Abstract
Background:
Liver-lung transplantation (LLT) is a rare procedure performed for patients with end-stage liver and lung disease. The lung allocation score (LAS), introduced in 2005, guides lung allocation including those receiving LLT. However, the impact of the LAS on outcomes in LLT is currently unknown.
Materials and methods:
The OPTN/United Network for Organ Sharing STAR file was queried for LLT candidates and recipients from 1988 to 2016. Demographic characteristics before (historic) and after (modern) the LAS were compared. Survival was analyzed with the Kaplan–Meier method and log-rank test.
Results:
In total, 167 candidates were listed for LLT, and 62 underwent LLT. The historic cohort had a higher FEV1% (48.22% versus 29.82%, P = 0.014), higher creatinine (1.22 versus 0.72, P < 0.001), and a higher percentage with pulmonary hypertension as the indication for transplantation (40% versus 0%, P = 0.003) compared with the modern cohort. LLT candidates in the historic cohort had a lower rate of transplant per 100 candidates (10.87 versus 33.33, P < 0.0001) and worse waitlist survival (1 y: 69.6% versus 80.9%, 3 y: 39.1% versus 66.8%, P = 0.004). Post-transplant survival was significantly lower in the historic cohort (1 y: 50.0% versus 82.7%, 5 y: 40.0% versus 69.0%, 10 y: 20.0% versus 55.5%, P = 0.0099).
Conclusions:
Most analyses of LLT have included patients before and after the introduction of the LAS. Our study shows that LLT candidates and recipients before the modern allocation system had distinct baseline characteristics and worse overall survival. Although many factors contributed to recent improved outcomes, these cohorts are significantly different and should be treated as such in future studies.
Keywords: Liver transplant, Lung transplant, Lung allocation score, UNOS, Survival, Outcomes
Simultaneous liver-lung transplantation (LLT) is indicated in patients with both end-stage liver and lung disease who are not projected to survive single-organ transplantation. However, there is debate whether the long-term outcomes of this procedure warrant dual allocation of both liver and lungs to a single patient. Much of the literature on survivability and outcomes in this rare cohort is limited to case series with relatively few large analyses.1–10 These case series span multiple decades without distinction between the different eras of transplantation.
The lung allocation score (LAS) was introduced in May 2005 and transformed the allocation of lungs from a system based primarily on wait-time to a system based primarily on medical need.11 The LAS estimates a candidate’s medical urgency and expected survival post-transplant compared with other potential recipients on the waitlist. The algorithm uses many factors to generate a score including diagnosis, patient demographics, comorbidities, lung function, patient functional status, pulmonary pressures, oxygen and/or ventilation requirements, and relevant laboratory clinical values.11 From this, an allocation score ranging from 0 to 100 is generated, with a higher score indicating a patient more likely to benefit from lung transplant. Although there have not been major studies examining the impact of end-stage liver disease on LAS, the algorithm includes total bilirubin and serum creatinine, and an increased score in LLT candidates may be likely.
Since the introduction of the LAS, there have been dramatic improvements in waitlist times, waitlist mortality, and post-transplant survival in both pediatric and adult lung transplant recipients.11–15 In addition, there has been a dramatic increase in the number of lung transplants performed.12–16 Notably, the increase in transplantation volume occurred despite a relatively stable pool of available donors over time. Thus, the introduction of the LAS has not increased organ availability but instead has redistributed the available organs to better prevent waitlist mortality.
We hypothesize that the introduction of the LAS resulted in a shift in morbidity and mortality in LLT candidates and recipients. If so, re-evaluation of long-term outcomes in this population may be warranted. We used the United Network for Organ Sharing database, which represents every solid organ transplant candidate and recipient in the United States since 1988. With this powerful tool, we present a retrospective analysis of survival in the LLT cohort before and after the introduction of the LAS.
