Abstract
The present study described the susceptibility of C4D guinea pigs to cutaneous infection with Treponema pallidum subsp. pertenue Haiti B strain. The general manifestations of the disease in adults and neonates differ, to a certain degree, from those induced by T. pallidum subsp. pallidum Nichols strain. Noticeable differences between the infections were reflected in the character of the skin lesions, their onset and persistence, and the kinetics of the humoral response. The incidence and dissemination of cutaneous yaws lesions in very young guinea pigs were remarkably different from the low frequency observed in a similar age group of syphilis infection, 100 versus 17%, respectively. Moreover, as opposed to T. pallidum subsp. pallidum, T. pallidum subsp. pertenue does not cross the placenta. Offspring born to yaws-infected mothers did not produce immunoglobulin M antibodies and their organs, examined by PCR and rabbit infectivity test (RIT), were all negative. Examination of a large number of tissues and organs in adult, neonate, and maternal yaws by PCR and RIT clearly demonstrated that, unlike syphilis, there was a low incidence and short persistence of the yaws pathogen in internal organs. These findings stress the dermotropic rather than the organotropic character of yaws and provide further evidence of distinctive biological and pathological differences between yaws and venereal syphilis.
Yaws is a nonvenereal treponematosis caused by Treponema pallidum subsp. pertenue. Its relationship to T. pallidum subsp. pallidum, the causative agent of syphilis, has been the subject of much debate for more than 300 years (see review of the literature in reference 27). Both T. pallidum subsp. pallidum and T. pallidum subsp. pertenue are non-cultivable and morphologically identical in that they cannot be distinguished by fluorescent or treponemicidal immobilization tests (13, 19). To date, no (6, 22), only minor (6, 18, 24, 35) or substantial (33) genetic differences between both pathogens have been reported. Clinically, however, yaws differs from syphilis in several aspects (10, 26). Transmission of yaws occurs by body contact predominantly at an early age (3 to 15 years), whereas syphilis has no age limitations and, except for congenital infection, is generally transmitted by sexual contact. Early stages of yaws and syphilis bear some similarities, but late lesions of yaws are thought to be limited to skin, bones, and joints. Late active syphilis, on the other hand, may involve any tissue or organ system. Congenital and neurosyphilis are the consequence of untreated or improperly treated syphilis, whereas in yaws reports of congenital, visceral, or central nervous system involvement are anecdotal (for a review of the literature, see reference 27) and, so far, experimentally unconfirmed. Indeed, the theory proposed by several investigators (7, 16) that the T. pallidum subsp. pallidum and T. pallidum subsp. pertenue have evolved from a common ancestor but are now in fact different diseases seems to be the most plausible one. This theory accounts for the varied clinical manifestations of yaws in natural and experimental infection and the failure to afford full cross-protection by infection of experimental animals with either pathogen (23, 32).
Rabbits (25, 34) and, especially, golden hamsters (14, 28, 31, 34) have been for years the animal of preference for exploring experimental yaws. Schell and coworkers contributed significantly to the exploration of the immune responses and the protective role of antibodies and immune cells in experimental yaws in hamsters (1, 2, 30–32).
Turner and Hollander (34) successfully infected guinea pigs intracutaneously with T. pallidum subsp. pertenue YD27 and maintained the T. pallidum subsp. pertenue strain through five passages in these animals. These experiments, however, were not further pursued. In fact, this may have contributed to the lack of recognition of the guinea pig as a susceptible model for T. pallidum subsp. pertenue by several investigators (15, 27, 29).
We have successfully elaborated the guinea pig model for studies of acquired (36), neonatal (38), and congenital syphilis (39). Using the same animal model and methods of investigation, we explored the clinical manifestations and immune response of yaws-infected adult and neonates and the possibility of transplacental transmission from yaws-infected pregnant sows.
MATERIALS AND METHODS
Treponemal strain.
For infection of all guinea pigs, T. pallidum subsp. pertenue strain Haiti B was used. This microorganism was transferred, in 1951, from an 11-year-old boy who had typical generalized frambesiform yaws of 5 weeks duration into rabbit testes (34) and propagated in rabbits in the laboratory of Thomas B. Turner, Johns Hopkins University, Baltimore, Md. We obtained the strain in 1983 from Paul Hardy, Jr., Johns Hopkins Hospital. The strain was immediately injected into rabbit testes with successful results. It was preserved at −70°C and propagated, when needed, into rabbit testes. For infection of guinea pigs, a fresh suspension was obtained from rabbit testes infected for 15 to 19 days. The suspension was prepared in phosphate-buffered saline containing 10% of inactivated guinea pig (C4D) serum.
Animals and infection.
Normal C4-deficient (C4D) guinea pigs were used for all experiments. The animals were obtained from the Wadsworth Center's animal facilities. The C4D strain, genetically related to inbred strain 13, is the strain most susceptible to T. pallidum subsp. pallidum infection (50% infective dose = 102 organisms [37]). The C4D animals have a genetically controlled total deficiency of the fourth component of complement (8); however, their immunologic competence at the cellular and humoral levels is similar to that of complement-sufficient strains (5, 11). The choice of the C4D guinea pig for the present study was based on the fact that 100% of adult animals are susceptible to cutaneous infection with T. pallidum subsp. pallidum, but approximately 75% of infected neonates remain temporarily (2 to 3 months) asymptomatic (38), whereas almost all offspring born to syphilitic mothers are congenitally infected (39).
