Abstract
Newborn screening (NBS) for severe combined immunodeficiency (SCID) can identify infants with non-SCID T cell lymphopenia (TCL). The purpose of this study was to characterize the natural history and genetic findings of infants with non-SCID TCL identified on NBS. We analyzed data from 80 infants with non-SCID TCL in the mid-Atlantic region between 2012 and 2019. 66 patients underwent genetic testing and 41 (51%) had identified genetic variant(s). The most common genetic variants were thymic defects (33%), defects with unknown mechanisms (12%) and bone marrow production defects (5%). The genetic cohort had significantly lower median initial CD3+, CD4+, CD8+ and CD4/CD45RA+ T cell counts compared to the non-genetic cohort. Thirty-six (45%) had either viral, bacterial, or fungal infection; only one patient had an opportunistic infection (vaccine strain VZV infection). Twenty-six (31%) of patients had resolution of TCL during the study period.
Keywords: T cell lymphopenia (TCL), Newborn screening (NBS), T cell receptor excision circle (TREC), Severe combined immunodeficiency (SCID), Genetic testing, Varicella-zoster virus (VZV), Pneumocystis jirovecii pneumonia (PJP)
Introduction
Severe combined immunodeficiency (SCID) is caused by a variety of genetic defects that affect bone marrow-based production or subsequent survival of T cells, resulting in increased susceptibility to recurrent infections, autoimmunity and failure to thrive [1, 2].Treatment options include hematopoietic cell transplant (HCT), enzyme replacement therapy and gene therapy, depending on the cause [3–5]. Early recognition is important as SCID becomes apparent early in life with only a short asymptomatic period after birth. Immediate and adequate treatment can significantly improve outcomes and prevent death [5–8]. Newborn screening (NBS) for severe SCID uses polymerase chain reaction (PCR) methodology to quantify T cell receptor excision circles (TRECs) as evidence of thymic T cell output, which is impaired in all patients with SCID [9]. In the mid-Atlantic region, states adopted the TREC assay for newborn screening over the last decade with Delaware starting screening in 2012, Pennsylvania in 2013, New Jersey and DC in 2014, Virginia in 2015, and Maryland in 2016 [10, 11]. As of December 10, 2018, all states in the United States have SCID newborn screening programs [12].
In addition to SCID, the TREC assay has identified infants with many forms of non-SCID T cell lymphopenia (TCL) which can include certain syndromes (DiGeorge, ataxia telangiectasia, Coloboma, Heart defect, Atresia choanae, Retarded growth and development, Genital hypoplasia, Ear anomalies/deafness (CHARGE), Jacobsen and Trisomy 21), secondary causes (congenital heart disease, gastrointestinal malformation, leukemia and prematurity) and idiopathic T cell lymphopenia (ITL) [13, 14].The incidence of SCID in the US is estimated to be approximately 1 in 58,000 live births while non-SCID TCL is thought to occur 3 to 4 times more frequently than SCID; however these remain estimates as published data are not yet available from all fifty states [13, 15–17].
Practices for preventing infection in infants with SCID include prophylactic antibiotics, intravenous immunoglobulin (IVIG), avoiding live viral immunizations and protective isolation while awaiting HCT [3, 18]. Unlike SCID, most infants with non-SCID TCL will not require HCT and best practices regarding the workup, preventive care needs, infection risks and long-term management of this population remains unclear despite limited data from regional studies[19–21]. Here, we describe the results of newborn screening utilizing the TREC assay in the mid-Atlantic US over a seven-year period, with a focus on the longitudinal evaluation of infants who have non-SCID TCL.
Material and methods
A retrospective chart review-based study was designed to study infants with abnormal or critical TREC assays evaluated at the following institutions (birth state): Children’s Hospital of Philadelphia (NJ/PA), Nemours Children’s Hospital (DE), Johns Hopkins University (MD), Children’s National Hospital (DC, MD, VA), University of Virginia Health (VA), and Virginia Commonwealth University (VA). Protocols were approved by the Institutional Review Boards of each institution and all research was done in compliance with the Declaration of Helsinki.
Infants evaluated from 2012 – 2019 due to abnormal TREC NBS and found to have non-SCID TCL, as defined by less than 2000 CD3+ cells/μL on flow cytometry, were included in the study. We selected a uniform cutoff value of <2000 cells/μL to maintain consistency within the study, despite highly variable values used in individual state programs [13]. Patients with syndromic features and low T cell counts based on institutional reference values were also included.
The data obtained included: sex, race, gestational age, age at newborn screening, TREC values/Ct, presence of congenital heart disease, exposure to maternal immunosuppressive agents, as well as presence of other hematologic abnormalities, dysmorphisms, neurodevelopmental features, growth abnormalities, and autoimmune disorders. Occurrence of infections and survival as of last follow-up were recorded. Age of resolution of lymphopenia was recorded if applicable. TCL was considered resolved if CD3+ T cells reached 2000 cells/μL on at least one occasion if the patient was less than two years of age, and 1500 cells/μL if the patient was greater than two years of age. Results of genetic evaluations were also recorded where performed.
Other data, if applicable, included age at follow up testing, lymphocyte subsets (CD3, CD4, CD8, CD4/CD45RA, CD19, CD3−/CD16 and/or CD56) from flow cytometry, infections, and treatment. Naive CD4+ and CD8+ T cells were identified using CD45RA as a marker. Additional markers of naive T cells (e.g. CCR7, CD31, CD27) were not universally available for clinical lymphocyte enumeration and so were not included in the analysis.
Descriptive statistics were calculated for demographic and laboratory characteristics. Patient variables included presence of an identified genetic condition, congenital heart disease, lymphocyte-losing state (e.g. protein losing enteropathy, lymphangiectasia, congenital chylothorax), and history of maternal immunosuppression. Laboratory values were compared with patient variables via 2-way ANOVA and multiple T-tests. Logistic regression was performed to compare variables including lymphocyte values, gestational age at birth, age of resolution of lymphopenia (if relevant), and initial TREC Ct. Statistical analyses were performed using Prism 9 (San Diego, CA) and SAS, version 9.4 (Cary, NC).
