Abstract
Background
The lack of standardized methods for clinical trial design and disease activity assessment has contributed to an absence of approved medical therapies for the prevention of postoperative Crohn’s disease (CD). We developed recommendations for regulatory trial design for this indication and for endoscopic assessment of postoperative CD activity.
Methods
An international panel of 19 gastroenterologists was assembled. Modified Research and Development/University of California Los Angeles methodology was used to rate the appropriateness of 196 statements using a 9-point Likert scale in 2 rounds of voting. Results were reviewed and discussed between rounds.
Results
Inclusion of patients with a history of completely resected ileocolonic CD in regulatory clinical trials for the prevention of postoperative recurrence was appropriate. Given the absence of approved medical therapies, a placebo-controlled design with a primary end point of endoscopic remission at 52 weeks was appropriate for drug development for this indication; however, there was uncertainty regarding the appropriateness of a coprimary end point of symptomatic and endoscopic remission and the use of currently available patient-reported outcome measures. The modified Rutgeerts Score, endoscopic assessment of the anastomosis, and a minimum of 5cm of neoterminal ileum were also appropriate; although the appropriateness of other indices including the Simple Endoscopic Score for CD for endoscopic assessment of postoperative CD activity was uncertain.
Conclusions
A framework for regulatory trial design for the prevention of postoperative CD recurrence and endoscopic assessment of disease activity has been developed. Research to empirically validate end points for these trials is needed.
Keywords: inflammatory bowel disease, medical therapy, randomized controlled trials, surgery
A framework for regulatory trial design and endoscopic assessment of postoperative recurrent Crohn’s disease has been developed. Regulatory trials assessing medical therapy for prevention of postoperative recurrence should be designed as randomized placebo-controlled studies with an endoscopic primary end point.
Introduction
Advances in the medical management of Crohn’s disease (CD) have been associated with a decline in the rates of surgical resection.1,2 However, surgery continues to provide an important therapeutic role for patients with obstructive symptoms, penetrating complications, or medically refractory disease. Up to 25% of patients with CD will require surgery within 5 years of diagnosis,3 and up to a quarter of these patients will undergo a second resection within 5 years of the first surgery.4 Surgery is rarely curative, and most patients will experience disease recurrence. Effective medical therapy to prevent postoperative disease recurrence is an unmet need, as no routinely available drugs for the treatment of CD have specifically received regulatory approval for this indication.
Although postoperative CD is not specifically addressed in regulatory guidelines, a coprimary end point of symptomatic and endoscopic remission is mandated for the registration of new medicinal products for the treatment of CD.5 However, the cardinal symptoms of CD evaluated by patient-reported outcome measures, such as abdominal pain and stool frequency, do not correlate well with endoscopic disease recurrence in the postoperative period. This may be a consequence of concomitant pathology such as intra-abdominal adhesions, small intestinal bacterial overgrowth, or bile acid diarrhea.6,7 Additionally, there may be a paucity of clinical symptoms or “clinically silent” disease despite endoscopic recurrence in the postoperative setting. The discrepancy between endoscopic and symptom-based outcomes in postoperative CD was highlighted in the PREVENT trial,8 where only 18.1% (20/110) of patients with endoscopic recurrence as defined by the Rutgeerts Score ≥i2 also had recurrence based on the Crohn’s Disease Activity Index (CDAI; defined by a total CDAI score >200 and a ≥70-point increase from baseline). As such, there was no significant difference in the risk of clinical recurrence among patients treated with infliximab compared with those treated with placebo, despite a statistically significant 28.9% reduction in the risk of endoscopic recurrence for patients treated with infliximab. This discordance among outcomes and current regulatory requirements for their use in CD trials presents a challenge for the conduct of trials of therapies for the prevention postoperative recurrence.
Endoscopic assessment is a more objective measure that can be integrated into a management algorithm to prevent CD recurrence, as demonstrated by the Post-Operative Crohn’s Endoscopic Recurrence (POCER) study, a randomized trial comparing early colonoscopy to standard of care.9 However, a fully validated endoscopic index for assessment of postoperative CD is lacking. Although partially validated, the Rutgeerts score is the only instrument specific to postoperative CD; however, this instrument was designed as a prognostic tool rather than a disease activity index.10 Accurate endoscopic assessment in postoperative CD is further complicated given that endoscopic features of recurrent CD may not overlap with or have the same relevance as those observed in native luminal CD. For example, the early development of aphthae in a postoperative patient may be of greater importance than when observed in a patient with longstanding disease; ulcers isolated to the anastomosis may be ischemic in origin11; and stenosis may be related to surgical technique rather than a disease-related fibrotic complication. Finally, some items of existing endoscopic indices (eg, stenosis) are unreliable.12
After recognizing the need for guidance on regulatory trial design for the prevention of postoperative CD in adult patients and endoscopic assessment of postoperative disease activity, we assembled an international panel of gastroenterologists for a 2-round evaluative process using modified Research and Development (RAND)/University of California Los Angeles (UCLA) methodology, the intent of which was to generate recommendations to facilitate drug development for this indication.
Methods
Statement Generation
The initial list for survey development consisted of statements related to facets of clinical trial design, including patient selection and eligibility, inclusion criteria, comparator groups, study duration, methods for end point assessment, outcome measures, definitions, and methods for endoscopic assessment. Statements were based on component items of existing endoscopic indices for CD including the CDEIS,13 SES-CD,14 POCER index,15 REMIND score,16 and the original and modified Rutgeerts score (see Supplementary Material for a description of the indices).10,17 The modified Rutgeerts score differentiates lesions confined to the ileocolonic anastomosis (scored as i2a) from those in the neoterminal ileum (scored as i2b). Additionally, items deemed relevant to the assessment of postoperative endoscopic CD not captured in the previously mentioned indices were also considered.
Expert Consensus Process
Recruitment of panelists
An international panel of 19 gastroenterologists from Australia, Belgium, Canada, France, Germany, the Netherlands, Spain, and the United States were invited to participate. Panelists were selected based on publication record, expertise in the use and/or development of indices for assessment of endoscopic CD activity, and experience in clinical drug development and clinical trial design. These criteria took precedence over geographical representation. The final selection of panelists was determined by C.M. and V.J. Potential conflicts of interest for all panelists are summarized in the Conflicts of Interest section.
Modified RAND/UCLA methodology was used to determine the appropriateness, content, and face validity of the statements generated by the panel.18 This method incorporates a modified Delphi panel approach with iterative rounds of voting and discussion to combine the best available evidence with the combined experience of the panel, without forcing consensus or attempting to reach a higher percentage of agreement. This process is widely accepted and evidence-based.
Initial assumptions for the creation of the list of relevant statements for assessment of appropriateness were presented and discussed during an introductory meeting with the panel. Panelists provided feedback on these assumptions and were invited to suggest modifications and/or generate new statements. Consistent with RAND/UCLA methodology, these modifications and additions were included in a final list of statements, which was circulated as an online survey.
Panelists anonymously rated the appropriateness of individual survey statements based on a 9-point scale (1, extremely inappropriate; 5, uncertain; 9, extremely appropriate). Each survey statement was classified as inappropriate (median score 1 to 3.5 without disagreement), uncertain (median score 3.5 to 6.5 without disagreement or any median score with disagreement), or appropriate (median score 6.5 to 9 without disagreement) as defined in the RAND/UCLA manual.18 Disagreement regarding statement appropriateness was defined as ratings by at least one-third of the panelists (ie, at least 6 ratings) in each of the extreme ends of the rating scale (1 to 3 and 6 to 9). The median rating for each statement and the distribution of ratings, expressed as the interquartile range, are reported.
