Abstract
Objectives
This study aimed to assess the nature and degree of association between exposure to potentially traumatic wartime experiences in early life, such as living in a heavily bombed region or witnessing death firsthand, and later-life frailty.
Method
The Vietnam Health and Aging Study included war survivors in Vietnam, 60+, who completed a survey and health exam between May and August 2018. Latent class analysis (LCA) is used to construct classes exposed to similar numbers and types of wartime experiences. Frailty is measured using a deficit accumulation approach that proxies biological aging. Fractional logit regression associates latent classes with frailty scores. Coefficients are used to calculate predicted frailty scores and expected age at which specific levels of frailty are reached across wartime exposure classes.
Results
LCA yields 9 unique wartime exposure classes, ranging from extreme exposure to nonexposed. Higher frailty is found among those with more heavy/severe exposures with a combination of certain types of experiences, including intense bombing, witnessing death firsthand, having experienced sleep disruptions during wartime, and having feared for one’s life during war. The difference in frailty-associated aging between the most and least affected individuals is more than 18 years.
Discussion
War trauma hastens aging and warrants greater attention toward long-term implications of war on health among vast postconflict populations across the globe.
Keywords: Armed conflict, Biological aging, Developing countries, Latent class analysis, Trauma
Frailty is the accumulation of deficits and declines across multiple physiologic systems (Cesari et al., 2017; Clegg et al., 2013; Fried et al., 2001; Gobbens, Luijkx, et al., 2010; Rockwood, 2005). Because accumulated physiological deficits signify a global deterioration in an organism’s normal and healthy function, those with high levels of frailty are vulnerable to any type of physical or psychological stressor, and are therefore at high risk of chronic disease, disability, cognitive disorders, falls, institutionalization, and ultimately death. Stylized measures of frailty quantify these physiological declines and vulnerabilities and are proxies for biological aging (Mitnitski et al., 2001; Rockwood & Howlett, 2019). In countries experiencing rapid population aging, frailty syndrome consequently becomes a concern for public health (Fedarko, 2011; Rodríguez-Artalejo & Rodríguez-Mañas, 2014). Vietnam is a prime example. Its population aged 60+ has grown from roughly 5.5 million, representing 8% in 1990, to 12 million and 12% in 2020, and should reach 30 million and 27% by 2050, with oldest-old being the fastest-growing subsegment (United Nations Department of Economic and Social Affairs, 2019). Population aging alone, therefore, will result in large numbers of Vietnamese with moderate and severe frailty.
However, the distribution of later-life frailty is heterogeneous (Woo et al., 2005). Early- and mid-life stress are particularly consequential and manifest through a series of biological processes (Bijker & Agyemang, 2016; Haapanen et al., 2018; Murphy et al., 2017). For one, exposure to stress brings about physiological responses that influence genetic stability and cell function, impacting biological age. Recent emphasis on telomere length is one example of the potential genetic manifestation of such responses (Schutte & Malouff, 2016). There is also a link between earlier-life stress and chronic inflammation, which is a result of a cascade of responses and secondary stressors, leading to later-life chronic disease (Ellis & Del Giudice, 2014; Finch & Crimmins, 2004). Cumulative inequality theory (Ferraro et al., 2009), which is born out of a more general life-course perspective (Ben-Shlomo & Kuh, 2002), provides a framework for understanding these associations. Among the propositions of cumulative inequality is that health trajectories are affected by onset, duration, and magnitude of stressful exposures and that accumulation of stress accelerates aging, even among those asymptomatic of disease for most of their lives. Pearlin and colleagues (2005) similarly propose that some types of early-life trauma result in a continual process of stress accumulation throughout life, which in turn has a long-range toll on later-life health.
Extant literature substantiates these propositions when it comes to early-life wartime experiences. In utero or experienced during early childhood, war trauma adversely influences early development and health (Akbulut-Yuksel, 2014; Akresh et al., 2012; Chamarbagwala & Morán, 2011; Lee, 2014). Studies of combat veterans have found substantial effects across a range of long-term health outcomes (Bedard & Deschênes, 2006; Brown et al., 2014; Buckley & Kaloupek, 2001; Cange, 2016; Johnson et al., 2010; Nice et al., 1996; Nichter et al., 2019; O’Toole & Catts, 2008; Spiro et al., 1994; Spiro & Settersten, 2012; Wilmoth, Landes, et al., 2018; Wilmoth, London, et al., 2018). Wartime traumatic events that are likely to have lasting effects on psychological resources include witnessing death, losing family, and living with inhospitable ambient conditions (Ellis et al., 2017; Haapanen et al., 2018; Roelfs et al., 2010). Studies also suggest that mechanisms such as impoverishment, availability of nutritional resources, adoption of harmful behaviors, changes and disruption in housing, living in unsanitary conditions, and other such social structural factors have long-term effects on health (Akbulut-Yuksel, 2017; Alastalo et al., 2009; Cange, 2016; Hardy & Reyes, 2016; Islam et al., 2017; Lindeboom & van Ewijk, 2015; Ramirez & Haas, 2021; Settersten Jr., 2006; Spiro & Settersten, 2012; Taylor et al., 2016).
Some studies, however, reveal contrary or more nuanced influences. Studying World War II survivors, Ramirez and Haas (2021) find timing critical with those born during, as opposed to prior to, war having later-life disadvantages. Others hypothesized sensitive periods during which traumatic events are more or less impactful (Dunn et al., 2018). Theories of resilience suggest milder levels of adversity, experienced at certain ages, lead to adjustment, adaptation, and coping (Gershon & High, 2015; Hardy & Reyes, 2016). For instance, Sagi-Schwartz and colleagues (2013), studying Holocaust survivors, observe some factors promoted by wars, like finding meaning and satisfaction in life, increase longevity.
The cohort of Vietnamese now entering older ages is illustrative of a population who lived through extensive periods of considerable earlier-life trauma, a direct result of the “American War” that peaked in the late 1960s and early 1970s (Mizoguchi, 2010; Young et al., 2021). Our study explores the nature and degree of association between exposure to potentially traumatic wartime experiences in early life and later-life frailty among a sample of war survivors in Vietnam. Frailty is measured using a cumulative deficit approach (Mitnitski et al., 2001). Wartime trauma is assessed using discrete groups exposed to common experiences derived statistically from a latent class analysis (LCA; McCuthcheon, 1987).
Vietnam presents a particularly interesting and unique example for such a study. First, it houses one of the world’s largest older populations exposed to potentially traumatic wartime experiences earlier in life. Second, Vietnamese were exposed to a wide array of experiences and wartime statuses. Some older Vietnamese were in the military and participated directly in combat; some participated in less formalized local militia; and some lived directly within war zones. Experiences are likely to have differed across these groups. Third, in Vietnam, many women engaged in combat and dangerous paramilitary operations (Gottschang Turner, 1998). A longstanding and consistent finding in the literature is the paradox that while women live longer than men, they do so at higher levels of frailty (Gordon et al., 2017; Verbrugge, 1984). There is also evidence of sex differences in the long-term physical and mental health consequences of military service (Batuman et al., 2011; Sternke, 2011; Street et al., 2009; Wilmoth et al., 2011).
Method
Data and Sample
The 2018 Vietnam Health and Aging Study (VHAS; https://vhas.utah.edu) involved 2,447 Vietnamese men and women aged 60+. It used a multistage, probability sample beginning with purposive choice of three provinces and four administrative Districts in northern Vietnam representing a range of wartime bombing intensities. Within Districts, geographic units called Communes were randomly selected. A list of eligible respondents in each Commune provided a sampling frame from which a stratified random sample of men and women, with and without military service, was chosen. Response rate was 84.7%. Data collection included in-person interviews and a health exam (for protocol details, see Korinek et al, 2019). The current study omits 75 cases fully completed by proxy and 79 missing data on wartime exposure, frailty, or other key study variables, for a valid sample size of 2,294.