Results
Demographics
Eighty patients without SCID were identified by positive TREC NBS and confirmed via flow cytometry to have TCL (< 2000 CD3+ cells/μL). Of note, six infants had initial CD3+ counts that were greater than 2000 cells/μL but on repeat testing had CD3+ counts less than 2000 cells/μL or had syndromic features and were therefore included. Of these infants, 45 (56%) were male and 39 (49%) identified as White (Figure 1A). The majority of the cohort (n = 60; 75%) were full term (Figure 1A). There were two sets of twins.
Figure 1. Demographics.
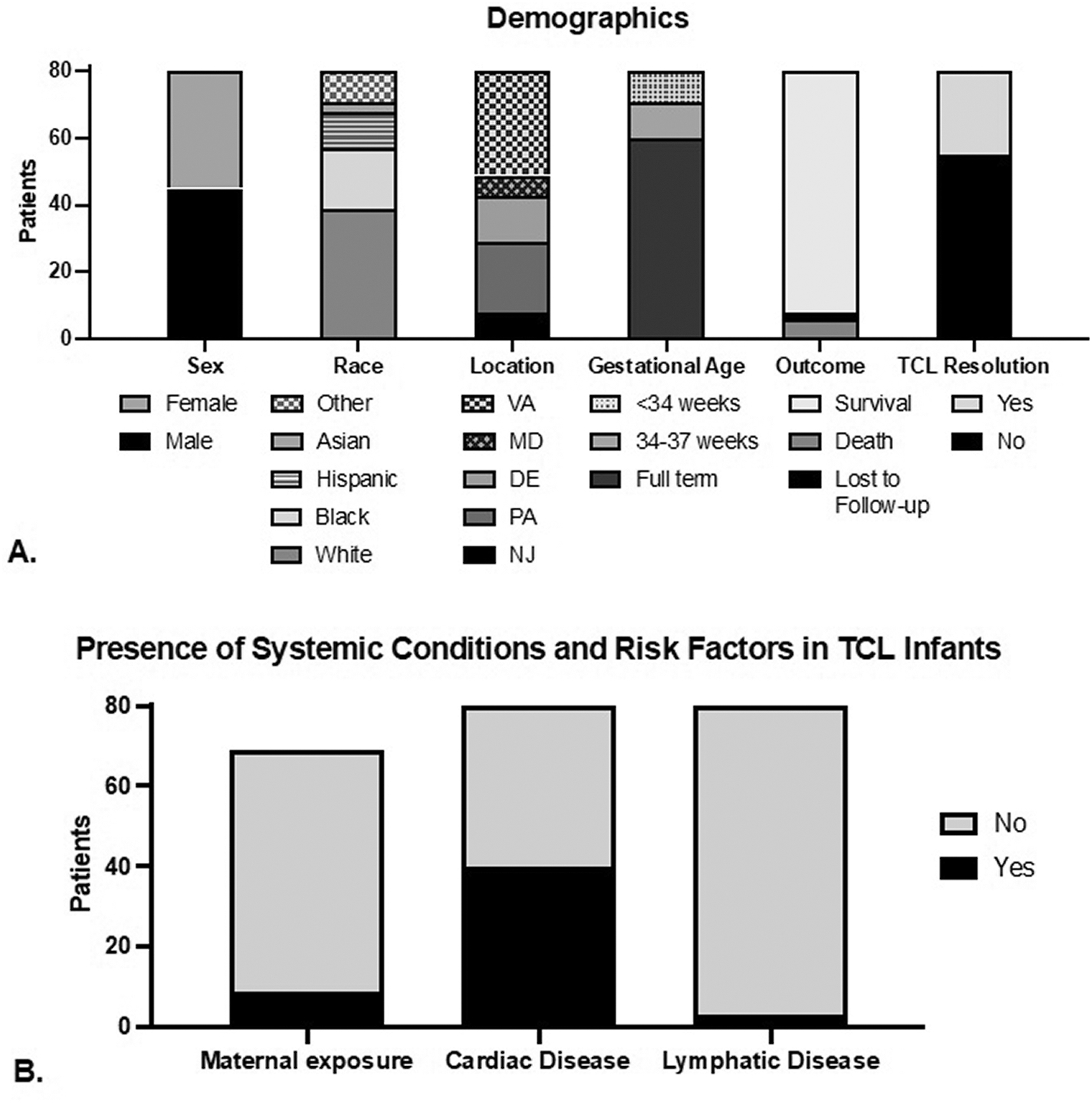
A. Demographics and outcomes in patients with non-SCID T cell lymphopenia in TREC-positive newborns.
B. Presence of Systemic Conditions and Risk Factors in TCL Infants.
Of the patients evaluated, 72 (90%) were alive at the time of data collection while 6 (8%) died of causes such as pulmonary hypertension, cardiogenic shock, cardiac arrest, and sepsis (Figure 1A). Two patients were lost to follow-up at time of record review. TCL was persistent for 55 patients (69%) at time of last evaluation. With regard to potential etiology of the TCL, 10 patients (12.5%) experienced maternal risk factor exposure such as maternal diabetes or immunosuppression, 40 (50%) had evidence of congenital heart disease at birth and/or during infancy and 3 patients (4%) had evidence of lymphatic disease at birth and/or during infancy (Figure 1B).
Immunologic evaluations
The initial median CD3+ T cell count was 1380 cells/μL, CD4+ T cell count was 917 cells/μL, CD8+ T cell count was 397 cells/μL, and CD4/CD45RA+ T cell count was 780 cells/μL. The initial median CD19+ B-cell count was 565 cells/μL while the initial median CD16/CD56+ NK cell count was 244 cells/μL. The initial absolute counts and relative frequencies of T cell subsets are shown in Table E3 in the Online Repository. The initial TREC Ct was available for 37 patients; the median Ct was 35.9. There was no correlation between the initial CD3+ T cell count and the TREC Ct (p = 0.745; Table I). T cell proliferation (thymidine assay or mitogen proliferation) results were available for 57 patients. Of those patients, 17 patients had initial abnormal testing. Eleven patients had repeat evaluation and 10 of those patients had subsequent normal testing.
Table I.
Impact of patient characteristics on initial T cell count and age of T cell lymphopenia resolution.
| Dependent variable | Covariate | t-value | p-value |
|
| |||
| Initial T cell count | Premature birth | −1.82 | 0.078 |
| Initial TREC Ct | 0.325 | 0.745 | |
|
| |||
| Dependent variable | Covariate | z-value | p-value |
|
| |||
| Age of TCL resolution | Maternal immunosuppression | 0.187 | 0.853 |
| Initial TREC Ct | −0.675 | 0.516 | |
| Male gender | −2.084 | 0.050* | |
| Race/White | −0.382 | 0.674 | |
| Race/Non-White | 0.426 | 0.707 | |
| CHD | −0.134 | 0.894 | |
Note:
Significant p-value.
