Abstract
Background.
Lobar resection is the gold standard therapy for medically fit patients with stage I non-small cell lung cancer (NSCLC). However, considerable variability exists in the use of surgical therapy. This study tested the hypothesis that center-based variation in the use of surgical therapy affects survival in NSCLC.
Methods.
We queried the National Cancer Database for patients with stage I NSCLC. Mixed-effects multivariable models were developed to establish the percenter adjusted rate of surgical therapy. Patients were stratified into quartiles based on the treating center’s adjusted rate of surgical therapy. Survival was estimated and then tested by using Kaplan-Meier and the log-rank test. Multivariable Cox proportional hazard models were developed to estimate the effect of rate of surgical therapy on overall survival.
Results.
A total of 139,802 patients met the criteria. There was wide variation in the per-center rate of surgical resection in the highest (80.8%) versus lowest (41.4%, p < 0.001) quartile. Across cohorts, patients were similar in age (mean 68.8 years in the highest quartile versus 69.7 in the lowest quartile) and Charlson-Deyo Score of 2 or greater (15.1% in the highest quartile versus 14.4% in the lowest quartile). Five-year survival was higher for patients treated at high-use centers (52.7% versus 36.7%, p < 0.001). After adjustment, an adjusted rate of surgical therapy in the lowest 25th percentile was associated with lower survival (adjusted hazard ratio 1.40, 95% confidence interval: 1.37 to 1.40, p < 0.001).
Conclusions.
Treatment at a center with a higher rate of surgical therapy confers a considerable survival advantage, even after adjustment for hospital volume, surgical approach, and other confounders. Targeted efforts to improve adherence to guidelines about provision of surgical therapy in early-stage NSCLC may represent a meaningful opportunity to improve outcomes.
With more than 200,000 newly diagnosed cases each year, lung cancer is the second most commonly diagnosed cancer in the United States and is the leading cause of cancer-related death in both men and women [1]. Guidelines from the National Comprehensive Cancer Network and American College of Chest Physicians recommend surgical resection with curative intent for all operable stage I non-small cell lung cancers (NSCLCs) in medically appropriate patients [2, 3].
Prior studies have investigated the relationship between surgical volume and outcomes across a range of surgical diseases [4, 5]. Higher volume centers have consistently demonstrated improved survival in those patients undergoing surgical resection [6, 7]. In addition, various factors such as geography, insurance status, and surgery consultation have been associated with increased use of definitive treatment, including surgery and radiation [8–10]. However, many of these studies focus on a specific patient population, evaluating the role of age or racial disparities on the treatment of lung cancer, thereby limiting the generalizability of the results.
Despite improved outcomes and national recommendations, there remains considerable variability in the use of surgical therapy for early-stage NSCLC [8, 11–13]. Disparities in the adherence to guideline-concordant delivery of surgical therapy may represent a modifiable behavior to improve oncologic outcomes. This technique has recently been applied to both small-cell lung cancer and pancreatic adenocarcinoma, demonstrating in each that higher adjusted per-center rates of use of surgical therapy are associated with improved survival [14, 15]. With the use of the National Cancer Database (NCDB), this study aims to identify the center-based variation in the rate of use of surgical therapy, how this variation affects overall survival (OS) in stage I NSCLC, and the extent to which this variation may help to better understand observed disparities in treatment outcomes for patients with NSCLC.
Patients and Methods
The university’s institutional review board provided exempt status for this analysis. This is a retrospective cohort review of the 2014 NCDB Participant User Files. The NCDB is a de-identified database jointly administered by the American Cancer Society and the American College of Surgeons Commission on Cancer. The 2014 file includes cases diagnosed between 2004 and 2014 and includes survival information for cases diagnosed through 2013.
The study population included all adult patients aged 85 years and younger with clinical stage I NSCLC treated at a single center reported to the NCDB between 2004 and 2014. Patients with undocumented clinical stage, those missing survival data, and those who underwent operation as a part of their treatment was undocumented were excluded. Primary outcomes were the receipt of curative-intent surgical therapy and OS. OS was measured from the time of diagnosis. Secondary end points included the following: 30- and 90-day mortality, 30-day readmission rates, surgical margin status, number of lymph nodes examined, and hospital length of stay.
Patient baseline demographic characteristics were extracted from the NCDB. The demographic information included patient age, sex, race, education, income quartile, treatment facility type, insurance status, and Charlson-Deyo comorbidity index. Tumor characteristics included pathologic tumor, margin, and nodal status. Treatment and surgical end points included the use of adjuvant radiation and chemotherapy, extent of surgical resection, surgical approach, number of lymph nodes examined, and surgical margin status. Extent of surgical resection included lobectomy and pneumonectomy. Surgical approach was categorized as either video-assisted thoracoscopic surgery (VATS), open procedure, robotic-assisted procedure, or those converted to open. Baseline characteristics were compared between groups by using the Kruskal-Wallis analysis of variance test for continuous variables and the χ2 test for categorical variables.
Hospital factors included in the analysis were hospital type (academic/research, community, comprehensive community, and integrated) and hospital volume. Hospital volume was calculated based on quartiles of the average annual number of clinical stage I NSCLC cases treated at that facility. Volume was based on the total number of patients treated at the facility, rather than the total number of surgical cases performed. Because each center may not have contributed data to the NCDB for each year of the study period, per-center volume was calculated based on the number of years data were contributed to the NCDB by each respective center. Hospital volume quartiles were established as follows: lowest quartile was 4.7 cases or fewer per year, second quartile was 10.9 cases or fewer per year, third quartile was 21.1 cases or fewer per year, and the highest volume quartile was more than 21.1 cases per year.
