Highlights
-
•
Simultaneous quantification of ten antimicrobials in human plasma by LC–MS/MS utilizing a SIL-IS for each analyte.
-
•
Therapeutic drug monitoring of antimicrobials in clinical laboratory.
-
•
Measurement of β-lactams by LC–MS/MS.
-
•
Extensive stability studies of β-lactams in plasma and whole blood at room temperature, refrigerated and frozen.
Keywords: Antimicrobials, LC–MS/MS, β-Lactams, Routine therapeutic drug monitoring
Abbreviations: CZO, cefazolin; CEP, cefepime; CTA, cefotaxime; CTZ, ceftazidime; CIP, ciprofloxacin; CV, coefficient of variation; ESI, electrospray ionization; FLU, flucloxacillin; HPLC, high performance liquid chromatography; ICU, intensive care unit; LIN, linezolid; LLOQ, lower limit of quantification; LC–MS/MS, liquid chromatography tandem mass spectrometry; MER, meropenem; MIC, minimum inhibitory concentration; MRM, multiple reaction monitoring; NOR, norfloxacin; PK, pharmacokinetic; PIP, piperacillin; QC, quality control; R, resistant organism; Rt, retention time; RT, room temperature; r2, coefficient of determination; S, susceptible, wild type organism; SIL-IS, stable isotope labelled internal standard; TAZ, tazobactam; TDM, therapeutic drug monitoring; ULOQ, upper limit of quantitation
Abstract
Background
Optimizing antimicrobial therapy to attain drug exposure that limits the emergence of resistance, effectively treats the infection, and reduces the risk of side effects is of a particular importance in critically ill patients, in whom normal functions are augmented or/and are infected with pathogens less sensitive to treatment. Achievement of these goals can be enhanced by therapeutic drug monitoring (TDM) for many antibiotics. A liquid chromatography tandem mass spectrometry (LC–MS/MS) method is presented here for simultaneous quantification of ten antimicrobials: cefazolin (CZO), cefepime (CEP), cefotaxime (CTA), ceftazidime (CTZ), ciprofloxacin (CIP), flucloxacillin (FLU), linezolid (LIN), meropenem (MER), piperacillin (PIP) and tazobactam (TAZ) in human plasma.
Methods
Plasma samples were precipitated with acetonitrile and injected into the LC–MS/MS. Chromatographic separation was on a Waters Acquity BEH C18 column. Compounds were eluted with water and acetonitrile containing 0.1 % formic acid, using a gradient (0.5–65 % B), in 3.8 min. The flow rate was 0.4 mL/min, and the run time was 5.8 min.
Results
The calibration curves were linear across the tested concentration ranges (0.5–250, CZO, CEP, CTA, CTZ and FLU; 0.2–100, MER and TAZ; 0.1–50, CIP and LIN and 1–500 mg/L, PIP). The intra and inter-day imprecision was < 11 %. Accuracy ranged from 95 to 114 %. CTZ and MER showed ionization suppression while CIP showed ionization enhancement, which was normalized with the use of the internal standard.
Conclusion
An LC–MS/MS method for simultaneous quantification of ten antimicrobials in human plasma was developed for routine TDM.
Introduction
Since the introduction of the first synthetic antibiotic, salvarsan, [1] in clinical use in 1910, and the subsequent discovery of penicillin in 1928, [2] antimicrobials have been used for the treatment of various infections, drastically improving patient survival. Their discovery has changed modern medicine, enabling the performance of many procedures, previously unthinkable, such as organ transplantation, cardiac surgery, as well as treatment of immunosuppressive cancer and auto-immune disorders. However, widespread overuse of antimicrobials, due to easy access and treatment effectiveness, has resulted in the emergence of multi-drug-resistant bacteria. The continuing rise of antimicrobial resistance in conjunction with the lack of new antimicrobials is an increasing and significant global concern [3].
Resistance to antibiotics can be acquired quickly since bacteria replicate rapidly. Long-term use of antibiotic at inadequate concentrations (sub-optimal exposure) is likely a contributing factor to resistance [4]. Another concern associated with inappropriate antimicrobial use, although perceived to be uncommon, is an increase in neurological toxicity [5] and seizures [6] associated with high β-lactam concentrations. Optimizing antibiotic dosing can minimize resistance emergence bacteria and reduce the side effects. This can be achieved by therapeutic drug monitoring (TDM), tailoring the drug dose to an individual. It is particularly important to individualize antimicrobial therapy in critically ill patients, in whom normal functions are augmented and standard treatment is less effective. These patients can be infected with pathogens that are less sensitive to treatment, resulting in poor clinical outcomes. Large variations in antimicrobial concentrations in plasma have been reported for patients in intensive care units (ICU) receiving guideline-directed, standard treatment. For example, sub-optimal exposure has been reported for flucloxacillin (FLU) [7], and increased neurological toxicity [5] and seizures [6] have been correlated with high meropenem (MER) and cefepime (CEP) concentrations, respectively, while both toxic and sub-therapeutic concentrations have been documented with linezolid (LIN) [8]. The literature indicates a relationship between serum concentrations of β-lactams and clinical outcomes in the critically ill [9], making TDM a potentially useful tool for dose optimization of this class of drugs.
Measuring drug concentration for various antimicrobials in clinical laboratories is generally performed by high performance liquid chromatography (HPLC), immunoassay or liquid chromatography tandem mass spectrometry (LC–MS/MS). HPLC instruments, although reliable, are relatively inefficient. The extraction procedure for HPLC analysis is generally lengthy, as the separation of the analytes is essential for quantitation, and the overall run time per sample is long, with results generally not available on the same day of sample collection. Immunoassays, on the other hand, have the advantage of a short turn-around time; however, these assays tend to lack specificity and commercial kits are not always available, particularly for newer classes of antibiotics. LC–MS/MS technology, which is becoming more readily available in clinical labs, can overcome these drawbacks with results being available much sooner than possible with HPLC. Simultaneous methods for measurement of many analytes, for example 10–15 antibiotics, allows for a broad range of compounds to be measured daily. This in turn reduces not only the turn-around time, but also pre-analytical and operator time, as well as the reagent and storage costs. Additionally, sicker patients tend to be prescribed more than one antibiotic at a time, but changes in their prescribed regimens are also frequent. It is, therefore, useful to have a multiplexing method that allows for the results to be available before the next dosing occasion. Given that quantification of antimicrobials, using either HPLC or LC–MS/MS technology, is generally limited to in-house developed methods, the selection of the analytes to be included in the method, generally, is tailored to the clinical need, the lab, or the research group. Consequently, the current published LC–MS/MS methods are quite diverse in the classes of antimicrobials chosen for measurement. Simultaneous quantification of five or more antimicrobials [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29] has been reported in the literature, with only few methods enabling quantitation of ten or more analytes [14], [16], [18], [19], [23], [25]. Among these, protein precipitation is the predominant sample extraction procedure with one method reporting the use of a solid phase extraction [15]. Analytical run time for the methods ranged from 4 [18] to 12 [13] minutes. Some of the current methods have narrow analytical ranges with either the lower limit of quantitation (LLOQ) being too high, above the minimum inhibitory concentration (MIC), or the upper limit of quantitation (ULOQ) being too low [16], [18], [19], thus requiring re-analysis of the samples after dilution, and thereby delaying the availability of results.
Among the current published methods that enable quantification of ≥ 10 antimicrobials, one used analyte analogue as the internal standard [13] rather than stable isotope labelled internal standard (SIL-IS), as recommended in LC–MS/MS [30] analysis, with the remaining using a SIL-IS for some but not all. Although most of the published methods state that the methods are fit for TDM purposes, none have reported on whether the methods have been implemented in the clinic for routine antimicrobial monitoring.
To date, no method has been published for measuring the set of antimicrobials presented here. The assay developed includes quantification of different classes of antibiotics (i.e., penicillins, carbapenems, cephalosporins, oxazolidinones and quinolones), commonly prescribed in ICUs. The aim of this work was to improve the current LC–MS/MS method used for routine monitoring of antimicrobials in our lab, particularly focusing on resolving the signal suppression (i.e., changes in signal intensity affected by the co-eluting interferences) and cross-signal contribution (i.e., interferences between the analyte and SIL-IS from the naturally occurring isotopes). The initial LC–MS/MS method included simultaneous quantitation of five antimicrobials using norfloxacin (NOR) as the internal standard. The LC–MS/MS method presented herein, simultaneously quantifies ten antimicrobials: cefazolin (CZO), CEP, cefotaxime (CTA), ceftazidime (CTZ), ciprofloxacin (CIP), FLU, LIN, MER, piperacillin (PIP) and tazobactam (TAZ) in human plasma and utilizes SIL-IS for each analyte.
