Abstract
Non-COVID hospital admissions decreased during the COVID-19 pandemic and follow-up of people in the lung cancer risk group was delayed. There are not enough studies on the effects of the pandemic period on the diagnosis of lung cancer. In this study, it was aimed to determine the characteristics of patients diagnosed with lung cancer in the pre-pandemic and pandemic period and to investigate the effects of the pandemic on the diagnosis of lung cancer. Patients with newly diagnosed lung cancer 16 months before and after the detection of the first COVID-19 case were retrospectively analyzed for their characteristics at the time of diagnosis. Age, gender, pathological diagnosis, distant organ metastasis status, and also pathological stages at the time of diagnosis of the patients were analyzed. Two hundred forty-six patients were included in the study. One hundred forty-five of the patients were diagnosed in the pre-pandemic period and 101 during the pandemic period. Mean age of patients was 64.24 years and 91.87% were male. Pathological diagnosis distributions were similar in the pre-pandemic group and the pandemic period group. Distant organ metastases were present in 59.31% of the pre-pandemic group and 65.35% of the pandemic group. There was no significant difference in terms of the stages of the patients at the time of diagnosis. Number of patients diagnosed with lung cancer during the pandemic period was lower. The characteristics of the patients were similar. These results may have resulted from the decrease in applications to health institutions due to social isolation and fear of COVID-19 infection, and limitations in accessing health services.
Keywords: COVID-19, Cancer stage, Lung cancer, Pandemic
Introduction
COVID-19 infection due to the SARS-CoV-2 agent emerged on 31 December 2019 in Wuhan, China and soon affected the whole world. The first case in Turkey was detected on 11 March 2020. Along with the pandemic measures, applications of elderly individuals and patients with chronic diseases to health institutions were affected due to reasons such as the worry of getting sick, social isolation, and lockdowns. A significant restructuring of healthcare delivery has occurred to cope with the increasing burden of patients requiring hospitalization and to minimize the risk of transmission to healthcare workers. The number of outpatient clinics and inpatient admissions were reduced. Interventional and non-urgent operations were postponed and access to healthcare outside of COVID-19 became more difficult.
Prior to the pandemic, lung cancer was the leading cause of cancer diagnosis and death worldwide; it is known that it constitutes 11.6% of new cases and 18.4% of deaths [1]. The high mortality rate is largely attributed to the aggressive course of the disease and its diagnosis at an advanced stage. With the pandemic period, the access to radiological examinations, nuclear medicine applications, and tissue biopsy methods has decreased due to the intense focus of healthcare providers on COVID-19 cases. Minimally invasive procedures that lead to aerosol generation, such as bronchoscopy and endobronchial ultrasound, were particularly affected because of their risk of transmission. In some guidelines published on the use of bronchoscopy during the pandemic process, it was argued that bronchoscopy should be postponed for non-urgent indications [2].
In this study, it was aimed to determine the age, gender, pathological diagnosis, stage at diagnosis, and distant organ metastasis status of patients diagnosed with lung cancer before and after the pandemic thus comparing the results of the two periods and investigating the effects of the pandemic on the diagnosis of lung cancer.
Materials and Methods
Patients with newly diagnosed lung cancer among the patients who applied to the thoracic oncology outpatient clinic of our hospital between 11 November 2018 and 11 July 2021 were included in the study. The period between 11 November 2018 and 10 March 2020 was determined as the pre-pandemic period, and the period between 11 March 2020 and 11 July 2021 was determined as the pandemic period. Patients diagnosed with lung cancer in the 16-month period before and after 11 March 2020, when the first COVID-19 case was reported in Turkey, were evaluated retrospectively and cross-sectionally in terms of their characteristics at the time of diagnosis. Ethical approval dated 01.12.2021 and numbered 20.478.486/1048 was obtained from the Health Sciences Ethics Committee.
