Abstract
Aeromonas hydrophila is an opportunistic pathogen and the leading cause of fatal hemorrhagic septicemia in rainbow trout. A gene encoding an elastolytic activity, ahyB, was cloned from Aeromonas hydrophila AG2 into pUC18 and expressed in Escherichia coli and in the nonproteolytic species Aeromonas salmonicida subsp. masoucida. Nucleotide sequence analysis of the ahyB gene revealed an open reading frame of 1,764 nucleotides with coding capacity for a 588-amino-acid protein with a molecular weight of 62,728. The first 13 N-terminal amino acids of the purified protease completely match those deduced from DNA sequence starting at AAG (Lys-184). This finding indicated that AhyB is synthesized as a preproprotein with a 19-amino-acid signal peptide, a 164-amino-acid N-terminal propeptide, and a 405-amino-acid intermediate which is further processed into a mature protease and a C-terminal propeptide. The protease hydrolyzed casein and elastin and showed a high sequence similarity to other metalloproteases, especially with the mature form of the Pseudomonas aeruginosa elastase (52% identity), Helicobacter pylori zinc metalloprotease (61% identity), or proteases from several species of Vibrio (52 to 53% identity). The gene ahyB was insertionally inactivated, and the construct was used to create an isogenic ahyB mutant of A. hydrophila. These first reports of a defined mutation in an extracellular protease of A. hydrophila demonstrate an important role in pathogenesis.
Aeromonas hydrophila is a gram-negative opportunistic pathogen in humans and several fish species, causing soft tissue wound infections and diarrhea in the former (1, 18, 21) and fatal hemorrhagic septicemia in the latter (2, 12, 15, 37). It has been speculated that A. hydrophila virulence could involve several extracellular enzymes including proteases, hemolysins, enterotoxins, and acetylcholinesterase. Some of the toxins have been biochemically characterized, but their precise roles in the pathogenicity of A. hydrophila have not yet been determined (8, 29, 35, 41, 42). The two major extracellular proteolytic activities of A. hydrophila that have been described so far, a 38-kDa thermostable metalloprotease (29, 41) and a 68-kDa temperature-labile serine protease (30, 42), are present in most A. hydrophila culture supernatants. In addition, a 19-kDa zinc proteinase was found in the growth medium of a strain of A. hydrophila isolated from the intestinal tract of the leech Hirudo medicinalis (31), and a 22-kDa serine proteinase, which is stable at 56°C for 10 min, was purified from A. hydrophila strain B32 culture supernatant (43). Several strategies have been used to examine the role of some A. hydrophila proteases in virulence, including Tn5-induced protease-deficient mutants of A. hydrophila (29) and direct inoculation of purified 22-kDa serine protease in rainbow trout (43), but with conflicting results. Two major secretion products of A. salmonicida, an extracellular serine protease (AspA) and a glycerophospholipid:cholesterol acyltransferase (SatA), had previously been thought to be responsible for the fish disease furunculosis (6, 10, 13); however, isogenic aspA and satA deletion mutants have recently been shown to have little, if any, effect on A. salmonicida pathogenesis (49).
Two A. hydrophila genes involved in protease production have been cloned and efficiently expressed in different bacteria. One of them, cloned from A. hydrophila SO2/2, encodes a 68-kDa temperature-labile serine protease (7, 42), which is very similar in molecular mass to the serine protease AspA produced by A. salmonicida. The other gene was cloned from the same bacterium and encoded a 38-kDa temperature-stable metalloprotease (41). Both proteases degraded azocasein, but no elastolytic activity was detected with elastin Congo red substrate (41, 42). However, many A. hydrophila strains, including SO2/2, secrete elastolytic activity into the culture medium when plated on insoluble elastin nutrient agar, although this activity has not been attributed to any extracellular protein. Generally, prokaryotes and eukaryotes synthesize proteases as inactive precursors (preproenzymes) that are activated only after proteolytic removal of a propeptide that is convalently attached to the N and/or C termini of mature protease sequence. This is the case, for example, with the elastase produced by Pseudomonas aeruginosa, a 33-kDa metalloprotease closely related to other proteases (24, 34) that is encoded by lasB and is synthesized as a preproenzyme (53.4 kDa) with a classical signal peptide and a covalently linked 18-kDa amino-terminal propeptide (25, 26, 27). This is also the case with LasA protease from P. aeruginosa, which is a 20-kDa zinc metalloendopeptidase with a high staphylolytic activity (26).
In this study we provide evidence that the A. hydrophila ahyB gene product contributes most of the elastolytic activity of this bacterium. Experiments were conducted to explore the processing of AhyB protease. We also constructed an A. hydrophila ahyB mutant by allelic replacement and found that the ahyB product is essential for virulence in rainbow trout.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
The bacterial strains and plasmids used in this study are detailed in Table 1. A. hydrophila and A. salmonicida strains were grown on Luria-Bertani (LB) broth or agar as before (41), or on tryptic soy agar or broth (Biolife), and incubated at 28°C. Escherichia coli strains were grown on any one of the media mentioned and incubated at 37°C. The media used were supplemented, when necessary, with the antibiotics ampicillin (100 μg/ml), kanamycin (40 μg/ml), and chloramphenicol (10 μg/ml), along with skim milk (2%, wt/vol) or insoluble elastin (1%, wt/vol) from bovine neck ligament (Sigma).
TABLE 1.
Characteristics of bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant properties | Reference or source |
|---|---|---|
| Strains | ||
| E. coli | ||
| C600 | Transformation recipient for plasmids | 38 |
| S17-1 | Mobilizing donor for conjugation | 45 |
| A. hydrophila AG2 | Virulent strain rainbow trout isolate | 17 |
| A. salmonicida subsp. masoucida | Nonproteolytic strain | CECT 896 |
| Plasmids | ||
| pUC18 | Apr; cloning vector | 50 |
| pJRD215 | Kanr, Smr; broad-host-range mobilizable vector | 42 |
| pSUP202 | Tcr, Apr, Cmr, ColE1 ori, Mob+; broad-host-range mobilizable suicide vector | 45 |
| pSUP202-1 | Tcr, Cmr, ColE1 ori, Mob+; broad-host-range mobilizable suicide vector | This study |
| pAHE5 | Apr; pUC18 containing ahpB gene | This study |
| pAHE6 | pJRD215 with 2.5-kb SalI-XhoI fragment from pAHE5 | This study |
| pAHE7 | pAHE5 with a Kanr cassette ligated into BglII site | This study |
| pAHE8 | pSUP202-1 with 3.8-kb SalI-XhoI fragment from pAHE7 ligated into SalII site | This study |
Chemicals and enzymes were obtained from Boehringer GmbH, Promega Corp., or Pharmacia and used as specified by the manufacturers.
