Abstract
Inflammation has been linked to clinical cognitive impairment, including Alzheimer’s disease. Less is known, however, about the relationship between inflammation and normal, age-associated cognitive decline. An understanding of the determinants of all types of cognitive decline is important for improving quality of life in an aging world. This study investigated whether biomarkers of inflammation were associated with cognitive function and decline in older Taiwanese adults.
Data were from the Taiwan Longitudinal Study of Aging and the Social Environment and Biomarkers of Aging Study. Inflammation was measured in 2000 and 2006 as C-reactive protein, interleukin-6, soluble e-selectin, soluble intercellular adhesion molecule-1, and white blood cell count. Cognition was assessed via ten cognitive and memory tasks, measured in 2006, 2007, and 2011. Growth curve models were used to examine the relationship between inflammation and cognitive score over this time period.
Higher levels of inflammation were associated with lower baseline cognitive scores, but not associated with longitudinal change in cognitive score. This study did not support a causal link between inflammation and cognitive decline among this older cohort. The observed cross-sectional relationship could reflect a causal relationship that arises earlier in life, or confounding; additional research across the life course is warranted.
1. Background
Several population-based studies have identified an association between biomarkers of systemic inflammation and incident dementia in older adults (Engelhart et al., 2004; Jenny et al., 2012; Schmidt et al., 2002), but less is known about the relationship between inflammation and age-related cognitive decline that falls short of diagnosed impairment. Some researchers argue that dementia represents a pathological course distinct from the cognitive change seen in “normal” aging (Deary et al., 2009). Others emphasize that the slow progression of dementias like Alzheimer’s disease make it extremely difficult to distinguish normal cognitive changes from the early stages of disease (Fjell, McEvoy, Holland, Dale, & Walhovd, 2014). Experts disagree about how exactly to make this distinction (Ownby, 2010), and the utility of mild cognitive impairment as an intermediate stage between health and dementia (Petersen, 2011) suggest a continuum of cognitive decline. If dementia represents one end of this continuum, then perhaps inflammation is associated with cognitive change across the entire spectrum.
Even in the absence of Alzheimer’s Disease or another dementia diagnosis, age-related cognitive decline has a negative impact on well-being (Andrews, Das, Cherbuin, Anstey, & Easteal, 2016; Wilson et al., 2013), limiting older adults’ health literacy (Kobayashi, Wardle, Wolf, & von Wagner, 2016), impairing their ability to perform everyday tasks such as paying bills and following medication instructions (Tucker-Drob, 2011), and leading to poor decision making (Boyle et al., 2012). Further, a large fraction of the variation in later life cognitive decline is not explained by pathological indices of Alzheimer’s Disease and other dementias (Boyle et al., 2013). With the aging of the global population, age-related cognitive decline will become a bigger—and more expensive—problem in coming decades (Hurd, Martorell, Delavande, Mullen, & Langa, 2013; Prince et al., 2013). Thus, an understanding of the determinants of cognitive decline is important for improving the quality of life for the growing number of older adults. Is systemic inflammation one such determinant of cognitive decline at older ages?
The limited evidence on the inflammation-cognitive decline link has been mixed. Cross-sectional studies of non-demented populations identified associations between worse cognitive function and higher levels of two biomarkers of inflammation, interleukin-6 levels and C-reactive protein, among older adults in the Netherlands (Schram et al., 2007) and among white and black elderly Americans (Yaffe et al., 2003). Other studies, however, failed to find this relationship among German older adults (measured as interleukin-6; Baune et al., 2008), older American women (C-reactive protein; Weuve, Ridker, Cook, Buring, & Grodstein, 2006) and high-functioning elderly Americans (both C-reactive protein and interleukin-6; Alley, Crimmins, Karlamangla, Hu, & Seeman, 2008).
Results from longitudinal studies of non-demented populations are similarly mixed. Baseline C-reactive protein predicted worse cognitive function after 12 years among older Finnish women (Komulainen et al., 2007) and cognitive decline among white and black elderly Americans after two years (Yaffe et al., 2003). Interleukin-6 predicted cognitive decline among Dutch older adults over five years (Schram et al., 2007), high-functioning elderly Americans over seven years (Weaver et al., 2002), white and black elderly Americans over two years (Yaffe et al., 2003), and older African-Caribbean adults living in London over three years (Jordanova, Stewart, Davies, Sherwood, & Prince, 2007). However, two other studies of older adults in Amsterdam (Dik et al., 2005) and in the Netherlands (Teunissen et al., 2003) found that neither biomarker predicted cognitive decline over three and six years, respectively.
In this study, I examined the relationship between inflammation and cognitive ability among non-impaired older Taiwanese adults. This study of inflammation and cognitive function among older Taiwanese adults contributes to the literature in two main ways: the richness of the data and the population under study. Most previous studies are limited by data: typically one or two biomarkers of inflammation were measured once at baseline, with one later measure of cognition as the outcome. This study included five biomarkers that capture different facets of the inflammatory response, perhaps providing a more comprehensive picture of the inflammation-cognition link. Biomarkers were measured twice over six years, providing a less noisy measure of chronic inflammation, and cognition was measured three times over five years, allowing the examination of changes in cognitive ability over this time period. Finally, this is the first study of which I am aware to study inflammation and subclinical cognitive decline in an East Asian population.
2. Methods
2.1. Data
Data are from the 2006, 2007, and 2011 waves of the Taiwan Longitudinal Study of Aging (TLSA) and the 2000 and 2006 waves of the Social Environment and Biomarkers of Aging Study (SEBAS). TLSA is a nationally representative longitudinal sociodemographic survey of Taiwanese adults aged 50 and above (including the institutionalized population). TLSA began in 1989; follow-up waves are ongoing. SEBAS collected health information on a random subsample of TLSA participants via a detailed health survey, functional tests, biomarker collection, and a medical examination. The 2000 SEBAS wave sampled participants who were at least 53 years old in the 1999 wave of TLSA. The 2006 wave of SEBAS included survivors of the 2000 SEBAS wave, as well as a refresher sample of younger participants drawn from the 2003 TLSA wave. Information on demographic characteristics and cognitive function was collected in TLSA; inflammation biomarkers were measured for the SEBAS subsample.
