Abstract
Background and Objective
Patients with acute symptomatic seizures (ASyS) after stroke are discharged on antiseizure medications (ASMs) and stay on them for an extended period. We analyzed the current ASM management practice, 6 months, and at the last follow-up after stroke-related ASyS concerns to identify chronic and long-term ASM use predictors.
Methods
A single-center, retrospective cohort study of adults who underwent continuous EEG monitoring for ASyS concerns after stroke (April 1, 2012 to March 31, 2018) with at least 6 months of follow-up was performed. ASM use beyond 6 months after the initial ASyS concern was defined as “chronic” among patients discharged on them. “Long-term” ASM use at the last follow-up in all patients with ASyS concerns was analyzed. Logistic regression and Cox regression multivariable modeling to analyze predictors of “chronic” and “long-term” ASM use, respectively, was performed.
Results
A total of 465 (mean age 61.7 ± 13.3 years and 52% female patients) patients (41.9% ischemic stroke, 36.1% intracerebral hemorrhage, and 21.9% subarachnoid hemorrhage) were included. Of the 179 (38.5%) patients discharged on ASMs, 132 (73.7%; 28.4% of study population) had chronic ASM use, despite 90% not experiencing any seizure (poststroke epilepsy [PSE]) during this time. The independent predictors of chronic ASM use were electrographic ASyS (odds ratio [OR] = 9.27, 95% CI = 2.53–60.4) and female sex (OR = 2.2, 95% CI = 1.02–4.83). After a median 61-month (5.1 years) follow-up, 101 (21.7%) patients in the study population were on long-term ASM use, including 67 (14.4%) who developed PSE. Long-term ASM use was associated with NIH Stroke Scale Score (OR = 1.5, 95% CI = 1.015–1.98), cortical involvement (OR = 1.28, 95% CI = 1.02–1.6), convulsive ASyS (OR = 1.46, 95% CI = 1.02–2.09), epileptiform findings on outpatient EEG (OR = 4.03, 95% CI = 1.28–12.76), and PSE development (OR = 7.06, 95% CI = 3.7–13.4).
Discussion
Chronic ASM use is highly associated with electrographic, rather than convulsive, ASyS. However, long-term ASM use is independently associated with PSE and its risk factors, including convulsive ASyS. With the ubiquity of stroke-related ASyS concerns in routine clinical practice, comparative effectiveness studies to guide ASM management are needed.

Convulsive acute symptomatic seizures (ASyS) account for 40% of all afebrile seizures with a lifetime risk of 3.6%.1,2 Acute strokes account for a substantial proportion of ASyS.1 Convulsive ASyS occurs in 4–15% of stroke patients.3-5 The tremendous growth in continuous EEG (cEEG) monitoring in the past 2 decades shows that a large proportion of ASyS is nonconvulsive.6-8 ASyS are treated with antiseizure medications (ASMs) during hospitalization, and experts recommend their use during the acute phase of brain injury.9 More than 90% of stroke patients with ASyS or epileptiform abnormalities (EAs) on cEEG are discharged on ASMs.10 In the absence of research and guidelines on ASM management after ASyS, an overwhelming majority continue them beyond their first poststroke clinic visit.10 With ASyS more commonly encountered in the times of ubiquitous cEEG use, an improved understanding of current ASM use is needed to inform future research and best practices. This study aims to assess the chronic ASM use and its predictors in patients with ASyS concerns after stroke.
