Abstract
Background
Neurodevelopmental delay is a significant long-term complication of childhood tuberculous meningitis (TBM). The objective of this study was to assess risk factors for neurodevelopmental delay in children with TBM.
Methods
We conducted a retrospective cohort study of children diagnosed with TBM at Tygerberg Hospital, Cape Town, South Africa, over a 30-year period between 1985 and 2015. We assessed the relationship between demographic, clinical, laboratory and neuro-imaging characteristics, and cognitive impairment at the conclusion of anti-tuberculous treatment. Poor outcome was defined as moderate-to severe cognitive impairment.
Results
A total of 327 TBM patients were included, 71 (21.7%) suffered a poor outcome. Multivariate analysis revealed that decreased level of consciousness (adjusted OR (aOR): 4.68; 95%CI: 2.43–13.88; p = 0.005), brainstem dysfunction (aOR: 3.20; 95%CI: 1.70–6.00; p < 0.001), and radiological infarction (aOR: 3.47; 95%CI: 1.87–6.45; p < 0.001) were associated with a poor developmental outcome. Left hemispherical (single and multiple) stroke and bilateral stroke were associated with poor developmental outcomes.
Conclusion
Certain neurological signs as well as radiological infarct characteristics are important predictors of poor developmental outcome. Anticipation of the likely level of cognitive impairment at diagnosis allows more accurate prognostication and prompt institution of supportive and rehabilitative measures, after the acute illness.
Keywords: Tuberculosis, Central nervous system, Risk factors, Neurodevelopmental delay
Introduction
Globally, an estimated 1.2 million children develop tuberculosis (TB) each year [1], of which the most devastating form of disease is tuberculous meningitis (TBM). TBM continues to be an important cause of neurological deficit for children in resource-constrained countries [2], placing a large strain on families and health systems. With the widespread rollout of infant vaccinations to pneumococcus, meningococcus, and haemophilus, Mycobacterium tuberculosis (Mtb) has emerged as one of the leading causes of bacterial meningitis in many high TB-burden settings [3].
The peak incidence of TBM in children is between 2 and 4 years of age [4–6] therefore, severe illness often coincides with a critical period in their neurological development, with motor, language, behavioural, and cognitive delay a common outcome [7, 8]. Most survivors of childhood TBM suffer from long-term complications of neurocognitive and functional impairment. Early diagnosis and treatment of TBM has long been recognised as the single most important factor determining outcome [9].
Poor neurodevelopmental outcome is associated with younger age, delayed presentation and treatment initiation, clinical severity, and hydrocephalus [7]. Schoeman and colleagues found that 80% of child TBM survivors had cognitive delay (median Intelligence Quotient (IQ): 71.5, range 36–102) while 43–53% of school-aged children showed poor scholastic progress, including grade repetition following TBM treatment [9]. Neurological outcome of childhood TBM remains poor despite advances in treatment, necessitating long-term follow-up studies to determine the need for future support and services [10]. TBM-associated progressive vasculitis increases the risk of ischaemic strokes due to progressive occlusion [11, 12], with the most commonly affected areas supplied by the terminal internal carotid artery, the proximal middle and anterior cerebral arteries, and the perforating branches of the proximal middle cerebral artery [13, 14]. Stroke is the leading cause of long-term disability in childhood TBM. The most widely used neuroimaging procedure in acute stroke is contrasted brain computed tomography (CT), which allows differentiation between haemorrhagic and ischaemic stroke, localization of the lesion, and assists in decision-making regarding allocation of intervention level. Studies have shown that the degree of motor impairment and the potential for motor recovery depends on the site of the lesion, the combination of lesions in cortical areas and fibre tracts, and the involvement of deep grey structures e.g. the basal ganglia, thalamus, and brainstem [15].
Even though it is well known that TBM causes significant neurocognitive and functional impairment in children and adults [16], there are limited global epidemiological data using well-validated assessment tools that document findings across different ages and populations. A major challenge is the limited standardised assessment measures that are developed for, or normed across, different geographical and cultural settings [17]. Although the Bayley and Griffiths Scales assess developmental quotient (DQ), and not IQ, in children less than 8 years of age, Schoeman and colleagues found excellent correlation between DQ performed at 6 months after onset of TBM and IQ obtained years later [9].
Neurological sequelae have lasting socio-economic implications for patients and their families. If the likely level of cognitive and functional impairment can be anticipated at diagnosis then supportive and rehabilitative measures can be instituted promptly, and optimized, after acute illness. We therefore sought to identify baseline clinical, laboratory, and neuro-imaging findings that predicted poor functional outcome in children with TBM.
Methods
Patient inclusion
We conducted a retrospective cohort study to assess risk factors of neurodevelopmental outcome in children with TBM at the conclusion of anti-tuberculous therapy, over a 30-year period between 1985 and 2015. The study was conducted at Tygerberg Hospital, a large tertiary academic centre which lies within the metropole of Cape Town, serving a population of over 2.6 million people from a geographical drainage area with a high TB burden.
All children with TBM were treated according to local standard of care with a short, intensive four-drug regimen consisting of daily isoniazid 20 mg/kg, rifampicin 20 mg/kg, pyrazinamide 40 mg/kg, and ethionamide 20 mg/kg for 6 months. HIV-associated TBM cases are treated for 9 months. Prednisone 2 mg/kg/day was given for the first month of treatment. Institutional practice is to perform lumbar puncture on all children with suspected TBM, even those with signs of raised intracranial pressure (ICP). Air encephalography is then performed to determine whether the hydrocephalus is of communicating or non-communicating nature. TBM cases with non-communicating hydrocephalus are treated by ventriculoperitoneal (VP) shunting or third ventriculostomy, while communicating hydrocephalus is managed medically with diuretics (50 mg/kg/day of acetazolamide and 1 mg/kg/ day of furosemide). Children presenting with signs of impending herniation are referred for emergency VP shunting (lumbar puncture contra-indicated).
