Abstract
Toxic shock syndrome (TSS) is primarily caused by toxic shock syndrome toxin-1 (TSST-1) and staphylococcal enterotoxin B (SEB). These toxins belong to a family of pyrogenic toxin superantigens (PTSAgs) produced by Staphylococcus aureus and exhibit several shared biological properties, including the induction of massive cytokine release and Vβ-specific T-cell proliferation. The crystal structures of most PTSAgs are now published, and they demonstrate a striking similarity in conformational architecture even though their primary protein sequences are different. Despite these structural and immunobiological similarities, no cross-reactivity between TSST-1 and other PTSAgs has been demonstrated in serological or neutralization assays. Our laboratory has developed a neutralizing murine anti-TSST-1 monoclonal antibody (MAb5) which displayed cross-reactivity with SEB by enzyme-linked immunosorbent assay. The aim of the present study was to evaluate whether MAb5 can also cross-neutralize SEB-induced superantigenic activities in vitro. MAb5 was found to partially inhibit SEB-induced T-cell mitogenesis (63%) and tumor necrosis factor alpha (TNF-α) secretion (70%) in human peripheral blood mononuclear cells (PBMC) in a dose-dependent manner, while an isotypic anti-TSST-1 monoclonal antibody showed no effect. Epitope mapping revealed that MAb5 bound to TSST-1 residues 47 to 56 (47FPSPYYSPAF56) and to SEB residues 83 to 92 (83DVFGANYYYQ92), sequences that located in different regions of these toxins and are structurally dissimilar. SEB peptide 83DVFGANYYYQ92 was synthesized and found to also inhibit SEB-induced mitogenesis and TNF-α secretion in human PBMC. Our results demonstrate for the first time that MAb5 binds to different epitopes on TSST-1 and SEB that appear functionally important in inducing T-cell mitogenesis and TNF-α secretion in vitro.
Staphylococcal and streptococcal toxins, including toxic shock syndrome toxin 1 (TSST-1), staphylococcal enterotoxin (SE) serotypes A, B, C1 to C3, D, E, G, and H, and streptococcal pyrogenic exotoxin (SPE) serotypes A, B, and C, are known as pyrogenic toxin superantigens (PTSAgs) (31). They can cause profound disturbances in the homeostasis of the immune system, including massive proliferation of T cells bearing specific Vβ elements on their receptors, and an uncontrolled release of proinflammatory cytokines such as interleukin-1α (IL-1α), IL-1β, IL-2, IL-4, IL-6, and IL-10, gamma interferon (IFN-γ), tumor necrosis factor alpha (TNF-α) and TNF-β, and others (9, 24, 28). These immunologic events may result in various disease states ranging from acute, self-limited food poisoning (25) to life-threatening toxic shock syndrome (2, 5, 13, 16).
Biochemically, all PTSAgs are small polypeptides of approximately 22 to 30 kDa, with a neutral to basic isoelectric point (31). They are generally resistant to acid, heat, and protease digestion (2). Unlike conventional antigens, PTSAgs bind to the major histocompatibility complex (MHC) class II molecules of accessory cells outside of the peptide binding groove and do not require prior processing for T-cell presentation (11, 22). In addition to these unique immunological and biochemical properties, PTSAgs also share the ability to induce fever and to enhance host susceptibility to endotoxic shock (2). Primary amino acid sequence alignment analysis also suggests the presence of conserved sequences among these PTSAgs. For example, based on these sequences, PTSAgs can be separated into two predominant groups in which members share at least 50% sequence similarity: group 1, consisting of SE serotype B (SEB), SEC1 to -3, and SPEA; and group 2, consisting of SEA, SED, and SEE (1, 31). In contrast, TSST-1, SPEB, and SPEC share little (generally <25%), if any, sequence similarity with the other toxins (31). However, despite considerable sequence dissimilarity between TSST-1 and the other SEs, their crystal structures reveal striking similarities in conformational architecture (26, 27, 36, 37). For example, TSST-1 and the other PTSAgs all exhibit a two-domain structure with a C-terminal β-grasp motif (domain A), a characteristic N-terminal claw-like β barrel (domain B), and a long diagonal α helix separating these two domains (30, 31, 38).
Historically, PTSAgs were regarded as being antigenically distinct (6). Cross-reactivity was noted between SEA and SEE and among SEC1, SEC2, and SEC3, but not the other PTSAgs (4, 29). However, with more sensitive assays such as immunoblotting and immunoprecipitation, Hynes et al. (10) demonstrated serologic cross-reactivity among SEB, SEC1, and SPEA. Others have also identified monoclonal antibodies (MAbs) which can cross-react with SEA, SEB, SEC, SED, and SEE by enzyme-linked immunosorbent assay (ELISA) (23, 24). Furthermore, Bohach et al. (3) showed that MAbs against SPEA and SEC1 could cross-neutralize mitogenicity induced by homologous and heterologous toxins (SPEA, SEC1, and SEB). However, none of these previously identified MAbs have shown cross-reactivity between TSST-1 and SEs or SPEs.
Recently, our laboratory developed a murine anti-TSST-1 MAb (MAb5; deposited in the American Type Culture Collection under accession no. HB11475) which neutralized various superantigenic activities induced by TSST-1, including T-cell proliferation, cytokine secretion, and lethality in two different animal models (17). Interestingly, MAb5 also demonstrated significant cross-reactivity with SEB by ELISA, suggesting the presence of cross-reactive epitopes on TSST-1 and SEB. In the present study, the ability of MAb5 to cross-neutralize similar functional activities induced by SEB was examined. The experimental approach included (i) assessment of the reactivity of MAb5 with TSST-1 and SEB by ELISA and Western immunoblotting; (ii) determination of the ability of MAb5 to cross-neutralize SEB-induced mitogenesis and TNF-α secretion in vitro; and (iii) characterization of the neutralizing domains recognized by MAb5 on TSST-1 and SEB by peptide mapping and competitive inhibition studies.
MATERIALS AND METHODS
Purification of TSST-1 and SEB.