Abbreviations: TREC, T cell receptor excision circle; Ct, cycle threshold; TCL, T cell lymphopenia; CHD, congenital heart disease.
Genetic evaluation
There was an almost equal number of non-SCID TCL patients with identified genetic findings that were likely contributory to the TCL (n = 41) versus patients with no genetic findings (n = 25 with negative genetic testing and n = 14 with no genetic testing performed that were categorized under patients with no genetic findings for the sake of further analysis). Genetic testing included chromosomal microarray (n=49, normal in 31), karyotype (n=17, normal in 15), targeted sequencing (n=36, normal in 10) and/or whole exome sequencing (n=7, normal in 4).
Males comprised 70% of the genetically undefined patients and 44% of those with a genetic diagnosis (P = 0.0178). White patients comprised 48% of the genetically undefined patients and 54% of those with a genetic diagnosis (nonsignificant). Very/extreme-preterm infants were more likely to lack an identified genetic cause of their TCL (8 non-genetic vs. 2 genetic; P = 0.0509).
Genetic variants thought to be the cause of non-SCID TCL were categorized into bone marrow production defects, thymic defects, and defects with unclear mechanisms of action. The largest category of genetic defects leading to non-SCID TCL were thymic defects (33%), followed by defects with unclear mechanisms (12%), and bone marrow production defects (5%) (Figure 2A). Genetic defects involved in bone marrow production included Cartilage Hair Hypoplasia, Noonan syndrome, and a variant of uncertain significance in RMRP and PTPRC (Figure 2B). Thymic defects included partial DiGeorge syndrome due to TBX1 mutation, 22q11.2 Deletion syndrome, Miller-Deiker syndrome, Vici syndrome, heterozygous FOXN1 mutations, CHARGE syndrome, and mutations in KRAS (Figure 2C). Genetic defects with unclear mechanisms included many variants of uncertain and likely pathogenic significance, Trisomy 21, Jacobson syndrome, and Turner syndrome (Table E1). Variants of uncertain significance were discovered in seven infants.
Figure 2. Genetic diagnoses.
