Abstract
Purpose
Epidemiologic evidence regarding the role of dietary vitamin A in the development of diabetes is limited and inconsistent. This study was to explore the association between vitamin A intake and diabetes risk in Chinese adults.
Methods
A prospective cohort study was conducted among 17 111 adults (8537 men and 8577 women) who participated in the China Health and Nutrition Survey between 1989 and 2015. Dietary intakes were assessed by 3 consecutive 24-hour dietary recalls combined with a household food inventory. Diabetes was determined by self-reported diagnosis, diabetes medication use, or additional criterion in 2009 of fasting blood glucose or hemoglobin A1c. We analyzed the association of vitamin A intake (total, β-carotene, retinol) with diabetes risk using Cox proportional hazards models.
Results
A total of 519 men and 531 women developed diabetes during a median of 11 years of follow-up. Higher dietary total vitamin A intakes were associated with a lower risk of diabetes in both men (quintile 5 [Q5] vs Q1: hazard ratio [HR] = 0.69, 95% CI, 0.49-0.97, P-trend = 0.079) and women (Q5 vs Q1: HR = 0.63; 95% CI, 0.45-0.89; P-trend = 0.039). An inverse relation with diabetes risk was observed for dietary intakes of β-carotene (Q5 vs Q1: HR = 0.71; 95% CI, 0.52-0.97) and retinol (Q5 vs Q1: HR = 0.58; 95% CI, 0.39-0.85) among men, but not women. Dose-response analyses showed the association of dietary intakes of total vitamin A, β-carotene, and retinol with diabetes risk in men was L-shaped (P-nonlinearity = 0.043), reverse J-shaped (P-nonlinearity = 0.001), and linear, respectively.
Conclusion
Our findings suggest that adequate intake of vitamin A may help protecting against diabetes, especially for men.
Keywords: risk of diabetes, dietary intake, vitamin A, β-carotene, retinol, prospective cohort study
Vitamin A is an indispensable micronutrient necessary for normal physiologic function, including maintenance of vision, reproduction, immune function and skin health, and involvement in cell differentiation and fetal development. Vitamin A in the diet comprises both preformed retinoids (predominantly retinol and retinyl esters) from animal sources (eg, meat, liver, eggs) and provitamin A carotenoids from plants (eg, fresh vegetables and fruits). β-carotene, the primary form of dietary provitamin-A carotenoids (1), can be converted to retinol and further metabolized to retinaldehyde and retinoic acid (RA) (Supplementary Fig. 1) (2). Emerging evidence indicates a role of vitamin A in the prevention and causation of metabolic disorders (3).
Diabetes, overwhelmingly type 2 diabetes, is a major health concern that has reached pandemic proportions, with a global prevalence of 10.5% among adults aged 20 to 79 years in 2021 according to the latest International Diabetes Federation Diabetes Atlas (4). Accumulating evidence suggests that vitamin A is involved in regulating pancreas development, β-cell functions, insulin secretion, hepatic glucose metabolism, and pancreatic stellate cells phenotypes (5), which are physiological processes associated with the development and progression of diabetes. However, previous observational studies have mainly focused on the association of circulating levels and dietary intake of provitamin A carotenoids with diabetes risk and reported contradictory findings (6). For instance, some studies have indicated higher intakes or blood levels of β-carotene with a lower risk of diabetes (7, 8), whereas others showed null association (9-12). Of note, most of these studies were conducted in American and European populations, whose diet is characterized by higher consumption of animal products and refined grains in conjunction with lower consumption of vegetables and fruits. Eastern populations, however, consume mainly plant-based diets. For example, the Chinese diets consists of large amounts of cereals, vegetables, and fruits, but small amounts of animal-based foods, which provide only 10% to 15% of total energy intake and 20% to 30% of total protein intake (13, 14). Thus, the findings from Western countries may not apply to Eastern countries, where dietary intake and food sources of vitamin A are different because of the differences in dietary patterns. In addition, evidence linking retinol to diabetes is scarce and has also produced inconsistent results, with some suggesting no association with diabetes risk, whereas others suggesting that retinol was a risk factor for diabetes (15). To our knowledge, merely 1 prospective cohort study assessed the relation between total vitamin A intake and 5-year risk of diabetes in 19 168 healthy Japanese adults to date (16). Nevertheless, this study was limited by a relatively short follow-up period, a single-time assessment of dietary intake, and not distinguishing between vitamin A subtypes (eg, retinol, β-carotene). Notably, compared with Chinese, Japanese consumed more retinol but less β-carotene. Therefore, few studies have focused on the influence of vitamin A intake mainly derived from dietary β-carotene on diabetes development.
Against this background, the current study aimed to comprehensively examine the long-term association between dietary total vitamin A, β-carotene, retinol intake and diabetes risk in a large prospective cohort in China over a 26-year study period.
Research Design and Methods
Study Population
Data of the current study were from the China Health and Nutrition Survey (CHNS). Established in 1989, CHNS is an ongoing open longitudinal study in China, approved by institutional review boards at the University of North Carolina, Chapel Hill (Chapel Hill, NC), and the National Institute of Nutrition and Food Safety, China Center for Disease Control and Prevention, and each participant provided written informed consent. Until 2015, a total of 10 rounds of surveys have already been completed. The survey design and methods have been described in detail elsewhere (17).
CHNS included 38 536 participants from 1989 to 2015. The current study only included adult participants aged ≥ 18 years, who were free from diabetes at baseline and were followed up at least once. We excluded participants with missing or implausible energy or vitamin A intake (in the top and bottom 1% of the intakes), as well as those who were pregnant, nursing, disabled, and excessively obese (body mass index [BMI] > 40 kg/m2). Finally, a total of 17 111 participants were included (Supplementary Fig. 2) (2).
Assessment of Dietary Intake
Dietary intake data were assessed using 3 consecutive 24-hour dietary recalls at the individual level in combination with a weighing inventory over the same 3 days at the household level. The weighing and measuring of products were used to obtain information on edible oils and condiments consumption over the same 3 days (2 weekdays and 1 weekend day). All investigators were trained nutritionists and underwent specialized training before the nutrition work in this survey. The accuracy of 24-hour dietary recall designed to assess energy and nutrient intake has been validated (18). Frequency of dietary recall examination in men and women ranged between 1 and 9 (median (interquartile range): 3 (1, 6) vs 3 (1, 6) times for men vs women, P = 0.779; Supplementary Fig. 3) (2).
Energy and nutrient intake were estimated by multiplying the consumed amount of each food item by the nutrient content of standard portion size (100 g) based on the Chinese Food Composition Tables (19-21), and then the intakes for all food items were summed. The vitamin A equivalent is expressed as retinol activity equivalent (RAE) and calculated by the following formula: vitamin A (μg RAE) = retinol (μg) + β-carotene (μg)/12 + other provitamin A carotenoids (μg)/24, accounting for the bioavailability of the different forms (22). Because total carotene in plant foods was measured by paper chromatography without distinguishing β-carotene from other carotenes, total vitamin A intake was calculated as the sum of retinol (μg) and total carotene/β-carotene (μg)/12 in the current study. After log-transformation, the intake of each nutrient or food group was adjusted for total energy (TE) intake for men (mean 2449 kcal/d) and women (mean 2035 kcal/d) using the residual method, separately (23). In the analyses, cumulative average intake values of each nutrient from baseline to the survey before outcome identified were used to reduce within-subject variation and to best represent long-term dietary intake.