The analytic sample was restricted to respondents with inflammation measures in both 2000 and 2006 and at least one complete cognitive assessment in 2006, 2007, or 2011. Of the 639 participants who completed the SEBAS examination in both 2000 and 2006, 596 respondents have inflammation measures in both 2000 and 2006, no missing covariates, and at least one cognitive assessment. About three quarters of the analytic sample (76%) complete cognitive assessments in all three years, while 20% complete two assessments, and 4% complete only one assessment. See Supplementary Materials (S1–S2) for more details on the construction of the analytic sample.
2.2. Measures
2.2.1. Inflammation
Blood samples were taken from SEBAS participants during hospital-based examinations in 2000 and 2006. Within hours of collection, samples were collected by Union Clinical Laboratories, which performed the laboratory analysis. Five biomarkers of inflammation were assayed from serum: C-reactive protein, interleukin-6, soluble e-selectin, soluble ICAM-1, and white blood cell count. C-reactive protein is an acute-phase protein: it increases quickly and substantially as part of the inflammatory response to injury and illness (Gabay & Kushner, 1999). It is considered a marker of systemic inflammation (Ridker, Hennekens, Buring, & Rifai, 2000). Interleukin-6 is a proinflammatory cytokine, or signaling molecule, that is largely responsible for increases in C-reactive protein and other acute-phase proteins (Gabay & Kushner, 1999). White blood cells proliferate during inflammation, leading to an increase in the white blood cell count. Soluble e-selectin and soluble ICAM-1 facilitate the adhesion of white blood cells to the endothelial cells that line the blood vessels, directing the immune response to the appropriate part of the body (Albelda, Smith, & Ward, 1994).
C-reactive protein was measured on the 2000 and 2006 samples using immunoturbidimetry; the 2000 levels were determined via frozen samples that were analyzed in 2009. Interleukin-6 was assayed with enzyme linked immunosorbent assays (ELISA), with 2000 levels measured from frozen samples in 2007. Soluble e-selectin and soluble ICAM-1 were measured via ELISA, with 2000 levels measured from frozen samples in 2009. White blood cell count was measured in 2000 and 2006 using the direct current method.
Each inflammatory biomarker was log transformed to better approximate a normal distribution (except soluble ICAM-1, which was square root transformed), then standardized based on values in 2000; thus, each measure in 2000 had a mean of zero and a standard deviation of one. The five standardized markers were averaged into a single index (itself standardized based on the 2000 values) to capture overall inflammatory activity. (Biomarker indexes are calculated in various ways in the literature (Seplaki, Goldman, Glei, & Weinstein, 2005). Results using alternative index definitions are shown in Supplementary Materials (S4). Results using each biomarker in separate models available upon request.)
The primary model specification included the average of the inflammation index over 2000 and 2006. This average was meant to capture chronic inflammation better than either year would separately, and reduced noise in the measure. A secondary specification added the difference in the inflammation index between 2000 and 2006 to assess whether recent change in inflammation was associated with cognitive score. Inflammation variables were interacted with age in order to determine whether inflammation was associated with the rate of cognitive change.
2.2.2. Cognition
Cognition was assessed in TLSA with ten cognitive and memory tasks—listed in Table 1—derived from the Short Portable Mental Status Questionnaire, the Rey Auditory Verbal Learning Test, and a modified Digits Backward test; for details, see Chang et al. (2012) and Glei et al. (2005). Following Herzog and Wallace (1997), I summed the ten tasks to create an overall cognition score (range 0–24). If a respondent did not answer a particular task, it was coded as incorrect (zero). Summary statistics of the cognition score are shown in Table 2.
Table 1:
Tasks included in the cognitive assessment
| Item | Max score | Source |
|---|---|---|
|
| ||
| Tell me your address | 1 | SPMSQ |
| What is today's date? (Year, month and day) | 3 | SPMSQ |
| What day of the week is it? | 1 | SPMSQ |
| How old are you this year? | 1 | SPMSQ |
| What is your mother's maiden name? | 1 | SPMSQ |
| Who is the current president? | 1 | SPMSQ |
| Who was the president before him? | 1 | SPMSQ |
| Serial 3s subtraction task (4 times, starting at 20) | 4 | SPMSQ |
| 10-item recall task (dog, cloth, watermelon, etc.) | 10 | RAVL |
| 5 numbers repeated in reverse order task | 1 | WAIS |
|
| ||
| Total | 24 | |
Note: SPMSQ = Short Portable Mental Status Questionnaire. RAVL = Rey Auditory Verbal Learning Test. WAIS = Wechsler Adult Intelligence Scale
Table 2:
Characteristics of study population for all respondents and separately by sex
| All respondents | Female respondents | Male respondents | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean or % | SD | Med. | N | Mean or % | SD | Med. | N | Mean or % | SD | Med. | N | |
|
|
||||||||||||
| Demographic characteristics | ||||||||||||
| Female | 43.1% | -- | -- | 596 | 100% | -- | -- | 257 | 0% | -- | -- | 339 |
| Age, 2000 | 66.2 | 7.6 | 65.0 | 596 | 65.9 | 7.7 | 65.0 | 257 | 66.4 | 7.6 | 66.0 | 339 |
| Age, 2006 | 72.3 | 7.6 | 72.0 | 596 | 72.0 | 7.7 | 71.0 | 257 | 72.5 | 7.6 | 72.0 | 339 |
| Age, 2007 | 72.7 | 7.6 | 72.0 | 577 | 72.5 | 7.7 | 71.0 | 247 | 72.9 | 7.6 | 72.0 | 330 |
| Age, 2011 | 75.9 | 7.3 | 75.0 | 500 | 75.8 | 7.5 | 75.0 | 223 | 76.1 | 7.3 | 75.0 | 277 |