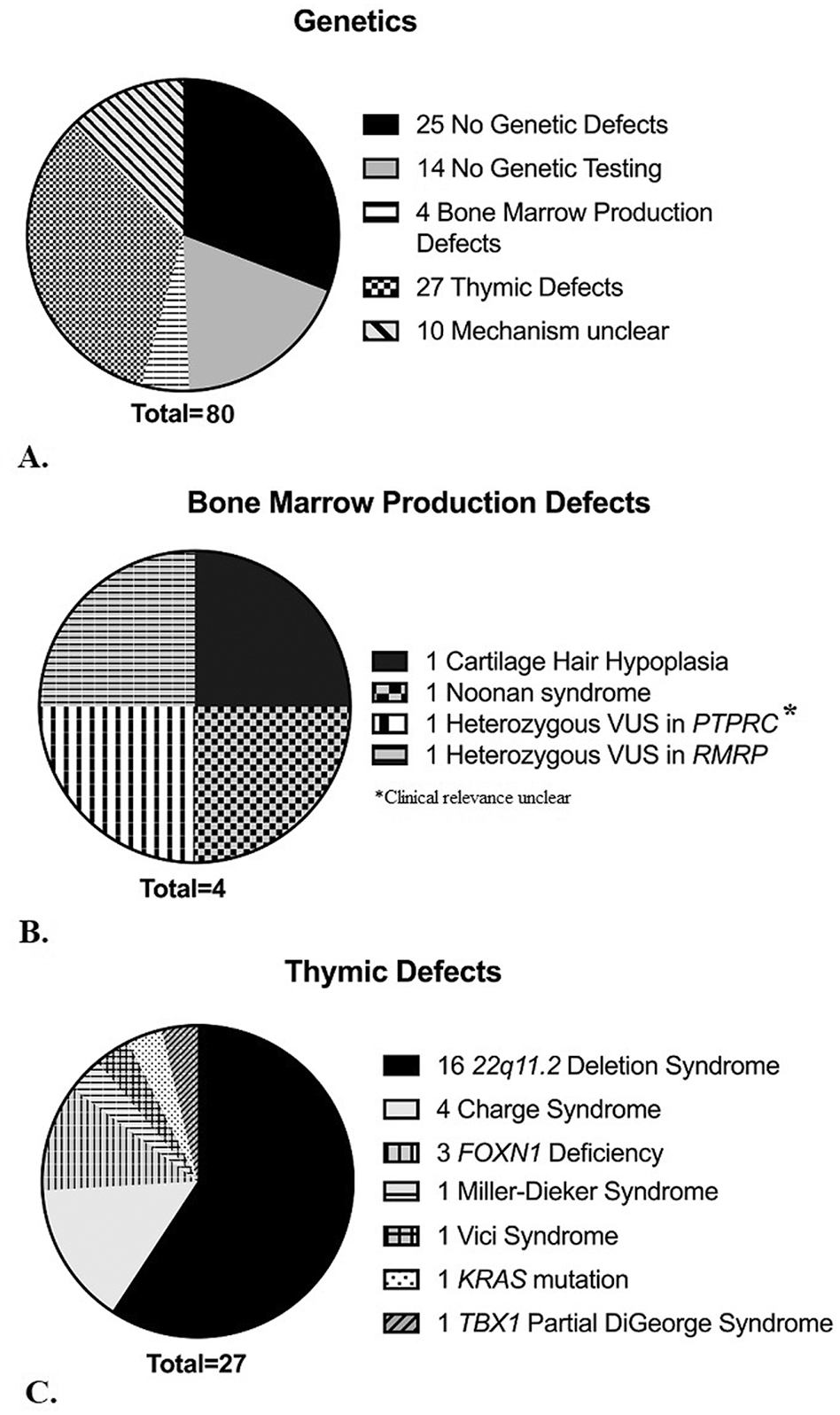
A. Categories of genetic findings of 80 infants with non-SCID T cell lymphopenia.
B. Genetic variants related to bone marrow production in 4 infants with T cell lymphopenia.
C. Genetic variants related to thymic defects in 27 infants with T cell lymphopenia.
Impact of specific genetic diagnosis on differences in initial and longitudinal lymphocyte analysis
The genetic cohort had significantly lower median initial CD3+ (967 vs. 1335 cells/μL; p = 0.0303) (Figure 3A) and CD4+ T cell counts (641 vs. 914 cells/μL; p = 0.0312) (Figure 3B). The genetic cohort also had lower median initial CD8+ (262 vs. 395 cells/μL; p=0.0302) (Figure 3C) and CD4/CD45RA+ T cell counts (485 vs. 747 cells/μL; p=0.0273) (Figure 3D). Initial CD19+ B-cell counts did not differ between the genetic and non-genetic cohorts (506 vs. 575 cells/μL; p = 0.458) (Figure 3E). However, initial CD16/56+ NK cell counts were higher in the genetic cohort (466 vs. 244 cells/μL; p = 0.0339) (Figure 3F). The mean initial TREC levels did not differ between the non-genetic and genetic cohorts (13 vs. 12.4 TREC/μL of blood; p = 0.449), nor did the Ct values (35.5 vs. 36; p = 0.619) (Figure E1).
Figure 3. Initial CD3+ T, CD4+ T, CD8+ T, CD4/CD45RA+ T, B, and NK lymphocyte counts.
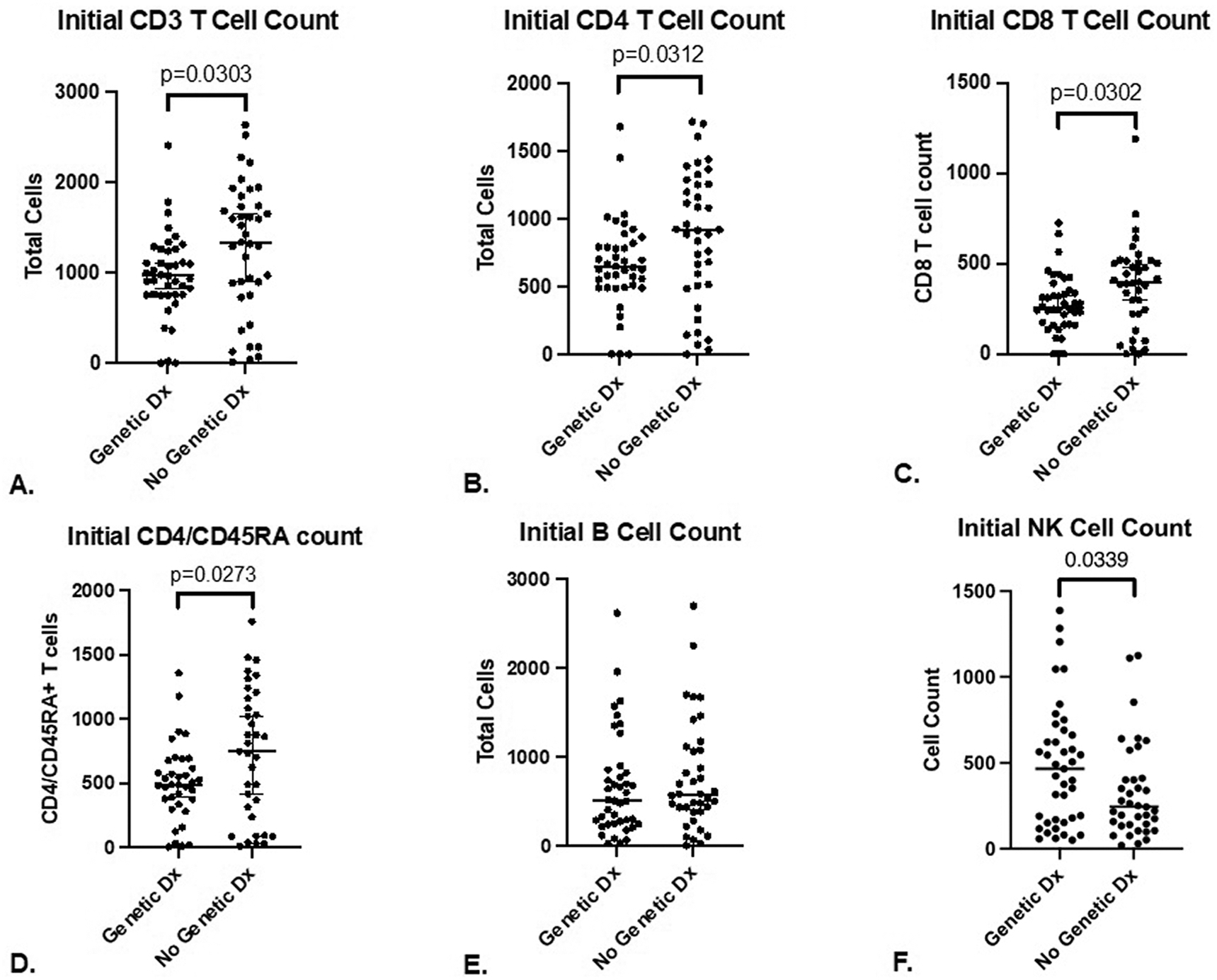
A. Initial CD3+ T cell count in 80 infants with T cell lymphopenia (median with 95% CI).
B. Initial CD4+ T cell count in 80 infants with T cell lymphopenia (median with 95% CI).
C. Initial CD8+ T cell count in 80 infants with T cell lymphopenia (median with 95% CI).
D. Initial CD4/CD45RA T cell count in 80 infants with T cell lymphopenia (median with 95% CI).
E. Initial B cell count in 80 infants with T cell lymphopenia (median with 95% CI).
F. Initial NK cell count in 80 infants with T cell lymphopenia (median with 95% CI).
Sub-analysis of T cell counts by subject age showed generally lower CD4+ T cell counts in infants with a genetic diagnosis at age 5–6 months (mean 686 vs. 1137 cells/μl; Figure 4A), though this was non-significant (p = 0.067). A difference was found in CD8+ T cell counts in infants with a genetic diagnosis versus those without at age 5–6 months (mean 237 vs. 524 cells/μl; p = 0.01; Figure 4B), which also held for infants at 7–9 months (mean 237 vs. 409 cells/μl; p = 0.036).