Materials and methods
Chemicals and reagents
Cefazolin sodium (98 % purity), cefazolin 13C215N (CZO-IS; 98.6 % isotopic purity), cefepime dihydrochloride monohydrate (98 % purity), cefepime 2H3 sulfate (CEP-IS; 98.6 % isotopic purity), cefotaxime 2H3 (CTA-IS; 99.8 % isotopic purity), ceftazidime pentahydrate (97 % purity), ceftazidime 2H5 (CTZ-IS; 99.4 % isotopic purity), ciprofloxacin (98 % purity), ciprofloxacin 2H8 (CIP-IS; 98.3 % isotopic purity), flucloxacillin sodium (97 % purity), flucloxacillin 13C4 (FLU-IS; 99.5 % isotopic purity), linezolid (98 % purity), linezolid 2H3 (LIN-IS; 98.3 % isotopic purity), meropenem trihydrate (97 % purity), meropenem 2H6 (MER-IS; 99.6 % isotopic purity), piperacillin 2H5 (PIP-IS; 98.9 % isotopic purity), tazobactam sodium (96 % purity), tazobactam sodium 13C215N (TAZ-IS; 97.9 % isotopic purity) were purchased from Toronto Research Chemicals (PM Separations, Australia). Cefotaxime sodium (96.4 % purity), piperacillin sodium (96 % purity) and formic acid, LC–MS grade, were obtained from Sigma-Aldrich (St Louise, MO, USA). LC–MS grade acetonitrile, methanol and water were from Thermo Fisher Scientific (Scoresby, VIC, Australia). Water was further filtered using a Simplicity UV purification system from Millipore Australia (North Ryde, NSW, Australia). Disodium hydrogen phosphate was obtained from Chem-Supply (Gillman, SA, Australia). Expired, drug free, human plasma was obtained from blood bank (SydPath, St Vincent’s Hospital, Sydney).
UHPLC–MS/MS equipment and conditions
The LC–MS/MS system included a Shimadzu Ultra-Fast Liquid Chromatography system coupled to a Shimadzu-8050 triple quadrupole mass spectrometer (Shimadzu Oceania, Rydalmere, NSW, Australia). The LC system consisted of a solvent delivery system (Nexera X2 LC-30AD), an autosampler (Nexera X2 SIL-30AC) maintained at 8 °C, a vacuum degasser (DGU −20A5R), a column oven (Prominence CTO-20A) set to 40 °C and a system controller (CBM-20A). Compounds were chromatographically separated on a Waters Acquity BEH C18 (2.1 × 50 mm, 1.7 µm) column with gradient elution using water and acetonitrile, each containing 0.1 % formic acid, as mobile phases A and B, respectively. The initial gradient starting at 0.5 % B was held for 0.2 min and then linearly increased to 65 % B over 3.8 min. The gradient was ramped up to 100 % B where it was held for one minute before returned to initial conditions and equilibration for a further minute. The flow rate was 0.4 mL/min, and the total run time was 5.8 min.
A Shimadzu 8050 tandem mass spectrometer equipped with an electrospray ionization (ESI) source interface, and operated in positive ion mode, was used for multiple reaction monitoring (MRM) analysis. Two product ions were selected for each analyte and one for each of the SIL-IS. Optimization parameters are shown in Table 1 and the mass spectra data are illustrated in Fig. 1. Mass resolution for first and third quadrupoles were set to “unit” with a full- width -half-mass of 0.51–0.80 Daltons. Data acquisition and processing used Shimadzu LabSolution software version 5.96. Optimized ESI parameters were as follows: 1) nitrogen was used as the nebulizing gas, set to 2.8 L/min, and also used as the heating and drying gas at flow rates of 9 L/min, 2) capillary voltage was set to 4 kV, and 3) interface, heating block and de-solvation line temperatures were set to 250 °C, 400 °C and 150 °C, respectively.
Table 1.
Optimization parameters, MRM transitions and retention times of all analytes and their stable isotope internal standards.
| Analyte | Rt (min) | MRM (m/z) | CE (eV) | Dwell time (ms) | SIL-IS | Rt (min) | MRM (m/z) | CE (eV) | Dwell time (ms) |
|---|---|---|---|---|---|---|---|---|---|
| CEP | 1.71 | 241.0 → 227.0 125.9 |
11 25 |
25 20 |
CEP-IS | 1.70 | 242.4 → 227.0 | 11 | 5 |
| TAZ | 1.77 | 300.9 → 207.1 168.2 |
16 15 |
30 10 |
TAZ-IS | 1.77 | 304.0 → 168.0 | 14 | 15 |
| CTZ | 1.82 | 273.9 → 80.1 126.0 |
25 24 |
20 20 |
CTZ-IS | 1.81 | 276.5 → 85.1 | 22 | 5 |
| MER | 1.83 | 384.0 → 68.0 141.1 |
41 16 |
15 10 |
MER-IS | 1.82 | 390.0 → 147.1 | 19 | 5 |
| CIP | 2.04 | 331.9 → 288.1 245.0 |
19 26 |
30 20 |
CIP-IS | 2.03 | 340.1 → 296.1 | 20 | 20 |
| CTA | 2.11 | 456.1 → 396.2 125.0 |
12 30 |
15 5 |
CTA-IS | 2.12 | 461.0 → 401.2 | 12 | 10 |
| CZO | 2.25 | 454.9 → 323.0 155.9 |
13 18 |
15 5 |
CZO-IS | 2.25 | 460.1 → 326.1 | 11 | 5 |
| LIN | 2.50 | 338.0 → 235.2 195.0 |
20 22 |
15 5 |
LIN-IS | 2.50 | 341.1 → 297.3 | 20 | 5 |
| PIP | 2.87 | 518.1 → 143.0 160.0 |
8 8 |
10 5 |
PIP-IS | 2.87 | 523.2 → 148.1 | 24 | 5 |
| FLU | 3.52 | 454.0 → 160.0 295.0 |
17 16 |
15 5 |
FLU-IS | 3.52 | 460.0 → 160.0 | 17 | 10 |
Rt, retention time; MRM, multiple reaction monitoring; CE, collision energy; SIL-IS, stable isotope labelled internal standard; CEP, cefepime; TAZ, tazobactam; CTZ, ceftazidime; MER, meropenem; CIP, ciprofloxacin; CTA, cefotaxime; CZO, cefazolin; LIN, linezolid; PIP, piperacillin; FLU, flucloxacillin.
Fig. 1.
Mass spectrometry fragmentation pattern for cefazolin (CZO), cefepime (CEP), cefotaxime (CTA), ceftazidime (CTZ), ciprofloxacin (CIP), flucloxacillin (FLU), linezolid (LIN), meropenem (MER), piperacillin (PIP) and tazobactam (TAZ).
Preparation of calibrators and quality control (QC) samples
Two independently weighed out stock powders of each analyte at concentrations of 2 g/L (CEP, CTA, CTZ, CIP, LIN and TAZ), 4 g/L (MER, PIP) and 10 g/L (CZO, FLU) were used for preparation of the working calibrators and QC. A minimum of 10 mg of each was accurately weighed-out and dissolved in either methanol (CEP, CTA, CTZ, LIN and PIP), acidified methanol (0.1 M hydrochloric acid in methanol (0.05/99.95 v/v)) (CIP), water (CZO, MER, and TAZ) or buffer (0.1 M di-sodium hydrogen phosphate, pH = 7.4, (adjusted with concentrated ortho-phosphoric acid) containing 0.3 % sodium chloride) (FLU). Stock solutions were sonicated for 10 min, except TAZ (20 min), and stored in glass vials at −80 °C. Working calibrators at concentrations of 0.1, 0.4, 1.0, 10, 20 and 50 mg/L (CIP, LIN); 0.2, 0.8, 2.0, 20, 40 and 100 mg/L (MER, TAZ); 0.5, 2.0, 5.0, 50, 100 and 250 mg/L (CZO, CEP, CTA, CTZ and FLU) and 1.0, 4.0, 10, 100, 200 and 500 mg/L (PIP) were prepared in drug free plasma and stored as aliquots (50 µL) in microfuge plastic tubes at −80 °C. Working QC samples at concentrations of 0.5, 4.0 and 40 mg/L (CIP, LIN); 1.0, 8.0 and 80 mg/L (MER, TAZ); 2.5, 20 and 200 mg/L (CZO, CEP, CTA, CTZ and FLU) and 5.0, 40 and 400 mg/L (PIP) were also prepared in plasma and stored at −80 °C. The highest calibrator and QC were prepared first and used to prepare the subsequent calibrators/QC, by dilution, with drug-free plasma. To ensure the integrity of the plasma, the final volume of water and methanol content in the working calibrators/QC was kept to ≤ 20 %. Internal standard stock solutions were prepared at 1 mg/mL in either methanol (CTZ-IS, MER-IS, LIN-IS, PIP-IS and TAZ-IS) or water (CZO-IS, CEP-IS, CTA-IS and FLU-IS). Working internal standard (WIS) mixture at concentrations of 0.25 mg/L (CIP, LIN), 0.5 mg/L (TAZ), 1.0 mg/L (MER, FLU), 1.5 mg/L (CTZ, PIP) and 3.0 mg/L (CZO, CEP and CTA) was prepared in acetonitrile and stored in glass vials at −80 °C until analysis.