Variation in the Use of Surgical Therapy
To establish the degree to which there exists variation at the hospital level in the use of surgical therapy for patients without contraindications or refusal, mixed effects logistic regression models that incorporated hospital identification as a random effect were constructed. The final model included patient-, tumor, and hospital-level factors to establish an adjusted per-center rate of surgical therapy that considers case mix in determination of the per-center rate of use of surgical therapy.
Survival Analysis
Unadjusted OS was estimated by using the Kaplan-Meier method and the log-rank test to compare survival between patients treated at hospitals stratified by the percenter adjusted rate of use of surgical therapy (adjusted for patient- and tumor-level factors) described in the preceding paragraph. Cox proportional hazard models were subsequently developed to further investigate the adjusted hazard for mortality associated with treatment at centers stratified by the adjusted rate of surgical therapy. The assumption of proportional hazards was verified graphically. Age and hospital volume were evaluated first in a nonlinear fashion with the use of restricted cubic splines, and they were treated as categorized variables in the final model.
All analyses were conducted with R version 3.4.3 (R Foundation for Statistical Computing, Vienna, Austria) with a p value less than 0.05 indicating statistical significance.
Results
Patient Demographic Characteristics
A total of 215,252 patients met the inclusion criteria and were determined to be suitable for analysis. Demographic characteristics were compiled and described based on the adjusted rate quartile of the treating hospital. Demographic characteristics are summarized in Table 1. A plurality of patients (65,331 or 30.7%) were cared for at centers representing the top adjusted rate quartile. Patients treated at centers with the highest rate of use of surgical therapy were clinically similar in age, albeit statistically younger (mean 68.8 years versus 69.7 years in the lowest quartile), were less frequently men (45.6% versus 49.0% in the lowest quartile), were more frequently white (88.3% versus 86.6% in the lowest quartile), and were less likely to have a Charlson-Deyo Score of 0 (50.6% versus 56.6% for the lowest rate quartile) (all p < 0.001). Patients treated at the highest rate centers were more likely to hold private insurance (28.2% versus 20.7% in the lowest rate centers) and were more likely to be in the top income quartile (40.3% versus 15.9% in the lowest income quartile) (all p < 0.001).
Table 1.
Patient Characteristics and Hospital Practices by Per-Center Adjusted Rate of Surgical Therapy Quartile
| Characteristic | Top 25th Percentile Rate (n = 65,331) | 50th-75th Percentile Rate (n = 63,109) | 25th-50th Percentile Rate (n = 56,679) | Bottom 25th Percentile Rate (n = 30,133) | p Value |
|---|---|---|---|---|---|
| Patient characteristics | |||||
| Age at diagnosis, years | 68.82 ± 9.77 | 69.06 ± 9.70 | 69.31 ± 9.71 | 69.68 ± 9.53 | <0.001 |
| Sex, male | 29,788 (45.6) | 29,385 (46.6) | 26,926 (47.5) | 14,758 (49.0) | <0.001 |
| Race | <0.001 | ||||
| White | 57,664 (88.3) | 55,731 (88.3) | 49,715 (87.7) | 26,100 (86.6) | |
| Black | 5,105 (7.8) | 5,295 (8.4) | 5,449 (9.6) | 3,348 (11.1) | |
| Other | 2,562 (3.9) | 2,083 (3.3) | 1,515 (2.7) | 685 (2.3) | |
| Charlson-Deyo Score | <0.001 | ||||
| 0 | 33,052 (50.6) | 32,321 (51.2) | 29,492 (52.0) | 17,057 (56.6) | |
| 1 | 22,427 (34.3) | 21,337 (33.8) | 18,534 (32.7) | 8,729 (29.0) | |
| ≥2 | 9,852 (15.1) | 9,451 (15.0) | 8,653 (15.3) | 4,347 (14.4) | |
| Insurance | <0.001 | ||||
| Private | 18,444 (28.2) | 16,061 (25.4) | 13,146 (23.2) | 6,231 (20.7) | |
| Medicare/Medicaid | 44,839 (68.6) | 44,354 (70.3) | 41,036 (72.4) | 22,142 (73.5) | |
| Other government | 432 (0.7) | 796 (1.3) | 819 (1.4) | 684 (2.3) | |
| Uninsured | 773 (1.2) | 1,016 (1.6) | 995 (1.8) | 624 (2.1) | |
| Income quartile | <0.001 | ||||
| Top | 25,974 (40.3) | 17,308 (27.8) | 14,130 (25.2) | 4,739 (15.9) | |
| Second | 12,815 (19.9) | 15,295 (24.6) | 14,813 (26.4) | 9,439 (31.7) | |
| Third | 16,564 (25.7) | 17,143 (27.5) | 16,248 (29.0) | 7,385 (24.8) | |
| Bottom | 9,061 (14.1) | 12,505 (20.1) | 10,825 (19.3) | 8,229 (27.6) | |
| Distance travelled, miles | 28.96 ± 110.57 | 31.61 ± 102.68 | 23.25 ± 79.30 | 21.38 ± 56.21 | <0.001 |
| Days from diagnosis to treatment | 34.87 ± 45.37 | 40.16 ± 46.43 | 42.29 ± 47.10 | 47.95 ± 51.23 | <0.001 |
