Abstract
The outbreak of coronavirus SARS-COV2 affected more than 180 countries necessitating fast and accurate diagnostic tools. Reverse transcriptase polymerase chain reaction (RT-PCR) has been identified as a gold standard test with Chest CT and Chest Radiography showing promising results as well. However, radiological solutions have not been used extensively for the diagnosis of COVID-19 disease, partly due to radiation risk. This study aimed to provide quantitative comparison of imaging radiation risk versus COVID risk. The analysis was performed in terms of mortality rate per age group. COVID-19 mortality was extracted from epidemiological data across 299, 004 patients published by ISS-Integrated surveillance of COVID-19 in Italy. For radiological risk, the study considered 659 Chest CT performed in adult patients. Organ doses were estimated using a Monte Carlo method and then used to calculate Risk Index that was converted into an upper bound for related mortality rate following NCI-SEER data. COVID-19 mortality showed a rapid rise for ages >30 years old (min: 0.30%; max: 30.20%), whereas only four deaths were reported in the analysed patient cohort for ages <20 years old. The rates decreased for radiation risk across age groups. The median mortality rate across all ages for Chest-CT and Chest-Radiography were 0.007% (min: 0.005%; max: 0.011%) and 0.0003% (min: 0.0002%; max: 0.0004%), respectively. COVID-19, Chest Radiography, and Chest CT mortality rates showed different magnitudes and trends across age groups. In higher ages, the risk of COVID-19 far outweighs that of radiological exams. Based on risk comparison alone, Chest Radiography and CT for COVID-19 care is justified for patients older than 20 and 30 years old, respectively. Notwithstanding other aspects of diagnosis, the present results capture a component of risk consideration associated with the use of imaging for COVID. Once integrated with other diagnostic factors, they may help inform better management of the pandemic.
Keywords: COVID-19, mortality, radiation risk, x-ray Computed Tomography, x-ray radiography
1. Introduction
Between December 2019 and 15 June 2020, 8 million cases of coronavirus SARS-COV2 disease (COVID-19) have been reported, with over 434 000 attributed deaths worldwide [1, 2]. More than 180 countries report an outbreak, with the US showing the largest number of cases. On 30 January 2020, the World Health Organization (WHO) declared the pandemic a ‘public emergency of international concern’. In this landscape, accurate and fast diagnosis is one of the most necessary tools to curb the spread of the disease.
To date, the gold standard diagnostic method for COVID-19 has been reverse transcriptase polymerase chain reaction [3]. However, this diagnostic test tends to be slow, requires a specific manufacturing process, is not always promptly available, and can have a varied sensitivity within the range of 60%–95% [4]. Because the infected patients often have lung involvements, radiological imaging has been used in many affected countries as an alternative for diagnosis of the disease. Frequent imaging findings are bilateral pulmonary ground-glass opacities and consolidations with peripheral predominance [5]. Chest CT and Chest Radiography have shown promising performance with CT sensitivity reported to be up to 98% [6–8]. The World Health Organization recently published a rapid advice guide in the use of chest imaging in Covid-19. The report identified 22 studies that evaluated the accuracy of Chest CT and Chest Radiography in the diagnosis of Covid-19. The median sensitivity and specificity were 0.92 and 0.56 for Chest CT; and 0.64 and 0.92 for Chest Radiography [9]. Imaging, therefore, can potentially offer an additional tool for the diagnosis or follow-up in the pandemic. Nevertheless, several international institutions are discouraging the use of radiological studies for the diagnosis of coronavirus infection. In particular, the American College of Radiology recommends that ‘CT should not be used to screen for or as a first-line test to diagnose COVID-19’ and the Center for Disease Control does not currently recommend the use of radiological imaging to diagnose COVID-19 [10].
