Abstract
In order to develop a new system for the analysis of capsular biosynthetic pathways we have explored the possibility of expressing type 3 capsular polysaccharide (CPS) from the pathogen Streptococcus pneumoniae in Lactococcus lactis, an unencapsulated lactic acid bacterium being developed as a vaccine delivery vehicle for mucosal immunization. Only three of the four type 3 CPS biosynthesis genes were found to be necessary for the abundant formation (120 mg liter−1) of an extracellular type 3 CPS in L. lactis, implying a role for the type 3-specific synthase in the extracellular transport of the CPS or implying the existence of an alternative export system in L. lactis. The authenticity of the expressed heterologous polysaccharide was established by chemical and immunological analyses. Proton and carbon nuclear magnetic resonance spectroscopy of CPSs purified from L. lactis and S. pneumoniae showed that the two CPS structures were identical. When mice were immunized intraperitoneally with 3.5 × 106 CFU of live recombinant lactococci expressing a total of approximately 0.5 μg of type 3 CPS, the immune responses elicited appeared identical to those observed in mice inoculated with 0.5 μg of type 3 CPS purified from S. pneumoniae. These findings show that L. lactis is a useful host in which to study the role and function of genes involved in the production of bacterial capsules. Additionally, L. lactis shows potential as a host for the safe production of capsule antigens and as a vaccine delivery vehicle for polysaccharide antigens.
Highly invasive bacteria, regardless of whether they are gram positive or gram negative, commonly have an organized, adherent layer of polysaccharide associated with their outer surface. Such encapsulated bacteria are major causes of morbidity and mortality worldwide (7, 25, 29). The polysaccharide(s) comprising a capsule can be multichained or composed of linear homopolymers or heteropolymers. Capsules mediate important roles during certain stages of infection. Early in infection, during colonization, they facilitate bacteria-to-bacteria adhesion (which may aid in quorum sensing); later, they enable bacteria to survive in the infected host despite attack by the complement- and phagocyte-mediated mechanisms associated with both innate and acquired immunity. Hence, virtually all bacteria capable of rapid proliferation in the bloodstream are encapsulated.
Because of this vital role in pathogenicity and because many capsular polysaccharides (CPSs) are immunogenic and used as vaccine antigens, it is important to improve our understanding of the biological properties of these bacterial polysaccharides and of the genes and enzymes involved in their biosynthesis. A number of recent studies (2, 13, 17, 18, 21, 24) have described the genetic organization of capsular biosynthetic operons. This paper describes a novel system of heterologous capsule expression which can be used to augment and refine these studies further. It also opens the way to certain unique possibilities, such as the production of CPSs in nonpathogenic bacteria and the analysis of the biological properties of such polysaccharides on bacterial surfaces in isolation from all other virulence determinants. Intriguingly, it also points to the possibility of recombining protective polysaccharide epitopes and generating chimeric vaccine antigens. Single vaccine antigens of this type could then be used to elicit protection against a range of bacterial serotypes.
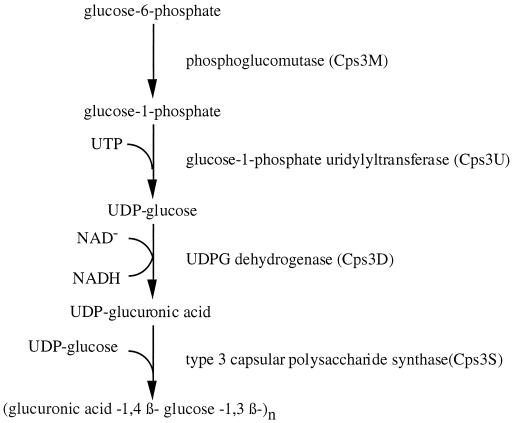
To establish this system we used as an initial model the expression in Lactococcus lactis of the capsule biosynthesis genes of Streptococcus pneumoniae type 3 (strain WU2). The type 3 capsule biosynthetic genes of S. pneumoniae have a cassette-like organization, a feature common to the capsule operons of all serotypes analyzed to date. Genetic and biochemical analyses of the type 3 capsule locus have revealed four type-specific genes (Fig. 1). Phosphoglucomutase (Cps3M) and glucose-1-phosphate uridylyltransferase (Cps3U) are required for the synthesis of one of the saccharide precursors, UDP-glucose. UDP-glucose dehydrogenase (Cps3D) is the enzyme necessary for conversion of UDP-glucose to the second saccharide precursor, UDP-glucuronic acid, and the type 3 synthase (Cps3S) is required for polymerization of the precursors into a repeating unit (2, 9, 10). The region flanking the left end of the cassette is repeated in the pneumococcal chromosome and found upstream of other operons. Downstream of the last type 3 capsule-specific gene lies an internal portion of a gene (designated tnpA) that has homology with putative transposases of insertion sequences. Adjacent to tnpA is a sequence homologous to plpA, a gene encoding a permease-like protein involved in the transport of oligopeptides that has undergone a deletion at the 5′ terminus (9, 10). Both tnpA and plpA are physically linked and present in single copy in other capsule types.
FIG. 1.
Biosynthetic pathway for type 3 pneumococcal CPS (10).
MATERIALS AND METHODS
Bacterial strains and growth conditions.
S. pneumoniae strain WU2 of serotype 3 (8) was grown at 37°C in Trypticase soy broth (Difco Laboratories, East Molesley, United Kingdom). The plasmid-cured L. lactis strain MG1363 (14) and recombinant L. lactis MG1363 were cultured at 30°C in M17 broth or on M17 agar plates (Difco) supplemented with 0.5% glucose (Sigma, Dorset, United Kingdom). For antibiotic selection erythromycin was used at a concentration of 5 μg ml−1 (Sigma). To purify CPS from L. lactis UCP1619 the bacteria were grown at 30°C in a minimal medium containing 120 mM morpholinepropanesulfonic acid (MOPS) (pH 7.2), 6.7 g of yeast nitrogen base (Difco) per liter, and 2 g of casein enzymatic hydrolysate (Sigma) per liter, supplemented with 0.5% glucose and 5 μg of erythromycin (Sigma) per ml.
DNA techniques.