Sample extraction procedure
Plasma calibrators and QC (25 µL) were precipitated with 225 µL of the WIS solution mixture in acetonitrile. Tubes were vortex mixed (2 min), centrifuged (10 min, 14,000×g) and the supernatant was diluted with water (1 in 5), prior to 1 µL of the sample being injected into the LC–MS/MS. A double blank sample, containing neither the analyte nor the internal standard, and a zero calibrator (blank plasma with the internal standard) were prepared with each calibration curve.
Validation protocol
The method was developed and validated in accordance with the NATA [30] guidelines in terms of linearity, accuracy and precision, specificity and selectivity, carry-over and stability.
Linearity
Drug-free plasma was spiked with each drug at six concentrations to produce a calibration curve and assayed on four different days. Linear regression analysis, weighted 1/x2, using the peak area ratio (analyte/IS) versus the concentration was used to determine the analyte concentration. Acceptance criteria for each calibrator were set to ± 15 % from the weigh-in values, except the LLOQ for which ± 20 % was allowed. The correlation coefficient (r2) of the calibration curves was set to ≥ 0.990.
Accuracy and precision
QC at three concentrations for each analyte were analyzed on four different days in quadruplicate to determine the intra- and inter-day precision (defined by the coefficient of variation, CV %= 100*Standard Deviation/Mean) and accuracy (mean obtained concentration/weighted-in concentration*100). An acceptance criterion for each QC was set to ± 15 % of the weighed-in values. The limit of detection (LOD) was defined as the lowest concentration that has a peak height 3x the height of the blank sample.
Specificity and selectivity
Cross-signal contribution
Cross-signal contribution between the analytes and SIL-IS from naturally occurring isotopes and isotopically impure SIL-IS was assessed. Individual analytes, prepared in pure solution at the ULOQ, and the SIL-IS at concentrations of 1 mg/L, were individually injected into the LC–MS/MS, while response of all analytes were monitored. Any peak observed at the retention time (Rt) and MRM, except for the analyte being injected, was considered a cross-signal contribution. Acceptance criteria were set to the response being ≤ 20 % of the analyte LLOQ and ≤ 5 % of the SIL-IS response.
Suppression and enhancement of ionization
To evaluate the effect on ionization efficiency of the endogenous and/or exogenous compounds present in the matrix, calibrators were prepared identically in pure solution (water) and in plasma. Extracted samples were analyzed and their slopes compared. Slope ratios (plasma /water) between 0.85 and 1.15 indicate absence of suppression or enhancement of ionization by the matrix. Additionally, patient samples (n = 35) requested for routine antimicrobial monitoring were spiked with a pure solution mixture containing all the analytes. The concentrations of the solution used for spiking were 2 mg/L (CIP, LIN), 5 mg/L (MER, TAZ), 25 mg/L (CEP, CTA, CTZ, CZO, and FLU) and 50 mg/L (PIP). Each sample was analyzed in duplicate; neat and spiked. The recovered concentrations were calculated by subtracting the concentrations of the neat samples from the spiked. The acceptance criteria were set to 85–115 % recovery from the spiked concentration with 67 % of the samples falling within the predefined criteria.
Carryover
Carryover was assessed by injecting the highest calibrator followed by two double blank plasma extracts. Acceptance criteria of the carryover, according to the guidelines, states that the response in the blank sample should not exceed 20 % of the response of analyte LLOQ.
Stability of the stock solutions and samples
Stability of the analytes in plasma and whole blood was assessed at three concentrations in triplicate at room temperature (RT) and at 4 °C for up to 24 and 48 h, respectively. Short- and long-term stability in plasma was also tested at −20 and −80 °C for two weeks and 8 months, respectively. The freeze–thaw stability over three cycles was evaluated (−80 °C to RT). Stability of the extracted samples in the autosampler at 8 °C was assessed for up to 24 h. The stability of the stock solutions was assessed at −80 °C for up to 11 months for all analytes and up to 20 and 24 months for linezolid and ceftazidime, respectively. Stability of the analyte at the defined condition was accepted, if the mean concentration was ± 15 % from the freshly prepared samples at the same concentration.
Ethical considerations
Samples described in the study were collected for measurement of antimicrobial concentrations for clinical purposes and retained by an accredited clinical pathology service and the identities of the donors were not necessary for the research described herein. The use of these samples for research purposes is consistent with the Australian National Statement on Research Ethics (section 3.2.6, https://www.nhmrc.gov.au/about-us/publications/national-statement-ethical-conduct-human-research-2007-updated-2018#toc__725).
Results
Analytes were eluted at Rt ranging from 1.7 to 3.5 min under the chromatographic conditions defined above. Rt for the analytes and their corresponding SIL-IS were as follow: 1.71, 1.77, 1.82, 1.83, 2.04, 2.11, 2.25, 2.50, 2.87 and 3.52 for CEP, TAZ, CTZ, MER, CIP, CTA, CZO, LIN, PIP and FLU, respectively (Table 1). A representative chromatogram for a LLOQ sample is shown in Fig. 2.
Fig. 2.
Chromatograms for cefepime (CEP, 1), tazobactam (TAZ, 2), ceftazidime (CTZ, 3), meropenem (MER, 4), ciprofloxacin (CIP, 5), cefotaxime (CTA, 6), cefazolin (CZO, 7), linezolid (LIN, 8), piperacillin (PIP, 9) and flucloxacillin (FLU, 10) in human plasma at lower limit of quantitation.
Linearity
The assays were linear across the tested concentration ranges (0.5–250, CZO, CEP, CTA, CTZ and FLU; 0.2–100, MER and TAZ; 0.1–50, CIP and LIN and 1–500 mg/L, PIP). An example of a calibration curve for each analyte is shown in Supplementary Figure S1. Mean r2 of the calibration curves were 0.995 or greater. Summary of the slope, intercept and r2 for each calibration curve is presented in the in supplementary Table S1.
Accuracy and precision
Precision and accuracy data are listed in Table 2. The maximum intra- and inter-day imprecision was < 7 % for all analytes at all concentrations, except CIP, which was 11.4 % at the low QC. Accuracy within batch and between the batches ranged from 95 to 110 %, except the inter-batch accuracy of CTA and CIP at the low QC was 112 and 114 %, respectively. All accuracies were within the predefined acceptance criteria of ± 15 %. The LOD for CZO, CEP, CTA, CTZ, CIP, FLU, MER, LIZ, PIP and TAZ was 18, 35, 30, 104, 62, 8, 7, 18, 9 and 43 µg/L, respectively.
Table 2.
Intra and inter-day precision and accuracy data.