Despite the reservations, the need for rapid diagnosis to mitigate a rapidly spreading pandemic is at an all-time high, and radiological examination is continuing to be used in many countries for the early diagnosis of the disease and its follow-up. Such use should be primarily dictated by the sensitivity and specificity of the procedure. But in addition, the use of radiological exams has an associated radiation risk. This risk is obviously not the primary factor that should be taken into account for justifying the use of imaging in COVID care; nonetheless, it is a factor that is of high public concern needing an explicit management of its own. This is particularly the case considering the differing radiation burdens of CT and Radiography, regardless of their diagnostic accuracy.
The purpose of this study was to compare, in real clinical populations, the COVID-19 infection mortality risk versus the intrinsic radiation risk associated with CT and Radiography. COVID-19 risk was extracted from epidemiological data and analysed in terms of mortality per age group. Analogously, the ionising radiation risk was evaluated in terms of mortality per age group considering the Risk Index (RI) from Monte Carlo based organ doses (ODs), converted to a 5-year relative mortality rate. This risk comparison, by necessity, did not include image quality and individual clinical risk factors. Rather, it was made to fill one necessary gap for consideration and to serve as a complementary analysis to be added to those of diagnostic accuracy and medical intervention. Such an integration could then provide a holistic picture to guide policy makers on the best strategy in managing COVID-19 patients.
2. Materials and methods
The study involved assessing and comparing the radiation risk associated with imaging exams to those associated with COVID. The study was performed in compliance with the Health Insurance Portability and Accountability Act and was determined to be exempt from Institutional Review Board requirements.
For COVID-19 associated risk, this study considered the COVID-19 mortality rate in the epidemiological data across 299.004 patients published by Istituto Superiore di Sanità—Integrated surveillance of COVID-19 in Italy [11]. We treated the reported observations as samples from a Bernoulli random variable and used maximum likelihood estimation to calculate the mortality rate in each age group. The associated uncertainties were then evaluated in MATLAB 2019b (Mathworks, Inc.) as binomial confidence intervals (CI) with a 95% significance level [12].
For radiological risk estimation, this study included 659 Chest CT examinations without contrast performed on adult patients (median age = 62.8 years old; range = [18.1–79.9] years old) in US between March 2018 and January 2019 using typical imaging protocols (120 kV tube voltage, 1.53 pitch, 7.6 mGy median CTDIvol, [2.07–37.44] mGy CTDIvol range). Patient-specific ODs were calculated using an established methodology [13]. Each patient was matched to a virtual human model from the XCAT phantom library (18–78 years old, 52–117 kg, 23/35 M/F) [14] based on patient lung height and patient diameter (figure 1). The process included the effect of tube current modulation by first estimating ODs under constant tube current into CTDIvol-to-organ-dose conversion coefficients (h factors) lookup tables [13, 15, 16] using Monte Carlo simulations (PENELOPE, version 2006, Universitat de Barcelona, Spain). To achieve relative errors smaller than 1%, each virtual human model was simulated with 8 × 107 photon histories. Dose spread functions (DSF) were likewise simulated for cylindrical phantoms of different diameters (8–50 cm with 2 cm step) at constant mA [17]. Size-specific DSF, normalised to the CTDI and convolved with the TCM, were used to scale the h factors to drive the TCM-specific OD values [17].
Figure 1.