Participants were selected based on the following eligibility criteria: male and female subjects with an age range of 3 months to 13 years; subjects with definite and probable TBM according to the uniform case definition of TBM [18], irrespective of HIV status; and with a completed neurodevelopmental assessment performed at conclusion of anti-tuberculous treatment.
Outcome definitions
Developmental quotient (DQ) or intelligence quotient (IQ) were categorized as normal ≥ 85, mild/borderline impairment 50–84, moderate impairment 35–49, and severe/profound impairment < 35. DQ is a measure of an infant or young child’s development across a range of competencies, including personal social development, attention, expressive and receptive language, visuo-perceptual skills, motor skills, initiative, independence, cognitive development, problem solving, and memory. IQ is typically measured in older children and represents a deviation score based on statistical comparison of an individual’s performance on set tasks under highly controlled test conditions with age-specific normative data [19]. For statistical analysis, we defined a normal or mild/borderline DQ/IQ as a “good” outcome whilst a moderate or severe DQ/IQ was considered a “poor” outcome.
We used the Bayley scales, Griffiths Mental Development Scales, and Junior South African Individual Scales (JSAIS) as tools to assess the outcome, depending on the age of the child. The Bayley scales of Infant and Toddler Development is a standardised tool that was used to assess developmental functioning in young children between the ages of 1 month and 3 years of age. This tool evaluates cognitive development, expressive and receptive language, and gross and fine motor development [20]. The first and second editions of the Bayley Scales were used to assess young children during the study period. The Griffiths Mental Development Scales (Griffiths) measure the rate of infant development and can be used for children from 1 month to 8 years of age. It consists of six sub-scales: locomotor, personal-social, language, eye hand co-ordination, performance, and practical reasoning [21]. Practical reasoning is only assessed in children older than 2 years of age. The Huntley version of the Griffiths scale was used in children under two years of age, and the Griffiths Mental Development Scales, including the Extended Revised Version, was used in children from two to eight years of age. The JSAIS tool assesses cognitive ability in older children. The full battery comprises of 22 tests but only 12 are used to assess the cognitive profile. These tests are grouped in verbal, performance, numerical and memory scales [22]. Each child, therefore, underwent age-appropriate assessments in order to calculate their IQ (using the JSAIS in those > 8 years) and DQ (using the Bayley or Huntley version of the Griffith scales in those < 2 years, and the Extended Revised version of the Griffith scales in those between 2 and 8 years) at the end of therapy. For this analysis; therefore, we used either IQ or DQ depending on age, and subsequently refer to this only as IQ.
Data collection
Demographic variables included age, sex, HIV status, and evidence of bacillus Calmette-Guérin immunization. Clinical variables included presenting symptoms, duration of symptoms before admission, TBM stage as per the revised British Medical Research Council (BMRC) criteria [23], focal neurological fallout, and nutritional status. Nutritional status was assessed based on serial weight measurements as per the Road to Health Chart (RTHC) or Road to Health Booklet (RTHB). Laboratory variables included characteristic cerebrospinal fluid (CSF) findings (based on white cell counts and protein and glucose concentrations) and Mtb isolated in CSF or from other samples. CT imaging variables included hydrocephalus, infarcts, and basal enhancement post contrast. Additional included variables were known TB exposure, tuberculin skin tests (positive as defined by guidelines of the World Health Organization: in high-risk children ≥ 5-mm induration and in all other children ≥ 10 mm), and chest radiography compatible with pulmonary TB.
Definitions
“Definite TBM” was defined as children with meningitis and acid-fast bacilli seen on CSF microscopy, positive CSF Mtb culture and/or detection by commercial nucleic acid amplification test (NAAT). “Probable” TBM was defined using a scoring system based on clinical presentation, CSF findings, neuroimaging, and evidence of extra-neural TB [18].
The refined BMRC criteria classified TBM severity as follows: Stage 1: Glasgow coma scale (GCS) of 15, without focal neurological deficit; Stage 2a: GCS of 15 with focal neurological deficit; Stage 2b GCS of 11–14 with or without focal neurological deficit and Stage 3: GCS < 11 with or without focal neurological deficit [23, 24]. Stage 1 and 2a was grouped together as representative of milder stage TBM and stage 2b and 3 together as advanced stage TBM. Depressed level of consciousness (LOC) was defined as GCS < 15.
Radiological infarctions on CT were categorised as single unilateral, multiple unilateral or bilateral hemispheric involvement [15]. Additionally, the anatomical location of the infarcts were categorised i.e. caudate nuclei/internal capsule, thalamic nuclei, internal capsule, and cerebral artery territory (posterior, anterior, and middle cerebral artery). Brainstem infarction(s) was excluded due to inadequate visualization on CT.
Statistical analysis
Statistical analysis was performed using Stata (Stata Statistical Software: Release 15. College Station, TX: StataCorp LLC) and (SPSS version 26 (SPSS Inc., Chicago, IL, USA). Numerical variables were summarized using means and standard deviations, when normally distributed, and medians and interquartile ranges (IQRs) when not. The Mann–Whitney U test was used to compare 2 non-parametric variables. Univariate binary logistic regression analysis was used to assess the association between categorical variables and Kruskal–Wallis test for non-normally distributed variables. All independent variables that had a p-value of ≤ 0.1 in the univariate model were added to the multivariable binary logistic regression model. We report odds ratios (ORs) with 95% confidence intervals (CI) for all variables that remained statistically significant in the final model, after removing non-significant risk factors (p > 0.05) in a backward stepwise approach. Missing categorical data were coded as missing and included in the model. Missing quantitative data were excluded from the model. The level of significance was set at p < 0.05 (2-sided).
The study was approved by the Stellenbosch University Human Research Ethics Committee (reference number: S20/10/294).