In addition, we calculated the Chinese Healthy Eating Index, a tool to assess the overall adherence to the Dietary Guidelines for Chinese residents (2016) (24). The higher the score, the better the diet quality.
Measurement of Nondietary Risk Factors for Diabetes
Information about demographics and lifestyles was obtained through structured questionnaires, including age, residential area (urban, rural), education level (low [primary school and below], middle [middle school, high school, and technical or vocational school], high [college, postgraduate degree and above]), smoking status (former or current, never), and current alcohol consumption (yes, no). Body weight and height were measured by well-trained investigators following standard measurement procedures (25). BMI was calculated by dividing weight in kilograms by height in meters squared. Physical activity level (PAL) was quantified as multiples of basal metabolic rate (BMR) according to the Chinese Dietary Reference Intakes (2000 edition) (26): 1.3 × BMR for very light (for both sexes), 1.6 and 1.5 × BMR for light, 1.7 and 1.6 × BMR for medium, 2.1 and 1.9 × BMR for heavy, and 2.4 and 2.2 × BMR for very heavy in men and women, respectively. Per capita annual household income was divided into quartiles (low, medium, high, very high). For all nondietary covariates, we used the measures at baseline.
Outcome Identification
The participants had been asked to report their previous history of diabetes with a questionnaire-based interview at each follow-up since 1989. Diabetes was defined as a self-reported diagnosis of diabetes by a specialist or use of any of the diabetes treatments, such as special diet, weight control, oral medicine, injection of insulin, Chinese traditional medicine, home remedies, or qigong (or spiritual treatment). In addition, blood samples were collected and assayed, but the data were available only in 2009. Therefore, an additional criterion (ie, fasting blood glucose ≥ 7.0 mmol/L or hemoglobin A1c ≥ 6.5% [48 mmol/mol]) was added for the diagnosis of diabetes in 2009. Information on incident diabetes before 1997 was indirectly derived from answers to subsequent questionnaires returned by the same individuals. If there were multiple or inconsistent records regarding incident diabetes, we retained only the first record to minimize recall bias.
Statistical Analysis
We performed all the analyses separately for men and women and divided male and female participants into 5 groups according to quintiles (Q) of dietary vitamin A intake. Participants’ characteristics were given as mean and standard or median and interquartile range for continuous variables and numbers and proportions for categorical variables, respectively. To test for the linear trend across quintiles, linear regression was used for continuous variables (with the median intake of each quintile as the variable included in the model) and χ 2 with linear-by-linear association test was used for categorical variables.
The follow-up person-time for each participant was calculated from the first entry into the survey to the first diabetes diagnosis, the last survey round before the participant departed from the survey, or the end of the latest survey (2015), whichever came first. We calculated the incidence of diabetes by dividing the number of new diabetes cases by follow-up person-years in each quintile. Cox proportional hazards regression models were used to assess the association between dietary total vitamin A, β-carotene, and retinol intake and diabetes risk. For the calculation of hazard ratios (HRs) and corresponding 95% CIs across quintiles, we took the lowest intake quintiles as reference. Models were adjusted for predefined covariates. Model 1 was adjusted for age, BMI, and dietary intake of TE. Model 2 was adjusted for the variables in model 1 plus other nondietary factors, including residence area, highest education level, household income level, PAL, smoking status, alcohol consumption, and history of hypertension at baseline. Model 3 was adjusted for the variables in model 2 plus dietary intake of carbohydrates, protein, the ratio of monounsaturated fat-to-saturated fat (SFA) intake, the ratio of polyunsaturated fat-to-saturated fat intake, cholesterol, zinc, dietary fiber, vegetables, fruits, and eggs. Tests for linear trend of HRs were conducted with the median value for each quintile of intake as a continuous variable. We also used restricted cubic splines (RCS) to test for nonlinearity of the dose-response association between dietary total vitamin A, β-carotene, retinol intake, and diabetes risk in the multivariable-adjusted Cox regression analyses (model 3). Multiple imputations were performed for missing values and the results showed no significant difference before and after the imputation.
All P values were 2-sided, and P < 0.05 was considered statistically significant. All analyses were conducted using SPSS software version 26.0 (SPSS Inc., Chicago, IL) and R version 4.1.2.
Results
Study Participants and Baseline Characteristics
A total of 17 111 participants (8534 men and 8577 women) with complete dietary data and without diabetes at baseline were included in the present analysis. The average age of men and women was 39.1 ± 15.7 years and 41.0 ± 15.4 years at baseline, respectively. The median intake of total vitamin A, β-carotene, and retinol was 259 (173, 390) μg RAE/d, 1708 (977, 2738) μg/d, and 95 (49, 167) μg/d among men, and 240 (158, 357) μg RAE/d, 1591 (900, 2565) μg/d, and 85 (44, 150) μg/d among women, respectively. At baseline, men and women with higher total vitamin A intake were older, had lower BMI and PAL, higher education and household income levels, and were more likely to be urban residents (Table 1). Additionally, women with higher total vitamin A intake were less likely to be former or current smokers but more likely to be current alcohol consumers. In both men and women, the intakes of almost all the nutrients increased with quintiles of total vitamin A intake, except that the intakes of carbohydrates and dietary fiber decreased. There was no linear trend in dietary intake of TE in both sexes, dietary intake of polyunsaturated fat in women and intake of dietary fiber in men across total vitamin A quintile.
Table 1.