Outcome
The deficit accumulation approach is used to measure frailty (Rockwood & Mitnitski, 2007; Searle et al., 2008). A deficit is a disease, illness, functional problem, or other indicator of physiological dysfunction. The approach provides an indicator that proxies biological aging (Mitnitski et al., 2001; Rockwood & Mitnitski, 2007; Searle et al., 2008), and as such, our results can be interpreted as the degree to which wartime trauma hastens aging. Fifty-eight items (see Supplementary Table 1) were identified across 15 health domains. A Frailty Index (FI) score is the proportion of deficits. A FI is calculated for those with valid responses for 80%+ of items (nine of the 79 observations deleted are due to a missing FI score). Table 1 shows mean FI is .293. There is sex variation; women’s mean is .316 versus .260 for men. Supplementary Figure 1 shows mean FIs by single years of age and sex and, because of substantial variation by single years due to small Ns, predicted FIs using a fractional regression. Results conform to expectations that women and older people have higher frailty (Gobbens, van Assen, et al., 2010).
Table 1.
Descriptive Statistics for Study Variables (Standard Deviations for Means in Parentheses)
| Total sample | |
|---|---|
| N a | 2,294 |
| Mean Frailty Index scores | 0.293 (0.161) |
| Exposure items (shortened descriptor in parentheses) | |
| % Witnessed death (death) | 48.9 |
| % Experienced sleep disruptions (sleep) | 36.6 |
| % Experienced fearing for own life (fear) | 32.4 |
| % Knew someone who died/was injured (knew someone) | 31.0 |
| % Was displaced from home (displacement) | 28.1 |
| % Had shortage of food and/or water (food/water) | 23.1 |
| % Lived in a region of intense bombing (bombing) | 22.8 |
| Mean age | 69.9 (8.3) |
| % Female | 58.8 |
| % Military experience | |
| Civilian | 56.8 |
| Formal | 19.6 |
| Militia | 23.6 |
| % Married | 69.1 |
| Mean household size | 3.74 (2.07) |
| Mean number living children | 4.42 (1.87) |
| % Agricultural occupation | 67.6 |
| Mean years of education | 7.53 (4.14) |
| % With children in college | 36.8 |
| Mean wealth index | 0.141 (1.748) |
| % Alcohol consumption | |
| None | 68.0 |
| Sometimes | 20.8 |
| Daily | 11.2 |
| % Smoking/betel nut status | |
| Never | 53.2 |
| Former | 25.7 |
| Current | 21.1 |
| Mean stressor index | 0.893 (0.980) |
| % Fair/poor health in childhood | 37.1 |
| % Experienced hunger in childhood | 33.2 |
Note:
aUnweighted.
Wartime Exposure
VHAS included a battery of questions derived from validated instruments to assess potential traumatic exposures experienced during the American war (Keane et al., 1989; King et al., 2006; Young et al., 2021). VHAS also recorded respondents’ geographic region of residence during the war. This is paired with records of bombing intensity (bombs per square kilometer) published by the Department of Defense (Miguel & Roland, 2011). Using paired data, we construct a measure for whether an individual lived in a region that experienced high bombing intensity, defined as 300+ bombs per square kilometer, between 1965 and 1975.
LCAs were used to define emergent patterns of experiences based on survey items plus bombing intensity. Some experiences overlapped in that they associated with the same latent class; overlapping items were combined. For instance, “how often did you encounter having to move due to evacuation?” was combined with “how often did you encounter moving due to bombing?” Experiencing either evacuation, or moving due to bombing, results in being coded as ‘was displaced from home.’ Some experiences did not statistically distinguish war exposure classes and were deleted. After reductions, there were seven possible wartime exposure items. Table 1 shows the percentage of the weighted sample that experienced each, and these are: witnessed death firsthand (48.9%), experienced sleep disruptions (36.6%), experienced fearing for own life (32.4%), knew someone personally who died/was injured (31.0%), was displaced from home (28.1%), experienced shortage of food or water (23.1%), and lived in a region of intense bombing (22.8%). Supplementary Table 2 shows distributions for these items and individual items combined to create the seven experiences.
Covariates
A base model controls for age, sex, and military status. Three categories of military status are civilian/nonmilitary (reference), formal military, or informal military. The latter includes the many, especially women, who as youth engaged in militias and other military support organizations such as the Youth Shock Brigades, a common way Vietnamese informally participated in war efforts (Guillemot, 2009). Additional covariates represent socioeconomic, demographic, and health indicators. Besides age and sex, these include marital status (married vs not), household size, and number of living children. Socioeconomic indicators include lifetime occupation (agriculture vs other), education, whether respondent has a child with college education, and wealth. A principal component analysis procedure introduced by Filmer and Pritchett (2001) was used to construct a wealth index comprising the linear combination of household assets, such as television and motor vehicle. Health includes several subdomains. Health behaviors include alcohol consumption in the last 12 months, categorized as none (reference), sometimes, and daily, and smoking or betel nut chewing (men tend to engage in the former and women the latter, with the effects similar; Ko et al., 1995), coded as none (reference), former, and current. An index of recent stressors sums six life events experienced in the last 3 years: accident that caused injury, marital disruption, residential move, death of child, severe illness of spouse, and financial difficulty. Two early-life health conditions are childhood health status (fair vs poor) and childhood hunger (yes vs no). Table 1 shows descriptive statistics for study variables.
Statistical Analysis
Using LCA, we first separate the sample into discrete exposure groups (McCuthcheon, 1987). LCA statistically structures the sample into classes occupied by individuals exposed to similar experiences by applying an iterative expectation–maximization algorithm to determine maximum likelihood parameters that best describe underlying latent classes. Compared to additive approaches, which tend to assume experiences have equal value (King et al., 2006), LCA permits a look at how common discrete patterns are linked to late-life frailty. There is no a priori reason to believe any experience affects frailty in a particular way, and identifiable combinations of factors may have specific implications. In addition, the LCA strategy provides a look at the structure of traumatic events experienced by Vietnamese and therefore experiences that go together. Results show the probability that an observation belongs to a class and the probability that a person who is a member of a class was exposed to an experience. An optimal number of classes is determined by Bayesian information criterion, Lo–Mendell–Rubin adjusted likelihood ratio test, and parametric bootstrapped likelihood ratio. Observations are assigned to the class for which they have the largest probability, with the average in our data being .80, a quantity indicating good fit.
Next, classes are used to model frailty. Because FI is a proportion with hard limits at .00 and 1.00, it is modeled using fractional regression with a logit link function. Three models are presented. The base controls for age and sex. Model 2 adds socioeconomic and demographic indicators. Model 3 adds health indicators. Adding covariates sequentially permits assessment of robustness of the association to confounders.
We test for interaction between exposure and three key characteristics across which we might expect the impact to vary: sex, age, and military status. With respect to sex, there are basic differences in the progression of frailty between men and women, and a long-investigated paradox that women have greater longevity while men have better health, which translates into men and women of the same age having different levels of frailty (Gordon et al., 2017; Verbrugge, 1984). As noted earlier, age of exposure to trauma may determine the degree to which this impacts health long term. Interactions with military status are of interest, given those in the formal military, militia, and civilians have experienced war and the events of war quite differently.