Results
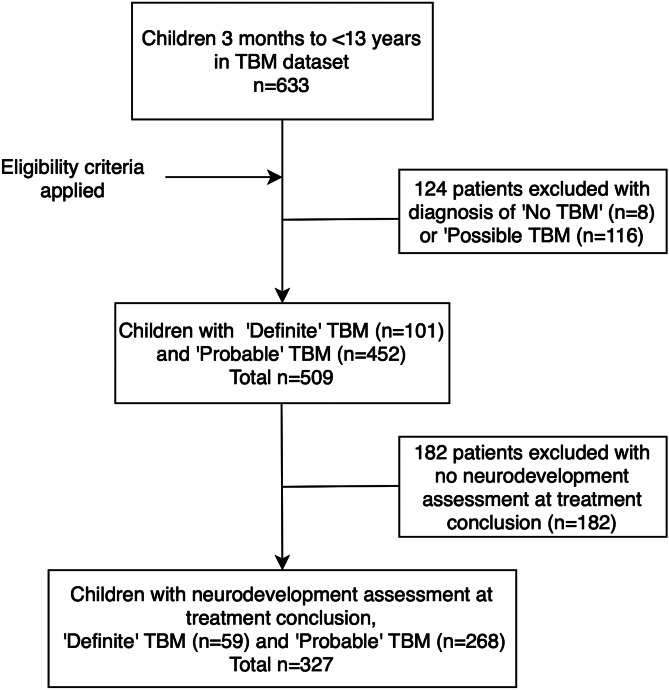
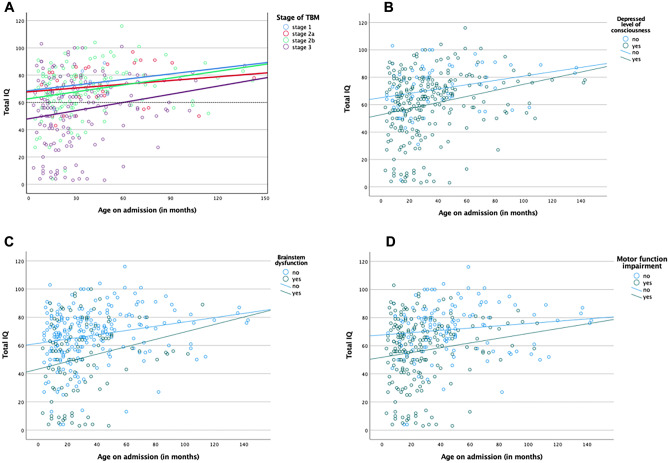
A total of 327 children were included in the study of which 59 had definite TBM and 268 probable TBM (Fig. 1). There was a slight male predominance 51.4% (n = 168) and the median age on admission was 28 months (IQR: 15–47 months). HIV prevalence was 4% (only 7 out of 177 children were tested for HIV). The median age on admission was lower for TBM survivors with moderate neurodevelopmental delay (22 months; IQR: 14–31 months) and severe neurodevelopmental delay (18 months; IQR 11–28 months) in comparison with mild neurodevelopmental delay (29.5 months; IQR: 15–48 months; Table 1). Scatterplots of total IQ/DQ versus age at admission for TBM stratified by TBM stage, depressed LOC, brainstem dysfunction, and motor function impairment illustrate a declining association in older children (Fig. 2A–D).
Fig. 1.
Participants flowchart
Table 1.
Background characteristics by intelligence/developmental quotient (IQ/DQ) level
|
Normal (n = 47) n (%) |
Mild/borderline (n = 209) n (%) |
Moderate (n = 29) n (%) |
Severe (n = 42) n (%) |
Total (n = 327) n (%) |
|
|---|---|---|---|---|---|
| Age on admission (months) | |||||
| Median (IQR) (n = 327) | 36 (22–59) | 29.5 (15–48) | 22 (14–31) | 18 (11–28) | 28 (15–47) |
| Sex | |||||
|
Female Male |
26 (55.3) 21 (44.7) |
101 (48.3) 108 (51.7) |
14 (48.3) 15 (51.7) |
18 (42.9) 24 (57.1) |
159 (48.6) 168 (51.4) |
| HIV status (n = 177 tested) | |||||
|
Negative Positive |
14 (93.3) 1 (6.7) |
110 (96.5) 4 (3.5) |
19 (90.5) 2 (9.5) |
27 (100.0) 0 |
170 (96.0) 7 (4.0) |
| Known TB contact | 24 (51) | 120 (57.4) | 14 (48.3) | 23 (54.8) | 181 (55.4) |
| BCG vaccination/scar | 32 (68.1) | 126 (60.3) | 18 (62.1) | 29 (69.1) | 205 (62.7) |
| Weight faltering/loss | 24 (51) | 77 (36.9) | 9 (31.0) | 23 (54.8) | 133 (40.7) |
| TBM stage grouping | |||||
|
1&2a (mild stage) 2b&3 (advanced stage) |
10 (21.3) 37 (78.7) |
28 (13.4) 181 (86.6) |
1 (3.5) 28 (96.5) |
0 42 (100.0) |
39 (11.9) 288 (88.1) |
IQR Interquartile range, HIV human immunodeficiency virus, TB tuberculosis, BCG Bacille Calmette-Guerin, TBM tuberculous meningitis
Fig. 2.
Scatterplots of total IQ/DQ versus age at admission for tuberculous meningitis (TBM) stratified by A TBM stage, B depressed level of consciousness (LOC), C brainstem dysfunction, and D motor function impairment
Table 2 compares the median IQ for clinical, cerebrospinal fluid investigations and neuroimaging characteristics. Univariate logistic regression revealed that age < 4 years (OR: 4.18; 95%CI: 1.73–10.08; p < 0.001), decreased LOC (OR: 5.28; 95%CI: 1.85–15.13; p < 0.001), brainstem dysfunction (OR: 4.13; 95%CI 2.38–7.15; p < 0.001), impaired motor function (OR: 4.52; 95%CI: 2.27–9.00; p < 0.001), advanced stage TBM (OR: 12.20; 95%CI: 1.86–90.50; p = 0.001), CSF culture positivity (OR: 0.37; 95%CI: 0.14–0.96; p = 0.038) and radiological infarction demonstrated on neuroimaging (OR: 4.02; 95%CI: 2.43–6.64; p < 0.001) were associated with a poor outcome (Table 3). After all non-statistically significant variables were removed, the final multivariable binary logistic regression model (Table 3) showed that the variables independently associated with a poor outcome were decreased LOC (adjusted OR (aOR): 4.68; 95%CI: 2.43–13.88; p = 0.005), brainstem dysfunction (aOR: 3.20; 95%CI: 1.70–6.00; p < 0.001), and radiological infarction (aOR: 3.47; 95%CI: 1.87–6.45; p < 0.001).