DNA preparation, manipulation, and gene library construction.
Chromosomal DNA from the pathogenic A. hydrophila AG2, the source of the ahpA gene, was obtained from an overnight culture grown at 28°C as reported elsewhere (38). Plasmids used in this study were propagated in E. coli and isolated by the alkali lysis method (3). Standard molecular cloning, transformation, and electrophoresis techniques were used (44). Southern blotting and hybridization were performed by random-primer DNA labeling with digoxigenin-dUTP, and hybrids were detected by an enzyme immunoassay as specified by the manufacturer (Boehringer).
Chromosomal DNA, prepared as described above, was partially digested with Sau3A, and a library consisting of 3- to 9-kb fragments was prepared in BamHI-digested dephosphorylated pUC18 (Pharmacia). The ligation mixture was precipitated with ethanol, resuspended in 10 μl of distilled water, and used to transform electroporated E. coli C600. Electroporation was performed with a Gene Pulser apparatus (Bio-Rad Laboratories) set at 2.5 kV, 25 μF, and 1,000 Ω (field strength, 12.5 kV/cm), as described previously (7). Transformants were selected on LB agar supplemented with ampicillin and skim milk. Nucleotide sequences were determined by the dideoxynucleotide chain termination method with double-stranded templates by means of the fmol DNA sequencing system (Promega). Gaps in the sequences were completed by using DNA primers synthesized by Promega.
PCR.
PCR was performed with a pair of primers annealing 5′ and 3′ regions of the A. hydrophila ahpB gene. The forward primer, F1, consisted of 22 nucleotides (5′-GGCAACGTCAAGACTGGCAAGT-3′) corresponding to positions 571 to 592 of the ahpB gene sequence; the reverse primer, R1, had a length of 20 nucleotides (5′-CGATCAGGGAGCCTGCGGCT-3′) corresponding to positions 338 to 1,357. Primers were synthesized by Promega. Samples to be analyzed by PCR were cultured bacteria. PCR amplification was carried out with a DNA thermal cycler (Perkin-Elmer Cetus) and a PCR kit (Boehringer) in accordance with the manufacturer's instructions, with some modifications. In brief, the reaction mixture consisted of 1 μl of DNA-containing sample, 1.25 U of Taq DNA polymerase, 5 μl of 10× PCR buffer (100 mM Tris-HCl, 20 mM MgCl2, 500 mM KCl [pH 8.3]), 1 μM each primer, 0.5 mM deoxynucleoside triphosphates, and double-distilled water to a final volume of 50 μl. To minimize evaporation, 50 μl of mineral oil was added to the mixture. DNA denaturation was carried out at 94°C for 2 min, and then a total of 40 cycles were run under the following conditions: DNA denaturation at 92°C for 1 min, primer annealing at 58°C for 30 s, and DNA extension at 72°C for 2 min. After the final cycle, reactions were terminated by a further run at 72°C for 5 min. Reactions were kept at 4°C until analyzed by endonuclease digestion and agarose gel electrophoresis (2.5% agarose gels, running Tris-borate-EDTA buffer).
Bacterial conjugation.
Conjugation was performed as described by others (47). In brief, donor (E. coli S17-1 with the appropriate plasmid) and recipient (A. salmonicida subsp. masoucida or A. hydrophila) strains were grown overnight in LB broth with shaking and incubated at 37 and 25°C, respectively. Then 10-μl aliquots of each of the overnight cultures of the donor and recipient strains were mixed on the surface of a sterile 0.45-μm-pore-size filter (Millipore), placed on the surface of a dried LB agar plate with no antibiotics, and incubated for 4 h at 25°C. The mixed bacteria were harvested in LB broth, and dilutions were spread on selective LB agar plates, which were then incubated at 25°C for 48 h.
Purification of protease.
The starting material for AhpB purification was culture supernatant from A. hydrophila AG2, or A. salmonicida masoucida containing plasmid pAHE5, which was fractionated with ammonium sulfate; 35 to 60% ammonium sulfate-insoluble materials containing a high proteolytic activity was used for further purification. A detailed procedure for AhpB purification was described by others (41). The purified protein was analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (28). The enzyme was stored at −20°C.
Proteolytic and elastolytic assays.
Proteolytic and elastolytic activities on solid medium were detected by patching bacteria on LB supplemented with 2% skim milk and 1% insoluble elastin, respectively, with clear zones around patches revealing activities. Total proteolytic activity in Aeromonas culture fluid was determined by adding 5 μl of filtered (0.45-μm-pore-size filter) culture supernatant (48 h of incubation in LB medium at 30°C) to a reaction mixture containing 0.4% azocasein and 25 mM Tris-HCl buffer (pH 7.5), in a final volume of 500 μl. The reaction mixture was incubated at 37°C for 1 h, and the reaction was stopped by adding 500 μl of 10% trichloroacetic acid (TCA). After centrifugation at 13,000 × g, 500 μl of supernatant was mixed with an equal volume of 1 N NaOH. One unit of caseinolytic activity is defined as the amount of enzyme causing an increase in A450 of 0.1 for 1 h of incubation. Elastolytic activity was determined in culture supernatant processes as above by elastin Congo red assays as described by others (4). One unit of elastolytic activity is defined as the amount of enzyme causing an increase in A495 of 0.01 for 1 h of incubation. Protein was determined by the method of Bradford (5). In both cases, enzymatic activity was linear for the whole duration of the assay and was proportional to the amount of enzyme added.
Antibodies.
Antibodies to AhpB were raised in New Zealand White rabbits by subcutaneous injection of 250 μg of pure AhpB protease mixed with complete Freund adjuvant, followed by two additional injections of 100 μg of the antigen at 1-week intervals. Antibodies against AhpB were affinity purified from the resultant antiserum and were used in immunoblots at a dilution ranging from 1/500 to 1/1,000.
SDS-PAGE and immunoblotting.
Proteins were separated by SDS-PAGE by the Laemmli method (28) with 4% stacking gel and 12% separating gel. Samples of culture supernatant (1 ml) were obtained under standard incubation conditions and prepared by centrifugation (10,000 × g for 15 min) of the cell suspensions at 4°C. Samples for SDS-PAGE and immunoblotting were immediately precipitated by adding TCA to a final concentration of 10%. After standing overnight at room temperature, TCA precipitates were pelleted, washed four times with acetone, air dried, and dissolved in 1/10 Laemmli sample buffer. Protein bands were visualized by silver staining (28). Proteins were transferred from the gel used for SDS-PAGE to nitrocellulose filter paper in a Trans-Blot apparatus (Bio-Rad) for 2 h at 160 mA and 4°C. Immunoblot detection of AhpB protease was performed using AhpB rabbit polyclonal immunoglobulin G as the primary antibody followed by a goat anti-rabbit immunoglublin G-peroxidase conjugate (Bio-Rad).