Results of the first-round survey were summarized, shared with panelists, and presented in a moderated video conference, with the aim of highlighting areas of disagreement on statement appropriateness and discussing the rationale for individual responses. The survey was then revised based on the panel meeting to improve clarity prior to recirculation and a second voting round. Statement appropriateness for the second round of voting was scored as described previously.
Results
Overall Rating of Statements
The first-round survey consisted of 188 statements. Overall, 55 (29%) statements were considered appropriate, 109 (58%) uncertain, and 24 (13%) inappropriate. After a moderated video conference to review and discuss the results of the first survey, an amended final survey consisting of 196 statements was distributed and rated. Overall, 69 (35%) statements were considered appropriate, 105 (54%) uncertain, and 22 (11%) inappropriate. Key statements and panelist ratings are summarized in Tables 1-3, and the results of the final survey are included as Supplementary Table 1.
TABLE 1.
Standardization of regulatory trial design for the prevention of postoperative Crohn’s disease.
| Statement | Median | IQR | Appropriateness |
|---|---|---|---|
| Only patients with ileocolonic CD that has been completely resected should be included in regulatory trials assessing the effectiveness of a therapy for prevention of postoperative recurrence. | 8 | 4–9 | Appropriate |
| For regulatory trials assessing the effectiveness of a therapy for prevention of postoperative CD recurrence shortly after surgery, patients should be randomized within 14 days of surgery. | 7 | 5–8 | Appropriate |
| For regulatory trials assessing the effectiveness of a therapy for prevention of postoperative CD recurrence shortly after surgery, patients should be randomized within 30 days of surgery. | 7 | 5–8 | Appropriate |
| For regulatory trials assessing the effectiveness of a therapy for prevention of postoperative CD recurrence shortly after surgery, patients should be randomized within 60 days of surgery. | 4 | 3–6 | Uncertain |
| For regulatory trials assessing the effectiveness of a therapy for prevention of postoperative CD recurrence shortly after surgery, patients should be randomized within 90 days of surgery. | 2 | 1–5 | Inappropriate |
| Patients in the control arm of a regulatory trial assessing the effectiveness of a therapy for prevention of postoperative CD recurrence should receive placebo. | 7 | 6–8 | Appropriate |
| Patients in the control arm of a regulatory trial assessing the effectiveness of a therapy for prevention of postoperative CD recurrence should receive a 5-aminosalicylate. | 3 | 1–5 | Inappropriate |
| Patients in the control arm of a regulatory trial assessing the effectiveness of a therapy for prevention of postoperative CD recurrence should receive metronidazole. | 3 | 2–5 | Inappropriate |
| Patients in the control arm of a regulatory trial assessing the effectiveness of a therapy for prevention of postoperative CD recurrence should receive a thiopurine. | 3 | 1–5 | Inappropriate |
| Patients in the control arm of a regulatory trial assessing the effectiveness of a therapy for prevention of postoperative CD recurrence should receive a combination of metronidazole and a thiopurine. | 2 | 1–4 | Inappropriate |
| Patients in the control arm of a regulatory trial assessing the effectiveness of a therapy for prevention of postoperative CD recurrence should receive a TNF antagonist. | 4 | 1–7 | Uncertain |
| The design of regulatory trials assessing the effectiveness of a therapy for prevention of postoperative CD recurrence should be “treat-straight-through” with a minimum duration of 26 weeks. | 5 | 4–7 | Uncertain |
| The design of regulatory trials assessing the effectiveness of a therapy for prevention of postoperative CD recurrence should be “treat-straight-through” with a minimum duration of 52 weeks. | 7 | 6–8 | Appropriate |
| The design of regulatory trials assessing the effectiveness of a therapy for prevention of postoperative CD recurrence should be “treat-straight-through” with a minimum duration of 78 weeks. | 6 | 4–6 | Uncertain |
| The design of regulatory trials assessing the effectiveness of a therapy for prevention of postoperative CD recurrence should be “treat-straight-through” with a minimum duration of 104 weeks. | 5 | 3–7 | Uncertain |
| The primary end point of regulatory trials assessing the effectiveness of a therapy for prevention of postoperative CD recurrence should be based on symptoms. | 3 | 2–4 | Inappropriate |
| The primary end point of regulatory trials assessing the effectiveness of a therapy for prevention of postoperative CD recurrence should be based on: endoscopic disease activity. | 8 | 8–9 | Appropriate |
| The primary end point of regulatory trials assessing the effectiveness of a therapy for prevention of postoperative CD recurrence should be based on both symptoms and endoscopic disease activity (coprimary). | 5 | 3–6 | Uncertain |
| The primary end point of regulatory trials assessing the effectiveness of a therapy for prevention of postoperative CD recurrence should be based on fecal calprotectin. | 4 | 3–5 | Uncertain |
| The primary end point of regulatory trials assessing the effectiveness of a therapy for prevention of postoperative CD recurrence should be based on C-reactive protein. | 3 | 1–3 | Inappropriate |
| The primary end point of regulatory trials assessing the effectiveness of a therapy for prevention of postoperative CD recurrence should be based on intestinal ultrasound. | 4 | 2–5 | Uncertain |
| The primary end point of regulatory trials assessing the effectiveness of a therapy for prevention of postoperative CD recurrence should be based on histological activity. | 3 | 2–5 | Inappropriate |
Abbreviations: CD, Crohn’s disease; TNF, tumor necrosis factor; IQR, interquartile range.
Rating of Statements According to Topic
Trial design
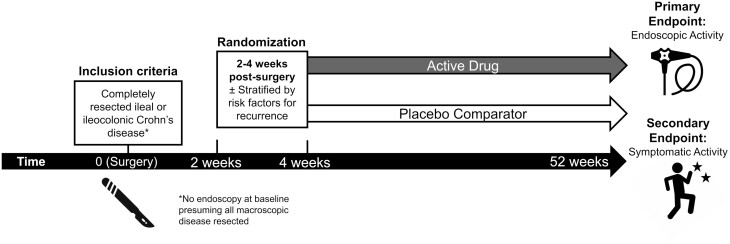
Final recommendations for trial design are summarized in Table 1 and Figure 1. Limiting inclusion to only patients with ileal or ileocolonic CD that has been completely resected in regulatory trials assessing the effectiveness of a therapy for prevention of postoperative recurrence was considered appropriate by the panel, as was randomization at 14 to 30 days postsurgery. Statements regarding randomization at later time points were considered either uncertain (60 days) or inappropriate (90 days), as early recurrence may occur prior to these time points. Use of a placebo comparator was considered appropriate, whereas there was uncertainty regarding the use of active comparators, including 5-aminosalicylates, thiopurines, metronidazole, or tumor necrosis factor-antagonists. Based on panel feedback, this uncertainty was driven by the lack of approved treatment options and limited or poor-quality evidence, supporting the efficacy of these agents for this indication. Although the panel acknowledged that the risk of postoperative recurrence is variable and may be dependent on certain clinical risk factors, they were uncertain whether these factors generally should be required for patient inclusion. A trial duration of 52 weeks was deemed appropriate; however, there was uncertainty about shorter (26 weeks) and longer (78, 104 weeks) studies. This was driven by concerns about trial feasibility given the potential for insufficient number of events occurring in shorter trials and the cost and resources required to perform longer trials. In discussion, the panelists acknowledged the possibility of evaluating the primary end point at 52 weeks and secondary end points at a later time point.
Figure 1:
Proposed approach to regulatory trials for the prevention of postoperative Crohn’s disease based on the recommendations of the RAND panel.