Methods
A single-center, retrospective cohort study after institutional review board (IRB) approval was conducted. In the absence of a definition for chronic ASM use after ASyS, ASM continuation beyond 6 months after initial ASyS concern was considered as “chronic” use. Prior findings guided this generous 6-month period because an overwhelming majority of patients continue ASMs beyond an average of 50 days postdischarge (first poststroke clinic visit).10 The inclusion criteria for the study population were adults (18 years or older) diagnosed with acute stroke from April 1, 2012 to March 31, 2018, underwent cEEG monitoring in the acute period (≤7 days of stroke) because of ASyS concerns, and had at least 6 months of follow-up. To reduce selection bias, we included patients if their ASMs were discontinued or they developed PSE within 6 months of follow-up (Figure 1). Patients with a history of epilepsy were excluded. The study population was identified using 3 prospectively maintained patient databases: institutional acute stroke database, EEG database, and Cleveland Clinic Knowledge Program database, a prospective registry of patient-reported outcomes collected as part of standard care.11 Every acute brain injury patient with convulsive seizures or suspected nonconvulsive seizure/status epilepticus (electrographic seizures) undergoes cEEG monitoring for at least 12 hours at our institution. Combined, these patients were defined as having “ASyS concern.” There is not institutional protocol to start all patients with hemorrhage on ASMs.
Figure 1. Study Population Flowchart for the Study Period (April 1, 2012, to March 31, 2018).

Percentages shown as proportion of the number of patients a step above in the flowchart. Mos = months.
The chronic ASM use after ASyS concern is most relevant in patients discharged on ASMs rather than the entire study population. Therefore, the chronic ASM use was investigated in this subgroup of the study population (“discharged on ASM” cohort). Given the clinical and research relevance of understanding ASM use at the last outpatient follow-up (referred to as “long-term” use), an additional analysis of the total study population was also performed. Patients with only dose reduction or deprescription of one of the multiple ASMs were considered as continuing ASM use.
Study variables include age, sex, stroke type (ischemic stroke [IS], subarachnoid hemorrhage [SAH], and intracerebral hemorrhage [ICH]), NIH Stroke Scale Score (NIHSS) at presentation, cortical involvement by the stroke, convulsive seizure before cEEG, electrographic seizures on cEEG (defined by the Salzburg criteria12), EAs on cEEG (including isolated sharp waves,13 lateralized periodic discharges, generalized periodic discharges, lateralized rhythmic delta activity as defined by American Clinical Neurophysiology Society14), stroke history, type of clinical service that provided first poststroke clinic visit (follow-up provider; neurology and neurosurgery), date of last clinical follow-up, ASMs at discharge and last follow-up (levetiracetam, nonlevetiracetam, levetiracetam + [ASM in addition to levetiracetam]; ASMs for nonseizure indication not included), and seizures since index hospital discharge (based on the clinical judgment and documentation of outpatient treating physician).
Statistical Methods
Descriptive statistics to summarize demographic/clinical characteristics were used. Frequency counts with percentages were used to present categorical data. Mean with standard deviation and median with interquartile range (IQR, first quartile–third quartile) were used to present continuous variables, as appropriate. Imputation using hot deck methodology was performed for missing NIHSS. For the primary analysis of chronic ASM use beyond 6 months in the “discharged on ASM” cohort, the association between predictor variables and the primary outcome variable (chronic ASM use) was initially determined with univariable logistic regression models. Subsequently, multivariable logistic regression modeling using the backward stepwise elimination approach based on the Akaike Information Criterion (AIC) was performed. The criterion estimates the fit of each statistical model, penalizes overfitting, and provides a means to select relevant variables that improve the model even if they do not reach the threshold for significance (p < 0.05).15 All predictors used in the above logistic regression model were used after checking for collinearity using variance inflation factor (VIF). The median time of ASM continuation in the “discharged on ASM” cohort was calculated using Kaplan-Meier survival analysis. The predictors of “long-term” ASM use at the last follow-up in the study population were analyzed using the Cox proportional hazard model. A stepwise variable selection algorithm based on AIC was used for the Cox proportional hazard model. Computations were performed in R, version 4.0.2.16
Data Availability
The data set analyzed in this study is not publicly available because of restricted access, but further information about the data sets is available from the corresponding author on reasonable request.
Standard Protocol Approvals, Registrations, and Patient Consents
The approval from an ethical standards committee (Institutional Review Board) on human experimentation was received.