Methods
Study design
The Institutional Review Board at Duke University Medical Center approved this retrospective analysis of the UNOS database. Eligible patients were >17 y of age at time of listing for simultaneous liver and lung transplantation for end-stage liver and lung disease in the United States from January 1, 1988, until December 31, 2016. Any patient receiving or listed for additional allografts at time of simultaneous LLT was excluded. The LAS was implemented in mid-2005, and to avoid overlap, patients listed or transplanted between January 1, 2005, and December 31, 2005 were excluded.
Study endpoints
The primary endpoint was overall survival. Secondary endpoints available in UNOS included pre-transplant, perioperative, and graft outcomes including waitlist mortality, time-to-transplant, graft failure, rejection episodes, re-transplantation, and 30-d postoperative mortality.
Statistical analysis
Baseline characteristics and unadjusted outcomes were computed using descriptive statistics utilizing the Kruskal–Wallis test for continuous variables and the Pearson χ2 test for categorical variables. Patients listed or transplanted before January 1, 2005, were deemed pre-LAS or the “historic” cohort. Patients listed or transplanted after December 31, 2005, were deemed the “modern” cohort or post-LAS. MELD-XI was calculated for each patient. MELD-XI is a predictive formula for 90-d pre-transplant mortality incorporating bilirubin and creatinine but omitting the international normalized ratio and has been validated in patients with thoracic disease and liver dysfunction.17–20
Survival was plotted using the Kaplane–Meier method,21 and curves were compared using the log-rank test. Cumulative incidence of transplants was calculated and used to determine a rate of transplant per 100 candidates within the first year of listing. Univariate proportional hazards regressions were calculated. To assess risk-adjusted mortality, covariates in the multivariate proportional hazards regression were chosen using forward stepwise selection.22
A P-value of less than 0.05 was deemed statistically significant. Statistical analysis was performed using R, version 3.4.3, (R Foundation for Statistical Computing, Vienna, Austria).
Results
Recipient demographics
A total of 62 adult recipients of LLT were identified and included. Of those, 10 underwent transplant before the introduction of LAS in May 2005. The rate of LLTs performed per year has increased after the implementation of the LAS from 1.29 LLTs per year pre-LAS to 6.6 LLTs post-LAS (Fig. 1). The mean LAS for modern era patients was 50.78 (SD 17.65). Average age (historic 37.50 y versus modern 37.00, P = 0.926) and male gender (80% versus 59.6%, P = 0.387) were not significantly different. The mean MELD-XI was statistically similar for both cohorts (14.30 versus 11.48, P = 0.064). Comparatively, the historic cohort had a higher FEV1% (48.22% versus 29.82%, P = 0.014) and higher creatinine at transplant (1.22 versus 0.72, P < 0.001). Complete baseline characteristics for the two groups are described in Table 1.
Fig. 1 –

The number of simultaneous liver-lung transplantation recipients by year.
Table 1 –
Baseline LLT recipient and donor characteristics between the historic (pre-LAS) and modern (post-LAS) cohorts.
