Abstract
Background
/Purposes: Studies have indicated that salivary molecules from patients with periodontitis and diabetes are confounded with pathological conditions associated with SARS-CoV-2 infection. The study aimed to address whether the abundance of Porphyromonas gingivalis which causes periodontitis, differed compared with that of Aggregatibacter actinomycetemcomitans (used as control) and to analyze the correlation of periodontitis with the expression levels of severe acute respiratory syndrome coronavirus 2 receptor (ACE2) and periodontitis inflammatory markers (TLR-2/TLR-4, TNFα, and miR-155).
Materials and Methods
A saliva sample (5 mL) was obtained from 23 hospitalized patients with COVID-19, categorized into two groups: diabetic (G1, n = 10) and non-diabetic (G2, n = 13). Saliva from patients with periodontitis without diabetes and coronavirus disease 2019 (COVID-19; n = 6) were included as control. The quantitative real-time polymerase chain reaction measured the levels of P. gingivalis and A. actinomycetemcomitans, as well as periodontitis markers in saliva. The obtained data were analyzed using one-way ANOVA and the Spearman correlation test.
Results
The abundance of P. gingivalis was observed to be higher (p < 0.05) in saliva of patients with diabetes (G1) than in those without diabetes (G2). A contradictory trend was observed for A. actinomycetemcomitans. The transcription level of ACE2 was comparable in all groups tested, while the expression of periodontitis markers varied. The relationships and sensitivity/specificity among P. gingivalis infection ACE2 expression, and inflammatory markers were also evaluated.
Conclusions
This study showed that the association between P. gingivalis infection and ACE2 expression might reflect the characteristics of saliva in COVID-19 patients with and without diabetes. However, the relationships between TLR-4 and miR-155 are more specific in discriminating against COVID-19 patients with and without diabetes.
Keywords: Porphyromonas gingivalis, Periodontitis, COVID-19, Angiotensin-converting enzyme 2, Diabetes
Abbreviations: ACE2 :, Angiotensin-Converting Enzyme 2; TLR-2 :, Tool Like Receptor-2; TLR-4 :, Tool Like Receptor-4; TNFα :, Tomour Necrosis Factor alpha; miR-155 :, microRNA-155
1. Introduction
Literature shows that periodontitis and coronavirus disease 2019 (COVID-19) are associated (Huang et al., 2021), suggesting that the oral cavity is the crucial area for transmitting SARS-CoV-2, the virus that causes COVID-19.
Periodontitis is highly prevalent in people with systemic diseases, including diabetes and those of viral origin (Baghbani et al., 2020). Periodontitis pathogenesis reflects the activation of local and systemic inflammatory processes triggered by the dysbiotic bacterial community and orchestrated by the keystone pathogen, P. gingivalis (Lamont et al., 2018). Since virus favors dysbiosis and disease progression (Radaic et al., 2021), early diagnosis is the most critical factor for the effective treatment of periodontitis in individuals with positive SARS-CoV-2.
Literatures show that microRNAs (miRNAs) are beneficial as diagnostic markers of oral diseases, and most of the detectable salivary miRNA is concentrated in exosomes (Kim et al., 2015, Mikamori et al., 2017). Moreover, a previous study has shown that miRNA-155 enhances ACE2 expression and modulates genes involved in host immunity (Roganovic 2021). In this study, we investigated whether the up-or down-regulation of salivary miR-155 is associated with the inflammatory markers associated with periodontitis patients with and without diabetes diagnosed with COVID-19. Therefore, we chose P. gingivalis and TLR2, TLR4, TNFα, and ACE2 as bacterial and host-related factors, respectively.
2. Materials and Methods
2.1. Patients and sample collection
This study was conducted at a University Hospital. Patients diagnosed with COVID-19 between June 15, 2021, and July 25, 2021, were included in this study. Of 23 patients with periodontitis and COVID-19, ten were reported to have type 2 diabetes. We only selected patients with no respiratory symptoms for more than two weeks.
This study was performed following the guidance of the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement (von Elm et al., 2014). All procedures were approved by the Ethical Review Committee of the Medical Ethics Committee of Rumah Sakit Universitas Indonesia (RSUI) and followed the principles of the Medical Research Involving Human Subject Act. All clinical data and information were obtained from medical reports of mildly symptomatic patients with COVID-19 (not shown). We recorded information on age, gender, and chronic medical history of comorbidities. However, our focus was on patients with both COVID-19 and periodontitis, with (G1) and without (G2) diabetes.