Patients older than 18 years of age who were diagnosed with new lung cancer between 11 November 2018 and 11 July 2021 and who were staged by positron emission tomography/computed tomography and brain magnetic resonance imaging at the time of diagnosis were included in the study. Patients who had no pathological diagnosis, whose radiological records could not be accessed, who had insufficient data for the study, whose diagnosis was suspicious, whose staging result was uncertain, and who were diagnosed with lung and extrapulmonary malignancies outside the study date range were excluded from the study. Age, gender, history of COVID-19, smoking history, pathological diagnosis, distant organ metastases, and stages at diagnosis were analyzed and the characteristics of the patients who applied in these two periods were compared. Eastern Cooperative Oncology Group (ECOG) performance scores of the patients were recorded [3]. Patients who could do their activities without restriction were classified as ECOG 0, patients experienced restriction during strenuous physical activity were ECOG 1, patients who were standing and could care for themselves but could not work were ECOG 2, patients who spent more than 50% of daylight hours in bed were ECOG 3, and patients who were completely bedridden were ECOG 4. The patients were evaluated in terms of disease stage according to the 8 TNM staging system for lung cancer [4]. In addition to these, the time from the radiological suspicion of lung cancer until the pathological sample was taken was obtained by file review. The groups diagnosed with lung cancer before and during the pandemic were evaluated in terms of cancer stage at the time of diagnosis and their access to diagnostic procedures.
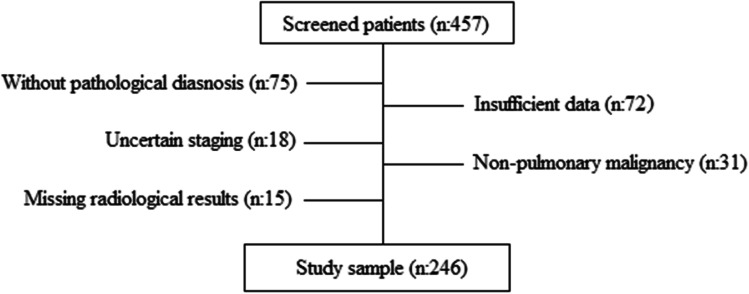
The records of 457 patients who applied to the thoracic oncology outpatient clinic of our hospital between 11 November 2018 and 11 July 2021 were reviewed. Seventy-five patients without a pathological diagnosis, 72 patients with insufficient data for the study, 31 patients with non-pulmonary malignancies, 15 patients whose radiological records could not be reached, and 18 patients with uncertain staging results were excluded from the study (Fig. 1).
Fig. 1.
Flow diagram
The data obtained in the study were evaluated statistically with the “SPSS Statistics 21” program. Frequency, percentage values, median (interquartile range), mean, and standard deviation values were determined as descriptive statistics. Numerical variables in the comparisons followed a normal distribution. Independent sample t-test was used for these variables. Comparisons between categorical variables were made using the chi-square test. Comparative correlation analyses (Pearson) were performed to determine the variables affecting mortality. In these statistical calculations, p < 0.05 was considered statistically significant.
Results
Two hundred forty-six patients were included in the study. The mean age of the patients was 64.24 (± 7.71) and 226 (91.87%) were male. The mean cigarette consumption of all patients was 43.81 (± 23.29) pack-years. Nine (8.91%) of the patients diagnosed with lung cancer during the pandemic period had COVID-19 infection history before diagnosis.
One hundred forty-five (58.94%) of the patients were diagnosed with lung cancer during the pre-pandemic period and 101 (41.06%) were diagnosed during the pandemic period (p < 0.001). The mean age of the patients diagnosed during the pre-pandemic period was 64.88 and those diagnosed during the pandemic period was 63.33 (p = 0.12). Eleven of 20 female patients were diagnosed during the pre-pandemic period and 9 of them were diagnosed during the pandemic period (p = 0.81). The age and gender distributions of the patients at the time of diagnosis were similar in the pre-pandemic and pandemic period. When the patients diagnosed before and during the pandemic were compared in terms of the amount of cigarette consumption, there was no significant difference (p = 0.45). The ECOG performance score of the majority of the patients was 1 (43.08%). The performance status of the groups diagnosed before and during the pandemic was similar at the time of diagnosis (p = 0.77) (Table 1).
Table 1.
