10.1007/BF01712451. [DOI] [PubMed] [Google Scholar]
- 27.Kreutzer D L, Dreyfus L A, Robertson D C. Interaction of polymorphonuclear leukocytes with smooth and rough strains of Brucella abortus. Infect Immun. 1979;23:737–742. doi: 10.1128/iai.23.3.737-742.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kronvall G. A rapid slide agglutination method for typing pneumococci by means of specific antibody adsorbed to protein A-containing staphylococci. J Med Microbiol. 1973;6:187–190. doi: 10.1099/00222615-6-2-187. [DOI] [PubMed] [Google Scholar]
- 29.Leong D, Díaz R, Milner K, Rudbach J, Wilson J B. Some structural and biological properties of Brucella endotoxin. Infect Immun. 1970;1:174–182. doi: 10.1128/iai.1.2.174-182.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Markwell M A, M. H S, Bieber L L, Tolbert N E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978;87:206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- 31.Martínez de Tejada G, Moriyón I. The outer membranes of Brucella spp. are not barriers to hydrophobic permeants. J Bacteriol. 1993;175:5273–5275. doi: 10.1128/jb.175.16.5273-5275.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martínez de Tejada G, Pizarro-Cerdá J, Moreno E, Moriyón I. The outer membranes of Brucella spp. are resistant to bactericidal cationic peptides. Infect Immun. 1995;63:3054–3061. doi: 10.1128/iai.63.8.3054-3061.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moll H, Sonesson A, Jantzen E, Marre R, Zähringer U. Identification of 27-oxo-octacosanoic acid and heptacosane-1,27-dioic acid in Legionella pneumophila. FEMS Microbiol Lett. 1992;76:1–6. doi: 10.1016/0378-1097(92)90354-q. [DOI] [PubMed] [Google Scholar]
- 34.Moller L V M, Arends J P, Harmsen H J M, Talens A, Terpstra P, Slooff M J H. Ochrobactrum intermedium infection after liver transplantation. J Clin Microbiol. 1999;37:241–244. doi: 10.1128/jcm.37.1.241-244.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moreno E. Evolution of Brucella. In: Plommet M, editor. Prevention of brucellosis in the Mediterranean countries. Wageningen, The Netherlands: Pudoc Scientific Publishers; 1992. pp. 198–218. [Google Scholar]
- 36.Moreno E, Jones L M, Berman D T. Immunochemical characterization of rough Brucella lipopolysaccharides. Infect Immun. 1984;43:779–782. doi: 10.1128/iai.43.3.779-782.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moreno E, Stackebrandt E, Dorsch M, Wolters J, Busch M, Mayer H. Brucella abortus 16S rRNA and lipid A reveal a phylogenetic relationship with members of the alpha-2 subdivision of the class Proteobacteria. J Bacteriol. 1990;172:3569–3576. doi: 10.1128/jb.172.7.3569-3576.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moriyón I, Berman D T. Effects of nonionic, ionic, and dipolar ionic detergents and EDTA on the Brucella cell envelope. J Bacteriol. 1982;152:822–828. doi: 10.1128/jb.152.2.822-828.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moriyón I, López-Goñi I. Structure and properties of the outer membranes of Brucella abortus and Brucella melitensis. Int Microbiol. 1998;1:19–26. [PubMed] [Google Scholar]
- 40.Neumeister B, Faigle M, Sommer M, Zähringer U, Stelter F, Menzel R, Schutt C, Northoff H. Low endotoxic potential of Legionella pneumophila lipopolysaccharide due to failure of interaction with the monocyte lipopolysaccharide receptor CD14. Infect Immun. 1998;66:4151–4157. doi: 10.1128/iai.66.9.4151-4157.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nikaido H, Vaara M. Molecular basis of bacterial outer membrane permeability. Microbiol Rev. 1985;49:1–32. doi: 10.1128/mr.49.1.1-32.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nummila K, Kilpelainen I, Zähringer U, Vaara M, Helander I M. Lipopolysaccharides of polymyxin B-resistant mutants of Escherichia coli are extensively substituted by 2-aminoethyl pyrophosphate and contain aminoarabinose in lipid A. Mol Microbiol. 1995;16:271–278. doi: 10.1111/j.1365-2958.1995.tb02299.x. [DOI] [PubMed] [Google Scholar]