Four groups of guinea pigs were included in the present report. They represented adults, neonates, and fetuses and/or offspring guinea pigs born to yaws-infected mothers (maternal yaws). The number of animals per group, the age, the route of infection, the time of experimentation, and the dose inoculated are given in Table 1.
TABLE 1.
Adults and neonates guinea pigs used in these studies
| Group | No. of animals | Age (mo) | Route of infectiona | Experimental period (wk) | Doseb |
|---|---|---|---|---|---|
| Adult yaws | 10 | 3–5 | i.d. | 14 | 4 × 107 |
| 5 | 3–5 | i.v. | 4 × 107 | ||
| Neonatal yaws | 10 | 1–7 (days) | i.d. | 14 | 4 × 107 |
| Maternal yaws (long-term study) | 5 | 3–5 | i.d. | 14 | 1 × 108 |
| 6 | 3–5 | i.v. | 1 × 108 | ||
| Maternal yaws (short-term study) | 10 | 3–5 | i.v. | 1–7 (days) | 1 × 108 |
i.d., intradermal; i.v., intravenous.
All animals were infected with T. pallidum subsp. pertenue Haiti B strain.
Specimen collection.
At the end of the experiment, or when appropriate, the animals were anesthetized (Ketaset; Bristol Laboratory, Syracuse, N.Y.), bled into heparin (30 U/ml of blood) or without anticoagulant for serology, and sacrificed by intravenous injection of euthanasia agent (Somlethol; American Hoechst, Sommerville, N.Y.). For adult and neonatal yaws experiments, specimens such as whole blood (WB), inguinal lymph nodes (ILN), spleen (SP), heart (HRT), and brain (BR), and in some animals the mesenteric lymph nodes (MLN), were removed immediately, thoroughly rinsed with sterile buffered saline, minced, and mixed with double-concentrated TE buffer (40). In addition, for yaws-infected mothers and their progeny, specimens from endometrium (END), placenta (PL), and umbilical cord (UC) were collected and processed similarly. Whole small organs such as ILN, MLN, and fetal SP, or a portion of 300 to 500 mg from larger organs, were used for PCR and rabbit infectivity test (RIT) analysis. The procedure for the removal of fetuses from a normal pregnancy or due to maternal dystocia and the precautions taken to avoid cross-contamination with treponemal DNA during collection of organ specimens have been fully described (42).
Darkfield analysis.
The presence of T. pallidum subsp. pertenue in the skin lesions was examined by darkfield examination.
PCR.
The presence of T. pallidum subsp. pertenue DNA in animal tissues was examined by nested PCR using the same primers and reagents used for the detection of T. pallidum subsp. pallidum (40). The specificity of the PCR products was further confirmed by Southern blot analysis (41).
RIT.
The RIT was frequently used to confirm the viability of the organism detected by PCR. This test was critical in the exploration of congenital infection and to confirm the presence of viable organisms at the skin site of infection, long after the lesion had subsided. This test was as described in detail previously (41).
All animal procedures used were approved by the Institutional Animal Care Committee of the Wadsworth Center.
ELISA with T. pallidum subsp. pallidum.
Levels of treponemal antibodies and their immunoglobulin isotypes were examined by enzyme-linked immunosorbent assay (ELISA) with T. pallidum subsp. pallidum using Percoll-purified 10% alcohol-fixed T. pallidum subsp. pallidum as antigen (43) and affinity-purified rabbit immunoglobulin G (IgG) antibodies to guinea pig IgM and IgG (ICN Biomedicals, Costa Mesa, Calif.) as described (39). Optical densities ≥0.100 for IgM and IgG were considered to be positive. These values were >2 standard deviations above the values for noninfected guinea pigs determined in sera from normal young (n = 10) and adult (n = 10) animals. Positive and negative controls consisted of serum pools containing anti-T. pallidum subsp. pallidum antibodies previously tested by ELISA with T. pallidum subsp. pallidum and immunoblotting.
FTA-ABS test.
The fluorescent treponemal antibody absorption test (FTA-ABS) was done as described earlier (39).
Immunoblotting.
Solubilized T. pallidum subsp. pallidum proteins were fractionated on gradient electrophoresis gels (4 to 20% Tris-glycine Ready Gels; Bio-Rad Laboratories, Inc., Hercules, Calif.) as previously reported (39). Nitrocellulose blots of the separated proteins were subsequently probed with affinity-purified rabbit IgG antibodies to guinea pig IgM and IgG and peroxidase-conjugated goat anti-rabbit IgG(ICN Biomedicals).
RESULTS
Course of infection with T. pallidum subsp. pertenue in adults and neonatal yaws.
