Abstract
Necroptosis is currently attracting the attention of the scientific community for its broad implications in inflammatory diseases and cancer. However, detecting ongoing necroptosis in vivo under both experimental and clinical disease conditions remains challenging. The technical barrier lies in four aspects, namely tissue sampling, real-time in vivo monitoring, specific markers, and distinction between different types of cell death. In this review, we presented the latest methodological advances for in vivo necroptosis identification. The advances highlighted the multi-parameter flow cytometry, sA5-YFP tool, radiolabeled Annexin V/Duramycin, Gallium-68-labeled IRDye800CW contrast agent, and SMART platform in vivo. We also discussed the up-to-date research models in studying necroptosis, particularly the mice models for manipulating and monitoring necroptosis. Based on these recent advances, this review aims to provide some advice on current necroptosis techniques and approaches.
Keywords: Methodological advance, Necroptosis
1. Overview of necroptosis
Necroptosis was discovered as an alternate cell death mechanism when apoptosis is blocked in response to tumor necrosis factor (TNF). Because of its proinflammatory nature, necroptosis has emerged as one the most important programmed cell death (PCD) pathways in various diseases. When the signaling pathway is engaged, the classic RIPK1-RIPK3-MLKL axis consequently triggers the release of damage-associated molecular patterns. The alternative TRIF- and/or ZBP1- mediated activation of RIPK3, on the other hand, plays a pivotal role in infectious diseases as well as tumors1 (Fig. 1). Necroptosis signaling has lately gained a much broader reach since it has been demonstrated in a variety of disease models, including ischemia/reperfusion damage, neurological diseases, retinal disorders, acute renal injury, and cancer.
Fig. 1.
Necroptosis signaling. Necroptosis can be engaged by the ligation of tumor necrosis factor receptor (TNFR) family proteins (including TNFR, FAS, TRAILR, and DR6). Upon ligation, receptor-interacting serine/threonine protein kinase 1 (RIPK1) and RIPK3 was recruited when caspase-8 activity is blocked. Activation of Toll-like receptor 3 (TLR3) or TLR4 by double-stranded RNA (dsRNA) or lipopolysaccharide (LPS) can also initiate necroptosis in macrophages through TIR-domain-containing adapter-inducing interferon-β (TRIF)-dependent activation of RIPK3. Intracellular DNA/RNA released from viral amplification or damaged mitochondria (mtDNA/mtRNA) can activate RIPK3 via Z-DNA binding protein 1 (ZBP1). Mixed lineage kinase domain-like pseudokinase (MLKL) is phosphorylated by activated RIPK3 and oligomerizes as a result. The oligomerized MLKL translocates to the plasma membrane, where it engages ion channels and mediates plasma membrane rupture.
While the significance of necroptosis has been well established, precise detection and dissection of this pathway in different research models is limited by the tools that are available. One of the challenges is to distinguish necroptosis from other forms of PCD in a heterogenous tissue, as many of the PCDs share similar morphological features.2,3 Therefore, specific positive indicators of necroptosis, coupled with cell viability assays and techniques to rule out apoptosis would be ideal.4 Different protein markers in the necroptosis regulatory pathway can be used for detection, including phosphorylated RIPK1, RIPK3, and MLKL. MLKL oligomerization and subsequent release of intracellular components is considered the most unique signature to claim necroptosis. Here, we summarized up-to-date methods for necroptosis detection, with emphasis to the methodologies employed for in vivo identification. In addition, we also discussed the existing genetic models in necroptosis study.