Fractional regression coefficients are not easily interpretable as a change in FI. Two additional calculations, done using the full estimated equation, provide more intuitive interpretations. First is the predicted FI for each class when covariates are controlled. This is equivalent to the expected FI when covariates are evaluated at their mean. The difference between two predicted values is a marginal effect of being in one class rather than another with respect to frailty. Second is the expected age at which an individual of a given sex and class reaches a specific FI, holding other covariates constant. Comparisons of how the age expected to reach a particular FI differs across exposure classes are useful when predicted ages are within the valid sample age range. We assess the age of reaching a FI of .30 and .35. Expected ages for these levels of frailty, for men and women, respectively, fall within the age range of the sample (60–98 years). The measure of frailty we use is continuous and there are no published guidelines that cut the FI into low versus high degrees of frailty. However, FIs of .30–.35 can generally be interpreted as severe levels. First, these scores are just slightly below the average frailty for those institutionalized, which is around .40 (Rockwood & Mitnitski, 2011). Second, FIs between .25 and .35 are levels at which mortality rates begin to accelerate and at which a 5-year risk of death exceeds 50% (Armstrong et al., 2015).
Results
Latent Class Analysis
Table 2 summarizes results for the best fitting LCA solution. There are nine statistically unique wartime exposure classes (WECs), listed as 1–9 (column 1) according to mean number of exposures experienced by those in the class (column 4), from highest to lowest. Means vary markedly, suggesting that part of the latent class structure is a function of number of experiences to which one was exposed. For instance, members of WEC 1 average 5.7 experiences, while those who are members of WEC 9 average 0.3. The column “class title” (column 2) provides a descriptor related to mean number of experiences to which individuals in that class were exposed. There is one class labeled “Extreme,” three “Heavy,” three “Moderate,” one “Light,” and one “Nonexposed.” From this point on, we refer to WECs by descriptor names.
Table 2.
Summary of Latent Class Analysis Results, Showing Mean Number of Items to Which Members of Class Were Exposed, Listing Experiences With High (.80–1.00) and Moderate (.50–.79) Probability of Exposure, and Percent in Class, by Wartime Exposure Class
| Column | ||||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 |
| Wartime exposure class |
Class title | % in class | Mean number experiences to which members of class were exposed | Experiences with very high probability of exposure (p = .75–1.00) |
| 1 | Extreme | 9.3 | 5.7 | Death Sleep Displacement Food/water Bombing |
| 2 | Heavy 1 | 4.1 | 4.3 | Death Sleep Displacement Bombing |
| 3 | Heavy 2 | 8.0 | 4.0 | Death Knew someone |
| 4 | Heavy 3 | 8.2 | 3.9 | Death Bombing |
| 5 | Moderate 1 | 9.1 | 3.0 | Sleep Fear |
| 6 | Moderate 2 | 4.1 | 2.7 | Displacement |
| 7 | Moderate 3 | 9.7 | 2.5 | Knew someone |
| 8 | Light | 7.2 | 1.7 | None |
| 9 | Nonexposed | 40.3 | 0.3 | None |
Note: Death = witnessed death firsthand; sleep = experienced sleep disruptions; fear = experienced fearing for own life; knew someone = knew someone personally who died/was injured; displacement = was displaced from home; food/water = had a shortage of food and/or water; bombing = lived in a region with intense bombing.
Column 3 displays percent of the weighted sample in each class. About 40% belong to the Nonexposed and about 9% to the Extreme class. The three Heavy classes combined contain about 20% of the sample. The Moderate classes combined are about 23%. The Light class has about 7% of the sample.
Further distinguishing classes are unique combinations of experiences common to each. Column 5 lists exposures that members of the class likely experienced, indicated by a probability of at least .75. Full results with specific probabilities for each item by class are found in Supplementary Table 3. Those in the Extreme class were likely exposed to five experiences (witnessed death, sleep disruptions, displacement, shortage of food or water, and intense bombing). Members of classes labeled Heavy 1–3 have a mean number of experiences of about 4. With means being similar, these classes are better distinguished by experiences common to each. Members of all three of these classes likely witnessed death. Members of classes titled Heavy 1 and Heavy 3 experienced intense bombing. What distinguishes these two is that those in Heavy 1 also experienced sleep disruptions and displacement. In contrast, those in Heavy 2 knew someone who died/was injured. The classes titled Moderate 1–3 average 2.5 to 3.0 experiences. Moderate 1 consists of those who experienced the combination of sleep disruptions and fear of death. The Moderate 2 consists of people who were displaced. Those in Moderate 3 knew someone who died/was injured. There is no specific experience characterizing the Light group, but their average is 1.7, so their exposure is nonzero. The Nonexposed group, in contrast, do not have a high probability of any experiences, and with a mean of 0.3, most in this group did not report any exposure.
Fractional Regression
Table 3 presents fractional regression models. The main result is that, across three models, those in the classes Extreme, Heavy 1–3, and Moderate 1 and 3 consistently have significantly higher frailty than those in the Nonexposed class across all models. The largest coefficient is associated with the “Extreme” class, and therefore these individuals have on average the highest levels of frailty. Comparatively, large coefficients are seen for classes Heavy 1 and Heavy 3. Light exposure is significant in Models 1 and 2 but not in Model 3. Those in the class titled Moderate 2 are not statistically different from those Nonexposed.
Table 3.
Fractional Regression Model for Frailty Index Scores, Showing Estimates and Standard Errors
| Model 1 Base | Model 2 + SES and demographics | Model 3 + health | ||||
|---|---|---|---|---|---|---|
| β | SE | β | SE | β | SE | |
| WECa | ||||||
| Extreme | .462** | 0.066 | .440** | 0.062 | .329** | 0.059 |
| Heavy 1 | .354** | 0.081 | .379** | 0.077 | .290** | 0.074 |
| Heavy 2 | .230** | 0.073 | .235** | 0.067 | .170** | 0.066 |
| Heavy 3 | .375** | 0.081 | .337** | 0.082 | .273** | 0.075 |
| Moderate 1 | .354** | 0.079 | .320** | 0.072 | .190** | 0.065 |
| Moderate 2 | .035 | 0.089 | .064 | 0.093 | −.026 | 0.095 |
| Moderate 3 | .0678* | 0.077 | .179* | 0.073 | .158* | 0.074 |
| Light | .185* | 0.077 | .158* | 0.079 | .108 | 0.078 |
| Nonexposed | — | — | — | — | — | — |
| Age | .025** | 0.003 | .018** | 0.003 | .019** | 0.003 |
| Female | .322** | 0.068 | .217** | 0.065 | .173* | 0.074 |
| Military experience (omitted Civilian) | ||||||
| Formal | .155* | 0.070 | .139* | 0.067 | .173** | 0.064 |
| Militia | .059 | 0.049 | .035 | 0.046 | .019 | 0.011 |
| Married | .040 | 0.046 | .010 | 0.045 | ||
| Household size | −.005 | 0.011 | .006 | 0.011 | ||
| Number children | .015 | 0.011 | .008 | 0.011 | ||
| Agricultural occupation | −.014 | 0.049 | −.034 | 0.047 | ||
| Years of education | −.021** | 0.006 | −.021** | 0.006 | ||
| Children in college | −.063 | 0.006 | −.051 | 0.042 | ||
| Wealth index | −.085** | 0.045 | −.057** | 0.014 | ||
| Alcohol consumption (omitted never) | ||||||
| Some | −.216** | 0.060 | ||||
| Daily | −.215** | 0.085 | ||||
| Smoking/betel nut status (omitted never) | ||||||
| Former | .137* | 0.060 | ||||
| Current | −.068 | 0.055 | ||||
| Index of stressors | .199** | 0.019 | ||||
| Health in childhood | −.011 | 0.038 | ||||
| Hunger in childhood | .064# | 0.037 | ||||
| Constant | −3.064 | 0.199 | −2.389 | 0.261 | −2.554 | 0.290 |
| Log likelihood | −1361.2 | −1326.3 | −1295.2 | |||
Notes: SES = socioeconomic status; WEC = wartime exposure class.
aExperiences with high probability of exposure by WEC: Extreme: death, sleep, displacement, food/water, and bombing; Heavy 1: death, sleep, displacement, and bombing; Heavy 2: death and knew someone; Heavy 3: death and bombing; Moderate 1: sleep and fear; Moderate 2: displacement; Moderate 3: knew someone; Light: none; Nonexposed: none.