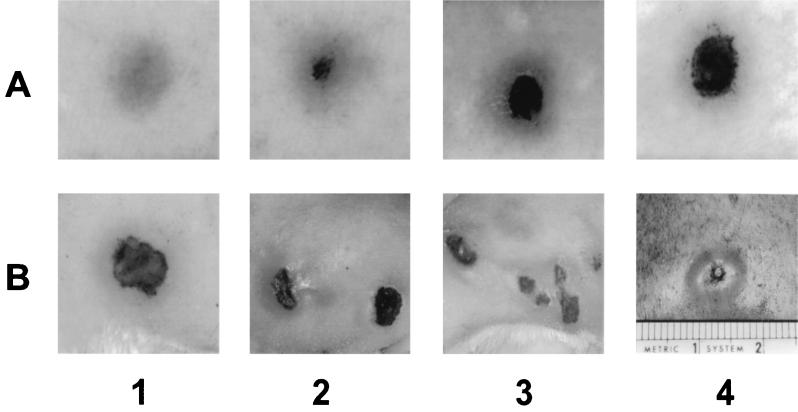
The number of experimental and control animals, the sizes of the inocula, the type of organism, and the route of inoculation are given in Table 1. Only adults and neonates infected intradermally developed darkfield-positive lesions on both buttocks. All but one adult, which never developed lesions, showed an erythematous reaction of 3 to 8 mm within the first week (Fig. 1, A1), which evolved at 14 days after infection into flat necrotic lesions 10 to 15 mm in diameter (Fig. 1, A2 to A4). These lesions were different from the characteristically high indurated syphilitic lesions shown in Fig. 1 (B4). The yaws lesions lasted from 7 to 12 weeks, which was longer than those for syphilitic lesions. In nine neonates the lesions started a few days later than in adult animals, and in one pup the incubation lasted 33 days. The character of the skin lesions in neonates was similar to those in the adults (Fig. 1, B1), but in 8 of 20 inoculated buttocks, multiple satellite lesions appeared (Fig. 1, B2 and B3). None of the intravenously infected animals developed obvious clinical signs of infection. Histopathological examination of yaws lesions showed a predominant degeneration and necrosis of the epidermis, with considerable infiltration of not only mononuclear cells but also neutrophils and eosinophils (data not shown).
FIG. 1.
Yaws lesions in adult C4D guinea pigs at 1 (A1) and 2 (A2 to A4) weeks after infection. Single and multiple lesions in C4D neonates at 20 to 30 days after intradermal inoculation (B1 to B3). The flat necrotic frambesia lesions contrast with the typical indurated syphilitic chancre (B4).
Maternal yaws.
In the long-term study, 5 sows infected intradermally during pregnancy produced 14 offspring; 5 were born apparently healthy, 4 were stillborn, and 5 were surgically removed because of maternal dystocia. The 6 intravenously inoculated mothers were the source of 12 offspring; 6 healthy, 3 stillborn, and 3 surgically removed because of abnormal labor. Not one of the 11 apparently healthy pups showed any sign of abnormalities during 14 weeks of observation. Two of seven stillborn animals were macerated and unsuitable for PCR testing or pathologic examination. None of the eight fetuses that were surgically removed showed any developmental abnormalities.
PCR and RIT.
The results of 30 tissue specimens from adult animals with yaws, 59 specimens from neonatal animals with yaws, and 120 specimens collected from 24 animals from the prepartum period to 14 weeks of life, born to 11 yaws-infected mothers, are shown in Table 2. In adult guinea pig yaws, T. pallidum DNA was present in all of the skin specimens long after the healing process was completed. Five internal organs were examined in each of five individual animals. The pathogen could be identified in only 7 of these 25 organs, including ILN, HRT, and BRN, all in the first 5 weeks after infection. In neonatal yaws, the persistence of T. pallidum subsp. pertenue in the skin was similar to that in adult animals. Except for the ILN collected from two pups sacrificed at 3 weeks after infection, all other organs were PCR and RIT negative, as were all 120 internal organs collected from the fetuses or pups born to the yaws-infected mothers. This was further confirmed in the next group of maternal yaws, in which 49 tissue specimens from 10 intravenously inoculated mothers and 189 samples from 27 fetuses surgically removed after 1 to 7 days of maternal infection were analyzed by PCR and, if deemed necessary, by RIT to confirm the viability of the pathogen. As shown in Table 3, viable organisms were present in only 5 of 10 maternal blood specimens and in 16 of 39 maternal internal organs, in most cases within 4 days of maternal infection. On the other hand, except for one END specimen, all fetal specimens collected 1 to 7 days after maternal infection were negative by PCR or RIT.
TABLE 2.
PCR and RIT examination of organs from guinea pigs infected as adults and neonates and from the fetuses and/or offspring born to yaws-infected mothers (long-term study)
| Guinea pig group | Animal no. | Infection duration (wk) | PCR and RIT analysis of organa
|
|||||
|---|---|---|---|---|---|---|---|---|
| SK | ILN | MLN | SPL | HRT | BR | |||
| Adult with yaws | 655 | 1 | + | + | − | + | + | + |
| 656 | 3 | + | − | − | − | − | + | |
| 657 | 5 | +* | − | − | − | + | +* | |
| 654 | 14 | +* | − | − | − | − | − | |
| 653 | 14 | +* | − | − | − | − | − | |
| Neonate with yaws | 696A | 3 | + | +* | − | − | − | − |
| 696B | 3 | + | +* | − | − | − | − | |
| 697A | 5 | + | − | − | − | − | − | |
| 697B | 5 | + | − | − | − | − | − | |
| 670A | 10 | +* | − | − | − | − | − | |
| 670B | 10 | + | − | − | − | − | − | |
| 670C | 10 | + | − | − | − | − | − | |
| 671A | 14 | +* | − | − | − | − | − | |
| 671B | 14 | +* | − | − | − | − | − | |
| 671C | 14 | ND | − | − | − | − | − | |
| Fetus and/or offspringb | 24 animals | 0 day to 14 wkc | ND | − | − | − | − | − |
SK, skin; ILN, inguinal lymph nodes; MLN, mesenteric lymph nodes; SP, spleen; HRT, heart; BR, brain. ∗, RIT positive. ND, not determined.