Sociodemographic data
| Mean (± SD) | ||
|---|---|---|
| Age (years) | 64.24 (± 7.71) | |
| Gender | Male | 226 (91.87%) |
| Smoking (pack-year) | 43.81 (± 23.29) | |
| Performance status | ECOG 0 | 64 (26.01%) |
| ECOG 1 | 106 (43.08%) | |
| ECOG 2 | 55 (22.35%) | |
| ECOG 3 | 20 (8.13%) | |
| ECOG 4 | 1 (0.41%) | |
One hundred seven (43.49%) of all patients had squamous cell carcinomas, 74 (30.08%) adenocarcinomas, 44 (17.88%) small cell carcinomas, 18 (7.31%) non-small cell carcinomas, and 3 (1.21%) had other pathological subtype diagnoses when evaluated in terms of pathological types. The most common diagnosis in both groups was squamous cell lung cancer. Pathological diagnosis distributions of patients diagnosed with lung cancer before and during the pandemic were similar (p = 0.42).
Distant metastases were present in 152 (61.79%) patients at the time of diagnosis. The mean ages of patients with and without distant metastases were similar. Distant organ metastases were present in 86 of 145 patients diagnosed in the pre-pandemic period and in 66 of 101 patients diagnosed during the pandemic period. Distant organ metastasis rates were similar during the pre-pandemic and pandemic period (p = 0.35). When all patients were examined, 27 (10.97%) patients had brain metastases, 80 (32.52%) patients had bone metastases, and 40 (16.26%) patients had contralateral lung metastases. There were no significant differences between the two groups in terms of the presence of brain, bone, and contralateral lung metastases at the time of diagnosis (p = 0.30, p = 0.78, p = 0.73, respectively) (Table 2).
Table 2.
Comparison of the two groups in terms of pathological diagnosis and metastasis status
| Pre-pandemic | Pandemic | p value | ||
|---|---|---|---|---|
| Pathological diagnosis | SCLC | 22 (15.17%) | 22 (21.78%) | 0.42 |
| NSCLC | 13 (8.96%) | 5 (4.95%) | ||
| SCC | 63 (43.45%) | 44 (43.56%) | ||
| Adenocarcinoma | 46 (31.72%) | 28 (27.72%) | ||
| Others | 1 (0.69%) | 2 (1.98%) | ||
| Presence of metastases | Brain | 13 (8.96%) | 14 (13.86%) | 0.30 |
| Bone | 46 (31.72%) | 34 (33.66%) | 0.78 | |
| Contralateral lung | 25 (17.24%) | 15 (14.85%) | 0.73 | |
| All metastases | 86 (59.31%) | 66 (65.35%) | 0.35 | |
SCLC small cell lung cancer, NSCLC non-small cell lung cancer, SCC squamous cell lung cancer
Considering the distant organ metastasis rates in terms of pathological types, the highest rate of distant organ metastasis was found in small cell lung cancer cases. Distant organ metastases were detected in 72.7% of patients with small cell lung cancer, 49.5% of patients with squamous cell lung carcinoma, and 68.9% of patients with adenocarcinoma (p = 0.006).
The rate of patients diagnosed with lung cancer at an early stage (stage 1a, 1b, 2a, 2b) according to the 8 TNM classification was 8.27% in the pre-pandemic group and 9.90% in the pandemic group. The rate of those diagnosed with locally advanced stage (stage 3a, 3b, 3c) lung cancer was 32.41% in the pre-pandemic group and 24.75% in the pandemic-period group. In those diagnosed with metastatic (stage 4a, 4b) lung cancer, the rate was found to be 59.31% in the pre-pandemic group and 65.34% in the pandemic-period group. There was no statistically significant difference between the two groups in terms of disease stage (p = 0.59) (Table 3).
Table 3.