| Education, years | 5.7 | 4.7 | 6.0 | 596 | 3.7 | 4.2 | 3.0 | 257 | 7.1 | 4.5 | 6.0 | 339 |
| Current smoker, 2006 | 18.8% | -- | -- | 596 | 3.1% | -- | -- | 257 | 30.7% | -- | -- | 339 |
| Body mass index, 2006 | 24.6 | 3.6 | 24.4 | 596 | 25.2 | 3.8 | 25.0 | 257 | 24.2 | 3.3 | 24.1 | 339 |
| Body mass index >30, 2006 | 6.4% | -- | -- | 596 | 10.1% | -- | -- | 257 | 3.5% | -- | -- | 339 |
| Health assessments (range: 1–5 = poor to excellent) | ||||||||||||
| Interviewer-rated health, 2006 | 3.7 | 1.0 | 4.0 | 596 | 3.5 | 1.0 | 4.0 | 257 | 3.8 | 0.9 | 4.0 | 339 |
| Self-rated health, 2006 | 3.0 | 1.0 | 3.0 | 589 | 2.9 | 1.0 | 3.0 | 254 | 3.1 | 1.0 | 3.0 | 335 |
| Cognitive function score (range: 0–24) | ||||||||||||
| 2006 | 15.8 | 3.7 | 17.0 | 588 | 15.1 | 4.4 | 16.0 | 254 | 16.4 | 3.0 | 17.0 | 334 |
| 2007 | 15.5 | 3.9 | 16.0 | 570 | 14.6 | 4.5 | 16.0 | 245 | 16.3 | 3.1 | 17.0 | 325 |
| 2011 | 14.6 | 4.3 | 16.0 | 462 | 13.7 | 4.8 | 15.0 | 205 | 15.3 | 3.8 | 16.0 | 257 |
| Inflammation, 2000 | ||||||||||||
| C-reactive protein (mg/L) | 2.7 | 6.4 | 0.8 | 596 | 2.6 | 4.1 | 1.0 | 257 | 2.8 | 7.7 | 0.7 | 339 |
| Interleukin-6 (pg/L) | 3.1 | 4.3 | 2.1 | 596 | 3.2 | 5.4 | 2.2 | 257 | 3.0 | 3.3 | 2.0 | 339 |
| Soluble e-selectin (ng/mL) | 46.7 | 23.9 | 40.7 | 596 | 47.5 | 25.4 | 41.5 | 257 | 46.1 | 22.7 | 40.1 | 339 |
| Soluble intercellular adhesion molecule-1 (ng/mL) | 246.9 | 97.2 | 234.5 | 596 | 251.8 | 101.4 | 241.2 | 257 | 243.2 | 93.9 | 229.2 | 339 |
| White blood cell count (x 103/|iL) | 6.0 | 1.5 | 5.8 | 596 | 6.0 | 1.5 | 5.7 | 257 | 6.0 | 1.4 | 5.8 | 339 |
| Inflammation, 2006 | ||||||||||||
| C-reactive protein (mg/L) | 2.9 | 7.0 | 1.1 | 596 | 2.2 | 3.8 | 1.2 | 257 | 3.5 | 8.6 | 1.1 | 339 |
| Interleukin-6 (pg/L) | 4.3 | 8.6 | 2.7 | 596 | 3.7 | 4.1 | 2.7 | 257 | 4.7 | 10.8 | 2.8 | 339 |
| Soluble e-selectin (ng/mL) | 42.4 | 28.9 | 35.2 | 596 | 40.2 | 29.8 | 33.3 | 257 | 44.0 | 28.1 | 36.9 | 339 |
| Soluble intercellular adhesion molecule-1 (ng/mL) | 282.0 | 101.3 | 268.3 | 596 | 281.2 | 94.6 | 269.9 | 257 | 282.5 | 106.2 | 266.2 | 339 |
| White blood cell count (x 103/|iL) | 6.1 | 1.8 | 5.9 | 596 | 5.9 | 1.7 | 5.6 | 257 | 6.2 | 1.8 | 6.1 | 339 |
| Inflammation index | ||||||||||||
| 2000 | 0.00 | 1.00 | −0.05 | 596 | 0.00 | 1.00 | −0.05 | 257 | 0.00 | 1.00 | −0.08 | 339 |
| 2006 | 0.00 | 1.00 | −0.07 | 596 | 0.00 | 1.00 | −0.07 | 257 | 0.00 | 1.00 | −0.05 | 339 |
| Average of 2000 & 2006 | 0.00 | 0.89 | −0.05 | 596 | 0.00 | 0.92 | −0.06 | 257 | 0.00 | 0.88 | −0.03 | 339 |
| Change between 2000 & 2006 | 0.00 | 0.89 | −0.02 | 596 | 0.00 | 0.80 | 0.00 | 257 | 0.00 | 0.95 | −0.04 | 339 |
2.2.3. Covariates
Sex and age were included as covariates in all models. To ease interpretation of results, age was centered at 70, close to the median age in 2006. Educational attainment, health behaviors, and general health, all measured in 2006, were included as possible confounders. Years of educational attainment was centered at six years, the median level of education.
While studies of inflammation and cognition typically control for health variables, there is debate regarding whether these variables should be controlled for as possible confounders or considered a step on the causal chain (Steptoe, 2012); inflammation may influence cognition via its effect on health behaviors and general health. For consistency with other studies, I include models that adjust for very basic measures of health behaviors and general health. Indicators were included for self-identified current smokers and those with measured body mass index (BMI) greater than 30. Interviewer-rated health—wherein the interviewer responds to the question “Regarding the respondent’s current state of health, do you feel it is excellent, good, average, not so good, or poor?”—measured overall health (Todd & Goldman, 2013).
2.3. Analytic strategy
Growth curve analysis was used to examine the relationship between inflammation in 2000 and 2006, and change in cognitive score over 2006, 2007, and 2011. These models allowed systematic and random differences between individuals in baseline cognitive score and the rate of cognitive change per year of age.
The growth curve model of cognitive score for person i at time t is given by:
| (1) |
where the intercept is:
| (2) |
and the slope is:
| (3) |
where
cog = cognitive score (measured in 2006, 2007, 2011)
infavg = inflammation (averaged over 2000 and 2006)
othercovariates = a vector of controls, including years of education, health behaviors, and general health (all measured in 2006)
subscripts M and F indicate sex-specific coefficients for male and female respondents, respectively
= random component of person i’s intercept
= random component of person i’s slope
= person i’s time-specific error term,
The intercept and slope vary between respondents systematically based on respondent characteristics; these systematic differences are represented by α’s and β’s. The intercept and slope also vary randomly over and above the influence of covariates, as represented by and . These random components and are assumed to come from a bivariate normal distribution with mean zero; due to data constraints, covariance between the random components is assumed to be zero. Individual-time-specific error is denoted , and is assumed to be normally distributed with mean zero and variance .
All analyses were conducted in Stata 12.1 (StataCorp., 2011).
3. Results
3.1. Descriptive statistics
Table 2 lists descriptive statistics for the entire analytic sample, and separately by sex. Pairwise correlation coefficients of each of the inflammatory measures in 2000 and 2006 are shown in Supplementary Materials (S3).