Table 2.
Comparison of median DQ/IQ per clinical, cerebrospinal fluid and neuroimaging criteria
| Median IQ/DQ (IQR) | P-value | ||
|---|---|---|---|
| Age < 4 years | Yes | 62.00 (38.60–73.75) | < 0.001 |
| No | 67.00 (41.25–74.13) | ||
| Fever | Present | 65.00 (37.90–74.00) | 0.795 |
| Absent | 61.00 (44.25–72.50) | ||
| Convulsions | Present | 65.50 (54.25–73.75) | 0.015 |
| Absent | 56.80 (28.75–74.33) | ||
| HIV infected | Yes | 65.00 (57.60–65.50) | 0.743 |
| No | 63.00 (38.00–74.00) | ||
| Household TB contact | Known | 63.00 (40.70–74.75) | 0.519 |
| Unknown | 64.00 (38.00–72.50) | ||
| BCG scar or documentation | Yes | 63.00 (38.00–73.50) | 0.902 |
| No | 72.00 (57.50–83.00) | ||
| Weight faltering/loss | Yes | 64.00 (40.40–74.00) | 0.413 |
| No | 63.00 (38.00–73.00) | ||
| Decreased LOC | Yes | 59.50 (37.90–73.25) | 0.001 |
| No | 69.50 (64.25–74.88) | ||
| Brainstem dysfunction | Yes | 29.00 (7.25–56.50) | < 0.001 |
| No | 66.00 (54.25–74.00) | ||
| Motor function impaired | Yes | 58.00 (33.00–72.00) | < 0.001 |
| No | 68.00 (57.60–75.30) | ||
| TBM stage | Advanced stage IIb/III | 59.00 (37.80–72.50) | 0.003 |
| Mild stage I/IIa | 73.00 (65.50–74.90) | ||
| CSF leucocytes 10–500 cells/L | Yes | 59.00 (40.70–72.00) | 0.011 |
| No | 64.00 (37.80–74.75) | ||
| CSF lymphocytes > 50% | Yes | 59.00 (37.80–72.50) | 0.903 |
| No | 65.00 (55.00–78.50) | ||
| CSF protein > 1 g/L | Yes | 66.00 (50.00–78.00) | 0.262 |
| No | 61.50 (31.25–73.75) | ||
| CSF glucose < 2.2 mmol/L | Yes | 65.00 (37.70–74.00) | 0.805 |
| No | 59.50 (40.85–72.50) | ||
| CSF- Mtb Culture | Positive | 66.00 (56.50–73.75) | 0.005 |
| Negative | 61.00 (37.80–74.00) | ||
| Radiological infarction | Yes | 48.00 (28.50–68.50) | < 0.001 |
| No | 66.00 (56.80–76.15) | ||
| Basal enhancement | Yes | 61.00 (37.60–73.00) | 0.813 |
| No | 66.00 (54.00–74.50) | ||
| Hydrocephalus | Yes | 59.00 (38.00–73.00) | 0.314 |
| No | 69.00 (64.75–74.70) | ||
| Tuberculoma(s) | Yes | 69.50 (48.90–75.22) | 0.772 |
| No | 60.50 (37.90–72.25) |
DQ developmental quotient, IQ intelligence quotient, IQR interquartile range, HIV human immunodeficiency virus, TB tuberculosis, BCG Bacille Calmette-Guerin, LOC level of consciousness, TBM tuberculous meningitis, CSF cerebrospinal fluid, Mtb Mycobacterium tuberculosis
Table 3.
Risk factors of poor outcome (moderate/severe/profound developmental delay) compared to a good outcome (borderline/mild developmental delay and normal development) using univariate and multivariable logistic regression models (n = 326)
| Univariate logistic regression | Multivariate logistic regression | |||
|---|---|---|---|---|
| OR (95% CI) | P-value | aOR (95% CI) | p-value | |
| Age < 4 years | 4.18 (1.73–10.08) | < 0.001 | 2.12 (0.80–5.62) | 0.130 |
| Fever | 1.15 (0.63–1.68) | 0.764 | ||
| Convulsions | 1.93 (1.11–1.87) | 0.025 | 1.39 (0.73–2.63) | 0.317 |
| HIV infected | 1.08 (0.20–5.75) | 1.000 | ||
| Household TB contact | 0.85 (0.50–1.43) | 0.590 | ||
| BCG scar or documentation | 1.22 (0.69–2.13) | 0.575 | ||
| Weight faltering/loss | 1.01 (0.45–2.29) | 1.000 | ||
| Decreased LOC | 5.28 (1.85–15.13) | < 0.001 | 4.68 (1.58–13.88) | 0.005 |
| Brainstem dysfunction | 4.13 (2.38–7.15) | < 0.001 | 3.20 (1.70–6.00) | < 0.001 |
| Motor function impaired | 4.52 (2.27–9.00) | < 0.001 | 1.72 (0.78–3.79) | 0.177 |
| Advanced TBM stage (2b and 3) | 12.20 (1.65–90.50) | 0.001 | 1.99 (0.23–17.05) | 0.530 |
| CSF leucocytes 10–500 cells/L | 0.75 (0.43–1.32) | 0.379 | ||
| CSF lymphocytes > 50% | 1.95 (0.79–4.82) | 0.173 | ||
| CSF protein > 1 g/L | 0.80 (0.443–1.48) | 0.515 | ||
| CSF glucose < 2.2 mmol/L | 1.46 (0.75–2.83) | 0.337 | ||
| CSF- Mtb Culture positive | 0.37 (0.14–0.96) | 0.038 | 0.37 (0.13–1.06) | 0.065 |
| Radiological infarction | 4.02 (2.43–6.64) | < 0.001 | 3.47 (1.87–6.45) | < 0.001 |
| Basal enhancement | 1.12 (0.53–2.36) | 0.853 | ||
| Hydrocephalus | 2.36 (0.69–7.18) | 0.224 | ||
| Tuberculoma(s) | 0.79 (0.35–1.79) | 0.694 | ||
OR odds ratio, CI confidence interval, aOR adjusted odds ratio, HIV human immunodeficiency virus, TB tuberculosis, BCG Bacille Calmette-Guerin, LOC level of consciousness, TBM tuberculous meningitis, CSF cerebrospinal fluid, Mtb Mycobacterium tuberculosis
The Kruskal–Wallis test demonstrated a difference between the presence or absence of radiological infarction and age on admission and (p = 0.001); the median age with radiological infarction was 23 months (IQR: 13.5–36.5 months) and in those without radiological infarction, 30 months (IQR: 16.0–50.0 months).