With respect to the primary end point of a regulatory trial for prevention of postoperative CD recurrence, only an endoscopic outcome was considered appropriate. The panel was uncertain about the appropriateness of a coprimary end point of symptoms and endoscopy. A symptoms-based primary end point was considered inappropriate, although evaluation of symptoms as a secondary end point was appropriate. The panel was uncertain about secondary end points based on biomarkers, histology, or intestinal ultrasound. There was also uncertainty regarding appropriate symptoms for inclusion in patient-reported outcome measures. For example, increased stool frequency and abdominal pain were discussed as not specific to inflammatory disease activity in the postoperative setting and possibly arising due to ileo-cecal valve resection and small bowel intestinal overgrowth or bile acid related diarrhea. Assessment of stool frequency as the number of stools above the postoperative baseline was considered appropriate for regulatory clinical trials. The panelists also acknowledged the difficulty associated with establishing a “normal” postoperative baseline, particularly if patients are randomized shortly after surgery, as regular stool frequency may take months to establish in this setting. None of the patient-reported outcome measures considered by the panel (PRO2, Numeric Rating Score, IBD-10 Score, survey-based CDAI, patient-based Harvey-Bradshaw Index, Mobile Health Index for CD, IBD-Control Questionnaire) were deemed appropriate.
Endoscopic Assessment
The requirement for a specific index to assess postoperative CD activity was considered appropriate by the panel. This need was further reflected in panel uncertainty regarding the suitability of existing indices (SES-CD, CDEIS, Rutgeerts Score) for this purpose (Table 2). The modified Rutgeerts score was the only index considered appropriate for assessment of postoperative endoscopic CD activity; however, the SES-CD component items were considered appropriate for evaluation of postoperative disease activity in the neoterminal ileum.
TABLE 2.
Standardization of endoscopic assessment in regulatory trials for the prevention of postoperative Crohn’s disease.
| Statement | Median | IQR | Appropriateness |
|---|---|---|---|
| The measurement of postoperative Crohn’s disease (CD) activity requires a specific index. | 8 | 8–9 | Appropriate |
| The Simple Endoscopic Score for Crohn’s Disease (SES-CD) is suitable to measure postoperative endoscopic CD activity in regulatory clinical trials. | 6 | 3–7 | Uncertain |
| The Crohn’s Disease Endoscopic Index of Severity (CDEIS) is suitable to measure postoperative endoscopic CD activity in regulatory clinical trials. | 5 | 3–6 | Uncertain |
| The Rutgeerts score is suitable to measure postoperative endoscopic CD activity in regulatory clinical trials. | 6 | 5–7 | Uncertain |
| The modified Rutgeerts score is suitable to measure postoperative endoscopic CD activity in regulatory clinical trials. | 7 | 5–8 | Appropriate |
| Postoperative endoscopic CD activity in regulatory clinical trials should be measured in the neoterminal ileum only. | 4 | 2–6 | Uncertain |
| Postoperative endoscopic CD activity in regulatory clinical trials should be measured in the neoterminal ileum and at the anastomosis. | 7 | 5–8 | Appropriate |
| Postoperative endoscopic CD activity in regulatory clinical trials should be measured in the neoterminal ileum, at the anastomosis, and in the colon segment distal to the anastomosis. | 4 | 2–7 | Uncertain |
| Postoperative endoscopic CD activity in regulatory clinical trials should be measured in the neoterminal ileum, at the anastomosis, and all colonic segments. | 4 | 2–7 | Uncertain |
| The ileocolonic anastomosis should be scored separately from the neoterminal ileum and right colon. | 7 | 6–8 | Appropriate |
| The configuration of the anastomosis should be described in the endoscopic scoring report. | 8 | 6–9 | Appropriate |
| End-to-end and side-to-side anastomoses can be scored using the same endoscopic criteria. | 7 | 6–8 | Appropriate |
| The neoterminal ileum is demarcated from the anastomosis by the point where there is circumferential ileal mucosa in a single circular lumen. | 7 | 6–8 | Appropriate |
| Lesions confined to the ileocolonic anastomosis are best defined as those extending <1cm proximally into the ileum. | 7 | 6–8 | Appropriate |
| A minimum of 5cm of ileal intubation must be achieved for adequate assessment of postoperative endoscopic CD activity. | 7 | 6–7 | Appropriate |
| A minimum of 10cm of ileal intubation must be achieved for adequate assessment of postoperative endoscopic CD activity. | 7 | 6–8 | Appropriate |
| Assessment of endoscopic activity in the neoterminal ileum should be based on the last 10cm proximal to the anastomosis. | 7 | 6–8 | Appropriate |
Abbreviations: CD, Crohn’s disease; SES-CD, Simplified Endoscopic Score for Crohn’s Disease; CDEIS; Crohn’s Disease Endoscopic Index of Severity; IQR, interquartile range.
Evaluation of postoperative recurrence in both the neoterminal ileum and the anastomosis was considered appropriate, as was scoring of the anastomosis separately from the neoterminal ileum and right colon. The panel was uncertain regarding the appropriateness of assessment in the distal colonic segment, as recurrence at sites other than the anastomosis or neo-terminal ileum is infrequent. The panel was also uncertain about assessing lesions in the blind loop of a side-to-side anastomosis. Including the anastomotic configuration (eg, end-to-end, end-to-side, or side-to-side) in the endoscopic report and use of the same endoscopic criteria for scoring end-to-end and side-to-side anastomoses were appropriate. Although the panel acknowledged that the endoscopic appearance may differ depending on the type of anastomosis, development of indices specific to anastomosis anatomy was regarded as unfeasible, impractical to apply, and unlikely to be meaningful for the assessment of disease activity. The development of standardized definitions of the anastomosis and neoterminal ileum were viewed as more beneficial. Circumferential ileal mucosa in a single circular lumen was considered an appropriate demarcation between the anastomosis and the neoterminal ileum. Anastomotic lesions were best defined as those extending <1cm into the ileum. Minimum ileal intubation thresholds of 5cm and 10cm were considered appropriate for adequate assessment of disease activity, as was assessment of endoscopic activity in the neoterminal ileum based on the last 10cm proximal to the anastomosis. Although the panelists agreed in discussion that a 10-cm intubation is ideal, they also acknowledged that this is not always feasible due to anatomic constraints, adhesions, or impassable strictures.
Items for Endoscopic Assessment
Evaluation of ulcers, percentage of ulcerated surface (including aphthous ulcers), percentage of affected surface, and endoscopically impassable strictures were considered appropriate to include in the assessment of postoperative CD in the neoterminal ileum; there was uncertainty about including passable stenosis (Table 3). Although the terms “stenosis” and “stricture” are often used interchangeably, statements to differentiate these 2 terms were rated appropriate by the panelists: “stenosis” was defined as a clear decrease in the diameter of the lumen compared with the proximal and distal bowel, which does not insufflate with gas, and which may be associated with trauma when attempting to pass the colonoscope; and “stricture” was defined as a stenosis that is impassable without prior dilatation and a reasonable amount of pressure when attempting to pass the colonoscope. The panelists acknowledged that the ability to pass a colonoscope would depend on the size of the instrument, which would vary by manufacturer and endoscopist preference for use of an adult or pediatric colonoscope.
TABLE 3.
Standardization of items for endoscopic assessment in regulatory trials for the prevention of postoperative Crohn’s disease.
| Item | Median | IQR | Appropriateness |
|---|---|---|---|
| Ulcers should be included in the assessment of postoperative endoscopic CD activity in the neoterminal ileum. | 8 | 5–8 | Appropriate |
| The percentage of ulcerated surface should be included in the assessment of postoperative endoscopic CD activity in the neoterminal ileum. | 7 | 6–8 | Appropriate |
| The percentage of affected surface should be included in the assessment of endoscopic postoperative CD activity in the neoterminal ileum. | 7 | 6–8 | Appropriate |
| Strictures should be included in the assessment of postoperative endoscopic CD activity. | 7 | 5–8 | Appropriate |
| The presence of stenosis should be included in the assessment of postoperative endoscopic CD activity in the neoterminal ileum. | 6 | 5–8 | Uncertain |
| Endoscopically normal ileal mucosa is best described by: the absence of erosions and ulcers. | 8 | 7–8 | Appropriate |
| Depth of ulceration should be estimated relative to a closed biopsy forceps. | 5 | 5–7 | Uncertain |
| Ulcers at the ileocolonic anastomosis should be assessed as ulcers, regardless of whether there is suspicion that these are ischemic. | 8 | 7–9 | Appropriate |
Abbreviations: CD, Crohn’s disease; IQR, interquartile range.