Comparison of two groups by cancer stage
| Stage | Pre-pandemic | Pandemic | p value |
|---|---|---|---|
| 1A | 4 | 3 | 0.59 |
| 1B | 2 | 2 | |
| 2A | 0 | 2 | |
| 2B | 6 | 3 | |
| 3A | 17 | 6 | |
| 3B | 19 | 14 | |
| 3C | 11 | 5 | |
| 4A | 44 | 35 | |
| 4B | 42 | 31 |
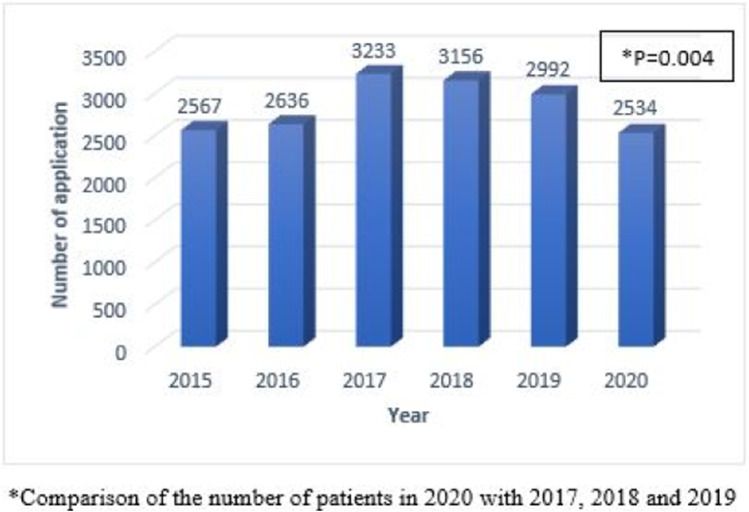
Our hospital’s thoracic oncology outpatient clinic was applied 3958 times in total during the pre-pandemic period covering 11 November 2018 to 10 March 2020 and 3291 times in total during the pandemic period covering 11 March 2020 and 11 July 2021. Considering the number of applications to the thoracic oncology outpatient clinic by years, it was found that 2567 in 2015, 2636 in 2016, 3253 in 2017, 3156 in 2018, 2292 in 2019, and 2534 in 2020. The number of outpatient visits in 2020, when the pandemic started, showed a sharper decline compared to 2018 and 2019. It was statistically significant (p = 0.004) (Fig. 2).
Fig. 2.
Number of applications to the thoracic oncology outpatient clinic by years
The mean time from radiological suspicion of lung cancer to obtaining a pathological sample was 10.06 (± 10.76) days in all patients. While this period was 9.06 (± 9.50) days in the pre-pandemic period, it was 11.50 (± 12.25) days during the pandemic period (p = 0.079). When the patients were evaluated in terms of accessibility to diagnostic procedures for lung cancer, this period was slightly longer in patients with the pandemic period, but it was not statistically significant.
Discussion
Compared to the 16-month period before the pandemic, it was determined that the number of patients diagnosed with new lung cancer in our clinic decreased significantly in the 16-month period starting on March 11, 2020, when the first case of COVID-19 was seen in Turkey. An increasing trend is observed in the number of patients admitted to the thoracic oncology outpatient clinic since 2015 until 2017 and a decreasing trend is observed from 2017 to 2021. Compared to the decrease in the number of patients admitted to the thoracic oncology outpatient clinic in previous years, it is observed that the decrease in the total number of patients admitted during the pandemic period is significantly higher.
These results support the hypothesis that patients are underdiagnosed due to decreased referrals to healthcare providers. London et al. investigated the effects of the COVID-19 pandemic on current and potential cancer patients using a network of 20 US institutions with more than 28 million patients. As a result of this study, it was determined that the number of patients diagnosed with cancer in April 2020 decreased by 65.2% compared to the number of patients diagnosed with cancer in April 2019, while the number of patients diagnosed with lung cancer decreased by 46.8% [5]. In a study conducted in China, it was found that patients with lung cancer who were scheduled to reapply to the hospital in January and February 2020 had to interrupt or delay their treatment [6]. These results are important in that the COVID-19 crisis shows the disruption in the follow-up of lung cancer and the hesitations of patients in terms of applying to health institutions.
This study shows that the pandemic has no effect on the stage of lung cancer at the time of diagnosis. Although fewer patients were diagnosed with lung cancer during the pandemic period in a similar period, it is noteworthy that there was no significant difference between the two periods in terms of disease stage. Factors such as the increase in hospital admissions with the suspicion of COVID-19 infection of patients with respiratory symptoms during the pandemic period and the increase in the use of imaging methods may have prevented patients from being diagnosed at a more advanced stage than before the pandemic.