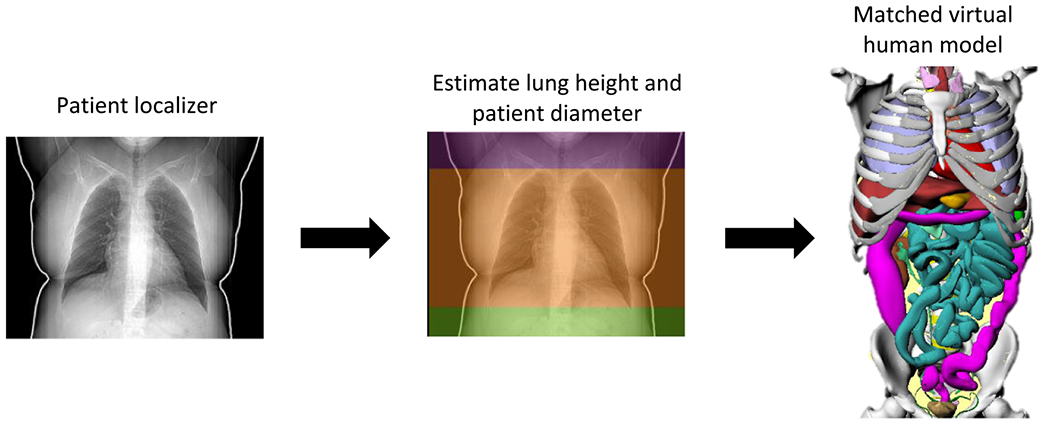
Example of patient matching to a virtual human model.
Following the National Research Council BEIR VII report, ODs were used to calculate the radiation RI for each patient as described by Li et al [18, 19], using , where HT is the equivalent dose for the organ or tissue T and rT is the gender-, age-, and tissue-specific lifetime attributable risk of cancer incidence reported in BEIR VII [18, 19]. Using the National Cancer Institute Surveillance, Epidemiology, and End Results Cancer Statistics Review 1975–2015 (NCI—SEER) [20], the RI was converted to a 5-year relative mortality rate for all cancers. The NCI-SEER data are based on real follow-up of patients into 2015 and the expected survival rates are derived from life tables by socio-economic status, geography, and race developed by the SEER program: its application to the calculated RI enables the estimation of an upper bound for the Chest CT mortality rate.
Radiography mortality was calculated by re-scaling the ODs estimated in the CT studies following Zhang et al methodology [21]. The study estimated ODs and effective doses for Chest Radiography and Chest CT using a Monte Carlo dose simulation program (PENELOPE, version 2006, Universitat de Barcelona, Spain) on a total of 59 computational anthropomorphic male and female adult phantoms (extended cardiac-torso, XCAT) and that reported the average lung dose in PA radiography examinations to be about 2% of that in Chest CT [21]. In particular, the study reported an average lung dose for posteroanterior Chest Radiography of 0.12 mGy (effective dose: 0.04 mSv) and of 5.8 mGy in Chest CT (effective dose: 3.2 mSv). The Radiography ODs were then used to calculate the Radiography-related radiation RI per each patient and the 5 year relative mortality rate for all cancers as described above, giving an upper bound for the Chest Radiography mortality rate. A schematic of the Chest CT and Chest Radiography mortality estimation method is provided in figure 2.
Figure 2.
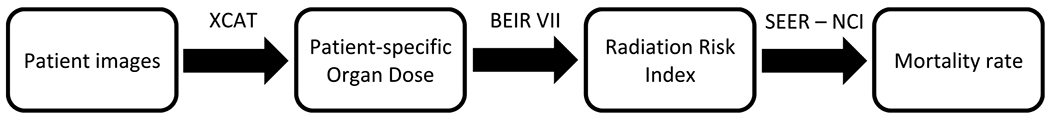
Schematic of radiological procedures risk estimation.
The risks associated with Chest Radiography, Chest CT, and COVID-19 were then compared in terms of mortality as a function of age.
3. Results
ODs were calculated for 25 organs in male and 26 organs in female patients [17]. Across all patients, median Lung dose was 15.3 mGy (mean: 16.1 mGy) and ranged between 3.5 mGy and 62.3 mGy. COVID-19 cases per age group, mortality rates per age group, and the related CI are reported in table 1. Chest CT and Chest Radiography mortality median and interquartile range per age group are also reported in table 1 and in figure 3. The COVID risk factors show strong age dependency. The radiation risks are less varied as a function of age but show a broad spread due to variations in patient size [22, 23].
Table 1.
