Abstract
Background
Increased p16 INK4a (p16) expression is directly related to cellular senescence and is a robust biomarker of aging in humans. Prior studies have shown that levels of p16 dramatically increase in breast cancer patients who have received adjuvant chemotherapy. This study investigated whether moderate physical activity during chemotherapy would attenuate the expected rise in p16 expression.
Methods
Participants were women with Stage I–III breast cancer enrolled in a walking study for the duration of their chemotherapy (NCT02167932, NCT02328313, NCT03761706). Participants were asked to walk at least 30 min or 6200 steps/day following a structured walking program and to wear an activity tracker. p16 mRNA levels were measured in peripheral blood T‐cells before chemotherapy initiation and at approximately 6 months after last chemotherapy treatment (mean 200 days, SD 40 days).
Results
In total, 141 participants met inclusion criteria and 10% (n = 14) averaged > 6200 steps/day. There was no significant association of daily steps with change in p16 levels pre‐ to post‐chemotherapy (Pearson correlation coefficient = 0.11, p = 0.17). After adjusting for age, stage, anthracycline‐based chemotherapy, and baseline p16, the change in log2 p16 for each 1000 steps was estimated to be 0.03 (p = 0.35). Most participants were sedentary prior to chemotherapy and achieved modest levels of physical activity during treatment.
Conclusion
A self‐guided walking program achieved only modest levels of physical activity and was unable to ameliorate chemotherapy‐induced change in p16 levels in women undergoing chemotherapy for early‐stage breast cancer. More structured and vigorous exercise programs should be tested for a more definitive exploration of their impact on post‐chemotherapy p16 levels.
Keywords: adjuvant chemotherapy, breast cancer, exercise, intervention, p16, walking
This study investigated whether a self‐guided walking program during chemotherapy would attenuate the expected rise in p16 expression in women with early breast cancer treated with chemotherapy. Most participants were unable to meet their daily step goal, and there was no significant association of daily steps with change in p16 levels pre‐ to post‐chemotherapy (p = 0.17), after adjusting for age, anthracycline‐based chemotherapy, and baseline p16 (p = 0.35). More structured and vigorous exercise programs should be tested for a more definitive exploration of their impact on post‐chemotherapy p16 levels.
1. INTRODUCTION
Aging is a heterogeneous process among humans, and chronological age is often an inconsistent predictor of health status. 1 Over the past decade, biomarkers have emerged as a novel method for measuring molecular age in pursuit of more accurate clinical assessments and personalized treatment plans. The p16 INK4a (p16) gene encodes a protein that leads to cellular senescence and has been identified as a critical correlate of cell‐intrinsic, molecular aging in humans. 2 , 3 , 4 , 5 Senescence is a cellular mechanism that promotes permanent inhibition of the cell cycle in response to cellular stress or DNA damage. Over time, the accumulation of senescent cells in tissue reduces replicative function and promotes inflammation. 6 , 7 The expression of p16 mRNA in peripheral blood T‐lymphocytes (PBTL) is an accurate indicator of this molecular aging process. 8
The expression of p16 has been studied in the context of multiple disease processes, 9 , 10 including breast cancer and chemotherapy treatments. 1 , 11 P16 RNA expression dramatically increases in early‐stage breast cancer patients following the initiation of (neo)adjuvant chemotherapy, especially in patients receiving anthracycline‐based regimens. 12 Anthracycline‐based chemotherapies have demonstrated accelerated molecular aging by 23–26 years, compared with non‐anthracycline‐based regimens, which accelerated molecular aging by 9–11 years. 11 Baseline (pre‐chemotherapy) p16 levels, but not chronological age or race, are also associated with the magnitude of increases in p16 levels after chemotherapy, specifically patients with lower p16 levels at baseline are more likely to experience larger increases in p16 levels post chemotherapy treatment. 11
p16 expression has also been studied in the context of lifestyle choices. Higher p16 levels positively correlate with physical inactivity and tobacco consumption. 8 These findings led us to hypothesize that physical activity during chemotherapy for early breast cancer might mitigate the rise in p16 levels observed in our previous research. Current Center for Disease Control and Prevention (CDC) and American Cancer Society guidelines recommend 150 min of physical activity per week for adults with cancer, with guidance from the treating oncologist for patients in active treatment such as chemotherapy. 13 , 14 , 15 , 16 As in adults without a cancer diagnosis, the majority of adults with cancer generally do not meet guideline‐recommended levels of physical activity. 17 , 18 , 19 , 20 , 21 For the current study, our primary aim was to evaluate whether adherence to a self‐directed physical activity intervention throughout chemotherapy treatment would be associated with smaller pre‐post chemotherapy rises in p16 levels compared with patients with lower physical activity adherence.