| Intra-batch precision and accuracy (n = 4) |
Inter-batch precision and accuracy (n = 16) |
|||||
|---|---|---|---|---|---|---|
| Analyte concentration (mg/L) | Mean concentration (mg/L) ± SD | CV (%) | Accuracy % |
Mean concentration (mg/L) ± SD | CV (%) | Accuracy % |
| Cefazolin (CZO) | ||||||
| 0.5 | 0.49 ± 0.009 | 1.8 | 99 | |||
| 3.0 | 3.01 ± 0.09 | 3.1 | 106 | 3.03 ± 0.10 | 3.6 | 106 |
| 20 | 21.7 ± 0.93 | 4.3 | 101 | 22.2 ± 1.3 | 6.0 | 103 |
| 200 | 203.6 ± 8.1 | 4.0 | 102 | 207.3 ± 12.5 | 6.1 | 104 |
| Cefepime (CEP) | ||||||
| 0.5 | 0.51 ± 0.004 | 0.8 | 101 | |||
| 2.5 | 2.59 ± 0.06 | 2.5 | 104 | 2.64 ± 0.12 | 4.8 | 106 |
| 20 | 20.3 ± 0.1 | 0.5 | 102 | 20.3 ± 0.6 | 2.7 | 102 |
| 200 | 200.2 ± 3.3 | 1.6 | 100 | 200.3 ± 6.7 | 3.3 | 100 |
| Cefotaxime (CTA) | ||||||
| 0.5 | 0.50 ± 0.005 | 1.0 | 100 | |||
| 2.5 | 2.76 ± 0.09 | 3.1 | 110 | 2.80 ± 0.11 | 3.9 | 112 |
| 20 | 21.4 ± 0.9 | 4.2 | 107 | 21.4 ± 0.6 | 2.8 | 107 |
| 200 | 206.9 ± 5.3 | 2.6 | 104 | 211.6 ± 9.7 | 4.6 | 106 |
| Ceftazidime (CTZ) | ||||||
| 0.5 | 0.51 ± 0.07 | 1.5 | 101 | |||
| 2.5 | 2.49 ± 0.04 | 1.7 | 99 | 2.62 ± 0.16 | 6.2 | 105 |
| 20 | 19.7 ± 0.9 | 4.5 | 99 | 19.7 ± 0.7 | 3.5 | 99 |
| 200 | 202.3 ± 13.0 | 6.4 | 101 | 203.1 ± 11.9 | 5.9 | 102 |
| Ciprofloxacin (CIP) | ||||||
| 0.1 | 0.10 ± 0.003 | 2.6 | 99 | |||
| 0.5 | 0.51 ± 0.05 | 10.6 | 102 | 0.57 ± 0.07 | 11.4 | 114 |
| 4 | 4.20 ± 0.20 | 4.8 | 105 | 4.15 ± 0.19 | 4.7 | 104 |
| 40 | 38.4 ± 2.2 | 5.7 | 96 | 39.6 ± 3.0 | 7.5 | 99 |
| Flucloxacillin (FLU) | ||||||
| 0.5 | 0.50 ± 0.005 | 1.0 | 100 | |||
| 2.5 | 2.66 ± 0.05 | 1.7 | 107 | 2.68 ± 0.09 | 3.4 | 107 |
| 20 | 20.3 ± 0.11 | 0.5 | 102 | 20.2 ± 0.36 | 1.8 | 101 |
| 200 | 203.6 ± 7.0 | 3.4 | 102 | 203.5 ± 7.7 | 3.8 | 102 |
| Linezolid (LIN) | ||||||
| 0.06 | 0.06 ± 0.01 | 0.8 | 101 | |||
| 0.5 | 0.52 ± 0.01 | 1.0 | 103 | 0.52 ± 0.01 | 2.7 | 104 |
| 4 | 3.86 ± 0.07 | 1.8 | 97 | 3.85 ± 0.12 | 3.3 | 96 |
| 40 | 38.4 ± 1.2 | 3.2 | 96 | 38.5 ± 1.3 | 3.4 | 96 |
| Meropenem (MER) | ||||||
| 0.2 | 0.20 ± 0.001 | 0.5 | 101 | |||
| 1 | 1.06 ± 0.03 | 2.5 | 106 | 1.08 ± 0.05 | 4.4 | 108 |
| 8 | 8.13 ± 0.36 | 4.4 | 102 | 8.11 ± 0.26 | 3.2 | 101 |
| 80 | 86.3 ± 3.7 | 4.3 | 108 | 83.8 ± 4.0 | 4.8 | 105 |
| Piperacillin (PIP) | ||||||
| 0.85 | 0.86 ± 0.005 | 0.6 | 101 | |||
| 5 | 4.89 ± 0.09 | 1.8 | 97.9 | 4.97 ± 0.12 | 2.5 | 99 |
| 40 | 38.2 ± 0.5 | 1.4 | 95.6 | 37.9 ± 0.7 | 1.9 | 95 |
| 400 | 402.4 ± 11.5 | 2.9 | 100.6 | 394 ± 16.2 | 4.1 | 99 |
| Tazobactam (TAZ) | ||||||
| 0.2 | 0.20 ± 0.001 | 0.5 | 100 | |||
| 1 | 1.09 ± 0.07 | 6.6 | 109 | 1.08 ± 0.07 | 6.6 | 108 |
| 8 | 8.19 ± 0.30 | 3.7 | 102 | 8.24 ± 0.36 | 4.4 | 103 |
| 80 | 78.5 ± 4.3 | 5.4 | 98 | 78.9 ± 3.2 | 4.1 | 99 |
CV, coefficient of variation; SD, standard deviation.
Specificity and selectivity
Cross-signal contribution
Cross-signal contribution from naturally occurring isotopes of the analytes at ULOQ for each analyte to the SIL-IS for the selected MRM transitions was < 5 % for all SIL-IS, except for CTA-IS and CZO-IS (5.4 and 8.6 %, respectively). The contribution effect at the second highest calibrator (concentration of 100 mg/L) was 2.3 and 4.5 % for CTA-IS and CZO-IS, respectively. No analyte cross-signal contribution was observed from the SIL-IS at concentrations of 1 mg/L.
Suppression and enhancement of ionization
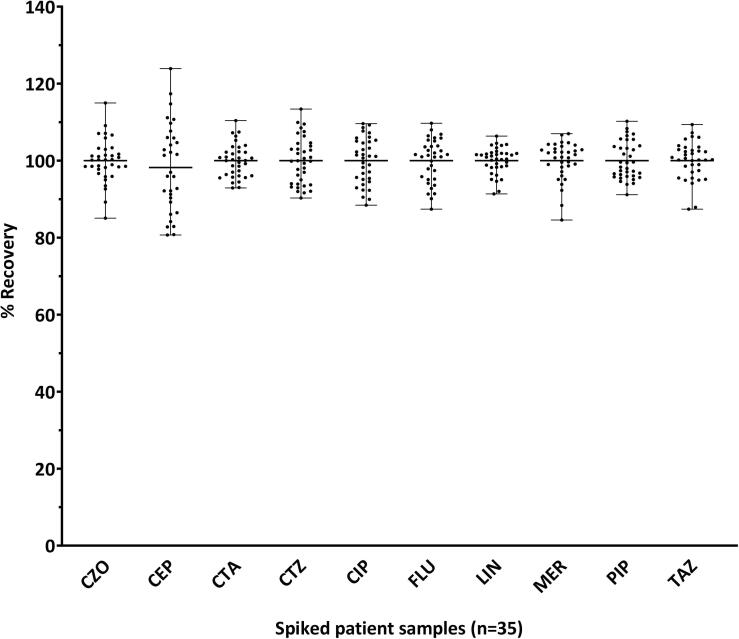
CTZ and MER showed ionization suppression while CIP showed ionization enhancement. Slope area ratios (plasma/water) were 0.21, 0.69 and 1.23 for CTZ, MER and CIP, respectively. The ionization effect was normalized with the use of the SIL-IS. Slope ratios (absolute and normalized for the IS) of the remaining analytes were in the range of 0.87–1.08. The recovery data for the spiked patient samples shown in Fig. 3 were within the predefined acceptance criteria (85–115 %) for all the analytes, except for CEP (6/35; recovery ranged from 81 to 124 %).
Fig. 3.
Recovery (%) of the spiked patient samples (n = 35). Samples from patients requested for antimicrobial monitoring were analyzed in duplicate: a) neat and b) spiked with a solution mixture containing all the analytes. The recovered concentrations were calculated by subtracting the concentration obtained for the neat sample from the spiked (b-a). The percent recovery (%) was calculated from recovered concentration divided by the concentration of the spiked solution mixture and multiplied by 100. The recovery (%) was withing 85–115 %, except for CEP (81–124 %). CZO, cefazolin; CEP, cefepime; CTA, cefotaxime; CTZ, ceftazidime; CIP, ciprofloxacin; FLU, flucloxacillin; LIN, linezolid; MER, meropenem; PIP, piperacillin; TAZ, tazobactam.
Carryover
The response observed in the first double blank plasma extract injected after the highest calibrator was ≤ 20 % of the area of the LLOQ sample for five out of ten antibiotics and ≤ 5 % for all the SIL-IS. CEP, CTZ, CTA, CIP and FLU showed carryover of 21, 48, 27, 220, and 25 % of the area of the LLOQ, respectively, corresponding to concentrations of 0.11, 0.24, 0.14, 0.22 and 0.13 mg/L, respectively. This was reduced to < 8 % in the second double blank injection for all analytes, except CTZ (16 %) and CIP (100 %).