- 43.Ocaktan A, Yoneyama H, Nakae T. Use of fluorescence probes to monitor function of the subunit proteins of the MexA-MexB-oprM drug extrusion machinery in Pseudomonas aeruginosa. J Biol Chem. 1997;272:21964–21969. doi: 10.1074/jbc.272.35.21964. [DOI] [PubMed] [Google Scholar]
- 44.Paulsen I T, Brown M H, Skurray R A. Proton-dependent multidrug efflux systems. Microb Rev. 1996;60:575–608. doi: 10.1128/mr.60.4.575-608.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Perry M B, Bundle D R. Lipopolysaccharide antigens and carbohydrates of Brucella. In: Adams L G, editor. Advances in brucellosis research. College Station, Tex: Texas A&M University Press; 1990. pp. 76–88. [Google Scholar]
- 46.Pizarro-Cerdá J, Meresse S, Parton R G, van der Goot G, Sola-Landa A, López-Goñi I, Moreno E, Gorvel J P. Brucella abortus transits through the autophagic pathway and replicates in the endoplasmic reticulum of nonprofessional phagocytes. Infect Immun. 1998;66:5711–5724. doi: 10.1128/iai.66.12.5711-5724.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pizarro-Cerdá J, Moreno E, Sanguedolce V, Mege J L, Gorvel J P. Virulent Brucella abortus prevents lysosome fusion and is distributed within autophagosome-like compartments. Infect Immun. 1998;66:2387–2392. doi: 10.1128/iai.66.5.2387-2392.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Qureshi N, Takayama K, Seydel U, Wang R, Cotter R J, Agrawal P K, Bush C A, Kurtz R S, Berman D T. Structural analysis of the lipid A from the lipopolysaccharide of Brucella abortus. J Endotoxin Res. 1994;1:137–148. [Google Scholar]
- 49.Rasool O, Freer E, Moreno E, Jarstrand C. Effect of Brucella abortus lipopolysaccharide on oxidative metabolism and lysozyme release by human neutrophils. Infect Immun. 1992;60:1699–1702. doi: 10.1128/iai.60.4.1699-1702.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Riley L K, Robertson D C. Brucellacidal activity of human and bovine polymorphonuclear leukocyte granule extracts against smooth and rough strains of Brucella abortus. Infect Immun. 1984;46:231–236. doi: 10.1128/iai.46.1.231-236.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rudbach J A, Milner K C, Ribi E. Hybrid formation between bacterial endotoxins. J Exp Med. 1967;126:63–79. doi: 10.1084/jem.126.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Saavedra J, Garrido C, Folgueira D, Torres M J, Ramos J T. Ochrobactrum anthropi bacteremia associated with a catheter in an immunocompromised child and review of the pediatric literature. Pediatr Infect Dis J. 1999;18:658–660. doi: 10.1097/00006454-199907000-00023. [DOI] [PubMed] [Google Scholar]
- 53.Schromm A B, Brandenburg K, Loppnow H, Zähringer U, Rietschel E T, Carroll S F, Koch M H, Kusumoto S, Seydel U. The charge of endotoxin molecules influences their conformation and IL-6-inducing capacity. J Immunol. 1998;161:5464–5471. [PubMed] [Google Scholar]
- 54.Seydel U, Lindner B, Wollenweber H W, Rietschel E T. Structural studies on the lipid A component of enterobacterial lipopolysaccharides by laser desorption mass spectrometry. Eur J Biochem. 1984;145:505–509. doi: 10.1111/j.1432-1033.1984.tb08585.x. [DOI] [PubMed] [Google Scholar]
- 55.Sola-Landa A, Pizarro-Cerdá J, Grilló M J, Moreno E, Moriyón I, Blasco J M, Gorvel J P, López-Goñi I. A two-component regulatory system playing a critical role in plant pathogens and endosymbionts is present in Brucella abortus and controls cell invasion and virulence. Mol Microbiol. 1998;29:125–138. doi: 10.1046/j.1365-2958.1998.00913.x. [DOI] [PubMed] [Google Scholar]