Plasmid pTREP, a derivative of the pTREX vector, was purified from L. lactis using the QIAprep spin plasmid preparation kit (Qiagen Inc., Valencia, Calif.) according to the method suggested by the manufacture, except that the bacteria were first recovered by centrifugation and incubated in 100 μl of Tris-EDTA buffer (10 mM Tris-HCl [pH 8.0]; 1 mM EDTA) containing 5 mg of lysozyme (Sigma) per ml and 100 U of mutanolysin (Sigma) per ml for 10 min at 37°C to partially digest the cell wall. General molecular cloning was carried out by techniques described by Maniatis et al. (20). The method for the transformation of L. lactis strain MG1363 has been described previously (32). Chromosomal DNA was isolated as described by Ausubel et al. (5).
Isolation of the type 3 locus DNA from S. pneumoniae WU2.
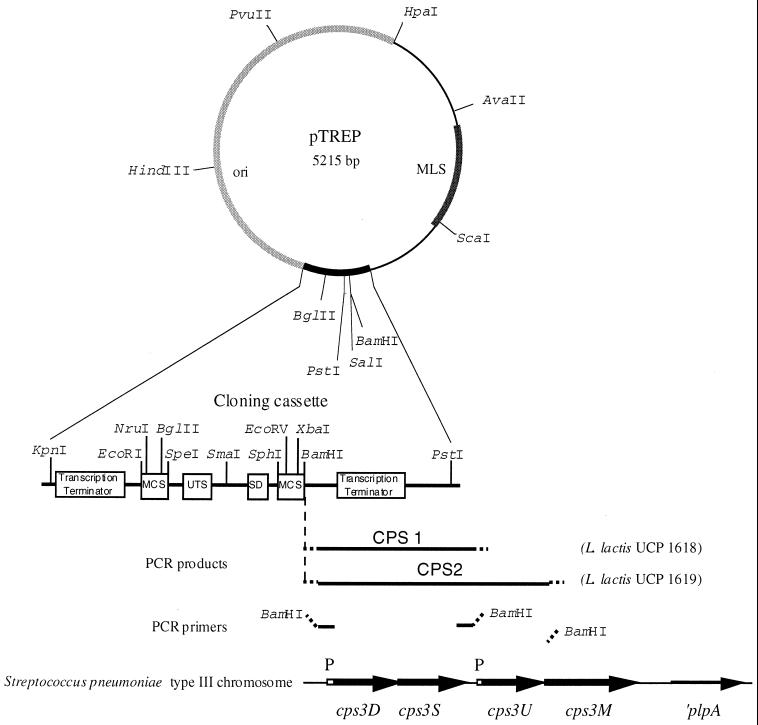
The DNA fragments CPS1 and CPS2 were amplified by PCR with the following oligonucleotides primers: forward primer CPS1 (5′-CGGGATCCGACCCTGGATAAACGAGCTGC-3′), reverse primer CPS1 (5′-CGGGATCCCTTACGTCTGAAGTGAGCC-3′), forward primer CPS2 (5′-CGGGATCCGACCCTGGATAAACGAGCTGC-3′), and reverse primer CPS2 (5′-CGGGATCCAATTCATAGGTGTCCACTTGACG-3′) (BamHI endonuclease restriction sites used for cloning are underlined). PCR reaction mixtures contained S. pneumoniae WU2 genomic DNA (50 ng), 20 pmole of each primer, 3 U of TaqPlus long PCR enzyme mix (Stratagene, La Jolla, Calif.) and Mg2+ at a final concentration of 2 mM in the buffer recommended for TaqPlus. DNA was amplified by PCR using 35 cycles of denaturation (95°C, 30 s), annealing (60°C, 1 min), and extension (72°C, 3 min), followed by a final extension period of 10 min at 72°C. The PCR-amplified fragments CPS1 and CPS2 were purified from an agarose gel, digested with BamHI, and ligated to BamHI-cut, dephosphorylated pTREP (Fig. 2).
FIG. 2.
Schematic representation of the plasmid pTREP and the expression plasmids pTREP-CPS1 and pTREP-CPS2. PCR-generated fragments CPS1 and CPS2 from S. pneumoniae type 3 chromosome were cloned into the BamHI site of the pTREP cloning cassette. The main features of the cloning cassette and key restriction sites are indicated. MLS, resistance to macrolides, lincosamines, and streptogramin B type antibiotics; MCS, multiple cloning site; UTS, universal translation stop signals; SD, Shine-Dalgarno sequence (ribosome binding site); P, promoter.
Purification of the CPS.
A 100-ml volume of an overnight culture of L. lactis UCP1619 expressing type 3 CPS was mixed with 25 ml of trichloracetic acid (100%), and the precipitated proteins were removed by centrifugation at 10,000 × g. An equal volume of acetone was then added to the supernatant to precipitate the polysaccharide. The precipitated polysaccharide was recovered by centrifugation, washed with acetone, and redissolved in 5% sodium acetate. The solution was extracted twice with a one-fifth volume of chloroform:butanol (5:1); then the polysaccharide was precipitated once more with an equal volume of acetone, and the pellet was washed with acetone and dissolved in deionized sterile water. The total neutral sugar content was determined by the phenol-sulfuric acid method (12). The polysaccharide was then freeze-dried.
NMR spectroscopy.
The freeze-dried polysaccharide and the purified pneumococcal CPS (Merck) were dissolved in D2O. The 600-MHz 1H and 13C nuclear magnetic resonance (NMR) experiments were performed with a Brucker DRX 600. The spectra were obtained at 320 K for the polysaccharide purified from L. lactis UCP1619 and at 335 K for the CPS purified from S. pneumoniae type 3 (Merck).
Cells extracts and protein determinations.
The optical density values of overnight cultures of L. lactis strains were recorded, and a 5-ml sample of each strain was centrifuged. The pellet was washed in NaH2PO4 buffer (50 mM, pH 7) and resuspended in 5 ml of the same buffer. Cells were broken with a French pressure cell and centrifuged to remove cells debris. The protein contents were determined on the supernatants by the method of Bradford (6) using bovine serum albumin (BSA) (Sigma) as the standard.
Mice.
Specific pathogen-free female (BALB/c) mice (6 weeks old) were purchased from Harlan UK Ltd. (Bicester, Oxon, United Kingdom).
Immunizations.