Figure 4. T cell trends (genetic diagnosis vs. no genetic diagnosis).
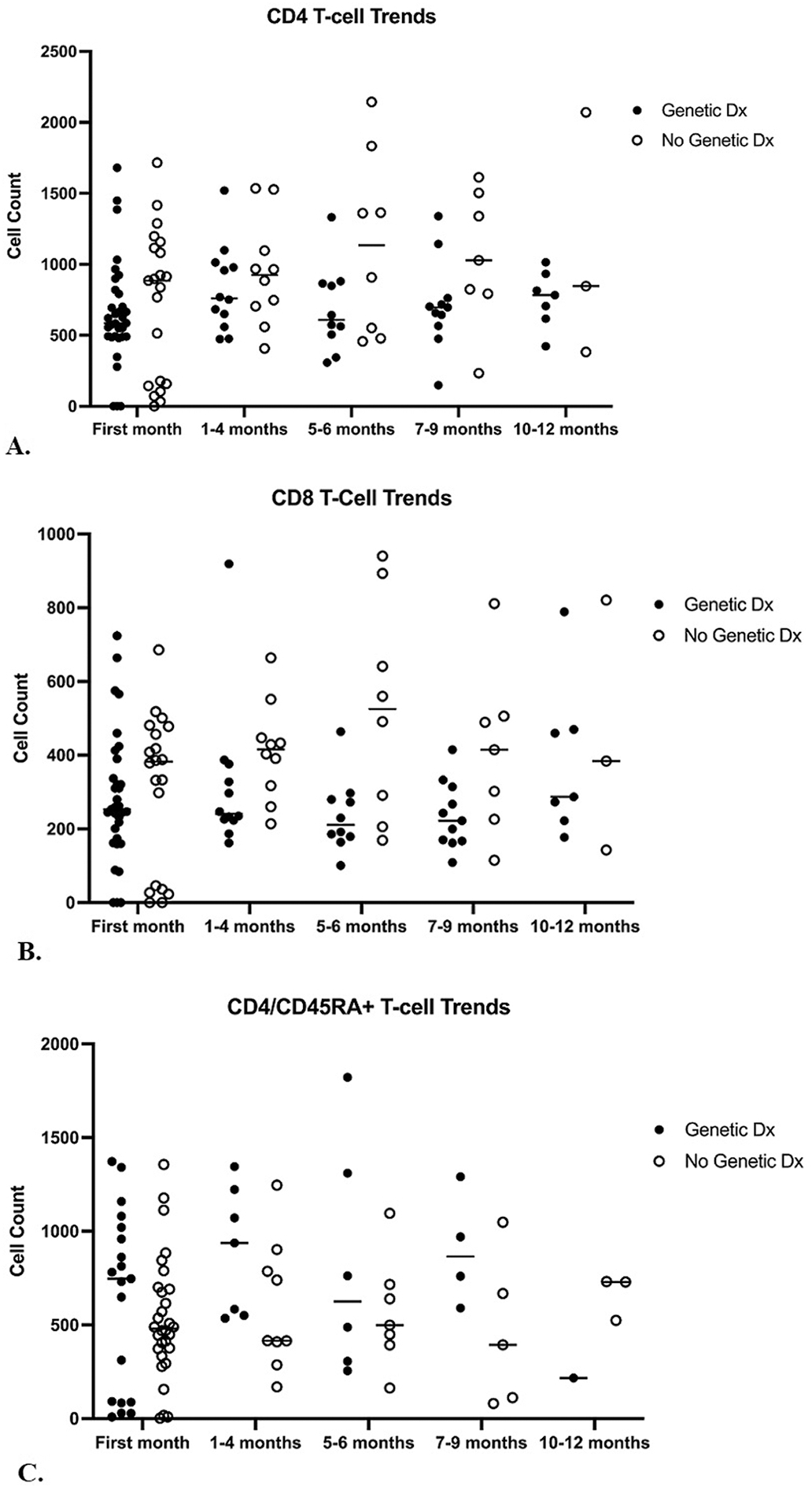
A. CD4+ T cell counts in T cell lymphopenia patients with a genetic diagnosis versus those without a genetic diagnosis over 0–1 months, 1–4 months, 5–6 months, 7–9 months and 10–12 months of life. There was no significant difference in CD4+ T cell counts in patients with a genetic diagnosis versus those without during any time point.
B. CD8+ T cell counts in T cell lymphopenia patients with a genetic diagnosis versus those without a genetic diagnosis over 0–1 months, 1–4 months, 5–6 months, 7–9 months and 10–12 months of life. There is a significant difference during age 5–6 months (mean 237 versus 524 cells/ul; p=0.01) and age 7–9 months (mean 237 vs 409 cells/ul; p=0.036).
C. CD4/CD45RA+ T cell counts in T cell lymphopenia patients with a genetic diagnosis versus those without a genetic diagnosis over 0–1 months, 1–4 months, 5–6 months, 7–9 months and 10–12 months of life. There was no significant difference in CD4/CD45RA+ T cell counts in patients with a genetic diagnosis versus those without during any time point.
Impact of non-genetic factors on initial T cell count
Variables including initial TREC Ct, maternal immunosuppression, premature birth, congenital heart disease, gender, and race were evaluated for their relative impact on T cell counts. Of these, only male gender correlated with a lower initial T cell count (p = 0.050; Table I). Only 6 of the 20 premature infants had resolution of their TCL while others persisted with idiopathic TCL or had a secondary diagnosis of TCL despite prematurity.
There were 5 patients in the cohort who had maternal exposure to immunosuppressive medication. Two were diagnosed with genetic conditions unrelated to this exposure (CHARGE syndrome and cartilage hair hypoplasia). There were also 5 patients who were infants of diabetic mothers, of whom 2 were diagnosed with congenital athymia. Of these 10 patients with TCL, only one had subsequent resolution (Table E2).
Infection risk and prophylaxis use in TCL patients
In our cohort of 80 patients, 36 (45%) experienced either viral, bacterial or fungal infection (Table II); 15 of these were considered to be serious infections. Upper respiratory infection was the most common viral infection (n = 15), acute otitis media (AOM) was the most common bacterial infection (n = 9) and oral thrush affected 5 infants.
Table II.