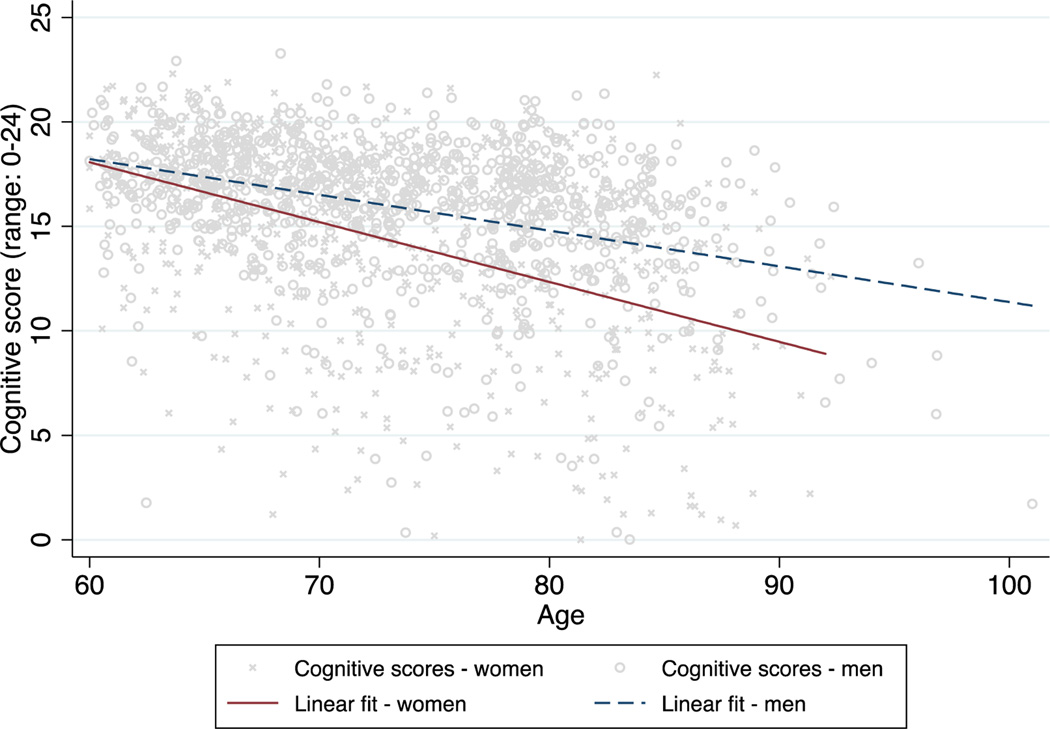
Figure 1 shows cognitive scores by age for men and women and fitted lines representing a linear growth curve model with no covariates. Across the age range of the sample population, women had significantly lower cognitive scores at a given age and their scores declined significantly more with each additional year of age compared to men (significance tests not shown).
Figure 1:
Scatterplot of cognitive scores by age for men and women, with fitted lines from a linear growth curve model with no covariates
Note: Markers of cognitive score values are jittered.
3.2. Growth curve models
Table 3 shows the results from growth curve models. Model 1 included no covariates, while Models 2 and 3 separately added education and health behavior variables. Model 4 included both education and health behavior variables; Model 5 added interviewer-rated health.
Table 3:
Results of growth curve models of average inflammation as a predictor of cognitive score
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Beta | 95% CI | Beta | 95% CI | Beta | 95% CI | Beta | 95% CI | Beta | 95% CI | |
|
|
||||||||||
| Intercept | ||||||||||
| Baseline cognitive score, male | 16.561 | (16.206, 16.916) | 16.167 | (15.841, 16.492) | 16.584 | (16.153, 17.015) | 16.112 | (15.717, 16.507) | 16.253 | (15.669, 16.836) |
| Female | −1.309 | (−1.842, −0.775) | −0.310 | (−0.821, 0.201) | −1.258 | (−1.835, −0.681) | −0.190 | (−0.742, 0.361) | −0.038 | (−0.572, 0.496) |
| Inflammation index, average | −0.621 | (−0.915, −0.326) | −0.345 | (−0.614, −0.076) | −0.593 | (−0.894, −0.292) | −0.332 | (−0.606, −0.057) | −0.310 | (−0.576, −0.043) |
| Slope | ||||||||||
| Age * male | −0.169 | (−0.212, −0.125) | −0.150 | (−0.191, −0.110) | −0.169 | (−0.214, −0.125) | −0.149 | (−0.191, −0.108) | −0.139 | (−0.179, −0.099) |
| Age * female | −0.278 | (−0.328, −0.229) | −0.245 | (−0.291, −0.199) | −0.278 | (−0.328, −0.229) | −0.245 | (−0.291, −0.199) | −0.227 | (−0.272, −0.182) |
| Age * inflammation index, average | 0.003 | (−0.036, 0.042) | 0.002 | (−0.035, 0.038) | 0.003 | (−0.036, 0.042) | 0.002 | (−0.035, 0.038) | −0.004 | (−0.039, 0.031) |
|
| ||||||||||
| SD(slope) | 0.172 | (0.129, 0.230) | 0.165 | (0.126, 0.216) | 0.175 | (0.132, 0.233) | 0.167 | (0.129, 0.218) | 0.158 | (0.120, 0.208) |
| SD(intercept) | 2.559 | (2.322, 2.822) | 2.180 | (1.951, 2.436) | 2.546 | (2.307, 2.808) | 2.165 | (1.936, 2.421) | 2.053 | (1.826, 2.307) |
| SD(residual) | 2.217 | (2.116, 2.323) | 2.239 | (2.137, 2.345) | 2.216 | (2.114, 2.322) | 2.238 | (2.136, 2.345) | 2.250 | (2.148, 2.357) |
|
| ||||||||||
| Includes education? | No | Yes | No | Yes | Yes | |||||
| Includes BMI? | No | No | Yes | Yes | Yes | |||||
| Includes current smoker? | No | No | Yes | Yes | Yes | |||||
| Includes interviewer-rated heath? | No | No | No | No | Yes | |||||
|
| ||||||||||
| Number of observations | 1620 | 1620 | 1620 | 1620 | 1620 | |||||
| Number of respondents | 596 | 596 | 596 | 596 | 596 | |||||
| Mean observations per respondent | 2.7 | 2.7 | 2.7 | 2.7 | 2.7 | |||||
|
| ||||||||||
| Chi squared (p-value), sex * baseline cog score | 23.092 | (<0.001) | 1.414 | (0.234) | 18.259 | (<0.001) | 0.458 | (0.498) | 0.019 | (0.890) |
| Chi squared (p-value), sex * age | 10.693 | (0.001) | 9.150 | (0.002) | 10.427 | (0.001) | 9.198 | (0.002) | 8.468 | (0.004) |
Model 1 estimated that a 70-year-old man had a cognitive score of 16.6 points (out of 24) on average; a 70-year-old woman had 1.3 fewer points, for an average cognitive score of 15.3 points. As respondents aged, cognitive scores declined by 0.17 points per year for men, and 0.28 points per year for women, on average. The sex difference in the rate of annual cognitive decline was statistically significant (p=0.001). The growth curve model allows the intercept (cognitive score at age 70) and the slope (change in cognitive score per year of age) to vary across respondents. The standard deviations shown in the table indicate that these values do vary significantly across respondents, beyond the systematic differences associated with the characteristics included in the model. The intercept has a standard deviation of 2.6 points and the slope has a standard deviation of 0.2 points. This model assumes independence between a respondent’s individual intercept and slope, meaning it is assumed that respondents who start out with a low baseline cognitive score do not see annual changes in cognition that are systematically higher or lower than respondents who start out with a high baseline cognitive score. (When the model is relaxed to freely estimate this correlation between intercept and slope, no significant correlation is found; results not shown.)