*.01 < p < .05. **p < .01. #.05 < p < .10.
The coefficient for the Extreme class is robust across all models (β = .462, .440, .329). The same is true for those in Heavy 1 and 3, both of which consist of individuals likely exposed to witnessing death and having lived in a region with intense bombing (Heavy 1 β = .354, .379, .290; Heavy 3 β = .375, .337, .273). The moderate decline in coefficients across models indicates that potentially confounding variables have some but moderate impact on the base association. The coefficient for Moderate 1 (for Model 3, β = .190) indicates that this class, composed of individuals who tended to encounter sleep disruptions and fear for their lives, also has comparatively high frailty. The strength of association drops for Heavy 2 and Moderate 3, but these classes remain statistically significant (for Model 3, β = .170 and β = .158).
Interactions between WEC and sex, age, and military status were tested and are all nonsignificant. Therefore, levels of frailty by WECs are consistent across these characteristics. Full results for interaction models are provided in Supplementary Table 4.
Other covariates indicate higher frailty is associated with older age, being female, being in the formal military, more numerous recent stressors, and reporting hunger in childhood. Lower frailty is associated with higher education, wealth, current and former alcohol consumption, and former smoking/betel nut chewing. A favorable result of alcohol consumption may indicate that alcohol intake in Vietnam is light, that those who do engage may be selected for health, and/or that alcohol consumption is associated with socioeconomic status in Vietnam. Former smokers/betel nut chewers, in contrast, have less favorable frailty than current users, which may indicate quitting such behavior is due to being in poor health.
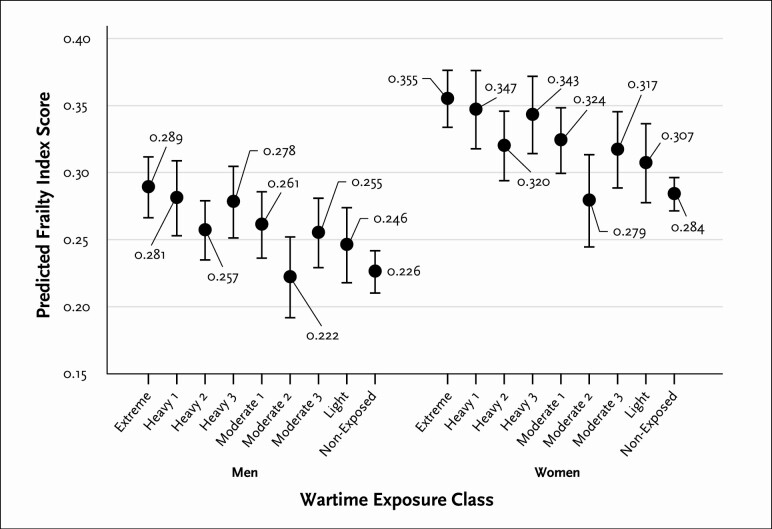
Predicted FIs, and 95% confidence intervals, by WECs, computed using Model 3 from Table 3, are provided in Figure 1. While the figure demonstrates potential overlap in FIs across a number of the WECs, it also shows that individuals who are members of several WECs have substantially and significantly higher levels of frailty than others. The predicted FI for men, for instance, is very high in the Extreme class at .289 (95% CI = 0.266, 0.312) and much lower for men in the Nonexposed class at .226 (95% CI = 0.210, 0.242). It is lowest for those in the Moderate 2 class, the group that experienced displacement, at .222 (95% CI = 0.191, 0.253). Being in the Extreme class, therefore, results in a predicted increase in FI, or marginal effect, of .289 – .226 = .063 in comparison to being in the Nonexposed class, and .289 – .222 = .067 in comparison to being in the Moderate 2 class. For women, the predicted FI is .355 (95% CI = 0.333, 0.377) for the Extreme group, .284 (95% CI = 0.271, 0.297) for the Nonexposed group, and .279 (95% CI = 0.244, 0.314) for the Moderate 2 class, representing marginal effects of .355 – .284 = 0.71 and .355 – .279 = .076, respectively. More generally, being in the Extreme, Heavy 1, or Heavy 3 classes is associated with comparatively high predicted levels of frailty, while being in Moderate 2, Light, or Nonexposed groups is associated with comparatively low levels.
Figure 1.
Predicted values for Frailty Index scores across wartime exposure classes, showing point estimates and high/low 95% confidence interval bars.
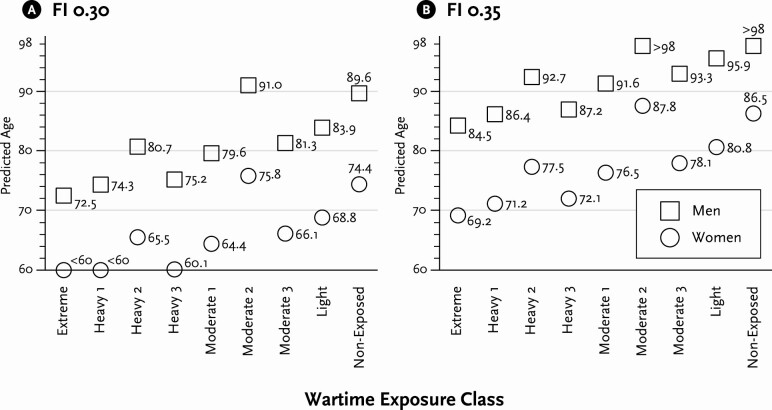
Figure 2 presents predicted age at which men and women in different WECs are expected to reach an FI of .30 or .35. The predicted age at which individuals are expected to reach these FIs, for men and women, respectively, across classes, falls within the age range of the sample, which is ages 60–98, allowing for valid comparison. As noted above, these FIs can also be interpreted as representing quite severe levels of frailty (Armstrong et al., 2015; Rockwood et al., 2017). If predicted age is below 60 or above 98, it is beyond the age range of the sample and is indicated by <60 and >98 (some classes of women reach an expected FI of .30 younger than 60, and some classes of men reach an expected FI of .35 older than 98). Depending upon the WEC, men can expect a frailty level of .30 between ages 72.5 (Extreme) and 91.0 (Moderate 2), suggesting that those in the most affected class look 18.5 years older than those in the least affected. Women can expect to reach a frailty level of .35 as young as 69.2 (Extreme) and as old as 87.8 (Moderate), indicating a difference. For another meaningful comparison, those in the Extreme group look about 17 years older than those Nonexposed. While these are the largest effects of wartime exposure on frailty-associated aging, there are noticeable differences even between adjacent WECs. For instance, for both men and women, those in Heavy 1 and Heavy 3 look about 2 years younger than counterparts in the Extreme class. Those in the Nonexposed class look between 5 and 6 years younger than those in the Light class.
Figure 2.
Predicted age to reach a Frailty Index (FI) score of 0.30 and 0.35 by exposure class and sex.