| Characteristic | Historic (pre-LAS) | Modern (post-LAS) | P |
|---|---|---|---|
| n | 10 | 52 | |
| Age | 37.50 (15.28) | 37.00 (15.58) | 0.926 |
| Male gender | 8 (80.0) | 31 (59.6) | 0.387 |
| White | 10 (100.0) | 50 (96.2) | 0.999 |
| Recipient BMI | 20.71 (3.63) | 21.54 (4.05) | 0.55 |
| Donor age | 30.90 (11.12) | 29.48 (13.59) | 0.757 |
| Donor BMI | 22.84 (2.04) | 23.32 (3.97) | 0.713 |
| Previous transplant | 0.00 (0.00) | 0.02 (0.14) | 0.665 |
| Diabetes | 2 (20.0) | 23 (44.2) | 0.281 |
| FEV1% | 48.22 (31.82) | 29.82 (17.42) | 0.014 |
| Total bilirubin | 1.58 (1.35) | 2.38 (6.46) | 0.699 |
| Serum albumin at transplant | 3.55 (0.13) | 3.25 (0.62) | 0.34 |
| Creatinine at transplant | 1.22 (0.70) | 0.72 (0.31) | <0.001 |
| MELD-XI | 14.30 (5.77) | 11.48 (4.01) | 0.064 |
| Lung diagnosis | 0.003 | ||
| Cystic fibrosis | 5 (50.0) | 30 (57.7) | |
| Primary pulmonary hypertension | 4 (40.0) | 0 (0.0) | |
| Secondary pulmonary hypertension | 0 (0.0) | 2 (3.8) | |
| Idiopathic pulmonary fibrosis | 0 (0.0) | 9 (17.3) | |
| Pulmonary fibrosis—other | 1 (10.0) | 3 (5.8) | |
| Alpha-1-antitrypsin deficiency | 0 (0.0) | 3 (5.8) | |
| COPD/emphysema | 0 (0.0) | 1 (1.9) | |
| Sarcoidosis | 0 (0.0) | 1 (1.9) | |
| Other | 0 (0.0) | 2 (3.8) | |
| Liver diagnosis | 0.10 | ||
| Cystic fibrosis | 5 | 30 | |
| Alpha-1-antitrypsin | 0 | 3 | |
| Alcoholic cirrhosis | 2 | 2 | |
| Biliary cirrhosis | 0 | 1 | |
| Cirrhosis (other) | 2 | 12 | |
| Non–nonalcoholic steatohepatitis | 0 | 1 | |
| Hepatocellular carcinoma (HCC) | 0 | 1 | |
| Other | 1 | 2 |
Categorical data are reported as n (%), and continuous data are reported as mean (SD) unless otherwise stated.
Recipient indications for lung transplant differed between the historic and modern cohorts (P = 0.003). The primary indication for lung transplant in both cohorts was cystic fibrosis (50% versus 57.7%). However, 40% of patients in the historic era were transplanted secondary to primary pulmonary hypertension, whereas 0% of patients in the modern era were transplanted for that indication. The second most prevalent indication in the modern era was idiopathic pulmonary fibrosis (17.3%), and no patients in the historic era were transplanted because of idiopathic pulmonary fibrosis. The indications for liver transplant remained relatively stable between the historic and modern cohorts. The most numerous primary indication for liver transplant was cystic fibrosis (50.0% versus. 57.7%) with cirrhosis as the second most common indication (20.0% versus 23.1%).
Waitlist outcomes
The historic cohort had significantly higher median days on the waitlist (382 versus 116 d, P < 0.0001) before transplant or removal. In addition, the historic cohort had a significantly lower rate of candidates transplanted within the first year after initial listing (Fig. 2, 10.87 transplants versus 33.33 transplants per 100 candidates, P < 0.0001) and a worse waitlist survival (Fig. 3, 6 mo: 82.1% versus 87.4%, 1 y: 69.6% versus 80.9%, 1.5 y: 58.9% versus 80.9%, and 3 y: 39.1% versus 66.8%, P = 0.004).