In Model 1, higher inflammation was associated with a lower baseline cognitive score: a one-standard-deviation increase in the average inflammation index was associated with a 0.62-point lower cognitive score at age 70. To put this in context, this model predicted that a one-standard-deviation increase in the average inflammation index was associated with a decrease in baseline cognitive score equivalent to what would be expected by aging about three and a half years for men or just over two years for women. The interaction between age and inflammation was estimated at approximately zero (0.003) and statistically insignificant: respondents with higher levels of inflammation did not experience more rapid decline in cognitive score than those with lower levels.
In Model 2, male and female baseline cognitive scores were not significantly different (p=0.23), indicating that education accounted for the sex differences in baseline cognitive scores seen in Model 1. The sex difference in cognitive change per year of age persisted in Model 2 (p=0.002), with women experiencing steeper declines. Once education was taken into account, the coefficient on average inflammation attenuated by almost 50%, to −0.35, but remained statistically significant. As in Model 1, the age-inflammation interaction was estimated at nearly zero (0.002), indicating that inflammation is not associated with the rate of cognitive change per year of age.
The health behavior and general health variables had little impact on the results. The results of Model 3 were nearly unchanged from Model 1, despite adding variables for smoking status and obesity. Similarly, the results of Model 4 were nearly identical to those of Model 2. Adding all of the covariates (education, BMI, smoking status, and interviewer-rated health) in Model 5 did not change the findings: inflammation remained associated with baseline cognitive score, but not with the rate of change in cognitive score.
Models in Table 4 included the averaged inflammation index, as in Table 3, and added the change in the inflammation index between 2000 and 2006. Across all models, change in inflammation was not significantly associated with either baseline cognitive score or with change in cognitive score per year of age. Additionally, the results for average inflammation were not altered by the addition of change in inflammation; the coefficients were remarkably similar to those in Table 3. While average inflammation was inversely associated with baseline cognitive score, it was not associated with change in cognitive score.
Table 4:
Results of growth curve models of average inflammation and change in inflammation as predictors of cognitive score
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Beta | 95% CI | Beta | 95% CI | Beta | 95% CI | Beta | 95% CI | Beta | 95% CI | |
|
|
||||||||||
| Intercept | ||||||||||
| Baseline cognitive score, male | 16.569 | (16.213, 16.925) | 16.179 | (15.854, 16.505) | 16.600 | (16.166, 17.035) | 16.139 | (15.741, 16.537) | 16.269 | (15.685, 16.853) |
| Female | −1.320 | (−1.857, −0.783) | −0.323 | (−0.835, 0.190) | −1.278 | (−1.859, −0.696) | −0.217 | (−0.771, 0.337) | −0.064 | (−0.600, 0.472) |
| Inflammation index, average | −0.620 | (−0.914, −0.325) | −0.341 | (−0.610, −0.072) | −0.591 | (−0.892, −0.289) | −0.325 | (−0.600, −0.050) | −0.303 | (−0.570, −0.037) |
| Inflammation index, change | 0.000 | (−0.308, 0.307) | 0.017 | (−0.259, 0.293) | −0.010 | (−0.317, 0.297) | 0.004 | (−0.271, 0.280) | 0.009 | (−0.258, 0.275) |
| Slope | ||||||||||
| Age * male | −0.168 | (−0.212, −0.124) | −0.149 | (−0.190, −0.108) | −0.169 | (−0.213, −0.124) | −0.148 | (−0.189, −0.107) | −0.137 | (−0.177, −0.097) |
| Age * female | −0.280 | (−0.330, −0.230) | −0.248 | (−0.294, −0.202) | −0.280 | (−0.330, −0.230) | −0.247 | (−0.294, −0.201) | −0.230 | (−0.276, −0.185) |
| Age * inflammation index, average | 0.003 | (−0.036, 0.042) | 0.000 | (−0.036, 0.037) | 0.002 | (−0.037, 0.041) | 0.000 | (−0.036, 0.037) | −0.005 | (−0.041, 0.030) |
| Age * inflammation index, change | −0.010 | (−0.049, 0.028) | −0.021 | (−0.057, 0.015) | −0.010 | (−0.049, 0.029) | −0.020 | (−0.056, 0.016) | −0.021 | (−0.056, 0.014) |
|
| ||||||||||
| SD(slope) | 0.173 | (0.129, 0.230) | 0.165 | (0.127, 0.216) | 0.176 | (0.133, 0.233) | 0.168 | (0.129, 0.218) | 0.158 | (0.120, 0.208) |
| SD(intercept) | 2.559 | (2.322, 2.821) | 2.178 | (1.950, 2.433) | 2.545 | (2.307, 2.807) | 2.163 | (1.934, 2.418) | 2.052 | (1.826, 2.306) |
| SD(residual) | 2.217 | (2.115, 2.323) | 2.238 | (2.136, 2.345) | 2.215 | (2.113, 2.321) | 2.237 | (2.135, 2.344) | 2.249 | (2.147, 2.356) |
|
| ||||||||||
| Includes education? | No | Yes | No | Yes | Yes | |||||
| Includes BMI? | No | No | Yes | Yes | Yes | |||||
| Includes current smoker? | No | No | Yes | Yes | Yes | |||||
| Includes interviewer-rated heath? | No | No | No | No | Yes | |||||
|
| ||||||||||
| Number of observations | 1620 | 1620 | 1620 | 1620 | 1620 | |||||
| Number of respondents | 596 | 596 | 596 | 596 | 596 | |||||
| Mean observations per respondent | 2.7 | 2.7 | 2.7 | 2.7 | 2.7 | |||||
|
| ||||||||||
| Chi squared (p-value), sex * baseline cog score | 23.227 | (<0.001) | 1.525 | (0.217) | 18.564 | (<0.001) | 0.590 | (0.442) | 0.055 | (0.815) |
| Chi squared (p-value), sex * age | 10.964 | (<0.001) | 9.898 | (0.002) | 10.716 | (0.001) | 9.894 | (0.002) | 9.224 | (0.002) |
A number of sensitivity analyses are described in Supplementary Materials (S4-S5).