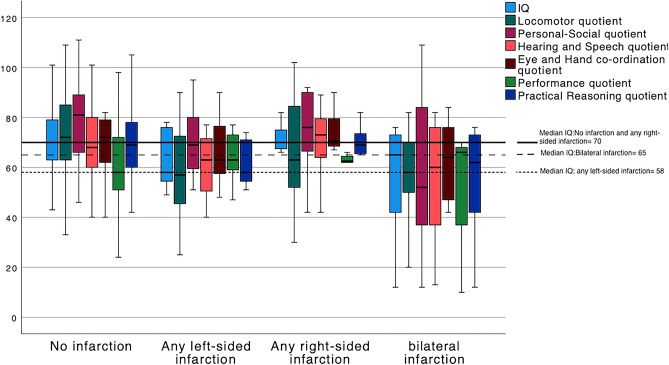
Data on site of radiological infarction were only available in 123 patients, all of whom were assessed using the Griffiths Mental Development Scales (Table 4). On univariate logistic regression, left-sided and bilateral radiological infarction, as compared to right-sided infarcts, were associated with a poor developmental outcome (Table 5). Figure 3 shows box plots of global developmental quotient and locomotor, personal/social, hearing/speech, eye/hand co-ordination, performance, and practical reasoning sub-quotients in groups of no radiological infarction, any left-sided infarction, any right-sided infarction, and bilateral infarction. There is clear illustration of lower quotients for any left-sided and bilateral infarction, with hearing/speech most closely approximating the global quotient.
Table 4.
Radiological infarction site and intelligence/developmental quotient (IQ/DQ) level (n = 123)
|
Total n/N (%) |
Normal IQ/DQ n/N (%) |
Borderline/mild IQ/DQ n/N (%) |
Moderate IQ/DQ n/N (%) |
Severe/profound IQ/DQ n/N (%) |
|
|---|---|---|---|---|---|
| Single left-sided | 26/123 (21.1) | 0 | 16/123 (13.0) | 3/123 (2.4) | 7/123 (5.7) |
| Single right-sided | 34/123 (27.6) | 1/123 (0.8) | 23/123 (18.7) | 1/123 (4.9) | 5/123 (4.1) |
| Multiple left-sided | 10/123 (8.1) | 0 | 2/123 (1.6) | 4/123 (3.6) | 4/123 (3.6) |
| Multiple right-sided | 12/123 (9.8) | 0 | 8/123 (6.5) | 1/123 (0.8) | 3/123 (2.4) |
| Bilateral | 24/123 (19.5) | 0 | 10/123 (8.1) | 5/123 (4.1) | 9/123 (7.3) |
| Left caudate/IC | 24/123 (19.5) | 0 | 11/123 (8.9) | 4/123 (3.6) | 9/123 (7.3) |
| Right caudate/IC | 31/123 (25.2) | 1/123 (0.8) | 21/123 (17.1) | 2/123 (1.6) | 7/123 (5.7) |
| Left thalamus | 23/123 (18.7) | 0 | 11/123 (8.9) | 3/123 (2.4) | 9/123 (7.3) |
| Right thalamus | 17/123 (13.8) | 0 | 12/123 (9.8) | 2/123 (1.6) | 3/123 (2.4) |
| Left MCA | 9/123 (7.3) | 0 | 6/123 (4.9) | 2/123 (1.6) | 1/123 (0.8) |
| Right MCA | 8/123 (6.5) | 0 | 4/123 (3.6) | 2/123 (1.6) | 2/123 (1.6) |
IC internal capsule, MCA middle cerebral artery
Table 5.
Association of radiological infarction site* and poor outcome compared to good outcome
| OR (95% CI) | P-value | |
|---|---|---|
| Single left-sided | 2.74 (1.07–7.03) | 0.036 |
| Single right-sided | 1.64 (0.67–4.05) | 0.280 |
| Multiple left-sided | 18.60 (3.67–94.28) | < 0.001 |
| Multiple right-sided | 1.81 (0.50–6.54) | 0.363 |
| Bilateral | 8.50 (3.16–22.85) | < 0.001 |
| Left caudate/IC | 6.62 (2.50–17.51) | < 0.001 |
| Right caudate/IC | 1.57 (0.62–3.97) | 0.338 |
| Left thalamus | 5.73 (2.16–15.22) | < 0.001 |
| Right thalamus | 1.50 (0.48–4.71) | 0.484 |
| Left MCA | 1.78 (0.42–7.63) | 0.437 |
| Right MCA | 3.79 (0.88–16.28) | 0.073 |
OR odds ratio, CI confidence interval, IC internal capsule, MCA middle cerebral artery
*Infarction at site compared to no infarction
Fig. 3.