The number of viral, bacterial, and fungal infections in 80 patients with T cell lymphopenia.
| Number of patients (%) | Sinopulmonary | Gastrointestinal | Skin/Mucosa | Systemic/Invasive | Urinary | |
|---|---|---|---|---|---|---|
| Viral infections | 20 (25%) | URI (n=15) Bronchiolitis (n=3) Croup (n=1) Pneumonia (n=1) |
Gastroenteritis (n=3) | Coxsackievirus (n=2) Molluscum contagiosum (n=1) |
Vaccine stain VZV infection (n=1) |
|
| Bacterial infections | 25 (31%) | AOM (n=9) Pneumonia (n=5) Conjunctivitis (n=2) Tracheitis (n=1) |
NEC (n=2) | MRSA abscess (n=1) | Sepsis/bacteremia (n=7) Meningitis (n=1) Ventriculitis (n=1) |
UTI (n=2) |
| Fungal infections | 10 (13%) | Candidal diaper dermatitis (n=3) | Oral thrush (n=5) | Candidal UTI (n=2) |
Abbreviations: URI, upper respiratory infection; AOM, acute otitis media; NEC, necrotizing enterocolitis, MRSA, Methicillin-resistant Staphylococcus aureus; VZV, Varicella-zoster virus; UTI, urinary tract infection.
Serious infection included viral pneumonia (n = 1), disseminated infection with vaccine strain VZV (n = 1), necrotizing enterocolitis (NEC; n = 2), bacteremia (n = 7), bacterial pneumonia (n = 5), tracheitis (n = 1), ventriculitis (n = 1) and meningitis (n = 1). There were no infections due to Epstein-Barr virus (EBV), cytomegalovirus (CMV) or Pneumocystis jirovecii (PJP). Of those with serious infections, 8 (53%) were born premature. One premature infant was documented to have medical necrotizing enterocolitis. Bacteremia was common in premature infants (n=4). Premature infants had fewer cases of viral upper respiratory infections compared to full-term infants (2 versus 13 patients, respectively). Full-term patients also had acute otitis media as the most common bacterial infection while no cases were documented in the premature infants.
Patients with infection(s) did not have significantly different initial CD3+ (1182 vs 1058 cells/μL; p = 0.18), CD4+ (789 vs 737 cells/μL; p = 0.29), CD8+ (350 vs 310 cells/μL; p = 0.19) or CD4/CD45RA+ (656 vs 573; p = 0.20) counts versus patients without infection(s). There was also no significant difference when comparing TCL infants with only viral or fungal infections versus without viral or fungal infections as well as serious versus non-serious infections.
There were 28 (35%) patients who were started on prophylactic antimicrobial treatment. The most common prophylaxis regimen was a combination of trimethoprim-sulfamethoxazole and fluconazole (n= 12). There were 9 patients with known length of duration for antimicrobial prophylaxis. The median length of duration was 4 weeks while the range was from 1 week to 48 weeks. Twelve infants received immunoglobulin replacement therapy. Of the 28 patients who were on prophylaxis, 10 (36%) developed infection. Of the 52 patients that were not on prophylaxis, 27 developed infection (52%). Of the 15 patients who had a serious infection, four (27%) were on prophylaxis at the time the infection was diagnosed.
T cell lymphopenia resolution
Roughly one third (n = 25) of patients with TCL had documented resolution of their TCL during the study period. The median age of resolution was 23 weeks (range 1 week to 156 weeks of age). Male subjects did show a trend toward a lower age of TCL resolution (p = 0.05). Variables including maternal immunosuppression, congenital heart disease (CHD), gender, and race were not found to have significant impact on the age of TCL resolution (Table I).
There was no significant difference in initial CD3+ counts in patients with T cell resolution versus those without T cell resolution (1115 vs 1113 cells/μL; p = 0.99) nor in initial CD4+ counts (757 vs 761 cells/μL; p = 0.98), initial CD8+ counts (330 vs 327; p = 0.97) or initial CD4/CD45RA+ counts (592 vs 614 cell/μL; p = 0.84) (Table III). CD4/CD45RA+ T cells showed no significant differences between the groups by age (Figure 4C). Of note, the initial TREC Ct value was not predictive of the initial T cell count (p = 0.745) nor the age of TCL resolution (p = 0.516; Table I).
Table III.
Initial mean CD3+, CD4+, CD8+ and CD4+/CD45RA+ T cell counts (cells/uL) in T cell lymphopenia patients with and without resolution of lymphopenia.
| Initial mean | Initial mean | Initial mean | Initial mean | |
|---|---|---|---|---|
| CD3+ (cells/μL) | CD4+ (cells/μL) | CD8+ (cells/μL) | CD4/CD45RA+ (cells/μL) | |
|
| ||||
| TCL w/resolution | 1115 | 757 | 330 | 592 |
| TCL w/out resolution | 1113 | 761 | 327 | 614 |
|
| ||||
| p-value | 0.99 | 0.98 | 0.97 | 0.84 |
Abbreviation: TCL, T cell lymphopenia.
Discussion
SCID newborn screening using the TREC assay allows rapid and efficient identification of SCID as well as non-SCID TCL. Non-SCID TCL occurs more frequently than SCID [13], however there are no published consensus guidelines on the management and follow up of newborns with non-SCID TCL. In this study, we characterized the demographics, laboratory characteristics, and the clinical outcomes of 80 infants with and without a genetic cause of non-SCID TCL identified by NBS in the Mid-Atlantic region over seven years.
Defining non-SCID TCL is itself a challenge, as cutoff values for TCL vary by age and between states, reference laboratories, and institutions [13]. In our study we defined TCL using a cutoff of 2000 cells/ul based on published reference intervals [13, 21, 22]. Various genetic syndromes have been identified that may lead to non-SCID TCL [20, 21]. In our 41 patients with a genetic diagnosis, the most common cause of TCL was thymic defects including 22q11.2 Deletion syndrome, CHARGE syndrome, FOXN1 deficiency, Miller-Dieker syndrome, Vici syndrome, and KRAS mutation. There were an additional 4 children with genetic defects associated with impaired bone marrow production of lymphocytes and 10 with genetic anomalies otherwise known to be associated with lymphopenia that is of unclear mechanism. Fourteen of our 80 patients did not receive genetic testing, which may have revealed additional genetic causes of non-SCID TCL in our population.