N-terminal amino acid sequence analysis.
The N-terminal amino acid sequence of the purified protease blotted from SDS-polyacrylamide gels to Immobilon-P (Millipore Corp., Bedford, Mass.) was determined by using an Applied Biosystems 470A gas-liquid-phase sequencer. Fourteen cycles were acquired, and the amino acid residues were identified by comparison with a β-lactoglobulin standard.
LD50 determinations.
Rainbow trout (Oncorhynchus mykiss; 10 to 15 cm in length) were obtained from a commercial fish farm. The animals were kept in 70-liter plastic tanks supplied with running well water at 15°C, maintained under constant photoperiod conditions (12 h of light/12 h of darkness), and fed with commercial trout pellets. Before manipulation, the fish were anesthetized with 1:15,000 tricaine methane sulfonate MS-222 (Sandoz) in water. For 50% lethal dose (LD50) determinations, six groups of 10 fish were intraperitoneally injected with 0.1 ml of washed culture of A. hydrophila AG2 and of A. hydrophila ahpB mutant, emulsified in sterile phosphate-buffered saline containing 104 to 109 CFU. The trout were observed for 7 days, and any dead specimen was removed for routine bacteriological examination. The experiment was carried out four times in duplicate, and the LD50 was calculated by the statistical approach of Reed and Muench (40).
Nucleotide sequence accession number.
The nucleotide sequence of ahpB gene was submitted to the GenBank nucleotide sequence database under accession no. AF193422.
RESULTS
Molecular cloning of the A. hydrophila ahpB gene.
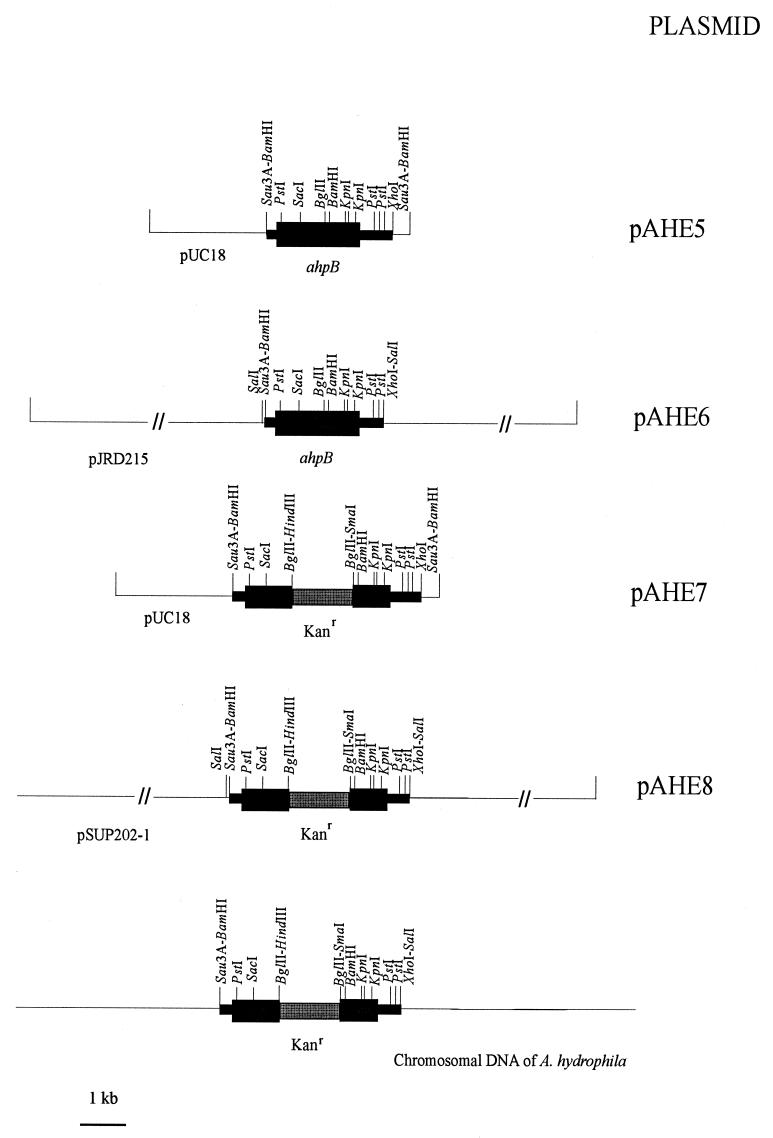
The ahpB gene was cloned from a genomic library of the pathogenic strain A. hydrophila AG2 (17) constructed in E. coli C600, using plasmid pUC18 as a vector. Approximately 3,000 ampicillin-resistant (Apr) transformants were selected on LB agar plates supplemented with ampicillin and skim milk. A clear halo, indicating degradation of milk proteins, surrounded one transformant of the AG2 genomic library after 48 h at 37°C. Plasmid pAHE5 (Fig. 1) was extracted from this transformant and used to transform E. coli C600 again. When these cells were grown on LB agar supplemented with ampicillin and skim milk, 100% of colonies were Apr and protease positive. The physical map of pAHE5 (Fig. 1) showed a 2.7-kbp DNA insert originating from A. hydrophila AG2 chromosomal DNA, as demonstrated by Southern blot hybridization (data not shown).
FIG. 1.
Restriction maps of the ahpB locus and construction of the ahpA::Kanr cassette, the base of allele exchange. Black boxes represent A. hydrophila AG2 cloned DNA; the thicker black box represents the A. hydrophila ahpB gene, which is oriented from 5′ (left) to 3′ (right). The shaded box represents the Kanr cassette. Horizontal lines represent different plasmid vectors or A. hydrophila ahpA mutant chromosomal DNA.
Nucleotide sequence analysis.