2. METHODS
2.1. Participants
Our sample was drawn from participants in three studies that included similar eligibility criteria and the same physical activity intervention — NCT02167932 (age 21–64), NCT02328313 (age 65 and older), NCT03761706 (age 22 and older). Patients provided written or electronic informed consent meeting all federal, state, and institutional guidelines, and the studies were approved by the University of North Carolina Lineberger Comprehensive Cancer Center Protocol Review Committee and the Institutional Review Boards for each study site (4). Descriptions of these studies have been previously published. 22 , 23 Eligibility criteria were: female, breast cancer diagnosis (Stage I, II or III), and scheduled for adjuvant or neoadjuvant chemotherapy. Patients with any form of prior chemotherapy were excluded. All participants had to have p16 values assessed pre‐chemotherapy (baseline) and post‐chemotherapy.
2.2. Intervention
The home‐based walking intervention was based on an adaptation of the Arthritis Foundation's evidence‐based Walk With Ease (WWE) program. 16 , 24 Specifically, the intervention consisted of walking guideline‐recommended 150 min/week (CDC and American Cancer Society guidelines) at a pace that was safe, comfortable, and sustainable for the duration of chemotherapy. We have previously described how we estimated that achievement of 150 min/week at a moderate pace amounted to approximately 6200 steps/day. 16 , 25 Participants received an activity tracker (Fitbit™ or GARMIN™) and were asked to wear the tracker throughout their waking hours. Every 2–3 weeks, during a scheduled chemotherapy visit, research assistants uploaded activity tracker steps directly into research computers. Research assistants also asked participants about their walking and strength training, while providing positive encouragement and reassurance to those who were not meeting goals at each chemotherapy visit. Study participants were also given a copy of the WWE workbook, which includes chapters on overcoming barriers to walking, developing a walking routine, and other resources for staying motivated. 26
2.3. p16 expression
Blood samples were collected from study participants within 1 week prior to chemotherapy initiation and at approximately 6 months after their last chemotherapy treatment (mean 200 days, SD 40 days, range 105–336 days). Blood was drawn into lavender top EDTA tubes, T cells were isolated, and expression of p16 mRNA in peripheral blood T lymphocytes was determined using TaqMan real‐time quantitative reverse transcription polymerase chain reaction. Expression analysis was performed by Sapere Bio (Research Triangle), using technology described previously. 12 Every assay included external and internal controls to monitor assay performance. Cycle threshold values over 37 were considered below detection and excluded from the analysis. The same assay and quality control procedures were used in every run. P16 is reported in log base 2 units.
2.4. Statistical analysis
Descriptive statistics are provided to summarize the study sample. Associations with the primary outcome of interest (change in log2 p16) were evaluated using Pearson correlation coefficients and linear regression modeling. Changes in log2 p16 were calculated and used as the outcome measure for analysis. 12 Linear regression requires a normality assumption; based on a histogram of the data, QQ plot for quantiles, and the Kolmogorov–Smirnov test for normality, it was concluded that using linear regression was appropriate for the outcome measure of change in log2 p16. Unadjusted and adjusted (for age, anthracycline, and baseline p16) modeling was performed. All analyses were conducted using SAS statistical software v9.4. All statistical tests were two‐sided, and a p value < 0.05 was considered statistically significant.
3. RESULTS
3.1. Patient characteristics
Table 1 shows the baseline pre‐chemotherapy characteristics of the 141 study participants. The mean age was 57 years (range 28–83 years), and the majority of participants were white. Almost 94% of participants had comorbidities, with arthritis and high blood pressure being the most common. Over half of participants had a body mass index/BMI > 25, and only 32% reported engaging in vigorous physical activity on a weekly basis pre‐chemotherapy. A majority of participants had a Stage II breast cancer diagnosis, with 28% having triple negative tumors. Roughly 41% of participants received neoadjuvant chemotherapy and 59% adjuvant chemotherapy, with 44% of regimens containing anthracycline.
TABLE 1.