2. Technical difficulties in detecting in vivo necroptosis
The biochemical investigation of necroptosis in cultured cells is well established, with phosphorylated RIPK3 and MLKL being the most utilized indicators. However, detecting necroptosis in animal models and patient samples is technically harder. The reason lies in the following four aspects: First, the tissue sampling in patient samples is very challenging. For animal models, tissue samples can be readily obtained after termination of the experiments. But for patients, the approaches to collect samples is very limited, mostly during surgery or biopsy. In addition, the bulk tissues collected during operation are usually a mixture of different components including blood. The heterogenous nature of clinical sampling might interfere the analysis and interpretation of results. Second, there lacks approaches to precisely monitor necroptosis in real-time. The established methods to detect cellular necroptosis mainly depend on immunoblot, immunofluorescence, and histochemistry. These methods are barely adequate in animal models, let alone conducting real-time measurements in patients. Third, while determining the level of necroptosis-related indicators, there are numerous confounding factors to consider. Tissues, for example, must be disseminated into single cell suspensions to determine cell viability and plasma membrane rupture. The tissue digesting techniques, on the other hand, may have a significant impact on these two parameters. Furthermore, assessing the release of cellular material in tissues is inaccurate. Lactate dehydrogenase (LDH) release, for example, is a useful indicator of necroptosis, but anemia, tissue injury, and infections5 can also cause an increase in LDH release. Lastly, and perhaps the most critically, it is difficult to rule out the possibility of apoptosis. In vitro, the most common strategy is to use pan-caspase inhibitors. However, inhibiting caspase activity in vivo can have off-target effects and cannot be tissue specific. Moreover, tissue specific caspase knockout is available in animal models, but impossible for patients.
3. Approaches to detect necroptosis in vivo
New approaches to detect necroptosis in vivo, coupled with traditional detection techniques such as morphological features and the release of intra-cellular components, should allow a more accurate assessment of necroptosis contribution in both experimental models and patients. Here we reviewed applicable approaches for necroptosis detection in vivo, especially the newly developed methods.
3.1. Cell viability assay
3.1.1. Traditional cell viability assays
Traditional cell-impermeant dyes that bind to DNA, such as 4′,6-diamidino-2-phenylindole (DAPI), propidium iodide (PI), or 7-amino-actinomycin D (7-AAD), identify the loss of plasma membrane integrity that occurs when cells die. Counter-staining with Annexin V and PI is frequently used to separate cells into healthy (Annexin V-negative and PI-negative), early apoptotic (Annexin-V positive, PI-negative), and late apoptotic/secondary necrotic and necroptotic (Annexin V-positive and PI-positive) groups. However, none of these parameters is specific for necroptosis. Hence, multi-parameter flow cytometry was developed for classification of cell death.
3.1.2. Multiparameter flow cytometry
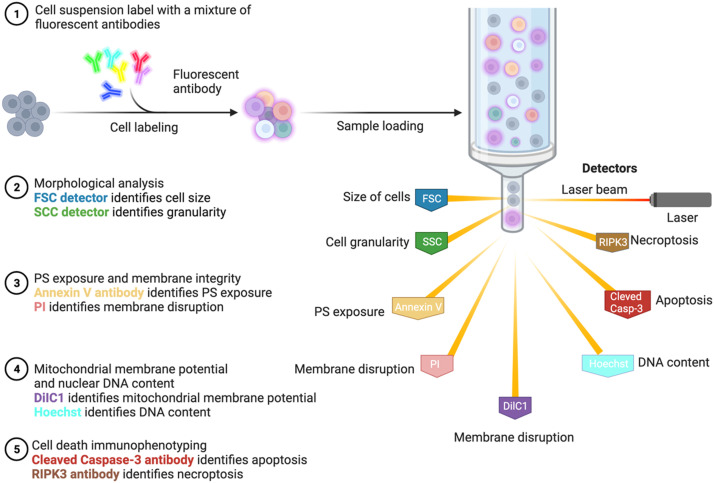
Cell death can be characterized by six cytofluorometric parameters (size, granularity, phosphatidylserine (PS) exposure, plasma membrane integrity, mitochondrial membrane potential and DNA content). Changes in forward scatter (FSC) and side scatter (SSC) provide information about cell size and cell granularity and show the viability of a cell population. An indication for apoptosis is decrease cell size and an increased cytoplasmic granularity or vacuolization. Thus, apoptotic cells are detected as a population with decreased FSC and increased SSC.6 By employing the Annexin V for PS exposure, PI for plasma membrane damage, 1,1′,3,3,3′,3′-hexamethylindodicarbocyanine iodide (DiIC) for mitochondrial membrane potential, and Hoechst for nuclear DNA content, this assay can be done in a single tube measurement.7 This method is capable to detect phenotypically different subpopulations during the progress of cell death. Cells were considered viable, if they do not expose PS on their surfaces, display high mitochondrial membrane potential, and exclude PI. Apoptotic cells are defined by exposure on their surfaces of PS and exclusion of PI. Cells are considered necroptotic if they display penetration of PI of their plasma membranes. Although the intensity of nucleus PI signal together with the change of morphology parameters helped distinguish apoptosis and necroptosis, it's not optimal for the late-stage cell death.