Characteristics of baseline demographic and lifestyle factors and cumulative average food and nutrient intake according to quintiles of dietary intake of vitamin A in men (n = 8534) and women (n = 8577)a
| Quintiles of vitamin A intake (μg RAE)b in men | P-trendc | Quintiles of vitamin A intake (μg RAE)b in women | P-trendc | |||||
|---|---|---|---|---|---|---|---|---|
| Q1 (n = 1707) | Q3 (n = 1707) | Q5 (n = 1706) | Q1 (n = 1715) | Q3 (n = 1714) | Q5 (n = 1715) | |||
| Age (y) | 37.6 ± 15.8 | 39.5 ± 15.8 | 39.4 ± 15.8 | 0.004 | 40.0 ± 15.9 | 40.9 ± 15.1 | 41.6 ± 15.5 | <0.001 |
| BMI (kg/m2) | 22.3 ± 3.2 | 22.4 ± 3.2 | 22.1 ± 3.1 | 0.046 | 22.6 ± 3.4 | 22.4 ± 3.3 | 22.5 ± 3.3 | 0.115 |
| PAL (× BMR) | 1.84 ± 0.29 | 1.75 ± 0.31 | 1.70 ± 0.30 | <0.001 | 1.66 ± 0.23 | 1.60 ± 0.23 | 1.57 ± 0.23 | <0.001 |
| Education level | <0.001 | <0.001 | ||||||
| Low | 708 (41.5) | 596 (34.9) | 536 (31.4) | 960 (56.0) | 846 (49.4) | 708 (41.3) | ||
| Middle | 926 (54.2) | 965 (56.5) | 1014 (59.4) | 705 (41.1) | 751 (43.8) | 871 (50.8) | ||
| High | 73 (4.3) | 146 (8.6) | 156 (9.1) | 50 (2.9) | 117 (6.8) | 136 (7.9) | ||
| Household income level | <0.001 | <0.001 | ||||||
| Low | 693 (40.6) | 481 (28.2) | 353 (20.7) | 682 (39.8) | 502 (29.3) | 313 (18.3) | ||
| Medium | 438 (25.7) | 452 (26.5) | 485 (28.4) | 448 (26.1) | 447 (26.1) | 453 (26.4) | ||
| High | 309 (18.1) | 395 (23.1) | 435 (25.5) | 320 (18.7) | 378 (22.1) | 469 (27.3) | ||
| Very high | 267 (15.6) | 379 (22.2) | 433 (25.4) | 265 (15.5) | 387 (22.6) | 480 (28.0) | ||
| Urban residence area | 406 (23.8) | 638 (37.4) | 816 (47.8) | <0.001 | 457 (26.6) | 682 (39.8) | 867 (50.6) | <0.001 |
| Former or current smoker | 1191 (69.8) | 1175 (68.8) | 1188 (69.6) | 0.706 | 172 (10.0) | 131 (7.6) | 101 (5.9) | <0.001 |
| Alcohol consumer | 961 (56.3) | 966 (56.6) | 966 (56.6) | 0.747 | 184 (10.7) | 263 (15.3) | 293 (17.1) | <0.001 |
| History of hypertension | 327 (19.2) | 320 (18.7) | 293 (17.2) | 0.205 | 287 (16.7) | 253 (14.8) | 245 (14.3) | 0.070 |
| Cumulative average dietary intakes | ||||||||
| TE (kcal/d) | 2464 ± 621 | 2445 ± 568 | 2439 ± 547 | 0.118 | 2028 ± 537 | 2041 ± 466 | 2020 ± 500 | 0.471 |
| Carbohydrates (%TE) | 64.1 ± 10.2 | 57.9 ± 9.8 | 54.4 ± 10.3 | <0.001 | 64.0 ± 10.0 | 58.2 ± 9.4 | 54.6 ± 10.1 | <0.001 |
| Protein (%TE) | 11.5 ± 1.8 | 12.2 ± 2.1 | 13.1 ± 2.6 | <0.001 | 11.6 ± 1.8 | 12.4 ± 2.1 | 13.6 ± 2.8 | <0.001 |
| SFA (%TE) | 5.1 ± 2.7 | 6.9 ± 2.7 | 7.9 ± 2.9 | <0.001 | 5.3 ± 2.7 | 7.0 ± 2.7 | 8.0 ± 2.9 | <0.001 |
| PUFA (%TE) | 6.8 ± 3.8 | 6.8 ± 3.7 | 6.7 ± 3.6 | 0.016 | 7.1 ± 3.9 | 7.2 ± 3.7 | 7.0 ± 3.7 | 0.448 |
| MUFA (%TE) | 9.2 ± 5.1 | 12.3 ± 4.6 | 13.9 ± 4.9 | <0.001 | 9.5 ± 5.0 | 12.5 ± 4.5 | 14.1 ± 5.0 | <0.001 |
| PUFA/SFA | 1.5 ± 0.8 | 1.1 ± 0.6 | 0.9 ± 0.5 | <0.001 | 1.5 ± 0.7 | 1.1 ± 0.6 | 0.9 ± 0.5 | <0.001 |
| MUFA/SFA | 1.8 ± 0.6 | 1.9 ± 0.5 | 1.8 ± 0.4 | 0.017 | 1.8 ± 0.6 | 1.9 ± 0.5 | 1.8 ± 0.5 | 0.236 |
| Vitamin A (μg RAE/d) | 113 (85, 135) | 259 (241, 280) | 586(491, 771) | <0.001 | 105 (79, 125) | 240 (222, 258) | 524 (445, 691) | <0.001 |
| β-carotene (μg/d) | 729 (482, 1027) | 1921 (1375, 2408) | 3441 (2250, 4872) | <0.001 | 678 (447, 955) | 1772 (1253, 2255) | 3311 (2115, 4559) | <0.001 |
| Retinol (μg/d) | 41 (19, 65) | 98 (62, 145) | 282 (137, 501) | <0.001 | 36 (17, 59) | 91 (53, 135) | 235 (116, 415) | <0.001 |
| Zinc (mg/d) | 11.20 ± 1.79 | 11.94 ± 2.08 | 12.93 ± 2.18 | <0.001 | 9.43 ± 1.63 | 10.12 ± 1.83 | 10.98 ± 1.87 | <0.001 |
| Dietary fiber (g/d) | 10.0 (7.9,13.7) | 10.1 (8.0,13.4) | 10.8 (8.4,13.6) | 0.805 | 13.0 (9.3,16.2) | 11.6 (9.1,15.1) | 10.8 (8.5,14.0) | <0.001 |
| Cholesterol (mg/d) | 106.63 (51.98, 175.25) | 238.77 (150.22, 353.12) | 291.31 (194.50, 429.94) | <0.001 | 97.34 (46.62, 157.12) | 223.02 (136.56, 315.45) | 269.09 (167.52, 400.69) | <0.001 |
| Vegetables (g/d) | 283.3 ± 164.3 | 337.3 ± 183.1 | 372.2 ± 215.2 | <0.001 | 256.9 ± 205.0 | 306.4 ± 210.7 | 341.9 ± 197.0 | <0.001 |
| Fruits (g/d) | 0.00 (0.00, 11.06) | 0.00 (0.00, 30.62) | 3.62 (0.00, 41.12) | <0.001 | 0.00 (0.00, 21.09) | 7.71 (0.00, 45.28) | 13.53 (0.00, 57.85) | <0.001 |
| Eggs (g/d) | 8.17 (0.00, 17.65) | 20.95 (7.88, 38.45) | 19.27 (6.77, 40.41) | <0.001 | 7.87 (0.00, 17.07) | 20.70 (8.73, 35.74) | 19.52 (5.69, 39.85) | <0.001 |
| CHEI | 45.90 (43.15, 48.99) | 47.11 (43.83, 50.94) | 47.10 (43.66, 51.38) | <0.001 | 46.02 (43.19, 49.26) | 47.02 (43.58, 50.89) | 47.22 (43.74, 52.34) | <0.001 |
Data are presented as mean ± SD or n (%).