Recombinant TSST-1 was purified from culture supernatants of Staphylococcus aureus RN4220 containing plasmid pBS(tst) by a combination of preparative isoelectric focusing and chromatofocusing as described previously (18, 19). SEB was purchased from Toxin Technology (Sarasota, Fla.) and further purified by chromatofocusing using a pH 6 to 9 gradient polybuffer exchanger (18). Reagents and glassware used in the purification of toxins were maintained pyrogen-free to prevent endotoxin contamination. Purity of the toxins was assessed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis on 14% acrylamide gels and silver staining.
MAb preparation.
Two murine anti-TSST-1 MAbs, MAb5 and MAb6, were previously developed in our laboratory (17). Both were of immunoglobulin G1 (IgG1) κ isotype with similar binding affinity to TSST-1, but only MAb5 neutralized TSST-1-induced superantigenic activities in vitro and in vivo (17). Therefore, MAb6 was included as an isotypic control for MAb5 in the present study. Additionally, a murine anti-human monocyte MAb with the same IgG1 κ isotype (63D3; American Type Culture Collection, Manassas, Va.) was chosen as an irrelevant isotypic MAb control for the immunoreactivity assays by ELISA (see below). Hybridomas were expanded in RPMI 1640 medium containing 15% heat-inactivated fetal bovine serum (HyClone Laboratories, Inc., Logan, Utah), and were injected intraperitoneally (5 × 106 cells in 0.5 ml of saline) into primed female BALB/c mice (8 weeks old; Charles River Biological Laboratory, Montreal, Quebec, Canada) as previously described (17). MAbs from ascitic fluids were precipitated with saturated ammonium sulfate and further purified by protein G chromatography using a MAb Trap kit (Pharmacia Fine Chemicals, Dorval, Quebec, Canada) according to the manufacturer's instructions.
Immunoreactivity of MAb5 with TSST-1 and SEB by ELISA and immunoblotting.
The relative binding affinity of each MAb that reacted with purified TSST-1 or SEB was assessed by ELISA as described by Lokker et al. (21). Toxins (10 μg/ml in 0.05 M bicarbonate-carbonate buffer [pH 9.6]) were coated onto 96-well microtiter plates overnight at room temperature. After washing with phosphate-buffered saline containing 1% Tween 20 (PBS-T), serial dilutions of MAb5 or an irrelevant isotypic control MAb in PBS-T, or PBS-T alone, were added in duplicate wells. Plates were incubated for 90 min at 37°C, washed with PBS-T, and incubated with alkaline phosphatase conjugated to goat anti-mouse IgG (1:1,000 dilution; Sigma Chemical Co., St. Louis, Mo.) for another 90 min at 37°C. Finally, wells were washed with PBS-T and incubated with 0.1 ml of p-nitrophenyl phosphate (1 mg/ml; Sigma), and the absorbance (optical density [OD] at 410 nm (OD410) was read in a Dynatech MR 5000 microplate reader (Dynatech Laboratories, Alexandria, Va.). The relative binding affinity was expressed as the reciprocal MAb concentration required to produce 50% saturation in binding to 10 μg of TSST-1. Next, the dose of each toxin for 50% inhibition of maximum binding of a constant amount of MAb (10 μg/ml) was also assessed by ELISA as described above, except that serial dilutions of each toxin were preincubated with a constant amount of MAb (10 μg/ml) before addition to the 96-well microtiter plates precoated with 10 μg of TSST-1. The relative inhibitory activity of each toxin was expressed as the concentration required to inhibit 50% maximum binding of TSST-1 to 10 μg of MAb5 per ml. Immunoblotting of toxins with MAb5 or IgG1 isotypic control was performed as previously described (18).
Inhibition of TSST-1- and SEB-induced mitogenesis by MAbs.
The ability of MAb5 and MAb6 to inhibit TSST-1- and SEB-induced mitogenesis was studied with human peripheral blood mononuclear cells (PBMC) as follows. A dose-response curve for each toxin was first determined. Fresh human PBMC were obtained from healthy adult donors and purified by Ficoll-Histopaque 1.077 (Sigma) buoyant density gradient centrifugation as previously described (32). Approximately 3 × 105 cells in 100 μl of RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum (HyClone), 2 mM l-glutamine (Gibco BRL, Burlington, Ontario, Canada), and 20 μg of polymyxin B sulfate (Sigma) per ml were cultured in 96-well round-bottom tissue culture plates (Falcon Labware; Becton-Dickinson Canada Inc., Mississauga, Ontario, Canada) and incubated with various concentrations of TSST-1 or SEB for 3 days at 37°C in 5% CO2. Cells were pulsed with 1 μCi of [3H]thymidine (5 Ci/mmol; Amersham, Arlington Heights, Ill.) and harvested 18 h later onto glass fiber filter paper by using an automatic harvester (Skatron, Sterling, Norway). All samples were in triplicates, and [3H]thymidine incorporation was quantitated in a liquid scintillation counter (LS1800; Beckman, Mississauga, Ontario, Canada). For the neutralization studies, various concentrations of MAbs in RPMI 1640 medium were preincubated with TSST-1 (45 pM) or SEB (1 pM) for 2 h at 37°C prior to the addition of cells.
Inhibition of TSST-1 and SEB-induced TNF-α secretion by MAbs.
Induction of TNF-α in human PBMC following toxin stimulation was assayed by ELISA as previously established in our laboratory (18). The sensitivity limit of the TNF-α assay was 35 pg/ml. A dose-response curve for each toxin was first determined. Approximately 3 × 105 human PBMC were cultured with various concentrations of TSST-1 or SEB in 200-μl volumes in 96-well flat-bottom tissue culture plates (Falcon Labware) for 24 h at 37°C in 5% CO2. Culture supernatants were collected, microcentrifuged at 800 × g for 5 min, and frozen at −70°C until analysis. Again, for the neutralization studies, serial dilutions of MAbs were incubated with either TSST-1 (45 pM) or SEB (10 pM) prior to the addition of cells.
Synthesis of overlapping support-coupled peptides.
To map the cross-reactive epitopes on TSST-1 and SEB, overlapping peptides were synthesized by the method of Geysen et al. (7), using a multipin peptide synthesis kit (Chiron Technologies, Clayton, Australia). All possible overlapping decapeptides homologous with the linear amino acid sequences of TSST-1 (residues 46 to 70) and SEB (residues 1 to 239) were synthesized in duplicate on prederivatized polyethylene pins, using N-α-fluorenylmethyloxycarbonyl chemistry according to the manufacturer's instructions. The peptides remained coupled to polyethylene pins after acetylation of the terminal amino groups and side chain deprotection.