L. lactis strains were cultured at 30°C in the MOPS minimal medium supplemented with 0.5% glucose (Sigma) and 5 μg of erythromycin (Sigma) per ml. The cultures were diluted directly into phosphate-buffered saline (PBS) to achieve a concentration of 3.5 × 106 cells in a 100-μl volume prior to immunization. Groups of 60 mice (180 mice in total) were immunized intraperitoneally with 3.5 × 106 live lactococci harboring the control vector pTREP or with 3.5 × 106 cells of L. lactis UCP1619 (expressing the equivalent of 0.5 μg of polysaccharide) or 0.5 μg of purified CPS from S. pneumoniae type 3 (Merck, Darmstadt, Germany). In each group, one subgroup of mice was bled daily from day 1 to 10 following the immunization to assay the immunoglobulin M (IgM) response. Subsequently, subgroups of 12 mice from each treatment group were bled weekly and tested for CPS-specific IgG responses. Moreover, a naive nonvaccinated control group was also included in the experiment.
Antibody detection of CPS on live recombinant strains of L. lactis.
Overnight cultures were centrifuged, and the pellets were resuspended in a volume of medium to achieve a concentration of 1010 live recombinant L. lactis cells per ml (an optical density at 600 nm of 1 was considered to indicate a concentration of 5 × 108 cells ml−1). A 10-μl volume of the suspension (108 live cells) was pipetted onto nitrocellulose, and the filter was blocked for 1 h at room temperature with 1% BSA in PBS (Sigma). The presence of CPS was detected by using rabbit anti-CPS type 3 polyclonal serum (Statens Seruminstitut, Copenhagen, Denmark) as the primary antiserum, followed by anti-rabbit immunoglobulin horseradish peroxidase conjugate (DAKO A/S, Glostrup, Denmark). The conjugate was detected by incubating the filter in a solution of 4-chloronaphthol (Sigma). Cell culture supernatants were applied to nitrocellulose in volumes of approximately 10-μl, with each application being allowed to dry thoroughly before subsequent applications were made.
ELISA for the detection of antigen-specific serum antibody.
Antibodies (of IgM, IgG, and IgG subclasses) to pneumococcal type 3 CPS were measured by enzyme-linked immunosorbent assay (ELISA). ELISA plates (Nunc-Immuno plates with MaxiSorp surfaces; Gibco, Paisley, Scotland) were coated with 10 μg of type 3 CPS (Merck) per ml in PBS and incubated for 5 h at 37°C and then overnight at 4°C. Wells were blocked for 1 h at room temperature with 1% BSA (Sigma). Primary antisera were tested in duplicate wells using a twofold dilution series (in PBS containing 1% BSA and 0.05% Tween 20), including replicate wells of a diluted (1/50) preimmune serum on every plate. After 2 h, secondary anti-mouse immunoglobulin-alkaline phosphatase conjugates diluted in PBS containing 1% BSA and 0.05% Tween 20 (Southern Biotechnology Associates, Inc., Birmingham, Alabama) were applied for 90 min. Reactions were developed by using n-nitrophenyl phosphate (Sigma) as a substrate, and the optical density was read at 405 nm in an ELISA reader (Titertek Multiskan MCC340; ICN Biomedicals Ltd., Thame, Oxfordshire, United Kingdom). Dilution curves were drawn for each sample, and the end-point titers were calculated as the dilution of the sample producing the same optical density as the 1/50 dilution of a pooled preimmune serum. Statistical comparisons between results for different groups were made by the Mann-Whitney U test. A P value of >0.05 was considered insignificant.
RESULTS
Cloning and expression of genes for pneumococcal type 3 capsule biosynthesis in L. lactis.
A schematic diagram of the pneumococcal type 3 locus and the genes required for capsule biosynthesis is shown in Fig. 2. Since UDP-glucose is necessary for many bacteria to produce essential cell constituents, including techoic acid and lipotechoic acid (4), we predicted that L. lactis would possess homologues of cps3U and cps3M. In order to investigate which of the pneumococcal type 3 enzymes would be required for capsule production in L. lactis, two different PCR fragments were generated by using oligonucleotide primers designed from the nucleic sequence of the genomic region involved in the capsule biosynthesis in S. pneumoniae type 3 (Fig. 2) (10). The PCR-amplified fragment CPS1 included the cps3D and cps3S genes and their cognate promoter but lacked the cps3U and cps3M genes. The PCR-amplified fragment CPS2 contained the cps3U gene in addition to cps3D and cps3S (shown in Fig. 2). DNA fragments CPS1 and CPS2 were ligated into the BamHI site of plasmid pTREP, a promoterless derivative of the vector pTREX (31) and then were used to transform electrocompetent L. lactis strain MG1363. Clones UCP1618 and UCP1619 harbored recombinant plasmids pTREP-CPS1 and pTREP-CPS2 containing the fragments CPS1 and CPS2, respectively (Fig. 2).
Pneumococcal type 3 capsule production in L. lactis.
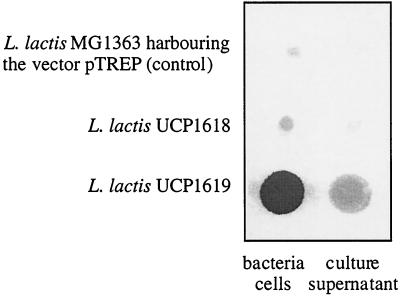
A suspension of 108 cells of live lactococci of strains UCP1618 and UCP1619 and a control strain harboring the vector pTREP were spotted onto nitrocellulose membranes (corresponding to 12, 13.4, and 12 μg of protein content, respectively). The presence of type 3 CPS was detected by immunoblotting with rabbit type 3 specific antisera (Fig. 3). A strong signal was detected with L. lactis UCP1619(pTREP-CPS2), indicating extracellular production of the type 3 capsule in this strain. Compared to the control nonexpressor strain, a signal was also observed with L. lactis UCP1618(pTREP-CPS1) but this signal was substantially weaker than that obtained with strain UCP1619. The low level of CPS production in strain UCP1618 was confirmed by the analysis of nine other independently selected clones harboring the plasmid pTREP-CPS2. The quantity of CPS produced by each of these nine clones was identical. These findings indicated that the Cps3M function can be complemented in L. lactis but that expression of the pneumococcal glucose-1-phosphate uridylyltransferase (Cps3U) is required for efficient production of the saccharide precursor UDP-glucose in L. lactis. In L. lactis the level (or activity) of cellular UDP-Glc-1-phosphate uridylyltransferase might be lower than the level (or activity) of pneumococcal cellular UDP-Glc-1-phosphate uridylyltransferase (GalU) (22). We expected the type 3 polysaccharide to be loosely associated with lactococcal cells since it is the only CPS which has not been shown to be covalently linked to the cell wall in pneumococcus (27). Interestingly, however, when L. lactis UCP1619 cells were mixed by vortexing and pelleted by centrifugation, the amount of CPS released in the supernatant of the culture was low compared to the amount of CPS that remained associated with cells (Fig. 3).