Periodontitis status (moderate to severe) was defined according to the criteria described elsewhere (Papapanou et al., 2018). The exclusion criteria included previous periodontal treatment within the last year, age < 18 years, use of antibiotics in the last six months, and pregnancy. To detect attachment loss, we measured the probing pocket depth (Bachtiar et al., 2021). We collected unstimulated whole saliva (2 mL) by spitting it into a sterile Falcon tube. For the control, saliva was collected from patients with periodontitis without COVID-19/diabetes (n = 6) registered at the periodontal clinic of RSUI. The collected saliva was placed on ice and delivered to the laboratory for further processing.
2.2. Exosome preparation and quantitative PCR (qPCR)
The exosomes from saliva specimens (0.5–1.0 mL) were extracted using ExoQuick-TCTM (EQ, System Bioscience Inc., Mountain View, CA, USA), as per the manufacturer’s recommendation with some modifications (Zlotogorski-Hurvitz et al., 2015). We used the GENEzolTM reagent (General, ltd, New Taipei City, Taiwan) to extract total RNA from exosomes according to the manufacturer’s instructions. RNA concentration and quality were assessed using the Qubit assay reagent (Invitrogen: Carlsbad, Ca, United States), followed by a reverse transcription kit (Applied Biosystems). The resulting cDNA was amplified in triplicate on an ABI StepOnePlus Real-Time PCR system, where an SYBR Green PCR Master Mix (Applied Biosystems) was used. The primers used are shown in Table 1, and the PCR conditions were as follows: 95 °C for 2 min, followed by 35 cycles of 95 °C for 5 s, 62 °C for 10 s, and 72 °C for 30 s, then 95 °C for 1 min, 62 °C for 1 min, and 95 °C for 15 s. The obtained data were analyzed using the 2-ΔΔCt method.
Table 1.
Primers used in this study.
| Name | Primer (5′-3′) | Reference |
|---|---|---|
| miR-155 | 5′-GGGCCTTCCCTGGAACAGGAGTCT- 3′ 5′-GGGAGATTCATGGTATCAAGCACCC- 3′ |
(Wu et al., 2021) |
| U6 | 5′-CTCGCTTCGGCAGCACA- 3′ 5′-AACGCTTCACGAATTTGCGT- 3′ |
(Wu et al., 2021) |
| ACE2 | 5′-ACAGTCCACACTTGCCCAAAT- 3′ 5′-TGAGAGCACTGAAGACCCATT- 3′ |
(Sakaguchi et al., 2020) |
| TLR-2 | 5′-GGCCAGCAAATTACCTGTGTG- 3′ 5′-AGGCGGACATCCTGAACCT- 3′ |
(Sha et al., 2004) |
| TLR-4 | 5′-GTCCTCAGTGTGCTTGTAG- 3′ 5′-ATCCTGGCTTGAGTAGATAAC- 3′ |
(Hedgpeth et al., 2015) |
| TNFα | 5′-AGGGAAGAGTTCCCCAGG- 3′ 5′-GGGAGTAGATGAGGTACAGGC- 3′ |
(McMahon et al., 2011) |
| GAPDH | 5′-AATGGAAATCCCATCACCATCT- 3′ 5′-CAG-ACTCGCACTTG-3′ |
(Zhang et al., 2015) |
| P. gingivalis | 5′-ATAGTAGCGTGTCCGGCTTC- 3′ 5′ -ATCGTAGGCGGATTGGAGA- 3′ |
(Tomas et al., 2017) |
| A. actinomycetemcomitans | 5′-CAATACTACGGTGGTGCAGTATCT- 3′ 5′-ATATTGTTGGCGGTAATGC- 3′ |
(Tomas et al., 2017) |
| Total bacteria | 5′-TTAAACTCAAAGGAATTGACGG- 3′ 5′-CTCACGACACGAGCTGACGAC-3′ |
(Sedgley et al., 2005) |
2.3. qPCR of ACE2, TLRs, and TNFα
Total RNA was extracted from salivary epithelial cells, followed by reverse transcription, as mentioned above. The mRNA expression of ACE2, TLRs, and TNFα, was analyzed by qPCR using primers shown in Table 1. The relative expression of mRNA was analyzed using the formula mentioned above.
2.4. Proportion of P. gingivalis and A. actinomycetemcomitans in the saliva microbiota
Bacterial DNA was extracted from the saliva samples using a similar procedure described above. The abundance of both P. gingivalis and A. actinomycetemcomitans and the total amount of bacteria in the saliva samples were determined using the relative calculation of the ΔΔ Ct formula (2- ΔΔCt) (Bachtiar et al., 2020; Navidshad et al., 2012). The PCR programs were set as previously reported (Bachtiar et al., 2020; Bachtiar et al., 2022), using primers listed in Table 1,
2.5. Statistical analysis
This examination did not include the patient's blood glucose levels. Hence, the relevance of such variables could not be determined in data analysis. The analysis was conducted using GraphPad PRISM 9.4 (GraphPad Software, San Diego, CA, USA). Data are presented as mean ± standard error (SE), and a p-value of < 0.05 was considered statistically significant. For comparison between groups, we used one-way ANOVA, while for comparison within groups, we used the unpaired Student’s t-test. For correlation, Spearman's correlation coefficient (r) was calculated, and linear regression was used to generate the line of best fit with 95 % confidence intervals. The receiver operating characteristic (ROC) curve analysis was performed to determine the relationship between two independent variables with high sensitivity and specificity.