COVID-19 total number of cases; COVID-19, Chest CT, and Chest Radiography mortality rate per age group, n/a values are related to age groups not included in the patient cohorts.
| Age group (years) | COVID-19 cases | Covid-19 mortality (% across age groups; 95% CI) | CT mortality × 10−2 (%) median; (IQ range) | Radiography mortality × 10−2 (%) median; (IQ range) |
|---|---|---|---|---|
| 0–9 | 5074 | 0.10; [0.00–0.05] | n/a | n/a |
| 10–19 | 10 261 | 0.00; [0.00–0.02] | 0.84; [0.47–1.16] | 0.03; [0.02–0.05] |
| 20–29 | 27 284 | 0.10; [0.04–0.20] | 0.86; [0.45–1.99] | 0.03; [0.02–0.08] |
| 30–39 | 28 366 | 0.20; [0.12–0.30] | 0.80; [0.33–2.04] | 0.03; [0.01–0.08] |
| 40–49 | 39 857 | 0.80; [0.68–0.93] | 1.10; [0.55–3.17] | 0.04; [0.02–0.13] |
| 50–59 | 51 318 | 2.40; [2.23–2.58] | 1.01; [0.42–1.80] | 0.04; [0.02–0.07] |
| 60–69 | 36 620 | 9.80; [9.43–10.18] | 0.72; [0.33–1.28] | 0.03; [0.01–0.05] |
| 70–79 | 37 016 | 25.20; [24.67–25.74] | 0.46; [0.17–0.93] | 0.02; [0.01–0.04] |
| 80–89 | 43 439 | 33.60; [33.03–34.17] | n/a | n/a |
| >90 | 19 747 | 33.10; [32.17–34.04] | n/a | n/a |
| Unknown | 22 | 4.5; [1.58–10.86] | n/a | n/a |
| TOTAL | 299 004 | 11.90; [11.74–12.06] | 0.72; [0.33–1.35] | 0.03; [0.01–0.05] |
Figure 3.
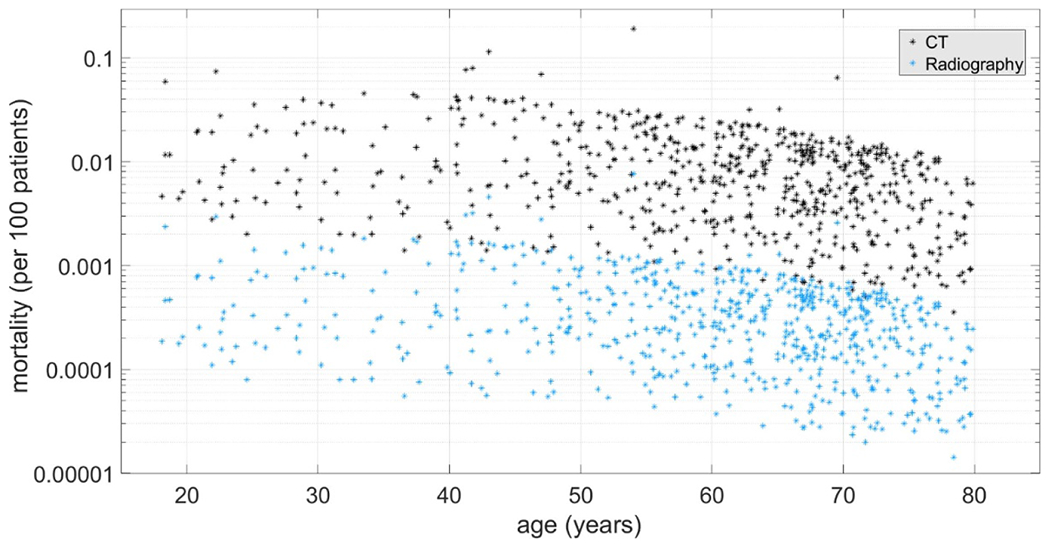
Distribution of Chest CT (black) and Chest Radiography (blue) mortalities per age. Each dot represents a patient undergoing Chest CT or Radiography.