FIG. 3.
Detection of pneumococcal type 3 capsule formation by recombinant L. lactis. Equal volumes of cultures of nonrecombinant (control) and recombinant L. lactis strains UCP1619 and 1618 were spotted onto nitrocellulose and blotted with anti-type 3 antiserum.
The CPS produced by L. lactis UCP1619 was purified from a minimal medium broth in order to avoid the presence of other polysaccharides in the culture (contaminant saccharides were precipitated from M17 rich medium). In minimal medium, an L. lactis culture harvested at stationary phase (at 30°C) yielded approximately 15 μg of polysaccharide per 108 CFU of lactococcal cells (corresponding to 120 mg liter−1). The calculated amount of polysaccharide produced by L. lactis may in fact be an underestimate due to inefficiencies in the extraction procedure and loss of material during the purification steps. Possible errors in our calculation of the amounts of polysaccharide present on L. lactis cells would not have influenced our immunogenicity studies (see Results), since similar immune responses were measured over a 10-fold range of L. lactis expressing type 3 CPS.
NMR spectroscopy.
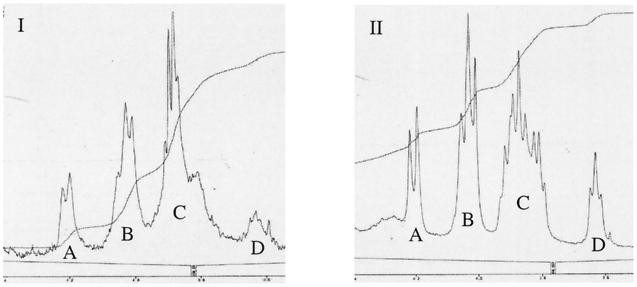
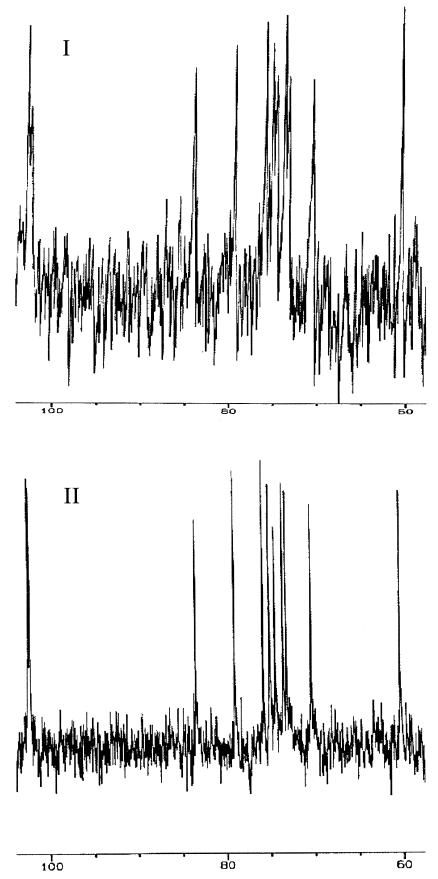
The proton NMR spectra (Fig. 4) and the carbon NMR spectra (Fig. 5) of CPSs purified from S. pneumoniae type 3 (Fig. 5I) and L. lactis strain UCP1619 (Fig. 5II) were very similar. On the proton spectra, the integration of the hydrogen peaks associated with the saccharides were comparable for the two polysaccharides. Moreover, two specific peaks corresponding to hydrogens involved in a β-link were observed in the proton NMR chromatograms for both S. pneumoniae and L. lactis (Fig. 5I and II) at 4.76 and 5.03 ppm (data not shown). All these results confirmed that L. lactis strain UCP 1619 was producing a surface CPS with a structure identical to that of the native CPS produced by S. pneumoniae type 3. No contaminants were evident from the proton and the carbon spectra of the type 3 CPS purified from L. lactis UCP1619.
FIG. 4.
Proton NMR spectroscopy of the CPSs purified from S. pneumoniae type 3 (I) and the L. lactis expressor strain UCP1619 (II). The levels of integration of the hydrogen peaks obtained are as follows. Peaks in panel I: A, 10.2%; B, 28.3%; C, 53.8%; and D, 7.7%. Peaks in panel II: A, 13.8%, B, 31.1%, C, 46.2%; and D, 8.9%.
FIG. 5.
Carbon NMR spectroscopy of the CPSs purified from S. pneumoniae type 3 (I) and the L. lactis expressor strain UCP1619 (II).
Immune responses to purified pneumococcal type 3 CPS and recombinant lactococci expressing type 3 CPS.
Three groups of 60 mice were inoculated with either live L. lactis UCP1619 (expressing in total 0.5 μg of type 3 polysaccharide), lactococci harboring the control vector pTREP, or 0.5 μg of purified pneumococcal type 3 CPS via the intraperitoneal route. Since Snippe et al. (26) had shown previously that IgM responses are short-lived and occur within the first 2 weeks of vaccination with CPS, 6 mice were selected at random on each of the first 10 days after inoculation from each of the three treatment groups, and blood samples were collected for IgM assay. In the subsequent period, 12 mice from each treatment group were bled weekly and tested for CPS-specific IgG responses.