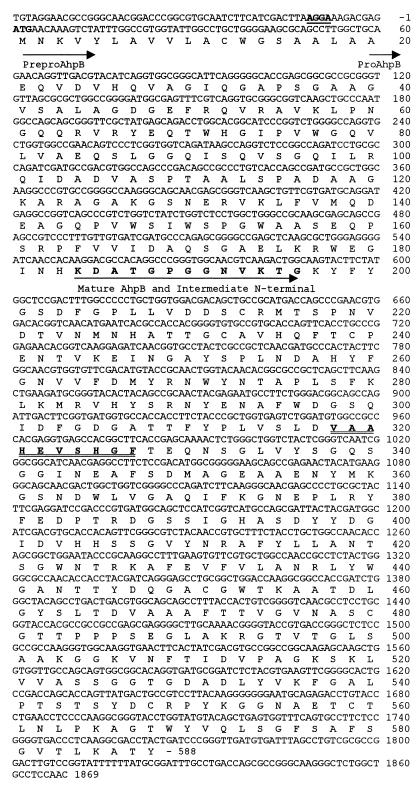
The nucleotide sequence of the 2.7-kbp insert revealed one major open reading frame of 1,764 bp with the capacity to encode a polypeptide of 588 amino acids and with a molecular size of 62,728 (Fig. 2). A protease-encoding gene that had previously cloned from another A. hydrophila strain, SO2/2 (41), was found to have an identical nucleotide sequence (data not shown). The predicted amino acid sequence of A. hydrophila AhpB showed homology with several metalloproteases from Vibrio spp. (9, 11, 16, 33), Helicobacter pylori hemagglutinin/proteinase fragment (46), Vibrio cholerae hemagglutinin/proteinase precursor (16), and P. aeruginosa elastase precursor (LasB) (14) (Fig. 3). Analysis of the A. hydrophila AhpB amino acid sequence using the PROSITE computer program (Swiss Institute of Bioinformatics) revealed a zinc-binding region at positions 318-VAAHEVSHGF-327. This result, together with effects of inhibitors (41), suggested that AhpB is a zinc metalloprotease.
FIG. 2.
Nucleotide sequence of the ahpB gene and amino acid sequence deduced from its open reading frame. DNA bases (top line) and amino acids (one-letter code) are numbered at the right. The ATG initiation codon (boldface) is preceded by a potential Shine-Dalgarno (boldface and underlined). Initiation of prepro-AhpB, pro-AhpB, and mature AhpB proteins is underlined by an arrow. The symbol—indicates the TGA termination codon; underlined boldfaced amino acid positions 184 to 196 correspond to the amino-terminal sequence determined for both the purified AhpB mature protease and the purified 43.4-kDa intermediate; double-underlined and boldfaced amino acid positions 318 to 327 correspond to a zinc-binding region signature.
FIG. 3.
Amino acids sequence alignment of the AhpB protease of A. hydrophila AG2 (AHPB), the hemagglutinin/proteinase precursor of V. cholerae (HAPT), the elastase, a zinc-metalloprotease of P. aeruginosa (LASB), and the hemagglutinin/proteinase fragment of H. pylori (HAP). Amino acids highlighted in black boxes are identical in three out of four proteins. Shaded boxes correspond to residues specifically conserved with AhpB protease of A. hydrophila AG2.
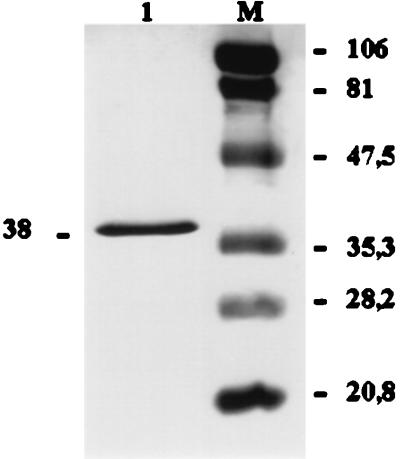
Nucleotide sequence analysis revealed a preproenzyme domain structure for the ahpB gene product. Although the mature secreted elastase, AhpB, is about 38,000 Da by SDS-PAGE, the predicted ahpB gene product is much larger (62,728 Da). The sequence immediately downstream from the initiator methionine is a typical signal peptide of 19 amino acids including several charged residues near the amino terminus and a potential signal peptidase cleavage site 17-A-X-A-19 (cleavage after the second A) (39). The region between the signal peptide and the mature protease sequences is a long propeptide of 164 amino acids (17,342 Da), as indicated by the fact that the sequence determined for the first 13 amino acids of the mature protease was 184-KDATGPGGNVKTG. However, the apparent molecular mass of mature protease (about 38,000 Da by SDS-PAGE [Fig. 4]) did not correspond with that deduced from the amino acids sequence (43,473 Da). These results would suggest that the 43.4 kDa is an intermediate that is further processed to the mature 38-kDa protease.
FIG. 4.
SDS-PAGE of purified AhpB protease from culture supernatant of A. hydrophila AG2 (lane 1), and molecular weight markers (lane 2); from top to bottom: phosphorylase b, bovine serum albumin, ovalbumin, carbonic anhydrase, soybean trypsin inhibitor, and lysozyme). Numbers at left and right are molecular sizes in kilodaltons.
Secretion and processing of AhpB.
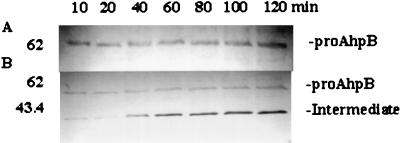
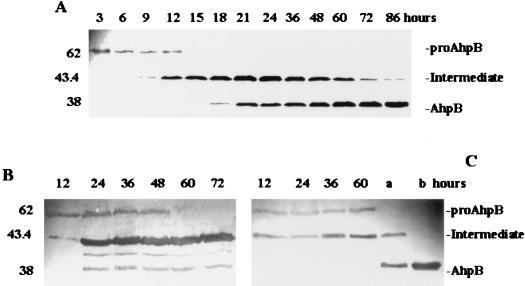
To understand the mechanisms underlying processing and secretion of AhpB protease, as well as its cellular location, we defined conditions that allowed for identification of short-lived secreted protein species. These protein species were analyzed by suspending late-exponential A. hydrophila AG2 cells in fresh LB medium. Samples were removed every 10 or 20 min up to 120 min, and TCA was immediately added to both cells and culture supernatants to prevent proteolysis. Immunoblots with antibodies to AhpB protease revealed that cells contained only proAhpB (Fig. 5A). However, two protein species were detected in the culture supernatant. One of them, a 62-kDa protein that was present in almost constant amount in each sample analyzed, presumably corresponds to proAhpB. The other species was a 43.4-kDa intermediate that appeared in increasing amounts from 10 min up to 120 min (Fig. 5B). The mature 38-kDa AhpB protein species did not appear at all throughout the time course of the experiment, suggesting that may be requires another protease in the culture supernatant.
FIG. 5.
Secretion and processing of AhpB protease in A. hydrophila AG2. SDS-PAGE and immunoblotting with antibodies to AhpB protease were performed as detailed in the text. (A) Whole-cell extracts; (B) cell culture supernatants. Samples were removed at 10- or 20-min intervals. Numbers at the left are molecular sizes in kilodaltons.