Figure 4 shows the three mortality risks. Only four deaths due to COVID-19 were reported in Italy across patients between 0 and 19 years old. The risk exhibited a rapid rise between 30 and 89 years old with a gradual decrease for patients older than 90 years old. Chest CT and Chest Radiography data followed the same trends across age groups with a median Radiography risk magnitude that was 4.3% of the CT risk. CT and Radiography mortality rates showed highest values for the 40–49 years old patients and a gradual decrease for older patients, who are less sensitive to radiation effects. The risk remain largely unchanged for younger patients as the applied protocols are appropriately adjusted based on the Image Gently guidelines. COVID-19 and Chest Radiography risk showed similar values for the 10–19 age group, whereas COVID-19 and Chest CT were comparable for patients between 20 and 30 years old.
Figure 4.
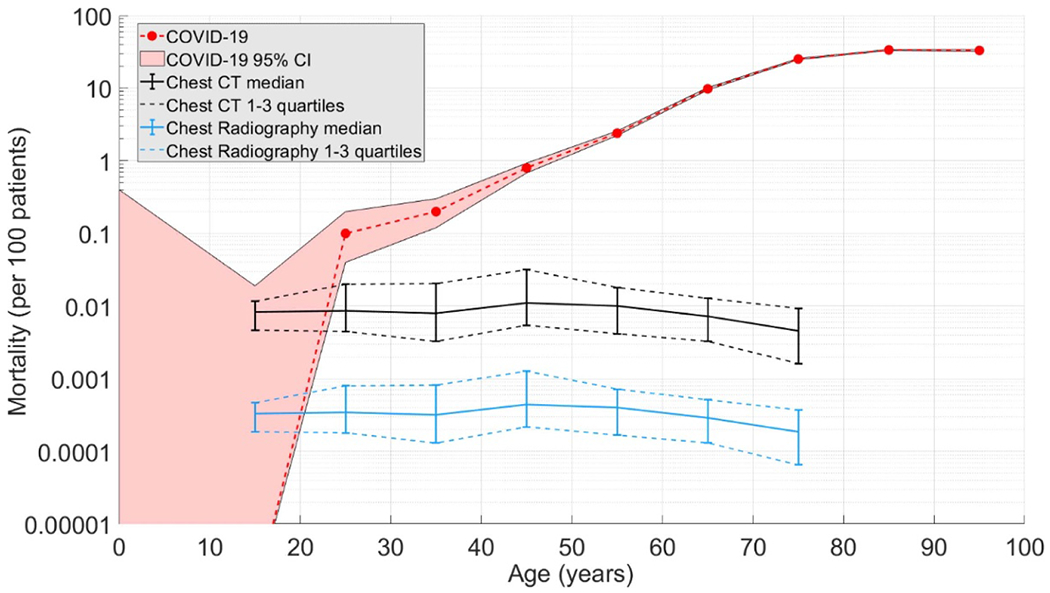
COVID-19 mortality per age (red) compared to those from radiation used in Chest CT (black) and Chest radiography (blue).
4. Discussion
This study compared the mortality rate associated with COVID-19 in almost 300 000 patients with an upper bound of mortality for Chest CT and Chest Radiography in adult clinical populations. To our knowledge, such a comparison has not previously been made. The data demonstrates that the risk associated with radiation burden in radiological procedures is low and can be appropriately taken into consideration in using imaging for pandemics. As COVID-19 mortality shows significant differences across age groups, the justification and the choice of the appropriate diagnostic strategy should include patient age considering younger patients that are more sensitive to ionising radiation [24]. In the 20–29-year old age group, COVID-19 mortality is higher than that estimated for Chest Radiography. Between 20 to 29 years old, CT and COVID-19 mortality rates are comparable, with the novel coronavirus risk rising rapidly for patients over 40 years old. Based on risk comparison alone, Chest Radiography and CT for COVID-19 care is justified for patients older than 20 and 30 years old, respectively. With a reported median time of 12 days between the onset of symptoms to the death of COVID-19 positive patients [25], diagnostic imaging can play a timely response for diagnostic assessment of the disease, as well as the means to assess the extent of the lung involvement for stratified treatments.