Epitope mapping.
Binding of synthetic peptides to anti-TSST-1 MAb was quantitated by ELISA. Support-coupled peptides were precoated with a mixture of ovalbumin (10%, Sigma), bovine serum albumin (10%, Sigma), and PBS-T for 1 h at room temperature to block nonspecific absorption and incubated with MAb5 (1 μg/ml) overnight at 4°C. After four washes with 0.5% PBS-T, goat anti-mouse IgG-alkaline phosphatase conjugate (1:1,000 dilution; Sigma) was added for 1 h at room temperature. After further washing with PBS-T, freshly prepared p-nitrophenyl phosphate (1 mg/ml; Sigma) was added, and the absorbance (OD410) was read at 410 nm using a Dynatech MR 5000 microplate reader. Color development was stopped when the absorbance of the most highly colored enzyme substrate solution reached an OD of approximately 2.0. This precaution was taken to reduce the possibility of nonlinearity in the relationship between the absorbance and the amount of enzyme conjugate. For data analysis, the background absorbance was defined as the mean values for the entire set of peptides. The epitope was defined as the group of peptides with the highest significant mean signals that were 3 standard deviations greater than the background level. Data are presented as means ± standard errors of the means (SEM).
Inhibition of SEB-induced mitogenesis and TNF-α secretion in human PBMC by synthetic peptides.
A decapeptide homologous to the linear sequence of SEB residues 83 to 92 (83DVFGANYYYQ92), which was identified to bind to MAb5 by epitope mapping, and a scrambled control peptide (QGAYDYNYFV) were synthesized (Nucleic Acid Protein Service, University of British Columbia). These synthetic peptides were examined for the ability to inhibit SEB-induced mitogenic activity and TNF-α secretion in human PBMC. Mitogenicity assays were performed as described earlier, except that human PBMC were incubated for 3 days with increasing peptide concentrations in the absence or presence of 1 pM SEB, before pulsing with 1 μCi of [3H]thymidine. The relative inhibitory activity was expressed as the concentration of peptide required to yield 50% maximum inhibition of mitogenic activity by human PBMC induced with 1 pM SEB. The induction and release of TNF-α from human PBMC were performed as described earlier, except the human PBMC were incubated with increasing peptide concentrations in the absence or presence of 10 pM SEB. The relative inhibitory activity was expressed as the concentration of peptide required to yield 50% maximum inhibition of TNF-α secretion by human PBMC induced with 10 pM SEB.
Statistical analysis.
GraphPad PRISM version 3.0 (GraphPad Software, Inc., San Diego, Calif.) was used for data analysis. The dose-dependent effect of the MAbs or SEB peptides in inhibiting various TSST-1 or SEB induced biological activities in vitro was assessed by one-way analysis of variance (ANOVA) with repeated measures. Differences were considered significant if the two-tailed probability of a null hypothesis was less than 5% (P < 0.05).
RESULTS
Immunoreactivity of MAb5 with TSST-1 and SEB.
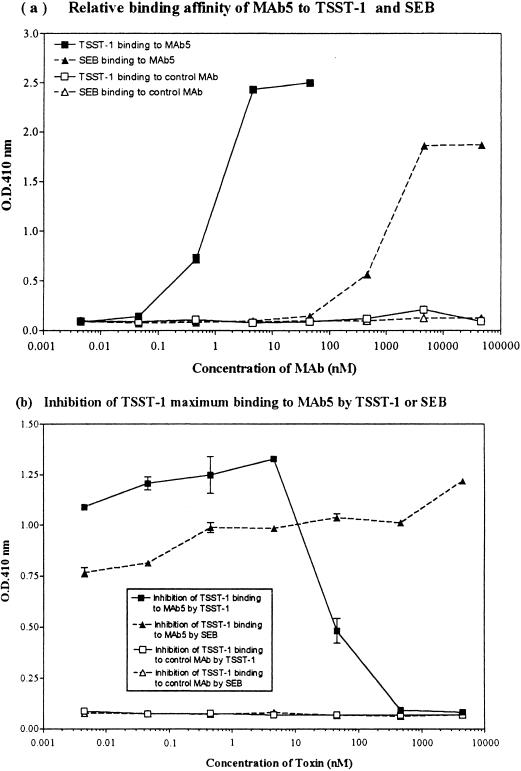
The immunoreactivity of MAb5 with TSST-1 and SEB was determined by ELISA and Western immunoblotting. MAb5 reacted strongly to TSST-1 by immunoblotting, as demonstrated by an intense 22-kDa band (data not shown). Additionally, MAb5 reacted strongly with TSST-1 by ELISA, with a relative binding affinity of 2.05 nM (Fig. 1a). MAb5 also cross-reacted with SEB by ELISA, but the relative binding affinity to SEB (0.00216 nM) was approximately 3 logs lower than that to TSST-1 (Fig. 1a). Although MAb5 reacted with SEB by ELISA, no reactivity was observed by immunoblotting. The IgG1 isotype control did not bind to either TSST-1 or SEB (Fig. 1a). The dose of TSST-1 required for 50% inhibition of maximum binding of TSST-1 to a constant amount (10 μg/ml) of MAb5 was 0.041 μM (Fig. 1b). SEB was unable to inhibit maximum TSST-1 binding to MAb5 (Fig. 1b).
FIG. 1.
(a) Relative binding affinity of MAb5 for TSST-1 and SEB by ELISA. MAb5 reacted strongly with TSST-1, with a relative binding affinity of 2.05 nM. MAb5 also reacted with SEB in a dose-dependent manner, with a relative binding affinity of 0.00216 nM (1,000-fold lower than for TSST-1). The IgG1 isotypic control MAb (63D3) did not bind to either TSST-1 or SEB. (b) Inhibition of maximum TSST-1 binding to a constant amount of MAb5 (10 μg/ml) by either TSST-1 or SEB. The dose of TSST-1 for 50% inhibition of maximum binding of TSST-1 to MAb5 was 0.041 μM. SEB was unable to inhibit TSST-1 binding to MAb5. The IgG1 isotype control did not bind to either TSST-1 or SEB.