There remains a question of when infants with TCL should undergo genetic testing, since some have resolution of their abnormalities without other sequelae. In our cohort, initial T cell counts were significantly lower in those with a genetic diagnosis, while initial TREC values, Ct values, and B-cell counts were not significantly different in those with a genetic diagnosis. Additionally, CD8+ T cells were significantly lower at 5–6 months of age and remain low through 7–9 months of age in infants with a genetic diagnosis. These data may help stratify patients who require genetic testing. We would propose that infants with initial T cell counts below the medians noted in our cohort with genetic diagnoses (approximately CD4+ < 650 cells/μl, CD8+ < 250 cells/μl; CD4/CD45RA+ < 500 cells/μl) are deserving of early genetic diagnostic testing, and furthermore, genetic testing should also be considered for infants within a standard deviation of the genetic cohort median (approximately CD4+ < 1000 cells/μl; CD8+ < 450 cells/μl, CD4/CD45RA+ < 800 cells/μl). Certainly, infants with other comorbidities or clinical features of a syndrome should have genetic evaluation sooner.
Predicting which patients will resolve their TCL is especially difficult. In a single center analysis of 43 infants identified with TCL on NBS in New York, infants with transient TCL (n = 22) had significantly higher median initial CD3+, CD4+ and CD8+ T cell counts compared to those infants with persistent TCL [20]. We found that 31% (n=2) of our patients had resolution of their TCL (CD3+ > 2000 for < 24 months of age and > 1500 for > 2 years of age). Notably, there was no correlation between initial median TREC Ct values, CD4+, CD8+ or CD4/CD45RA+ T cell counts and resolution. There was also no correlation between maternal immunosuppression, premature birth, congenital heart disease and TCL resolution. Male gender did show a trend towards a lower age of TCL resolution. In addition, the age of resolution varied widely from 1 week to 154 weeks. Therefore, determining timing for re-assessments can be challenging. However, given the association between persistent lymphopenia and genetic findings in our cohort, we would propose close monitoring of well infants with TCL until resolution occurs and if persistent CD8+ TCL by age 9 months, considering genetic testing.
Deciding which patients should be placed on antimicrobial prophylaxis or replacement immunoglobulin differs between institutions. We found that 35% of our cohort with TCL was on prophylaxis while 15% received replacement immunoglobulin. Despite most not being on prophylaxis, the rate of opportunistic infections was exceedingly low with only one patient developing vaccine strain VZV infection. Notably, there were no infants with PJP pneumonia nor EBV or CMV infection. These findings suggest that most infants with TCL may not require antimicrobial prophylaxis.
This study was limited due to its retrospective design, loss of several patients to follow up before a possible resolution or genetic diagnosis was made, and the variability in genetic testing modalities used by clinicians. There was limited data about the dosing and timing of antimicrobial prophylaxis and immunoglobulin replacement so were unable to draw conclusions regarding dosing and length of therapy in prevention of infection. Future prospective studies with uniform follow-up and testing strategies will be beneficial in developing diagnostic and management guidelines.
Conclusions
Newborn screening for SCID has been valuable in detecting infants with SCID in addition to non-SCID TCL, which can be caused by a myriad of genetic disorders, comorbid conditions or be idiopathic. These infants with non-SCID TCL are at low risk of opportunistic infection even in the absence of antimicrobial prophylaxis or immunoglobulin replacement. Initial T cell counts as well as persistently low CD8+ T cells at 5–6 months of life may be a useful marker of a genetic cause of TCL, which if identified can be very helpful in guiding decision making for further prognosis and treatment.
Supplementary Material
Newborn screening for severe combined immunodeficiency can detect T cell lymphopenia
There is limited data on the cause or natural history of idiopathic T lymphopenia
51% of infants had genetic findings; these infants had lower initial T cell counts
45% had infection but only one patient had opportunistic infection
31% had resolution of lymphopenia during the study
Funding:
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Abbreviations:
- NBS
Newborn screening
- TREC
T cell receptor excision circle
- Ct
Cycle threshold
- TCL
T cell lymphopenia
- qPCR
Quantitative polymerase chain reaction
- HCT
Hematopoietic cell transplant
- ITL
Idiopathic T cell lymphopenia
- IVIG
Intravenous immunoglobulin
- NK
Natural killer
- AOM
Acute otitis media
- NEC
Necrotizing enterocolitis
- EBV
Epstein-Barr virus
- CMV
Cytomegalovirus
Footnotes
Declarations of Interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Tangye SG, Al-Herz W, Bousfiha A, Chatila T, Cunningham-Rundles C, Etzioni A, Franco JL, Holland SM, Klein C, Morio T, Ochs HD, Oksenhendler E, Picard C, Puck J, Torgerson TR, Casanova JL, Sullivan KE, Human Inborn Errors of Immunity: 2019 Update on the Classification from the International Union of Immunological Societies Expert Committee, J Clin Immunol, 40 (2020) 24–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Kwan A, Puck JM, History and current status of newborn screening for severe combined immunodeficiency, Semin Perinatol, 39 (2015) 194–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Gaspar HB, Qasim W, Davies EG, Rao K, Amrolia PJ, Veys P, How I treat severe combined immunodeficiency, Blood, 122 (2013) 3749–3758. [DOI] [PubMed] [Google Scholar]