Fig. 2 –
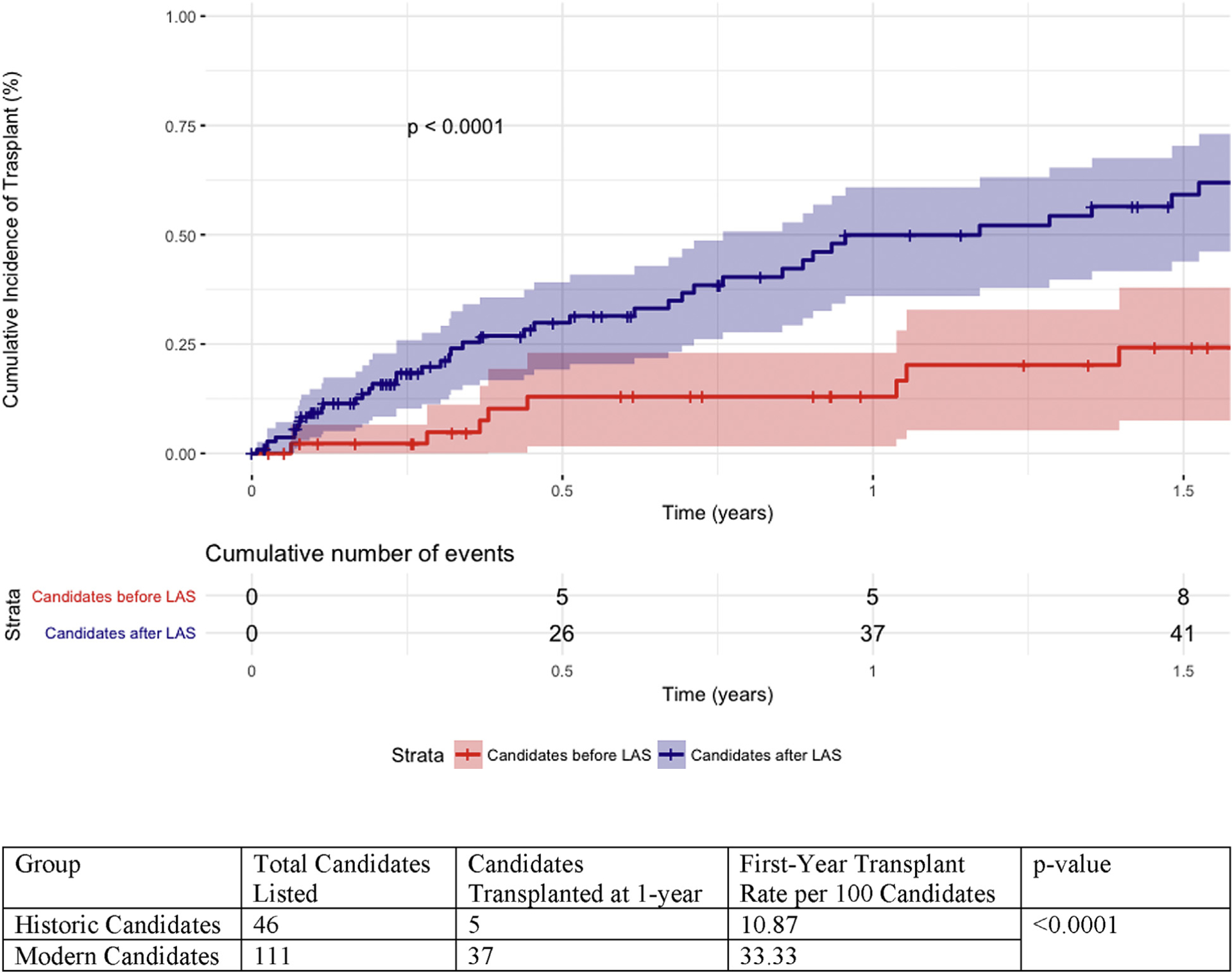
Cumulative incidence of transplant over 1.5 y after initial listing.
Fig. 3 –

LLT candidate waitlist survival probability within 3 y of initial listing.
Post-transplant outcomes
The median hospital stay between historic and modern cohorts was not significantly different (34 versus 28 d, P = 0.71). The percentage of patients with graft failure at the last known time point was significantly different between the cohorts (historic 70.0% versus modern 11.5%, P < 0.001). No patients received a liver retransplant. Instances of acute rejection, lung retransplant rates, and 30-d mortality were not significantly different (Table 2).