Inhibition of TSST-1- and SEB-induced mitogenesis by MAb5.
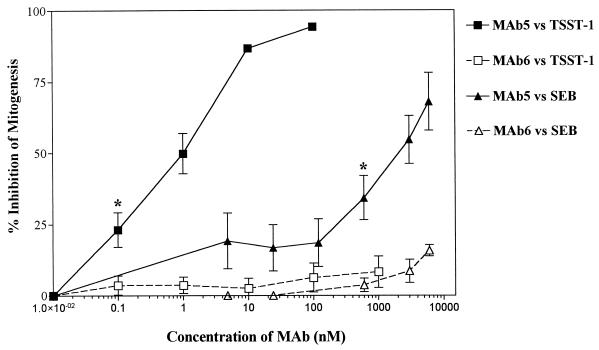
The dose-response relationship of TSST-1 and SEB in stimulating T-cell mitogenesis in human PBMC was first determined in order to choose the appropriate toxin doses for the neutralization experiments. Both TSST-1 and SEB were potent inducers for T-cell mitogenesis in human PBMC, with stimulation at doses as low as 1 and 10 fM, respectively. However, SEB induced proliferation to a greater extent, and the linear dose response occurred over a broader range (4 orders of magnitude higher) than for TSST-1 (i.e., 10 fM to 10 nM for SEB versus 1 fM to 1 pM for TSST-1). Based on these data, 45 pM TSST-1 and 1 pM of SEB were chosen for the inhibition studies. Serial dilutions of MAb5 and MAb6 were assessed for the ability to inhibit TSST-1- and SEB-induced T-cell mitogenesis in human PBMC (Fig. 2). MAb5 almost completely inhibited TSST-1-induced mitogenesis at a concentration of 100 nM. MAb5 also inhibited SEB-induced mitogenesis in a dose-dependent manner (P < 0.05), but approximately 3 logs more MAb5 was required, and only partial inhibition was achieved (63% inhibition at 6 μM). MAb6 had no effect on either TSST-1- or SEB-induced mitogenesis (Fig. 2). Neither MAb5 nor MAb6 was cytotoxic to human PBMC (as determined by trypan blue exclusion) or had intrinsic activity in inducing apoptosis (as detected by staining with 7-aminoactinomycin D) or mitogenesis of human PBMC compared to RPMI medium control, even at concentrations up to 6 μM (data not shown).
FIG. 2.
Effects of MAb5 and MAb6 on TSST-1- and SEB-induced mitogenesis in human PBMC. Data represent the mean ± SEM of triplicate measurements for four donors. ∗, lowest concentration of MAb5 which significantly inhibited TSST-1- or SEB-induced mitogenesis compared to stimulated donor cells in the absence of MAb5 (P < 0.05; one-way ANOVA, two tailed).
Inhibition of TSST-1 and SEB-induced TNF-α secretion by MAb5.
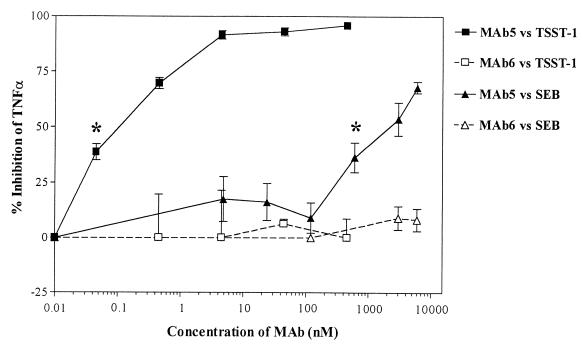
The dose-response relationship of TSST-1 and SEB on TNF-α secretion from human PBMC was first determined in order to choose the appropriate toxin doses for the neutralization experiments. TSST-1 appeared to induce much higher levels of TNF-α compared to SEB, but these data were not conclusive since different donors were used for these dose-response experiments. Based on these data, 45 pM TSST-1 and 10 pM SEB were chosen for the inhibition studies. Serial dilutions of MAb5 and MAb6 were assessed for the ability to inhibit TSST-1- and SEB-induced TNF-α production in human PBMC (Fig. 3). MAb5 almost completely inhibited TSST-1-induced TNF-α secretion at a concentration of 5 nM. MAb5 also inhibited SEB-induced TNF-α secretion in a dose-dependent manner (P < 0.05), but approximately 3 logs more MAb5 was required and only partial inhibition was achieved (70% inhibition at 5 μM). MAb6 had no effect on either TSST-1 or SEB-induced TNF-α secretion (Fig. 3). Neither MAb5 nor MAb6 was cytotoxic to human PBMC or had intrinsic activity in inducing apoptosis or TNF-α secretion from human PBMC compared to the RPMI medium control, even at concentrations of 6 μM (data not shown).
FIG. 3.
Effects of MAb5 and MAb6 on TSST-1- and SEB-induced TNF-α secretion in human PBMC. Results represent the mean ± SEM of triplicate measurements in five donors for TSST-1 and four donors for SEB. ∗, lowest concentration of MAb5 which significantly inhibited TSST-1- or SEB-induced TNF-α secretion compared to stimulated donor cells in the absence of MAb5 (P < 0.05; one-way ANOVA, two tailed).
Characterization of the TSST-1 and SEB epitopes recognized by MAb5.