Normal ileal mucosa was appropriately defined as the absence of erosions and ulcers. For the assessment of ulcers in the neoterminal ileum, appropriate ordinal categories included size (<0.5cm, 0.5–2cm, >2cm) and the percentage of ulcerated surface (<10%, 10%–30%, >30%). There was uncertainty about the appropriateness of assessing ulcer depth in 2-dimensional endoscopic images, even when reference methods such as a closed biopsy forceps are used to assist evaluation of this item. Evaluation of the circumferential extent and size of ulcers at the ileocolonic anastomosis on an ordinal scale was considered appropriate, whereas there was uncertainty regarding assessment of their number and depth. The panelists proposed scoring of anastomotic ulcers irrespective of purported etiology, as ischemic and inflammatory ulcers cannot be reliably distinguished endoscopically. However, because of potential differences in etiology, a greater weight for lesions in the neoterminal ileum compared with those found in the anastomosis was considered appropriate when assessing their contribution to an overall endoscopic activity score. The appropriateness of including granularity, erythema, and friability in the assessment of postoperative recurrence of CD was uncertain, especially because some of these features are unlikely to be reliably assessed or valid measures of disease activity in the small bowel. Assessment of pseudopolyps and healed ulcerations was considered inappropriate, as these findings are not considered representative of active inflammation.
Discussion
Given the high risk for disease recurrence, effective medical treatment for prevention of postoperative CD is a substantial unmet need. Drug development for this indication will be dependent upon standardized methods for the design and conduct of clinical trials. Specifically, a valid configuration for a regulatory trial of postoperative prevention of CD has proven difficult to define because of considerable heterogeneity in multiple facets related to trial design for this indication, including appropriate eligibility criteria, study duration, outcomes, efficacy end points, and conventions for disease activity assessment. This variability, which is evident in the current literature, confounds drug development; this in turn inhibits investment into pivotal trials for this indication. To this end, we have employed RAND/UCLA methods to develop recommendations to standardize the design of randomized controlled trials (RCTs) for evaluation of medical therapy for prevention of postoperative CD recurrence, in addition to those focused on appropriate methods for assessment of endoscopic disease activity.
Patients with completely resected ileal or ileocolonic CD should be included in regulatory trials of postoperative prevention of CD; this is in contrast to trials of luminal CD, which enroll patients with evidence of active inflammation at baseline. Clinical decisions in the postoperative period depend on risk factors for disease recurrence such as smoking, disease phenotype (penetrating or fistulizing disease relative to stricturing disease), and prior resection.19 However, it is unclear whether these risk factors should be employed as inclusion criteria for clinical trials, as their relative importance and potential interactions between them are unknown. An alternative strategy may be to control for the differential risk of postoperative CD recurrence by stratifying patient randomization according to the presence of risk factors. To ensure an adequate number of recurrence events, the ideal population for a trial of prophylaxis for early postoperative CD includes patients with multiple risk factors for recurrence. Both the POCER and PREVENT trials stratified randomization by baseline risk of relapse, defined by ≥1 vs <1 risk factor, including smoking, penetrating phenotype, or previous surgery. Although differences in CDAI-based remission were not observed in either trial, in the POCER study this risk model did differentiate the probability of clinical relapse among high- vs low-risk patients receiving active care (42% vs 19%). It remains unclear if the optimal method of controlling for confounding risk factors is to evaluate their absence or presence or whether multiple risk factors are relevant. For example, a recent study by Joustra et al showed that any combination of ≥3 risk factors was associated with endoscopic recurrence.20
Acknowledging that histologic inflammation can recur within days of surgery21 and that endoscopic recurrence rates may approach 75% at 3 months postresection,22 the RAND panel supported early randomization within 2 to 4 weeks of surgery. This recommendation has practical implications for trial recruitment, as the time required for clearance from postoperative complications and washout of preoperative medical therapies prior to enrolment may jeopardize patient eligibility, although it is currently unclear whether the requirement for the latter is relevant for trials designed for the prevention of postoperative recurrence. A trial duration of 52 weeks was considered appropriate to balance feasibility, trial cost, and the need for a sufficient number of recurrence events. The potential for assessment of secondary end points beyond 1 year was also proposed. Given that an endoscopic primary end point was voted as appropriate in the final round, an interim end point at an earlier time point (eg, 3 to 6 months) was also considered in discussion after voting; this would allow patients in the placebo group with early endoscopic recurrence to cross over to the active treatment arm.
There was substantial debate amongst the panel regarding an appropriate comparator group in a prevention trial. Ultimately, the absence of clear standard of practice, validated stratification schemes for interventions, or regulatory approved medical therapies for the prevention of postoperative CD recurrence justified the inclusion of a placebo arm. In support of this position, a recent network meta-analysis indicated that 5-nitroimidazole antibiotics and 5-aminosalicylates were no better than placebo at preventing endoscopic recurrence at 12 months, whereas thiopurines were only marginally more effective.23 Moreover, a Cochrane review focusing on thiopurines in postoperative CD found no difference in endoscopic recurrence compared with placebo, but 9 of 10 included trials were considered to have either unclear or high risk of bias.24 Although recent investigator-initiated studies have included active arms,25 panelists were uncertain about adopting this approach in regulatory trials based upon the lack of approved comparators and the relatively poor quality of data supporting the candidate “active” agents. Furthermore, placebo arms remain essential for evaluation of safety outcomes.
Despite regulatory recommendations for coprimary end points of symptomatic and endoscopic remission, the panelists were uncertain about this approach in trials for the prevention of CD recurrence and favored an endoscopic primary end point. The inconsistent relationship between the presence of symptoms and endoscopic disease activity at a given time in postoperative patients contributed to hesitancy to include symptomatic remission in the primary end point. Previous cross-sectional studies have mostly described a weak correlation between these outcomes,26,27 and although a trend towards higher CDAI scores in patients with endoscopic recurrence has been observed, the use of the CDAI alone would result in substantial underestimation of endoscopic disease recurrence.28 This limitation is supported by data from the PREVENT trial where the rate of endoscopic recurrence substantially surpassed the rate of CDAI-based recurrence.8 These uncertainties require further discussion with regulatory authorities. The previous position that the primary end point for postoperative CD trials include a coprimary end point defined by both symptomatic and endoscopic outcomes has likely led to a (near complete) lack of investment in drug development for this indication and, consequently, no approved therapies. An evolution to an achievable primary endoscopic outcome would likely enable substantial future investments in trials studying this indication.
In contrast to the ability of symptoms to predict endoscopic recurrence, multiple studies have consistently shown a strong association between endoscopic recurrence and the risk for symptomatic relapse. In a meta-analysis of 8 RCTs, patients who experienced endoscopic relapse were 10 times more likely to experience subsequent clinical relapse (relative risk 10.77; 95% confidence interval [CI], 4.08–28.40). In cohort studies, the corresponding relative risk was 21.33 (95% CI, 9.55–47.66). This association underscores the potential importance of endoscopic disease activity as a surrogate measure for clinical recurrence, a concept that has been partially validated in the POCER trial. In this trial, medical therapy was escalated based on endoscopic findings and associated with a subsequent (albeit nonsignificant) reduction in the risk of clinical relapse among patients undergoing early colonoscopy. In the absence of trials definitively validating a surrogate measure and given the limitations of symptom-based instruments described earlier, only a primary end point based on endoscopic disease activity was considered appropriate for regulatory trials assessing the effectiveness of a therapy for prevention of postoperative CD recurrence.