Table 2 –
Post-transplant LLT recipient outcomes comparing the historic (pre-LAS) and modern (post-LAS) cohorts.
| Outcome | Total (n = 62) | Historic (pre-LAS) (n = 10) | Modern (post-LAS) (n = 52) | P-value |
|---|---|---|---|---|
| Length of stay (median [IQR]) | 29.00 (17.00–58.00) | 34.00 (29.25–50.75) | 28.00 (17.00–60.50) | 0.71 |
| Acute rejection | 9.4% (5) | 0.0% (0) | 9.5% (5) | >0.999 |
| Graft failure | 21.0% (13) | 70.0% (7) | 11.5% (6) | <0.001 |
| Lung retransplant | 6.5% (4) | 10.0% (1) | 5.8% (3) | 0.618 |
| 30-d mortality | 20.0% (2) | 3.8% (2) | 6.5% (4) | 0.057 |
Categorical data are reported as n (%), and continuous data are reported as mean (SD) unless otherwise stated.
Patient survival analysis
Overall, patient survival was significantly worse in the historic cohort before the introduction of LAS compared with the modern cohort after LAS (Fig. 4, 1 y: 50.0% versus 82.7%, 5 y: 40.0% versus 69.0%, and 10 y: 20.0% versus 55.5%, P = 0.0099). Adjusting for MELD-XI, the use of LAS was associated with decreased risk of mortality (HR 0.32, 95% CI 0.14–0.76, P = 0.01; Table 3).
Fig. 4 –

Kaplan-Meier survival analysis of simultaneous liver-lung transplantation recipients comparing before and after the LAS.
Table 3 –
Cox proportional hazards model of overall survival for LLT recipients.
| Variable | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| Hazard ratio (95% confidence interval) | P-value | HR (95% CI) | P-value | |
| Age | 1.01 (0.98–1.04) | 0.601 | ||
| Gender (ref = male) | 1.05 (0.46–2.39) | 0.900 | ||
| Ethnicity (ref = white) | 1.11 (0.15–8.27) | 0.918 | ||
| Donor age | 0.997 (0.97–1.03) | 0.836 | ||
| FEV1% | 1.01 (0.99–1.03) | 0.215 | ||
| MELD-XI | 1.09 (1.01–1.18) | 0.025 | 1.10 (1.02–1.19) | 0.018 |
| Serum albumin | 0.63 (0.29–1.39) | 0.25 | ||
| Presence of LAS | 0.34 (0.15–0.80) | 0.013 | 0.32 (0.14–0.76) | 0.01 |
| Time on waitlist | 0.999 (0.998–1.001) | 0.532 | ||
| Length of stay | 1.003 (0.999–1.007) | 0.172 | ||
Discussion
LLT is a life-saving procedure used to help a rare patient population with end-stage liver and end-stage lung disease that would not be expected to survive isolated single-organ transplantation. There is currently debate in the field of transplantation whether the long-term outcomes for this procedure warrant allocation of two organs to a single recipient.23 However, much of the previous work studying this population was limited to small case series that span multiple eras of transplantation.1–10 Our study is the first to show the introduction of the LAS was associated with significant changes in the demographics and outcomes in the LLT population. Following the introduction of the LAS, patients receiving LLT in the modern era have had decreased waitlist time, decreased waitlist mortality, and improved overall survival compared with their historic counterparts. These findings suggest that this critical change in allocation has benefited this population of patients, and that these cohorts should be examined separately in future analyses.
In recent years, the frequency of LLT has increased, and thus the importance of understanding long-term outcomes in this population is imperative. Previous case series in LLT show 1-y survival ranging from 56% to 100% and 3-y survival ranging from 62% to 70%.5–10,24 These series have consisted of between 5 and 15 patients, including pediatric patients and patients receiving organs additional to a liver and lungs, such as an en bloc heart transplant. The 1- and 5-y survival probability in the modern cohort was similar to previously reported survivals; however, the historic cohort demonstrated significantly worse overall survival. The modern outcomes of LLT compare similarly with the outcomes following isolated lung transplantation.23
In the second largest study within this population, Wolf et al.23 examined the UNOS database to determine whether patients benefited from LLT versus remaining on the waitlist. They found 42 LLT recipients from 1987 to 2010. Similar to our study, most patients listed for LLT had cystic fibrosis as their primary indication. Wolf’s manuscript reported a mean LAS was 35.9, which was notably different from our mean LAS of 50.78; however their reported mean MELD for LLT patients was 10.9, which compared similarly to our mean MELD-XI (historic 14.30 and modern 11.48). Post-transplant survival in their study at 1-y was 75.5%, which was similar to our modern cohort (83%) but notably different from the historic cohort (50%). A more pronounced trend is seen in the long-term results compared with their 5-y survival of 59.0%. Likewise, their waitlist survival at 1-y (65.7%) and 3-y (41.0%) were similar to our historic cohort’s 1- and 3-y waitlist survival but far lower than the modern cohort’s waitlist survival at both time points. Although this study was an excellent look at survival versus remaining on the waitlist in this population, it does not evaluate changes to this cohort over time as demonstrated in our present study.