Discussion
We examined the association between war exposure earlier in life and later-life frailty in Vietnam, a country with an aging population that lived through long and harsh wartime periods, including both direct engagement in military operations and civilian exposure to bombing and other stressors (Mizoguchi, 2010). The results strongly suggest wartime trauma hastens aging. Nine classes were distinguished by number and combination of war experiences. The highest predicted levels of frailty were found among those in the Extreme exposure class, a class consisting of individuals who experienced most if not all of the exposure items used in the LCA. In comparison, low FIs were found for those in the Nonexposed and in the Moderate 2 classes, the latter being a class of individuals with high probability of displacement during war. The difference quantified as predicted age at which a specific level of frailty is reached indicates the Extreme class is about 17 years older, with respect to age-related frailty, than the Nonexposed, and more than 18 years older than the Moderate 2 class. The highest predicted frailty classes share characteristics of having high probability of having resided in an intensely bombed region during the war and having witnessed death firsthand. Therefore, these may be particularly consequential experiences for long-term health. Additionally, those in Moderate 1, occupied by individuals who did not have a large number of experiences but did tend to experience sleep disruptions and fearing for their life, also have comparatively high levels of frailty. The Moderate 1 class looks about 10 years older than the Nonexposed class.
The class that contains those who were displaced (Moderate 2), but exposed to few other experiences, had favorable FIs not significantly different from those in the Nonexposed and Light classes. This result contrasts research that has suggested displacement impacts mental and physical health (Daoud et al., 2012; Husain et al., 2011). The current result may be a function of unique contextual features of northern Vietnam in which war displacement involved short distances and relocation to familiar environments near family and other social connections, rather than to refugee camps (Vong, 2020). Those fleeing localized violence may have moved into relatively supportive settings more favorable than typical wartime displacement.
In conclusion, those in classes that contain individuals characterized as having a larger number of experiences generally have higher frailty, while there are also differences in frailty across specific combinations of experiences. These two findings combined indicate that, net of other covariates, both number and composition of wartime experiences predict frailty. We tested for interactions between exposure and three variables thought to potentially influence how exposure impacts frailty: sex, age, and type of military experience. None of these were significant, indicating that effects are consistent across these population subgroups.
In several ways, the associations support the cumulative inequality theory of health as presented by Ferraro and colleagues (2009), and related propositions on the long-range impacts of trauma suggested by Pearlin and colleagues (2005). These perspectives postulate that trauma earlier in life results in extreme stress, which accumulates over time, and that the accumulation of severe stress accelerates the aging process. This occurs because early-life trauma alters the lifelong ability to regulate stress, thus contributing to a disruption of human development through immune, metabolic, mental, and even genetic systems (Ellis & Del Giudice, 2014; Greenblatt Kimron et al., 2019; Roelfs et al., 2010; Schneiderman et al., 2005). The potentially traumatic experiences that combined to have the greatest impact on late-life frailty in our analysis may in fact be those that spawn severe and long-term stress. For instance, heavy bombing intensity has been shown to lead to weakened immune systems and increase susceptibility to some forms of disease (Singhal, 2019). Witnessing death, fearing death, and experiencing sleep disruptions during war likely have similar connections.
Strengths of the study include a large sample of individuals in a developing country exposed to a variety of wartime experiences and retrospective data that allow for examination of long-term life-course effects. There are also advantages to using the LCA approach to categorize exposure. LCA allows examination of discrete categories and assessment of the structure of exposure in Vietnam. An advantage over an additive approach is that the latter assumes every experience has an equal impact on health, which is clearly not the case. We suggest future analyses to examine the impact of each exposure factor separately in order to gain insights into what types of wartime experiences specifically lead to higher frailty in later life.
The study has limitations. Exposures do not cover all possible traumatic experiences. In particular, veterans may have been exposed to additional experiences such as being shot or captured. We use the term “potential traumatic experience” in this paper because not all experiences will be traumatic, or equally traumatic, for all people. While there are advantages to LCA, individuals are not perfectly matched in a class. For instance, those in classes with higher levels of frailty tended to have experienced witnessing death and living in a region with intense bombing, but some in other classes were also exposed to these experiences. The LCA is a method for obtaining common overarching patterns in data, but given the huge number of potential combinations of experiences, it is impossible to derive classes without overlap. Then again, posterior probabilities for the classes created in our analysis are very high (.80), indicating that the LCA solution reached represents a very good fit. While results provide evidence of correlation, there are unexamined factors that could threaten causality and therefore causal inference needs to be assessed carefully. For instance, more heavily bombed areas of Vietnam may, as a result or coincidentally, have developed economically at a slower pace. Thus, those who experienced intense bombing may have experienced poorer access to health resources. Retrospective survey data may be subject to recall bias. Given that frailty is associated with cognitive decline, it is possible that frailer respondents are more apt to misreport. While the direction of the misreporting is uncertain, if cognitive problems result in forgetting experiences, it would lead to an underestimate of the associations.
Hirschman and colleagues (1995) estimate numbers of war-related deaths in Vietnam reached one million or more. Merli (2000) shows that, contrary to other wars, those in higher socioeconomic positions, measured by paternal education, bore the burden of war disproportionately and were more likely to die relative to those in lower positions in Vietnam. These findings suggest that survival probability to an age where one could become part of the current sample may result in sample selectivity. However, there are also reasons to believe that selectivity may not be of great concern. First, most of the deaths attributed to the American War occurred between 1965 and 1975, a result of acute causes rather than the type of long-term accumulation of stress that is the focus of the current analysis. Second, if one assumes that those who were most affected by the war are the ones who died between the end of the war and the beginning of our data collection, then selectivity would underestimate impacts. Third, life-course perspective suggests that the accumulation of harmful effects may remain latent throughout life and appear only late in life when individuals are more vulnerable to frailty (Cange, 2016).
A great number of individuals around the world, in developing countries, were exposed to war in and near their own homes and communities. Many were not prepared or trained for combat, but were exposed to trauma. Military personnel from more developed countries sent to fight wars in other countries often have some level of reintegrative health resources available, but those in developing countries may endure these experiences without availability of good health care. Therefore, our results point to health implications associated with frailty in a large number of postconflict settings globally where exposure to early-life trauma was commonplace. Several populations come to mind, such as Rwandans who lived through genocide in the 1990s, Koreans who experienced wars in the 1950s, and Bosnians who suffered war in the 1990s. Results of our study may be useful for understanding current and future health needs of such populations and for developing strategies to mitigate the long-term effects of early-life wartime trauma.
Funding
This work was supported by the National Institute of Aging at the National Institutes of Health grant #1R01AG052537 “Health and Aging Post Conflict: War’s Enduring Effects Among Survivors in Vietnam” and The Canadian Institutes for Health Research, project title “The Long-Term Effects of War on Biological Aging: The Case of Vietnam,” Project Number 175309.
Conflict of Interest
None declared.
Supplementary Material
Acknowledgments
The lead author acknowledges funding from the Social Sciences and Humanities Research Council of Canada through the Canada Research Chairs program. The authors thank three anonymous reviewers for their thoughtful comments on earlier versions of the paper.
Contributor Information
Zachary Zimmer, Department of Gerontology and Family Studies and Global Aging and Community Initiative, Mount Saint Vincent University, Halifax, Nova Scotia, Canada.
Kim Korinek, Department of Sociology, University of Utah, Salt Lake City, Utah, USA.
Yvette Young, Department of Sociology, University of Utah, Salt Lake City, Utah, USA.
Bussarawan Teerawichitchainan, Department of Sociology and Centre for Family and Population Research, National University of Singapore, Singapore.
Tran Khanh Toan, Department of Family Medicine, Hanoi Medical University, Hanoi, Vietnam.