In another study conducted with a similar purpose to our study, the number of newly diagnosed lung cancer cases and clinical features during the pandemic period were compared with lung cancer cases diagnosed in the 3 years before the pandemic. In this study, it was shown that the rate of patients with stage III–IV non-small cell lung cancer increased significantly during the pandemic period compared to previous years [7]. In another study, patients diagnosed with lung cancer between May and October 2020 were compared with the same months of 2019 and 2018 in terms of diagnosis and disease stage. Although there was a significant decrease in the number of diagnoses in 2020 compared to the previous 2 years, no significant difference was observed in the distribution of disease stages between years [8].
In order to clarify the effect of accessibility to diagnostic procedures on the results, the time spent by the patients in our clinic from admission to the diagnostic procedure before and during the pandemic was found to be similar. In our clinic, bronchoscopic procedures and imaging-guided biopsy procedures have continued since the first wave of the COVID-19 pandemic. Failure to interrupt diagnostic procedures may explain the fact that the stage of patients diagnosed with lung cancer diagnosed in our clinic is similar to that before the pandemic.
Bronchoscopy, which is frequently used as a diagnostic method in lung cancer, is also known as an aerosol-generating procedure, resulting in a high risk of infection for healthcare workers during the COVID-19 pandemic [9]. Different measures have been taken in countries regarding these procedures. A nationwide study conducted in Germany in the first wave of the pandemic showed that 16% of bronchoscopy units, particularly smaller ones, canceled more than 80% of planned interventions [10]. In a retrospective study evaluating routine bronchoscopy between 11 March and 15 May 2020 in Turkey, no appointment cancelations were reported. At the end of the 14-day follow-up period after the procedure, it was stated that no patient had COVID-19. Based on this, it was argued that endobronchial ultrasonography and/or bronchoscopy should not be delayed in patients with known or suspected lung cancer [11].
A recent study reported a 72% decrease in the annual volume of low-dose CT scans for screening compared to the pre-COVID-19 era [12]. In a study evaluating changes in future mortality rates using UK National Health System data, 29,305 patients with lung cancer were screened and it was estimated that there would be an increase of 4.8–5.3% in lung cancer deaths in the coming years when compared to the pre-pandemic figures [13]. Although there is no significant difference in the stage of the disease, the decrease in the number of lung cancer diagnoses during the pandemic period may mean that lung cancer patients who have not yet been diagnosed will apply to the hospital in the late period and there will be an increase at the rate of advanced lung cancer. It can be predicted that patients diagnosed with lung cancer and deaths from lung cancer may increase, especially in the period after the outbreak rate has decreased with longer-term studies.
The main limitations of this study are that the sample size is relatively small, the results of a single center are included, and the time interval of the study does not include the data for the next period due to the long-term decrease in the outbreak rate and the ongoing pandemic. This study to determine whether the COVID-19 pandemic causes a delay in the diagnosis of lung cancer and to compare the characteristics of the patients at the time of diagnosis will contribute to taking more precautions for the diagnosis of the disease at an early stage during epidemic periods and to prevent the increase in lung cancer-related mortality rates. In this period, when studies investigating the effects of the pandemic on the diagnosis of lung cancer are limited in the literature, this study will shed light on the 32-month period before and after the pandemic.
Conclusions
As a result of this study, the characteristics of the patients diagnosed with lung cancer in the 16-month period before and during the pandemic were found to be similar at the time of diagnosis. Although characteristics such as disease stage, presence of metastases, and health performance status were not affected in patients with lung cancer during the pandemic period, a decrease was observed in the number of patients diagnosed with lung cancer. This may be due to the total decrease in applications to healthcare institutions and limitations in accessing healthcare services due to reasons such as social isolation and fear of COVID-19 infection.
Although the study group was relatively small, this study showed the effects of the pandemic on lung cancer. By learning lessons from the COVID-19 pandemic, plans should be made in order not to delay the diagnosis and treatment of deadly diseases in possible future epidemics.