Abbreviations: CHEI: Chinese Healthy Eating Index; MUFA, monounsaturated fat; PAL, physical activity level; PUFA, polyunsaturated fat; SFA, saturated fat; TE, total energy.
a Information of nondietary factors was collected at baseline, and dietary data were estimated as energy-adjusted cumulative average intake from baseline and follow-up periods.
b Quintiles are based on energy-adjusted vitamin A intake (μg RAE), using the residual method.
c We used linear regressions to test the linear trends for continuous variables (with the median intake of vitamin A as continuous variables included in the regression models). We used χ 2 with linear-by-linear association tests to test the linear trends for categorical variables.
Association Between Dietary Total Vitamin A Intake and Diabetes
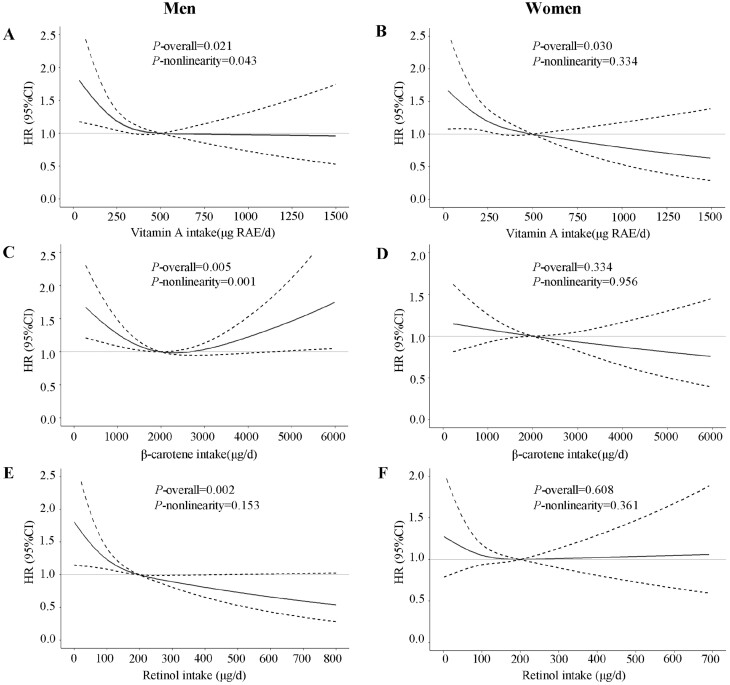
During a median of 11 years of follow-up (217 917 person-years), we ascertained that 519 men and 531 women developed diabetes. The average age for men and women when first diagnosed with diabetes was 56.3 years and 60.1 years, respectively. As shown in Table 2, we observed that higher total vitamin A intakes were significantly associated with a lower risk of diabetes in both sexes after adjusting for nondietary and dietary factors (Q5 vs Q1: HR = 0.69, 95% CI, 0.49-0.97, P-trend = 0.079 in men; HR = 0.63, 95% CI, 0.45-0.89, P-trend = 0.039 in women). RCS analysis further showed an L-shaped association between total vitamin A intake and diabetes risk among men (Fig. 1A, P-nonlinearity = 0.043). Diabetes risk reduced with total vitamin A intake pronouncedly when total vitamin A intake was relatively low; however, the HRs were not further decreased when total vitamin A intake exceed 500 μg RAE/d. No nonlinear association was found between total vitamin A intake and diabetes risk among women (Fig. 1B, P-nonlinearity = 0.334).
Table 2.
Diabetes risk according to quintiles of cumulative average dietary intakes of vitamin A, β-carotene and retinol in men (n = 8,534) and women (n = 8577)a
| Quintiles of dietary intake in men | P-trend | Quintiles of dietary intake in women | P-trend | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 1 | 2 | 3 | 4 | 5 | |||
| Vitamin A | ||||||||||||
| Median intake (μg RAE/d) | 113.2 | 191.3 | 259.1 | 354.2 | 585.5 | 105.0 | 173.5 | 240.5 | 327.3 | 524.3 | ||
| Cases/person-years | 108/20 969 | 104/23 350 | 110/22 315 | 104/22 554 | 93/22 012 | 126/20 238 | 107/22 278 | 114/22 572 | 103/21 471 | 81/20 157 | ||
| Model 1 | 1.00(ref) | 0.83(0.64-1.09) | 0.94(0.72-1.22) | 0.89(0.68-1.16) | 0.85(0.64-1.12) | 0.422 | 1.00(ref) | 0.78(0.60-1.01) | 0.82(0.64-1.06) | 0.85(0.65-1.01) | 0.72(0.54-0.95) | 0.070 |
| Model 2 | 1.00(ref) | 0.76(0.58-1.00) | 0.83(0.64-1.09) | 0.75(0.57-0.98) | 0.70(0.52-0.93) | 0.037 | 1.00(ref) | 0.72(0.55-0.93) | 0.76(0.59-0.98) | 0.73(0.56-0.96) | 0.63(0.47-0.84) | 0.009 |
| Model 3 | 1.00(ref) | 0.78(0.58-1.03) | 0.85(0.64-1.14) | 0.77(0.56-1.05) | 0.69(0.49-0.97) | 0.079 | 1.00(ref) | 0.73(0.56-0.96) | 0.78(0.59-1.04) | 0.75(0.55-1.04) | 0.63(0.45-0.89) | 0.039 |
| β-carotene | ||||||||||||
| Median intake (μg/d) | 558.5 | 1115.6 | 1707.5 | 2474.4 | 3955.5 | 515.1 | 1029.7 | 1591.4 | 2318.2 | 3698.3 | ||
| Cases/person-years | 119/18 059 | 98/22 794 | 107/23 801 | 107/24 503 | 88/22 046 | 95/16 676 | 127/22 166 | 116/23 455 | 117/24 442 | 76/19 976 | ||
| Model 1 | 1.00(ref) | 0.59(0.45-0.77) | 0.61(0.47-0.79) | 0.61(0.47-0.80) | 0.64(0.49-0.85) | 0.038 | 1.00(ref) | 0.85(0.65-1.10) | 0.76(0.58-1.00) | 0.72(0.55-0.95) | 0.72(0.53-0.98) | 0.032 |
| Model 2 | 1.00(ref) | 0.56(0.43-0.73) | 0.58(0.45-0.76) | 0.60(0.46-0.78) | 0.65(0.49-0.86) | 0.061 | 1.00(ref) | 0.84(0.64-1.10) | 0.77(0.58-1.01) | 0.73(0.56-0.96) | 0.74(0.55-1.01) | 0.059 |
| Model 3 | 1.00(ref) | 0.59(0.45-0.77) | 0.64(0.49-0.84) | 0.66(0.50-0.87) | 0.71(0.52-0.97) | 0.315 | 1.00(ref) | 0.85(0.65-1.12) | 0.79(0.59-1.04) | 0.76(0.57-1.02) | 0.77(0.55-1.07) | 0.639 |
| Retinol | ||||||||||||
| Median intake (μg/d) | 20.8 | 58.7 | 95.2 | 147.1 | 301.5 | 18.5 | 51.4 | 85.1 | 132.7 | 265.2 | ||
| Cases/person-years | 109/23 327 | 99/23 775 | 106/23 277 | 100/19 391 | 105/21 431 | 112/21 568 | 117/23 910 | 104/22 607 | 93/18 772 | 105/19 859 | ||
| Model 1 | 1.00(ref) | 0.88(0.67-1.15) | 0.92(0.70-1.21) | 1.02(0.771-1.34) | 0.98(0.75-1.29) | 0.720 | 1.00(ref) | 0.90(0.70-1.17) | 0.88(0.68-1.16) | 0.96(0.72-1.26) | 1.04(0.80-1.36) | 0.435 |
| Model 2 | 1.00(ref) | 0.80(0.61-1.05) | 0.76(0.58-1.00) | 0.75(0.56-1.00) | 0.69(0.52-0.93) | 0.052 | 1.00(ref) | 0.86(0.66-1.12) | 0.75(0.56-0.99) | 0.76(0.57-1.02) | 0.82(0.61-1.09) | 0.404 |
| Model 3 | 1.00(ref) | 0.79(0.59-1.06) | 0.72(0.53-0.99) | 0.66(0.46-0.95) | 0.58(0.39-0.85) | 0.022 | 1.00(ref) | 0.92(0.70-1.21) | 0.81(0.59-1.12) | 0.84(0.58-1.22) | 0.90(0.61-1.34) | 0.909 |
Data are HR (95% CI) calculated by using Cox proportional hazard analyses. Model 1: adjusted for age, BMI, dietary intake of TE. Model 2: model 1 + residence area, highest education level, household income level, PAL, smoking status, alcohol consumption, and history of hypertension at baseline. Model 3: model 2 + dietary intake of carbohydrates, protein, the ratio of MUFA-to-SFA intake, ratio of PUFA-to-SFA intake, cholesterol, zinc, dietary fiber, vegetables, fruits, eggs, and CHEI. Tests for linear trend for HRs were conducted by using the median value for each quintile of intake as a continuous variable.