Boxplots of Griffiths total IQ and sub-quotients compared to presence and site of radiological infarction
Discussion
In accordance with previous studies, this study found that decreased LOC, brainstem dysfunction, and radiological infarction on CT are risk factors for poor long-term neurological outcome in children with TBM.
Detection of brainstem dysfunction is challenging but of utmost importance in comatose children with TBM both to guide therapy and to support outcome prediction. Brainstem dysfunction may lead to sensory and motor deficits, cranial nerve palsies, loss of consciousness, dysautonomia, and respiratory failure. There is often an overlap between the signs of raised ICP/hydrocephalus and brainstem dysfunction which necessities the need for neuro-imaging. A further complicating factor for accurate diagnosis is the fact that CT has a low sensitivity at diagnosing brainstem ischemia.
Similar to previous TBM outcome studies, this study also identified a trend towards poorer outcomes in younger children. Studies have shown that tuberculous stroke in young children have worse cognitive outcomes [15]. One explanation is that the classical signs of TBM are often absent in young children, and symptoms and signs tend to be non-specific. It has been shown that the younger the child, the less specific the signs and symptoms are of meningitis [25]. This invariably leads to diagnostic delays with a higher likelihood of advanced (severe) TBM disease. In addition, the first few years of life are a particularly important period of critical brain development and any injury during this period is likely to have irreversible longtime consequences. A key difference between young children and teenagers is that young childhood stroke results primarily in a changed ability to achieve, rather than lose, functional independence. The extent and severity of deficits across motor, sensory, cognitive, social, and behavioural domains may not be apparent in the short-term and it is likely that the development outcome in this group may even be worse. The mechanisms of infarction in TBM is not well understood and several varying pathogeneses have been suggested including (but not limited to) vasculitis, arterial thrombosis, and vascular proliferation. Perforating artery infarcts occurring in the middle cerebral artery (MCA) territory play an important role in TB stroke and the smaller diameter of such vessels in young children may increase vulnerability to vessel stenosis and eventually occlusion [26]. Despite infarctions being an important predictor of outcome in TBM, no effective therapeutic strategy is agreed on for their prevention. Both corticosteroids and aspirin have been evaluated, and to date neither have been shown to be an effective preventive therapy against infarctions [27–31].
An interesting finding from the study is the poorer outcome in TBM children with left hemispherical stroke. This can be attributed to the fact that subcortical structures and mechanisms, specifically the basal ganglia and thalamus, actually underlie speech and language processing. There are only few studies in the literature that have investigated language outcomes after childhood stroke; none of them included children with TBM. In some of the studies the side of the lesion did not influence language, while others reported that considerable language or verbal memory difficulties were observed in left hemisphere lesions [32]. Haemodynamic differences have been demonstrated between the right and left carotid artery circulations [33]. In a study of premature babies followed up until 2 years of age, increased left cerebral blood flow velocity was associated with a better Bayley Mental Developmental Index score, with a postulation that adequate left cerebral blood flow velocity is especially important for language development [34]. Although our study did not demonstrate increased frequency of left-sided infarctions, there was significant association with worse IQ, indicating the importance of adequate left cerebral blood flow to the dominant hemisphere for later development, including language. Studies also show that bilateral cerebral infarction negatively impacts on neuroplasticity; a finding which explains the poorer outcome in study TBM children with bilateral cerebral infarcts.
Few controlled prospective studies exist regarding hydrocephalus outcomes; in their absence, largely retrospective studies must be used to evaluate the long-term consequences of hydrocephalus and its treatments. Tuberculous hydrocephalus (raised intracranial pressure), a very common complication of TBM, was not a predictor of poor developmental outcome in our study. This can be ascribed to the fact that most cases of tuberculous hydrocephalus communicating (non-obstructive) and responsive to diuretic therapy. A study at our institution utilizing transcranial Doppler imaging and serial lumbar CSF opening pressures at baseline, 3 days, and 7 days found that intracranial pressure normalized within 7 days after initiation of acetazolamide and furosemide in children with communicating hydrocephalus [35].
There are limited data to guide standardized long-term neurocognitive management of both adults and children with TBM, partly due to the heterogeneity of assessment methods [36]. Other than for motor and sensory impairment, various standardized tools to assess neurodevelopment, neurocognition, functional ability, and behavior have been proposed [37]. It is essential that standardized tools should be used in prospective studies, including comprehensive assessment of language development in young children, in order to build a body of data to inform evidence-based guidelines. What is undisputed is that the long-term management of TBM in children is multidisciplinary and should be initiated as soon as possible to ensure better outcomes and quality of life.
Limitations of this study included a very small HIV-positive sample size which can be attributed to lack of HIV testing during the early period of the study. Even though the study period was prior to the Covid-19 pandemic, it would be worthwhile studying the impact of TBM and Covid-19 co-infection on stroke and long-term outcome due to both resulting in a hypercoagulable state [38]. Accurate determination of treatment initiation time in TBM is notoriously difficult to assess as it relies on caregiver recall as well as the fact that the initial symptoms and signs of TBM are often subtle and non-specific. This was thus not assessed, which may have resulted in possible confounding. The study was conducted at a single site only which could have affected the generalizability of our findings. Outcomes relating to radiological infarction was assessed using only baseline CT imaging and small infarctions may have gone undetected. Only a sub-set had neuroimaging available for analysis. In addition, approximately 25% of children who have TBM without evidence of radiological infarction have been shown to develop new infarcts or enlargement of infarcts at 1 month of age [39]. This may have led to an underestimation of the number of infarcts. High resolution structural MRI is superior at identifying even small stroke lesions, but relating the size of lesions to clinical impairment and functional outcome is difficult especially since small lesions of the subcortical white matter or the brainstem can produce disproportionate clinical disturbances. The study focused purely on DQ/IQ as outcome measurement and the associations thus identified were non-specific to other outcome measures such as behaviour profiles and sensory impairments. Despite the abovementioned limitations, to the best of our knowledge, the study represents the largest data set (spanning 3 decades), to date, of cognitive outcome data in children with TBM. Additionally, only probable and definite TBM cases (according to TBM research case definition stipulations) were included.