Pretreatment patient characteristics
| Variable | Overall N = 141 |
|---|---|
| Age, year | 56.9 (SD, 13) |
| Race | |
| White | 106 (75%) |
| Black | 24 (17%) |
| Other | 11 (8%) |
| Comorbidities | 132 (94%) |
| Arthritis/Rheumatism | 42 (31%) |
| High blood pressure | 45 (33%) |
| Heart disease | 6 (4%) |
| Diabetes | 9 (7%) |
| Stroke | 2 (2%) |
| Marital status | |
| Single, never married | 22 (16%) |
| Married | 78 (55%) |
| Separated, divorced, or widowed | 41 (29%) |
| Live alone | 32 (23%) |
| BMI | |
| <18.5 (underweight) | 2 (1%) |
| 18.5–25 (normal) | 42 (30%) |
| 25–30 (overweight) | 43 (31%) |
| 30+ (obese) | 54 (38%) |
| Breast cancer stage | |
| I | 29 (20%) |
| II | 74 (53%) |
| III | 38 (27%) |
| Hormone receptor status | |
| Negative | 60 (43%) |
| Positive | 81 (57%) |
| HER 2 | |
| Negative | 100 (71%) |
| Positive | 41 (29%) |
| Triple negative | 40 (28%) |
| Surgery | |
| None | 3 (2%) |
| Lumpectomy | 63 (45%) |
| Mastectomy | 75 (53%) |
| Therapy | |
| Radiation | 102 (72%) |
| Endocrine | 68 (50%) |
| Chemotherapy timing | |
| Neoadjuvant | 58 (41%) |
| Adjuvant | 83 (59%) |
| Chemotherapy regimen | |
| AC‐T (Doxorubicin/Cyclophosphamide plus Paclitaxel) | 45 (32%) |
| AC‐TC (Doxorubicin/Cyclophosphamide plus Paclitaxel/Carboplatin) | 16 (11%) |
| TC (Docetaxel/Cyclophosphamide +/− HER‐2) | 34 (24%) |
| TCH (Docetaxel/Carboplatin/anti‐HER‐2) | 29 (21%) |
| Other | 17 (12%) |
| Chemotherapy containing anthracycline | 62 (44%) |
| Frequency of vigorous physical activity a | |
| Never | 36 (26%) |
| A few times per month | 28 (21%) |
| 1–2 times per month | 27 (20%) |
| 3–4 times per month | 33 (24%) |
| 5 or more times/week | 12 (9%) |
| Smoking history | |
| Never smoked | 84 (60%) |
| Used to smoke | 42 (30%) |
| Currently smoke | 15 (10%) |
| Alcohol consumer | |
| Yes | 51 (37%) |
| No | 52 (37%) |
| Almost never | 36 (26%) |
Defined as at least 10 min of physical activity that causes heavy sweating or large increases in heart rate or breathing.
3.2. Physical activity associations with change in p16
The average baseline log2 p16 was 9.62 (SD 0.89, 95% CI: 9.47–9.77). During chemotherapy treatment, the average steps/day was 3025 (95% CI: 2651–3398 steps/day). Only 14 participants were able to average ≥ 6200 steps/day (corresponding to 150 min/week of walking at a moderate pace). There was no association between average daily activity tracker steps and change in p16 (Pearson correlation coefficient = 0.11, p = 0.17, Figure 1). For each 1000 step increase in average steps/day, the estimated change in log2 p16 was increased 0.047. This non‐significant finding did not change after adjustment for age, stage, anthracycline chemotherapy, baseline log2 p16. In a model adjusted for age, stage, anthracycline chemotherapy, and baseline p16, the estimated increase in log2 p16 change for each 1000 steps was reduced to 0.03 (p = 0.35).
FIGURE 1.
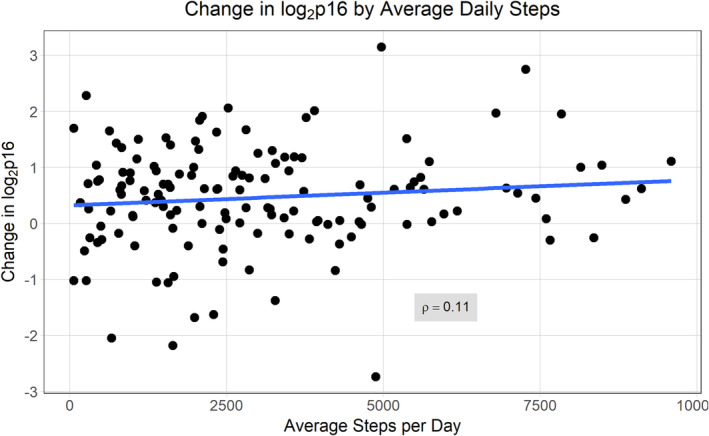
Association of activity tracker steps with p16 change over time
For the entire cohort, the average change in log2 p16 was 0.46 (SD 0.92; 95% CI: 0.30–0.61), which is similar to previous findings for women treated with adjuvant chemotherapy. 11 Baseline p16 was a strong predictor of change over time, with a Pearson correlation coefficient of −0.45, p < 0.0001. Higher p16 at baseline was associated with less change in p16 over time (Figure 2), consistent with previous findings. 11
FIGURE 2.