Abbreviations: BMI, body mass index; CHEI, Chinese Healthy Eating Index; HR, hazard ratio; MUFA, monounsaturated fat; PAL, physical activity level; PUFA, polyunsaturated fat; RAE, retinol activity equivalent; ref, reference; SFA, saturated fat; TE, total energy.
a Intakes were estimated as energy-adjusted cumulative average intake from baseline and follow-up periods.
Figure 1.
Multivariable-adjusted HRs (black solid lines) and 95% CIs (dotted lines) for risk of diabetes according to dietary intakes of vitamin A (A and B), β-carotene (C and D), and retinol (E and F) in men and women, respectively, in model 3. The median intakes were set as references (gray solid lines).
Association Between Dietary β-carotene Intake and Diabetes
In men, HRs (95% CIs) across quintiles of β-carotene intake were 1.00 (ref), 0.59 (0.45-0.77), 0.64 (0.49-0.84), 0.66 (0.50-0.87), and 0.71 (0.52-0.97), suggesting an inverse but not linear association (Table 2, P-trend = 0.315). RCS analysis indicated that there was a significant nonlinear dose-response association between β-carotene intake and diabetes risk in men, which followed a reverse J-shape (Fig. 1C, P-nonlinearity = 0.001). When β-carotene intake was below 2000 μg/d, it was negatively associated with diabetes risk in men, whereas when β-carotene intake exceeded 2000 μg/d, there was a trend of increase in diabetes risk despite not significant. Nevertheless, in women, HRs across quintiles of β-carotene intake were not statistically significant (Table 2, Fig. 1D).
Association Between Dietary Retinol Intake and Diabetes
For retinol intake, the results of HRs across quintiles were similar to that of β-carotene intake, but the P-trend of 0.022 suggested that there was a significant linear association between retinol intake and diabetes risk in men (Table 2). Compared with men in the lowest quintile of retinol intake, those in the highest quintile have a 42% lower risk of developing diabetes (HR = 0.58; 95% CI, 0.39-0.85; P-trend = 0.022). RCS analysis further confirmed the linear association between retinol intake and diabetes risk among men (Fig. 1E, P-nonlinearity = 0.153). However, retinol intake was not significantly associated with diabetes risk among women (Fig. 1F, P-overall = 0.608).
Discussion
This prospective analysis examined the long-term association between dietary total vitamin A, β-carotene, retinol intake, and the risk of diabetes among a large sample of Chinese adults with predominately plant-based diets characterized by low total vitamin A and retinol intake but relatively high β-carotene intake. To our knowledge, this study is the first to report a nonlinear association between dietary intake of vitamin A subtypes and diabetes risk in consideration of sex differences. Our findings show that dietary total vitamin A intake was negatively associated with diabetes risk in both sexes, whereas a ceiling effect was observed in men that the risk of diabetes was not reduced any longer when daily total vitamin A intake reached the threshold of 500 μg RAE (ie, L-shaped association). In addition, dietary β-carotene and retinol intake were significantly associated with diabetes risk only in men but not in women. The association with diabetes risk was reverse J-shaped for β-carotene intake and inversely linear for retinol intake among male adults.
Several observational studies have examined the association of dietary β-carotene intake (median/mean intake ranging from 1105 to 5498 μg/d) with type 2 diabetes risk in the Europe and United States but have produced inconsistent results (7-12). A recent meta-analysis of 77 643 participants from these 6 prospective cohort studies (all conducted in Western populations) summarized that higher dietary β-carotene intakes were associated with a lower risk of type 2 diabetes (highest vs lowest: pooled relative risk = 0.78; 95% CI, 0.70-0.87) (6). Similar to these findings, we also found that dietary β-carotene intake was inversely associated with diabetes risk, but the inverse association was only significant in men (highest vs lowest: HR = 0.71; 95% CI, 0.52-0.97; P-overall = 0.005) but not in women (highest vs lowest: HR = 0.77; 95% CI, 0.55-1.07; P-overall = 0.334). Circulating levels of β-carotene were shown to be well correlated with dietary β-carotene intake after adjustment for serum cholesterol in a controlled feeding study (Pearson r = 0.53; R2 = 0.28) (27). A 27-year follow-up study reported that both serum levels and dietary intake of β-carotene were inversely associated with incident type 2 diabetes in Swedish men (12). Consistently, the EPIC-InterAct study, a large prospective case-cohort study nested within the European Prospective Investigation into Cancer and Nutrition (EPIC) study, also showed an inverse association between plasma β-carotene levels and the risk of type 2 diabetes (highest vs lowest: HR = 0.45; 95% CI, 0.39-0.52) (28). When pooling the results from the EPIC-InterAct study and 6 other prospective observational studies (n = 34 234), the inverse association between circulating β-carotene concentrations and type 2 diabetes risk remained significant (highest vs lowest: HR = 0.60; 95% CI, 0.46-0.78) (6), which strengthen the inverse association we observed for dietary β-carotene.