3.1.3. Simultaneous flow cytometric immunophenotyping of necroptosis
The use of anti-RIPK-3 (clone B-2, Cat. No. sc-374,639, Santa Cruz, USA) and anti-active caspase-3 fluorescently tagged antibodies (anti-active caspase-3-BV650, clone C92–605, Cat. No. 564,096, BD Biosciences, USA) can be used as a helpful supplement to multi-parameter flowcytometry.8 This allows the determination of the degree of necroptosis, apoptosis, and RIPK1-dependent apoptosis within live and dead populations. Apoptotic cells were defined by an active caspase-3 positive /RIPK3 negative phenotype, while RIPK1-dependent apoptosis was described by double positive for RIPK3/active caspase-3 events in live and dead populations. In this study, necroptosis was determined by the upregulation of RIPK3 and negative for caspase-3, which does not necessarily reflect the actual occurrence of necroptosis since upregulation of RIPK3 is not always associated with the execution of necroptosis. To specify the presence of necroptosis, translocation of MLKL to the plasma membrane is an inviting option. However, Deli H. and his colleagues found that neither the N terminus nor the C terminus of the plasma translocated MLKL oligomers span outside of plasma membrane.9 Only when integrity of plasma membrane is compromised, MLKL can be detected, which lacked efficacy in immunophenotyping. Therefore, once a phospho-MLKL specific antibody for flow cytometry is available, we recommend adding it in the detection cocktail (Fig. 2).
Fig. 2.
Multiparameter flow cytometry and simultaneous immunotyping. This method can detect phenotypically different subpopulations during the progress of cell death. The six morphological parameters (size, granularity, PS exposure, plasma membrane integrity, mitochondrial membrane potential and DNA content) can be measured with FSC/SSC detectors and fluorescent labeling (Annexin V antibody, PI, DilC1, and Hoechst) in a single tube measurement. Meanwhile, the engagement of apoptosis or necroptosis can be identified by cleaved-caspase-3 or RIPK3 antibody, respectively. DiIC, 1,1′,3,3,3′,3′-hexamethylindodicarbocyanine iodide; FSC, forward scatter; PI, propidium iodide; PS, phosphatidylserine; SSC, side scatter.
3.2. In vivo detection of PCD
3.2.1. Utilization of sA5-YFP mouse model
Although multi-parameter flow cytometry is suitable for necroptosis detection in single cells suspension, it is impractical in vivo, expect for peripheral blood. To overcome this limitation, Kristel and colleagues generated transgenic mouse models expressing secreted Annexin V- yellow fluorescent protein (sA5- YFP) under control of the CAG promoter. This enables in vivo visualization and quantification of PCD in real time. Thus, the sA5-YFP mouse line provides novel insight into the incidence and relevance of various types of cell death in tissues, although incapable of specifying the necroptosis.10
3.2.2. In vivo radiolabel of Annexin V and duramycin
Exposure of PS or phosphatidylethanolamine (PE) on the outer plasma membrane was observed prior to loss of cell integrity during necroptosis.11 Annexin V and duramycin tracers radiolabeled with 99mTc12,13 can be utilized to image necroptotic cells based on the affinity between Annexin V and PS or duramycin and PE. Whole-body 99mTc single photon emission computed tomography (SPECT/CT) imaging was performed after intravenously tracer injection. Volume-of-interest analysis with PMOD software can be used to quantify tracer uptake data in various organs. Terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) staining was performed to validate the cell death biodistribution within SPECT imaging data. The main downside of the Annexin V probe is the significant off-target radiation, which has slowed its acceptance in clinical practice. Radiolabeled duramycin may obviate this shortcoming of the radionuclide imaging. Clinical necroptosis imaging in both acute myocardial infarction and cancer suggests a promising application of this tool. It should be noted that the exposure of PS/PE on the outer plasma membrane was also witnessed in apoptosis.14, 15, 16 Therefore, the imaging data with Annexin V/duramycin probes might not be specific to necroptosis. To explicit the participation of necroptosis and apoptosis, recruiting cell death inhibitors and genetically modified animal models would be helpful.