The LAS has been shown to decrease waitlist mortality and improve short- and long-term outcomes in isolated lung transplant recipients, which our waitlist and post-transplant survival analyses support in this unique cohort. The increases in waitlist survival and rate of candidates receiving a transplant support that LAS had a significant impact in this candidate population. One of the main indications for LLT both before and after LAS was cystic fibrosis. In one study of 704 single-organ lung transplant recipients with cystic fibrosis, Thabut et al.16 found that higher LAS correlated with a greater survival benefit from transplant. Similar to our results, national analyses have found an increase in idiopathic pulmonary fibrosis as a primary indication for transplantation after the introduction of the LAS.12,14 A recent national analysis also noted similar results to our data with significantly increased 1-y survival after the introduction of the LAS.14 The introduction of the LAS in LLT mirrors the results seen in single-organ lung transplantation with changes to primary indication, improved waitlist survival, and improved long-term outcomes.
Although the introduction of the LAS score may have helped improve outcomes in this cohort, it was most likely not the sole mediator of improved survival. Since 2005, new immunosuppressive regimens have become available, the field has garnered increased understanding of HLA mismatch, and novel technologies, such as normothermic machine perfusion, have become more prevalent.25–27 Indeed, these improvements in patient care and technical capabilities have contributed to temporal changes in survival beyond the shift in allocation with the implementation of the LAS. Several studies have demonstrated temporal changes in survival in both lung- and liver-alone transplant recipients.28,29 As such, it is difficult to separate the individual contributions of the LAS system from other improvements in liver and lung transplantation over time to the overall survival of LLT recipients. With the new allocation system, these have all contributed significantly to changes in patient care. Overall, the introduction of the LAS score represents a dramatic shift within transplantation in a variety of forms. Therefore, the LLT populations before and after 2005 should be considered different when performing retrospective, long-term analyses.
Our study has several limitations. This is a retrospective review of the national UNOS database. As such, it may suffer from selection bias for LLT or coding errors inherent in the database. Owing to a difference in the measures captured in the UNOS database before and after the implementation of the LAS and the measures needed for the LAS algorithm, it is unfortunately impossible to retrospectively calculate an allocation score for the historic cohort. We were unable to include cause of mortality or reason for removal from the waitlist to our results. A notable portion of patients have a date of death recorded but do not have a cause of death coded in the database. Our study has a small number of patients due to the rarity of this procedure, especially in the historic cohort. Although this is the largest cohort of LLT recipients and candidates studied in the literature, our study is not powered to perform large multivariate analyses, and generalized inferences from the data are difficult to make. The introduction of LAS serves as a marker differentiating eras of transplantation, but it is impossible to discern the exact impact that LAS had on survival in this small population. Our study is not powered to pinpoint the exact causes of decreased survival in the earlier cohort.
Conclusions
LLT has increased in frequency over the last 2 decades, and an improved understanding of outcomes following LLT is imperative to determine whether dual allocation of organs to a single recipient is justifiable. However, most national and single institution analyses have included patients from multiple eras of transplantation, including before and after the introduction of the LAS. Our study shows that the LLT cohort after the introduction of the LAS has significantly different baseline characteristics, was more likely to receive a transplant, and had increased post-transplant and waitlist survival. Although many factors contributed to the changes in mortality, the cohorts before and after the introduction of LAS are significantly different and should be treated as such when conducting future studies in the population receiving simultaneous thoracic and abdominal organ transplants.