Author Contributions
Z. Zimmer initially conceptualized the article and the analysis, ran the software to analyze the data, and wrote the first draft. K. Korinek, Y. Young, B. Teerawichitchainan, and T. K. Toan rewrote sections of the paper, contributed to the interpretation of the results, read and edited various versions, added text and analysis for revised versions, and assisted in responding to reviewer comments. Z. Zimmer, K. Korinek, B. Teerawichitchainan, and T. K. Toan developed and planned all aspects of the originating study including developing questionnaires and sampling frames. T. K. Toan oversaw the study operations in Vietnam including the day-to-day handling of the survey operation, and contributed in the cultural interpretation of the findings. Y. Young conceptualized, created, and edited figures.
References
- Akbulut-Yuksel, M. (2014). Children of war: The long-run effects of large-scale physical destruction and warfare on children. Journal of Human Resources, 49(3), 634–662. doi: 10.3368/jhr.49.3.634 [DOI] [Google Scholar]
- Akbulut-Yuksel, M. (2017). War during childhood: The long run effects of warfare on health. Journal of Health Economics, 53, 117–130. doi: 10.1016/j.jhealeco.2017.02.005 [DOI] [PubMed] [Google Scholar]
- Akresh, R., Lucchetti, L., & Thirumurthy, H. (2012). Wars and child health: Evidence from the Eritrean–Ethiopian conflict. Journal of Development Economics, 99(2), 330–340. doi: 10.1016/j.jdeveco.2012.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alastalo, H., Raikkonen, K., Pesonen, A. K., Osmond, C., Barker, D. J., Kajantie, E., Heinonen, K., Forsen, T. J., & Eriksson, J. G. (2009). Cardiovascular health of Finnish war evacuees 60 years later. Annals of Medicine, 41(1), 66–72. doi: 10.1080/07853890802301983 [DOI] [PubMed] [Google Scholar]
- Armstrong, J. J., Mitnitski, A., Launer, L. J., White, L. R., & Rockwood, K. (2015). Frailty in the Honolulu–Asia Aging Study: Deficit accumulation in a male cohort followed to 90% mortality. The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences, 70(1), 125–131. doi: 10.1093/gerona/glu089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batuman, F., Bean-Mayberry, B., Goldzweig, C., Huang, C., Miake-Lye, I. M., Washington, D. L., Yano, E. M., Zephyrin, L. C., & Shekelle, P. G. (2011). Health effects of military service on women veterans. Department of Veterans Affairs. http://europepmc.org/abstract/med/21796832 [PubMed] [Google Scholar]
- Bedard, K., & Deschênes, O. (2006). The long-term impact of military service on health: Evidence from World War II and Korean War veterans. The American Economic Review, 96(1), 176–194. doi: 10.1257/000282806776157731 [DOI] [PubMed] [Google Scholar]
- Ben-Shlomo, Y., & Kuh, D. (2002). A life course approach to chronic disease epidemiology: Conceptual models, empirical challenges and interdisciplinary perspectives. International Journal of Epidemiology, 31(2), 285–293. doi: 10.1093/ije/31.2.285 [DOI] [PubMed] [Google Scholar]
- Bijker, R., & Agyemang, C. (2016). The influence of early-life conditions on cardiovascular disease later in life among ethnic minority populations: A systematic review. Internal and Emergency Medicine, 11(3), 341–353. doi: 10.1007/s11739-015-1272-y [DOI] [PubMed] [Google Scholar]
- Brown, M. T., Wilmoth, J. M., & London, A. S. (2014). Veteran status and men’s later-life cognitive trajectories: Evidence from the Health and Retirement Study. Journal of Aging and Health, 26(6), 924–951. doi: 10.1177/0898264314534893 [DOI] [PubMed] [Google Scholar]
- Buckley, T. C., & Kaloupek, D. G. (2001). A meta-analytic examination of basal cardiovascular activity in posttraumatic stress disorder. Psychosomatic Medicine, 63(4), 585–594. doi: 10.1097/00006842-200107000-00011 [DOI] [PubMed] [Google Scholar]
- Cange, C. W. (2016). The life course model as a framework for post-conflict health analysis: Reflections on the Gulf War critical period. Medicine, Conflict, and Survival, 32(4), 282–294. doi: 10.1080/13623699.2016.1269284 [DOI] [PubMed] [Google Scholar]
- Cesari, M., Calvani, R., & Marzetti, E. (2017). Frailty in older persons. Clinics in Geriatric Medicine, 33(3), 293–303. doi: 10.1016/j.cger.2017.02.002 [DOI] [PubMed] [Google Scholar]
- Chamarbagwala, R., & Morán, H. E. (2011). The human capital consequences of civil war: Evidence from Guatemala. Journal of Development Economics, 94(1), 41–61. doi: 10.1016/j.jdeveco.2010.01.005 [DOI] [Google Scholar]
- Clegg, A., Young, J., Iliffe, S., Rikkert, M. O., & Rockwood, K. (2013). Frailty in elderly people. Lancet (London, England), 381(9868), 752–762. doi: 10.1016/S0140-6736(12)62167-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daoud, N., Shankardass, K., O’Campo, P., Anderson, K., & Agbaria, A. K. (2012). Internal displacement and health among the Palestinian minority in Israel. Social Science & Medicine (1982), 74(8), 1163–1171. doi: 10.1016/j.socscimed.2011.12.041 [DOI] [PubMed] [Google Scholar]
- Dunn, E. C., Nishimi, K., Gomez, S. H., Powers, A., & Bradley, B. (2018). Developmental timing of trauma exposure and emotion dysregulation in adulthood: Are there sensitive periods when trauma is most harmful? Journal of Affective Disorders, 227, 869–877. doi: 10.1016/j.jad.2017.10.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis, B. J., & Del Giudice, M. (2014). Beyond allostatic load: Rethinking the role of stress in regulating human development. Development and Psychopathology, 26(1), 1–20. doi: 10.1017/S0954579413000849 [DOI] [PubMed] [Google Scholar]
- Ellis, B. J., Oldehinkel, A. J., & Nederhof, E. (2017). The adaptive calibration model of stress responsivity: An empirical test in the Tracking Adolescents’ Individual Lives Survey study. Development and Psychopathology, 29(3), 1001–1021. doi: 10.1017/S0954579416000985 [DOI] [PubMed] [Google Scholar]
- Fedarko, N. S. (2011). The biology of aging and frailty. Clinics in Geriatric Medicine, 27(1), 27–37. doi: 10.1016/j.cger.2010.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraro, K. F., Shippee, T. P., & Schafer, M. H. (2009). Cumulative inequality theory for research on aging and the life course. In V. L. Bengston, D. Gans, N. M. Pulney, & M. Silverstein (Eds.), Handbook of theories of aging (pp. 413–433). Springer Publishing Company.