4. Conclusion
The aim of this study was to assess the relationship between inflammation and cognitive function among older Taiwanese adults. I found that higher levels of inflammation were associated with lower baseline cognitive scores, but there was no association between inflammation and subsequent change in cognitive score. Using models that considered a measure of recent change in inflammation and controlled for a number of potential confounders had no effect on these results.
This study found no evidence of a relationship between inflammation and the rate of cognitive decline among older adults. These results are consistent with the findings of several studies of longitudinal cognitive change (Alley et al., 2008; Dik et al., 2005; Teunissen et al., 2003), but conflicting with others. Alley et al. (2008) pointed out that many of the studies that did find an association measured cognitive decline dichotomously as a decline greater than some threshold (Jordanova et al., 2007; Weaver et al., 2002; Wichmann et al., 2014; e.g., Yaffe et al., 2003), suggesting that this association may have been due to incident dementia, rather than age-related cognitive decline. Perhaps, then, inflammation plays a role in pathologic cognitive decline such as Alzheimer’s disease, but is less relevant for normal age-related cognitive decline.
The present finding that inflammation is associated with lower baseline cognitive score, but not with the rate of age-related cognitive decline, suggests that the inflammation-cognition link is not causal, at least at these ages. Life course research is critically needed. Inflammation may causally influence cognitive ability, but at a younger age than is represented in this study, or perhaps exposure to inflammation matters on a scale of decades rather than the years captured here. Studies of inflammation and cognition are typically conducted in cohorts of older adults; this is logical, since dementia and cognitive decline are hallmarks of aging, but it may miss important effects occurring earlier in life. Alternatively, it is possible that inflammation influences cognitive ability more rapidly than this study can capture, such that declines in cognition would had already occurred by the time an increase in inflammation could be registered. Both long and short-term investigation across the life course may be particularly important because the physiological mechanisms underlying a potential inflammation-cognition link are not well understood.
Compared with health behaviors and general health, education was responsible for a larger share of the attenuation in the inflammation-baseline cognition relationship, suggesting that socioeconomic status may play a role. A link between socioeconomic status and inflammation has been documented in several studies (Loucks et al., 2010; Pollitt et al., 2007; Ranjit et al., 2007; Steptoe, Owen, Kunz-Ebrecht, & Mohamed-Ali, 2002). Education is consistently linked to cognitive function, though whether this is due to socioeconomic status or to the direct effect of education on cognition itself—or the effect of cognition on education—is debated (Cagney & Lauderdale, 2002; Lee, Kawachi, Berkman, & Grodstein, 2003). Life course analyses could improve understanding of the mechanisms.
This study was not without limitations. A larger sample size, longer time frame, and more frequent measurement would add power and precision. Selection may have contributed to an underestimate of the inflammation-cognition relationship. The analytic sample was restricted to respondents with inflammation measures in both 2000 and 2006; this means respondents who died, were lost to follow-up, or were ineligible for examination in either the 2000 or 2006 SEBAS wave were excluded. This exclusion likely led to an analytic sample that was healthier, with perhaps better cognitive abilities, compared to the entire TLSA sample. Further, respondents with rapidly declining cognitive abilities may be more likely to have been interviewed by proxy, and thus be missing cognitive scores, in later waves. If these respondents also had higher levels of inflammation, the present sample would be biased toward an underestimate of the inflammation-cognitive decline association. Finally, inflammation may influence only specific aspects of cognitive ability, e.g., working memory or executive function, nuance that might be lost with the general cognitive measure used here.
This study has several benefits over previous studies of inflammation and cognition. Rather than just one or two measures of inflammation, this study used five biomarkers, including measures of blood vessel inflammation. Additionally, it used growth curve analysis to take advantage of several measures of cognitive function—up to three per respondent. These advantages add strength to the conclusions of this study. Inflammation did not predict cognitive decline in this sample of health older adults, suggesting that the observed cross-sectional relationship between inflammation and cognitive score was not causal at these older ages. While a link between inflammation and subsequent incident dementia has been demonstrated in some previous work, the results of this study indicate that this relationship may not be universal, and likely does not generalize to normal cognitive changes associated with aging.
Supplementary Material
Acknowledgments
I gratefully acknowledge the hard work and dedication of the staff at the Center for Population and Health Survey Research, Bureau of Health Promotion, Taiwan Department of Health, who were instrumental in the design and implementation of the Social Environment and Biomarkers of Aging Study and supervised all aspects of the fieldwork and data processing. I would like to thank Noreen Goldman, Jennifer Dowd, Germán Rodríguez, Scott Lynch, Maxine Weinstein, Dana Glei, and the Fellowship of Woodrow Wilson Scholars at Princeton University for helpful suggestions on this study.
Funding
This work was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health (grant number P2CHD047879) and the National Institute on Aging of the National Institutes of Health (grant number R01AG016790). Funders had no role in the design, methods, interpretation, or preparation of the manuscript.