Acknowledgment
Authors’ contribution: K.F. participated in the writing of the article, data analysis, and research design. P.M.S., B.E., and M.S.M. participated in the writing of the article and data analysis. M.L.C. participated in the writing of the article. M.G.H. participated in the writing of the article and research design. S.J.K. participated in the writing of the article, data analysis, and research design.
Footnotes
Data contained in this article were presented as an oral presentation at the Academic Surgical Congress in February 2018 in Jacksonville, Florida.
Disclosures
The data used in this study are derived from a de-identified UNOS file. The Organ Procurement and Transplantation Network have not verified and are not responsible for the analytic or statistical methodology employed or the conclusions drawn from these data by the investigators. The authors have no disclosures.
REFERENCES
- 1.Zimmerman AA, Howard TK, Huddleston CB. Combined lung and liver transplantation in a girl with cystic fibrosis. Can J Anaesth. 1999;46:571–575. [DOI] [PubMed] [Google Scholar]
- 2.Corno V, Dezza MC, Lucianetti A, et al. Combined double lung-liver transplantation for cystic fibrosis without cardiopulmonary by-pass. Am J Transplant. 2007;7:2433–2438. [DOI] [PubMed] [Google Scholar]
- 3.Backman S, Javela K, Koivusalo AM, et al. Successful liver and lung transplantation in patients with severe IgA deficiency, high anti-IgA concentration and a history of anaphylactic transfusion reaction. Transfus Med. 2014;24:251–253. [DOI] [PubMed] [Google Scholar]
- 4.Ceulemans LJ, Monbaliu D, Verslype C, et al. Combined liver and lung transplantation with extended normothermic lung preservation in a patient with end-stage emphysema complicated by drug-induced acute liver failure. Am J Transplant. 2014;14:2412–2416. [DOI] [PubMed] [Google Scholar]
- 5.Couetil JP, Houssin DP, Soubrane O, et al. Combined lung and liver transplantation in patients with cystic fibrosis. A 4 1/2-year experience. J Thorac Cardiovasc Surg. 1995;110:1415–1422. discussion 1422–3. [DOI] [PubMed] [Google Scholar]
- 6.Arnon R, Annunziato RA, Miloh T, et al. Liver and combined lung and liver transplantation for cystic fibrosis: analysis of the UNOS database. Pediatr Transplant. 2011;15:254–264. [DOI] [PubMed] [Google Scholar]
- 7.Praseedom RK, McNeil KD, Watson CJ, et al. Combined transplantation of the heart, lung, and liver. Lancet. 2001;358:812–813. [DOI] [PubMed] [Google Scholar]
- 8.Barshes NR, DiBardino DJ, McKenzie ED, et al. Combined lung and liver transplantation: the United States experience. Transplantation. 2005;80:1161–1167. [DOI] [PubMed] [Google Scholar]
- 9.Grannas G, Neipp M, Hoeper MM, et al. Indications for and outcomes after combined lung and liver transplantation: a single-center experience on 13 consecutive cases. Transplantation. 2008;85:524–531. [DOI] [PubMed] [Google Scholar]
- 10.Yi SG, Burroughs SG, Loebe M, et al. Combined lung and liver transplantation: analysis of a single-center experience. Liver Transpl. 2014;20:46–53. [DOI] [PubMed] [Google Scholar]
- 11.Egan TM, Murray S, Bustami RT, et al. Development of the new lung allocation system in the United States. Am J Transplant. 2006;6(5 Pt 2):1212–1227. [DOI] [PubMed] [Google Scholar]
- 12.Kozower BD, Meyers BF, Smith MA, et al. The impact of the lung allocation score on short-term transplantation outcomes: a multicenter study. J Thorac Cardiovasc Surg. 2008;135:166–171. [DOI] [PubMed] [Google Scholar]