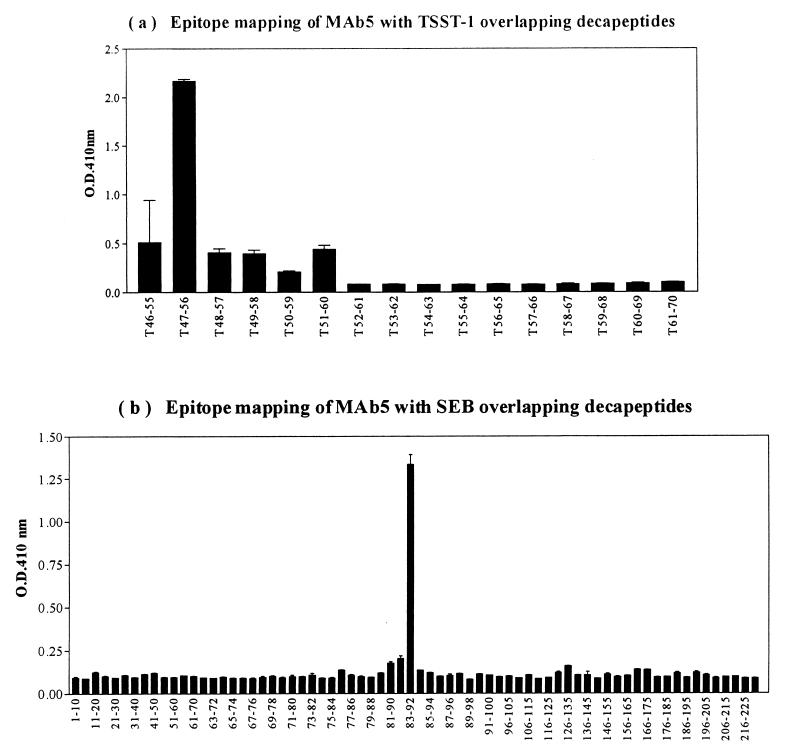
To identify the epitope on TSST-1 defined by MAb5, scanning was performed with overlapping decapeptides spanning TSST-1 residues 46 to 70, since this region was previously identified to bind to MAb5 (17). The results indicated that MAb5 reacted strongly with one single decapeptide, 47FPSPYYSPAF56 (Fig. 4a), while an isotypic IgG1 control (R&D Systems) did not react with any of the decapeptides (data not shown). To identify the antigenic epitope on SEB that cross-reacted with MAb5, peptide scanning was also performed using overlapping decapeptides spanning the entire SEB (residues 1 to 239). Only one decapeptide, 83DVFGANYYYQ92, reacted with MAb5 with a strong signal-to-background ratio (Fig. 4b), while the IgG1 isotype control did not react with any of the peptides (data not shown).
FIG. 4.
Epitope mapping of MAb5 with overlapping TSST-1 decapeptides spanning TSST-1 residues 46 to 70 (a) and overlapping SEB decapeptides spanning the entire SEB sequence (residues 1 to 239) (b).
Peptide inhibition studies.
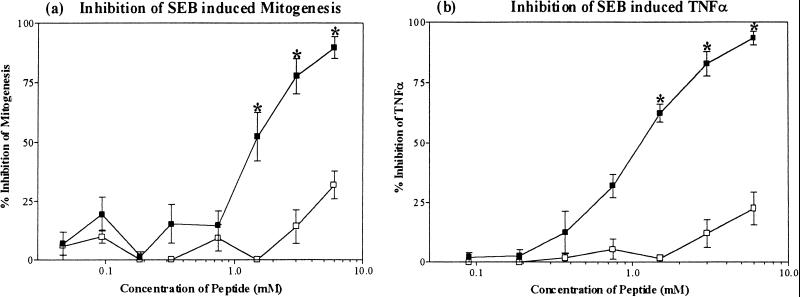
To determine whether the cross-reactive SEB epitope recognized by MAb5 has inhibitory activity, the decapeptide 83DVFGANYYYQ92 corresponding to SEB(83-92) was synthesized and tested for its ability to inhibit SEB-induced T-cell proliferation and TNF-α secretion in human PBMC. A scrambled decapeptide based on the same amino acid residues (QGAYDYNYFV) served as a negative control. The concentration of SEB used for the mitogenesis and TNF-α secretion inhibition studies (1 and 10 pM, respectively) were predetermined to be within the linear range of their respective dose-response curves as described above. Neither SEB(83-92) nor the scrambled control peptide exhibited any intrinsic mitogenic or TNF-α-secreting activity (data not shown). However, SEB(83-92) significantly inhibited both SEB-induced mitogenesis (Fig. 5a) and TNF-α production (Fig. 5b) in a dose-dependent manner, while the scrambled control peptide had no effect. However, relatively high concentrations (1.5 mM or higher) of SEB(83-92) were required to demonstrate these inhibitory activities.
FIG. 5.
Effect of decapeptide SEB(83-92) (■) and scrambled control peptide (□) on inhibition of SEB-induced T-cell mitogenesis (a), and inhibition of SEB-induced TNF-α secretion (b) in human PBMC. Data represent the mean ± SEM for duplicate measurements in three different donors. ∗, statistically significant inhibition compared to SEB alone (P < 0.05; one-way ANOVA, two tailed).
DISCUSSION
Anti-TSST-1 MAb5 was found by ELISA to cross-react with SEB, although the relative binding affinity was 1,000-fold lower than for TSST-1. Furthermore, this cross-reactivity with SEB could not be detected by immunoblotting, suggesting that the SEB determinant recognized by MAb5 was a discontinuous rather than a linear epitope and that this conformation was disrupted by denaturation during the immunoblotting procedure. An alternative explanation is that the cross-reactive epitope was inaccessible to MAb5 during Western immunoblotting, or that the immunoblot was not sensitive enough to detect this low-affinity interaction. This cross-reactivity of MAb5 with SEB was not due to nonspecific binding by IgG1, since the isotypic control MAb (63D3) had no effect (Fig. 1). Furthermore, MAb5 also neutralized SEB-induced mitogenicity and TNF-α release in a dose-dependent manner (Fig. 2 and 3). MAb5 was not cytotoxic and did not have intrinsic activity in inducing apoptosis in human PBMC. Thus, we believe this to be the first demonstration of a cross-reactive SEB epitope recognized by an anti-TSST-1 MAb.