Conclusion
The study identified three factors, at the time of the TBM diagnosis, that were predictive of a poor developmental outcome. Cerebral infarcts in TBM are especially predictive of a poor outcome, a finding supported by several previous studies. There is a need to evaluate further the role of TBM itself in causation of these infarcts through age-matched controlled studies. Prediction of outcome, especially after stroke is important for setting realistic and attainable treatment goals, informing caregivers properly, facilitating discharge planning, and anticipating possible consequences for home and school adjustments.
Author contribution
Caro-Lee Saal: conceptualization, methodology, writing – original draft. Priscilla Springer: methodology, writing – original draft. James A Seddon: methodology, writing – original draft, review and editing. Ronald van Toorn: methodology, supervision, writing – original draft, review and editing. Tonya Esterhuizen: conceptualization, methodology, supervision, writing –review and editing. Regan S Solomons: conceptualization, methodology, supervision, writing – original draft, review and editing. All the authors read and approved the final manuscript.
Funding
Regan Solomons is supported by the National Research Foundation of South Africa (109437). James A Seddon is supported by a Clinician Scientist Fellowship jointly funded by the UK Medical Research Council (MRC) and the UK Department for International Development (DFID) under the MRC/DFID Concordat agreement (MR/R007942/1).
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The study was approved by the Health Research Ethics Committee of Stellenbosch University (study nr. S20/10/294). Waiver of consent was granted as this was a retrospective study.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Global Tuberculosis Report . World Health Organization. Geneva: Switzerland; 2020. [Google Scholar]
- 2.Graham SM, Donald PR. Death and disability: the outcomes of tuberculous meningitis. Lancet Infect Dis. 2014;14(10):902–904. doi: 10.1016/S1473-3099(14)70872-2. [DOI] [PubMed] [Google Scholar]
- 3.Donald PR, Cotton MF, Hendricks MK, Schaaf HS, de Villiers JN, Willemse TE. Pediatric meningitis in the Western Cape Province of South Africa. J Trop Pediatr. 1996;42(5):256–261. doi: 10.1093/tropej/42.5.256. [DOI] [PubMed] [Google Scholar]
- 4.van Well GT, Paes BF, et al. Twenty years of pediatric tuberculous meningitis: a retrospective cohort study in the western cape of South Africa. Pediatrics. 2009;123(1):e1–8. doi: 10.1542/peds.2008-1353. [DOI] [PubMed] [Google Scholar]
- 5.Nelson LJ, Schneider E, Wells CD, Moore M. Epidemiology of childhood tuberculosis in the United States, 1993–2001: the need for continued vigilance. Pediatrics. 2004;114(2):333–341. doi: 10.1542/peds.114.2.333. [DOI] [PubMed] [Google Scholar]
- 6.Wolzak NK, Cooke ML, Orth H, van Toorn R. The changing profile of pediatric meningitis at a referral centre in Cape Town. South Africa J Trop Pediatr. 2012;58(6):491–495. doi: 10.1093/tropej/fms031. [DOI] [PubMed] [Google Scholar]
- 7.Rohlwink UK, Donald K, et al. Clinical characteristics and neurodevelopmental outcomes of children with tuberculous meningitis and hydrocephalus. Dev Med Child Neurol. 2016;58(5):461–468. doi: 10.1111/dmcn.13054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wait JW, Stanton L, Schoeman JF. Tuberculosis meningitis and attention deficit hyperactivity disorder in children. J Trop Pediatr. 2002;48(5):294–299. doi: 10.1093/tropej/48.5.294. [DOI] [PubMed] [Google Scholar]
- 9.Schoeman J, Wait J, et al. Long-term follow up of childhood tuberculous meningitis. Dev Med Child Neurol. 2002;44(8):522–526. doi: 10.1111/j.1469-8749.2002.tb00323.x. [DOI] [PubMed] [Google Scholar]
- 10.Chiang SS, Khan FA, et al. Treatment outcomes of childhood tuberculous meningitis: a systematic review and meta-analysis. Lancet Infect Dis. 2014;14(10):947–957. doi: 10.1016/S1473-3099(14)70852-7. [DOI] [PubMed] [Google Scholar]
- 11.Sheu JJ, Chiou HY, Kang JH, Chen YH, Lin HC. Tuberculosis and the risk of ischemic stroke: a 3-year follow-up study. Stroke. 2010;41(2):244–249. doi: 10.1161/STROKEAHA.109.567735. [DOI] [PubMed] [Google Scholar]
- 12.Per H, Unal E, et al. Childhood stroke: results of 130 children from a reference center in Central Anatolia. Turkey Pediatr Neurol. 2014;50(6):595–600. doi: 10.1016/j.pediatrneurol.2013.12.023. [DOI] [PubMed] [Google Scholar]
- 13.Thwaites GE, van Toorn R, Schoeman J. Tuberculous meningitis: more questions, still too few answers. Lancet Neurol. 2013;12(10):999–1010. doi: 10.1016/S1474-4422(13)70168-6. [DOI] [PubMed] [Google Scholar]
- 14.van Toorn R, Solomons R. Update on the diagnosis and management of tuberculous meningitis in children. Semin Pediatr Neurol. 2014;21(1):12–18. doi: 10.1016/j.spen.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 15.Springer P, Swanevelder S, van Toorn R, van Rensburg AJ, Schoeman J. Cerebral infarction and neurodevelopmental outcome in childhood tuberculous meningitis. Eur J Paediatr Neurol. 2009;13(4):343–349. doi: 10.1016/j.ejpn.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 16.Kalita J, Misra UK, Ranjan P. Predictors of long-term neurological sequelae of tuberculous meningitis: a multivariate analysis. Eur J Neurol. 2007;14(1):33–37. doi: 10.1111/j.1468-1331.2006.01534.x. [DOI] [PubMed] [Google Scholar]