Since little information was obtained from a short-lived secreted protein species, a long-lived secreted protein species was analyzed by inoculating fresh LB medium with an overnight culture of A. hydrophila AG2, and samples, removed from 3 h up to 72 h, were processed as before (Fig. 6). Immunoblots with antibodies to AhpB protease revealed that cells contained only pro-AhpB as in the previous experiment (data not shown). No other AhpB-related proteins with smaller molecular weight were detected, suggesting that no processing of pro-AhpB occurred within the cells. When culture supernatants were analyzed by immunoblots with the same antibodies (Fig. 6A), three AhpB-related proteins were detected. A 62-kDa protein, which should be pro-AhpB, was observed up to a 12-h period of incubation (the amount of extracellular proAhpB protein was fairly constant from 3- to 12-h period, and then presumably it is processed). The level of a 43.4-kDa processing intermediate increased up to 24-h and then decreased from 24 h onward. The 43.4-kDa intermediate was purified, and the N-terminal amino acid sequence was determined and found to be identical to that of the mature AhpB protein. The third protein species detected was the mature 38-kDa AhpB form, appearing in increasing amounts from 18 h onward. These results suggested that the 43.4-kDa intermediate is further processed to the mature AhpB protein by cleaving a C-terminal propeptide of about 6 kDa and generating the mature form of 38-kDa AhpB protease. Collectively, these results indicate that pro-AhpB is exported in its unprocessed form and both the N- and C-terminal propeptides are removed extracellularly by the action of some other protease(s) or by AhpB protease itself.
FIG. 6.
SDS-PAGE and immunoblotting with antibodies to AhpB protease from cell culture supernatants of A. hydrophila AG2 (A), A. hydrophila ahpB mutant (B), and A. salmonicida masoucida containing plasmid pAHE6 (C) in a long-lived experiment. Lanes are culture supernatant samples taken at different hours. Lanes a and b are filtered culture supernatants after incubation 48 and 72 h, respectively, at 37°C. Numbers at the left are molecular masses in kilodaltons.
It is known that A. hydrophila secretes to the culture supernatant a serine protease which was previously characterized (42). To determine whether this serine protease (named AhpA; previously called P2) played a role in pro-AhpB processing, we constructed an A. hydrophila AhpA isogenic mutant by insertional inactivation of the ahpA gene with a kanamycin resistance (Kanr) cassette (7). A. hydrophila ahpA mutant cells were grown at 28°C on LB medium, samples were removed from 12 h onward, and TCA was added to the supernatants to prevent proteolysis. Immunoblots with antibodies to AhpB indicated that the 43.4-kDa intermediate accumulated in the culture supernatant of the mutant compared with that of the wild type (Fig. 6B). Also, the amount of 43.4-kDa intermediate in the culture of the mutant strain that was processed to mature AhpB protease was lower than that in the culture of the wild-type strain. The pro-AhpB protein was maintained in the culture supernatant for a longer period of time (up to 48 h). We also observed a second intermediate protein species of approximately 41 kDa, probably generated as a consequence of the lack of AhpA serine protease. Elastolytic activity of the AhpA mutant was similar to the wild-type level (Table 2). However, the caseinolytic activity was considerably less than the wild-type level. These results indicate that the AhpA serine protease is partially involved in processing the 43.4-kDa intermediate to the mature AhpB protein species and that this intermediate possesses elastolytic activity. AhpA serine protease may also speed up the processing of proAhpB to the 43.4-kDa intermediate but apparently is not necessary for this step. Nevertheless, minimal amounts of the mature AhpB protease were detected in the culture supernatant of AG2 mutant strain (from 24 h onward), indicating that other secreted proteases, which have not yet been characterized in A. hydrophila AG2, may be involved in processing proAhpB protein. Alternatively, proAhpB may be processed to the mature AhpB protease by itself. To investigate this latter possibility, we expressed the ahpB gene in the nonproteolytic A. salmonicida subsp. masoucida (see below). Samples of A. salmonicida subsp. masoucida(pAHE6) culture supernatants were obtained from 12 h onward. Immunoblots with antibodies to AhpB protease demonstrated proAhpB processing to the 43.4-kDa intermediate (Fig. 6C). No mature AhpB protease was detected after 60 h of incubation. However, all of the 43.4-kDa intermediate was processed to the mature 38-kDa AhpB protease after incubation of the filtered culture supernatant at 37°C for 48 h (Fig. 6C, lanes a and b). These results suggested that complete processing of the A. hydrophila AG2 pro-AhpB protease is a slow process carried out by itself and probably speeded up by AhpA serine protease.
TABLE 2.
Proteolytic activities of Aeromonas strains
| Strains | % Caseinolytic activity | % Elastolytic activity |
|---|---|---|
| A. hydrophila AG2 | 100 | 100 |
| AG2 ahpA mutant | 15 | 80 |
| A. salmonicida subsp. masoucida | 80 | 10 |
| AG2 ahpB mutant | 0 | 0 |
| A. salmonicida subsp. masoucida ahpA | 50 | 17 |
| A. salmonicida subsp. masoucida ahpB | 16 | 90 |
Contribution of AhpB protease to elastolytic activity.
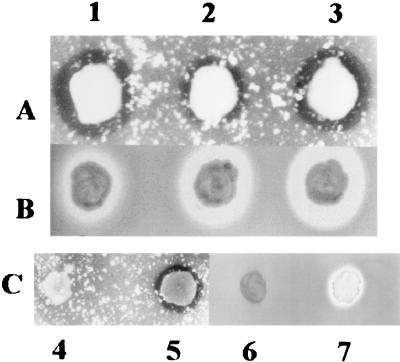
To demonstrate the precise proteolytic activity of the ahpB gene product (AhpB), the gene was expressed in the nonproteolytic A. salmonicida subsp. masoucida. The 2.5-kbp SalI-XhoI fragment containing the ahpB gene from plasmid pAHE5 was cloned in the broad-host-range pJRD215 plasmid at the unique SalI dephosphorylated endonuclease site, obtaining plasmid pAHE6 (Fig. 1), which was used to transform E. coli S17-1. Plasmid pAHE6 was transferred from the E. coli S17-1 donor strain to the nonproteolytic A. salmonicida subsp. masoucida recipient strain. Transconjugants were selected on LB agar plates supplemented with ampicillin and kanamycin. Kanr and Apr colonies were transferred to LB agar plates supplemented with kanamycin and skim milk or insoluble elastin. A clear zone around A. salmonicida subsp. masoucida patches containing pAHE6 (Fig. 7C, lanes 5 and 7) denoted secretion of both caseinolytic and elastolytic activities. Proteolytic activity was also determined in culture supernatants of A. hydrophila AG2, AG2 ahpA mutant, the nonproteolytic A. salmonicida subsp. masoucida, and A. salmonicida subsp. masoucida containing plasmid pAHE6 (Table 2). A. hydrophila culture supernatant contained high levels of both elastolytic and caseinolytic activities as expected; however, A. salmonicida ssp. masoucida containing plasmid pAHE6, which efficiently expresses AhpB protease, exhibited a high elastolytic activity, very similar to that produced by the wild type, but low caseinolytic activity. These results demonstrate that AhpB protease from A. hydrophila AG2 contributes mainly to the elastolytic activity.