- 13.Russo MJ, Worku B, Iribarne A, et al. Does lung allocation score maximize survival benefit from lung transplantation? J Thorac Cardiovasc Surg. 2011;141:1270–1277. [DOI] [PubMed] [Google Scholar]
- 14.Egan TM, Edwards LB. Effect of the lung allocation score on lung transplantation in the United States. J Heart Lung Transplant. 2016;35:433–439. [DOI] [PubMed] [Google Scholar]
- 15.Lancaster TS, Miller JR, Epstein DJ, et al. Improved waitlist and transplant outcomes for pediatric lung transplantation after implementation of the lung allocation score. J Heart Lung Transplant. 2017;36:520–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thabut G, Christie JD, Mal H, et al. Survival benefit of lung transplant for cystic fibrosis since lung allocation score implementation. Am J Respir Crit Care Med. 2013;187:1335–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wernly B, Lichtenauer M, Franz M, et al. Model for end-stage liver disease excluding INR (MELD-XI) score in critically ill patients: easily available and of prognostic relevance. PLoS One. 2017;12:e0170987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deo SV, Al-Kindi SG, Altarabsheh SE, et al. Model for end-stage liver disease excluding international normalized ratio (MELD-XI) score predicts heart transplant outcomes: evidence from the registry of the United Network for Organ Sharing. J Heart Lung Transplant. 2016;35:222–227. [DOI] [PubMed] [Google Scholar]
- 19.Grimm JC, Shah AS, Magruder JT, et al. MELD-XI score predicts early mortality in patients after heart transplantation. Ann Thorac Surg. 2015;100:1737–1743. [DOI] [PubMed] [Google Scholar]
- 20.Heuman DM, Mihas AA, Habib A, et al. MELD-XI: a rational approach to “sickest first” liver transplantation in cirrhotic patients requiring anticoagulant therapy. Liver Transpl. 2007;13:30–37. [DOI] [PubMed] [Google Scholar]
- 21.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 22.Vittinghoff E, McCulloch CE. Relaxing the rule of ten events per variable in logistic and Cox regression. Am J Epidemiol. 2007;165:710–718. [DOI] [PubMed] [Google Scholar]
- 23.Wolf JH, Sulewski ME, Cassuto JR, et al. Simultaneous thoracic and abdominal transplantation: can we justify two organs for one recipient? Am J Transplant. 2013;13:1806–1816. [DOI] [PubMed] [Google Scholar]
- 24.Ceulemans LJ, Strypstein S, Neyrinck A, et al. Combined liver-thoracic transplantation: single-center experience with introduction of the “Liver-first” principle. Transpl Int. 2016;29:715–726. [DOI] [PubMed] [Google Scholar]
- 25.Vincenti F, Rostaing L, Grinyo J, et al. Belatacept and long-term outcomes in kidney transplantation. N Engl J Med. 2016;374:333–343. [DOI] [PubMed] [Google Scholar]
- 26.Cypel M, Yeung JC, Liu M, et al. Normothermic ex vivo lung perfusion in clinical lung transplantation. N Engl J Med. 2011;364:1431–1440. [DOI] [PubMed] [Google Scholar]
- 27.Kotloff RM, Thabut G. Lung transplantation. Am J Respir Crit Care Med. 2011;184:159–171. [DOI] [PubMed] [Google Scholar]
- 28.Yusen RD, Christie JD, Edwards LB, et al. The registry of the international society for heart and lung transplantation: thirtieth adult lung and heart-lung transplant report–2013; focus theme: age. J Heart Lung Transplant. 2013;32:965–978. [DOI] [PubMed] [Google Scholar]
- 29.Lodhi SA, Lamb KE, Meier-Kriesche HU. Solid organ allograft survival improvement in the United States: the long-term does not mirror the dramatic short-term success. Am J Transplant. 2011;11:1226–1235. [DOI] [PubMed] [Google Scholar]