The value of standardized conventions for endoscopic assessment and central reader training to improve the reliability of scoring of disease activity has been described in prior studies. In addition to developing a framework for RCT design in postoperative CD, the panel explored important components of an endoscopic index for this indication. As existing indices were designed for CD outside the context of surgery or to assess postoperative prognosis, the panel identified the need for the development and validation of a specific endoscopic index for postoperative CD as a research priority. Several considerations for the development of a postoperative CD endoscopic instrument were considered. First, an appropriate definition of endoscopically normal ileal mucosa included the absence of erosions or ulcers, which reflects the fact that ulcers remain the defining feature of active disease in the postoperative setting, despite the possibility of alternative causes of ulceration. Because erythema may be the result of anastomotic configuration, the panelists did not consider the absence of this finding appropriate for inclusion in the definition of normal ileal mucosa. In contrast to CD outside the postoperative setting, where the assessment of ulcers is associated with high inter-rater reliability,12 numerically lower reliability was observed with the modified Rutgeerts score for the assessment of ulcers,29 potentially reflecting the requirement for counting ulcers or ambiguity when distinguishing ileal from anastomotic lesions.
Surgically altered anatomy is a considerable barrier to accurate and reliable endoscopic assessment. The panel determined that recurrence should be assessed in the neoterminal ileum and at the ileocolonic anastomosis, although there was uncertainty about scoring distal colonic segments in case of disease extension and for lesions in the blind loop of a side-to-side anastomosis. Intubation of at least 5cm of the neoterminal ileum was considered appropriate; the panel acknowledged that deeper intubation may not always be possible due to surgical factors. To further standardize assessment and increase reliability, the panel identified entrance into a single circular lumen with circumferential ileal mucosa as an appropriate demarcation between the anastomosis and the neoterminal ileum. In this regard, there may also be an advantage in further exploring the endoscopic procedure itself, including the mandated use of a pediatric colonoscope to facilitate intubation of the terminal ileum and formal training regarding routinely retroflexing at the anastomosis to facilitate identification and intubation of side-to-side anastomoses. The panelists supported the description of anastomotic configuration in the endoscopy report; an endoscopic index for assessment of disease activity, regardless of the type of anastomosis, was considered appropriate to improve feasibility. Studies are currently underway to explore the relationship between anastomotic configuration and postsurgical outcomes, and this recommendation may change based on the results of this ongoing research. The panel recognized the paucity of evidence on the natural history of endoscopic appearance of novel anastomosis types, such as the Kono-S anastomosis.30
The inclusion of additional characteristics of anastomotic lesions for assessment, such as size and circumferential extent, was also appropriate. These findings are included in the POCER index15 and REMIND score.16 These novel indices and the modified Rutgeerts score all imply that ileal recurrence is potentially more relevant than anastomotic recurrence. However, evidence to support this dichotomy is conflicting at best, with accurate interpretation confounded by the use of variable outcome definitions and duration of follow-up.16,31–33 Although the panel supported the possibility of differential weighting of lesions in the anastomosis and the neoterminal ileum, the uncertainty of evidence was acknowledged, and empirical data are required.
Our study has several strengths. We included an international panel of inflammatory bowel disease (IBD) gastroenterologists with extensive experience in trial design and endoscopic assessment of both native bowel and postoperative CD in adult patients. We addressed a broad range of issues pertaining to trials in postoperative CD and established a context for development of a novel endoscopic index for use in the postsurgical setting. Nonetheless, some important limitations should be acknowledged. First, voting for many of the statements was based on expert opinion, as there was little evidence to guide decision-making. This circumstance is reflected in the high proportion of statements for which appropriateness was uncertain. Second, the recommendations were focused on regulatory trials and may not be generalizable to observational trials. Third, the modified RAND/UCLA methodology does not force consensus; therefore, some ratings of appropriateness may appear contradictory to others (eg, both 5cm and 10cm were considered appropriate minimum acceptable depths of neoterminal ileal intubation). Fourth, given the breadth of the initiative, the large number of complex statements may have contributed to panelist fatigue; however, there was no increase in uncertain statements towards the end of the survey, and all panelists voted on all the statements. Additionally, a further round of voting was not considered, as there was only 1 statement voted as uncertain based on disagreement (the other uncertain statements were based on the median panel rating). Finally, given the scope and context of this work, the panel consisted of gastroenterologists with specific expertise in CD and clinical trial design. Although this group of internationally recognized leaders may have been well positioned to provide expert guidance, we did not include colorectal surgeons, radiologists, IBD nurses, or histopathologists.
Conclusions
In summary, we developed a framework for regulatory trial design for the prevention of postoperative CD and endoscopic assessment of postoperative disease activity in collaboration with a large international panel. The main finding from this exercise is that regulatory trials assessing medical therapy for the prevention of postoperative CD should be designed as randomized placebo-controlled studies with an endoscopic primary end point. These recommendations will also help inform the development of a new index specific for assessment of postoperative endoscopic CD activity. Use of a valid, reliable, and responsive endoscopic index will improve disease activity assessment in future trials and lead to more efficient drug development in this field.
Supplementary Material
Glossary
Abbreviations
- CD
Crohn’s disease
- CDAI
Crohn’s Disease Activity Index
- POCER
Postoperative Crohn’s Endoscopic Recurrence
- RAND
Research and Development
- UCLA
University of California Los Angeles
- CDEIS
Crohn’s Disease Endoscopic Index of Severity
- SES-CD
Simplified Endoscopic Score for Crohn’s Disease
- RCT
randomized controlled trial
- CI
confidence interval
- IBD
inflammatory bowel disease
Contributor Information
Jurij Hanzel, Department of Gastroenterology, UMC Ljubljana, Ljubljana, Slovenia; Alimentiv Inc., London, Ontario, Canada.
Vipul Jairath, Alimentiv Inc., London, Ontario, Canada; Division of Gastroenterology, Western University, London, Ontario, Canada; Department of Epidemiology and Biostatistics, Western University, London, Ontario, Canada.
Peter De Cruz, Department of Gastroenterology, The Austin Hospital, Melbourne, Australia; Department of Medicine, Austin Academic Centre, University of Melbourne, Melbourne, Australia.
Leonardo Guizzetti, Alimentiv Inc., London, Ontario, Canada.
Lisa M Shackelton, Alimentiv Inc., London, Ontario, Canada.
Peter Bossuyt, Department of Gastroenterology, Imelda General Hospital, Bonheiden, Belgium.
Marjolijn Duijvestein, Department of Gastroenterology and Hepatology, Amsterdam University Medical Centers, Amsterdam Gastroenterology Endocrinology Metabolism (AGEM), University of Amsterdam, Amsterdam, the Netherlands.
Parambir S Dulai, Division of Gastroenterology, University of California San Diego, La Jolla, California, USA.
Johannes Grossmann, Department of Internal Medicine I, Bethesda Hospital, Johanniter GmbH, Mönchengladbach, Germany.
Robert P Hirten, Icahn School of Medicine, The Susan & Leonard Feinstein IBD Center Division of Gastroenterology, Mount Sinai, New York City, New York, USA.
Reena Khanna, Division of Gastroenterology, Western University, London, Ontario, Canada.
Julian Panes, Hospital Clinic de Barcelona, IDIBAPS, CIBERehd, Barcelona, Spain.