The scientific community already understands that the risks induced by most radiological procedures are small, compared with other lifetime risks from various sources [26] and that the risk-to-benefit ratio is favourable when such techniques are used for symptomatic patients, even in the case of CT, the modality with the highest associated radiation burden [27]. Despite this consolidate knowledge, several months after the first reported COVID-19 case, utilising diagnostic imaging for diagnosis is not unanimously accepted. Few countries (i.e. Italy and China) are using diagnostic imaging [5–8, 28], whereas others do not [10]. A recent Multinational Consensus Statement from the Fleischner Society evaluated the utility of diagnostic imaging representing different factors [29]. We emphasise that radiation burden is obviously not the only factor under scrutiny for determining the role of imaging in COVID-19 care. However, it is an essential factor to be considered. Our observation enables and emphasises the need to apply optimisation principles to define specific imaging protocols that maximise the diagnostic outcome of the exam while keeping its dose in check [30].
Objective optimisation in radiology based on the risk-to-benefit ratio requires the quantification of both the diagnostic benefit as well as the disease and radiological risks. Both the benefit and the risk are relevant and important, and ideally assessed in the context of individual patients; e.g. our estimation of radiological risk was made using Monte Carlo methods accounting for individual patient factors of age, sex, and organ radio-sensitivity [17, 18, 31, 32]. This study focused primarily on the disease and radiological risk. We in fact specifically did not account for diagnostic accuracy, co-morbidities, or other clinical considerations that can strongly affect the individual justification of medical procedures for COVID patients. That is not to imply that those factors are not important. Rather, in this work, we squarely focused on radiation risk in an isolated fashion so that the estimated risk component can be incorporated in follow-up risk-to-benefit analyses. In that way, the outcome of our analysis can provide crucial information to decision and policy makers towards a more patient-centric use of imaging in COVID care.
Some limitations to this study merit discussion. First, the risk was compared in cohorts from two different countries (Italy and US). This was due to the limited availability of the data at this time. However, the reported methodology can be extended to more uniform populations when the new epidemiological data will be available. Second, the conversion from RI to mortality following NCI—SEER data [20] did not take into account the timeframe of the risk: COVID-risk is associated with death in the matter of weeks or months while radiation risk is in years and decades. There is currently no method to compare risks that differ in their time horizon. Moreover, there is a large uncertainty in the impact of patient age in the estimation of radiation related risk. Such uncertainties can be mitigated using BEIR VII gender-, age-, and tissue-specific lifetime attributable risk of cancer incidence factors as described in the presented methodology. Such consideration can be applied also if the cancer mortality is calculated applying the conversion factors reported in Table 12D-2 Lifetime Attributable Risk of Cancer Mortality of the BEIR VII report [18]. Third, Radiography ODs were calculated by re-scaling the ODs estimated in CT studies based on published data [21, 33]. Fourth, the radiation risk analysis was limited to one scanner model from one vendor. It is known that different vendors pursue different strategies in the management of dose and image quality across clinical populations [22, 23]. However, the differences in dose magnitude are not large enough to affect a population risk study. Lastly, as noted above, this study only focused on radiation risk associated with imaging. Future studies should extend the methodology to include the sensitivity and specificity associated with CT and Radiography towards a more comprehensive risk-to-benefit evaluation.
5. Conclusions
The data reported in this study offer a first approach to risk-to-benefit evaluation in the use of radiological procedures for diagnosis of COVID-19. Because the mortality associated with the pandemic disease and the statistical risk associated with radiological procedures change with patient age and show different trends, the use of CT and Radiography to support the diagnosis of COVID-19 should not be a priori excluded. Care should be used in the justification of the best modality, as well as in the optimisation of the device parameters considering patient age.