- Filmer, D., & Pritchett, L. H. (2001). Estimating wealth effects without expenditure data—Or tears: An application to educational enrollments in states of India. Demography, 38(1), 115–132. doi: 10.1353/dem.2001.0003 [DOI] [PubMed] [Google Scholar]
- Finch, C. E., & Crimmins, E. M. (2004). Inflammatory exposure and historical changes in human life-spans. Science (New York, N.Y.), 305(5691), 1736–1739. doi: 10.1126/science.1092556 [DOI] [PubMed] [Google Scholar]
- Fried, L. P., Tangen, C. M., Walston, J., Newman, A. B., Hirsch, C., Gottdiener, J., Seeman, T., Tracy, R., Kop, W. J., Burke, G., & McBurnie, M. A.; Cardiovascular Health Study Collaborative Research Group . (2001). Frailty in older adults: Evidence for a phenotype. The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences, 56(3), M146–M156. doi: 10.1093/gerona/56.3.m146 [DOI] [PubMed] [Google Scholar]
- Gershon, N. B., & High, P. C. (2015). Epigenetics and child abuse: Modern-day darwinism—The miraculous ability of the human genome to adapt, and then adapt again. American Journal of Medical Genetics Part C: Seminars in Medical Genetics, 169(4), 353–360, doi: 10.1002/ajmg.c.31467 [DOI] [PubMed] [Google Scholar]
- Gobbens, R. J., Luijkx, K. G., Wijnen-Sponselee, M. T., & Schols, J. M. (2010). In search of an integral conceptual definition of frailty: Opinions of experts. Journal of the American Medical Directors Association, 11(5), 338–343. doi: 10.1016/j.jamda.2009.09.015 [DOI] [PubMed] [Google Scholar]
- Gobbens, R. J., van Assen, M. A., Luijkx, K. G., Wijnen-Sponselee, M. T., & Schols, J. M. (2010). Determinants of frailty. Journal of the American Medical Directors Association, 11(5), 356–364. doi: 10.1016/j.jamda.2009.11.008 [DOI] [PubMed] [Google Scholar]
- Gordon, E. H., Peel, N. M., Samanta, M., Theou, O., Howlett, S. E., & Hubbard, R. E. (2017). Sex differences in frailty: A systematic review and meta-analysis. Experimental Gerontology, 89, 30–40. doi: 10.1016/j.exger.2016.12.021 [DOI] [PubMed] [Google Scholar]
- Gottschang Turner, K. (1998). Even the women must fight. Wiley. [Google Scholar]
- Greenblatt Kimron, L., Marai, I., Lorber, A., & Cohen, M. (2019). The long-term effects of early-life trauma on psychological, physical and physiological health among the elderly: The study of Holocaust survivors. Aging & Mental Health, 23(10), 1340–1349. doi: 10.1080/13607863.2018.1523880 [DOI] [PubMed] [Google Scholar]
- Guillemot, F. (2009). Death and suffering at first hand: Youth shock brigades during the Vietnam war (1950–1975). Journal of Vietnamese Studies, 4(3), 17–60. doi: 10.1525/vs.2009.4.3.17 [DOI] [Google Scholar]
- Haapanen, M. J., Perälä, M. M., Salonen, M. K., Kajantie, E., Simonen, M., Pohjolainen, P., Pesonen, A. K., Räikkönen, K., Eriksson, J. G., & von Bonsdorff, M. B. (2018). Early life stress and frailty in old age: The Helsinki Birth Cohort Study. BMC Geriatrics, 18(1), 179. doi: 10.1186/s12877-018-0873-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy, M. A., & Reyes, A. M. (2016). The longevity legacy of World War II: The intersection of GI status and mortality. The Gerontologist, 56(1), 104–114. doi: 10.1093/geront/gnv041 [DOI] [PubMed] [Google Scholar]
- Hirschman, C., Preston, S., & Loi, V. M. (1995). Vietnamese casualties during the American war: A new estimate. Population and Development Review, 21(4), 783–812. doi: 10.2307/2137774 [DOI] [Google Scholar]
- Husain, F., Anderson, M., Lopes Cardozo, B., Becknell, K., Blanton, C., Araki, D., & Vithana, E. K. (2011). Prevalence of war-related mental health conditions and association with displacement status in postwar Jaffna District, Sri Lanka. Journal of the American Medical Association, 306(5), 522–531. doi: 10.1001/jama.2011.1052 [DOI] [PubMed] [Google Scholar]
- Islam, A., Raschky, P., & Smyth, R. (2017). The long-term health effects of mass political violence: Evidence from China’s cultural revolution. Social Indicators Research, 132(1), 257–272. doi: 10.1007/s11205-015-1030-6 [DOI] [Google Scholar]
- Johnson, A. M., Rose, K. M., Elder, G. H.Jr., Chambless, L. E., Kaufman, J. S., & Heiss, G. (2010). Military combat and risk of coronary heart disease and ischemic stroke in aging men: The Atherosclerosis Risk in Communities (ARIC) study. Annals of Epidemiology, 20(2), 143–150. doi: 10.1016/j.annepidem.2009.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keane, T. M., Fairbank, J. A., Caddell, J. M., Zimering, R. T., Taylor, K. L., & Mora, C. A. (1989). Clinical evaluation of a measure to assess combat exposure. Psychological Assessment, 1(1), 53. doi: 10.1037/1040-3590.1.1.53 [DOI] [Google Scholar]
- King, L. A., King, D. W., Vogt, D. S., Knight, J., & Samper, R. E. (2006). Deployment Risk and Resilience Inventory: A collection of measures for studying deployment-related experiences of military personnel and veterans. Military Psychology, 18(2), 89–120. doi: 10.1207/s15327876mp1802_1 [DOI] [Google Scholar]
- Ko, Y. C., Huang, Y. L., Lee, C. H., Chen, M. J., Lin, L. M., & Tsai, C. C. (1995). Betel quid chewing, cigarette smoking and alcohol consumption related to oral cancer in Taiwan. Journal of Oral Pathology & Medicine, 24(10), 450–453. doi: 10.1111/j.1600-0714.1995.tb01132.x [DOI] [PubMed] [Google Scholar]
- Korinek, K., Teerawichitchainan, B., Zimmer, Z., Brindle, E., Chuc, N. T., Minh, N. H., & Toan, T. K. (2019). Design and measurement in a study of war exposure, health, and aging: Protocol for the Vietnam Health and Aging Study. BMC Public Health, 19(1), 1–11. doi: 10.1186/s12889-019-7680-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, C. (2014). In utero exposure to the Korean War and its long-term effects on socioeconomic and health outcomes. Journal of Health Economics, 33, 76–93. doi: 10.1016/j.jhealeco.2013.11.002 [DOI] [PubMed] [Google Scholar]
- Lindeboom, M., & van Ewijk, R. (2015). Babies of the war: The effect of war exposure early in life on mortality throughout life. Biodemography and Social Biology, 61(2), 167–186. doi: 10.1080/19485565.2015.1047489 [DOI] [PubMed] [Google Scholar]
- McCuthcheon, A. L. (1987). Latent class analysis. Sage Publications. [Google Scholar]
- Merli, M. G. (2000). Socioeconomic background and war mortality during Vietnam’s wars. Demography, 37(1), 1–15. doi: 10.2307/2648092 [DOI] [PubMed] [Google Scholar]
- Miguel, E., & Roland, G. (2011). The long-run impact of bombing Vietnam. Journal of Development Economics, 96(1), 1–15. doi: 10.1016/j.jdeveco.2010.07.004 [DOI] [Google Scholar]
- Mitnitski, A. B., Mogilner, A. J., & Rockwood, K. (2001). Accumulation of deficits as a proxy measure of aging. TheScientificWorldJournal, 1, 323–336. doi: 10.1100/tsw.2001.58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizoguchi, N. (2010). The consequences of the Vietnam war on the Vietnamese population [ UC Berkeley Electronic Theses and Dissertations, University of California at Berkeley; ]. http://escholarship.org/uc/item/0sh7j7s9 [Google Scholar]
- Murphy, M. O., Cohn, D. M., & Loria, A. S. (2017). Developmental origins of cardiovascular disease: Impact of early life stress in humans and rodents. Neuroscience and Biobehavioral Reviews, 74(Pt B), 453–465. doi: 10.1016/j.neubiorev.2016.07.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nice, D. S., Garland, C. F., Hilton, S. M., Baggett, J. C., & Mitchell, R. E. (1996). Long-term health outcomes and medical effects of torture among US Navy prisoners of war in Vietnam. Journal of the American Medical Association, 276(5), 375–381. [PubMed] [Google Scholar]