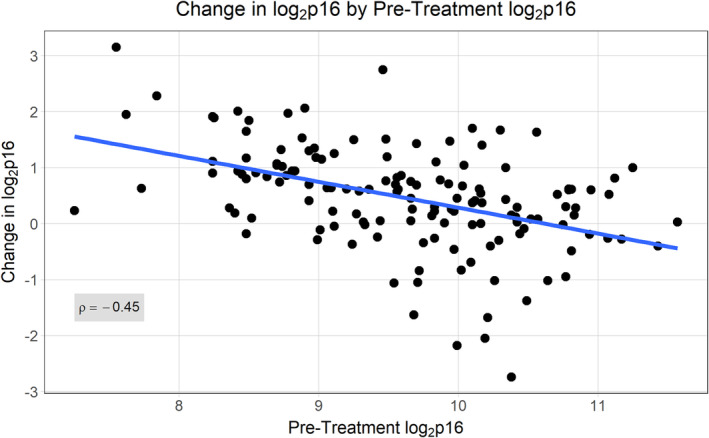
Association of baseline p16 with p16 change over time
4. DISCUSSION
Although we hypothesized that increased physical activity levels would slow the dramatic increase in p16 expression following chemotherapy treatment, that relationship was not observed in our cohort. It is important to note that only 9% of participants had engaged in vigorous physical activity a few days a week prior to their participation in our study, and only 10% were able to meet their physical activity goal of 6200 steps/day during chemotherapy despite encouragement. This sedentary patient population prior to chemotherapy is representative of the general population of women diagnosed with breast cancer. 18 , 27 , 28 In addition, in a prior study by our group using an identical walking intervention for early breast cancer patients less than 65 years of age, only 19% of participants were fully adherent to the walking goal. 16 Said study further examined patient‐specific factors associated with step count including BMI, education level, race, and baseline physical activity. 16 Factors such as race were not included in our analysis given the low number of non‐white participants (n = 35). Activity tracker data have shown that during the first week of chemotherapy, only 33% of this patient population is walking 100 min/week. 29
Our study underscores the challenges to implementing even a simple exercise regimen in breast cancer patients undergoing chemotherapy. We had hypothesized that a simple walking program meeting the activity criteria of the CDC and American Cancer Society would have ameliorated the increase p16 expression levels that we have found in up to 75% of women receiving adjuvant chemotherapy. 12
Our study has some limitations. Frist, the intervention period was limited to the duration of chemotherapy, while the p16 “change” period was from pre‐chemotherapy to approximately 6 months post chemotherapy completion. It is possible that a longer intervention period mirroring the p16 measurements would have shown greater impact from physical activity. Second, more intense or supervised encouragement of the walking exercise and the inclusion of weight training could be considered for future studies. 30 , 31
In our present study, we were not able to achieve even modest walking activity during chemotherapy, and it remains uncertain if a more vigorous and personalized exercise intervention might ameliorate the p16 rise seen with chemotherapy. Despite these findings, exercise should still be encouraged in this patient population as walking has been shown to decrease mortality in adults with and without chronic medical conditions 32 , 33 and to have positive health benefits and improved quality of life for women with breast cancer. 34 , 35
FUNDING INFORMATION
Support for this study was provided to Dr. Hyman Muss by the Kay Yow Cancer Fund (Cary, NC) and the Breast Cancer Research Foundation (New York, NY). The funders played no role in the study design, data collection, analysis, or publication of this manuscript.
CONFLICT OF INTEREST
NM is a cofounder of Sapere Bio, holds equity in the company, and is an inventor of intellectual property applications. The other authors have no conflicts to report.
PATIENT CONSENT
Before recruitment and enrollment in this study, the patient was given a full explanation of the study and the opportunity to review the consent form. Informed, written consent was obtained from all participants in compliance with the Good Clinical Practice (GCP) and to ethical principles that have their origin in the Declaration of Helsinki.
CLINICAL TRIAL REGISTRATION
The study was registered with clinicaltrials.gov (NCT02167932, NCT02328313, NCT03761706).