Stability
Stability data for each antibiotic is summarized in Table 3 and supplementary Table S2. CEP, TAZ, MER and PIP were each more stable in whole blood than in plasma. CZO, CTA and LIN were stable in both matrices, whereas CIP and FLU were more stable in plasma. Long term storage in plasma at −80 °C was acceptable for all analytes for at least eight months. Extracted samples stored in the autosampler at 8 °C were stable for up to 24 h. The three freeze–thaw cycles did not affect the stability of the analytes. Individual stocks were stable for up to eleven months at −80 °C, except CTZ (3 months). LIN stock solution was stable for up 20 months.
Table 3.
Summary of the stability data for all analytes in plasma and whole blood matrices. The stability of the analyte is assumed for the length of time at the specified temperature.
| Plasma | Whole blood | Plasma | Whole blood | ||
|---|---|---|---|---|---|
| Cefazolin (CZO) | Flucloxacillin (FLU) | ||||
| 24h RT | 24h RT | 8h RT | 8h RT | ||
| 48h 4°C | 48h 4°C | 24h 4°C | 8h 4°C | ||
| 2W−20°C | 2W−20°C | ||||
| 8M−80°C | 8M−80°C | ||||
| Cefepime (CEP) | Linezolid (LIN) | ||||
| 5h RT | 24h RT | 24h RT | 24h RT | ||
| 8h 4°C | 48h 4°C | 48h 4°C | 48h 4°C | ||
| 1W−20°C | 2W−20°C | ||||
| 8M−80°C | 8M−80°C | ||||
| Cefotaxime (CTA) | Meropenem (MER) | ||||
| 8h RT | 24h RT | 8h RT | 8h RT | ||
| 48h 4°C | 48h 4°C | 24h 4°C | 48h 4°C | ||
| 2W−20°C | 1W−20°C | ||||
| 8M−80°C | 8M−80°C | ||||
| Ceftazidime # (CTZ) | Piperacillin (PIP) | ||||
| 8h 4°C | 5h RT | 24h RT | |||
| 24h 4°C | 48h 4°C | ||||
| 1W−20°C | |||||
| 8M−80°C | |||||
| Ciprofloxacin (CIP) | Tazobactam (TAZ) | ||||
| 24h RT | 24h RT | 8h RT | 24h RT | ||
| 48h 4°C | 24h 4°C | 48h 4°C | |||
| 1W−20°C | 1W−20°C | ||||
| 8M−80°C | 8M−80°C |
RT, room temperature; W, week; M, month; # Stability only evaluated at RT and 4 °C for up to 8 h in plasma.
Discussion
Method development: Singly-charged protonated ions were selected for all analytes, except for CEP and CTZ, including their SIL-IS, where doubly charged ions were used due to a higher response. The PIP peak intensity was the highest of all so, to prevent detector signal saturation, non-optimal collision energy was used and the response was reduced by 30 %.
The initial in-house LC–MS/MS method, developed and used for routine monitoring of antimicrobials, included simultaneous measurement of five analytes: CIP, MER, FLU, PIP and TAZ with NOR as the IS. When additional compounds were incorporated into the method and the analytical ranges for the current analytes were expanded, ionization suppression was observed for MER, CTZ and CEP, with the latter two also displaying a non-linear mode of regression. Meanwhile, FLU and PIP became non-linear after the ULOQ was increased from 100 to 250 mg/L and 300 to 500 mg/L, respectively, implying that NOR was not a suitable internal standard. Further, when SIL-IS were incorporated into the assay, the issue of cross-signal contribution between the analyte and the SIL-IS, arising from the presence of naturally occurring isotopes, needed to be addressed. Cross-signal contribution was observed for CTA-IS, CZO-IS and FLU-IS resulting in non-linear calibration curves. To mitigate the effect, the approach of utilizing a less abundant SIL-IS isotope with a mass that has the least contribution from the analyte isotopes was implemented [31]. For CTA-IS, CZO-IS and FLU-IS, the isotopes m/z 461, 460 and 460, respectively, were used instead of the most abundant isotopes (m/z = 459, 459 and 458, respectively). However, this only mitigated the effect for FLU, while CTA and CZO required a baseline separation, in addition to utilizing a less abundant SIL-IS isotope, to prevent the cross-signal contribution between the two. Changing the gradient from 5 to 95 % B in 3.5 min to 0.5–65 % B in 3.8 min resolved the issue. Despite the mitigating strategies undertaken to counteract the effect, cross-signal contribution for CTA and CZO to the SIL-IS remained greater (5.4 and 8.6 %, respectively) than the predefined criteria (≤5 % of the IS response). While this could have been further reduced by increasing the amount of the SIL-IS used in the extraction, at the expense of increasing the cost, calibration curves remained linear, implying that the amount of the SIL-IS used was sufficient to counteract the effect. This was further reflected by the accuracy and precision data obtained for the two analytes (Table 2). A chromatogram for FLU, CTA and CZO and their corresponding SIL-IS is presented in supplementary Figure S2, demonstrating the effect of the cross-signal contribution from the analyte isotopes to the SIL-IS.
Various mobile phases (0.1 % formic acid in water, ammonium formate and ammonium acetate buffers, pH 2.5–7) combined with the gradient (60–100 % B) were trialed for their suitability. Using ammonium formate buffer (25 mM, pH = 3) and acetonitrile as mobile phases A and B, respectively, allowed for quantitation of an additional compound, ceftriaxone. However, the overall response was greatly reduced for all compounds, while the LLOQ, for some, became too high. The gradient profile 0–90 and 0–100 % B resulted in sharper peaks with less tailing. However, compound resolution was poorer, while hydrophilic compounds had a lower column retention. With the aim of utilizing the simplest extraction protocol, protein precipitation was the method of choice, because of simplicity and speed. Also, antimicrobial concentrations are relatively high (mg/L), allowing for direct sample dilution prior to injection into the LC–MS/MS, without the need for sample concentration.
Given that carryover for CEP, CTZ, CTA, CIP and FLU was > 20 % of the LLOQ response, in attempt to mitigate the effect, various combinations of wash solutions and rinse protocols were investigated. Using a 70 % isopropanol:water mix containing 0.1 % formic acid to wash the needle (internally and externally), and the port and the pump, reduced the carryover to < 20 %, except for CIP (64 %). This, however, resulted in an additional 2.7 min per injection extending the total run time to almost 9 min. Alternatively, re-injection of low concentration samples that happen to be analyzed after a high concentration sample, may be performed, to account for potential carryover issues. The actual increases in the LLOQ concentrations for the analytes are marginal and unlikely to be clinically significant, except for CIP for which the carryover was equivalent to 0.22 mg/L, increasing the LLOQ to 0.3 mg/L. It is noteworthy the CIP ULOQ was excessively high (5 times higher than required) and given that CIP is measured as a peak rather than trough, the actual concentrations in patient samples are unlikely to exceed 10 mg/L, and, therefore, the percent of carryover will be significantly lower. The carryover for CIP was estimated to be approximately 0.4 % of the analyte concentration, resulting in a concentration increase equivalent to 0.02, 0.032, 0.04, 0.1 and 0.2 mg/L at 5, 8, 10, 25 and 50 mg/L CIP concentrations, respectively. To maintain the potential carryover to < 20 %, patient samples with CIP concentrations of ≤ 0.1 (LLOQ), ≤ 0.25 and ≤ 0.5 mg/L analyzed after samples containing CIP ≥ 5, ≥ 10 and ≥ 20 mg/L, respectively, would need to be reinjected.
Stability of β-lactam antibiotics has been documented in the literature [32]. Generally, they are unstable in plasma and whole blood matrices at RT; hence proper sample handling is required to ensure result credibility. The stability data outlined in Table 3 and supplementary Table S2 shows that PIP, CEP and, particularly, CTZ are highly unstable at RT in a plasma matrix. However, if stored as whole blood, whether refrigerated or not, the analytes appear to be more stable. While plasma matrix is used for analysis, samples are collected as whole blood after a venipuncture. Knowing the stability in this matrix simplifies the sample handling process if no urgent centrifugation of the blood is required. It also reduces the cost of sample transport from the collection centers and external laboratories if no dry ice is needed. Additionally, the available samples stored under certain conditions may be used later, if needed, without the need for recollection. Our results concurred with the data reported in the literature [17], [32], [33], but were also contrary [19] to some. Barco et al. [33] reported similar observations to ours that PIP and CTZ were unstable in plasma at RT beyond 4 h, with lower concentrations being more affected. However, another research group’s observations were contradictory [19]. The reported length of analyte stability when stored at RT and 4 °C were identical. CEP and CTZ were deemed stable at RT for at least 48 h while MER, PIP and TAZ for at least 24 h. The reported stability, though, agreed with our results for the refrigerated storage conditions for PIP, MER, TAZ, CTA, and CIP (RT and 4 °C), but not for CEP and CTZ. Similarly, our results agreed to those of Decosterd et al. [17] for MER, PIP, TAZ, FLX (not for RT at 24 h) and CEP in whole blood, but not for CEP and CTZ in plasma at RT. The inconsistency in the reported stability was more evident at RT conditions, possibly contributed by sample handling, the concentrations used for the assessment and the defined criteria. Some papers reported on using a single concentration point to test stability [34] and, with analytes being more unstable at lower concentrations, the reported data becomes inconclusive. CTZ stability in our study was only performed in plasma for up to eight hours, as the compound was not included in the solution mixture at the time the samples for stability analyses were prepared.