The yaws-infected mothers had their SK positive by PCR, and those sacrificed before 5 weeks of infection had also HRT and BR positive by PCR.
“0-day” fetuses were removed prepartum because of maternal dystocia.
TABLE 3.
Lack of transmissibility of T. pallidum subsp. pertenue from intravenously infected sows to their progeny as demonstrated by PCR and RIT
| Mother no.a | Days gestationa | No. of fetuses | Days of infection | Pregnant sows
|
Fetuses
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PCRb
|
RIT (pooled tissues)d | Group | PCRc
|
RIT (pooled tissues)e | ||||||||||||||
| WB | ILN | SP | HRT | BR | END | PL | UC | ILN | SP | HRT | BR | |||||||
| 1 | 30–34 | 3 | 1 | + | + | + | NDf | + | + | A–C | − | − | − | − | − | − | − | − |
| 2 | 28–32 | 2 | 1 | + | + | − | + | + | + | A, B | − | − | − | − | − | − | − | ND |
| 3 | 40–45 | 3 | 3 | + | + | − | + | − | + | A–C | − | − | − | − | − | − | − | − |
| 4 | 40–45 | 1 | 3 | − | + | + | − | + | + | A | − | − | − | − | − | − | − | − |
| 5 | 42–47 | 2 | 4 | + | − | − | + | + | + | A | + | − | − | − | − | − | − | − |
| 6 | 25–30 | 4 | 5 | − | − | − | − | − | − | A–D | − | − | − | − | − | − | − | ND |
| 7 | 40–45 | 3 | 5 | − | − | − | − | − | − | A–C | − | − | − | − | − | − | − | − |
| 8 | 58–62 | 4 | 5 | − | + | − | + | + | + | A–D | − | − | − | − | − | − | − | − |
| 9 | 55–60 | 3 | 7 | + | − | − | − | − | − | A–C | − | − | − | − | − | − | − | − |
| 10 | 53–58 | 2 | 7 | − | − | − | − | − | − | A, B | − | − | − | − | − | − | − | − |
Pregnant sows infected intravenously with 108 virulent T. pallidum subsp. pertenue at the indicated time of gestation.
WB, whole blood; ILN, inguinal lymph node; SP, spleen; HRT, heart; BR, brain.
END, endometrium; PL, placenta; UC, umbilical cord.
Consisted of ILN, SP, HRT, and BR.
Pooled organs from two to three fetuses.
ND, not determined.
Immune response. (i) ELISA with T. pallidum subsp. pallidum.
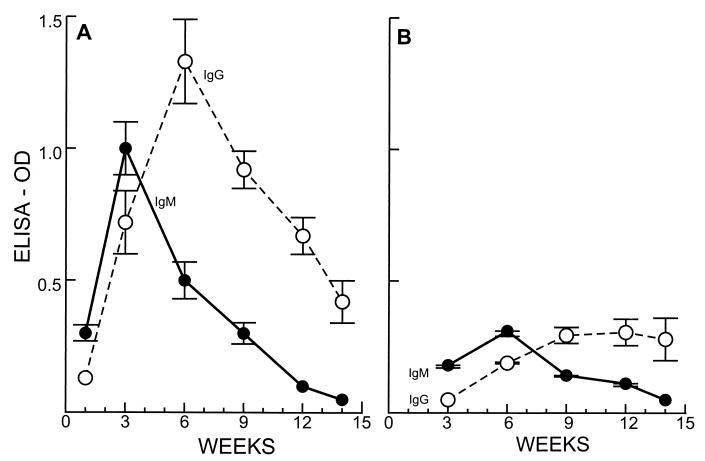
Regardless of the route of inoculation, adults and neonates responded to the infection with different kinetics and different patterns of IgM and IgG antibody activities (Fig. 2). In adult yaws, IgM and, to lesser degree, IgG were already detected at 1 week of infection (Fig. 2A), whereas in the neonates, detectable levels of IgM and IgG appeared in the third week of infection (Fig. 2B). The peak of the humoral response in the adults occurred at 3 and 6 weeks for IgM and IgG, respectively, whereas in the neonates a maximum response occurred at 6 and 9 weeks for the same isotypes. Moreover, the levels of IgM and IgG antibodies were more than 5 times higher in adults compared to neonates and sharply decreased after the peak. The humoral response in yaws-infected mothers was not different from that of other adult animals with yaws. However, none of the 11 live offspring born to mothers infected intravenously or intradermally demonstrated production of IgM antibodies by ELISA throughout the 14 weeks of observation. The sera of these young animals did show the presence of a very low level of IgG, apparently of maternal origin since it disappeared by between 3 and 9 weeks of age (data not shown).
FIG. 2.
ELISA with T. pallidum subsp. pallidum. IgM and IgG antibodies reacting with 10% alcohol-treated treponemes. Kinetics of the humoral response in 10 adult (A) and 10 neonates (B) C4D guinea pigs. Optical density values of ≥0.100 for IgM and IgG are significantly elevated.
(ii) Immunoblot.