Results
A total of 465 patients (mean age 61.7 ± 13.3 years and 52% female patients) qualified for this study, including 195 (41.9%) with IS, 168 (36.1%) with ICH, and 102 (21.9%) with SAH. Figure 1 shows the study population flowchart. Among them, 179 (38.5%) patients were discharged on ASMs. At the last follow-up (median = 61 [IQR 33–82] months), 101 (21.7%) were on ASMs. Figure 2 shows ASMs at discharge and the last follow-up. NIHSS was imputed for 104 (22.4%) patients.
Figure 2. ASMs at the Time of Hospital Discharge and Last Follow-up.

(Note: 11 patients on ASM for nonseizure indication at last follow-up not included.) ASM = antiseizure medication.
Chronic ASM Use in “Discharged on ASM” Cohort
Among 179 patients discharged on ASMs, 132 (73.7%; 28.4% of study population) were on ASMs 6 months after the ASyS concern. Table 1 presents demographical, clinical, and EEG characteristics of the “discharged on ASM” cohort. Outpatient EEG was performed in 26 (14.5%) patients within 6 months of ASyS concern, and 2 (7.7%) patients showed an EA. PSE developed in 14 (7.8%) patients during this time. Of the 132 patients receiving chronic ASM therapy, 119 (90.2%) did not develop PSE during the 6-month follow-up (1 PSE patient had ASM discontinued before 6 months). In this cohort (n = 179), at the time of last follow-up (median = 59 [4.9 years; IQR 30–82] months), 79 (44.1%) patients were on ASMs and 44 (24.6%) developed PSE. Based on the survival analysis function, this cohort's median ASM continuation time was 33 (95% CI 18–50) months. Figure 3 shows a Kaplan-Meier curve of ASM continuation probability in this patient cohort. Of note, most ASM discontinuation occurred within the first 18 months of follow-up.
Table 1.
Univariable Analysis of Characteristics Associated With Chronic (6 Months) ASM Continuation in “Discharged on ASM” Cohort
Figure 3. Kaplan-Meier Curve of ASM Continuaton After Discharge.

ASM = antiseizure medication.
Univariable analysis (Table 1) found chronic ASM use to be significantly associated with higher NIHSS (odds ratio [OR] = 1.06, 95% confidence interval [CI] = 1.00–1.12), presence of electrographic seizures (OR = 8.44, 95% CI = 2.43–53.34), EAs (OR = 2.24, 95% CI = 1.11–4.77), and longer admission duration (OR = 1.04, 95% CI = 1.003–1.09). Convulsive ASyS before cEEG were not associated with chronic ASM use in this population. As presented in Table 2, the multivariable logistic regression model based on lowest AIC included sex, stroke type, convulsive ASyS, electrographic ASyS, and admission duration. The chronic ASM use in the discharged on ASM cohort was significantly associated with sex (female patients; OR = 2.2, 95% CI = 1.02–4.83) and electrographic ASyS (OR = 9.27, 95% CI = 2.53–60.4). The C statistic of the model presented in Table 2 is 0.73 (95% CI = 0.65–0.81).
Table 2.