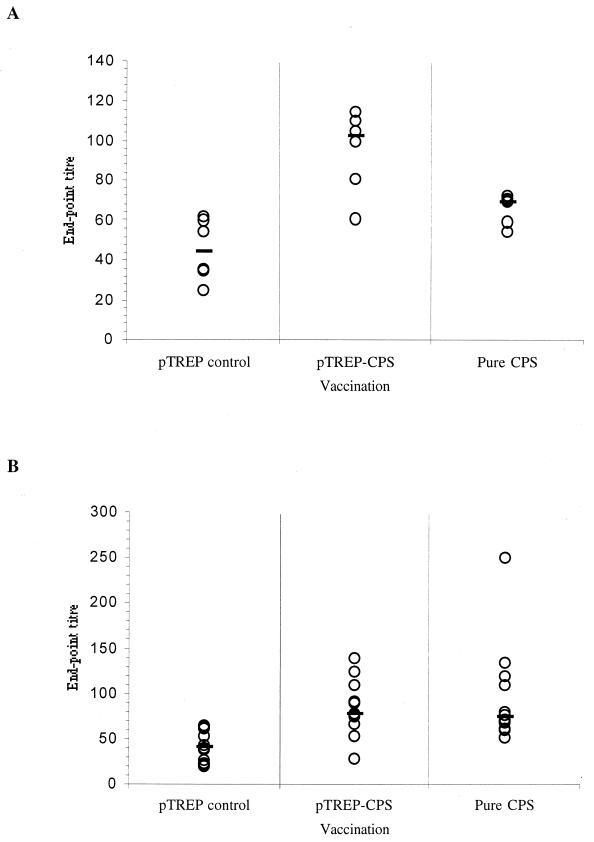
On days 4 to 9 following the primary immunization a slightly elevated type 3 CPS-specific IgM response was detected in mice given lactococci expressing CPS or purified CPS, compared with that detected in mice given the control lactococcal strain (Fig. 6A). On day 6, the IgM response elicited by lactococci expressing CPS reached a peak (median titer, 102) and this was significantly higher (P = 0.001) than the IgM responses of the control group whose median titer at this point was 45. The peak IgM response elicited by vaccination with pure CPS occurred a little earlier, on day 4, when the median titer reached 72. This was also significantly higher than the response of the control group (P = 0.032) at this time, with a median titer of 55. On days 4 to 8 the IgM responses of mice given pure CPS or lactococci expressing CPS were not significantly different (P > 0.05).
FIG. 6.
Anti-type 3 CPS serum antibody responses following intraperitoneal administration of recombinant L. lactis or purified pneumococcal CPS. Three groups of 60 mice were inoculated with either 3.5 × 106 CFU of L. lactis UCP1619 expressing 0.5 μg of type 3 polysaccharide (pTREP-CPS2), the same dose of lactococci harboring the control vector pTREP (pTREP control), or 0.5 μg of purified pneumococcal type 3 CPS (pure CPS) via the intraperitoneal route. (A) Anti-CPS IgM responses of 6 mice selected at random from each of the three treatment groups on day 6 postimmunization. (B) Anti-CPS IgG responses of 12 mice selected at random from each of the three treatment groups at 4 weeks postimmunization. The end-point titers were calculated as the dilution of serum producing the same optical density as a 1/50 dilution of a pooled preimmune serum taken at the start of the experiment. End-point titers of individual samples (open circles) and the group median values (bars) are shown.
After 4 weeks the low-level IgG responses elicited by pure CPS or lactococci expressing CPS reached a peak (Fig. 6B). The serum IgG titers of the mice given pure CPS (median titer, 74) were significantly higher (P = 0.001) than those of the control group (median titer, 41). The responses of mice inoculated with lactococci expressing CPS (median titer, 77) were also significantly higher than those of the control group (P = 0.001). The IgG responses of mice inoculated with either CPS formulation were not significantly different from each other (P > 0.05). After the initial primary responses had declined, at 7 weeks, a booster inoculation was given to each group to determine whether any of the immunized groups of mice had acquired immunological memory. For T-cell-dependent antigens the induction of memory B- and T-cells ensures that serum antibody responses to a secondary antigenic challenge appear more quickly, attain a higher titer, and consist predominantly of IgG. In contrast, T-cell-independent antigens such as bacterial polysaccharides cannot recruit specific T-cell help and therefore a second exposure to these antigens elicits an antibody response of similar isotype, kinetics, and magnitude to the primary response.
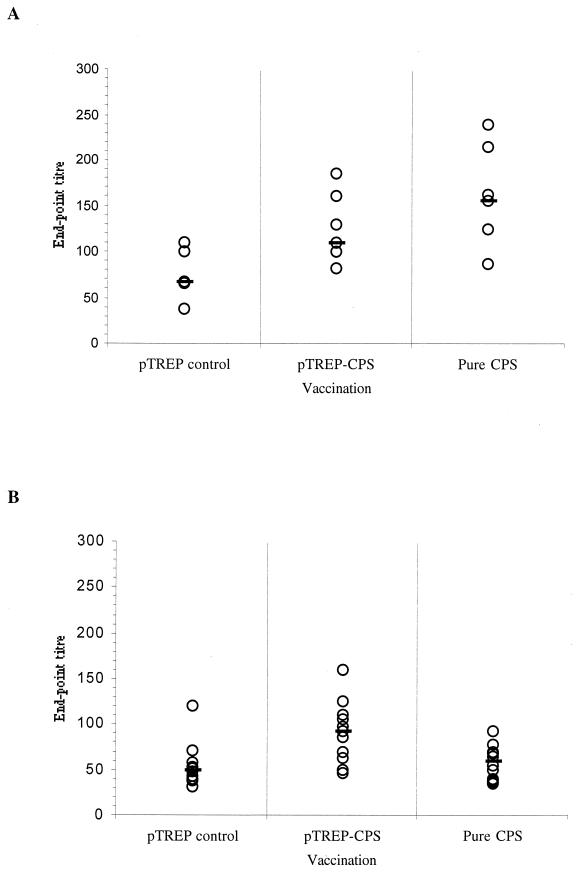
As after the primary inoculations, mice were bled daily for 10 days postboost so that IgM responses could be detected; they were subsequently bled every week for a further 6 weeks. A rise in CPS-specific serum IgM titers was noted between days 3 and 10 postboost. The peak in the IgM responses of mice given either form of CPS occurred on day 9 (Fig. 7A), when the titers of the group given lactococci expressing CPS (median titer, 110) and those elicited by pure CPS (median titer, 155) were significantly higher than those of the control group (P = 0.015 and P = 0.009 respectively).
FIG. 7.