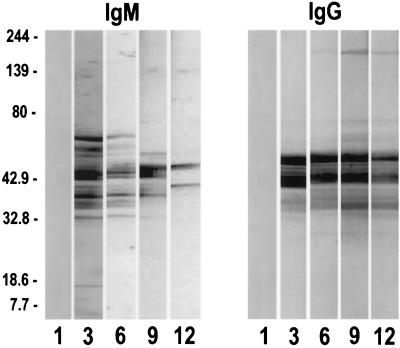
Immunoblot analysis confirmed the dynamic process of the ELISA with T. pallidum subsp. pallidum antibody response. Three to five pooled sera of adult animals with yaws obtained 1 to 12 weeks after infection were reacted with blots of peptides from T. pallidum subsp. pallidum. Both IgM and IgG obtained from animals 3 to 9 weeks after infection reacted with 13 to 16 individual peptides, ranging in weight from 30 to 70 kDa (Fig. 3). A decrease, which was sharper for IgM than for IgG, was evident at 12 weeks. IgM reactivity with the 17-kDa protein was noted 3 weeks after infection, whereas the 15-kDa polypeptide was not recognized by any of the antisera. The 17- and 15-kDa T. pallidum subsp. pallidum proteins were not recognized by the IgG antibodies which are regularly seen in syphilis infection. Immunoblot analysis of neonate sera showed a similar pattern as in the adults despite the fact that the reactions were less intense (data not shown).
FIG. 3.
Blots of T. pallidum were reacted with a pool of three to five sera from adult guinea pigs collected at from 1 to 12 weeks postinfection with T. pallidum subsp. pertenue. The reaction was developed with rabbit antibodies specific to guinea pig IgM and IgG and peroxidase-conjugated goat anti-rabbit IgG.
(iii) FTA-ABS.
The pattern of antibody response in FTA-ABS using anti-guinea pig immunoglobulins showed a peak of antibody response (mean titers of 320 in adults and 160 in neonates) at 9 weeks after infection but remained practically unchanged for 14 weeks of observation (data not shown).
DISCUSSION
We have attempted to reproduce the clinical manifestations of acquired, neonatal, and congenital syphilis in the C4D guinea pig by infecting these animals with the T. pallidum subsp. pertenue Haiti B strain. As observed earlier by Turner and Hollander (34), but contrary to what is generally reported in the literature (15, 27, 29), we showed that the guinea pig is equally susceptible to cutaneous infection with T. pallidum subsp. pertenue and T. pallidum subsp. pallidum.
Some similarities and some important differences between yaws and syphilis infections were observed in the guinea pig model. As opposed to rabbits (34) but like hamsters (31), the guinea pig does not show obvious testicular lesions after T. pallidum subsp. pallidum or T. pallidum subsp. pertenue infection. In adult guinea pigs, however, the character of the yaws skin lesions was different. The lesions have a tendency to appear later and persist longer than syphilitic lesions. Like rabbit yaws (25), no major involvement of draining lymph nodes was noticed in the guinea pig. In hamsters, however, the draining lymph nodes are the main target of infection and pathogenicity (32).
Even more remarkable were the differences in cutaneous susceptibility displayed by C4D neonates to yaws and syphilis infection. Yaws infection of C4D neonates not only caused extensive ulcerated lesions in all 10 infected pups but also, in 8 of 20 (40%) sites of infection, multiple satellite necrotic lesions occurred (Fig. 1B). The incidence and degree of cutaneous dissemination in the guinea pig model closely resembles that of secondary natural infection, which has been attributed to the pruritic properties of the skin lesions (26). It must be stressed, however, that the high incidence of lesions (100%) in the yaws-infected C4D pups is quite opposite to the high resistance (∼83%) of this age group to syphilis infection (38). This takes on meaning in view of the fact that, as a rule, children between 3 and 15 years of age are the most commonly infected with yaws. This particular age preference in human yaws has been attributed to the presence of immunity in adults and the lack of it in children (26). However, the possibility that genetically associated changes in immunological recognition also play a critical role in susceptibility and resistance to yaws must not be excluded, as has been demonstrated in the guinea pig model of acquired and neonatal syphilis. Two strains of guinea pigs, the C4D genetically related to inbred strain 13 and the Albany line haplotype identical to inbred strain 2, showed an opposite susceptibility to cutaneous infection with T. pallidum subsp. pallidum, first as neonates and then as adults (38), which cannot be attributed to the presence or absence of immunity. Similarly, an age-associated difference in susceptibility to cutaneous infection with T. pallidum subsp. pallidum has also been shown in the rabbit (12). On the other hand, genetic polymorphism within the same species may also account for the variable expression described in human yaws (26) and in the hamster model (31).
No major distinction between the persistence of T. pallidum subsp. pertenue and T. pallidum subsp. pallidum at the skin site of inoculation was noticed. A distinctive difference, however, was seen in the degree of systemic dissemination and the persistence of both treponemes in various internal organs. In yaws-infected adult and neonate guinea pigs the pathogen was present in the skin for the whole experimental period of 14 weeks. But, in contrast to syphilis (41), after T. pallidum subsp. pertenue infection only 3 of 5 adults and 2 of 10 neonates contained organs that were PCR or RIT positive and then only for very short period of time (3 to 5 weeks; Table 2). Additional organs, including WB, BR, and HRT, were targeted in yaws-infected mothers (Table 3) but also for no longer than 5 to 7 days after intravenous infection. The presence of T. pallidum subsp. pertenue in BR and HRT, while seemingly of no major consequence in the experimental model, may be of relevance in humans where infection with yaws has been implicated in the development of cardiovascular and neurological complications of late yaws (see the review in reference 27).