FIG. 7.
Proteolytic activity detected on solid media. Macrocolonies of A. hydrophila strains and A. salmonicida masoucida were grown on LB medium supplemented with elastin (A, C4, and C5) or casein (B, C6, and C7) and incubated for 48 h at 28°C. 1, A. hydrophila ahpA mutant; 2, A. hydrophila ahpB mutant; 3, A. hydrophila AG2 wild type; 4 and 6, A. salmonicida masoucida containing plasmid pJRD215; 5 and 7, A. salmonicida masoucida containing plasmid pAHE6. The medium for the later was also supplemented with kanamycin.
Another way to demonstrate the precise activity and role of AhpB protease in A. hydrophila AG2 virulence was by constructing an isogenic mutant. The wild-type ahpB gene was replaced on the A. hydrophila AG2 chromosome with an allele containing a Kanr marker (Fig. 1). The mobilizable suicide vector pSUP202-1, a pSUP202 derivative (45) in which ampicillin resistance was eliminated by blunt ending and ligating at the only PstI site, was used. To determine successful gene replacement, PCR amplification was carried out on a 800-bp fragment of ahpB from both the wild-type strain (AG2) and the AG2 ahpB mutant. The size of the PCR-amplified product from the mutant was 2.1 kbp, corresponding to the amplification of ahpB plus the Kanr marker (1.3 kbp). The PCR-amplified product from the wild type was 800 bp as expected (data not shown). The A. hydrophila ahpB mutant growth on LB agar supplemented with insoluble elastin had notably less clearing around the patch than the wild type (Fig. 7A, lanes 2 and 1, respectively); however, clearing around the patch of the A. hydrophila ahpB mutant grown on LB agar supplemented with casein was very similar to that for the wild type (Fig. 7B, lanes 2 and 1, respectively). Caseinolytic and elastolytic activities were determined in the 48-h culture supernatants of both the wild-type and ahpB mutant strains (Table 2). Mutant caseinolytic activity was 20% less than in the wild-type strain, but mutant elastolytic activity was 90% lower than in the wild-type strain. Complementation studies were carried out by conjugal transference of plasmid pAHE6 from E. coli S17-1 to A. hydrophila ahpB. The transconjugants had the same proteolytic activity levels as wild-type A. hydrophila. Again, these results suggest that AhpB is chiefly involved in elastolytic activity, while the caseinolytic activity should be attributed mainly to another protease, presumably the temperature-labile serine AhpA protease which we characterized earlier (42).
Inoculation into rainbow trout.
To ascertain the role of AhpB protease in the pathogenesis of A. hydrophila AG2, the LD50 was determined for A. hydrophila AG2 and AG2 ahpB mutant by intraperitoneal challenge of rainbow trout (Table 3). In this model system, the ahpB gene product was clearly a virulence factor. LD50 for the wild-type AG2 strain was 6 × 105 CFU, while the LD50 for the ahpB mutant was 3 × 107 CFU, about 102 times higher (Table 3). Fish injected with the parental strain died more rapidly than those injected with the isogenic ahpB mutant. All recorded deaths occurred within 3 days when the fish were injected with the wild type; however, deaths were recorded up to 6 days following injection when the fish were injected with ahpB mutant.
TABLE 3.
Calculatons of LD50 strain AG2 and the aphB mutant
| Bacteria/0.1 m.l | No. of fish that died
|
|||
|---|---|---|---|---|
| AG2
|
aphB mutant
|
|||
| Exp 1 | Exp 2 | Exp 2 | Exp 1 | |
| 109 | 10 | 10 | 9 | 10 |
| 108 | 9 | 10 | 6 | 5 |
| 107 | 8 | 8 | 3 | 3 |
| 106 | 6 | 6 | 2 | 2 |
| 105 | 3 | 3 | 1 | 1 |
| 104 | 0 | 0 | 0 | 0 |
| 103 | 0 | 0 | 0 | 0 |
| LD50 | 7 × 105 | 6 × 105 | 2.9 × 107 | 3.3 × 107 |
Examination of mortality showed typical clinical signs of hemorrhagic septicemia, mainly external lesions (abdominal distension and skin ulceration at the injection site) and internal hemorrhages as previously observed (17). No discernible difference in disease pathology caused by the wild-type and ahpB mutant strains was observed. To confirm stability of the insertional inactivated ahpB mutant gene, bacteria were isolated from dead fish inoculated with the AG2 ahpB mutant, all conferring a Kanr phenotype. PCR amplification of ahpB mutant with specific primers for the ahpB gene resulted in a 2.1-kbp fragment, confirming the stability of the mutated gene.
DISCUSSION
Molecular cloning and sequencing of the metalloprotease gene, ahpB, revealed an open reading frame of 1,767 nucleotides with the capacity to encode a polypeptide of 588 amino acids with a molecular weight of 62,728. However, the mature encoded protease, AhpB, is only 38 kDa by SDS-PAGE, suggesting that the protease is synthesized as a preproprotein composed of four domains: a 19-amino-acid signal peptide, a 164-amino-acid N-terminal propeptide, a mature protein which is smaller than 43.4 kDa (184K-588Y), with a molecular mass of 38 kDa and a C-terminal propeptide of about 6 kDa. Most proteases from prokaryotes and eukaryotes are synthesized as inactive precursors which have various lengths and locations in the precursor proteins. Precursor activation often requires proteolytic cleavage of a propeptide covalently attached to the amino and/or carboxyl termini of the mature protease sequence (26, 48). In our case, based on the small amount of pro-AhpB detected in the culture supernatants, the enzyme should be immediately autoprocessed to the 43.4-kDa intermediate, which is further processed to the mature AhpB protease by the AhpA serine protease. However, processing of the 43.4-kDa intermediate can be carried out by itself in the absence of AhpA serine protease, although very slowly.
A. hydrophila AG2 AhpB protease had both caseinolytic and elastolytic activities; however, the chief activity of AhpB is on elastin. When the ahpB gene was insertionally inactivated, 90% of elastolytic activity was lost (Table 2). Most A. hydrophila strains secrete two proteases into the culture medium, a thermostable metalloprotease (this work and reference 41) and the temperature-labile serine protease AhpA encoded by ahpA (7, 42). When the ahpA gene was insertionally inactivated in the same way as the mutant ahpB, most of the elastolytic activity was retained, with the caseinolytic activity being chiefly diminished (Table 3).