3. Results
3.1. Abundance of P. gingivalis and A. actinomycetemcomitansand transcription levels of ACE2
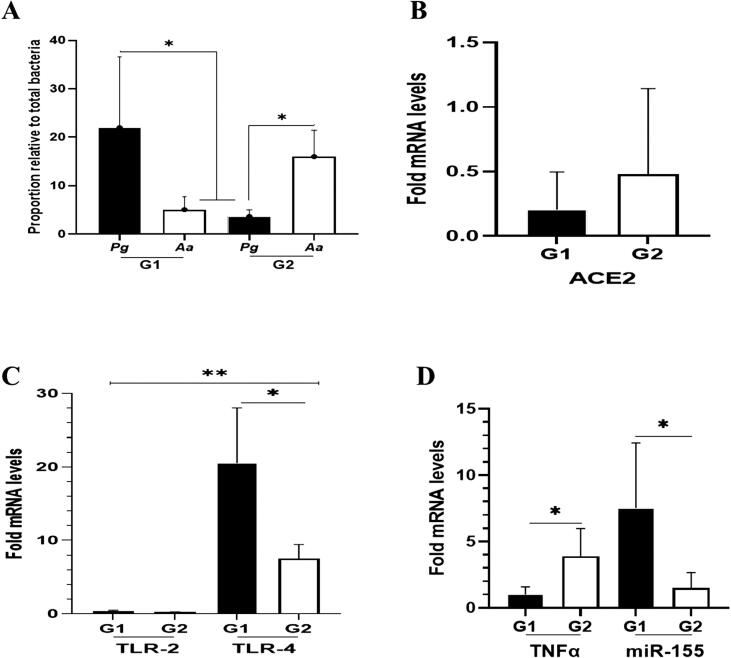
We found that the abundance of P. gingivalis (>20 %) was significantly higher in G1 than that in G2 (<10 %) (Fig. 1A, p < 0.05). Conversely, the amount of A. actinomycetemcomitans was higher in G2 than that in G1 group. Further, the transcription levels of ACE2 mRNA in G1 and G2 were different but statistically not significant (Fig. 1B, p > 0.05).
Fig. 1.
Quantification of bacterial proportions and mRNA expression of ACE2, as well as periodontitis-related inflammatory markers (TLR-2/TLR-4, TNFα, miR-155) in patients with periodontitis with (G1) and without diabetes (G2), diagnosed with COVID-19. (A) Value represents the relative proportion of Pg and Aa in G1 and G2. (B) Data represent the relative expression of ACE2 mRNA to control (patients with only periodontitis) that was set to 1. However, the relative transcription was not significant in either patient group (G1 or G2). (C) In both groups tested (G1 and G2), the transcription level of TLR-2 was lower than TLR-4. However, TLR-4 expression was higher in G1 than in G2 group. (D). Relative fold increases in expressions of TNFα mRNA and miR-155 are shown for G1 and G2. TNFα was up-regulated in G2, while miR-155 expression was accelerated in G1. All data are expressed as mean ± SE. * significant difference in gene expression within the group (G1 and G2). ** significant difference in gene expression between independent variables (TLR-2 and TLR-4). Pg = P. gingivalis. Aa = A. actinomycetemcomitans.
3.2. TLRs/TNFα mRNA and miR-155 transcription levels
In determining the inflammatory conditions, mRNA levels of TLR-2 and TLR-4 were measured. By comparing with that in the control group, the TLR-4 transcript level was higher by 20 times in G1 (p < 0.05), whereas the level was lower by ten times in G2. For TLR-2, the mRNA expression in both groups was comparable (Fig. 1C). For TNFα and miR-155, a higher expression of TNFα mRNA was found in G2 (p < 0.05) compared with those in G1, while the expression of miR-155 was greater (>5 times) in G1 than those in G2 (p < 0.05) (Fig. 1D).