By contrast, previous intervention studies failed to show a beneficial effect of β-carotene supplementation on preventing diabetes. Two randomized clinical trials conducted in the United States observed no significant effect of 50 mg β-carotene treatment every other day for 9.2 and 12 years on reducing the risk of type 2 diabetes among 8171 women and 22 071 men, respectively (29, 30). Similarly, the Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study also showed that 20 mg/d of β-carotene supplementation did not influence the incidence of type 2 diabetes after 6.1 years of intervention among 29 133 male smokers (31). In our study, we observed a reverse J-shaped association between dietary β-carotene intake and diabetes risk in men. In other words, the protective effect of β-carotene on prevention of diabetes was only significant when men consumed less than 2000 μg/d of dietary β-carotene. In accordance with our findings from dietary β-carotene, the EPIC-InterAct study revealed a L-shaped association between plasma β-carotene levels and the risk of type 2 diabetes (28). The nonlinear associations were subsequently confirmed by a meta-analysis study (6), which suggest that the inverse association with type 2 diabetes are stronger at lower circulating levels of β-carotene, but weaker at higher levels. Whereas the intervention dosage of β-carotene in the aforementioned trials was much larger than usual dietary intake, which may partially explain the inconsistent findings from the clinical trials and our study or other prospective cohort studies. β-carotene, as an antioxidant, can eliminate free radicals. However, free radicals play a dual role in the development of many diseases and overelimination of free radicals through increasing β-carotene intake may result in adverse effects, which may explain the possible association between excessive β-carotene intake and higher risk of diabetes among men in our study.
Existing data on total vitamin A and retinol intake and diabetes risk are scarce. To our knowledge, only 1 prospective cohort study to date reported a nonsignificant inverse association between dietary total vitamin A intake and type 2 diabetes risk during 5 years among 19 168 healthy Japanese aged 40 to 79 years (16). In contrast to the Japanese study, we found that higher dietary intakes of total vitamin A were associated with a lower risk of diabetes in both men and women, and dietary retinol intake was inversely related to diabetes risk in men. However, it is noteworthy that Japanese participants had a much higher total vitamin A intake than our participants (~1000 vs ~250 μg RAE). Additionally, retinol accounted for the majority of dietary vitamin A in Japanese, whereas less than 40% of vitamin A was retinol in the Chinese diet. The inconsistent results between the 2 studies, that the inverse association between vitamin A intake and diabetes risk was only significant in the Chinese population with low vitamin A intake, suggests there may be a ceiling effect of total vitamin A on prevention of diabetes.
Other dietary factors, such as higher intake of fiber and vegetables and lower intake of carbohydrates, as well as adherence to healthy dietary patterns, have been linked with a lower diabetes risk (32). Although participants with higher intake of vitamin A also tend to consume more fiber, monounsaturated fat, and vegetables and less carbohydrates, and have better diet quality in this study, the results did not materially change after controlling for these confounding factors.
Many studies have explained the possible mechanisms that vitamin A affects the development of diabetes. Animal studies showed that vitamin A deficiency could lead to loss of pancreatic β-cell mass, reduced insulin secretion and thereby hyperglycemia through inducing β-cell apoptosis (33). On the other hand, retinol-binding protein 4 (RBP4), the primary circulating retinol transporter that delivers retinol from the liver to peripheral target tissues (34), had been found to be positively associated with type 2 diabetes risk (35, 36). RBP4 partially primes the NOD-like receptor thermal protein domain associated protein 3 inflammasome for IL-1β release through Toll-like receptor 4, and partially inhibits insulin synthesis by activating membrane-specific receptor, the stimulated by retinoic acid 6 (37, 38). Although all-trans RA, the transcriptionally active form of vitamin A, can reduce serum RBP4 levels and enhance insulin sensitivity, which results in diabetes risk reduction (39). Moreover, vitamin A can exert antioxidant functions by activating the transcriptional network controlled by retinoic acid receptors and retinoic acid X receptors via RA (40).
In the current study, a threshold effect of total vitamin A intake on preventing diabetes was observed at around 500 μg RAE in men, but no such association was found in women. This kind of sex discrepancy may be due to the differences in vitamin A metabolism between the sexes and the interaction between sex hormones and vitamin A. As far back as the 1950s, researchers have noted that men are more sensitive to vitamin A deficiency than women (41). When receiving the same amount of vitamin A capsules orally, men had significantly higher rises in postabsorptive plasma retinyl ester concentrations than women (42), whereas females retained more vitamin A in the liver than males regardless of food sources of vitamin A (eg, carrots, green leaves, halibut liver oil) (43, 44). As observed in our study, no individual effect of dietary β-carotene and retinol intakes was found for protecting against diabetes among women. Accordingly, compared with women, it may be much easier for men to reach the plateau of circulating vitamin A levels and more responsive to low vitamin A intakes. In addition, given that estradiol increased retinoic acid receptor α gene expression (45), we speculate that the distribution and expression of vitamin A-related receptors may be different in men and women, leading to a greater benefit in women than in men from higher intake of vitamin A. The wide range of the frequency of dietary recall analyses (from 1 to 9) might also introduce bias into the sex-specific association between vitamin A intake and diabetes risk. Nevertheless, there is no significant difference in the number of dietary recalls between men and women in this study, so the sex discrepancy is likely not related to method bias. In consideration of the lack of literature on sex differences in vitamin A metabolism in recent decades, the findings of the sex-specific association between vitamin A and diabetes risk warrant further investigation.
There are several strengths in our study, including large sample size, the population-based prospective design, and a relatively long follow-up period. Moreover, dietary intake of vitamin A subtypes was assessed repeatedly by using 3 consecutive 24-hour dietary recalls, which accounts for changes in diet during the follow-up. Additionally, a large number of dietary and nondietary covariates were extensively collected and adjusted to minimize residual confounding.
Some limitations also need to be considered. First, self-reported diabetes was used as the primary outcome, which might lead to underestimation of the disease because participants should be aware of their condition before answering the questions. Unfortunately, almost 2 in 3 Chinese adults with diabetes are undiagnosed according to the estimation from China Chronic Disease and Risk Factors Surveillance (46). Second, we could not distinguish between type 1 and type 2 diabetes, which has different pathogenesis. Nevertheless, type 2 diabetes is the most common type of diabetes among adults, which accounts for more than 90% of diabetes in China (47). Third, our study could not assess the relation of other provitamin A carotenoids except for β-carotene with diabetes risk owing to the lack of relevant data in Chinese Food Composition Tables. Furthermore, we assumed that total carotenes from plant sources measured using paper chromatography are all β-carotene, which may overestimate β-carotene intake, as well as total vitamin A intake, given that other provitamin A carotenoids have lower provitamin A activity than β-carotene. Fourth, this study failed to collect information on vitamin A supplement use. However, a nationally representative survey reported that only 0.19% and 0.03% of the Chinese populations took vitamin A and carotene supplements, respectively (48). Fifth, the observational study design precludes causal inference. Sixth, blood levels of vitamin A subtypes, which could provide objective measurement of vitamin A intake and strengthen the association observed, were not measured in this study. Finally, the study population mainly consumed a plant-based diet, which may limit the generalization and extrapolation of the results to other regions.