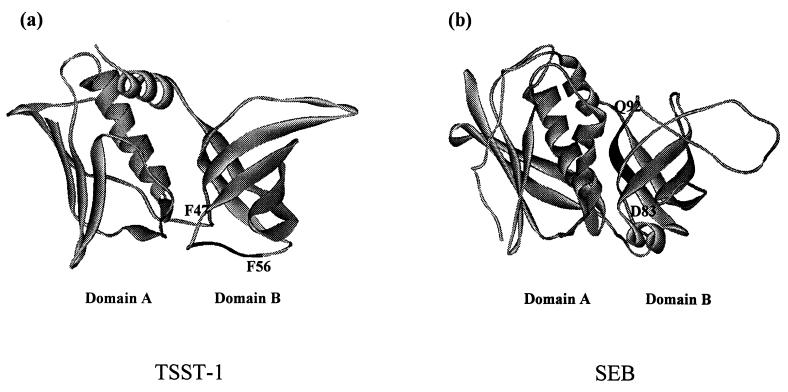
The presence of cross-reactive epitopes between TSST-1 and SEB is also supported by the recent work of Ulrich and coworkers (39). These investigators demonstrated that following immunization with SEB in BALB/c mice, high antibody titers were induced against SEB as well as the closely related SEC1 (approximately 80% sequence homology) (titer > 25,000) and that low but significant cross-reactive antibodies (titer > 1,600) were also generated against TSST-1 (<20% sequence homology) and SEA (35% sequence homology). Furthermore, these investigators observed similar but lower cross-reactive antibodies to SEC1 and TSST-1 in rhesus monkeys immunized with a site-directed SEB mutant vaccine. These data and our own results suggest that antibodies produced against one PTSAg may also recognize epitopes present in other PTSAgs even though there may be little linear sequence homology among these PTSAgs. However, the precise nature and location of these cross-reactive antigenic epitopes remain unknown, and it is uncertain whether this cross-reactivity of antisera to different PTSAgs is due to shared or similar structural motifs of these toxins. In the present study, we describe the localization and functional characterization of these cross-reactive epitopes on TSST-1 and SEB, using peptide scanning and synthetic peptide inhibition studies. These data revealed that MAb5 preferentially reacted with peptides corresponding to TSST-1(47FPSPYYSPAF56) and SEB(83DVFGANYYYQ92), respectively. These epitopes on TSST-1 and SEB are structurally dissimilar and are located within different regions of domain B of the crystal structure of these toxins (Fig. 6). Whereas TSST-1(47FPSPYYSPAF56) mapped to the β3/β4 loop at the base of domain B of TSST-1, SEB(83DVFGANYYYQ92) mapped to the β strand forming the interdomain cleft of SEB. Interestingly, we previously found TSST-1(51-56), a hexapeptide contained within TSST-1(47-56), to inhibit TSST-1-induced T-cell mitogenesis and TNF-α secretion in human PBMC (17), suggesting that the active epitope is within this hexapeptide. SEB(83-92) was demonstrated in the present study to inhibit SEB-induced T-cell mitogenesis and TNF-α secretion. Additional studies revealed that SEB(84-89) is the epitope within SEB(83-92) responsible for binding to MAb5 (data not shown). Since MAb5 inhibited both TSST-1- and SEB-induced mitogenicity and TNF-α secretion, our data suggest that both of these epitopes that react with MAb5 are functionally important for the superantigenic activity of these two toxins.
FIG. 6.
Location of decapeptide sequences recognized by MAb5 on the crystal structure of TSST-1 (a) and SEB (b).
In our study, MAb5 was 1,000-fold less active in neutralizing SEB-induced mitogenesis and TNF-α secretion in human PBMC compared to TSST-1, and only partial inhibition in these biologic activities was achieved. This relatively low inhibitory activity of MAb5 against SEB correlated with its 1,000-fold-lower relative binding affinity to SEB in ELISAs compared to TSST-1. Furthermore, SEB was unable to inhibit TSST-1 binding to MAb5 in ELISA, suggesting that the cross-reactive epitopes between TSST-1 and SEB have dissimilar structures even though both bind to MAb5. This is confirmed by our peptide mapping studies which revealed that the TSST-1 and SEB peptides recognized by MAb5 (47FPSPYYSPAF56 and 83DVFGANYYYQ92, respectively) are indeed located within different regions of these toxins and are structurally dissimilar.
The exact mechanism by which MAb5 inhibited TSST-1- and SEB-induced T-cell mitogenesis and TNF-α secretion is unclear. However, based on previous studies with other neutralizing MAbs, it appears that inhibition of PTSAg-induced activity may be accomplished through blocking the toxins from binding to either MHC class II molecules or the T-cell receptor (TCR) (8, 33). The TSST-1 epitope recognized by MAb5, 47FPSPYYSPAF56, mapped to the β3/β4 loop at the base of domain B of TSST-1 (Fig. 6a). This region was previously found by several groups (14, 35), including ourselves (17), to mediate TSST-1 binding to MHC class II molecules. Soos et al. (35) found that TSST-1 synthetic peptide T(39-68), which encompasses 47FPSPYYSPAF56, was able to displace 125I-TSST-1 binding to Raji and A20 cells. Kim et al. (14) also demonstrated by cocrystallization studies that this region resides within the TSST-1–HLA-DR1 interface. Thus, the TSST-1-inhibitory activity of MAb5 is likely mediated by blocking TSST-1 binding to MHC class II receptors within 47FPSPYYSPAF56. In contrast, the SEB epitope recognized by MAb5, 83DVFGANYYYQ92, mapped to the β strand bordering the interdomain cleft of SEB (Fig. 6b). This region has been shown by others to mediate both TCR binding via residues Y90 and Y91 and MHC class II binding via residues Y89, Q92, Y94, and S96 (20, 27, 37). Jett et al. (12) previously found that SEB-induced lymphocyte proliferation could be inhibited by the peptide SEB(61-92) but not SEB(41-70), suggesting that the region for SEB-induced T-cell proliferation involved residues within SEB(70-90). Our findings that the decapeptide SEB(83-92) inhibited SEB-induced T-cell proliferation confirmed this prediction. Although we did not evaluate the ability of SEB(83-92) to block SEB binding to MHC class II molecules, similar experiments by Komisar et al. (15) did demonstrate that the synthetic peptide SEB(90-114) was highly efficient in inhibiting SEB binding to HLA-DR1. From these data taken together, it seems plausible that MAb5 inhibited SEB-induced superantigenic activity by blocking both SEB binding to the TCR as well as MHC class II molecules via regions within or adjacent to SEB(83-92), either directly or through steric hindrance.
In summary, we identified the regions TSST-1(47-56) and SEB(83-92) as the cross-reactive epitopes recognized by MAb5. Since MAb5 can cross-neutralize both TSST-1- and SEB-induced T-cell proliferation and TNF-α secretion, these MAb5 binding sites are worth further detailed investigation. If these binding sites are found to be functionally important epitopes, they could provide useful tools for the creation of polyvalent vaccines or receptor inhibitors effective for the prevention or treatment of both TSST-1- and SEB-associated diseases.