Multivariable Logistic Regression Model for Chronic ASM Continuation in “Discharged on ASM” Cohort

Long-term ASM Use at the Last Follow-up
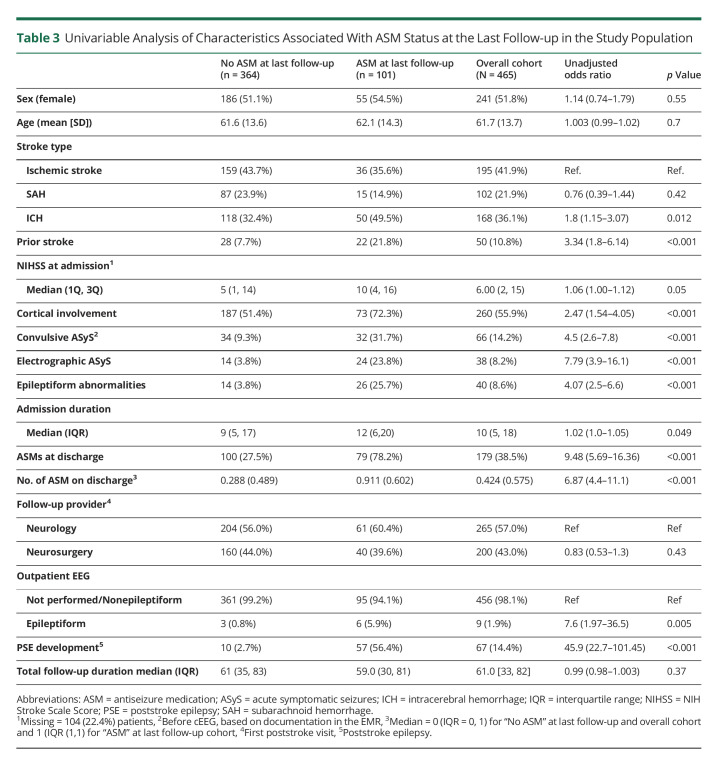
At the last follow-up, a median of 61 months (5.1 years; IQR 33–82) later, 101 of 465 (21.7%) patients were on ASMs and 67 (14.4%) developed PSE. The latter included 23 of 286 (8%; 4.9% of the study population) patients not discharged on ASMs. ASMs were restarted in 2 patients after the initial wean off because of PSE. Three patients who were not discharged on ASMs developed PSE and were prescribed ASMs, which were eventually discontinued by the time of their last follow-up. Outpatient EEG was performed in 101 (21.7%) patients. Table 3 presents the univariable analysis of the study population based on their last follow-up ASM status.
Table 3.
Univariable Analysis of Characteristics Associated With ASM Status at the Last Follow-up in the Study Population
The number of ASMs at discharge was not included into the Cox proportional hazard model because of collinearity with ASM status at discharge (yes vs no) based on high VIF. Predictors included in the final model based on lowest AIC are presented in Table 4. Proportional hazard assumption was not violated in the model. Long-term ASM use was significantly associated with NIHSS (OR = 1.5, 95% CI = 1.015–1.98), cortical involvement (OR = 1.28, 95% CI = 1.02–1.6), convulsive ASyS (OR = 1.46, 95% CI = 1.02–2.09), epileptiform findings on outpatient EEG (OR = 4.03, 95% CI = 1.28–12.76), and PSE development (OR = 7.06, 95% CI = 3.7–13.4).
Table 4.
Multivariable Cox Regression Model of ASM Use at the Last Follow-up in the Population

Outcomes Based on Stroke Type
The proportion of patients on chronic and long-term ASM therapy based on stroke types (Tables 1 and 3) was stable for IS (31.8% and 35.6%, respectively), SAH (17.4% and 14.9%, respectively), and ICH (50.8% and 49.5%, respectively) over time. Among patients included in chronic ASM therapy analysis (n = 179), PSE developed within 6 months of initial ASyS concern in 5 (8.2%) IS patients and 9 (10.5%) ICH patients. None of the SAH patients developed PSE during the 6-month period. By the end of the study follow-up, PSE developed in 24 (12.3%) IS, 5 (4.9%) SAH, and 38 (22.6%) ICH patients.