3.3. Correlation of P. gingivalis levels with ACE2, TLRs, TNFα, and miR-155
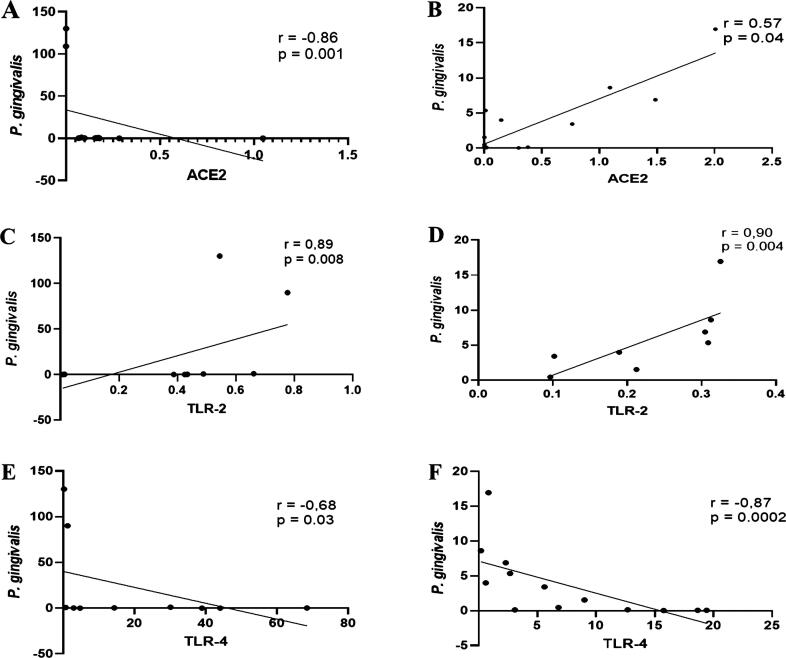
The Spearman correlation analysis indicated that the amount of P. gingivalis inversely correlated with transcription levels of ACE2 in G1 (r = -0. 86, p = 0.001), but a direct correlation (r = 0.57, p = 0.04) was observed in G2 (Fig. 2A and B). Further, in both G1 (r = 0.85, p = 0.001) and G2 (r = 0.90, p = 0.004), a significant positive correlation was observed between the abundance of P. gingivalis and the transcription levels of TLR-2. Conversely, a significant negative correlation was observed between the abundance of P. gingivalis and the mRNA level of TLR-4. The coefficient correlations were r = − 0.67, p = 0.003, and r = − 0.87, p = 0.0002, respectively (Fig. 2C–F).
Fig. 2.
Relationship between P. gingivalis and ACE2, TLR-2, and TLR-4 in saliva samples of patients with periodontitis with (G1) and without diabetes (G2) diagnosed with COVID-19. Spearman’s rho indicates a statistically inverse and significant positive relationship between the relative abundance of P. gingivalis and relative transcript levels of ACE2 in G1 and G2, respectively (A and B). In both groups tested, the relationship between P. gingivalis proportion and TLR-2 transcripts was significantly positive (C and D). The converse was observed in the relationship between P. gingivalis proportion and mRNA expression of TLR-4 (E and F). Spearman correlation coefficient (r) and exact p-value are given.
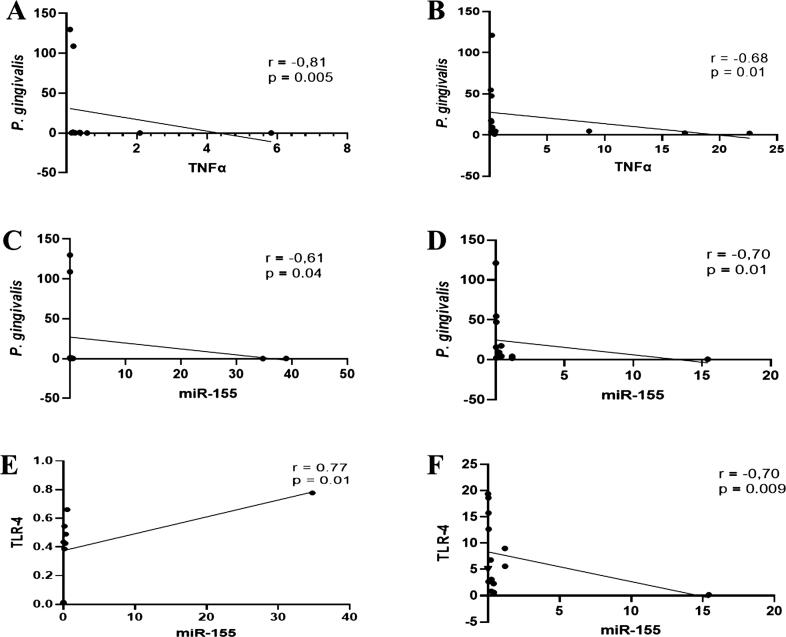
Subsequently, our data indicated that irrespective of the patient group, the inverse association between the amount of P. gingivalis and transcription levels of TNFα mRNA was significant. The coefficient correlations for G1 and G2 were r = -0.81, p = 0.005 and r = -0.68, p = 0.01, respectively (Fig. 3A and B). Similarly, the bacterium had a significant negative association with the expression level of miR-155, both in G1 (r = -0.61, p = 0.04) and G2 (r = -0.70, p = 0.01), respectively (Fig. 3C and D). The expression level of salivary miR-155 in G1 had a significant positive correlation with the mRNA expression of TLR-4 (r = 0.77, p = 0.01), while it had a significant negative correlation with the mRNA expression of TLR-4 in G2 (r = -0.70, p = 0,009).