Upon comparing the persistence of T. pallidum subsp. pallidum and T. pallidum subsp. pertenue in the same guinea pig host, it seems to correlate with the degree of pathogenicity of the organisms. The T. pallidum subsp. pertenue remains in the soft organs of the guinea pig for no longer than 5 weeks and the T. pallidum subsp. pallidum persists, depending on the organ, for 1 to 2 years (41, 42) and possibly for life as occurred in cases of untreated human syphilis.
The kinetics of the humoral responses to T. pallidum subsp. pallidum and T. pallidum subsp. pertenue also differ when measured by ELISA, using T. pallidum subsp. pallidum as the antigen. In yaws-infected guinea pigs, bell-shaped curves for both IgM and IgG responses were observed. The antibody titer peaked at between 3 (IgM) and 6 (IgG) weeks and dropped sharply after 3 months of infection, whereas in the same strain and age of animals infected with comparable inoculum of T. pallidum subsp. pallidum Nichols, the highest levels of antibody were reached in approximately 3 months but persisted at a similar level for at least 5 months after infection (4). Whether these patterns of immune response reflect the temporary versus the prolonged systemic infection with T. pallidum subsp. pertenue and T. pallidum subsp. pallidum, respectively, or reflect a selective recognition by the anti-yaws antiserum of specific antigens in the alcohol-fixed T. pallidum subsp. pallidum is unknown. Support for the first assumption is provided by the hamster model, where infection with T. pallidum subsp. pertenue causes extensive node involvement and chronic skin lesions, which usually resolve within approximately 6 to 7 months of infection. In these animals, the antitreponemal antibodies, as determined by the Sera-Tek treponema antibody test, reach their peak at 8 to 9 weeks but remain elevated for 9 months after infection (31).
The Haiti B strain of yaws did not cause congenital infection in the guinea pig. Neither IgM antibodies nor the presence of treponemas in tissues were detected in 24 offspring born to yaws-infected mothers examined for up to 14 weeks of age or in 27 fetuses removed from infected dams between 1 to 7 days after maternal infection. However, all 21 mothers were serologically and PCR and RIT positive. The above findings provide for the first time solid evidence that, unlike T. pallidum subsp. pallidum Nichols (39, 42), the Haiti B strain of yaws does not cross the placenta. These findings are at odds with several reports of human congenital yaws. Most of the latter reports, however, are in the old literature (3, 9, 17, 20), when serodiagnosis of congenital syphilis was based on the presence of antiphospholipid antibodies. Interestingly, except in reference 9, IgM antibodies were not yet recognized as the only antibody genuinely produced by the infected infant. It is also questionable whether in those early years the available knowledge and techniques were sufficiently reliable to make the clinical distinction between T. pallidum subsp. pallidum and T. pallidum subsp. pertenue infection or to recognize a coinfection with both treponemes.
The results of our studies with the Haiti B strain of T. pallidum subsp. pertenue are apparently in disagreement with the recent report by Centurion-Lara et al. (6). These investigators, examining the flanking region sequences of the 15-kDa lipoprotein gene of a number of strains of pathogenic treponemas, concluded that the Haiti B strain was the only one of the four T. pallidum subsp. pertenue strains examined that followed the pattern determined for T. pallidum subsp. pallidum. The authors speculated that either T. pallidum subsp. pallidum Nichols strain might have been mislabeled as Haiti B at some point during laboratory passage or the patient's abdominal skin lesions, the origin of the organism, were not frambesian but syphilitic lesions. The results obtained from the guinea pig and those of Schell and associates in the hamster model (31, 32) stand in opposition to those assumptions. The Haiti B strain of T. pallidum subsp. pertenue used in both laboratories came from the same source, Paul Hardy's laboratory, and the same patient alluded to by Centurion-Lara et al. If the T. pallidum subsp. pertenue Haiti B strain were a mislabeled T. pallidum subsp. pallidum, a different clinical response in the hamster would definitely have been observed earlier by Schell and associates. If mislabeling can definitely be excluded in the laboratory of Centurion-Lara et al., an alternative explanation for their results may involve a genetic shift from T. pallidum subsp. pertenue to T. pallidum subsp. pallidum. There have been early reports that yaws treponemes passaged for some time in rabbits gradually shift the character of the lesions toward those of syphilis and may even undergo true mutation (21, 34). Apparently, these changes have not occurred neither in Schell's nor in our laboratory. Furthermore, additional genetic differences have been recently observed by Izard et al. (18), who compared the sequences of the cytoplasmic filament polypeptides (CfpA) of T. pallidum subsp. pallidum Nichols and T. pallidum subsp. pertenue Haiti B strain, both provided by our laboratory. Significant sequence differences within homologous genes (TprJ) of T. pallidum subsp. pallidum and the T. pallidum subsp. pertenue Gauthier strain has also been reported by Stamm et al. (33). Obviously, more extensive genetic studies are needed to solve the controversy surrounding the relationship between yaws and syphilis and within different strains of yaws. Regardless of the molecular relationship between T. pallidum subsp. pertenue Haiti B and T. pallidum subsp. pallidum Nichols, the guinea pig model has unquestionably recognized distinctive differences in their pathogenicities.
ACKNOWLEDGMENTS
Doris Collins and Diane Decker are acknowledged for the histopathological examination, Nancy Payne for the final preparation of the manuscript, and Katherine Henrikson for helpful editorial comments.
This work was supported by Public Health Service grant AI21833 from the National Institute of Allergy and Infectious Diseases (V.W.) and by funds from the U.S. Department of Veterans Affairs (R.E.B.).