Notable signal suppression observed for MER and CTZ could be explained by the compounds co-eluting. Where gradient modification could have potentially enabled baseline separation of the two, at the expense of increased analysis time, the effect was normalized by using SIL-IS. In contrast, ionization enhancement observed for CIP is likely to be due to the analyte solubility in pure solution (water) rather than a true signal enhancement by the matrix, which was also normalized by using a SIL-IS.
The use of a SIL-IS for each analyte is a major strength of this new method compared to the other published methods [11], [20], [24] that reported simultaneous quantification of the same, or fewer, number of analytes. This is particularly important for hydrophilic compounds, which retain poorly on analytical columns eluting close to, or with, the solvent front and are more likely to be affected by the signal suppression caused by the coeluting analytes present in the matrix. In the paper published by Cazorla-Reyez et al. [14], 20 antimicrobials were quantified without utilizing an internal standard (neither an analogue nor a SIL-IS) with nearly 1/3 of the analytes eluting with, or near, the solvent front, which is likely to be the cause of the signal suppression observed for more than half of the analytes. Similarly, a recently published method by Barco et al., [33] used only a limited number of SIL-IS, even though nearly half of the analytes were not retained on the analytical column.
The method allows for a wide range of concentrations of various classes of antimicrobials to be quantified in <6 min eliminating the need for sample reanalysis after dilution. The assay’s high LOD can allow for the free-drug concentrations to be measured simultaneously. Therefore, a batch of 20 patient samples, including calibrators and controls, can be analyzed in approximately 3 h, ensuring result availability before the next dosing schedule, thus, impacting clinical decisions. Although, the method published by Colin et al. [16], had a total run time of 4 min enabling analysis of 12 compounds, the analytical range was very narrow, particularly for PIP, FLU, LIN and MER, potentially resulting in many samples needing reanalysis.
In terms of sample volume, only 25 µL was used in the extraction, making it attractive for PK analysis and pediatric populations, where a small sample size is desired. The other methods used, on average, 50–100 µL [17], [33] and even 1 mL [14].
Another advantage of this method, compared to the existing ones, is the use of plasma samples from patients treated with antimicrobials, for patient spike analysis. This is the first report in the literature for antimicrobial quantification. Generally, for method validation, 6–10 different plasma samples obtained from healthy volunteers are used to perform matrix effect and specificity and selectivity experiments. However, samples obtained from patients admitted to the ICU are unlikely to be similar in composition to the plasma of healthy volunteers. Using samples from this population group for validation purposes is more likely to identify potential interferences arising from drug coadministration.
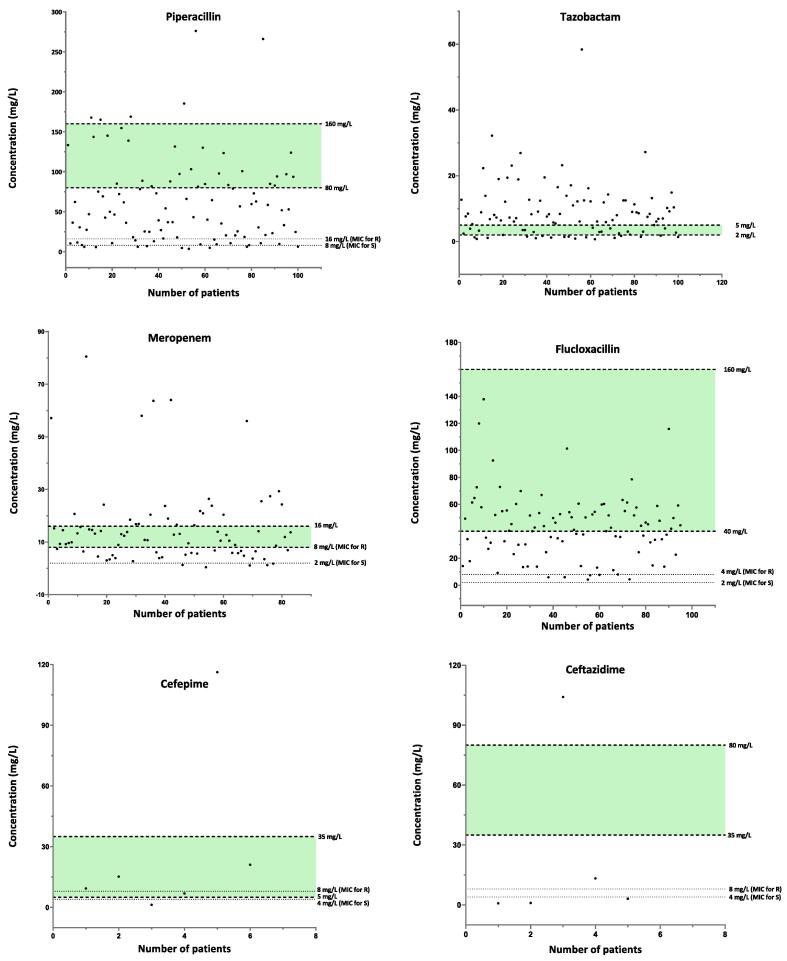
Method applicability: The developed method has been implemented in the clinical lab for daily antimicrobial monitoring. Over the three-month period, (August –October 2016), 412 patient episodes were requested for monitoring of at least one of the antimicrobials. PIP with TAZ were the most frequently requested (24 %), followed by FLU (23 %), MER (20 %), LIN (13 %), CIP (7 %), CZO (6 %), CTA (5 %), CEP and CTZ (1 %). Antimicrobial concentrations for each patient episode are depicted in Fig. 4. Based on the target ranges set for each antibiotic, a substantial percentage of patients did not achieve the desired targets. This, however, may be misleading as the MIC, the infection type and the pathogen were not correlated with the individual concentration, while samples may not have been collected at trough concentrations either. Also, the very low antibiotic concentrations observed in some cases may not be a true representation of a sub-optimal dosing, but rather confirmatory checks to validate antibiotic flushing after changing to a different drug class. The MIC in Fig. 4 were set based on EUCAST PK/PD clinical breakpoints [35], which are not species related. The MIC breakpoints for the susceptible (S) and resistant (R) organisms were 4 and 8 mg/L (CEP, CTZ); 1 and 2 mg/L (CZO, CTA); 0.25 and 0.5 mg/L (CIP); 2 and 4 (FLU); 2 and 8 mg/L (MER); 2 and 2 mg/L (LIN) and 8 and 16 mg/L (PIP), respectively. For the optimal bactericidal activity, an individual MIC should be determined; otherwise, clinical breakpoints defined by EUCAST for various pathogens may be used. Studies [36] have shown that maximal bacterial efficacy for β-lactams is achieved when 40–70 % of the time, within the dosing interval, the concentrations of the free fraction are 4–5 times above the MIC of the target pathogen. However, for clinically ill patients, the recommendation by the French Society of Pharmacology and Therapeutics and the French Society of Anesthesia and Intensive Care Medicine is plasma concentration of the free fraction of 4–8 times the MIC of the causative pathogen for 100 % of the dosing interval [37]. For antimicrobials exhibiting concentration dependent bactericidal effect, such as CIP, the target concentration is defined as a ratio of Cmax (maximum drug plasma concentration)/MIC > 10.
Fig. 4.