Discussion
ASyS often recur within 24 hours of initial presentation, and around 15% of patients have additional seizure recurrence within 3 weeks of initial ASyS.17 ASyS increase mortality in the first 30 days, and the mortality rate of poststroke ASyS patients is almost twice compared with age, sex, and stroke severity–matched controls.18,19 These are all valid reasons for preventing ASyS recurrence using ASMs after stroke, a position endorsed by the American Stroke Association.20,21 One-third of stroke patients with ASyS develop epilepsy.22 ASMs lack antiepileptogenic properties, and experts recommend their use for 1–2 weeks after acute brain injury–related ASyS.9 The current analysis shows that almost three-fourths of stroke patients discharged on ASMs because of stroke-related ASyS concerns use them for longer than 6 months. Among the 179 patients discharged on ASMs with at least 6 months of follow-up, the median ASM continuation time can be close to 3 years. At the 6-month point, 90% of patients on ASM had not had a seizure since hospital discharge. Among patients continuing chronic ASM therapy, only a third (35.6%) had convulsive ASyS before cEEG and a quarter (27.3%) were found to have electrographic seizures on cEEG. These findings suggest a very conservative and cautious approach toward ASM withdrawal in these patients. After adjusting for factors such as stroke type, stroke severity, cortical involvement, and hospital admission duration, the multivariable analysis shows that only electrographic ASyS and female sex independently predict chronic ASM use in patients discharged on them. Electrographic ASyS increases the odds of chronic ASM use by up to 9-fold after adjusting for convulsive ASyS, which is not an independent predictor. It is likely because outpatient providers prefer to rely on the objective electrographic ASyS on the cEEG report rather than historical reportage of convulsive ASyS when making ASM use decisions. Female patients had little more than two times odds of chronic ASM use. It may indicate differences in risk tolerance of seizure recurrence after ASM discontinuation or advocacy for sooner discontinuation of ASMs between the 2 sexes.
One of 5 (21.7%) patients with initial ASyS concern after stroke were on ASMs at the last follow-up, a median of 5 years later, and 1 in 7 (14.4%) developed PSE. In contrast to the total study population, close to half (44.1%) of patients discharged on ASMs were still on them at the last follow-up (similar median duration of 4.9 years) and a quarter (24.6%) developed PSE. Development of PSE is associated with the highest odds of long-term ASM continuation, which is not surprising. However, it is interesting to note that the well-known predictors of PSE, namely stroke severity, cortical involvement, and convulsive ASyS, remain independent predictors of long-term ASM use, even after adjusting for PSE development.23,24 It suggests that outpatient providers remain sensitive to these risk factors, independent and irrespective of PSE development when deciding on ASM continuation. This ASM management aligns with the SELECT study findings that these 3 stroke-related characteristics independently increase PSE risk up to 6 years after the stroke.25 The analysis also show that the presence of EAs on outpatient EEG, although only performed in 20% of the study population, is independently associated with long-term ASM continuation. No clear evidence exists, correlating EAs on outpatient EEG to PSE development, which does not mean evidence of absence of correlation. The outpatient providers likely extrapolate the predictive association of EAs on EEG with seizure recurrence after ASM withdrawal in patients with epilepsy.26
Compared with the role of outpatient EEG, the acute cEEG findings, especially electrographic ASyS, do not remain associated with long-term ASM use. It contrasts electrographic ASyS independently predicting chronic ASM use beyond the initial 6 months after ASyS concern. This differential temporal association of ASyS type on ASM suggests a gradation in electroclinical findings' association with outpatient ASM use, especially striking when the recent findings are considered.10 Both EAs and ASyS (electrographic and convulsive) are independently associated with ASM continuation in the immediate, posthospitalization follow-up.10 The chronic ASM use beyond the initial 6 months is only associated with electrographic ASyS and not acute EAs. The eventual long-term ASM use, a median of 5 years after initial poststroke ASyS-concern, is only associated with convulsive ASyS and not the acute EEG findings. Overall, it seems like posthospital discharge ASM management is initially concerned with preventing symptomatic seizures in the acute to subacute “healing” period of stroke, influenced by cEEG findings, which, later over the years, shifts to managing PSE and patients at high risk of PSE.