Fig. 3.
Association between the levels of P. gingivalis and TNFα/ miR-155 and between TLR-4 and miR-155 in patients with periodontitis with (G1) and without diabetes (G2) diagnosed with COVID-19. The scatter diagram depicts that in both groups, the correlation between P. gingivalis abundance and mRNA expression of TNFα or miR-155 was significantly negative (A-D). At the same time, the relationship between transcription levels of TLR-4 and miR-155 was significantly positive in G1 and negative in G2 (E-F). Spearman correlation coefficient (r) and exact p-value are shown.
3.4. Evaluation of the ROC curve
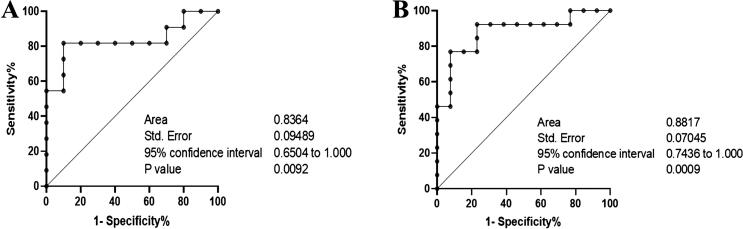
Since the correlation between the transcription levels of miR-155 and TLR-4 was inconsistent between G1 and G2, we further evaluated the incremental value to determine the diagnostic accuracy of their interaction using ROC curve analysis. The area under the ROC curve (AUC) of the predicted periodontitis activities was 0.83 with 80 % sensitivity and 100 % specificity for G1 and 0.88 with 90 % sensitivity and 100 % specificity for G2 (Fig. 4A and B).
Fig. 4.
The association between the transcription level of miR-155 and TLR-4 assessed by the receiver operating characteristic curve (ROC) The ROC illustrates the plot and best cut-off of the association between the transcription levels of TLR-4 and miR-155 in G1 (A) and G2 (B).
4. Discussion
The present study revealed that P. gingivalis in the saliva of our patients has a critical role in modulating the immune responses of COVID-19 pathophysiology. As we found, the abundance of P. gingivalis was 20 times higher (p < 0.05) in saliva samples of G1 than that in G2. A contradictory result was observed for A. actinomycetemcomitans, wherein this species was significantly lower in G1 than in G2. The recovery of P. gingivalis in high numbers in saliva samples of G1, suggested an active inflammatory disease in the periodontal microenvironment of COVID-19 patients with diabetes. Therefore, we speculated that the saliva samples of G1 reflected the activation of local and systemic inflammatory processes, which indicated oral dysbiosis and the dysregulation of the immune system in the periodontal niche.
Our finding is in accordance with a previous study, which reported that low levels of ACE2 expression are a common phenomenon in chronic pathologies (Pagliaro and Penna 2020), including type 2 diabetes and periodontitis. However, a previous in vitro study reported an elevated expression of ACE2 after treatment with the lipopolysaccharide from P. gingivalis (Pg-LPS) (Sena et al., 2021). As our results are mainly based on saliva samples, the observation of ACE2 expression could indicate the presence of SARS-CoV-2-infected cells in saliva (Tang et al., 2021). Notably, significant negative correlations between the bacterium and virus receptor were found in patients with diabetes, but positive correlations were found in patients without diabetes. These findings suggested that in the saliva of patients with diabetes, the two determinant factors associated with periodontitis and COVID-19 were relatively independent.