| Tumor characteristics | |||||
| Diagnostic confirmation | <0.001 | ||||
| Microscopic evaluation of tissue specimen | 61,388 (94.0) | 57,488 (91.1) | 51,048 (90.1) | 26,378 (87.5) | |
| Microscopic evaluation of cytologic specimen | 3,212 (4.9) | 4,491 (7.1) | 4,651 (8.2) | 3,099 (10.3) | |
| Imaging diagnosis only | 599 (0.9) | 896 (1.4) | 779 (1.4) | 529 (1.8) | |
| Clinical stage | <0.001 | ||||
| 1 | 2,074 (3.2) | 2,896 (4.6) | 3,027 (5.3) | 2,284 (7.6) | |
| 1A | 44,752 (68.5) | 41,327 (65.5) | 35,999 (63.5) | 17,724 (58.8) | |
| 1B | 18,505 (28.3) | 18,886 (29.9) | 17,653 (31.1) | 10,125 (33.6) | |
| Pathologic stage | <0.001 | ||||
| 1A | 26,526 (40.6) | 21,712 (34.4) | 16,428 (29.0) | 6,295 (20.9) | |
| 1B | 12,834 (19.6) | 11,418 (18.1) | 9,247 (16.3) | 3,701 (12.3) | |
| 2A | 2,513 (3.8) | 2,140 (3.4) | 1,762 (3.1) | 643 (2.1) | |
| 2B | 2,042 (3.1) | 1,638 (2.6) | 1,338 (2.4) | 529 (1.8) | |
| 3A | 1,987 (3.0) | 1,689 (2.7) | 1,289 (2.3) | 431 (1.4) | |
| 3B | 491 (0.8) | 356 (0.6) | 261 (0.5) | 97 (0.3) | |
| 4 | 304 (0.5) | 318 (0.5) | 224 (0.4) | 88 (0.3) | |
| No pathologic staging | 17,667 (27.0) | 22,723 (36.0) | 25,153 (44.4) | 17,797 (59.0) | |
| Hospital characteristics | |||||
| Facility type | <0.001 | ||||
| Academic | 27,763 (42.7) | 24,808 (39.5) | 13,939 (24.7) | 7,686 (25.6) | |
| Community | 3,926 (6.0) | 4,291 (6.8) | 4,196 (7.4) | 5,270 (17.6) | |
| Comprehensive | 29,424 (45.3) | 26,498 (42.2) | 30,250 (53.6) | 15,608 (52.0) | |
| Integrated | 3,845 (5.9) | 7,144 (11.4) | 8,026 (14.2) | 1,460 (4.9) | |
| Facility volume quartile | <0.001 | ||||
| Top 25th percentile | 43,260 (66.2) | 40,761 (64.6) | 35,065 (61.9) | 12,462 (41.4) | |
| 50th-75th percentile | 12,987 (19.9) | 14,189 (22.5) | 14,601 (25.8) | 8,843 (29.3) | |
| 25th-50th percentile | 7,889 (12.1) | 6,956 (11.0) | 5,235 (9.2) | 5,231 (17.4) | |
| Bottom 25th percentile | 1,195 (1.8) | 1,203 (1.9) | 1,778 (3.1) | 3,597 (11.9) | |
| Surgical treatment characteristics and secondary outcome measures | |||||
| Reason for no operation | <0.001 | ||||
| Operation performed | 52,850 (80.9) | 44,126 (69.9) | 34,092 (60.1) | 13,284 (44.1) | |
| Operation not part of planned first course treatment | 7,657 (11.7) | 13,519 (21.4) | 17,292 (30.5) | 14,055 (46.6) | |
| Operation contraindicated | 3,757 (5.8) | 4,143 (6.6) | 3,970 (7.0) | 2,015 (6.7) | |
| Operation refused | 945 (1.4) | 1,160 (1.8) | 1,083 (1.9) | 630 (2.1) | |
| Surgical approach | <0.001 | ||||
| Open | 16,507 (25.3) | 15,929 (25.2) | 12,405 (21.9) | 5,395 (17.9) | |
| No operation | 7,702 (11.8) | 11,907 (18.9) | 13,656 (24.1) | 9,785 (32.5) | |
| Robotic assisted | 2,340 (3.6) | 2,356 (3.7) | 1,311 (2.3) | 388 (1.3) | |
| VATS | 10,385 (15.9) | 6,663 (10.6) | 5,220 (9.2) | 1,288 (4.3) | |
| Surgical procedure | <0.001 | ||||
| No operation | 12,481 (19.1) | 18,983 (30.1) | 22,587 (39.9) | 16,849 (55.9) | |
| Lobar resection | 36,911 (56.5) | 32,362 (51.3) | 25,949 (45.8) | 10,190 (33.8) | |
| Pneumonectomy | 992 (1.5) | 960 (1.5) | 765 (1.3) | 356 (1.2) | |
| Sublobar resection | 14,947 (22.9) | 10,804 (17.1) | 7,378 (13.0) | 2,738 (9.1) | |
| Length of stay, days | 6.14 ± 6.36 | 6.83 ± 6.74 | 6.94 ± 6.67 | 7.20 ± 6.53 | <0.001 |
| 30-day mortality | 859 (1.9) | 896 (2.3) | 682 (2.3) | 315 (2.7) | <0.001 |
| 90-day mortality | 1,629 (3.5) | 1,562 (4.1) | 1,222 (4.1) | 552 (4.8) | <0.001 |
| Nonsurgical treatment characteristics | |||||
| Radiation therapy provided | <0.001 | ||||
| Beam radiation | 9,581 (14.7) | 14,075 (22.3) | 16,410 (29.0) | 11,693 (38.8) | |
| Not specified or unknown | 1,630 (2.5) | 1,211 (1.9) | 791 (1.4) | 373 (1.2) | |
| Radiation not administered | 54,120 (82.8) | 47,823 (75.8) | 39,478 (69.7) | 18,067 (60.0) | |
| Regional dose, cGy, if provided | 4,058.18 ± 17,632.46 | 4,566.53 ± 18,093.49 | 4,580.76 ± 17,176.86 | 5,657.80 ± 18,590.24 | <0.001 |
| Chemotherapy provided | |||||
| Chemotherapy not administered | 46,852 (71.7) | 44,961 (71.2) | 40,216 (71.0) | 20,979 (69.6) | <0.001 |
| Chemotherapy recommended but contraindicated | 2,849 (4.4) | 2,518 (4.0) | 2,442 (4.3) | 1,151 (3.8) | |
| Chemotherapy recommended but refused | 3,969 (6.1) | 3,900 (6.2) | 3,291 (5.8) | 1,626 (5.4) | |
| Multi-agent chemotherapy | 7,584 (11.6) | 7,482 (11.9) | 7,123 (12.6) | 4,154 (13.8) | |
| Single-agent chemotherapy | 1,688 (2.6) | 2,006 (3.2) | 2,109 (3.7) | 1,383 (4.6) |
Values are expressed as mean ± SD or n (%) unless otherwise indicated.