REFERENCES
- 1.Alder J D, Daugherty N, Harris O N, Liu H, Steiner B M, Schell R F. Phagocytosis of Treponema pallidum pertenue by hamster macrophages on membrane filters. J Infect Dis. 1989;160:289–297. doi: 10.1093/infdis/160.2.289. [DOI] [PubMed] [Google Scholar]
- 2.Azadegan A A, Schell R F, Steiner B M, Coe J E, Chan J K. Effect of immune serum and its immunoglobulin fractions on hamster challenged with Treponema pallidum subsp. pertenue. J Infect Dis. 1986;153:1007–1013. doi: 10.1093/infdis/153.6.1007. [DOI] [PubMed] [Google Scholar]
- 3.Bandyopadhyay B N. Congenital yaws. Indian Med Gaz. 1926;61:120–121. [PMC free article] [PubMed] [Google Scholar]
- 4.Baughn R E, Wicher V, Jakubowski A, Wicher K. Humoral response to Treponemal pallidum-infected guinea pigs. II. Circulating immune complexes. J Immunol. 1987;136:1406–1414. [PubMed] [Google Scholar]
- 5.Burger R, Shevach E M. Evaluation of the role of C4D in the cellular and immune response in vitro. J Immunol. 1979;122:2388–2394. [PubMed] [Google Scholar]
- 6.Centurion-Lara A, Castro C, Castillo R, Shaffer J M, Van Voorhis W C, Lukehart S A. The flanking region sequences of the 15-kDa lipoprotein gene differentiate pathogenic treponemas. J Infect Dis. 1998;177:1036–1040. doi: 10.1086/515247. [DOI] [PubMed] [Google Scholar]
- 7.Cockburn T A. The origin of the treponematoses. Bull W H O. 1961;24:221–228. [PMC free article] [PubMed] [Google Scholar]
- 8.Ellman L, Green I, Frank M M. Genetically controlled total deficiency of the fourth component of complement in the guinea pig. Science. 1970;170:74–75. doi: 10.1126/science.170.3953.74. [DOI] [PubMed] [Google Scholar]
- 9.Engelhardt H K. A study of yaws. Does congenital syphilis occur? J Trop Med Hyg. 1959;62:238–240. [PubMed] [Google Scholar]
- 10.Engelkens H J H, Ten Kate F J W, Judanarso J, Vuzevski V D, van Lier J B H J, Godschalk J C J, van der Sluis J J, Stolz E. The localization of treponemas and characterization of the inflammatory infiltrate in skin biopsies from patients with primary and secondary syphilis, or early infectious yaws. Genitourin Med. 1993;69:102–107. doi: 10.1136/sti.69.2.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frank M M, May J, Gaither T, Ellman L. In vitro studies of complement function in sera of C4D-guinea pigs. J Exp Med. 1971;134:176–187. doi: 10.1084/jem.134.1.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gamboa D, Miller J N. Experimental neonatal syphilis. I. Evidence of resistance to symptomatic infection in neonatal rabbits following intradermal inoculation with Treponema pallidum (Nichols) Pediatr Res. 1984;18:965–971. doi: 10.1203/00006450-198410000-00012. [DOI] [PubMed] [Google Scholar]
- 13.Garner M F, Backhouse J L, Daskalopoulos G, Walsh J L. Use of T. pertenue in the fluorescent and immobilization test. Br J Vener Dis. 1974;50:264–266. doi: 10.1136/sti.50.4.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Geiman I M, McKee R W. A symposium on the latest advances in the study of venereal diseases. Paper no. 3. Washington, D.C.: U.S. Public Health Service, Division of Venereal Diseases; 1950. Experimental studies with pathogenic spirochetes. [Google Scholar]
- 15.Gutman L T. The spirochetes. In: Joklik W K, Willet H P, Amos D B, Wilfert C M, editors. Zinzer microbiology. 20th ed. Norwalk, Conn: Appleton and Lange; 1992. pp. 657–675. [Google Scholar]
- 16.Hackett C J. On the origin of the treponematoses. Bull W H O. 1963;29:7–41. [PMC free article] [PubMed] [Google Scholar]
- 17.Hunt D, Johnson A L. Yaws, a study based on over 2,000 cases treated in American Samoa. US Navy Med Bull. 1923;18:599–607. [Google Scholar]
- 18.Izard J, Samsonoff W A, Kinoshita M B, Limberger R J. Genetic and structural analysis of the cytoplasmic filaments of wild type and a flagellar filament-deficient mutant of Treponema phagedenis. J Bacteriol. 1999;181:6739–6746. doi: 10.1128/jb.181.21.6739-6746.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khan S A, Nelson R A, Jr, Turner T B. Immunological relationship among species of virulent treponemes as determined with the treponema immobilization test. Am J Hyg. 1951;53:296–316. doi: 10.1093/oxfordjournals.aje.a119455. [DOI] [PubMed] [Google Scholar]
- 20.Leon R. Hereditary yaws. Am J Trop Med. 1929;9:439–443. [Google Scholar]
- 21.Manteufel P, Herzberg K. Zur Syphilis-Framboesiefrage. Zentbl Haut Geschl Kr. 1929;30:299–301. [Google Scholar]
- 22.Miao R M, Fieldsteel A H. Genetic relationship between Treponema pallidum and Treponema pertenue, two noncultivable human pathogens. J Bacteriol. 1980;141:427–429. doi: 10.1128/jb.141.1.427-429.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller J N. Immunity in experimental syphilis. VI. Successful vaccination of rabbits with Treponema pallidum Nichols strain, attenuated by γ-irradiation. J Immunol. 1973;110:1206–1215. [PubMed] [Google Scholar]