References
- Albelda SM, Smith CW, & Ward PA (1994). Adhesion molecules and inflammatory injury. The FASEB Journal, 8(8), 504–512. [PubMed] [Google Scholar]
- Alley DE, Crimmins EM, Karlamangla A, Hu P, & Seeman TE (2008). Inflammation and Rate of Cognitive Change in High-Functioning Older Adults. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences, 63(1), 50–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews SJ, Das D, Cherbuin N, Anstey KJ, & Easteal S. (2016). Association of genetic risk factors with cognitive decline: the PATH through life project. Neurobiology of Aging, 41, 150–158. 10.1016/j.neurobiolaging.2016.02.016 [DOI] [PubMed] [Google Scholar]
- Baune BT, Ponath G, Golledge J, Varga G, Arolt V, Rothermundt M, & Berger K. (2008). Association between IL-8 cytokine and cognitive performance in an elderly general population—The MEMO-Study. Neurobiology of Aging, 29(6), 937–944. 10.1016/j.neurobiolaging.2006.12.003 [DOI] [PubMed] [Google Scholar]
- Boyle PA, Wilson RS, Yu L, Barr AM, Honer WG, Schneider JA, & Bennett DA (2013). Much of late life cognitive decline is not due to common neurodegenerative pathologies. Annals of Neurology, 74(3), 478–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle PA, Yu L, Wilson RS, Gamble K, Buchman AS, & Bennett DA (2012). Poor Decision Making Is a Consequence of Cognitive Decline among Older Persons without Alzheimer’s Disease or Mild Cognitive Impairment. PLOS ONE, 7(8), e43647. 10.1371/journal.pone.0043647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cagney KA, & Lauderdale DS (2002). Education, Wealth, and Cognitive Function in Later Life. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences, 57(2), P163–P172. 10.1093/geronb/57.2.P163 [DOI] [PubMed] [Google Scholar]
- Chang M-C, Lin H-S, Chuang Y-L, Goldman N, Peterson CE, Glei DA, … Wu S-I (2012). Social Environment and Biomarkers of Aging Study (SEBAS) in Taiwan, 2000 and 2006: main documentation for SEBAS longitudinal public use data. Ann Arbor, MI: Inter-University Consortium for Political and Social Research. Retrieved from http://www.icpsr.umich.edu/icpsrweb/ICPSR/studies/3792 [Google Scholar]
- Das A. (2013). How does race get “under the skin”? Inflammation, weathering, and metabolic problems in late life. Social Science & Medicine, 77, 75–83. 10.1016/j.socscimed.2012.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deary IJ, Corley J, Gow AJ, Harris SE, Houlihan LM, Marioni RE, … Starr JM (2009). Age-associated cognitive decline. British Medical Bulletin, 92(1), 135–152. 10.1093/bmb/ldp033 [DOI] [PubMed] [Google Scholar]
- Dik MG, Jonker C, Hack CE, Smit JH, Comijs HC, & Eikelenboom P. (2005). Serum inflammatory proteins and cognitive decline in older persons. Neurology, 64(8), 1371–1377. 10.1212/01.WNL.0000158281.08946.68 [DOI] [PubMed] [Google Scholar]
- Engelhart MJ, Geerlings MI, Meijer J, Kiliaan A, Ruitenberg A, van Swieten JC, … Breteler MMB (2004). Inflammatory proteins in plasma and the risk of dementia: the rotterdam study. Archives of Neurology, 61(5), 668–672. 10.1001/archneur.61.5.668 [DOI] [PubMed] [Google Scholar]
- Fjell AM, McEvoy L, Holland D, Dale AM, & Walhovd KB (2014). What is normal in normal aging? Effects of aging, amyloid and Alzheimer’s disease on the cerebral cortex and the hippocampus. Progress in Neurobiology, 117, 20–40. 10.1016/j.pneurobio.2014.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabay C, & Kushner I. (1999). Acute-Phase Proteins and Other Systemic Responses to Inflammation. New England Journal of Medicine, 340(6), 448–454. 10.1056/NEJM199902113400607 [DOI] [PubMed] [Google Scholar]
- Glei DA, Landau DA, Goldman N, Chuang Y-L, Rodríguez G, & Weinstein M. (2005). Participating in social activities helps preserve cognitive function: an analysis of a longitudinal, population-based study of the elderly. International Journal of Epidemiology, 34(4), 864–871. 10.1093/ije/dyi049 [DOI] [PubMed] [Google Scholar]
- Goldman N, Turra CM, Rosero-Bixby L, Weir D, & Crimmins E. (2011). Do biological measures mediate the relationship between education and health: A comparative study. Social Science & Medicine, 72(2), 307–315. 10.1016/j.socscimed.2010.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog AR, & Wallace RB (1997). Measures of Cognitive Functioning in the AHEAD Study. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences, 52B(Special), 37–48. 10.1093/geronb/52B.Special_Issue.37 [DOI] [PubMed] [Google Scholar]
- Hurd MD, Martorell P, Delavande A, Mullen KJ, & Langa KM (2013). Monetary Costs of Dementia in the United States. New England Journal of Medicine, 368(14), 1326–1334. 10.1056/NEJMsa1204629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenny NS, French B, Arnold AM, Strotmeyer ES, Cushman M, Chaves PHM, … Newman AB (2012). Long-term Assessment of Inflammation and Healthy Aging in Late Life: The Cardiovascular Health Study All Stars. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences, 67(9), 970–976. 10.1093/gerona/glr261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordanova V, Stewart R, Davies E, Sherwood R, & Prince M. (2007). Markers of inflammation and cognitive decline in an African-Caribbean population. International Journal of Geriatric Psychiatry, 22(10), 966–973. 10.1002/gps.1772 [DOI] [PubMed] [Google Scholar]
- Kobayashi LC, Wardle J, Wolf MS, & von Wagner C. (2016). Aging and Functional Health Literacy: A Systematic Review and Meta-Analysis. The Journals of Gerontology: Series B, 71(3), 445–457. 10.1093/geronb/gbu161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komulainen P, Lakka TA, Kivipelto M, Hassinen M, Penttilä IM, Helkala E-L, … Rauramaa R. (2007). Serum high sensitivity C-reactive protein and cognitive function in elderly women. Age and Ageing, 36(4), 443–448. 10.1093/ageing/afm051 [DOI] [PubMed] [Google Scholar]