Anti-type 3 CPS serum antibody responses following secondary intraperitoneal administration of recombinant L. lactis or purified pneumococcal CPS. Three groups of 60 mice were inoculated with either 3.5 × 106 CFU of L. lactis UCP1619 expressing 0.5 μg of type 3 polysaccharide (pTREP-CPS2), the same dose of lactococci harboring the control vector pTREP (pTREP control), or 0.5 μg of purified pneumococcal type 3 CPS (pure CPS) via the intraperitoneal route and boosted with a second identical injection after 7 weeks. (A) Anti-CPS IgM responses of 6 mice selected at random from each of the three treatment groups on day 9 postboost. (B) Anti-CPS IgG responses of 12 mice selected at random from each of the three treatment groups at 2 weeks postboost. The end-point titers were calculated as the dilution of serum producing the same optical density as a 1/50 dilution of a pooled preimmune serum taken at the start of the experiment. End-point titers of individual samples (open circles) and the group median values (bars) are shown.
The peak in the postboost anti-CPS IgG responses occurred 2 weeks postboost (Fig. 7B), when the titers of the group given lactococci expressing CPS (median titer, 91) were significantly higher than those of the control group (P = 0.010) and remained at this level for the ensuing 3 weeks of the experiment.
The peak IgM and IgG responses elicited by either form of CPS were not significantly different from those obtained after primary immunization, indicating that the responses were characteristically T cell independent in nature. The IgG serum antibodies to CPS measured 2 weeks after the boost were primarily of the IgG1 and IgG3 subclasses. (From pTREP control, pTREP-CPS, and pure CPS groups the median IgG1 titers were 66, 122, and 80, respectively. Median IgG3 titers were 213, 360, and 480, respectively). The titer of IgG3 in sera from pTREP control mice increased to 213 compared with the preimmune level of 50. This might be due to the cross-reaction of antibodies elicited by L. lactis with S. pneumoniae polysaccharide. Moreover, it is generally known that anti-mouse whole-molecule IgG reagents do not recognize all IgG subclasses equally, and IgG3 may not have been detected with the same sensitivity as the other IgG subclasses.
DISCUSSION
We have shown that it is possible to express a heterologous type 3 CPS from S. pneumoniae in L. lactis. This was achieved by cloning fragments of the pneumococcal type 3 CPS biosynthetic operon into the lactococcal cloning vector pTREP and transforming L. lactis. Reports of the instability of pneumococcal DNA in Escherichia coli are well documented (11, 19), but in L. lactis the pneumococcal DNA could be stably manipulated and was efficiently expressed using the native pneumococcal promoters. The suitability of L. lactis as a host for the cloning and expression of pneumococcal operons is presumed to reflect the fact that pneumococci and lactococci are more closely related phylogenetically to each other than they are to E. coli and suggests that L. lactis may be a useful alternative host to E. coli for the manipulation and expression of streptococcal genes. Previous attempts to express type 3 CPS in E. coli failed to yield an extracellular capsule; instead, the polysaccharide accumulated in the periplasm, leading to poor growth (3, 17). In contrast, the growth rates of the lactococcal CPS expressor strain were identical to those of the host strain and plasmid instability was not observed during repeated subcultures.
On the basis of antibody detection of CPS on the surface of intact recombinant lactococcal cells, it was apparent that type 3 CPS was produced in low amounts when only two (cps3D and cps3S) of the four pneumococcal biosynthetic genes were introduced into L. lactis. The two enzymes encoded by cps3M and cps3U genes are not required for the expression of type 3 CPS (9, 10), but their roles in the synthesis of the precursor requirements for CPS biosynthesis (glucose-1-phosphate, UDP-glucose) must be fulfilled by essential enzymes involved in the cell metabolism. In L. lactis, two distinct forms of phosphoglucomutase have been described and their activities may fulfill the role of Cps3M (23). Recently, a glucose-1-phosphate uridylyltransferase, GalU, has been identified in L. lactis (15), and it may fulfill the role of Cps3U. However, the amount of CPS produced by L. lactis was dramatically increased by incorporating the pneumococcal Cps3U gene into L. lactis along with the genes encoding Cps3D and Cps3S. This indicated that the normal levels of lactococcal GalU activity appear to be too low to match the activities of the Cps3D and Cps3S enzymes expressed in L. lactis and become rate-limiting for the production of significant quantities of type 3 CPS. These results are in agreement with a recent publication reporting that the cloning and functional overexpression of the lactococcal galU gene resulted in increased levels of UDP-glucose and UDP-galactose in L. lactis (16).
The amount of CPS produced by L. lactis strain UCP1619 was estimated to be around 120 mg liter−1 in test-tube culture. It may well be possible to increase these yields still further by engineering strains that express functional phosphoglucomutase (Cps3M-like) in addition to the other biosynthetic enzymes. Proton and carbon NMR studies on purified type 3 CPS from S. pneumoniae and recombinant L. lactis strain UCP1619 showed that the composition and structure of the two polysaccharides were identical.
By determining the minimum number of genes required for a good expression level of pneumococcal type 3 capsule in L. lactis, we have shown that specific pneumococcal genes providing transport functions are not required for type 3 polysaccharide synthesis, assembly, and transport in L. lactis. A common feature of pneumococcal capsule biosynthetic operons is the presence of conserved sequences at both ends of the operon that contain genes thought to be involved in the export and polymerization of CPS. It has been shown that a number of type 3 CPS operons contain deletions in these conserved regions, suggesting that type 3 capsule synthesis may not require the lipid intermediates and additional transport proteins involved in the export of other capsular serotypes across the cell membrane (9). Our results support the hypothesis that the type 3 synthase encoded by the gene cps3S carries out both the polymerization and export functions necessary for type 3 capsule production. However, it remains possible that an alternative transport mechanism exists in both L. lactis and S. pneumoniae that can export type 3 CPS. Previous work on the expression of a plasmid encoded exopolysaccharide in L. lactis indicated that this organism may indeed possess a lipid carrier involved in the export of exopolysaccharide (28, 30).