As reported in the literature, the secretion of pro- and anti-inflammatory cytokines induced by P. gingivalis strain was mediated by either TLR-2 or TLR-4 (Zhou et al., 2005). These pattern-recognition receptors are also involved in sensing pathogen-associated molecular patterns of SARS-CoV-2 (Jung and Lee 2021). We observed that in the saliva of both groups (G1 and G2), the transcription level of TLR-4 was higher (>10 times) than observed in TLR-2 transcripts, whose level was similar to that of the control. Additionally, in both patient groups, we observed a significant positive association between the expression levels of TLR-2 mRNA and the amount of P. gingivalis, while a contradictory relationship was found between the amount of bacterium and expression levels of TLR-4. On the one hand, the negative association between P. gingivalis and TLR-4 could indicate the low immune stimulatory capability of P gingivalis lipid A (Jain and Darveau 2010). On the other hand, since TLR4 is a significant contributor to SARS-CoV-2 infection and pathogenesis (Choudhury and Mukherjee 2020), SARS-CoV-2 was likely present in the oral cavity of our patients, as previously reported (Huang et al., 2021). Therefore, the expression level of TLR4 mRNA was probably accelerated owing to the stimulating activity of SARS-CoV-2. In turn, the decreased expression of ACE2 mRNA may be attributable to the deprivation of this receptor by its ligand (SARS-CoV-2) (Li et al., 2020).
Cytokines and miRNAs regulate inflammatory responses (Jiang et al., 2022), and TNFα is a proinflammatory cytokine involved in the pathogenesis of the periodontal disease (Andrukhov et al., 2011). Our data indicated that in patients with diabetes, the transcript encoding TNFα was down-regulated, while the result was contradictory in patients without diabetes. In contrast, the expression levels of salivary miR-155 were significantly elevated in patients with diabetes compared with those without diabetes. Importantly, in both patient groups, the transcription level of TNFα and miR-155 was negatively associated with the amount of P. gingivalis. Although the correlation was insignificant, we suggest that the cytokine production is neither affected by the bacterium nor the diabetes condition. This result contradicted other study findings, which demonstrated that the production of TNFα was induced by plaque bacteria, including P. gingivalis, and was increased by diabetes (Kato et al., 2014). We suggest that TNFα is not pivotal in prolonged immune function in COVID-19 patients with periodontitis.
The most prominent result of the present study was that the expression levels of salivary exosomal miR-155 differed between patients with diabetes (up-regulated > 5-fold) and without diabetes. This observation suggests that systemic conditions, including diabetes, affect miR-155 expression in the saliva of patients with COVID-19. However, the association between miRNA-155 and P. gingivalis is unclear. Based on the data mentioned above, we further present evidence of the correlation between P. gingivalis and the specific markers of inflammation associated with periodontitis (TLR-4, TNFα, and miR-155) in patients with COVID-19 and with or without diabetes. We observed that the salivary presence of P. gingivalis has a significant inverse association with either the inflammatory markers or miR-155. Since miR-155 is a master regulator of the inflammatory response (Mahesh and Biswas 2019) and Pg-LPS is a specific ligand for TLR4 (Nativel et al., 2017), it is likely that in patients with COVID-19 and diabetes, higher TLR-4 expression and lower miR-155 expression cause the survival of SARS-CoV-2 in saliva. Therefore, our results indicate the involvement of P. gingivalis in accelerating the expression level of ACE2 in vitro (Sena et al., 2021). We further noted that the association between the transcription level of miR-155 and the main ligand of P. gingivalis (TLR-4) could discriminate diabetes (AUC: 0.83, p = 0.009) and non-diabetes conditions (AUC: 0.88, p = 0.0009) in our patients. This result indicates that the exosomal miR-155 detected in saliva is associated with a specific disorder, periodontal inflammation. Therefore, this study demonstrated an indirect association between P. gingivalis and miR-155.
In addition to the small sample size, this study's limitation is that we could not determine whether the amount of P. gingivalis observed in the saliva was the effect of SARS-CoV-2 replication or pathogenesis.
5. Conclusions
This study found that the average expression level of the periodontitis markers (TLR-4, TNFα, and miR-155) inversely changes in response to the amount of P. gingivalis. We propose that this finding be evaluated to determine whether the cause or effect of the higher amount of P. gingivalis in patients with both COVID-19 and diabetes is due to SARS-CoV-2.
Ethical statement
This study, 'Porphyromonas gingivalis association with inflammatory markers and exosomal miRNA-155 in saliva of periodontitis patients with and without diabetes diagnosed with COVID-19′ obtained ethical approval from the Ethical Review Committee of Medical Ethics Committee of Rumah Sakit Universitas Indonesia (protocol number: 2021/04/052).
CRediT authorship contribution statement
Boy M. Bachtiar: Writing – original draft, Methodology, Formal analysis, Conceptualization. Endang W. Bachtiar: Writing – review & editing, Formal analysis, Conceptualization. Ardiana Kusumaningrum: Data curation, Investigation, Supervision. Hari Sunarto: Methodology, Data curation, Conceptualization. Yuniarti Soeroso: Methodology, Data curation, Conceptualization. Benso Sulijaya: Methodology, Data curation, Investigation. Efa Apriyanti: Data curation, Investigation. Citra Fragrantia Theodorea: Data curation, Investigation. Irandi Putra Pratomo: Data curation, Investigation, Supervision. Yudhistira: Data curation, Investigation, Supervision. Defi Efendi: Data curation, Investigation.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
All the authors would like to acknowledge all the study participants, the Director of RSUI, and the Ethical clearance committee for providing permission and ethical clearance to conduct this research project. This study was supported by a grant provided by the Ministry of Education, Culture, Research and Technology, Republic of Indonesia (No.NKB-815/UN2.RST/HKP.05.00/2022).