3.2.3. Gallium-68-labeled IRDye800CW as a necrosis avid contrast agent
IRDye800CW, a cyanine-based probe, was proved for noninvasive imaging of cell death in preclinical evaluation.17 Gallium-68-labeled IRDye800CW has been advanced as a potential positron emission tomography (PET) tracer for in vivo imaging of tumor necrosis. The prominent dead cell binding of fluorescence and radioactivity from [68Ga] Ga-1 was confirmed with 4T1-Luc2 cells. [68Ga] Ga-1 was injected in 4T1-Luc2 tumor-bearing mice, and specific fluorescence and PET signal were observed in the spontaneously developing tumor necrosis.18 The intraperitoneal injection of D-luciferin enabled simultaneous bioluminescence imaging of the viable tumor regions. Tumor necrosis binding was confirmed ex vivo by colocalization of fluorescence uptake with TUNEL dead cell staining and radioactivity uptake in isolated tumors (Fig. 3).
Fig. 3.
Radiolabeling assisted contrast tracing. Exposure of PS or PE on the outer plasma membrane was observed during necroptosis. Hence, Annexin V and duramycin tracers radiolabeled with 99mTc can be utilized to image necroptotic cells in vivo based on the affinity between Annexin V and PS or duramycin and PE. In addition, radiolabeled IRDye800CW, a noninvasive cell death tracer, was capable of in vivo contrast tracing. After injection of these radiolabeled tracers, whole-body SPECT/CT or PET imaging was performed to quantify tracer uptake data. Bioluminescence imaging can be achieved simultaneously in luciferase reporter tumor bearing models. PE, phosphatidylethanolamine; PS, phosphatidylserine; PET, positron emission tomography; SPECT/CT, single photon emission computed tomography.
3.2.4. Imaging cell death-sensing ligand by nanoparticles
Currently, nanoparticles are being developed to image PCD without radiation and enable better topographic localization. Conjugation of the protein to superparamagnetic iron oxide nanoparticles allowed detection of this binding using magnetic resonance imaging (MRI). Ming and his colleagues conjugated C2 domain of synaptotagmin I, which binds to anionic phospholipids in cell membranes, to the nanoparticles. This tool was shown to successfully bind to the plasma membrane of apoptotic cells by both flow cytometry and confocal microscopy.19 One of the strengths of this approach is the ability to localize dead cells, determine its extent, and correlate it with morphology and functional consequences. Moreover, the application was demonstrated both in vitro, with isolated apoptotic tumor cells, and in vivo, in a tumor treated with chemotherapeutic drugs. However, because nanoparticles can be taken up by macrophages, such techniques may be limited in pathological conditions with significant macrophage infiltration.
3.2.5. Necroptosis detection via cellular content release
Although not conclusively, the release of high mobility group box 1 protein (HMGB1), interleukin-1, interleukin-33, cyclophilin A, LDH, or mitochondrial DNA, may indicate necroptosis. Inspired by this idea, HMGB1-Lucia™ construct/cells20 are designed to monitor cell death characterized by the release of the alarmin HMGB1 upon cell membrane rupture.21,22 The release of the HMGB1-Lucia protein in the extracellular milieu upon necroptosis, pyroptosis, or necrosis, can be readily measured using the QUANTI-Luc™ detection reagent. With the help of HMGB1-Lucia™ construct, this technique is readily applicable in generating transgenic mouse and orthotopic tumor models with easy adaption.