Preliminary experiments in our laboratory showed that 5-μg doses of CPS in mice elicited antibody titers similar to those elicited by 0.5-μg doses (data not shown). Therefore, 0.5-μg doses of CPS (or equivalent L. lactis cell doses) were used to study the immune responses in detail. After a single inoculation, groups of mice immunized with L. lactis expressing in total 0.5 μg of type 3 polysaccharide or 0.5 μg of purified type 3 CPS from pneumococcus elicited similar titers of anti-CPS IgM antibody in the serum. The primary immune responses were monitored over time, and once antibody levels had declined to background levels, the groups of mice were boosted with a second dose of either CPS-expressing lactococci or of purified pneumococcal CPS. A T-cell-dependent antigen would be expected to induce memory B and T cells during the primary immunization, leading to more rapid secondary antibody responses characterized by higher titers and consisting mainly of IgG. After boosting, the anti-CPS antibody responses in the groups of mice immunized with CPS-expressing lactococci or with purified CPS were not elevated above the levels observed for the initial primary antibody responses. These responses were therefore characteristic of those elicited by a classic T-cell-independent antigen such as an unconjugated bacterial polysaccharide, which cannot recruit antigen specific helper T cells. Immunization with L. lactis UCP1619 therefore did not affect the T-cell-independent nature of the anti-CPS responses. Although IgG antibodies to CPS could be detected in the serum of mice immunized with purified CPS or lactococci expressing CPS they were either of the IgG1 or IgG3 subclass, as observed for other T-cell-independent antigens in mice (1).
The antibody titers elicited in our experiments were relatively low. Nevertheless, previous workers have shown that inoculation of BALB/c mice with the same amount (0.5 μg) of purified type 3 CPS as used here (without adjuvant) can protect against challenge with 25 U of a mean lethal dose of S. pneumoniae type 3 (26). In our experiments, the rise in mean titer of anti-CPS antibody observed in the majority of mice was only twofold. However, following the boost with pure CPS, two of six mice showed a rise in IgM titer of four- to fivefold and a further two mice showed a three- to fourfold rise. Following boosting with strain UCP1619 only two of six mice displayed more than threefold rises in antibody titers. The increases observed here in IgM titer were not as great as those reported by Snippe et al. (26), who detected peak titers of 2-mercaptoethanol sensitive antibodies in vaccinated mice (5 μg of pure CPS) that were eightfold higher than those in the controls (26). They used haemagglutination assays rather than ELISAs to measure the antibody responses, and differences between the sensitivity of the assays may at least partly account for the lower titers recorded in our experiments. Snippe et al. showed that immunization with 0.5 μg of CPS resulted in complete protection against a lethal dose of S. pneumoniae type 3. The work of Snippe et al. indicates that we are likely to obtain protection from challenge with virulent pneumococci in a mouse model following immunization with L. lactis expressing between 0.5 to 5 μg of type 3 CPS. Future work is aimed at investigating the dose-dependent response to immunization with type 3 CPS L. lactis by using different routes of administration and testing the capacity of the immune responses elicited to protect against pneumococcal colonization and respiratory challenge.
Overall, these results clearly show the advantage of using L. lactis rather than E. coli as a heterologous host to clone, express, and study the genes involved in the biosynthesis of capsules in S. pneumoniae. They also confirm that L. lactis is a potential host for the safe production and delivery of capsule vaccine antigens. The L. lactis model described here for pneumococcal capsule expression in L. lactis would permit significant quantities of capsule antigen to be manufactured without the need to culture large quantities of pathogenic bacteria, thereby reducing the biological hazard involved with the growth of S. pneumoniae strains in order to purify the polysaccharides.
The question of whether immune clearance of lactococci was inhibited by CPS expression was not addressed; however, vaccinated mice were monitored following the immunizations. No obvious adverse effects could be observed.
The encouraging results described in this paper indicate that further investigations into the delivery of recombinant and chimeric capsule antigens via systemic and mucosal routes of administration using recombinant lactococci are now warranted.
ACKNOWLEDGMENTS
We thank Peter Grice (University Chemical Laboratory, University of Cambridge) for performing the NMR analysis of the polysaccharides.
At the time these studies were conducted, J.M.W. held an Advanced Research Fellowship from the Biotechnology and Biological Sciences Research Council, United Kingdom, and C.G. held a Fellowship from Cortecs plc, whose financial support is gratefully acknowledged.
REFERENCES
- 1.Alonso DeVelasco E, Verheul A F M, Verhoef J, Snippe H. Streptococcus pneumoniae: virulence factors, pathogenesis and vaccines. Microbiol Rev. 1995;59:591–603. doi: 10.1128/mr.59.4.591-603.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arrecubieta C, Garcia E, López R. Sequence and transcriptional analysis of a DNA region involved in the production of capsular polysaccharide in Streptococcus pneumoniae type 3. Gene. 1995;167:1–7. doi: 10.1016/0378-1119(95)00657-5. [DOI] [PubMed] [Google Scholar]
- 3.Arrecubieta C, López R, Garcia E. Type 3-specific synthase of Streptococcus pneumoniae (Cap3B) directs type 3 polysaccharide biosynthesis in Escherichia coli and in pneumococcal strains of different serotypes. J Exp Med. 1996;184:449–455. doi: 10.1084/jem.184.2.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Austrian R, Bernheimer H P, Smith E E B, Mills G T. Simultaneous production of two capsular polysaccharides by pneumococcus. II. The genetic and biochemical basis of binary capsulation. J Exp Med. 1959;110:585–602. doi: 10.1084/jem.110.4.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons; 1987. [Google Scholar]
- 6.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 7.Breiman F F, Butler J C, Tenover F C, Elliott J A, Facklam R R. Emergence of drug-resistant pneumococcal infections in the United States. JAMA. 1994;271:1831–1835. [PubMed] [Google Scholar]