Acknowledgments
The authors would like to thank the Editor, the IOP peer-review team, and the Reviewers for their insightful comments and efforts towards improving the manuscript.
Footnotes
Ethical statement
Institutional Review Board approval was obtained and written informed consent was waived by the Institutional Review Board.
References
- [1].Naming the coronavirus disease (COVID-19) and the virus that causes it https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/naming-the-coronavirus-disease-(covid-2019)-and-the-virus-that-causes-it (Accessed 26 March 2020)
- [2].Coronavirus COVID-19 global cases by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University https://coronavirus.jhu.edu/map.html (Accessed 15 June 2020)
- [3].Center for Disease Control and Prevention Interim guidelines for collecting, handling, and testing clinical specimens from persons for coronavirus disease 2019 (COVID-19) https://www.cdc.gov/coronavirus/2019-ncov/lab/guidelines-clinicalspecimens.html (Accessed 26 March 2020)
- [4].Yang Y. et al. Evaluating the accuracy of different respiratory specimens in the laboratory diagnosis and monitoring the viral shedding of 2019-nCoV infections (submitted) doi: 10.1101/2020.02.11.20021493. [DOI] [Google Scholar]
- [5].Chung M, Bernheim A, Mei X, Zhang N, Huang M and Zeng X 2020. CT imaging features of 2019 novel coronavirus (2019-nCoV) Radiology 295 202–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Bai HX et al. 2020. Performance of radiologists in differentiating COVID-19 from viral pneumonia on chest CT Radiology 296 E46–E54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Fang Y, Zhang H, Xie J, Lin M, Ying L, Pang P and Ji W 2020. Sensitivity of Chest CT for COVID-19: comparison to RT-PCR Radiology 296 E115–E117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ai T et al. 2020. Correlation of Chest CT and RT-PCR testing in Coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases Radiology 296 E32–E40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].WHO 2020. Use of Chest Imaging in COVID-19: A Rapid Advice Guide (Geneva: World Health Organization; ) [Google Scholar]
- [10].ACR Recommendations for the use of Chest Radiography and Computed Tomography (CT) for suspected COVID-19 infection https://www.acr.org/Advocacy-and-Economics/ACR-Position-Statements/Recommendations-for-Chest-Radiography-and-CT-for-Suspected-COVID19-Infection (Accessed 26 March 2020)
- [11].Task force COVID-19 del Dipartimento Malattie Infettive e Servizio di Informatica, Istituto Superiore di Sanità Epidemia COVID-19, Aggiornamento nazionale: 18settembre https://www.epicentro.iss.it/coronavirus/bollettino/Bollettino-sorveglianza-integrata-COVID-19_22-settembre-2020.pdf (Accessed 30 September 2020)
- [12].Trivedi KS 2002. Probability and Statistic with Reliability, Queuing, and Computer Science Applications 2nd edn (New York: Wiley; ) [Google Scholar]
- [13].Tian X, Segars WP, Dixon RL and Samei E 2016. Convolution-based estimation of organ dose in tube current modulated CT Phys. Med. Biol 61 3935–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Segars WP. et al. Population of anatomically variable 4D XCAT adult phantoms for imaging research and optimization. Med. Phys. 2013;40:043701. doi: 10.1118/1.4794178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Sahbaee P, Segars WP and Samei E 2014. Patient-based estimation of organ dose for a population of 58 adult patients across 13 protocol categories Med. Phys 41 072104–1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Tian X, Segars WP, Paulson EK, Frush DP and Samei E 2014. Pediatric chest and abdominopelvic CT: organ dose estimation based on 42 patient models Radiology 270 535–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Fu W, Ria F, Segars WP, Choudhury KR, Wilson JM, Kapadia A and Samei E 2019. Patient-informed organ dose estimation in clinical computed tomography: implementation and effective dose assessment in 1048 patients Am. J. Roentgenol (submitted) [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].National Research Council 2006. Health risks from exposure to low levels of ionizing radiation: BEIR VII Phase 2 (Washington, DC: The National Academies Press; ) [PubMed] [Google Scholar]