4. Necrosome formation and distinct markers
4.1. Measurement of RIPK1, RIPK3 and MLKL
In vivo necroptosis induction is sometimes associated with increased RIPK1, RIPK3 or MLKL mRNA or protein expression levels. Tissues and primary isolated cells with increased RIPK3 and MLKL expression is detected by antibody-based methods, including western blot, immunofluorescence (IF), immunohistochemistry (IHC), and flow cytometry. Many studies have assessed total RIPK3 or MLKL levels in tissues, but increased RIPK3 or MLKL abundance does not necessarily indicate pathway activation. Better indicators of necroptosis signaling are RIPK3 autophosphorylation and phosphorylation of MLKL. Phosphorylation of RIPK3 on S227,23 and MLKL on Thr357 and Ser358, reflect activation towards necroptosis in human cells,24,25 which could be used as a diagnostic and prognostic biomarker. Better yet, the lab of Wang et al. recently developed a phospho-human MLKL antibody and found it robust on liver biopsies.26 Different from human phosphorylation sites, Thr-231 and Ser-232 in mouse RIP327 and Ser-345, Ser-347, and Thr-349 in mouse MLKL28 are the specific phosphorylation sites in mouse. Thus, species specific phospho-RIPK3 and phospho-MLKL antibodies should be used. In our previous studies, mouse specific phospho-MLKL antibody (Abcam, ab196436), was valid for both western blot and immunohistochemical assays.29 As a result, in addition to the western blot assay, the detection of necroptosis using phospho-MLKL immunohistochemical labeling facilitated the necroptosis assay in tissue sections in both patients and mouse models.
4.2. Interaction between RIPK1, RIPK3, and MLKL
Interaction between the necroptosis key components (RIPK1, RIPK3, and MLKL) biochemically or microscopically can be used to identify necroptosis. Upon stimulation, RIPK1 and RIPK3 were reported to form amyloid-like oligomers,30 while MLKL was shown to form disulfide bond-dependent tetramers and octamers. Immuno-precipitation followed by nonreducing gel electrophoresis31 is helpful in detecting RIPK3 and MLKL interaction during necrosome formation.32 This approach was employed in experimental cholestasis and multiple sclerosis models.33 Moreover, liquid chromatography-tandem mass spectrometry (LC-MS/MS) is a powerful methodology for the unbiased analysis of complex protein assemblies.34 Cross-linking LC-MS and native mass spectrometry, followed by a cross-linking mass spectrometry (XL-MS) analytical model, can be used to investigate RIPK3 and MLKL oligomerization.35
To achieve a real-time monitoring approach in vivo, a fluorescence resonance energy transfer (FRET) biosensor has been developed, termed SMART (a sensor for MLKL activation by RIPK3 based on FRET). SMART is focused on conformation changes of MLKL induced by RIPK3 binding. Various FRET biosensors containing a series of fragments of MLKL between modified YFP and enhanced cyan fluorescent protein (CFP) were constructed. SMART enabled us to detect necroptosis by monitoring an increase in the FRET/CFP ratio36 in all kinds of animal models.
4.3. Fluorescence labeling of necrosome formation
The established methods are mainly suitable for necrosome formation in cultured cells or tissue homogenates. We are still in desperate need for an in vivo detection. Therefore, visualizing intracellular localization of necrosome components, RIPK3 and MLKL, and their interaction by confocal microscopy is of great value. A toolbox of antibodies for immunofluorescent detection of the core necroptosis effectors, RIPK1, RIPK3, and MLKL, and their phosphorylated forms, in human and mouse cells was established by Samson and his colleagues.37 To exemplify the advantages of visualizing endogenous necroptotic events in situ, samples can be co-stained for non-phosphorylated and phosphorylated MLKL. In necroptotic cells, phosphorylated MLKL was observed to assemble into macromolecular structures at the plasma membrane, rather than docking uniformly with the cell periphery. The MLKL-specific signals and the phosphorylation -specific signals overlap at the cytoplasmic clusters, but not at the plasma membrane, suggesting a signaling chronology analogous to the activation of MLKL, which can be dissected via IF using different antibodies.38
4.4. Duolink proximity ligation assay
The heterodimer of RIPK1/RIPK3 and the homodimer of RIPK1 or RIPK3 can also be determined using a Duolink in situ proximity ligation assay (PLA). The proximity of RIPK1 and RIPK3 was detected using two primary antibody probes. Only when these two probes bound in proximity did a pair of oligonucleotide-labeled secondary antibodies provide a fluorescent signal, indicating physical binding of RIPK1 and RIPK3 in cells. PLA signals were recognized as green/red fluorescent spots by calculating the average fluorescent spots in each cell in the image.39 By detecting the PLA signals, we can also identify the specific subcellular location of RIPK1/RIPK3 homodimers and heterodimers based on microscopy images.