ACKNOWLEDGMENTS
Funding and support for this study are listed elsewhere.
Kammire MS, Deal AM, Damone EM, et al. Does walking during chemotherapy impact p16INK4a levels in women with early breast cancer. J Clin Lab Anal. 2022;36:e24753. doi: 10.1002/jcla.24753
DATA AVAILABILITY STATEMENT
Designated staff entered all study data into a REDCap™ database. All procedures were IRB and HIPAA‐compliant. Database contains information for individual patients and is not publicly available. Investigators interested in these data should contact the corresponding author.
REFERENCES
- 1. Muss HB, Smitherman A, Wood WA, et al. p16 a biomarker of aging and tolerance for cancer therapy. Transl Cancer Res. 2020;9(9):5732‐5742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Krishnamurthy J, Torrice C, Ramsey MR, et al. Ink4a/Arf expression is a biomarker of aging. J Clin Invest. 2004;114(9):1299‐1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Herbig U, Ferreira M, Condel L, Carey D, Sedivy JM. Cellular senescence in aging primates. Science. 2006;311(5765):1257. [DOI] [PubMed] [Google Scholar]
- 4. Zindy F, Quelle DE, Roussel MF, Sherr CJ. Expression of the p16INK4a tumor suppressor versus other INK4 family members during mouse development and aging. Oncogene. 1997;15(2):203‐211. [DOI] [PubMed] [Google Scholar]
- 5. Melk A, Schmidt BMW, Takeuchi O, Sawitzki B, Rayner DC, Halloran PF. Expression of p16INK4a and other cell cycle regulator and senescence associated genes in aging human kidney. Kidney Int. 2004;65(2):510‐520. [DOI] [PubMed] [Google Scholar]
- 6. Campisi J, Andersen JK, Kapahi P, Melov S. Cellular senescence: a link between cancer and age‐related degenerative disease? Semin Cancer Biol. 2011;21(6):354‐359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. He S, Sharpless NE. Senescence in health and disease. Cell. 2017;169(6):1000‐1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liu Y, Sanoff HK, Cho H, et al. Expression of p16(INK4a) in peripheral blood T‐cells is a biomarker of human aging. Aging Cell. 2009;8(4):439‐448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tsygankov D, Liu Y, Sanoff HK, Sharpless NE, Elston TC. A quantitative model for age‐dependent expression of the p16INK4a tumor suppressor. Proc Natl Acad Sci U S A. 2009;106(39):16562‐16567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen H, Gu X, Su I, et al. Polycomb protein Ezh2 regulates pancreatic beta‐cell Ink4a/Arf expression and regeneration in diabetes mellitus. Genes Dev. 2009;23(8):975‐985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shachar SS, Deal AM, Reeder‐Hayes KE, et al. Effects of breast cancer adjuvant chemotherapy regimens on expression of the aging biomarker, p16INK4a. JNCI Cancer Spectr. 2020;4(6):pkaa082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sanoff HK, Deal AM, Krishnamurthy J, et al. Effect of cytotoxic chemotherapy on markers of molecular age in patients with breast cancer. J Natl Cancer Inst. 2014;106(4):dju057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kushi LH, Doyle C, McCullough M, et al. American Cancer Society guidelines on nutrition and physical activity for cancer prevention: reducing the risk of cancer with healthy food choices and physical activity. CA Cancer J Clin. 2012;62(1):30‐67. [DOI] [PubMed] [Google Scholar]
- 14. Schmitz KH, Courneya KS, Matthews C, et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc. 2010;42(7):1409‐1426. [DOI] [PubMed] [Google Scholar]
- 15. Walking. Physical Activity. CDC [Internet]. cited 2021 Jun 8. https://www.cdc.gov/physicalactivity/walking/index.htm
- 16. Nyrop KA, Deal AM, Choi SK, et al. Measuring and understanding adherence in a home‐based exercise intervention during chemotherapy for early breast cancer. Breast Cancer Res Treat. 2018;168(1):43‐55. [DOI] [PubMed] [Google Scholar]
- 17. Irwin ML, McTiernan A, Bernstein L, et al. Physical activity levels among breast cancer survivors. Med Sci Sports Exerc. 2004;36(9):1484‐1491. [PMC free article] [PubMed] [Google Scholar]
- 18. Blanchard CM, Courneya KS, Stein K. Cancer survivors' adherence to lifestyle behavior recommendations and associations with health‐related quality of life: results from the American Cancer Society's SCS‐II. J Clin Oncol. 2008;26(13):2198‐2204. [DOI] [PubMed] [Google Scholar]