- Lee S, Kawachi I, Berkman LF, & Grodstein F. (2003). Education, Other Socioeconomic Indicators, and Cognitive Function. American Journal of Epidemiology, 157(8), 712–720. 10.1093/aje/kwg042 [DOI] [PubMed] [Google Scholar]
- Loucks EB, Pilote L, Lynch JW, Richard H, Almeida ND, Benjamin EJ, & Murabito JM (2010). Life course socioeconomic position is associated with inflammatory markers: The Framingham Offspring Study. Social Science & Medicine, 71(1), 187–195. 10.1016/j.socscimed.2010.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDade TW, Burhop J, & Dohnal J. (2004). High-Sensitivity Enzyme Immunoassay for C-Reactive Protein in Dried Blood Spots. Clinical Chemistry, 50(3), 652–654. 10.1373/clinchem.2003.029488 [DOI] [PubMed] [Google Scholar]
- Ownby RL (2010). Neuroinflammation and Cognitive Aging. Current Psychiatry Reports, 12(1), 39–45. 10.1007/s11920-009-0082-1 [DOI] [PubMed] [Google Scholar]
- Petersen RC (2011). Mild Cognitive Impairment. New England Journal of Medicine, 364(23), 2227–2234. 10.1056/NEJMcp0910237 [DOI] [PubMed] [Google Scholar]
- Pollitt RA, Kaufman JS, Rose KM, Diez-Roux AV, Zeng D, & Heiss G. (2007). Early-life and adult socioeconomic status and inflammatory risk markers in adulthood. European Journal of Epidemiology, 22(1), 55–66. 10.1007/s10654-006-9082-1 [DOI] [PubMed] [Google Scholar]
- Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W, & Ferri CP (2013). The global prevalence of dementia: A systematic review and metaanalysis. Alzheimer’s & Dementia, 9(1), 63–75.e2. 10.1016/j.jalz.2012.11.007 [DOI] [PubMed] [Google Scholar]
- Ranjit N, Diez-Roux AV, Shea S, Cushman M, Ni H, & Seeman T. (2007). Socioeconomic Position, Race/Ethnicity, and Inflammation in the Multi-Ethnic Study of Atherosclerosis. Circulation, 116(21), 2383–2390. 10.1161/CIRCULATIONAHA.107.706226 [DOI] [PubMed] [Google Scholar]
- Ridker PM, Hennekens CH, Buring JE, & Rifai N. (2000). C-Reactive Protein and Other Markers of Inflammation in the Prediction of Cardiovascular Disease in Women. New England Journal of Medicine, 342(12), 836–843. 10.1056/NEJM200003233421202 [DOI] [PubMed] [Google Scholar]
- Schmidt R, Schmidt H, Curb JD, Masaki K, White LR, & Launer LJ (2002). Early inflammation and dementia: A 25-year follow-up of the Honolulu-Asia aging study. Annals of Neurology, 52(2), 168–174. 10.1002/ana.10265 [DOI] [PubMed] [Google Scholar]
- Schram MT, Euser SM, De Craen AJM, Witteman JC, Frölich M, Hofman A, … Westendorp RGJ (2007). Systemic Markers of Inflammation and Cognitive Decline in Old Age. Journal of the American Geriatrics Society, 55(5), 708–716. 10.1111/j.1532-5415.2007.01159.x [DOI] [PubMed] [Google Scholar]
- Seplaki CL, Goldman N, Glei D, & Weinstein M. (2005). A comparative analysis of measurement approaches for physiological dysregulation in an older population. Experimental Gerontology, 40(5), 438–449. 10.1016/j.exger.2005.03.002 [DOI] [PubMed] [Google Scholar]
- StataCorp. (2011). Stata Statistical Software: Release 12. College Station, Texas: StataCorp LP. [Google Scholar]
- Steptoe A. (2012). Socioeconomic Status, Inflammation, and Immune Function. In Segerstrom S. (Ed.), The Oxford Handbook of Psychoneuroimmunology. Oxford University Press. [Google Scholar]
- Steptoe A, Owen N, Kunz-Ebrecht S, & Mohamed-Ali V. (2002). Inflammatory cytokines, socioeconomic status, and acute stress responsivity. Brain, Behavior, and Immunity, 16(6), 774–784. 10.1016/S0889-1591(02)00030-2 [DOI] [PubMed] [Google Scholar]
- Teunissen CE, van Boxtel MPJ, Bosma H, Bosmans E, Delanghe J, De Bruijn C, … de Vente J. (2003). Inflammation markers in relation to cognition in a healthy aging population. Journal of Neuroimmunology, 134(1–2), 142–150. [DOI] [PubMed] [Google Scholar]
- Todd MA, & Goldman N. (2013). Do Interviewer and Physician Health Ratings Predict Mortality?: A Comparison with Self-Rated Health. Epidemiology, 24(6), 913–920. 10.1097/EDE.0b013e3182a713a8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker-Drob EM (2011). Neurocognitive functions and everyday functions change together in old age. Neuropsychology, 25(3), 368. 10.1037/a0022348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver JD, Huang M-H, Albert M, Harris T, Rowe JW, & Seeman TE (2002). Interleukin-6 and risk of cognitive decline MacArthur Studies of Successful Aging. Neurology, 59(3), 371–378. 10.1212/WNL.59.3.371 [DOI] [PubMed] [Google Scholar]
- Weuve J, Ridker PM, Cook NR, Buring JE, & Grodstein F. (2006). High-Sensitivity C-Reactive Protein and Cognitive Function in Older Women. Epidemiology, 17(2), 183–189. 10.1097/01.ede.0000198183.60572.c9 [DOI] [PubMed] [Google Scholar]
- Wichmann MA, Cruickshanks KJ, Carlsson CM, Chappell R, Fischer ME, Klein BEK, … Schubert CR (2014). Long-Term Systemic Inflammation and Cognitive Impairment in a Population-Based Cohort. Journal of the American Geriatrics Society, 62(9), 1683–1691. 10.1111/jgs.12994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RS, Boyle PA, Segawa E, Yu L, Begeny CT, Anagnos SE, & Bennett DA (2013). The influence of cognitive decline on well-being in old age. Psychology and Aging, 28(2), 304. 10.1037/a0031196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe K, Lindquist K, Penninx BW, Simonsick EM, Pahor M, Kritchevsky S, … Harris T. (2003). Inflammatory markers and cognition in well-functioning African-American and white elders. Neurology, 61(1), 76–80. 10.1212/01.WNL.0000073620.42047.D7 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.