5. Methods to rule out apoptosis
A key challenge with identifying necroptosis is the need to distinguish it from apoptosis and secondary necrosis. Cells can readily shift from apoptosis to necroptosis and vice versa, and secondary necrosis was often found in the later stage PCD. Therefore, detection of cell death should be complemented with real-time morphological or viability analysis and approaches targeting death signaling pathways.4
5.1. Caspase inhibition
Caspase-8 is a key inhibitor of necroptosis, as it has been shown to cleave and inactivate RIPK1 and RIPK3. When caspase-8 is active, it forms a complex with RIPK1 and FAS-associated protein with death domain (FADD) to initiate apoptosis. On the other hand, when caspase-8 is inhibited, RIPK1 and RIPK3 interact with RIP homotype interaction motifs (RHIMs) to initiate necroptosis.40
Pan-caspase chemical inhibitors, such as zVAD-fmk or Q-VD-OPh, are frequently used to impede caspase activation to cell death and/or confirm the occurrence of necroptosis.41,42 Although these caspase inhibitors can block most of the extrinsic signals for apoptosis, they may not completely block the intrinsic pathway of apoptosis, in which the role of caspases is to accelerate cell death following the irreversible loss of mitochondrial membrane potential.43 Therefore, genetic knockdown, knockout,44 or transgenic methods is required for the detection of necroptosis in most cases to assess necroptosis.
5.2. Unique spectral signatures of the nucleic acid dye acridine orange
Acridine orange (AO), a nucleic acid dye with unique spectral properties, reveals apoptosis versus necroptosis spectral fingerprints.45 Healthy cells had a cytoplasmic red AO emission and sharply defined nucleus with enriched red signal lining the entirety of the nucleus. Apoptotic cells could be distinguished by diminished red AO signal and the fragmentation and condensation of nuclei that were easily distinguished by their irregular outline. In contrast to apoptosis, during early stages of necroptosis, there was an overall loss of red AO structure, nuclear shrinkage, and a loss of nuclear demarcation, but no fragmentation. At the final stages of necroptosis, the nuclei were small and spherical and often had an increase in intensity or a smooth ungranulated appearance. Thus, necrosis/necroptosis was distinguished from apoptosis by nucleus morphology and cytoplasmic red AO emission.
6. In vivo necroptosis models
6.1. Pharmacological necroptosis inhibitors
Necrostatins is a group of compounds named for their capability to suppress necroptosis, among which Nec-1 has been used to study the contribution of necroptosis and target RIPK1 kinase activity in a wide range of pathological cell death events. Nec-1 inhibits RIPK1 autophosphorylation,46 which is a critical mechanism during TNF-induced necroptosis signaling,47 by interacting with the T-loop, an important structure for death domain receptor interaction. Several disease models, including cardiovascular, neurological, and renal ailments, have been shown to benefit from Nec-1 protection. However, it should be noted that necrostatins also blocked RIPK1-mediated apoptosis. Therefore, results from necrostatins are not definitive for necroptosis.
GSK’840, GSK’843, and GSK’872 bind to the kinase domain of RIPK3 and block its kinase activity with high specificity, targeting a broader range of pronecrotic stimuli.48 GSK'872 blocks TNF-induced necroptosis in a concentration-dependent manner in various cell lines and primary isolated mammalian cells.49 In vivo, GSK'872 therapy reduces necroptosis-related lesions following ischemia damage.50
Necrosulfonamide (NSA) is a necroptosis inhibitor, which specifically blocks MLKL, a critical substrate of RIPK3 during necroptosis. NSA inhibits MLKL-mediated necroptosis by blocking its N-terminal CC domain function through disulfide bond formation.24 The necroptosis inhibitory effect of NSA has been confirmed in multiple human cell lines, including TNF-α/Smac mimetic/z-VAD treated HT-29, TNF-α treated FADD-null Jurkat, and TNF-α/Smac mimetic treated RIPK3-Hela cells. However, NSA only efficiently blocks necroptosis in human cells, but not mouse cells, which may limit its preclinical evaluation.