- 8.Briles D E, Nahm M, Schroder K, Davie J, Baker P, Kearney J, Barletta R. Antiphosphocholine antibodies found in normal mouse serum are protective against intravenous infection with type 3 Streptococcus pneumoniae. J Exp Med. 1981;153:694–705. doi: 10.1084/jem.153.3.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caimano M J, Hardy G G, Yother J. Capsule genetics in Streptococcus pneumoniae and a possible role for transposition in the generation of the type 3 locus. Microb Drug Resist. 1998;4:11–23. doi: 10.1089/mdr.1998.4.11. [DOI] [PubMed] [Google Scholar]
- 10.Dillard J P, Vandersea M W, Yother J. Characterization of the cassette containing genes for type 3 capsular polysaccharide biosynthesis in Streptococcus pneumoniae. J Exp Med. 1995;181:973–983. doi: 10.1084/jem.181.3.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dillard J P, Yother J. Analysis of Streptococcus pneumoniae sequences cloned into Escherichia coli: effect of promoter strength and transcription terminators. J Bacteriol. 1991;173:5105–5109. doi: 10.1128/jb.173.16.5105-5109.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dubois M A, Gilles K A, Hamilton J K, Rebers P A, Smith F. Colorimetric method for determination of sugars and related substances. Anal Chem. 1956;28:350–356. [Google Scholar]
- 13.Garcia E, Arrecubieta C, Munoz R, Mollerach M, López R. A functional analysis of the Streptococcus pneumoniae genes involved in the synthesis of type 1 and type 3 capsular polysaccharides. Microb Drug Resist. 1997;3:73–88. doi: 10.1089/mdr.1997.3.73. [DOI] [PubMed] [Google Scholar]
- 14.Gasson M J. Plasmid compliments of Streptococcus lactis NCDO 712 and other lactic streptococci after protoplast-induced curing. J Bacteriol. 1983;154:1–9. doi: 10.1128/jb.154.1.1-9.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grossiord B, Vaughan E E, Luesink E J, de Vos W M. Genetics of galactose-utilization via the Leloir pathway in lactic acid bacteria. Lait. 1998;78:77–84. [Google Scholar]
- 16.Kleerebezem M, van Kranenburg R, Tuinier R, Boels I C, Zoon P, Looijesteijn E, Hugenholtz J, de Vos W M. Exopolysaccharides produced by Lactococcus lactis: from genetic engineering to improved rheological properties? Antonie Leeuwenhoek. 1999;76:357–365. [PubMed] [Google Scholar]
- 17.Kolkman M A B, van der Zeijst B A M, Nuijten P J M. Functional analysis of glycosyltransferases encoded by the capsular polysaccharide biosynthesis locus of Streptococcus pneumoniae serotype 14. J Biol Chem. 1997;272:19502–19508. doi: 10.1074/jbc.272.31.19502. [DOI] [PubMed] [Google Scholar]
- 18.Llull D, López R, Garcia E, Munoz R. Molecular structure of the gene cluster responsible for the synthesis of the polysaccharide capsule of Streptococcus pneumoniae type 33F. Biochim Biophys Acta. 1998;1443:217–224. doi: 10.1016/s0167-4781(98)00213-9. [DOI] [PubMed] [Google Scholar]
- 19.López P, Espinosa M, Greenberg B, Lacks S A. Generation of deletions in pneumococcal mal genes cloned in Bacillus subtilis. Proc Natl Acad Sci USA. 1984;80:5189–5193. doi: 10.1073/pnas.81.16.5189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maniatis T, Fritsch E F, Sambrook J. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1982. [Google Scholar]
- 21.Morona J K, Morona R, Paton J C. Molecular and genetic characterization of the capsule biosynthesis locus of Streptococcus pneumoniae type 19B. J Bacteriol. 1997;179:4953–4958. doi: 10.1128/jb.179.15.4953-4958.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mollerach M, López R, Garcia E. Characterization of the galU gene of Streptococcus pneumoniae encoding a uridine diphosphoglucose pyrophosporylase: a gene essential for capsular polysaccharide biosynthesis. J Exp Med. 1998;188:2047–2056. doi: 10.1084/jem.188.11.2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qian N, Stanley G A, Hahn-Hägerdahl B, Radström P. Purification and characterization of two phosphoglucomutases from Lactococcus lactis subsp. lactis and their regulation in maltose- and glucose-utilizing cells. J Bacteriol. 1994;176:5304–5311. doi: 10.1128/jb.176.17.5304-5311.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ramirez M, Tomasz A. Molecular characterization of the complete 23F capsular polysaccharide locus of Streptococcus pneumoniae. J Bacteriol. 1998;180:5273–5278. doi: 10.1128/jb.180.19.5273-5278.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shann F. Pneumococcal vaccine: time for another controlled trial. Lancet. 1998;351:1600–1601. doi: 10.1016/S0140-6736(05)77683-2. [DOI] [PubMed] [Google Scholar]
- 26.Snippe H, van Houte A-J, van Dam J E G, de Reuver M J, Jansze M, Willers J M N. Immunogenic properties in mice of hexasaccharide from the capsular polysaccharide of Streptococcus pneumoniae type 3. Infect Immun. 1983;40:856–861. doi: 10.1128/iai.40.3.856-861.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sorensen U B, Henrichsen J, Chen H C, Szu S C. Covalent linkage between the capsular polysaccharide and the cell wall peptidoglycan of Streptococcus pneumoniae revealed by immunochemical methods. Microb Pathog. 1990;8:325–334. doi: 10.1016/0882-4010(90)90091-4. [DOI] [PubMed] [Google Scholar]
- 28.Stingele F, Neeser J-R, Mollet B. Identification and characterization of the eps (exopolysaccharide) gene cluster from Streptococcus thermophilus Sfi6. J Bacteriol. 1996;178:1680–1690. doi: 10.1128/jb.178.6.1680-1690.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.The Lancet. Acute respiratory infections in under-fives: 15 million deaths a year. Lancet. 1985;ii:699–701. [PubMed] [Google Scholar]
- 30.van Kranenbur R, Marugg J D, van Swam I I, Willem N J, de Vos W M. Molecular characterization of the plasmid-encoded eps gene cluster essential for exopolysaccharide biosynthesis in Lactococcus lactis. Mol Microbiol. 1997;24:387–397. doi: 10.1046/j.1365-2958.1997.3521720.x. [DOI] [PubMed] [Google Scholar]
- 31.Wells J M, Schofield K M. Cloning and expression vectors for lactococci. In: Bozoglu T F, Ray B, editors. Lactic acid bacteria: current advances in metabolism, genetics and applications. NATO ASI Series. H98. Berlin, Germany: Springer-Verlag; 1996. pp. 37–62. [Google Scholar]
- 32.Wells J M, Wilson P W, Le Page R W F. Improved cloning vectors and transformation procedure for Lactococcus lactis. J Appl Bacteriol. 1993;74:629–636. doi: 10.1111/j.1365-2672.1993.tb05195.x. [DOI] [PubMed] [Google Scholar]