The pathogenicity of A. hydrophila (and related aeromonads) has been attributed to several characterized extracellular enzymes including hemolysins, enterotoxins, and proteases (20, 22, 23). However, the precise role as virulence factors have not been established. It has been suggested that proteolytic enzymes excreted by Aeromonas spp. play an important role in invasiveness and establishment of infection by overcoming initial host defenses and by providing nutrients for cell proliferation (19, 30). However, isogenic deletion mutants for GCAT (glycerophospholipid:cholesterol acyltransferase) and AspA (serine protease) demonstrated that these two major secreted toxins of A. salmonicida are not essential for virulence (49). Our study is the first to demonstrate that a secreted protease (AhpB) from A. hydrophila, with a high elastolytic activity, should be considered as a virulence factor. The LD50 of the A. hydrophila ahpB mutant is about 100 times higher than that of the wild type.
ACKNOWLEDGMENTS
This work was supported by DGICYT grants PB94-0136 and AGF98-0186 from the Spanish Ministerio de Educación y Cultura.
REFERENCES
- 1.Altwegg M, Geiss H K. Aeromonas as a human pathogen. Crit Rev Microbiol. 1989;16:253–286. doi: 10.3109/10408418909105478. [DOI] [PubMed] [Google Scholar]
- 2.Austin B, Austin D A. Bacterial fish pathogens. 2nd ed. Chichester, United Kingdom: Ellis Horwood; 1993. [Google Scholar]
- 3.Birnboim H C, Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979;7:1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bjorn M J, Sokol P A, Iglewski B H. Influence of iron on yields of extracellular products in Pseudomonas aeruginosa cultures. J Bacteriol. 1979;138:193–200. doi: 10.1128/jb.138.1.193-200.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 6.Buckley J T, Halasaand L N, McIntyre S. Purification and partial characterization of a bacterial phospholipid: cholesterol acyltransferase. J Biol Chem. 1982;255:3320–3325. [PubMed] [Google Scholar]
- 7.Cascón A, Fregeneda J, Aller M, Yugueros J, Temprano A, Hernanz C, Sánchez M, Rodríguez-Aparicio L, Naharro G. Cloning, characterization, and insertional inactivation of a major extracellular serine protease gene with elastolytic activity from Aeromonas hydrophila. J Fish Dis. 2000;23:1–11. [Google Scholar]
- 8.Chakrabarty T, Huhle B, Hof H, Bergbauer H, Goebel W. Marker exchange mutagenesis of the aerolysin determinant in Aeromonas hydrophila demonstrates the role of aerolysin in A. hydrophila-associated systemic infections. Infect Immun. 1987;55:2274–2280. doi: 10.1128/iai.55.9.2274-2280.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng J C, Shao C P, Hor L I. Cloning and nucleotide sequencing of the protease gene of Vibrio vulnificus. Gene. 1996;183:255–257. doi: 10.1016/s0378-1119(96)00488-x. [DOI] [PubMed] [Google Scholar]
- 10.Coleman G, Whitby P W. A comparison of the amino acid sequence of the serine protease of the fish pathogen Aeromonas salmonicida subsp. salmonicida with those of subtilisin-type enzymes relative to their substrate-binding sites. J Gen Microbiol. 1993;139:245–249. doi: 10.1099/00221287-139-2-245. [DOI] [PubMed] [Google Scholar]
- 11.David V, David V A, Deutch A H, Sloma A, Pawlyk D, Ally A, Durham D R. Cloning, sequencing and expression of the gene encoding the extracellular neutral protease, vibriolysin, of Vibrio proteolyticus. Gene. 1992;112:107–112. doi: 10.1016/0378-1119(92)90310-l. [DOI] [PubMed] [Google Scholar]
- 12.Del Corral F, Shotts E B, Jr, Brown J. Adherence, haemagglutination, and cell surface characteristics of motile aeromonads virulent for fish. J Fish Dis. 1990;13:255–268. [Google Scholar]
- 13.Ellis A E. The extracellular toxins of Aeromonas salmonicida ssp. salmonicida. In: Bernoth E-M, Ellis A E, Midtlyng P J, Olivier G, Smith P, editors. Furunculosis. Multidisciplinary fish disease research. London, United Kingdom: Academic Press Ltd.; 1997. pp. 248–268. [Google Scholar]
- 14.Fukushima J, Yamamoto S, Morihara K, Atsumi Y, Takeuchi H, Kawamoto S, Okuda K. Structural gene and complete amino acid sequence of Pseudomonas aeruginosa IFO 3455 elastase. J Bacteriol. 1989;171:1698–1704. doi: 10.1128/jb.171.3.1698-1704.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Handfield M, Simard P, Couillard M, Letarte R. Aeromonas hydrophila isolated from food and drinking water: hemagglutination, hemolysis, and cytotoxicity for a human intestinal cell line (HT-29) Appl Environ Microbiol. 1996;62:3459–3461. doi: 10.1128/aem.62.9.3459-3461.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hase C C, Finkelstein R A. Cloning and nucleotide sequence of the Vibrio cholerae hemagglutinin/protease (HA/protease) gene and construction of an HA/protease-negative strain. J Bacteriol. 1991;173:3311–3317. doi: 10.1128/jb.173.11.3311-3317.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hernanz C, Flaño E, López P, Villena A, Anguita J, Cascón A, Sánchez M, Razquín B, Naharro G. Molecular characterization of the Aeromonas hydrophila aroA gene and potential use of an auxotrophic aroA mutant as a live attenuated vaccine. Infect Immun. 1998;66:1813–1821. doi: 10.1128/iai.66.5.1813-1821.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holmberg S K, Schell W L, Ganning G R. Aeromonas intestinal infections in the United States. Ann Intern Med. 1986;150:683–689. doi: 10.7326/0003-4819-105-5-683. [DOI] [PubMed] [Google Scholar]
- 19.Hsu T C, Waltman W D, Shots E B. Correlation of extracellular enzymatic activity and biochemical characteristics with regard to virulence of Aeromonas hydrophila. Dev Biol Stand. 1981;49:101–111. [Google Scholar]
- 20.Janda J M. Biochemical and exoenzymatic properties of Aeromonas species. Diagn Microbiol Infect Dis. 1985;3:223–232. doi: 10.1016/0732-8893(85)90034-3. [DOI] [PubMed] [Google Scholar]
- 21.Janda J M, Duffey P S. Mesophilic aeromonads in human disease: current taxonomy, laboratory identification, and infectious disease spectrum. Rev Infect Dis. 1988;10:980–997. doi: 10.1093/clinids/10.5.980. [DOI] [PubMed] [Google Scholar]