- [19].Li X, Samei E, Segars WP, Sturgeon GM, Colsher JG, Toncheva G, Yoshizumi TT and Frush DP 2011. Patient-specific radiation dose and cancer risk estimation in CT: part II. Application to patients Med. Phys 38 408–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Noone AM et al. SEER cancer statistics review, 1975–2015. (Bethesda, MD: National Cancel Institute; ) https://seer.cancer.gov/csr/1975_2015/ [Google Scholar]
- [21].Zhang Y, Li X, Segars WP and Samei E 2014. Comparison of patient specific dose metrics between chest radiography, tomosynthesis, and CT for adult patients of wide ranging body habitus Med. Phys 41 0293901-1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Ria F, Davis JT, Solomon JB, Wilson JM, Smith TB, Frush DP and Samei E 2019. Expanding the concept of diagnostic reference levels to noise and dose reference levels in CT Am. J. Roentgenol 213 889–94 [DOI] [PubMed] [Google Scholar]
- [23].Ria F, Solomon JB, Wilson JM and Samei E 2020. Technical note: validation of TG 233 phantom methodology to characterize noise and dose in patient CT data Med. Phys 47 1633–9 [DOI] [PubMed] [Google Scholar]
- [24].Li X, Samei E, Segars WP, Sturgeon GM, Colsher JG and Frush DP 2011. Patient-specific radiation dose and cancer risk for pediatric chest CT Radiology 259 862–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Istituto Superiore di Sanità 2020. Characteristics of SARS-CoV-2 patients dying in Italy report based on available data on July 22nd, 2020 https://www.epicentro.iss.it/en/coronavirus/bollettino/Report-COVID-2019_22_july_2020.pdf (Accessed 29 July 2020)
- [26].McCollough CH, Giumaraes L and Fletcher JG 2009. In defence of body CT Am. J. Roentgenol 193 28–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Albert JM 2013. Radiation risk from CT: implications for cancer screening Am. J. Roentgenol 201 W81–W87 [DOI] [PubMed] [Google Scholar]
- [28].Larici AR 2020. COVID-19: cosa il medico radiologo deve sapere SIRM - Italian Society of Medical and Interventional Radiology https://www.sirm.org/2020/02/28/coronavirus-disease-2019-covid-19-cosa-il-medico-radiologo-deve-sapere/ (Accessed 28 March 2020)
- [29].Rubin GD, Haramati LB, Kanne JP, Shluger NW, Yim JJ and Anderson DJ 2020. The role of chest imaging in patient management during the COVID-19 pandemic: a multinational consensus statement from the Fleischner Society Radiology 296 172–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Dangis A et al. 2020. Accuracy and reproducibility of low-dose submillisievert chest CT for the diagnosis of Covid-19 Cardiothorac. Imaging 2 e200196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Samei E, Järvinen H, Kortesniemi M, Simantirakis G, Goh C, Wallace A, Vano E, Bejan A, Rehani M and Vassileva J 2018. Medical imaging dose optimization from ground up: expert opinion of an international summit J. Radiol. Prot 38 967–89 [DOI] [PubMed] [Google Scholar]
- [32].Ria F, Fu W, Zhang Y, Hoye J, Segars P, Kapadia A and Samei E Characterization of radiation risk across a clinical CT patient population: comparison across 12 risks metrics CIV RSNA Annual Meeting 2018 (Chicago, IL) [Google Scholar]
- [33].ACR-AAPM-SPR practice parameter for diagnostic reference levels and achievable doses in medical x-ray imaging Revised 2018. (Resolution 40) (Reston, VA: ACR; ) [Google Scholar]