Laurent Peyrin-Biroulet, Department of Gastroenterology and Inserm NGERE U1256, University Hospital of Nancy, University of Lorraine,Vandoeuvre-lès-Nancy, France.
Miguel Regueiro, Department of Gastroenterology, Hepatology, and Nutrition, Cleveland Clinic, Cleveland, Ohio, USA.
David T Rubin, University of Chicago Medicine Inflammatory Bowel Disease Center, Chicago, Illinois, USA.
Siddharth Singh, Division of Gastroenterology, University of California San Diego, La Jolla, California, USA.
Ryan W Stidham, Department of Internal Medicine, Division of Gastroenterology and Hepatology, University of Michigan Medical School, Ann Arbor, Michigan, USA; Department of Computational Medicine and Bioinformatics. University of Michigan Medical School, Ann Arbor, Michigan, USA.
William J Sandborn, Alimentiv Inc., London, Ontario, Canada; Division of Gastroenterology, University of California San Diego, La Jolla, California, USA.
Brian G Feagan, Alimentiv Inc., London, Ontario, Canada; Division of Gastroenterology, Western University, London, Ontario, Canada; Department of Epidemiology and Biostatistics, Western University, London, Ontario, Canada.
Geert R D’Haens, Department of Gastroenterology and Hepatology, Amsterdam University Medical Centers, Amsterdam Gastroenterology Endocrinology Metabolism (AGEM), University of Amsterdam, Amsterdam, the Netherlands.
Christopher Ma, Alimentiv Inc., London, Ontario, Canada; Division of Gastroenterology and Hepatology, University of Calgary, Calgary, Alberta, Canada; Department of Community Health Sciences, Cumming School of Medicine, University of Calgary, Calgary, Alberta, Canada.
Conflicts of Interest
JH has received speaker’s fees from Abbvie, Janssen, and Takeda; consulting fees from Alimentiv Inc (formerly Robarts Clinical Trials, Inc.).
VJ has received consulting fees from AbbVie, Eli Lilly, GlaxoSmithKline, Arena pharmaceuticals, Genentech, Pendopharm, Pfizer, Fresenius Kabi, Bristol Myers Squibb, Roche, Ferring, Sandoz, Merck, Takeda, Janssen, Alimentiv Inc. (formerly Robarts Clinical Trials Inc.), Topivert, Celltrion, Mylan, and Gilead; and speaker’s fees from Takeda, Janssen, Shire, Ferring, Abbvie, and Pfizer.
PDC has received research grant support from AbbVie, Janssen, Takeda and Schering-Plough and has been an advisory board member and speaker at symposia sponsored by Janssen, Celltrion and AbbVie.
LG is an employee of Alimentiv Inc. (formerly Robarts Clinical Trials, Inc.).
LMS has received consulting fees from Alimentiv Inc. (formerly Robarts Clinical Trials, Inc.).
PB has received grant support for research from Abbvie, Amgen, Janssen, Mundipharma, Mylan, Pfizer and personal fees (consulting, lecture) from Abbvie, Arena pharmaceuticals, Bristol Myers Squibb, Celltrion, Hospira, Galapagos, Gilead, Guidepont, Janssen, Merck Sharp & Dohme, Mundipharma, Pentax Medical, Pfizer, PSI-CRO, Roche, Sandoz, Takeda.
MD has received advisory fees from Echo Pharma and Alimentiv, Inc (formerly Robarts Clinical Trials, Inc); speaker fees from Janssen, Merck & Co., Inc., Pfizer, Takeda and Tillotts Pharma; and nonfinancial support from Dr. Falk Pharm. These activities were all outside of the submitted work.
PSD has received research and/or consulting fees from Takeda, Pfizer, BMS, Janssen, Abbvie, Gilead, Prometheus.
JG has received speakers fees from Abbvie, Janssen, Falk, consulting fees from Alimentiv, Janssen, Falk, Abbvie; stock from Takeda, Gilead, Roche, Fresenius.
RPH has received consulting fees from HealthMode,Inc, Janssen Pharmaceuticals. Research support from Intralytix Inc. and a Crohn’s and Colitis Foundation Career Development Award (grant number 607934).
RK has received consulting/speaking fees from AbbVie, Amgen, Encycle, Innomar, Gilead, Janssen, Lilly, Merck, Pendopharm, Pfizer, Roche/Genetech, Alimentiv (formerly Robarts Clinical Trials), Shire, and Takeda Canada outside the submitted work; research fees from Roche/Genentech.
JP has received consulting fees from AbbVie, Arena Pharmaceuticals, Boehringer Ingelheim, Celgene, Celltrion, Ferring, Genentech, GlaxoSmithKline, Janssen, Nestle, Origo, Pandion, Progenity, Pfizer, Robarts, Roche, Takeda, Theravance, and Wassermann; speaker fees from AbbVie, Biogen, Ferring, Janssen, Pfizer, and Takeda; and research funding from AbbVie and Pfizer.
LP-B has received speakers, consulting, or advisory board member fees from Merck Sharp and Dohme, AbbVie, Janssen, Genentech, Mitsubishi, Ferring, Norgine, Tillotts, Vifor, Hospira/Pfizer, Celltrion, Takeda, Biogaran, Boerhinger-Ingelheim, Lilly, HAC Pharma, Index Pharmaceuticals, Amgen, Sandoz, Forward Pharma, Celgene, Biogen, Lycera, Samsung Bioepis, and Theravance.
MR has received advisory fees from Abbvie, Janssen, UCB, Takeda, Pfizer, Miraca Labs, Amgen, Celgene, Seres, Allergan, Genentech, Gilead, Salix, Prometheus, Lilly, TARGET Pharma Solutions, ALFASIGMA, S.p.A., Bristol Meyer Squibb (BMS).
DTR has received grant support from Takeda; and has served as a consultant for Abbvie, Altrubio, Allergan Inc., Arena Pharmaceuticals, Bellatrix Pharmaceuticals, Boehringer Ingelheim Ltd., Bristol-Myers Squibb, Celgene Corp/Syneos, Connect BioPharma, GalenPharma/Atlantica, Genentech/Roche, Gilead Sciences, InDex Pharmaceuticals, Ironwood Pharmaceuticals, Iterative Scopes, Janssen Pharmaceuticals, Lilly, Materia Prima, Pfizer, Prometheus Biosciences, Reistone, Takeda, and Techlab Inc.
SS has received research grants from AbbVie and Janssen; personal fees from Pfizer for ad hoc grant review.
RWS has received research grants from Abbvie and Janssen; served as a consultant or advisory boards for AbbVie, Janssen, Takeda, Gilead, Eli Lilly, Exact Sciences, Evergreen Pharmaceuticals, and CorEvitas; hold intellectual property on endoscopic analysis technologies licensed by the University of Michigan to AMI, Inc.