- Nichter, B., Norman, S., Haller, M., & Pietrzak, R. H. (2019). Physical health burden of PTSD, depression, and their comorbidity in the U.S. veteran population: Morbidity, functioning, and disability. Journal of Psychosomatic Research, 124, 109744. doi: 10.1016/j.jpsychores.2019.109744 [DOI] [PubMed] [Google Scholar]
- O’Toole, B. I., & Catts, S. V. (2008). Trauma, PTSD, and physical health: An epidemiological study of Australian Vietnam veterans. Journal of Psychosomatic Research, 64(1), 33–40. doi: 10.1016/j.jpsychores.2007.07.006 [DOI] [PubMed] [Google Scholar]
- Pearlin, L. I., Schieman, S., Fazio, E. M., & Meersman, S. C. (2005). Stress, health, and the life course: Some conceptual perspectives. Journal of Health and Social Behavior, 46(2), 205–219. doi: 10.1177/002214650504600206 [DOI] [PubMed] [Google Scholar]
- Ramirez, D., & Haas, S. A. (2021). The long arm of conflict: How timing shapes the impact of childhood exposure to war. Demography, 58(3), 951–974. doi: 10.1215/00703370-9114715 [DOI] [PubMed] [Google Scholar]
- Rockwood, K. (2005). What would make a definition of frailty successful? Age and Ageing, 34(5), 432–434. doi: 10.1093/ageing/afi146 [DOI] [PubMed] [Google Scholar]
- Rockwood, K., Blodgett, J. M., Theou, O., Sun, M. H., Feridooni, H. A., Mitnitski, A., Rose, R. A., Godin, J., Gregson, E., & Howlett, S. E. (2017). A frailty index based on deficit accumulation quantifies mortality risk in humans and in mice. Scientific Reports, 7, 43068. doi: 10.1038/srep43068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockwood, K., & Howlett, S. E. (2019). Age-related deficit accumulation and the diseases of ageing. Mechanisms of Ageing and Development, 180, 107–116. doi: 10.1016/j.mad.2019.04.005 [DOI] [PubMed] [Google Scholar]
- Rockwood, K., & Mitnitski, A. (2007). Frailty in relation to the accumulation of deficits. The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences, 62(7), 722–727. doi: 10.1093/gerona/62.7.722 [DOI] [PubMed] [Google Scholar]
- Rockwood, K., & Mitnitski, A. (2011). Frailty defined by deficit accumulation and geriatric medicine defined by frailty. Clinics in Geriatric Medicine, 27(1), 17–26. doi: 10.1016/j.cger.2010.08.008 [DOI] [PubMed] [Google Scholar]
- Rodríguez-Artalejo, F., & Rodríguez-Mañas, L. (2014). The frailty syndrome in the public health agenda. BMJ Publishing Group Ltd. [DOI] [PubMed] [Google Scholar]
- Roelfs, D., Shor, E., Davidson, K., & Schwartz, J. (2010). War-related stress exposure and mortality: A meta-analysis. International Journal of Epidemiology, 39(6), 1499–1509. doi: 10.1093/ije/dyq132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagi-Schwartz, A., Bakermans-Kranenburg, M. J., Linn, S., & van Ijzendoorn, M. H. (2013). Against all odds: Genocidal trauma is associated with longer life-expectancy of the survivors. PLoS One, 8(7), e69179. doi: 10.1371/journal.pone.0069179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneiderman, N., Ironson, G., & Siegel, S. D. (2005). Stress and health: Psychological, behavioral, and biological determinants. Annual Review of Clinical Psychology, 1, 607–628. doi: 10.1146/annurev.clinpsy.1.102803.144141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schutte, N. S., & Malouff, J. M. (2016). The relationship between perceived stress and telomere length: A meta-analysis. Stress and Health, 32(4), 313–319. doi: 10.1002/smi.2607 [DOI] [PubMed] [Google Scholar]
- Searle, S. D., Mitnitski, A., Gahbauer, E. A., Gill, T. M., & Rockwood, K. (2008). A standard procedure for creating a frailty index. BMC Geriatrics, 8, 24. doi: 10.1186/1471-2318-8-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Settersten Jr., R. A. (2006). When nations call: How wartime military service matters for the life course and aging. Research on Aging, 28(1), 12–36. doi: 10.1177/0164027505281577 [DOI] [Google Scholar]
- Singhal, S. (2019). Early life shocks and mental health: The long-term effect of war in Vietnam. Journal of Development Economics, 141, 1–15. doi: 10.1016/j.jdeveco.2018.06.002 [DOI] [Google Scholar]
- Spiro, A.3rd, Schnurr, P. P., & Aldwin, C. M. (1994). Combat-related posttraumatic stress disorder symptoms in older men. Psychology and Aging, 9(1), 17–26. doi: 10.1037//0882-7974.9.1.17 [DOI] [PubMed] [Google Scholar]
- Spiro, A., & Settersten, R. A. (2012). Long-term implications of military service for later-life health and well-being. Research in Human Development, 9(3), 183–190. doi: 10.1080/15427609.2012.705551 [DOI] [Google Scholar]
- Sternke, L. M. (2011). Measurement of military combat exposure among women: Analysis and implications. Women’s Health Issues, 21(suppl. 4), S168. doi: 10.1016/j.whi.2011.04.020 [DOI] [PubMed] [Google Scholar]
- Street, A. E., Vogt, D., & Dutra, L. (2009). A new generation of women veterans: Stressors faced by women deployed to Iraq and Afghanistan. Clinical Psychology Review, 29(8), 685–694. doi: 10.1016/j.cpr.2009.08.007 [DOI] [PubMed] [Google Scholar]
- Taylor, M. G., Ureña, S., & Kail, B. L. (2016). Service-related exposures and physical health trajectories among aging veteran men. The Gerontologist, 56(1), 92–103. doi: 10.1093/geront/gnv662 [DOI] [PubMed] [Google Scholar]
- United Nations Department of Economic and Social Affairs. (2019). World population prospects 2019. U.N. population.un.org/wpp/
- Verbrugge, L. M. (1984). Longer life but worsening health? Trends in health and mortality of middle-aged persons. Milbank Memorial Fund Quarterly, 62(3), 475–519. doi: 10.2307/3349861 [DOI] [PubMed] [Google Scholar]
- Vong, S. (2020). “Assets of war”: Strategic displacements, population movements, and the uses of refugees during the Vietnam War, 1965–1973. Journal of American Ethnic History, 39(3), 75–100. doi: 10.5406/jamerethnhist.39.3.0075 [DOI] [Google Scholar]
- Wilmoth, J. M., Landes, S. D., & London, A. S. (2018). The health of male veterans in later life. Annual Review of Gerontology and Geriatrics, 39(1), 23–48. [Google Scholar]
- Wilmoth, J. M., London, A. S., & Oliver, W. J. (2018). Military service experiences and older men’s trajectories of self-rated health. In Spiro A., Settersten R. A., & Aldwin C. M. (Eds.), Long-term outcomes of military service: The health and well-being of aging veterans. American Psychological Association. doi: 10.1037/0000061-000 [DOI] [Google Scholar]
- Wilmoth, J. M., London, A. S., & Parker, W. M. (2011). Sex differences in the relationship between military service status and functional limitations and disabilities. Population Research and Policy Review, 30(3), 333–354. doi: 10.1007/s11113-010-9191-0 [DOI] [Google Scholar]
- Woo, J., Goggins, W., Sham, A., & Ho, S. C. (2005). Social determinants of frailty. Gerontology, 51(6), 402–408. doi: 10.1159/000088705 [DOI] [PubMed] [Google Scholar]
- Young, Y., Korinek, K., Zimmer, Z., & Toan, T. K. (2021). Assessing exposure to war-related traumatic events in older Vietnamese war survivors. Conflict and Health, 15(1), 14. doi: 10.1186/s13031-021-00343-y [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.