Antimicrobials concentrations of patients requested for therapeutic drug monitoring. MIC breakpoints for each agent were set based on the EUCAST PK/PD clinical breakpoints and are non-species related. The breakpoints for the susceptible (S) and resistant (R) organisms were 4 and 8 mg/L (CEP, CTZ); 1 and 2 mg/L (CZO, CTA); 0.25 and 0.5 mg/L (CIP); 2 and 4 (FLU); 2 and 8 mg/L (MER); 2 and 2 mg/L (LIN) and 8 and 16 mg/L (PIP), respectively. The target plasma concentration ranges set as 4 and 8 times the MIC breakpoint of the R organism were as per recommendation of the French Society of Pharmacology and Therapeutics (SFPT) and the French Society of Anesthesia and Intensive Care Medicine (SFAR) [37]. The targets of the total drug concentrations for CZO, CEP, CTA, CTZ, PIP and FLU, were based on the free drug fraction (%) and the MIC of the pathogen. For CZO, for example, the target free plasma concentration of 4 to 8 times the MIC of R (2 mg/L) is 8 and 16 mg/L, respectively. As the free fraction of CZO is approximately 20 % of the total dose, the target total plasma concentration is 40–80 mg/L. The estimated free fraction (%) for CZO, CEP, CTA, CTZ, PIP and FLU were approximately 15–20, 80, 60–80, 90, 80 and 5–10 %, respectively. For CEP, the target plasma concentration (5–35 mg/L) were taken from the SEPT and SFAR guidelines where calculations were based on the MIC of 1 mg/L (Enterobacteriaceae) and not 8 mg/L (P. aeruginosa), since this would have resulted in a concentration above the defined toxic threshold. For CTA and MER, the target ranges were also from the SEPT and SFAR guidelines and were based on the MIC of 4 mg/L for S. aureus and 2 mg/L for P. aeruginosa, respectively. For LIN and FLU, the targets were based on the 2–4 times the MIC [38]. For CIP, the target range was set as Cmax/MIC > 10. TAZ range was 2–5 mg/L at 500 mg given with PIP. MIC, minimum inhibitory concentration; EUCAST, European Committee on Antimicrobial Susceptibility Testing; PK/PD, pharmacokinetic/pharmacodynamic; Cmax, maximum concentration; CZO, cefazolin; CEP, cefepime; CTA, cefotaxime; CTZ, ceftazidime; CIP, ciprofloxacin; FLU, flucloxacillin; MER, meropenem; LIN, linezolid; PIP, piperacillin; TAZ, tazobactam; S, susceptible, wild-type organism; R, resistant organism.
Limitations and future work: Measuring the unbound fraction of highly protein-bound drugs, such as FLU, may be of great importance. Small changes in the plasma protein concentration, often observed in the severely ill, will lead to a significant increase or decrease of the unbound fraction. Similarly, measuring antimicrobial concentrations in other fluids and tissues, may better represent the drug concentration at the site of infection.
Conclusion
Overall simplicity of the method, in terms of extraction and a short run time, allowed for the method to be used daily for antimicrobial monitoring, providing results before the next dosing interval. Additional compounds could be incorporated into the method in the future, given the sample preparation and chromatography have already proved to be adequate for various classes of antimicrobials. Moreover, the wide assay analytical range allows for determination of free-drug concentrations, useful for some highly protein bound antimicrobials, and for pharmacokinetic peak levels to be measured simultaneously in the same method.
Funding
The authors received no financial support for the research, authorship and/or publication of this article.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
The provision of a Shimadzu 8050 LC–MS/MS by Shimadzu Australia to undertake this work is greatly appreciated. The work in the manuscript was presented at the International Association of Therapeutic Drug Monitoring and Clinical Toxicology (IATDMCT) 2018 Congress, September 16–19, Brisbane, Australia in a poster format.
Footnotes
Peer review under responsibility of “MSACL”.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jmsacl.2022.11.001.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Jones H.W. Report of a Series of Cases of Syphilis Treated by Ehrlich's Arsenobenzol at the Walter Reed General Hospital, District of Columbia. Boston Med. Surg. J. 1911;164:381–383. [Google Scholar]
- 2.Zaffiri L., Gardner J., Toledo-Pereyra L.H. History of Antibiotics. From Salvarsan to Cephalosporins. J. Invest. Surg. 2012;25:67–77. doi: 10.3109/08941939.2012.664099. [DOI] [PubMed] [Google Scholar]
- 3.WHO, Global antimicrobial resistance and use surveillance system (GLASS) report: 2021. https://apps.who.int/iris/handle/10665/341666, 2021 (Accessed February 18, 2022).
- 4.Sumi C.D., Heffernan A.J., Lipman J., Roberts J.A., Sime F.B. What Antibiotic Exposures Are Required to Suppress the Emergence of Resistance for Gram-Negative Bacteria? A Systematic Review. Clin. Pharmacokinet. 2019;58:1407–1443. doi: 10.1007/s40262-019-00791-z. [DOI] [PubMed] [Google Scholar]
- 5.Beumier M., Casu G.S., Hites M., Wolff F., Cotton F., Vincent J.L., Jacobs F., Taccone F.S. Elevated β-lactam concentrations associated with neurological deterioration in ICU septic patients. Minerva Anestesiol. 2015;81:497–506. [PubMed] [Google Scholar]
- 6.Smith N.L., Freebairn R.C., Park M.A., Wallis S.C., Roberts J.A., Lipman J. Therapeutic drug monitoring when using cefepime in continuous renal replacement therapy: seizures associated with cefepime. Crit. Care Resusc. 2012;14:312–315. [PubMed] [Google Scholar]
- 7.Abdul-Aziz M.H., McDonald C., McWhinney B., Ungerer J.P.J., Lipman J., Roberts J.A. Low Flucloxacillin Concentrations in a Patient With Central Nervous System Infection: The Need for Plasma and Cerebrospinal Fluid Drug Monitoring in the ICU. Ann. Pharmacother. 2014;48:1380–1384. doi: 10.1177/1060028014540610. [DOI] [PubMed] [Google Scholar]
- 8.Zoller M., Maier B., Hornuss C., Neugebauer C., Döbbeler G., Nagel D., Holdt L., Bruegel M., Weig T., Grabein B., Frey L., Teupser D., Vogeser M., Zander J. Variability of linezolid concentrations after standard dosing in critically ill patients: a prospective observational study. Crit. Care. 2014;18(4):R148. doi: 10.1186/cc13984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roberts J.A., Paul S.K., Akova M., Bassetti M., De Waele J.J., Dimopoulos G., Kaukonen K.M., Koulenti D., Martin C., Montravers P., Rello J., Rhodes A., Starr T., Wallis S.C., Lipman J. DALI: defining antibiotic levels in intensive care unit patients: are current β-lactam antibiotic doses sufficient for critically ill patients? Clin. Infect. Dis. 2014;58:1072–1083. doi: 10.1093/cid/ciu027. [DOI] [PubMed] [Google Scholar]
- 10.Ahsman M.J., Wildschut E.D., Tibboel D., Mathot R.A. Microanalysis of beta-lactam antibiotics and vancomycin in plasma for pharmacokinetic studies in neonates. Antimicrob. Agents Chemother. 2009;53:75–80. doi: 10.1128/AAC.00636-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abdulla A., Bahmany S., Wijma R.A., van der Nagel B.C.H., Koch B.C.P. Simultaneous determination of nine β-lactam antibiotics in human plasma by an ultrafast hydrophilic-interaction chromatography-tandem mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2017;1060:138–143. doi: 10.1016/j.jchromb.2017.06.014. [DOI] [PubMed] [Google Scholar]
- 12.Barco S., Bandettini R., Maffia A., Tripodi G., Castagnola E., Cangemi G. Quantification of piperacillin, tazobactam, meropenem, ceftazidime, and linezolid in human plasma by liquid chromatography/tandem mass spectrometry. J. Chemother. 2015;27:343–347. doi: 10.1179/1973947814Y.0000000209. [DOI] [PubMed] [Google Scholar]
- 13.Carlier M., Stove V., De Waele J.J., Verstraete A.G. Ultrafast quantification of β-lactam antibiotics in human plasma using UPLC-MS/MS. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2015;978–979:89–94. doi: 10.1016/j.jchromb.2014.11.034. [DOI] [PubMed] [Google Scholar]