Patient, tumor, and treatment center characteristics were stratified by the per-center adjusted rate of surgery. Treating centers were stratified into quartiles as described in Methods. Rate of use of surgery was adjusted for patient-, tumor-, and center-level factors as described in Methods.
VATS = video-assisted thoracoscopic surgery.
Patients treated at centers in the highest rate quartile were more frequently treated at academic centers (42.7% versus 25.6% in the lowest rate quartile). These patients were more likely to have a diagnostic workup that included microscopic evaluation of a tissue specimen (94% versus 87.5% in the lowest rate quartile) and less likely by review of a cytologic specimen (4.9% versus 10.3% in the lowest rate quartile) (all p < 0.001). Days from diagnosis to the start of treatment were lowest in the highest use quartile (mean 34.87 days versus 51.23 in the lowest use quartile), and days from diagnosis to definitive operation were likewise lowest in the highest use quartile (mean 32.51 days versus 39.31 days) (all p < 0.001).
Patients treated at the highest rate centers were more frequently clinical stage 1A (68.5% versus 58.8% in the lowest rate quartile), were more frequently clinically N0 (97.4% versus 96.3% in the lowest rate quartile), and were more frequently clinical M0 (98.3% versus 97.6% in the lowest rate quartile). Of those patients with pathologic tissue for histologic examination, sub-types varied modestly. Highest rate centers had the lowest rate of adenocarcinoma (37.0% versus 38.2% in the lowest rate centers) and a lower rate of squamous cell carcinoma (24% versus 31.1%) (all p < 0.001).
Of patients who underwent surgical therapy, those who did at centers in the highest rate quartile were more likely to undergo a minimally invasive (VATS) approach (18.2% versus 6.3%) and were more likely to receive sublobar resection (28.2% versus 20.6%). Length of stay for patients undergoing surgical therapy was shortest in the highest use quartile (mean 6.14 days versus 7.20 days in the lowest use quartile). Both 30- and 90-day mortality were lowest in the highest use quartile (30-day mortality: 1.9% versus 2.7%, 90-day mortality: 3.5% versus 4.8%) (all p < 0.001).
With respect to alternate or complementary treatment approaches, patients treated in the highest rate centers were less likely to receive beam radiation (14.7% versus 38.8% in the lowest rate centers) and less likely to receive multi-agent chemotherapy (11.6% versus 13.8%) (all p < 0.001).
Hospital-Level Variation in the Use of Surgical Therapy
Of the 215,252 patients included in the analytic cohort, a total of 144,352 (67%) underwent surgical resection. These patients were treated at a total of 1,227 unique facilities. Substantial difference existed in the per-center rate of use of surgical therapy for the study cohort. Sequential mixed-effects logistic regression models were fit as described above to adjust for observed per-center differences in patient-, tumor-, and hospital-level characteristics. Median per-center rate of surgical therapy was 67.11%. Patients were then stratified by their treating center’s adjusted rate of surgical therapy quartile for further analysis. Variation in the per-center adjusted rate of surgical therapy and related quartile determinations are depicted in Figure 1.
Fig 1.