WJS has received research grants from Abbvie, Abivax, Arena Pharmaceuticals, Boehringer Ingelheim, Celgene, Genentech, Gilead Sciences, Glaxo Smith Kline, Janssen, Lilly, Pfizer, Prometheus Biosciences, Seres Therapeutics, Shire, Takeda, Theravance Biopharma; consulting fees from Abbvie, Abivax, Admirx, Alfasigma, Alimentiv (previously Robarts Clinical Trials, owned by Alimentiv Health Trust), Alivio Therapeutics, Allakos, Amgen, Applied Molecular Transport, Arena Pharmaceuticals, Bausch Health (Salix), Beigene, Bellatrix Pharmaceuticals, Boehringer Ingelheim, Boston Pharmaceuticals, Bristol Meyers Squibb, Celgene, Celltrion, Cellularity, Cosmo Pharmaceuticals, Escalier Biosciences, Equillium, Forbion, Genentech/Roche, Gilead Sciences, Glenmark Pharmaceuticals, Gossamer Bio, Immunic (Vital Therapies), Index Pharmaceuticals, Intact Therapeutics, Janssen, Kyverna Therapeutics, Landos Biopharma, Lilly, Oppilan Pharma, Otsuka, Pandion Therapeutics, Pfizer, Progenity, Prometheus Biosciences, Prometheus Laboratories, Protagonists Therapeutics, Provention Bio, Reistone Biopharma, Seres Therapeutics, Shanghai Pharma Biotherapeutics, Shire, Shoreline Biosciences, Sublimity Therapeutics, Surrozen, Takeda, Theravance Biopharma, Thetis Pharmaceuticals, Tillotts Pharma, UCB, Vendata Biosciences, Ventyx Biosciences, Vimalan Biosciences, Vivelix Pharmaceuticals, Vivreon Biosciences, Zealand Pharma; stock or stock options from Allakos, BeiGene, Gossamer Bio, Oppilan Pharma, Prometheus Biosciences, Prometheus Laboratories Progenity, Shoreline Biosciences, Ventyx Biosciences, Vimalan Biosciences, Vivreon Biosciences; and is an employee at Shoreline Biosciences. Spouse: Iveric Bio—consultant, stock options; Progenity—stock; Oppilan Pharma—consultant, stock options; Prometheus Biosciences—employee, stock, stock options; Prometheus Laboratories—stock, stock options, consultant; Ventyx Biosciences—stock, stock options; Vimalan Biosciences—stock, stock options.
BGF has received grant/research support from AbbVie Inc., Amgen Inc., AstraZeneca/MedImmune Ltd., Atlantic Pharmaceuticals Ltd., Boehringer-Ingelheim, Celgene Corporation, Celltech, Genentech Inc/Hoffmann-La Roche Ltd., Gilead Sciences Inc., GlaxoSmithKline (GSK), Janssen Research & Development LLC., Pfizer Inc., Receptos Inc./Celgene International, Sanofi, Santarus Inc., Takeda Development Center Americas Inc., Tillotts Pharma AG, and UCB; consulting fees from Abbott/AbbVie, Akebia Therapeutics, Allergan, Amgen, Applied Molecular Transport Inc., Aptevo Therapeutics, Astra Zeneca, Atlantic Pharma, Avir Pharma, Biogen Idec, BioMx Israel, Boehringer-Ingelheim, Bristol-Myers Squibb, Calypso Biotech, Celgene, Elan/Biogen, EnGene, Ferring Pharma, Roche/Genentech, Galapagos, GiCare Pharma, Gilead, Gossamer Pharma, GSK, Inception IBD Inc, JnJ/Janssen, Kyowa Kakko Kirin Co Ltd., Lexicon, Lilly, Lycera BioTech, Merck, Mesoblast Pharma, Millennium, Nestle, Nextbiotix, Novonordisk, Pfizer, Prometheus Therapeutics and Diagnostics, Progenity, Protagonist, Receptos, Salix Pharma, Shire, Sienna Biologics, Sigmoid Pharma, Sterna Biologicals, Synergy Pharma Inc., Takeda, Teva Pharma, TiGenix, Tillotts, UCB Pharma, Vertex Pharma, Vivelix Pharma, VHsquared Ltd., and Zyngenia; speakers bureau fees from Abbott/AbbVie, JnJ/Janssen, Lilly, Takeda, Tillotts, and UCB Pharma; is a scientific advisory board member for Abbott/AbbVie, Allergan, Amgen, Astra Zeneca, Atlantic Pharma, Avaxia Biologics Inc., Boehringer-Ingelheim, Bristol-Myers Squibb, Celgene, Centocor Inc., Elan/Biogen, Galapagos, Genentech/Roche, JnJ/Janssen, Merck, Nestle, Novartis, Novonordisk, Pfizer, Prometheus Laboratories, Protagonist, Salix Pharma, Sterna Biologicals, Takeda, Teva, TiGenix, Tillotts Pharma AG, and UCB Pharma; and is the Senior Scientific Officer of Alimentiv Inc. (formerly Robarts Clinical Trials, Inc.).
GRD has received consultancy and/or speakers’ fees from AbbVie, ActoGeniX, AIM, Allergan, Amgen, Arena Pharmaceuticals, Boehringer Ingelheim, Celgene/Receptos, Celltrion, Cosmo Technologies, Elan Pharmaceuticals, enGene, Falk, Ferring, Galapagos, Genentech, Gilead Sciences, Giuliani SpA, Given Imaging, Gossamer Bio, GSK, Janssen, Lilly, MSD, Neovacs, Novo Nordisk, Otsuka, PDL BioPharma, Pfizer, Progenity, Prometheus Laboratories, Robarts Clinical Trials, Salix, Schering-Plough, Seres/Nestlé, SetPoint, Shire, Takeda, Tillotts, UCB Pharma, Versant, and Vifor Pharma; research grants from AbbVie, Falk, Given Imaging, Janssen, MSD, and PhotoPill; and speaking honoraria from AbbVie, Ferring, MSD, Norgine, Shire, Tillotts, Tramedico, and UCB Pharma.
CM has received consulting fees from AbbVie, Amgen, AVIR Pharma Inc, Bristol Myers Squibb, Ferring, Fresenius Kabi, Janssen, McKesson, Mylan, Takeda, Pfizer, Roche, Alimentiv (formerly Robarts Clinical Trials Inc.); speaker’s fees from AbbVie, AVIR Pharma Inc, Janssen, Takeda, and Pfizer; research support from Pfizer.
In total, 8 of the 19 panelists (JH, V.J., JG, RK, WJS, B.G.F., GRD, C.M.) are consultants with Alimentiv Inc. (formerly Robarts Clinical Trials, Inc.) a contract research organization that provides centralized endoscopy and histopathology imaging solutions for clinical trials. The current study was designed to ensure that a majority of the panelists were not affiliated with Alimentiv Inc.
Author Contributions
Concept and design: J.H., V.J., B.G.F., and C.M.
Data analysis and interpretation: J.H., V.J., L.G., L.M.S., B.G.F., and C.M.
Manuscript drafting: J.H., V.J., L.M.S., B.G.F., and C.M.
Manuscript editing for important intellectual content: all authors.
All authors have reviewed, contributed to, and approved the final version of this manuscript.
Data Availability
Data, analytic methods, and study materials will be made available to other researchers upon request (christopher.ma@ucalgary.ca).