As a single-center study, the current findings could be influenced by institutional practices. The electroclinical predictors associated with outpatient ASM management were analyzed, but the data on individual clinical decisions guiding ASM use were lacking. Outpatient ASM use could be influenced by concern about driving restrictions after ASM withdrawal, especially in patients tolerating them well. Sometimes, a patient's risk tolerance for seizure recurrence could be low, whereby ASMs are continued on their request. Such factors deserve exploration using a prospective study design. Although various stroke types do not show a definitive association with outpatient ASM management, future studies with larger patient populations need to investigate stroke-specific ASM use practices. It is underscored by SAH patients showing a trend toward less frequent use of chronic ASMs. The proportion of ICH patients is substantially higher than routine clinical practice, highlighting the higher ASyS concern in these patients compared with IS. This observational, retrospective analysis does not lend itself to chronic and long-term ASM management recommendations or guidelines in patients with stroke-related ASyS concerns. Nonetheless, it provides novel information about the real-world practice of outpatient ASM management in these patients at a tertiary care referral center. Pertinent to the current times of cEEG use in critically ill patients, this study provides evidence that findings of this diagnostic modality influence outpatient ASM management. The findings suggest that outpatient providers take a cautious approach toward ASM withdrawal in the first year after ASyS concern, especially among women. However, eventually, only PSE, its risk factors, and the outpatient EEG findings remain significant predictors of long-term ASM use. This knowledge of the current “lay of the land” would inform future comparative effectiveness studies and intervention trials investigating optimal ASM use in managing ASyS in stroke and other acute brain injuries.
Appendix. Authors

Study Funding
The authors report no targeted funding.
Disclosure
The authors report no relevant disclosures. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.
TAKE-HOME POINTS
→ More than a quarter (28.4%) of 465 stroke patients continued antiseizure medications (ASMs) 6 months after acute symptomatic seizure (ASyS) concerns.
→ Among patients discharged on ASMs, the independent predictors of chronic ASM use (6 months) are electrographic ASyS and female sex.
→ After a 5-year median follow-up, one-fifth (21.7%) of the study population was on ASMs (long-term use).
→ Poststroke epilepsy (diagnosed in 14.4%) and its known predictors along with epileptiform findings on outpatient EEG are independently associated with long-term ASM use.
→ These findings highlight current ASM use practice in patients with stroke-related ASyS concern and can inform future comparative effectiveness studies.
References
- 1.Annegers JF, Hauser WA, Lee JR, Rocca WA. Incidence of acute symptomatic seizures in Rochester, Minnesota, 1935-1984. Epilepsia. 1995;36(4):327-333. [DOI] [PubMed] [Google Scholar]
- 2.Hauser WA, Annegers JF, Rocca WA. Descriptive epidemiology of epilepsy: contributions of population-based studies from Rochester, Minnesota. Mayo Clin Proc. 1996;71(6):576-586. [DOI] [PubMed] [Google Scholar]
- 3.Beghi E, D'Alessandro R, Beretta S, et al. Incidence and predictors of acute symptomatic seizures after stroke. Neurology. 2011;77(20):1785-1793. [DOI] [PubMed] [Google Scholar]
- 4.So EL, Annegers JF, Hauser WA, O'Brien PC, Whisnant JP. Population-based study of seizure disorders after cerebral infarction. Neurology. 1996;46(2):350-355. [DOI] [PubMed] [Google Scholar]
- 5.Labovitz DL, Hauser WA, Sacco RL. Prevalence and predictors of early seizure and status epilepticus after first stroke. Neurology. 2001;57(2):200-206. [DOI] [PubMed] [Google Scholar]
- 6.Claassen J, Jette N, Chum F, et al. Electrographic seizures and periodic discharges after intracerebral hemorrhage. Neurology. 2007;69(13):1356-1365, . [DOI] [PubMed] [Google Scholar]