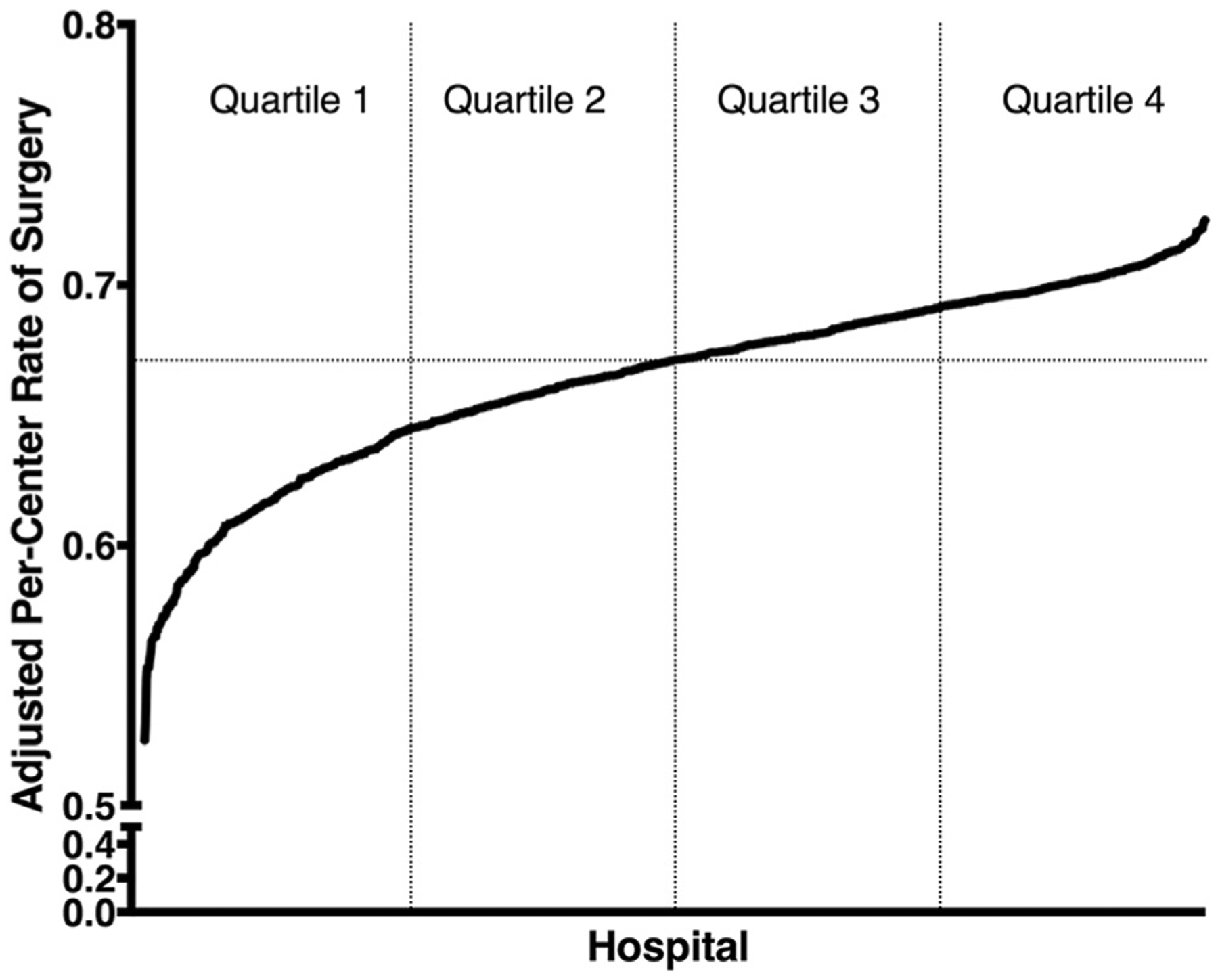
Adjusted per-center rates of surgical therapy, by hospital.
Relationship Between Use of Surgical Therapy and OS
OS was estimated in an unadjusted fashion by using the Kaplan-Meier method, with patients stratified by the treating center’s adjusted rate of surgical therapy. Because the 2014 NCDB Participant User File did not include survival data for those patients diagnosed in the year 2014, these patients (n = 28,076) were excluded from survival analysis. Patients who were treated at centers with a higher adjusted rate of surgical therapy had higher OS at both 1 and 5 years (1-year survival 87.0% versus 78.6% for the lowest adjusted rate quartile, 5-year survival 52.7% versus 36.7% for the lowest adjusted rate quartile, pairwise log-rank p < 0.001; Fig 2).
Fig 2.
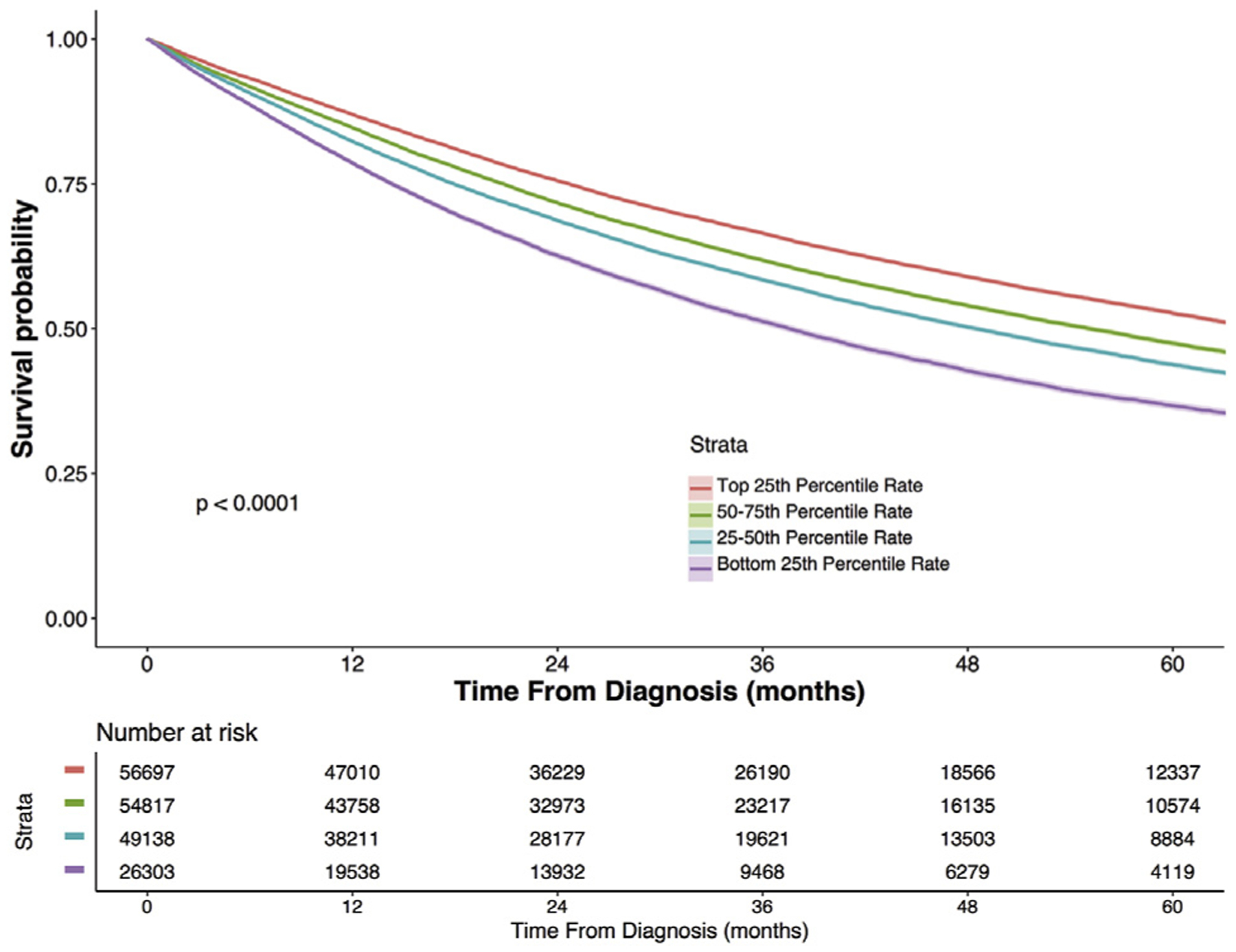