Footnotes
Peer review under responsibility of King Saud University. Production and hosting by Elsevier.
References
- Andrukhov O., Ulm C., Reischl H., et al. Serum cytokine levels in periodontitis patients in relation to the bacterial load. J. Periodontol. 2011;82:885–892. doi: 10.1902/jop.2010.100425. [DOI] [PubMed] [Google Scholar]
- Bachtiar, BM. Bachtiar, EW. Sunarto, H. Soeroso Y. Sulijaya, B. Theodorea, C.F., et al, 2022. ACE2 gene expression and inflammatory conditions in periodontal microenvironment of COVID-19 patients with and without diabetes evaluated by qPCR. MdRxiv. [Preprint.] March 14, 2022. Available from: https://doi.org/10.1101/2022.03.10.22271304.
- Bachtiar B.M., Theodorea C.F., Tahapary D.L., et al. A pilot study of red complex and three genera subgingival microbiome in periodontitis subjects with and without diabetes, evaluated by MinION platform. F1000Res. 2021;10:79. doi: 10.12688/f1000research.28216.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baghbani T., Nikzad H., Azadbakht J., et al. Dual and mutual interaction between microbiota and viral infections: a possible treat for COVID-19. Microb. Cell Fact. 2020;19:217. doi: 10.1186/s12934-020-01483-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhury A., Mukherjee S. In silico studies on the comparative characterization of the interactions of SARS-CoV-2 spike glycoprotein with ACE-2 receptor homologs and human TLRs. J. Med. Virol. 2020;92:2105–2113. doi: 10.1002/jmv.25987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedgpeth D.C., Zhang X., Jin J., et al. Periodontal CD14 mRNA expression is downregulated in patients with chronic periodontitis and type 2 diabetes. BMC Oral Health. 2015;15:145. doi: 10.1186/s12903-015-0118-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang N., Perez P., Kato T., et al. SARS-CoV-2 infection of the oral cavity and saliva. Nat. Med. 2021;27:892–903. doi: 10.1038/s41591-021-01296-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain S., Darveau R.P. Contribution of Porphyromonas gingivalis lipopolysaccharide to periodontitis. Periodontol. 2010;2000(54):53–70. doi: 10.1111/j.1600-0757.2009.00333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y., Xu X., Xiao L., et al. The role of microRNA in the inflammatory response of wound healing. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.852419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung H.E., Lee H.K. Current understanding of the innate control of toll-like receptors in response to SARS-CoV-2 infection. Viruses. 2021;13 doi: 10.3390/v13112132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato Y., Hagiwara M., Ishihara Y., et al. TNF-alpha augmented Porphyromonas gingivalis invasion in human gingival epithelial cells through Rab5 and ICAM-1. BMC Microbiol. 2014;14:229. doi: 10.1186/s12866-014-0229-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.H., Lee S.Y., Lee Y.M., et al. MicroRNAs as biomarkers for dental diseases. Singapore Dent. J. 2015;36:18–22. doi: 10.1016/j.sdj.2015.09.001. [DOI] [PubMed] [Google Scholar]
- Lamont R.J., Koo H., Hajishengallis G. The oral microbiota: dynamic communities and host interactions. Nat. Rev. Microbiol. 2018;16:745–759. doi: 10.1038/s41579-018-0089-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Zhou W., Yang L., et al. Physiological and pathological regulation of ACE2, the SARS-CoV-2 receptor. Pharmacol. Res. 2020;157 doi: 10.1016/j.phrs.2020.104833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahesh G., Biswas R. MicroRNA-155: a master regulator of inflammation. J. Interferon Cytokine Res. 2019;39:321–330. doi: 10.1089/jir.2018.0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon L., Schwartz K., Yilmaz O., et al. Vitamin D-mediated induction of innate immunity in gingival epithelial cells. Infect. Immun. 