- 7.Bentes C, Martins H, Peralta AR, et al. Post-stroke seizures are clinically underestimated. J Neurol. 2017;264(9):1978-1985. [DOI] [PubMed] [Google Scholar]
- 8.Mecarelli O, Pro S, Randi F, et al. EEG patterns and epileptic seizures in acute phase stroke. Cerebrovasc Dis. 2011;31(2):191-198. [DOI] [PubMed] [Google Scholar]
- 9.Mauritz M, Hirsch LJ, Camfield P, et al. Acute symptomatic seizures: an educational, evidence-based review. Epileptic Disord. 2022;24(1):26-49. [DOI] [PubMed] [Google Scholar]
- 10.Punia V, Honomichl R, Chandan P, et al. Long-term continuation of anti-seizure medications after acute stroke. Ann Clin Transl Neurol. 2021;8(9):1857-1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Katzan I, Speck M, Dopler C, et al. The Knowledge Program: an innovative, comprehensive electronic data capture system and warehouse. AMIA Annu Symp Proc AMIA Symp. 2011;2011:683-692. [PMC free article] [PubMed] [Google Scholar]
- 12.Beniczky S, Hirsch LJ, Kaplan PW, et al. Unified EEG terminology and criteria for nonconvulsive status epilepticus. Epilepsia. 2013;54(suppl 6):28-29. [DOI] [PubMed] [Google Scholar]
- 13.Noachtar S, Binnie C, FAU - Ebersole J, et al. A glossary of terms most commonly used by clinical electroencephalographers and proposal for the report form for the EEG findings. Electroencephalogr Clin Neurophysiol. 1999;52:21-41. [PubMed] [Google Scholar]
- 14.Hirsch LJ, LaRoche SM, Gaspard N, et al. American Clinical Neurophysiology Society's standardized critical care EEG terminology: 2012 version. J Clin Neurophysiol. 2013. 30(1):1-27. [DOI] [PubMed] [Google Scholar]
- 15.Bozdogan H. Model selection and Akaike's Information Criterion (AIC): the general theory and its analytical extensions. Psychometrika. 1987;52(3):345-370. [Google Scholar]
- 16.R Development Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; 2020. r-project.org/ [Google Scholar]
- 17.Leung H, Man CBL, Hui ACF, Kwan P, Wong KS. Prognosticating acute symptomatic seizures using two different seizure outcomes. Epilepsia. 2010;51(8):1570-1579. [DOI] [PubMed] [Google Scholar]
- 18.Hesdorffer DC, D'Amelio M. Mortality in the first 30 days following incident acute symptomatic seizures. Epilepsia. 2005;46(suppl 1):43-45. [DOI] [PubMed] [Google Scholar]
- 19.Zöllner JP, Misselwitz B, Kaps M, et al. National Institutes of Health Stroke Scale (NIHSS) on admission predicts acute symptomatic seizure risk in ischemic stroke: a population-based study involving 135, 117 cases. Sci Rep. 2020;10(1):3779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hemphill JC III, Greenberg SM, Anderson CS, et al. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2015;46(7):2032-2060. [DOI] [PubMed] [Google Scholar]
- 21.Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association Stroke. 2019;50:e344-e418. [DOI] [PubMed] [Google Scholar]
- 22.Hesdorffer DC, Benn EK, Cascino GD, Hauser WA. Is a first acute symptomatic seizure epilepsy? Mortality and risk for recurrent seizure. Epilepsia. 2009;50(5):1102-1108. [DOI] [PubMed] [Google Scholar]
- 23.Bladin CF, Alexandrov AV, Bellavance A, et al. Seizures after stroke: a prospective multicenter study. Arch Neurol. 2000;57(11):1617-1622. [DOI] [PubMed] [Google Scholar]
- 24.Galovic M, Ferreira-Atuesta C, Abraira L, et al. Seizures and epilepsy after stroke: epidemiology, biomarkers and management. Drugs Aging. 2021;38:285-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Galovic M, Döhler N, Erdélyi-Canavese B, et al. Prediction of late seizures after ischaemic stroke with a novel prognostic model (the SeLECT score): a multivariable prediction model development and validation study. Lancet Neurol. 2018;17(2):143-152. [DOI] [PubMed] [Google Scholar]
- 26.Lamberink HJ, Otte WM, Geerts AT, et al. Individualised prediction model of seizure recurrence and long-term outcomes after withdrawal of antiepileptic drugs in seizure-free patients: a systematic review and individual participant data meta-analysis. Lancet Neurol. 2017;16(7):523-531. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data set analyzed in this study is not publicly available because of restricted access, but further information about the data sets is available from the corresponding author on reasonable request.