Five-year unadjusted Kaplan-Meier overall survival for stage I non-small cell lung cancer (NSCLC), stratified by treating center adjusted rate of surgical therapy. Bands around each line represent the 95% confidence interval.
A multivariable Cox proportional hazards model was developed to adjust for patient-, tumor-, and hospital-level factors as described above. After adjustment for age, Charlson-Deyo Score, sex, race, insurance status, facility type, clinical stage, histologic findings, and hospital volume quartile, adjusted rate of surgical therapy remained significantly associated with survival, with treatment at a lowest rate quartile hospital associated with an adjusted hazard ratio of 1.40 (95% confidence interval: 1.37 to 1.43, p < 0.001; Fig 3).
Fig 3.
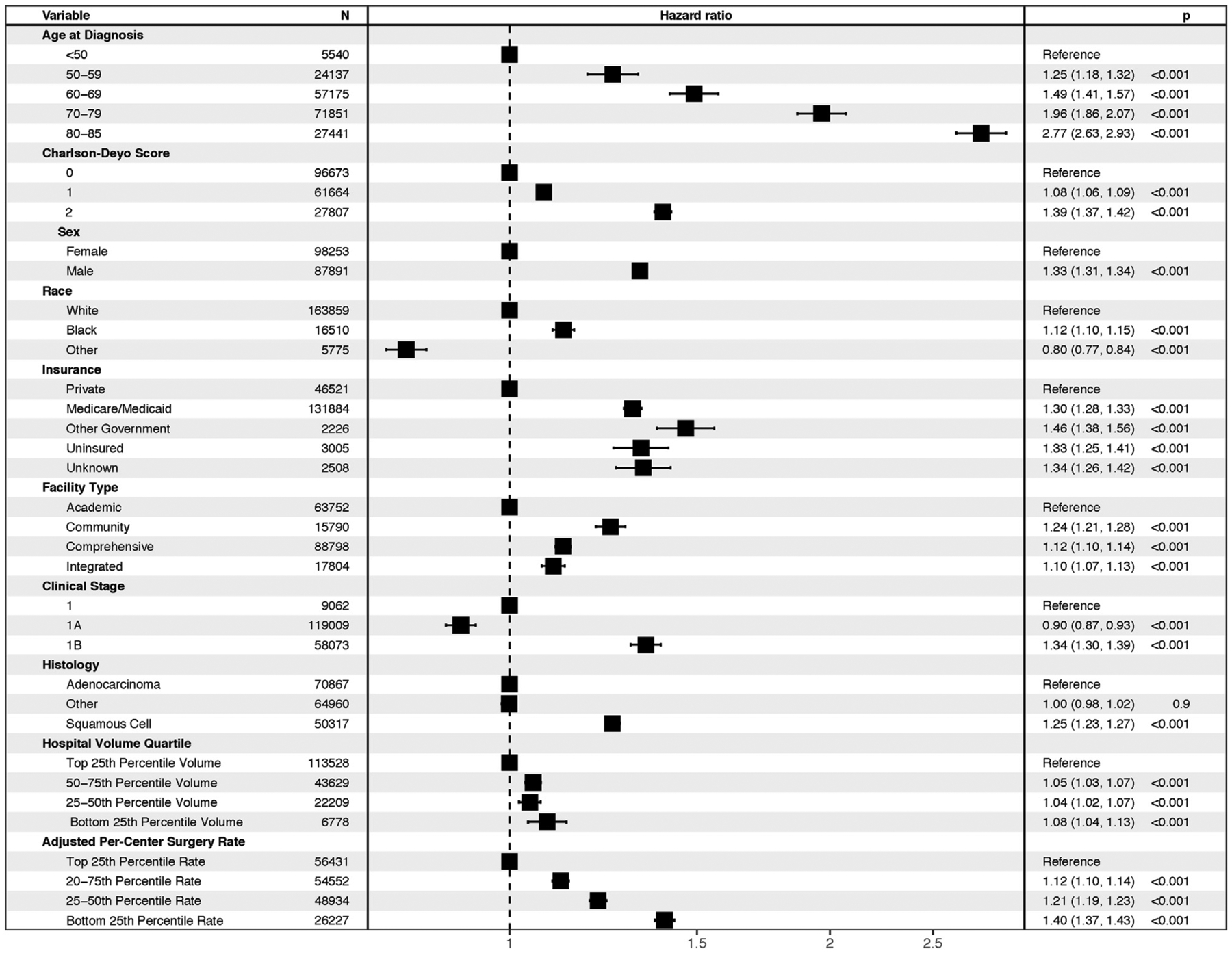
Results of Cox model with error bars representing 95% confidence interval.