- 19. Harrison S, Hayes SC, Newman B. Level of physical activity and characteristics associated with change following breast cancer diagnosis and treatment. Psychooncology. 2009;18(4):387‐394. [DOI] [PubMed] [Google Scholar]
- 20. Emery CF, Yang H‐C, Frierson GM, Peterson LJ, Suh S. Determinants of physical activity among women treated for breast cancer in a 5‐year longitudinal follow‐up investigation. Psychooncology. 2009;18(4):377‐386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Eheman C, Henley SJ, Ballard‐Barbash R, et al. Annual report to the nation on the status of cancer, 1975‐2008, featuring cancers associated with excess weight and lack of sufficient physical activity. Cancer. 2012;118(9):2338‐2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nyrop KA, Deal AM, Reeve BB, et al. Congruence of patient‐ and clinician‐reported toxicity in women receiving chemotherapy for early breast cancer. Cancer. 2020;126(13):3084‐3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nyrop KA, Deal AM, Shachar SS, et al. Patient(Pt)‐reported toxicities during breast cancer (BC) chemotherapy (CRx): associations with pre‐treatment (Tx) measures of quality of life (QOL) and Tx discontinuation. J Clin Oncol. 2018;36(15_suppl):546. [Google Scholar]
- 24. Callahan LF, Shreffler JH, Altpeter M, et al. Evaluation of group and self‐directed formats of the Arthritis Foundation's walk with ease program. Arthritis Care Res (Hoboken). 2011;63(8):1098‐1107. [DOI] [PubMed] [Google Scholar]
- 25. Tudor‐Locke C, Craig CL, Brown WJ, et al. How many steps/day are enough? For adults. Int J Behav Nutr Phys Act. 2011;8:79.21798015 [Google Scholar]
- 26. Arthritis Foundation . Walk with Ease: your Guide to Walking for Better Health, Improved Fitness and Less Pain. Third ed. Arthritis Foundation; 2010. [Google Scholar]
- 27. Centers for Disease Control and Prevention (CDC) . Adult participation in aerobic and muscle‐strengthening physical activities—United States, 2011. MMWR Morb Mortal Wkly Rep. 2013;62(17):326‐330. [PMC free article] [PubMed] [Google Scholar]
- 28. Centers for Disease Control and Prevention . U.S. Physical Activity Stat. 2007.
- 29. Wagoner CW, Choi SK, Deal AM, et al. Establishing physical activity in breast cancer: self‐report versus activity tracker. Breast Cancer Res Treat. 2019;176(2):395‐400. [DOI] [PubMed] [Google Scholar]
- 30. Van Waart H, Stuiver MM, Van Harten WH, et al. Effect of low‐intensity physical activity and moderate‐ to high‐intensity physical exercise during adjuvant chemotherapy on physical fitness, fatigue, and chemotherapy completion rates: results of the PACES randomized clinical trial. J Clin Oncol. 2015;33(17):1918‐1927. [DOI] [PubMed] [Google Scholar]
- 31. Courneya KS, Segal RJ, Mackey JR, et al. Effects of aerobic and resistance exercise in breast cancer patients receiving adjuvant chemotherapy: a multicenter randomized controlled trial. J Clin Oncol. 2007;25(28):4396‐4404. [DOI] [PubMed] [Google Scholar]
- 32. Zhao W, Ukawa S, Kawamura T, et al. Health benefits of daily walking on mortality among younger‐elderly men with or without major critical diseases in the new integrated suburban seniority investigation project: a prospective cohort study. J Epidemiol. 2015;25(10):609‐616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Warburton DER, Nicol CW, Bredin SSD. Health benefits of physical activity: the evidence. Can Med Assoc J. 2006;174(6):801‐809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Friedenreich CM, Gregory J, Kopciuk KA, Mackey JR, Courneya KS. Prospective cohort study of lifetime physical activity and breast cancer survival. Int J Cancer. 2009;124(8):1954‐1962. [DOI] [PubMed] [Google Scholar]
- 35. Bicego D, Brown K, Ruddick M, Storey D, Wong C, Harris SR. Effects of exercise on quality of life in women living with breast cancer: a systematic review. Breast J. 2009;15(1):45‐51. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Designated staff entered all study data into a REDCap™ database. All procedures were IRB and HIPAA‐compliant. Database contains information for individual patients and is not publicly available. Investigators interested in these data should contact the corresponding author.