2011;79:2250–2256. doi: 10.1128/IAI.00099-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikamori M., Yamada D., Eguchi H., et al. MicroRNA-155 controls exosome synthesis and promotes gemcitabine resistance in pancreatic ductal adenocarcinoma. Sci. Rep. 2017;7:42339. doi: 10.1038/srep42339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nativel B., Couret D., Giraud P., et al. Porphyromonas gingivalis lipopolysaccharides act exclusively through TLR4 with a resilience between mouse and human. Sci. Rep. 2017;7:15789. doi: 10.1038/s41598-017-16190-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navidshad B., Liang J.B., Jahromi M.F. Correlation coefficients between different methods of expressing bacterial quantification using real time PCR. Int. J. Mol. Sci. 2012;13:2119–2132. doi: 10.3390/ijms13022119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagliaro P., Penna C. ACE/ACE2 ratio: a key also in 2019 coronavirus disease (Covid-19)? Front. Med. (Lausanne) 2020;7:335. doi: 10.3389/fmed.2020.00335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papapanou P.N., Sanz M., Buduneli N., et al. Periodontitis: consensus report of workgroup 2 of the 2017 World workshop on the classification of periodontal and peri-implant diseases and conditions. J. Periodontol. 2018;89(Suppl 1):S173–S182. doi: 10.1002/JPER.17-0721. [DOI] [PubMed] [Google Scholar]
- Radaic A., Ganther S., Kamarajan P., et al. Paradigm shift in the pathogenesis and treatment of oral cancer and other cancers focused on the oralome and antimicrobial-based therapeutics. Periodontol. 2021;2000(87):76–93. doi: 10.1111/prd.12388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roganovic J. Downregulation of microRNA-146a in diabetes, obesity and hypertension may contribute to severe COVID-19. Med. Hypotheses. 2021;146 doi: 10.1016/j.mehy.2020.110448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi W., Kubota N., Shimizu T., et al. Existence of SARS-CoV-2 entry molecules in the oral cavity. Int. J. Mol. Sci. 2020;21 doi: 10.3390/ijms21176000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedgley C.M., Nagel A.C., Shelburne C.E., et al. Quantitative real-time PCR detection of oral Enterococcus faecalis in humans. Arch. Oral Biol. 2005;50:575–583. doi: 10.1016/j.archoralbio.2004.10.017. [DOI] [PubMed] [Google Scholar]
- Sena K., Furue K., Setoguchi F., et al. Altered expression of SARS-CoV-2 entry and processing genes by Porphyromonas gingivalis-derived lipopolysaccharide, inflammatory cytokines and prostaglandin E2 in human gingival fibroblasts. Arch. Oral Biol. 2021;129 doi: 10.1016/j.archoralbio.2021.105201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sha Q., Truong-Tran A.Q., Plitt J.R., et al. Activation of airway epithelial cells by toll-like receptor agonists. Am. J. Respir. Cell Mol. Biol. 2004;31:358–364. doi: 10.1165/rcmb.2003-0388OC. [DOI] [PubMed] [Google Scholar]
- Tang Q., Wang Y., Ou L., et al. Downregulation of ACE2 expression by SARS-CoV-2 worsens the prognosis of KIRC and KIRP patients via metabolism and immunoregulation. Int. J. Biol. Sci. 2021;17:1925–1939. doi: 10.7150/ijbs.57802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomas I., Regueira-Iglesias A., Lopez M., et al. Quantification by qPCR of pathobionts in chronic periodontitis: development of predictive models of disease severity at site-specific level. Front. Microbiol. 2017;8:1443. doi: 10.3389/fmicb.2017.01443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Elm E., Altman D.G., Egger M., et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Int. J. Surg. 2014;12:1495–1499. doi: 10.1016/j.ijsu.2014.07.013. [DOI] [PubMed] [Google Scholar]
- Wu P., Feng J., Wang W. Expression of miR-155 and miR-146a in the saliva of patients with periodontitis and its clinical value. Am. J. Transl. Res. 2021;13:6670–6677. [PMC free article] [PubMed] [Google Scholar]
- Zhang M.S., Niu F.W., Li K. Proflavin suppresses the growth of human osteosarcoma MG63 cells through apoptosis and autophagy. Oncol. Lett. 2015;10:463–468. doi: 10.3892/ol.2015.3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q., Desta T., Fenton M., et al. Cytokine profiling of macrophages exposed to Porphyromonas gingivalis, its lipopolysaccharide, or its FimA protein. Infect. Immun. 2005;73:935–943. doi: 10.1128/IAI.73.2.935-943.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlotogorski-Hurvitz A., Dayan D., Chaushu G., et al. Human saliva-derived exosomes: comparing methods of isolation. J. Histochem. Cytochem. 2015;63:181–189. doi: 10.1369/0022155414564219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachtiar, E.W. Bachtiar, BM. 2020. Effect of cell-free spent media prepared from Aggregatibacter actinomycetemcomitans on the growth of Candida albicans and Streptococcus mutans in co-species biofilms. European Journal of Oral Sciences, 2020, 128(5), 395–404 [DOI] [PubMed]