- [4].Buckley RH, Transplantation of hematopoietic stem cells in human severe combined immunodeficiency: longterm outcomes, Immunol Res, 49 (2011) 25–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Pai SY, Logan BR, Griffith LM, Buckley RH, Parrott RE, Dvorak CC, Kapoor N, Hanson IC, Filipovich AH, Jyonouchi S, Sullivan KE, Small TN, Burroughs L, Skoda-Smith S, Haight AE, Grizzle A, Pulsipher MA, Chan KW, Fuleihan RL, Haddad E, Loechelt B, Aquino VM, Gillio A, Davis J, Knutsen A, Smith AR, Moore TB, Schroeder ML, Goldman FD, Connelly JA, Porteus MH, Xiang Q, Shearer WT, Fleisher TA, Kohn DB, Puck JM, Notarangelo LD, Cowan MJ, O’Reilly RJ, Transplantation outcomes for severe combined immunodeficiency, 2000–2009, N Engl J Med, 371 (2014) 434–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Gaspar HB, Hammarstrom L, Mahlaoui N, Borte M, Borte S, The case for mandatory newborn screening for severe combined immunodeficiency (SCID), J Clin Immunol, 34 (2014) 393–397. [DOI] [PubMed] [Google Scholar]
- [7].Brown L, Xu-Bayford J, Allwood Z, Slatter M, Cant A, Davies EG, Veys P, Gennery AR, Gaspar HB, Neonatal diagnosis of severe combined immunodeficiency leads to significantly improved survival outcome: the case for newborn screening, Blood, 117 (2011) 3243–3246. [DOI] [PubMed] [Google Scholar]
- [8].Puck JM, The case for newborn screening for severe combined immunodeficiency and related disorders, Ann N Y Acad Sci, 1246 (2011) 108–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].van der Spek J, Groenwold RH, van der Burg M, van Montfrans JM, TREC Based Newborn Screening for Severe Combined Immunodeficiency Disease: A Systematic Review, J Clin Immunol, 35 (2015) 416–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Chan K, Puck JM, Development of population-based newborn screening for severe combined immunodeficiency, J Allergy Clin Immunol, 115 (2005) 391–398. [DOI] [PubMed] [Google Scholar]
- [11].Dorsey MJ, Puck JM, Newborn Screening for Severe Combined Immunodeficiency in the United States: Lessons Learned, Immunol Allergy Clin North Am, 39 (2019) 1–11. [DOI] [PubMed] [Google Scholar]
- [12].Sheller R, Ojodu J, Griffin E, Edelman S, Yusuf C, Pigg T, Huston A, Fitzek B, Boyle JG, Singh S, The Landscape of Severe Combined Immunodeficiency Newborn Screening in the United States in 2020: A Review of Screening Methodologies and Targets, Communication Pathways, and Long-Term Follow-Up Practices, Front Immunol, 11 (2020) 577853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kwan A, Abraham RS, Currier R, Brower A, Andruszewski K, Abbott JK, Baker M, Ballow M, Bartoshesky LE, Bonilla FA, Brokopp C, Brooks E, Caggana M, Celestin J, Church JA, Comeau AM, Connelly JA, Cowan MJ, Cunningham-Rundles C, Dasu T, Dave N, De La Morena MT, Duffner U, Fong CT, Forbes L, Freedenberg D, Gelfand EW, Hale JE, Hanson IC, Hay BN, Hu D, Infante A, Johnson D, Kapoor N, Kay DM, Kohn DB, Lee R, Lehman H, Lin Z, Lorey F, Abdel-Mageed A, Manning A, McGhee S, Moore TB, Naides SJ, Notarangelo LD, Orange JS, Pai SY, Porteus M, Rodriguez R, Romberg N, Routes J, Ruehle M, Rubenstein A, Saavedra-Matiz CA, Scott G, Scott PM, Secord E, Seroogy C, Shearer WT, Siegel S, Silvers SK, Stiehm ER, Sugerman RW, Sullivan JL, Tanksley S, Tierce M.L.t., Verbsky J, Vogel B, Walker R, Walkovich K, Walter JE, Wasserman RL, Watson MS, Weinberg GA, Weiner LB, Wood H, Yates AB, Puck JM, Bonagura VR, Newborn screening for severe combined immunodeficiency in 11 screening programs in the United States, JAMA, 312 (2014) 729–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kobrynski LJ, Identification of non-severe combined immune deficiency T-cell lymphopenia at newborn screening for severe combined immune deficiency, Ann Allergy Asthma Immunol, 123 (2019) 424–427. [DOI] [PubMed] [Google Scholar]
- [15].Verbsky JW, Baker MW, Grossman WJ, Hintermeyer M, Dasu T, Bonacci B, Reddy S, Margolis D, Casper J, Gries M, Desantes K, Hoffman GL, Brokopp CD, Seroogy CM, Routes JM, Newborn screening for severe combined immunodeficiency; the Wisconsin experience (2008–2011), J Clin Immunol, 32 (2012) 82–88. [DOI] [PubMed] [Google Scholar]
- [16].Currier R, Puck JM, SCID newborn screening: What we’ve learned, J Allergy Clin Immunol, 147 (2021) 417–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Gans MD, Gavrilova T, Retrospective Analysis of a New York Newborn Screen Severe Combined Immunodeficiency Referral Center, J Clin Immunol, 40 (2020) 456–465. [DOI] [PubMed] [Google Scholar]
- [18].Verbsky J, Thakar M, Routes J, The Wisconsin approach to newborn screening for severe combined immunodeficiency, J Allergy Clin Immunol, 129 (2012) 622–627. [DOI] [PubMed] [Google Scholar]
- [19].Albin-Leeds S, Ochoa J, Mehta H, Vogel BH, Caggana M, Bonagura V, Lehman H, Ballow M, Rubinstein A, Siegel S, Weiner L, Weinberg GA, Cunningham-Rundles C, Idiopathic T cell lymphopenia identified in New York State Newborn Screening, Clin Immunol, 183 (2017) 36–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Jongco AM 3rd, Sporter R, Hon E, Elshaigi O, Zhang S, Daian F, Bae E, Innamorato A, Capo C, Navetta-Modrov B, Rosenthal DW, Bonagura VR, Characterization of Infants with Idiopathic Transient and Persistent T Cell Lymphopenia Identified by Newborn Screening-a Single-Center Experience in New York State, J Clin Immunol, 41 (2021) 610–620. [DOI] [PubMed] [Google Scholar]
- [21].Amatuni GS, Currier RJ, Church JA, Bishop T, Grimbacher E, Nguyen AA, Agarwal-Hashmi R, Aznar CP, Butte MJ, Cowan MJ, Dorsey MJ, Dvorak CC, Kapoor N, Kohn DB, Markert ML, Moore TB, Naides SJ, Sciortino S, Feuchtbaum L, Koupaei RA, Puck JM, Newborn Screening for Severe Combined Immunodeficiency and T-cell Lymphopenia in California, 2010–2017, Pediatrics, 143 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Shearer WT, Rosenblatt HM, Gelman RS, Oyomopito R, Plaeger S, Stiehm ER, Wara DW, Douglas SD, Luzuriaga K, McFarland EJ, Yogev R, Rathore MH, Levy W, Graham BL, Spector SA, Pediatric ACTG, Lymphocyte subsets in healthy children from birth through 18 years of age: the Pediatric AIDS Clinical Trials Group P1009 study, J Allergy Clin Immunol, 112 (2003) 973–980. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