Comment
Although complementary treatment modalities continue to be examined and rigorously tested, surgical resection remains the guideline-recommended therapy for medically appropriate patients with stage I NSCLC. Mirroring findings in other surgical specialties, a robust relationship exists between higher per-center surgical volume in thoracic oncology and long-term survival [7]. This volume–outcome relationship remains a critical insight into the provision of high-quality cancer care. Although this volume–outcome relationship relates to disparate outcomes for patients treated across institutions, it does not reflect a behavior that is necessarily modifiable at the center level [16].
The present analysis demonstrates that beyond differences in per-center surgical volume, considerable variation exists in the rate at which individual centers provide surgical resection to patients with stage I NSCLC, a finding that is not entirely explained by patient-, tumor-, and center-level behaviors. This finding, although frequently associated with surgical volume, represents a distinct measure of a center’s tendency to treat stage I NSCLC in a particular manner. This finding is important and is maintained after controlling for other covariates, including hospital volume. Although distillation of care to higher-volume centers has both advantages and disadvantages (with respect to improved care at high-volume centers at the expense of decreased access to care), improvements in the rate at which centers provide surgical therapy need not diminish access to care, instead ensuring that patients become more likely to receive guideline-concordant care regardless of their treating center’s volume. Modifications to the rate of use of surgical therapy may be more actionable at the center level and, as such, may offer an important metric to use in the improvement of cancer care across institutions.
This analysis has attempted to identify factors associated with a higher rate of use of surgical therapy at the center level. We identify important differences with respect to patient- and tumor-level characteristics at high- and low-use centers, such as differences in age, sex, socioeconomic status, and insurance status. These findings are consistent with prior studies that have identified disparities in access to surgical care for minorities, the poor, and the uninsured [5, 10, 17, 18]. These data suggest that treatment at centers that use surgical therapy less frequently may contribute to the observed lower rates of surgical therapy for the black population. Assessment of centers in terms of the rate at which they offer surgical therapy is potentially a worthwhile strategy as a means to re-evaluate the consequences of further regionalization of care to high-volume centers that may hinder access to care. We find only modest (<10 mile) differences in the distance travelled for patients traveling to high- and low-use centers, suggesting that high-use centers may be more accessible to a broader population.
Although this analysis is not designed to compare treatment strategies for stage I NSCLC, the nature of the per-center comparison permits some degree of assessment of the outcome of different treatment approaches. Patients treated at lower-use centers are more likely to receive external beam radiation but only modest differences in the rate of multi-agent chemotherapy. This suggests that the decreased rate of surgical therapy does not represent therapeutic nihilism, but it may instead reflect a tendency to choose nonsurgical treatment options. These data may warrant further studies to better elucidate why low-use centers prefer nonstandard therapeutic modalities for this disease. These data also provide additional context with which to evaluate previously described changes in the prevailing care provided for stage I NSCLC [12, 19, 20]. Likewise, the higher rate of pathologic staging data available for patients who undergo surgical therapy at centers with a high rate of use of surgical therapy may help appropriately guide therapy for these patients and ensure that patients who are upstaged are appropriately treated.
Taken together, these data may provide useful targets for quality improvement attempts at the center level. Specifically, scrutiny of centers with low adjusted rates of surgical therapy may be warranted to better elucidate practices that result in low use of surgical therapy at these centers, followed by modifications to practice patterns such that more patients may either be provided with surgical therapy or transferred to centers that are better able to provide surgical therapy for this patient population. This may include a diagnostic approach, in that high-use centers were more likely to use tissue-based diagnostic techniques, rather than rely on cytologic or radiographic diagnosis. Attention to the rate of use of surgical therapy may represent a superior quality metric in the examination of per-center differences in care of patients with thoracic malignancies.
A few limitations bear consideration in discussion of this work. As with all retrospective studies that use large national databases, there exists the possibility of potential unmeasured confounders for which we cannot account. The study is limited by granularity provided by the data set, particularly with respect to understanding the factors that may contribute to the per-center approach to offering surgical therapy or determining medical fitness for surgical resection. These findings again support the argument that additional comorbidity granularity, such as the inclusion of pulmonary function testing, may be warranted in the NCDB. Comorbidities such as chronic obstructive pulmonary disease are highly relevant to the study of thoracic oncology (and to the specific analysis at hand, in which fitness for surgical therapy is evaluated) and are known to contribute to negative outcomes in patients with lung cancer, but to date they remain unmeasured in this data source [21].
We show that in patients with stage I NSCLC treatment at centers with higher use of surgical therapy confers a survival advantage over treatment at centers with lower propensity to provide surgical therapy. Improvements to the rate at which surgical therapy is provided at the institution level are required to improve outcomes in this patient population.