ACKNOWLEDGMENTS
This work was supported in part by grant MT-7630 from the Medical Research Council of Canada to A.W.C.
We thank Donna Hogge and the staff of the Vancouver Hospital Cell Separator Unit for providing human PBMC from different healthy adult donors, and we thank Herman Ziltner and Lawrence McIntosh for critical review of this study.
REFERENCES
- 1.Betley M J, Borst D W, Regassa L B. Staphylococcal enterotoxins, toxic shock syndrome toxin and streptococcal pyrogenic exotoxins: a comparative study of their molecular biology. Chem Immunol. 1992;55:1–35. [PubMed] [Google Scholar]
- 2.Bohach G A, Fast D J, Nelson R D, Schlievert P M. Staphylococcal and streptococcal pyrogenic toxins involved in toxic shock syndrome and related illness. Crit Rev Microbiol. 1990;17:251–272. doi: 10.3109/10408419009105728. [DOI] [PubMed] [Google Scholar]
- 3.Bohach G A, Hovde C J, Handley J P, Schlievert P M. Cross-neutralization of staphylococcal and streptococcal pyrogenic toxins by monoclonal and polyclonal antibodies. Infect Immun. 1988;56:400–404. doi: 10.1128/iai.56.2.400-404.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borja C R, Bergdoll M S. Purification and partial characterization of enterotoxin C produced by Staphylococcus aureus strain 137. Biochemistry. 1967;6:1467–1473. doi: 10.1021/bi00857a032. [DOI] [PubMed] [Google Scholar]
- 5.Davies H D, McGeer A, Schwartz B, Green K, Cann D, Simor A E, Low D E. Invasive group A streptococcal infections in Ontario, Canada. Ontario group A streptococcal study group. N Engl J Med. 1996;335:547–554. doi: 10.1056/NEJM199608223350803. [DOI] [PubMed] [Google Scholar]
- 6.Fleischer B. Superantigens. APMIS. 1994;102:3–12. doi: 10.1111/j.1699-0463.1994.tb04839.x. (Review). [DOI] [PubMed] [Google Scholar]
- 7.Geysen H M, Rodda S J, Mason T J, Tribbick G, Schoofs P G. Strategies for epitope analysis using peptide synthesis. J Immunol Methods. 1987;102:259–274. doi: 10.1016/0022-1759(87)90085-8. [DOI] [PubMed] [Google Scholar]
- 8.Hamad A R A, Herman A, Marrack P, Kappler J W. Monoclonal antibodies defining functional sites on the toxin superantigen staphylococcal enterotoxin B. J Exp Med. 1994;180:615–621. doi: 10.1084/jem.180.2.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Herman A, Kappler J W, Marrack P, Pullen A M. Superantigens: mechanism of T cell stimulation and role in immune responses. Annu Rev Immunol. 1991;9:745–772. doi: 10.1146/annurev.iy.09.040191.003525. [DOI] [PubMed] [Google Scholar]
- 10.Hynes W L, Weeks C R, Iandolo J J, Ferretti J J. Immunologic cross-reactivity of type A streptococcal exotoxin (erythrogenic toxin) and staphylococcal enterotoxins B and C1. Infect Immun. 1987;55:837–838. doi: 10.1128/iai.55.3.837-838.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jardetzky T S, Brown J H, Gorga J C, Stern L J, Urban R G, Chi Y I, Stauffacher C, Strominger J L, Wiley D C. Three-dimensional structure of a human class II histocompatibility molecule complexed with superantigen. Nature. 1994;368:711–718. doi: 10.1038/368711a0. [DOI] [PubMed] [Google Scholar]
- 12.Jett M, Neill R, Welch C, Boyle T, Bernton E, Hoover D, Lowell G, Hunt R E, Chatterjee S, Gemski P. Identification of staphylococcal enterotoxin B sequences important for induction of lymphocyte proliferation by using synthetic peptide fragments if the toxin. Infect Immun. 1994;62:3408–3415. doi: 10.1128/iai.62.8.3408-3415.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kain K C, Schulzer M, Chow A W. Clinical spectrum of nonmenstrual toxic shock syndrome (TSS): comparison with menstrual TSS by multivariate discriminant analyses. Clin Infect Dis. 1993;16:100–106. doi: 10.1093/clinids/16.1.100. [DOI] [PubMed] [Google Scholar]
- 14.Kim J, Urban R G, Strominger J L, Wiley D C. Toxic shock syndrome toxin-1 complexed with a class II major histocompatibility molecule HLA-DR 1. Science. 1994;266:1870–1878. doi: 10.1126/science.7997880. [DOI] [PubMed] [Google Scholar]
- 15.Komisar J L, Small-Harris S, Tseng J. Localization of binding sites of staphylococcal enterotoxin B (SEB), a superantigen, for HLA-DR by inhibition with synthetic peptides of SEB. Infect Immun. 1994;62:4775–4780. doi: 10.1128/iai.62.11.4775-4780.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kotzin B L, Leung D Y M, Kappler J, Marrack P. Superantigens and their potential role in human disease. Adv Immunol. 1993;54:99–166. doi: 10.1016/s0065-2776(08)60534-9. [DOI] [PubMed] [Google Scholar]
- 17.Kum W W S, Laupland K B, Chow A W. Defining a novel domain of staphylococcal toxic shock syndrome toxin-1 critical for major histocompatibility complex class II binding, superantigenic activity, and lethality. Can J Microbiol. 2000;46:171–179. [PubMed] [Google Scholar]
- 18.Kum W W S, Laupland K B, See R H, Chow A W. Improved purification and biologic activities of staphylococcal toxic shock syndrome toxin 1. J Clin Microbiol. 1993;31:2654–2660. doi: 10.1128/jcm.31.10.2654-2660.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kum W W S, Wood J A O, Chow A W. A mutation at glycine residue 31 of toxic shock syndrome toxin-1 defines a functional site critical for major histocompatibility complex class II binding and superantigenic activity. J Infect Dis. 1996;174:1261–1270. doi: 10.1093/infdis/174.6.1261. [DOI] [PubMed] [Google Scholar]