- 24.Noordhoek G T, Wieles B, Van der Sluis J J, van Embden J D. Polymerase chain reaction and synthetic DNA probes: a means of distinguishing the causative agent of syphilis and yaws? Infect Immun. 1990;58:2011–2013. doi: 10.1128/iai.58.6.2011-2013.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pearce L, Brown W H. Distinctive characteristics of infections produced by Treponema pertenue in the rabbit. J Exp Med. 1925;41:673–690. doi: 10.1084/jem.41.5.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perine P L, Hopkins D R, Niemel P L A, St. John R K, Causse G, Antal G M. Handbook of endemic treponematoses: yaws, endemic syphilis, and pinta. Geneva, Switzerland: World Health Organization; 1984. pp. 1–36. [Google Scholar]
- 27.Roman G C, Roman L N. Occurrence of congenital, cardiovascular, visceral, neurologic, and neuro-ophthalmologic complications in late yaws: a theme for future research. Rev Infect Dis. 1986;8:760–770. [PubMed] [Google Scholar]
- 28.Rosenau B J. Treatment and immune reactions of experimental yaws in hamster. Ph.D. thesis. Cambridge, Mass: Harvard University; 1953. [Google Scholar]
- 29.Smibert R M. Treponema. In: Krieg N R, Holt J G, editors. Bergey's manual of systematic bacteriology. Vol. 1. Baltimore, Md: Williams & Wilkins; 1984. pp. 50–57. [Google Scholar]
- 30.Schell R F, LeFrock J L, Babu J P. Passive transfer of resistance to frambesial infection in the hamster. Infect Immun. 1978;21:430–435. doi: 10.1128/iai.21.2.430-435.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schell R F, LeFrock J L, Babu J P, Chan J K. Use of CB hamsters in the study of Treponema pertenue. Br J Vener Dis. 1979;55:316–319. doi: 10.1136/sti.55.5.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schell R F, Azadegan A A, Nitskansky S G, LeFrock J L. Acquired resistance of hamsters to challenge with homologous and heterologous virulent treponemas. Infect Immun. 1982;37:617–621. doi: 10.1128/iai.37.2.617-621.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stamm L V, Greene S R, Bergen H L, Hardham J M, Barnes N Y. Identification and sequence analysis of Treponema pallidum tprJ, a member of a polymorphic multigene family. FEMS Microbiol Lett. 1998;169:155–163. doi: 10.1111/j.1574-6968.1998.tb13312.x. [DOI] [PubMed] [Google Scholar]
- 34.Turner T B, Hollander D H. Biology of the treponematoses. Geneva, Switzerland: World Health Organization; 1957. [PubMed] [Google Scholar]
- 35.Walker E M, Howell J K, You Y, Hoffmaster A R, Heath J D, Weinstock G M, Norris S J. Physical map of the genome of Treponema pallidum subsp. pallidum (Nichols) J Bacteriol. 1995;177:1797–1804. doi: 10.1128/jb.177.7.1797-1804.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wicher K, Wicher V. Experimental syphilis in guinea pig. CRC Crit Rev Microbiol. 1989;16:181–234. doi: 10.3109/10408418909104471. [DOI] [PubMed] [Google Scholar]
- 37.Wicher K, Wicher V. Median infective dose of Treponema pallidum determined in a highly susceptible strain of guinea pig. Infect Immun. 1991;59:453–456. doi: 10.1128/iai.59.1.453-456.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wicher V, Wicher K, Rudofsky U, Zabek J, Jakubowski A, Nakeeb S. Experimental neonatal syphilis in a susceptible (C4D) and a resistant (Albany) strain of guinea pig. Clin Immun Immunopathol. 1990;55:23–40. doi: 10.1016/0090-1229(90)90066-y. [DOI] [PubMed] [Google Scholar]
- 39.Wicher K, Baughn R E, Wicher V, Nakeeb S. Experimental congenital syphilis: guinea pig model. Infect Immun. 1992;60:271–277. doi: 10.1128/iai.60.1.271-277.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wicher K, Noordhoek G T, Abbruscato F, Wicher V. Detection of Treponema pallidum in early syphilis by DNA amplification. J Clin Microbiol. 1992;30:497–500. doi: 10.1128/jcm.30.2.497-500.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wicher K, Abbruscato F, Wicher V, Baughn R E, Noordhoek G T. Target organs of infection in guinea pigs with acquired or congenital syphilis. Infect Immun. 1996;64:3174–3179. doi: 10.1128/iai.64.8.3174-3179.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wicher K, Baughn R E, Abbruscato F, Wicher V. Vertical transmission of Treponema pallidum to various litters and generations of guinea pigs. J Infect Dis. 1999;179:1206–1212. doi: 10.1086/314718. [DOI] [PubMed] [Google Scholar]
- 43.Zeltzer P M, Pepose J S, Bishop N H, Miller J N. Microassay for immunoglobulin G antibodies to Treponema pallidum with radioiodinated protein A from Staphylococcus aureus: immunoglobulin G response in experimental syphilis in rabbits. Infect Immun. 1978;21:163–170. doi: 10.1128/iai.21.1.163-170.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]