- 14.Cazorla-Reyes R., Romero-González R., Frenich A.G., Rodríguez Maresca M.A., Martínez Vidal J.l. Simultaneous analysis of antibiotics in biological samples by ultra high performance liquid chromatography-tandem mass spectrometry. J. Pharm. Biomed. Anal. 2014;89:203–212. doi: 10.1016/j.jpba.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 15.Cohen-Wolkowiez M., White N.R., Bridges A., Benjamin D.K., Jr., Kashuba A.D. Development of a liquid chromatography-tandem mass spectrometry assay of six antimicrobials in plasma for pharmacokinetic studies in premature infants. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2011;879:3497–3506. doi: 10.1016/j.jchromb.2011.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Colin P., De Bock L., T'Jollyn H., Boussery K., Van Bocxlaer J. Development and validation of a fast and uniform approach to quantify β-lactam antibiotics in human plasma by solid phase extraction-liquid chromatography-electrospray-tandem mass spectrometry. Talanta. 2013;103:285–293. doi: 10.1016/j.talanta.2012.10.046. [DOI] [PubMed] [Google Scholar]
- 17.Decosterd L.A., Mercier T., Ternon B., Cruchon S., Guignard N., Lahrichi S., Pesse B., Rochat B., Burger R., Lamoth F., Pagani J.L., Eggimann P., Csajka C., Choong E., Buclin T., Widmer N., André P., Marchetti O. Validation and clinical application of a multiplex high performance liquid chromatography - tandem mass spectrometry assay for the monitoring of plasma concentrations of 12 antibiotics in patients with severe bacterial infections. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2020;1157 doi: 10.1016/j.jchromb.2020.122160. [DOI] [PubMed] [Google Scholar]
- 18.El-Najjar N., Hösl J., Holzmann T., Jantsch J., Gessner A. UPLC-MS/MS method for therapeutic drug monitoring of 10 antibiotics used in intensive care units. Drug Test. Anal. 2018;10:584–591. doi: 10.1002/dta.2253. [DOI] [PubMed] [Google Scholar]
- 19.Lefeuvre S., Bois-Maublanc J., Hocqueloux L., Bret L., Francia T., Eleout-Da Violante C., Billaud E.M., Barbier F., Got L. A simple ultra-high-performance liquid chromatography-high resolution mass spectrometry assay for the simultaneous quantification of 15 antibiotics in plasma. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2017;1065–1066:50–58. doi: 10.1016/j.jchromb.2017.09.014. [DOI] [PubMed] [Google Scholar]
- 20.Neugebauer S., Wichmann C., Bremer-Streck S., Hagel S., Kiehntopf M. Simultaneous Quantification of Nine Antimicrobials by LC-MS/MS for Therapeutic Drug Monitoring in Critically Ill Patients. Ther. Drug Monit. 2019;41:29–37. doi: 10.1097/FTD.0000000000000570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ohmori T., Suzuki A., Niwa T., Ushikoshi H., Shirai K., Yoshida S., Ogura S., Itoh Y. Simultaneous determination of eight β-lactam antibiotics in human serum by liquid chromatography-tandem mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2011;879:1038–1042. doi: 10.1016/j.jchromb.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 22.Paal M., Zoller M., Schuster C., Vogeser M., Schütze G. Simultaneous quantification of cefepime, meropenem, ciprofloxacin, moxifloxacin, linezolid and piperacillin in human serum using an isotope-dilution HPLC-MS/MS method. J. Pharm. Biomed. Anal. 2018;152:102–110. doi: 10.1016/j.jpba.2018.01.031. [DOI] [PubMed] [Google Scholar]
- 23.Rigo-Bonnin R., Ribera A., Arbiol-Roca A., Cobo-Sacristán S., Padullés A., Murillo Ò., Shaw E., Granada R., Pérez-Fernández X.L., Tubau F., Alía P. Development and validation of a measurement procedure based on ultra-high performance liquid chromatography-tandem mass spectrometry for simultaneous measurement of β-lactam antibiotic concentration in human plasma. Clin. Chim. Acta; Int. J. Clin. Chem. 2017;468:215–224. doi: 10.1016/j.cca.2017.03.009. [DOI] [PubMed] [Google Scholar]
- 24.Sime F.B., Roberts M.S., Roberts J.A., Robertson T.A. Simultaneous determination of seven β-lactam antibiotics in human plasma for therapeutic drug monitoring and pharmacokinetic studies. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2014;960:134–144. doi: 10.1016/j.jchromb.2014.04.029. [DOI] [PubMed] [Google Scholar]
- 25.H. Woksepp, L. Karlsson, A. Ärlemalm, A. Hällgren, T. Schön, B. Carlsson, Simultaneous Measurement of 11 Antibiotics for use in the Intensive Care Unit by Ultra Performance Liquid Chromatography-Tandem Mass Spectrometry, Ther. Drug Monit., doi: 10.1097/ftd.0000000000000911(2021). [DOI] [PubMed]
- 26.Wolff F., Deprez G., Seyler L., Taccone F., Hites M., Gulbis B., Vincent J.L., Jacobs F., Cotton F. Rapid quantification of six β-lactams to optimize dosage regimens in severely septic patients. Talanta. 2013;103:153–160. doi: 10.1016/j.talanta.2012.10.024. [DOI] [PubMed] [Google Scholar]
- 27.Zander J., Maier B., Suhr A., Zoller M., Frey L., Teupser D., Vogeser M. Quantification of piperacillin, tazobactam, cefepime, meropenem, ciprofloxacin and linezolid in serum using an isotope dilution UHPLC-MS/MS method with semi-automated sample preparation. Clin. Chem. Lab. Med. 2015;53:781–791. doi: 10.1515/cclm-2014-0746. [DOI] [PubMed] [Google Scholar]
- 28.Rehm S., Rentsch K.M. A 2D HPLC-MS/MS method for several antibiotics in blood plasma, plasma water, and diverse tissue samples. Anal. Bioanal. Chem. 2020;412:715–725. doi: 10.1007/s00216-019-02285-0. [DOI] [PubMed] [Google Scholar]
- 29.Bellouard R., Deslandes G., Morival C., Li J., Boutoille D., Jolliet P., Dailly É., Grégoire M. Simultaneous determination of eight β-lactam antibiotics in human plasma and cerebrospinal fluid by liquid chromatography coupled to tandem mass spectrometry. J. Pharm. Biomed. Anal. 2020;178 doi: 10.1016/j.jpba.2019.112904. [DOI] [PubMed] [Google Scholar]
- 30.NATA, Natinal Association of Testing Authorities Australia General Accreditation Guidance —Validation and verification of quantitative and qualitative test methods. 2018. https://nata.com.au/files/2021/05/Validation-and-Verification-of-Quantitative-and-Qualitative-Test-Methods.
- 31.Radovanovic M., Jones G., Day R.O., Galettis P., Norris R.L.G. Mitigating analyte to stable isotope labelled internal standard cross-signal contribution in quantitative liquid chromatography-tandem mass spectrometry. J. Mass Spectrometry Adv. Clin. Lab. 2022;24:57–64. doi: 10.1016/j.jmsacl.2022.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mortensen J.S., Jensen B.P., Zhang M., Doogue M. Preanalytical Stability of Piperacillin, Tazobactam, Meropenem, and Ceftazidime in Plasma and Whole Blood Using Liquid Chromatography-Tandem Mass Spectrometry. Ther. Drug Monit. 2019;41:538–543. doi: 10.1097/FTD.0000000000000650. [DOI] [PubMed] [Google Scholar]
- 33.Barco S., Mesini A., Barbagallo L., Maffia A., Tripodi G., Pea F., Saffioti C., Castagnola E., Cangemi G. A liquid chromatography-tandem mass spectrometry platform for the routine therapeutic drug monitoring of 14 antibiotics: Application to critically ill pediatric patients. J. Pharm. Biomed. Anal. 2020;186 doi: 10.1016/j.jpba.2020.113273. [DOI] [PubMed] [Google Scholar]
- 34.Rehm S., Rentsch K.M. LC-MS/MS method for nine different antibiotics. Clin. Chim. Acta. 2020;511:360–367. doi: 10.1016/j.cca.2020.11.001. [DOI] [PubMed] [Google Scholar]
- 35.The European Committee on Antimicrobial Susceptibility Testing - EUCAST 2022. Breakpoint tables for interpretation of MICs and zone diameters https://www.eucast.org/clinical_breakpoints/, 2022 (Accessed February 18, 2022).
- 36.Mouton J.W., den Hollander J.G. Killing of Pseudomonas aeruginosa during continuous and intermittent infusion of ceftazidime in an in vitro pharmacokinetic model. Antimicrob. Agents Chemother. 1994;38:931–936. doi: 10.1128/aac.38.5.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guilhaumou R., Benaboud S., Bennis Y., Dahyot-Fizelier C., Dailly E., Gandia P., Goutelle S., Lefeuvre S., Mongardon N., Roger C., Scala-Bertola J., Lemaitre F., Garnier M. Optimization of the treatment with beta-lactam antibiotics in critically ill patients—guidelines from the French Society of Pharmacology and Therapeutics (Société Française de Pharmacologie et Thérapeutique—SFPT) and the French Society of Anaesthesia and Intensive Care Medicine (Société Française d’Anesthésie et Réanimation—SFAR) Crit. Care. 2019;23(1) doi: 10.1186/s13054-019-2378-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pea F., Viale P., Cojutti P., Del Pin B., Zamparini E., Furlanut M. Therapeutic drug monitoring may improve safety outcomes of long-term treatment with linezolid in adult patients. J. Antimicrob. Chemother. 2012;67:2034–2042. doi: 10.1093/jac/dks153. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.