- 22.Joanne M R, Houston C W, Kurosky A. Bioactivity and immunological characterization of a cholera toxin-cross-reactive cytolytic enterotoxin from Aeromonas hydrophila. Infect Immun. 1989;57:1170–1179. doi: 10.1128/iai.57.4.1170-1176.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Joanne M R, Houston C W, Coppenhaver D H, Dixon J D, Kurosky A. Purification and chemical characterization of a cholera toxin-cross-reactive cytolytic enterotoxin produced by a human isolate of Aeromonas hydrophila. Infect Immun. 1989;57:1165–1169. doi: 10.1128/iai.57.4.1165-1169.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kessler E, Ohman D E. Pseudolysin. In: Barret A J, Rawling N D, Woessner F Jr, editors. Handbook of proteolytic enzymes. London, United Kingdom: Academic Press; 1998. p. 357. [Google Scholar]
- 25.Kessler E, Safrin M. Synthesis, processing, and transport of Pseudomonas aeruginosa elastase. J Bacteriol. 1988;170:5241–5247. doi: 10.1128/jb.170.11.5241-5247.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kessler E, Safrin M, Gustin J K, Ohman D E. Elastase and the LasA protease of Pseudomonas aeruginosa are secreted with their propeptides. J Biol Chem. 1998;273:30225–30231. doi: 10.1074/jbc.273.46.30225. [DOI] [PubMed] [Google Scholar]
- 27.Kessler E, Safrin M, Peretz M, Burstein Y. Identification of cleavage sites involved in proteolytic processing of Pseudomonas aeruginosa pre-proelastase. FEBS Lett. 1992;299:291–293. doi: 10.1016/0014-5793(92)80134-3. [DOI] [PubMed] [Google Scholar]
- 28.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 29.Leung K Y, Stevenson R M. Characteristics and distribution of extracellular proteases from Aeromonas hydrophila. J Gen Microbiol. 1988;134:151–160. [Google Scholar]
- 30.Leung K Y, Stevenson R M W. Tn5-induced protease-deficient strains of Aeromonas hydrophila with reduced virulence for fish. Infect Immun. 1988;56:2639–2644. doi: 10.1128/iai.56.10.2639-2644.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Loewy A G, Santer U V, Wieczorek M, Blodgett J K, Jones S W, Cheronis J C. Purification and characterization of a novel zinc-proteinase of Aeromonas hydrophila. J Biol Chem. 1993;268:9071–9078. [PubMed] [Google Scholar]
- 32.McIver K S, Kessler E, Ohman D E. Substitution of active site His-223 in Pseudomonas aeruginosa elastase and expression of the mutated lasB alleles in Escherichia coli show evidence for autoproteolytic processing of proelastase. J Bacteriol. 1991;173:7781–7789. doi: 10.1128/jb.173.24.7781-7789.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Milton D L, Norqvist A, Wolf-Watz H. Cloning of a metalloprotease gene involved in the virulence mechanism of Vibrio anguillarum. J Bacteriol. 1992;174:7235–7244. doi: 10.1128/jb.174.22.7235-7244.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morihara K. Pseudolysin and other pathogen endopeptidases of thermolysin family. Methods Enzymol. 1998;248:242–253. doi: 10.1016/0076-6879(95)48017-x. [DOI] [PubMed] [Google Scholar]
- 35.Nieto T P, Santos Y, Rodríguez L A, Ellis A E. An extracellular acetylcholinesterase produced by Aeromonas hydrophila is a major lethal toxin for fish. Microb Pathog. 1991;11:101–110. doi: 10.1016/0882-4010(91)90003-s. [DOI] [PubMed] [Google Scholar]
- 36.Oakley B R, Kirsch D R, Morris N R. A simplified ultrasensitive silver stain for detecting proteins in polyacrylamide gels. Anal Biochem. 1980;105:361–363. doi: 10.1016/0003-2697(80)90470-4. [DOI] [PubMed] [Google Scholar]
- 37.Paniagua C, Rivero O, Anguita J, Naharro G. Pathogenicity factors and virulence for rainbow trout (Salmo gairdneri) of motile Aeromonas spp. isolated form a river. J Clin Microbiol. 1990;28:350–355. doi: 10.1128/jcm.28.2.350-355.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Priefer U, Simonand R, Puhler A. Cloning with cosmids. In: Puhler A, Timmis K N, editors. Advanced molecular genetics. Berlin, Germany: Springer-Verlag KG; 1984. pp. 190–201. [Google Scholar]
- 39.Pugsley A P. The complete general secretory pathway in gram-negative bacteria. Microbiol Rev. 1993;57:50–108. doi: 10.1128/mr.57.1.50-108.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reed L J, Muench H. A simple method of estimating fifty percent end points. Am J Hyg. 1938;27:493–497. [Google Scholar]
- 41.Rivero O, Anguita J, Paniagua C, Naharro G. Molecular cloning and characterization of an extracellular protease gene from Aeromonas hydrophila. J Bacteriol. 1990;172:3905–3908. doi: 10.1128/jb.172.7.3905-3908.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rivero O, Anguita J, Mateos D, Paniagua C, Naharro G. Cloning and characterization of an extracellular temperature-labile serine protease gene from Aeromonas hydrophila. FEMS Microbiol Lett. 1991;81:1–8. doi: 10.1016/0378-1097(91)90461-i. [DOI] [PubMed] [Google Scholar]
- 43.Rodríguez L A, Ellis A E, Nieto T P. Purification and characterization of an extracellular metalloprotease, serine protease and haemolysin of Aeromonas hydrophila strain B32: all are lethal for fish. Microb Pathog. 1992;13:17–24. doi: 10.1016/0882-4010(92)90028-m. [DOI] [PubMed] [Google Scholar]
- 44.Sambrook J, Fritsch E F, Maniatis T. Molecular Cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 45.Simon R, Priefer U, Pühler A. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in gram negative bacteria. Bio/Technology. 1983;1:784–791. [Google Scholar]
- 46.Smith A W, Chahal B, French G L. The human gastric pathogen Helicobacter pylori has a gene encoding an enzyme first classified as a mucinase in Vibrio cholerae. Mol Microbiol. 1994;13:153–160. doi: 10.1111/j.1365-2958.1994.tb00410.x. [DOI] [PubMed] [Google Scholar]
- 47.Taylor R K, Manoil C, Mekalanos J J. Broad-host-range vectors for delivery of TnphoA: use in genetic analysis of secreted virulence determinants of Vibrio cholerae. J Bacteriol. 1989;171:1870–1878. doi: 10.1128/jb.171.4.1870-1878.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Teufel P, Götz F. Characterization of an extracellular metalloprotease with elastase activity from Staphylococcus epidermidis. J Bacteriol. 1993;175:4218–4224. doi: 10.1128/jb.175.13.4218-4224.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vipond R, Bricknell I R, Durant E, Bowden T J, Ellis A E, Smith M, McIntyre S. Defined deletion mutants demonstrate that the major secreted toxins are not essential for the virulence of Aeromonas salmonicida. Infect Immun. 1998;66:1990–1998. doi: 10.1128/iai.66.5.1990-1998.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yanisch-Perron C, Vieira J, Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]