In summary, higher dietary intakes of total vitamin A, β-carotene, and retinol were associated with a lower risk of diabetes in men, following an L-shaped trend for total vitamin A, a reverse J-shaped trend for β-carotene, and a linear trend for retinol, respectively. Among women, there was an inverse association of diabetes risk with dietary total vitamin A intake, but not with dietary β-carotene or retinol intake. Adequate intake of vitamin A from both plant-based and animal-derived foods may help protect against diabetes, especially in men.
Acknowledgments
This research uses data from China Health and Nutrition Survey (CHNS). We thank the National Institute for Nutrition and Health, China Center for Disease Control and Prevention, Carolina Population Center (P2C HD050924, T32 HD007168), the University of North Carolina at Chapel Hill, the NIH (R01-HD30880, DK056350, R24 HD050924, and R01-HD38700), and the NIH Fogarty International Center (D43 TW009077, D43 TW007709) for financial support for the CHNS data collection and analysis files from 1989 to 2015 and future surveys, and the China-Japan Friendship Hospital, Ministry of Health for support for CHNS 2009, Chinese National Human Genome Center at Shanghai since 2009, and Beijing Municipal Center for Disease Prevention and Control since 2011.
Glossary
Abbreviations
- BMI
body mass index
- BMR
basal metabolic rate
- CHNS
the China Health and Nutrition Survey
- HR
hazard ratio
- PAL
physical activity level
- Q
quintile
- RA
retinoic acid
- RAE
retinol activity equivalent
- RBP4
retinol-binding protein 4
- RCS
restricted cubic splines
- TE
total energy
Contributor Information
Lei Su, Department of Nutrition, School of Public Health, Sun Yat-sen University, Guangzhou 510080, P.R. China; Guangdong Provincial Key Laboratory of Food, Nutrition and Health, Guangzhou 510080, P.R. China.
Jingjing He, Key Laboratory of Precision Nutrition and Food Quality, Department of Nutrition and Health, China Agricultural University, Beijing 100083, P.R. China.
Zhaoyan Liu, Department of Nutrition, School of Public Health, Sun Yat-sen University, Guangzhou 510080, P.R. China; Guangdong Provincial Key Laboratory of Food, Nutrition and Health, Guangzhou 510080, P.R. China.
Shangling Wu, Department of Clinical Nutrition, The First Affiliated Hospital of Sun Yat-Sen University, Guangzhou 510080, P.R. China.
Peiyan Chen, Department of Clinical Nutrition, The First Affiliated Hospital of Sun Yat-Sen University, Guangzhou 510080, P.R. China.
Keji Li, Department of Nutrition and Food Hygiene, School of Public Health, Peking University, Beijing 100191, P.R. China.
Aiping Fang, Department of Nutrition, School of Public Health, Sun Yat-sen University, Guangzhou 510080, P.R. China; Guangdong Provincial Key Laboratory of Food, Nutrition and Health, Guangzhou 510080, P.R. China.
Funding
This study received no sources of support from any grants, fellowships, or gifts of materials.
Author Contributions
L.S. performed the analyses and wrote the manuscript. A.F. designed the research. A.F., J.H., and K.L. reviewed the manuscript. J.H., Z.L., S.W., and P.C. contributed to data collating. A.F. and K.L are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Conflict of Interest
There are no conflicts of interest to declare.
Data Availability
The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Weber D, Grune T. The contribution of β-carotene to vitamin A supply of humans. Mol Nutr Food Res. 2012;56(2):251-258. [DOI] [PubMed] [Google Scholar]
- 2. Su L, He J, Liu Z, et al. Data from: Dietary total vitamin A, β-carotene, and retinol intake and the risk of diabetes in Chinese adults with plant-based diets. figshare, Deposited July 28, 2022. 10.6084/m9.figshare.20391678. [DOI] [PMC free article] [PubMed]
- 3. Blaner WS. Vitamin A signaling and homeostasis in obesity, diabetes, and metabolic disorders. Pharmacol Ther. 2019;197:153-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sun H, Saeedi P, Karuranga S, et al. IDF Diabetes Atlas: global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. 2022;183:109119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhou Y, Wang H, Zhou J, et al. Vitamin A and its multi-effects on pancreas: recent advances and prospects. Front Endocrinol (Lausanne). 2021;12:620941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jiang YW, Sun ZH, Tong WW, et al. Dietary intake and circulating concentrations of carotenoids and risk of type 2 diabetes: a dose-response meta-analysis of prospective observational studies. Adv Nutr. 2021;12(5):1723-1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sluijs I, Cadier E, Beulens JWJ, et al. Dietary intake of carotenoids and risk of type 2 diabetes. Nutr Metab Cardiovasc Dis. 2015;25(4):376-381. [DOI] [PubMed] [Google Scholar]
- 8. Prentice RL, Pettinger M, Neuhouser ML, et al. Application of blood concentration biomarkers in nutritional epidemiology: example of carotenoid and tocopherol intake in relation to chronic disease risk. Am J Clin Nutr. 2019;109(4):1189-1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Basualdo CG, Wein EE, Basu TK. Vitamin A (retinol) status of first nation adults with non-insulin-dependent diabetes mellitus. J Am Coll Nutr. 1997;16(1):39-45. [DOI] [PubMed] [Google Scholar]
- 10. Otto MCD, Alonso A, Lee DH, et al. Dietary intakes of zinc and heme iron from red meat, but not from other sources, are associated with greater risk of metabolic syndrome and cardiovascular disease. J Nutr. 2012;142(3):526-533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kataja-Tuomola MK, Kontto JP, Mannisto S, et al. Intake of antioxidants and risk of type 2 diabetes in a cohort of male smokers. Eur J Clin Nutr. 2011;65(5):590-597. [DOI] [PubMed] [Google Scholar]
- 12. Arnlov J, Zethelius B, Riserus U, et al. Serum and dietary beta-carotene and alpha-tocopherol and incidence of type 2 diabetes mellitus in a community-based study of Swedish men: report from the Uppsala Longitudinal Study of Adult Men (ULSAM) study. Diabetologia. 2009;52(1):97-105. [DOI] [PubMed] [Google Scholar]
- 13. Ju L, Yu D, Fang H, et al. [Trends and food sources composition of energy, protein and fat in Chinese residents, 1992-2012]. Wei Sheng Yan Jiu. 2018;47(5):689-704. [PubMed] [Google Scholar]
- 14. Zhao L, Fang Y, He Y, et al. [Trends of food consumption among Chinese population in 1992-2012]. Wei Sheng Yan Jiu. 2016;45(4):522-526. [PubMed] [Google Scholar]
- 15. Olsen T, Blomhoff R. Retinol, retinoic acid, and retinol-binding protein 4 are differentially associated with cardiovascular disease, type 2 diabetes, and obesity: an overview of human studies. Adv Nutr. 2020;11(3):644-666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Eshak ES, Iso H, Muraki I, et al. Fat-soluble vitamins from diet in relation to risk of type 2 diabetes mellitus in Japanese population. Br J Nutr. 2019;121(6):647-653. [DOI] [PubMed] [Google Scholar]