Author Contribution
Study design: Deniz Kızılırmak, Yavuz Havlucu, Pınar Çelik. Data collection: Deniz Kızılırmak, Zeynep Yılmaz. Literature research: Deniz Kızılırmak, Yavuz Havlucu. Drafting manuscript: Deniz Kızılırmak, Zeynep Yılmaz, Pınar Çelik. Critical review: Yavuz Havlucu, Pınar Çelik.
Data Availability
Not applicable.
Code Availability
Not applicable.
Declarations
Ethics Approval
Approval was obtained from Health Sciences Ethics Committee of Manisa Celal Bayar University Medicine Faculty (decision date: 01.12.2021, decision number: 20.478.486/1048).
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Footnotes
This article is part of the Topical Collection on Covid-19
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Deniz Kızılırmak, Email: dr_dkizilirmak@yahoo.com.
Zeynep Yılmaz, Email: zeynep96yilmaz@gmail.com.
Yavuz Havlucu, Email: dyhavlucu@yahoo.com.
Pınar Çelik, Email: pinarcelik@yahoo.com.
References
- 1.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistic 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–442. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Wahidi MM, Shojaee S, Lamb CR, et al. The use of bronchoscopy during the COVID-19 pandemic: CHEST/AABIP guideline and expert panel report. Chest. 2020;158(3):1268–1281. doi: 10.1016/j.chest.2020.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oken M, Creech R, Tormey D, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–655. doi: 10.1097/00000421-198212000-00014. [DOI] [PubMed] [Google Scholar]
- 4.Kim SS. The IASLC lung cancer staging project: background data and proposed criteria to distinguish separate primary lung cancers from metastatic foci in patients with two lung tumors in the the forthcoming eight edition of the TNM classification for lung cancer. J Thorac Oncol. 2016;11:651–65. doi: 10.1016/j.jtho.2016.01.025. [DOI] [PubMed] [Google Scholar]
- 5.London JW, Fazio-Eynullayeva E, Palchuk MB, et al. Effects of the COVID-19 pandemic on cancer-related patient encounters. JCO Clinical Cancer Inform. 2020;4:657–665. doi: 10.1200/CCI.20.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sha Z, Chang K, Mi J, et al. The impact of the COVID-19 pandemic on lung cancer patients. Ann Palliat Med. 2020;9(5):3373–3378. doi: 10.21037/apm-20-1662. [DOI] [PubMed] [Google Scholar]
- 7.Park JY, Lee YJ, Kim T, et al. Collateral effects of the coronavirus disease 2019 pandemic on lung cancer diagnosis in Korea. BMC Cancer 29. 2020;20(1):1040. doi: 10.1186/s12885-020-07544-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mitjà PS, Àvila M, García-Olivé I. Impact of the COVID-19 pandemic on lung cancer diagnosis and treatment. Med Clin 26. 2021;0025–7753(21):00432–2. doi: 10.1016/j.medcle.2021.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tran K, Cimon K, Severn M, et al. Aerosol-generating procedures and risk of transmission of acute respiratory infections: a systematic review. CADTH Technol Overv. 2013;3(1):e3101. [PMC free article] [PubMed] [Google Scholar]
- 10.Heidemann CS, Garbe J, Damm M, et al. German bronchoscopy unit readiness for the COVID-19 pandemic: a nationwide survey. ERJ Open Res. 2020;6(3):00396–2020. doi: 10.1183/23120541.00396-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ozturk A, Sener MU, Yılmaz A. Bronchoscopic procedures during COVID-19 pandemic: experiences in Turkey. J Surg Oncol. 2020;122(6):1020–1026. doi: 10.1002/jso.26164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lang M, Yeung T, Shepard JO, et al. Operational challenges of a low-dose CT lung cancer screening program during the COVID-19 pandemic. Chest. 2021;159(3):1288–1291. doi: 10.1016/j.chest.2020.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maringe C, Spicer J, Morris M, et al. The impact of the COVID-19 pandemic on cancer deaths due to delays in diagnosis in England, UK: a national, population-based, modelling study. Lancet Oncol. 2020;21(8):1023–1034. doi: 10.1016/S1470-2045(20)30388-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.
Not applicable.