- 20.Li H, Llera A, Tsuchiya D, Leder L, Ysern X, Schlievert P M, Karjalainen K, Mariuzza R A. Three dimensional structure of the complex between a T cell receptor beta chain and the superantigen staphylococcal enterotoxin B. Immunity. 1998;9:807–816. doi: 10.1016/s1074-7613(00)80646-9. [DOI] [PubMed] [Google Scholar]
- 21.Lokker N A, Strittmatter U, Steiner C, Fagg B, Graff P, Kocher H P, Zenke G. Mapping the epitopes of neutralizing anti-human IL-3 monoclonal antibodies. Implications for structure-activity relationship. J Immunol. 1991;146:893–898. [PubMed] [Google Scholar]
- 22.Marrack P, Kappler J. The staphylococcal enterotoxins and their relatives. Science. 1990;248:705–711. doi: 10.1126/science.2185544. [DOI] [PubMed] [Google Scholar]
- 23.Meyer R F, MacNeill E M, Bennett R W, MacMillan J D. Development of a monoclonal antibody capable of interacting with five serotypes of Staphylococcus aureus enterotoxin. Appl Environ Microbiol. 1984;47:283–287. doi: 10.1128/aem.47.2.283-287.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miethke T, Duschek K, Wahl C, Heeg K, Wagner H. Pathogenesis of the toxic shock syndrome: T cell mediated lethal shock caused by the superantigen TSST-1. Eur J Immunol. 1993;23:1494–1500. doi: 10.1002/eji.1830230715. [DOI] [PubMed] [Google Scholar]
- 25.Minor T E, Marth E H. Staphylococcus aureus and staphylococcal food intoxications. A review. II. Enterotoxins and epidemiology. J Milk Food Technol. 1971;35:21–29. [Google Scholar]
- 26.Papageorgiou A C, Brehm R D, Leonidas D D, Tranter H S, Acharya K R. The refined crystal structure of toxic shock syndrome toxin-1 at 2.07 Å resolution. J Mol Biol. 1996;160:553–569. doi: 10.1006/jmbi.1996.0421. [DOI] [PubMed] [Google Scholar]
- 27.Papageorgiou A C, Tranter H S, Acharya K R. Crystal structure of microbial superantigen staphylococcal enterotoxin B at 1.5Å resolution: implications for superantigen recognition by MHC class II molecules and T-cell receptors. J Mol Biol. 1998;277:61–79. doi: 10.1006/jmbi.1997.1577. [DOI] [PubMed] [Google Scholar]
- 28.Parsonnet J. Mediators in the pathogenesis of TSS: overview. Rev Infect Dis. 1989;11(Suppl. 1):S263–S269. doi: 10.1093/clinids/11.supplement_1.s263. [DOI] [PubMed] [Google Scholar]
- 29.Reiser R F, Robbins R N, Noleto A L, Khoe G P, Bergdoll M S. Identification, purification, and some physicochemical properties of staphylococcal enterotoxin C3. Infect Immun. 1984;45:625–630. doi: 10.1128/iai.45.3.625-630.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schad E M, Papageorgiou A C, Svensson L A, Acharya K R. A structural and functional comparison of staphylococcal enterotoxins A and C2 reveals remarkable similarity and dissimilarity. J Mol Biol. 1997;269:270–280. doi: 10.1006/jmbi.1997.1023. [DOI] [PubMed] [Google Scholar]
- 31.Schlievert P M, Bohach G A, Ohlendorf D H, Stauffacher C V, Leung D Y, Murray D L, Prasad G S, Earhart C A, Jablonski L M, Hoffmann M L, Chi Y I. Molecular structure of staphylococcus and streptococcus superantigens. J Clin Immunol. 1995;15(Suppl.):4S–10S. doi: 10.1007/BF01540887. [DOI] [PubMed] [Google Scholar]
- 32.See R H, Chow A W. Staphylococcal toxic shock syndrome toxin 1-induced tumor necrosis factor alpha and interleukin-1b secretion by human peripheral blood monocytes and T lymphocytes is differentially suppressed by protein kinase inhibitors. Infect Immun. 1992;60:3456–3459. doi: 10.1128/iai.60.8.3456-3459.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shimonkevitz R, Boen E, Malmstrom S, Brown E, Hurley J M, Kotzin B L, Matsumura M. Delineation by use of specific monoclonal antibodies of the T-cell receptor and major histocompatibility complex interaction sites on the superantigen toxic shock syndrome toxin 1. Infect Immun. 1996;64:1133–1139. doi: 10.1128/iai.64.4.1133-1139.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shinagawa K, Kanazawa T, Matsusaka N, Sugii S, Nagata S. Murine monoclonal antibodies reactive with staphylococcal enterotoxins A, B, C2, D, E. FEMS Microbiol Lett. 1991;64:35–39. doi: 10.1016/0378-1097(91)90205-o. [DOI] [PubMed] [Google Scholar]
- 35.Soos J M, Russell J K, Jarpe M A, Pontzer C H, Johnson H M. Identification of binding domains on the superantigen, toxic shock syndrome-1, for class II MHC molecules. Biochem Biophys Res Commun. 1993;191:1211–1217. doi: 10.1006/bbrc.1993.1346. [DOI] [PubMed] [Google Scholar]
- 36.Sundstrom M, Abrahmsen L, Antonsson P, Mehindate K, Mourad W, Dohlsten M. The crystal structure of staphylococcal enterotoxin type D reveals Zn2+-mediated homodimerization. EMBO J. 1996;15:6832–6840. [PMC free article] [PubMed] [Google Scholar]
- 37.Swaminathan S, Furey W, Pletcher J, Sax M. Crystal structure of staphylococcal enterotoxin B, a superantigen. Nature. 1992;359:801–806. doi: 10.1038/359801a0. [DOI] [PubMed] [Google Scholar]
- 38.Tranter H S, Brehm R D, Acharya K R. Molecular topology is important for the function of staphylococcal superantigens. In: Thibodeau J, Sekaly R, editors. Bacterial superantigens: structure, function and therapeutic potential. Heidelberg, Germany: Springer-Verlag; 1995. pp. 5–23. [Google Scholar]
- 39.Ulrich R G, Olson M A, Bavari S. Development of engineered vaccines effective against structurally related bacterial superantigens. Vaccine. 1998;16:1857–1864. doi: 10.1016/s0264-410x(98)00176-5. [DOI] [PubMed] [Google Scholar]