References
- 1. Tsai L, Ma C, Dulai PS, et al. Contemporary risk of surgery in patients with ulcerative colitis and Crohn’s disease: a meta-analysis of population-based cohorts. Clin Gastroenterol Hepatol 2021;19:2031–2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ma C, Moran GW, Benchimol EI, et al. Surgical rates for Crohn’s disease are decreasing: a population-based time trend analysis and validation study. Am J Gastroenterol. 2017;112:1840–1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Frolkis AD, Dykeman J, Negrón ME, et al. Risk of surgery for inflammatory bowel diseases has decreased over time: a systematic review and meta-analysis of population-based studies. Gastroenterology. 2013;145:996–1006. [DOI] [PubMed] [Google Scholar]
- 4. Frolkis AD, Lipton DS, Fiest KM, et al. Cumulative incidence of second intestinal resection in Crohn’s disease: a systematic review and meta-analysis of population-based studies. Am J Gastroenterol. 2014;109:1739–1748. [DOI] [PubMed] [Google Scholar]
- 5. European Medicines Agency. Guideline on the development of new medicinal products for the treatment of Crohn’s Disease. 2016. Accessed August 25, 2018. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2016/07/WC500211430.pdf
- 6. Vítek L. Bile acid malabsorption in inflammatory bowel disease. Inflamm Bowel Dis. 2015;21:476–483. [DOI] [PubMed] [Google Scholar]
- 7. Klaus J, Spaniol U, Adler G, et al. Small intestinal bacterial overgrowth mimicking acute flare as a pitfall in patients with Crohn’s disease. Bmc Gastroenterol. 2009;9:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Regueiro M, Feagan BG, Zou B, et al. ; PREVENT Study Group . Infliximab reduces endoscopic, but not clinical, recurrence of Crohn’s disease after ileocolonic resection. Gastroenterology. 2016;150:1568–1578. [DOI] [PubMed] [Google Scholar]
- 9. De Cruz P, Kamm MA, Hamilton AL, et al. Crohn’s disease management after intestinal resection: a randomised trial. Lancet. 2015;385:1406–1417. [DOI] [PubMed] [Google Scholar]
- 10. Rutgeerts P, Geboes K, Vantrappen G, et al. Predictability of the postoperative course of Crohn’s disease. Gastroenterology. 1990;99:956–963. [DOI] [PubMed] [Google Scholar]
- 11. Hirten RP, Mashiana S, Cohen BL, et al. Ileocolic anastomotic inflammation after resection for Crohn’s disease indicates disease recurrence: a histopathologic study. Scand J Gastroenterol. 2020;55:795–799. [DOI] [PubMed] [Google Scholar]
- 12. Jairath V, Zou G, Parker CE, et al. Systematic review and meta-analysis: placebo rates in induction and maintenance trials of ulcerative colitis. J Crohns Colitis. 2016;10:607–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mary JY, Modigliani R. Development and validation of an endoscopic index of the severity for Crohn’s disease: a prospective multicentre study. Groupe d’Etudes Thérapeutiques des Affections Inflammatoires du Tube Digestif (GETAID). Gut. 1989;30:983–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Daperno M, D’Haens G, Van Assche G, et al. Development and validation of a new, simplified endoscopic activity score for Crohn’s disease: the SES-CD. Gastrointest Endosc. 2004;60:505–512. [DOI] [PubMed] [Google Scholar]
- 15. De Cruz P, Kamm MA, Hamilton AL, et al. P226. The first validated post-operative endoscopic Crohn’s disease index: the POCER index— identification of key endoscopic prognostic factors. J Crohn’s Colitis. 2016;Suppl_1:S206. [Google Scholar]
- 16. Hammoudi N, Auzolle C, Tran Minh ML, et al. Postoperative endoscopic recurrence on the neoterminal ileum but not on the anastomosis is mainly driving long-term outcomes in Crohn’s disease. Am J Gastroenterol. 2020;115:1084–1093. [DOI] [PubMed] [Google Scholar]
- 17. Domènech E, Mañosa M, Bernal I, et al. Impact of azathioprine on the prevention of postoperative Crohn’s disease recurrence: results of a prospective, observational, long-term follow-up study. Inflamm Bowel Dis. 2008;14:508–513. [DOI] [PubMed] [Google Scholar]
- 18. Fitch K, Bernstein S, Aguilar M, et al. The RAND/UCLA Appropriateness Method User’s Manual. Santa Monica, CA: RAND Corporation; 2001. Accessed April 8, 2021. https://www.rand.org/pubs/monograph_reports/MR1269.html [Google Scholar]
- 19. Auzolle C, Nancey S, Tran-Minh ML, et al. ; REMIND Study Group Investigators . Male gender, active smoking and previous intestinal resection are risk factors for postoperative endoscopic recurrence in Crohn’s disease: results from a prospective cohort study. Aliment Pharmacol Ther. 2018;48:924–932. [DOI] [PubMed] [Google Scholar]
- 20. Joustra V, Duijvestein M, Mookhoek A, et al. Natural history and risk stratification of recurrent Crohn’s disease after ileocolonic resection: a multicenter retrospective cohort study. Inflamm Bowel Dis. 2022;28:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. D’Haens GR, Geboes K, Peeters M, et al. Early lesions of recurrent Crohn’s disease caused by infusion of intestinal contents in excluded ileum. Gastroenterology. 1998;114:262–267. [DOI] [PubMed] [Google Scholar]
- 22. Rutgeerts P, Hiele M, Geboes K, et al. Controlled trial of metronidazole treatment for prevention of Crohn’s recurrence after ileal resection. Gastroenterology. 1995;108:1617–1621. [DOI] [PubMed] [Google Scholar]
- 23. Burr NE, Hall B, Hamlin PJ, et al. Systematic review and network meta-analysis of medical therapies to prevent recurrence of post-operative Crohn’s disease. J Crohns Colitis. 2019;13:693–701. [DOI] [PubMed] [Google Scholar]
- 24. Gjuladin-Hellon T, Iheozor-Ejiofor Z, Gordon M, Akobeng AK. Azathioprine and 6-mercaptopurine for maintenance of surgically-induced remission in Crohn’s disease. Cochrane Database Syst Rev. 2019;8:CD010233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. López-Sanromán A, Vera-Mendoza I, Domènech E, et al. Adalimumab vs azathioprine in the prevention of postoperative Crohn’s disease recurrence. A GETECCU randomised trial. Journal of Crohn’s and Colitis. 2017;11(11):1293-1301. [DOI] [PubMed] [Google Scholar]
- 26. Viscido A, Corrao G, Taddei G, Caprilli R. “Crohn’s disease activity index” is inaccurate to detect the post-operative recurrence in Crohn’s disease. A GISC study. Gruppo Italiano per lo Studio del Colon e del Retto. Ital J Gastroenterol Hepatol. 1999;31:274–279. [PubMed] [Google Scholar]
- 27. Regueiro M, Kip KE, Schraut W, et al. Crohn’s disease activity index does not correlate with endoscopic recurrence one year after ileocolonic resection. Inflamm Bowel Dis. 2011;17:118–126. [DOI] [PubMed] [Google Scholar]
- 28. Walters TD, Steinhart AH, Bernstein CN, et al. Validating Crohn’s disease activity indices for use in assessing postoperative recurrence. Inflamm Bowel Dis. 2011;17:1547–1556. [DOI] [PubMed] [Google Scholar]
- 29. Ma C, Gecse KB, Duijvestein M, et al. Reliability of endoscopic evaluation of postoperative recurrent Crohn’s disease. Clin Gastroenterol Hepatol. 2020;18:2139–2141.e2. [DOI] [PubMed] [Google Scholar]
- 30. Luglio G, Rispo A, Imperatore N, et al. Surgical prevention of anastomotic recurrence by excluding mesentery in Crohn’s disease: the SuPREMe-CD study - a randomized clinical trial. Ann Surg. 2020;272:210–217. [DOI] [PubMed] [Google Scholar]
- 31. Bayart P, Duveau N, Nachury M, et al. Ileal or anastomotic location of lesions does not impact rate of postoperative recurrence in Crohn’s disease patients classified i2 on the rutgeerts score. Dig Dis Sci. 2016;61:2986–2992. [DOI] [PubMed] [Google Scholar]
- 32. Ollech JE, Aharoni-Golan M, Weisshof R, et al. Differential risk of disease progression between isolated anastomotic ulcers and mild ileal recurrence after ileocolonic resection in patients with Crohn’s disease. Gastrointest Endosc. 2019;90:269–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rivière P, Vermeire S, Irles-Depe M, et al. No change in determining Crohn’s disease recurrence or need for endoscopic or surgical intervention with modification of the rutgeerts’ scoring system. Clin Gastroenterol Hepatol. 2019;17:1643–1645. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data, analytic methods, and study materials will be made available to other researchers upon request (christopher.ma@ucalgary.ca).