- 17. Zhang B, Zhai FY, Du SF, et al. The China Health and Nutrition Survey, 1989-2011. Obes Rev. 2014;15:2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Xue H, Yang M, Liu Y, et al. Relative validity of a 2-day 24-hour dietary recall compared with a 2-day weighed dietary record among adults in South China. Nutr Diet. 2017;74(3):298-307. [DOI] [PubMed] [Google Scholar]
- 19. Institute for Nutrition and Food Safety of the Chinese Center for Disease Control and Prevention. China Food Composition Table 2004. 1st ed. Beijing, China: Peking University Medical Press; 2005. [Google Scholar]
- 20. Institute of Health of the Chinese Academy of Medical Sciences, Food Composition Table. Beijing, China: People’s Medical Publishing House; 1980. [Google Scholar]
- 21. Institute for Nutrition and Food Hygiene of the Chinese Academy of Preventive Medicine, Food Composition Table. Beijing, China: People’s Medical Publishing House; 1991. [Google Scholar]
- 22. Institute of Medicine Panel on Micronutrients. In Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. Washington (DC): National Academies Press (US); 2001. [PubMed] [Google Scholar]
- 23. Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr. 1997;65(4 Suppl):1220S-1228S; discussion 1229S. [DOI] [PubMed] [Google Scholar]
- 24. Yuan YQ, Li F, Dong RH, et al. The development of a Chinese Healthy Eating Index and its application in the general population. Nutrients. 2017;9(9): 977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang Y, Ge K, Popkin BM. Tracking of body mass index from childhood to adolescence: a 6-y follow-up study in China. Am J Clin Nutr. 2000;72(4):1018-1024. [DOI] [PubMed] [Google Scholar]
- 26. Chinese Nutrition Society. Chinese Dietary Reference Intakes 2000. Beijing, China: China Light Industry Press; 2000. (In Chinese). [Google Scholar]
- 27. Lampe JW, Huang Y, Neuhouser ML, et al. Dietary biomarker evaluation in a controlled feeding study in women from the Women’s Health Initiative cohort. Am J Clin Nutr. 2017;105(2):466-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zheng JS, Sharp SJ, Imamura F, et al. Association of plasma biomarkers of fruit and vegetable intake with incident type 2 diabetes: EPIC-InterAct case-cohort study in eight European countries. BMJ. 2020;370:m2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Liu S, Ajani U, Chae C, et al. Long-term beta-carotene supplementation and risk of type 2 diabetes mellitus: a randomized controlled trial. JAMA. 1999;282(11):1073-1075. [DOI] [PubMed] [Google Scholar]
- 30. Song Y, Cook NR, Albert CM, et al. Effects of vitamins C and E and beta-carotene on the risk of type 2 diabetes in women at high risk of cardiovascular disease: a randomized controlled trial. Am J Clin Nutr. 2009;90(2):429-437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kataja-Tuomola M, Sundell JR, Männistö S, et al. Effect of alpha-tocopherol and beta-carotene supplementation on the incidence of type 2 diabetes. Diabetologia. 2008;51(1):47-53. [DOI] [PubMed] [Google Scholar]
- 32. Neuenschwander M, Ballon A, Weber KS, et al. Role of diet in type 2 diabetes incidence: umbrella review of meta-analyses of prospective observational studies. BMJ. 2019;366:l2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Trasino SE, Benoit YD, Gudas LJ. Vitamin A deficiency causes hyperglycemia and loss of pancreatic β-cell mass. J Biol Chem. 2015;290(3):1456-1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lee SA, Yuen JJ, Jiang H, et al. Adipocyte-specific overexpression of retinol-binding protein 4 causes hepatic steatosis in mice. Hepatology. 2016;64(5):1534-1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Takebayashi K, Suetsugu M, Wakabayashi S, et al. Retinol binding protein-4 levels and clinical features of type 2 diabetes patients. J Clin Endocrinol Metab. 2007;92(7):2712-2719. [DOI] [PubMed] [Google Scholar]
- 36. Fan J, Yin S, Lin D, et al. Association of serum retinol-binding protein 4 levels and the risk of incident type 2 diabetes in subjects with prediabetes. Diabetes Care. 2019;42(8):1574-1581. [DOI] [PubMed] [Google Scholar]
- 37. Huang R, Bai X, Li X, et al. Retinol-binding protein 4 activates STRA6, provoking pancreatic β-cell dysfunction in type 2 diabetes. Diabetes. 2021;70(2):449-463. [DOI] [PubMed] [Google Scholar]
- 38. Moraes-Vieira PM, Yore MM, Sontheimer-Phelps A, et al. Retinol binding protein 4 primes the NLRP3 inflammasome by signaling through Toll-like receptors 2 and 4. Proc Natl Acad Sci USA. 2020;117(49):31309-31318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Manolescu DC, Sima A, Bhat PV. All-trans retinoic acid lowers serum retinol-binding protein 4 concentrations and increases insulin sensitivity in diabetic mice. J Nutr. 2010;140(2):311-316. [DOI] [PubMed] [Google Scholar]
- 40. Saeed A, Dullaart RPF, Schreuder T, et al. Disturbed vitamin A metabolism in Non-Alcoholic Fatty Liver Disease (NAFLD). Nutrients. 2017;10(1):29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hoffmann R, Schneider A, Quamo Y. The sex difference in vitamin A metabolism. J Invest Dermatol. 1950;15(6):409-419. [DOI] [PubMed] [Google Scholar]
- 42. Johnson EJ, Krasinski SD, Russell RM. Sex differences in postabsorptive plasma vitamin A transport. Am J Clin Nutr. 1992;56(5):911-916. [DOI] [PubMed] [Google Scholar]
- 43. Booth VH. The influence of sex on the storage of vitamin A. Biochem J. 1950;47(4):xliii. [PubMed] [Google Scholar]
- 44. Booth VH. Liver storage of vitamin A by male and female rats. J Nutr. 1952;48(1):13-30. [DOI] [PubMed] [Google Scholar]
- 45. Ribeiro MP, Santos A. E., and Custódio J. B., Interplay between estrogen and retinoid signaling in breast cancer--current and future perspectives. Cancer Lett. 2014;353(1):17-24. [DOI] [PubMed] [Google Scholar]
- 46. Li Y, Teng D, Shi X, et al. Prevalence of diabetes recorded in mainland China using 2018 diagnostic criteria from the American Diabetes Association: national cross sectional study. BMJ. 2020;369:m997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tang X, Yan X, Zhou H, et al. Prevalence and identification of type 1 diabetes in Chinese adults with newly diagnosed diabetes. Diabetes Metab Syndr Obes. 2019;12:1527-1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gong W, Liu A, Yao Y, et al. Nutrient supplement use among the Chinese population: a cross-sectional study of the 2010-2012 China Nutrition and Health Surveillance. Nutrients. 2018;10(11):1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.