Acknowledgments
This work was supported by the National Institutes of Health (NIH)-funded Cardiothoracic Surgery Trials Network grant 5U01HL088953-05 (B.A.Y.) and the NIH TL-1 Clinical and Translational Science Award (CTSA) 1UL1-TR001117-01 (National Center for Advancing Translational Sciences; NCATS). The American College of Surgeons and the Commission on Cancer have not verified and are not responsible for the analytic or statistical methodology used or the conclusions drawn from these data by the investigator.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015;65:5–29. [DOI] [PubMed] [Google Scholar]
- 2.National Comprehensive Cancer Network. 2017. NCCN clinical practice guidelines in oncology: non-small cell lung cancer. Available at www.nccn.org. Accessed December 29, 2017. [DOI] [PubMed]
- 3.McCrory DC, Lewis SZ, Heitzer J, Colice G, Alberts WM. American College of Chest Physicians. Methodology for lung cancer evidence review and guideline development: ACCP evidence-based clinical practice guidelines (2nd edition). Chest 2007;132(3 Suppl):23S–8S. [DOI] [PubMed] [Google Scholar]
- 4.Gray SW, Landrum MB, Lamont EB, McNeil BJ, Jaklitsch MT, Keating NL. Improved outcomes associated with higher surgery rates for older patients with early stage nonsmall cell lung cancer. Cancer 2012;118:1404–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaniski F, Enewold L, Thomas A, Malik S, Stevens JL, Harlan LC. Temporal patterns of care and outcomes of non-small cell lung cancer patients in the United States diagnosed in 1996, 2005, and 2010. Lung Cancer 2017;103:66–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Samson P, Crabtree T, Broderick S, et al. Quality measures in clinical stage I non-small cell lung cancer: improved performance is associated with improved survival. Ann Thorac Surg 2017;103:303–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luchtenborg M, Riaz SP, Coupland VH, et al. High procedure volume is strongly associated with improved survival after lung cancer surgery. J Clin Oncol 2013;31:3141–6. [DOI] [PubMed] [Google Scholar]
- 8.Demmy TL, Curtis JJ. Minimally invasive lobectomy directed toward frail and high-risk patients: a case-control study. Ann Thorac Surg 1999;68:194–200. [DOI] [PubMed] [Google Scholar]
- 9.Esnaola NF, Gebregziabher M, Knott K, et al. Underuse of surgical resection for localized, non-small cell lung cancer among whites and african americans in South Carolina. Ann Thorac Surg 2008;86:220–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Groth SS, Al-Refaie WB, Zhong W, et al. Effect of insurance status on the surgical treatment of early-stage non-small cell lung cancer. Ann Thorac Surg 2013;95:1221–6. [DOI] [PubMed] [Google Scholar]
- 11.Fry WA, Phillips JL, Menck HR. Ten-year survey of lung cancer treatment and survival in hospitals in the United States: a national cancer data base report. Cancer 1999;86: 1867–76. [DOI] [PubMed] [Google Scholar]
- 12.Valle LF, Jagsi R, Bobiak SN, et al. Variation in definitive therapy for localized non-small cell lung cancer among National Comprehensive Cancer Network institutions. Int J Radiat Oncol Biol Phys 2016;94:360–7. [DOI] [PubMed] [Google Scholar]
- 13.Nur U, Quaresma M, De Stavola B, Peake M, Rachet B. Inequalities in non-small cell lung cancer treatment and mortality. J Epidemiol Community Health 2015;69:985–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wakeam E, Byrne JP, Darling GE, Varghese TK Jr. Surgical treatment for early small cell lung cancer: variability in practice and impact on survival. Ann Thorac Surg 2017;104: 1872–80. [DOI] [PubMed] [Google Scholar]
- 15.Swords DS, Mulvihill SJ, Skarda DE, et al. Hospital-level variation in utilization of surgery for clinical stage I-II pancreatic adenocarcinoma. Ann Surg 2017. Jul 11; doi: 10.1097/SLA.0000000000002404 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 16.Vinod SK, O’Connell DL, Simonella L, et al. Gaps in optimal care for lung cancer. J Thorac Oncol 2008;3:871–9. [DOI] [PubMed] [Google Scholar]
- 17.Slatore CG, Au DH, Gould MK; American Thoracic Society Disparities in Healthcare Group. An official American Thoracic Society systematic review: insurance status and disparities in lung cancer practices and outcomes. Am J Respir Crit Care Med 2010;182:1195–205. [DOI] [PubMed] [Google Scholar]
- 18.Cykert S, Dilworth-Anderson P, Monroe MH, et al. Factors associated with decisions to undergo surgery among patients with newly diagnosed early-stage lung cancer. JAMA 2010;303:2368–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kapadia NS, Valle LF, George JA, et al. Patterns of treatment and outcomes for definitive therapy of early stage non-small cell lung cancer. Ann Thorac Surg 2017;104:1881–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yerokun BA, Yang CJ, Gulack BC, et al. A national analysis of wedge resection versus stereotactic body radiation therapy for stage IA non-small cell lung cancer. J Thorac Cardiovasc Surg 2017;154:675–86. [DOI] [PubMed] [Google Scholar]
- 21.Bugge A, Lund MB, Brunborg C, Solberg S, Kongerud J. Survival after surgical resection for lung cancer in patients with chronic obstructive pulmonary disease. Ann Thorac Surg 2016;101:2125–31. [DOI] [PubMed] [Google Scholar]