- 17.Davis AG, Nightingale S, Springer PE, et al. Neurocognitive and functional impairment in adult and paediatric tuberculous meningitis. Wellcome Open Res. 2019;4:178. doi: 10.12688/wellcomeopenres.15516.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marais S, Thwaites G, et al. Tuberculous meningitis: a uniform case definition for use in clinical research. Lancet Infect Dis. 2010;10(11):803–12. doi: 10.1016/S1473-3099(10)70138-9. [DOI] [PubMed] [Google Scholar]
- 19.Iresearchnet. Psychology Research and Reference. Available from: http://psychology.iresearchnet.com/papers/developmental-quotient. Accessed 5 Aug 2022
- 20.Bayley N. The Bayley scales of infant and toddler development. 3. San Antonio, TX: Harcourt Assessment Inc; 2006. [Google Scholar]
- 21.Laughton B, Springer P, et al. Longitudinal developmental profile of children from low socio-economic circumstances in Cape Town, using the 1996 Griffiths Mental Development Scales. SAJCH. 2010;4(4):106–111. [PMC free article] [PubMed] [Google Scholar]
- 22.Theron LC (2013) Assessing School readiness using the Junior South African individual scales: a pathway to resilience. Psychological Assessment in South Africa: Research and Applications, edited by Sumaya Laher and Kate Cockcroft, Wits University Press pp. 60–73. 10.18772/22013015782.10. Accessed 29 Apr 2022
- 23.STREPTOMYCIN treatment of tuberculous meningitis Lancet. 1948;1(6503):582–596. [PubMed] [Google Scholar]
- 24.van Toorn R, Springer P, Laubscher JA, Schoeman JF. Value of different staging systems for predicting neurological outcome in childhood tuberculous meningitis. Int J Tuberc Lung Dis. 2012;16(5):628–632. doi: 10.5588/ijtld.11.0648. [DOI] [PubMed] [Google Scholar]
- 25.Solomons R, Grantham M, Marais BJ, van Toorn R. IMCI indicators of childhood TBM at primary health care level in the Western Cape Province of South Africa. Int J Tuberc Lung Dis. 2016;20(10):1309–1313. doi: 10.5588/ijtld.16.0062. [DOI] [PubMed] [Google Scholar]
- 26.Lammie GA, Hewlett RH, Schoeman JF, Donald PR. Tuberculous cerebrovascular disease: a review. J Infect. 2009;59(3):156–166. doi: 10.1016/j.jinf.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 27.Advice R. Treatment of tuberculosis in children. Geneva: WHO Guidelines Approved by the Guidelines Review Committee; 2010. [Google Scholar]
- 28.Prasad K, Singh MB, Ryan H (2016) Corticosteroids for managing tuberculous meningitis. Cochrane Database Syst Rev 4:CD002244 [DOI] [PMC free article] [PubMed]
- 29.Misra UK, Kalita J, Nair PP (2010) Role of aspirin in tuberculous meningitis: a randomized open label placebo controlled trial. J Neurol Sci 293(1–2):12–7 [DOI] [PubMed]
- 30.Mai NT, Dobbs N et al (2018) A randomised double blind placebo controlled phase 2 trial of adjunctive aspirin for tuberculous meningitis in HIV-uninfected adults. Elife 7 [DOI] [PMC free article] [PubMed]
- 31.Schoeman JF, Janse van Rensburg A, Laubscher JA, Springer P (2011) The role of aspirin in childhood tuberculous meningitis. J Child Neurol 26(8):956–62 [DOI] [PubMed]
- 32.Price CJ, Seghier ML, Leff AP. Predicting language outcome and recovery after stroke: the PLORAS system. Nat Rev Neurol. 2010;6(4):202–210. doi: 10.1038/nrneurol.2010.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rodriguez Hernandez SA, et al. Is there a side predilection for cerebrovascular disease? Hypertension. 2003;42(1):56–60. doi: 10.1161/01.HYP.0000077983.66161.6F. [DOI] [PubMed] [Google Scholar]
- 34.Arditi H, Feldman R, Hammerman C, Eidelman AI. Cerebral blood flow velocity asymmetry, neurobehavioral maturation, and the cognitive development of premature infants across the first two years. J Dev Behav Pediatr. 2007;28(5):362–368. doi: 10.1097/DBP.0b013e318114315d. [DOI] [PubMed] [Google Scholar]
- 35.van Toorn R, Schaaf HS, Solomons R, Laubscher JA, Schoeman JF. The value of transcranial Doppler imaging in children with tuberculous meningitis. Childs Nerv Syst. 2014;30(10):1711–1716. doi: 10.1007/s00381-014-2435-2. [DOI] [PubMed] [Google Scholar]
- 36.Marais BJ, Heemskerk AD, et al. Standardized methods for enhanced quality and comparability of tuberculous meningitis studies. Clin Infect Dis. 2017;64(4):501–509. doi: 10.1093/cid/ciw757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.WHO operational handbook on tuberculosis . Module 5: management of tuberculosis in children and adolescents. Geneva: World Health Organization; 2022. [PubMed] [Google Scholar]
- 38.Essajee F, Solomons R, Goussard P, Van Toorn R. Child with tuberculous meningitis and COVID-19 coinfection complicated by extensive cerebral sinus venous thrombosis. BMJ Case Rep. 2020;13(9):e238597. doi: 10.1136/bcr-2020-238597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Solomons RS, Nieuwoudt ST, Seddon JA, van Toorn R. Risk factors for ischemic stroke in children with tuberculous meningitis. Childs Nerv Syst. 2021;37(8):2625–2634. doi: 10.1007/s00381-021-05163-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.