- 56.Stinavage P, Martin L E, Spitznagel J. O antigen and lipid A phosphoryl groups in resistance of Salmonella typhimurium LT-2 to nonoxidative killing in human polymorphonuclear neutrophils. Infect Immun. 1989;57:3894–3900. doi: 10.1128/iai.57.12.3894-3900.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Suárez C E, Pacheco G A, Vigliocco A M. Immunochemical studies of oligosaccharides obtained from the lipopolysaccharide of Brucella ovis. Vet Microbiol. 1990;22:329–334. doi: 10.1016/0378-1135(90)90019-r. [DOI] [PubMed] [Google Scholar]
- 58.Vaara M. Agents that increase the permeability of the outer membrane. Microbiol Rev. 1992;56:395–411. doi: 10.1128/mr.56.3.395-411.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Velasco J, Díaz R, Grilló M J, Barberán M, Marín C M, Blasco J M, Moriyón I. Antibody and delayed-type hypersensitivity responses to Ochrobactrum anthropi cytosolic and outer membrane antigens in infections by smooth and rough Brucella spp. Clin Diagn Lab Immunol. 1997;4:279–284. doi: 10.1128/cdli.4.3.279-284.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Velasco J, Moll H, Knirel Y A, Sinnwell V, Moriyón I, Zähringer U. Structural studies on the lipopolysaccharide from a rough strain of Ochrobactrum anthropi containing a 2,3-diamino-2,3-dideoxy-d-glucose disaccharide lipid A backbone. Carbohydr Res. 1998;306:283–290. doi: 10.1016/s0008-6215(97)10029-5. [DOI] [PubMed] [Google Scholar]
- 61.Velasco J, Moll H, Vinogradov E V, Moriyón I, Zähringer U. Determination of the O-specific polysaccharide structure in the lipopolysaccharide of Ochrobactrum anthropi LMG 3331. Carbohydr Res. 1996;287:123–126. doi: 10.1016/0008-6215(96)00068-7. [DOI] [PubMed] [Google Scholar]
- 62.Velasco J, Romero C, López-Goñi I, Leiva J, Díaz R, Moriyón I. Evaluation of the relatedness of Brucella spp. and Ochrobactrum anthropi and description of Ochrobactrum intermedium sp. nov., a new species with a closer relationship to Brucella spp. Int J Syst Bacteriol. 1998;48:759–768. doi: 10.1099/00207713-48-3-759. [DOI] [PubMed] [Google Scholar]
- 63.Wiese A, Münstermann M, Gutsmann T, Lindner B, Kawahara K, Zähringer U, Seydel U. Molecular mechanisms of polymyxin B-membrane interactions: direct correlation between surface charge density and self-promoted transport. J Membr Biol. 1998;162:127–138. doi: 10.1007/s002329900350. [DOI] [PubMed] [Google Scholar]
- 64.Wollenweber H W, Rietschel E T. Analysis of lipopolysaccharide (lipid A) fatty acids. J Microbiol Methods. 1990;11:195–211. [Google Scholar]
- 65.Yanagi M, Yamasato K. Phylogenetic analysis of the family Rhizobiaceae and related bacteria by sequencing of 16S rRNA gene using PCR and DNA sequencer. FEMS Microbiol Lett. 1993;107:115–120. doi: 10.1111/j.1574-6968.1993.tb06014.x. [DOI] [PubMed] [Google Scholar]
- 66.Yu W L, Lin C W, Wang D Y. Clinical and microbiologic characteristics of Ochrobactrum anthropi bacteremia. J Formos Med Assoc. 1998;97:106–112. [PubMed] [Google Scholar]
- 67.Zähringer U, Knirel Y A, Lindner B, Helbig J H, Sonesson A, Marre R, Rietschel E T. The lipopolysaccharide of Legionella pneumophila serogroup 1 (strain Philadelphia 1): chemical structure and biological significance. Prog Clin Biol Res. 1995;392:113–139. [PubMed] [Google Scholar]
- 68.Zähringer U, Lindner B, Rietschel E T. Molecular structure of lipid A, the endotoxic center of bacterial lipopolysaccharide. Adv Carbohydr Chem Biochem. 1994;50:211–276. [PubMed] [Google Scholar]
- 69.Zygmunt M S, Dubray G, Bundle D R, Perry M B. Purified native haptens of Brucella abortus B19 and B. melitensis 16M reveal the lipopolysaccharide origin of the antigens. Ann Inst Pasteur Microbiol. 1988;139:421–433. doi: 10.1016/0769-2609(88)90105-6. [DOI] [PubMed] [Google Scholar]