6.2. Genetically modified animal models
Although chemical inhibitors are widely used in studying necroptosis, the limitation of drug delivery and the possible off-target effect complicates further mechanistic exploration. Using genetically modified models is considered the best method for confirming the presence of necroptosis.51
The functional contribution of RIPK1, RIPK3 or MLKL to pathologies has been analyzed in knockout (KO) animals. Unfortunately, Ripk1-deficient mice die perinatally,52 but this could be rescued by crossing them with Ripk3/caspase-8 double KO mice, illustrating that RIPK1 controls both apoptosis and necroptosis induction.52, 53, 54, 55 Recently, Rip1K45A/K45A kinase dead knock-in (KI) animals became available to the scientific community,52,53 allowing the distinction between RIPK1 kinase-independent functions (NF-κB and MAPKs activation and cell survival), and the kinase-dependent ones. Finally, organ specific conditional Ripk1 deficient mice have also been developed. Although RIP1 kinase-inactive mutants and necrostatins are nontoxic, they do not prevent all forms of RIPK3 necroptosis.53 Furthermore, the absence of any clear spontaneous phenotypic change in non-challenged Ripk3-deficient mice56 offered an appealing alternative to the lethality of Ripk1 KO mice.57,58 Viable and fertile Rip3K51A/K51A kinase-inactive KI mice were generated. Rip3K51A/K51A cannot support necroptosis but are fully immunocompetent and retain the ability to generate a robust, virus-specific T cell response as well as control infection by a natural mouse pathogen.48 We anticipate these mice will reveal additional insights into the contribution of necroptosis in inflammatory disease.
Recently, Mlkl-deficient mice have also been developed.59 Interestingly, Mlkl deficiency inhibited necroptosis in an acute pancreatitis mouse model but did not seem to affect NF-κB or MAPKs signaling or pro-inflammatory cytokine induction following TNF or LPS treatment.28,59 Because MLKL has less pleiotropic functions than RIPK1 and RIPK3, we may consider these animals to be the most appropriate system for assessing the contribution of necroptosis in experimental disease models.
6.3. Optogenetics model
The capacity to cause targeted death with minimal side effects by cell type-specific induction of cell death holds great promise, particularly for the treatment of cancer. As one of the controllable approaches, optogenetically controlled cell death effectors (optoCDEs) exhibit very specific cytotoxicity when expressed in cells. Based on the knowledge of PCD pathways, different optoCDEs can kill the target cells through apoptosis, pyroptosis, or necroptosis.60 For instance, when the photosensitive protein Cry2olig was fused to the necroptosis kinase RIPK3 or the executor MLKL, light activation can trigger oligomerization of RIPK3 and MLKL, respectively, and leads to necroptosis in targeted cells. Such optogenetics tools can be easily translated to in vivo application for studying the role of necroptosis at the resolution of single cells (or at least a selected group of cells) within tissue.
7. Future directions
7.1. From tissue sampling to fluid biopsy
The biggest challenge of necroptosis detection in vivo still lies in the sampling restriction. Since the crosstalk between different tissues can be achieved via multiple pathways, scientists believe that by analyzing extracellular communications carried in blood, saliva, urine, and other bodily fluids, we should be able to intercept messages indicating health and illness.61 Bodily fluids and peripheral blood are easily accessible and can be collected non-invasively, which is suitable for both clinical and research applications.62 Not only bodily fluids are easily accessible, but they also contained plenty of biological information. Abnormal nucleic acids and/or proteins have been identified in patients’ bodily fluids such as blood, urine, and cerebrospinal fluid, and have been demonstrated to be effective biomarkers for diagnostic use.63, 64, 65 However, heterogeneity and background noise must be carefully considered when employing bodily fluids and peripheral blood to detect necroptosis.
7.2. Real-time live imaging
There were five live imaging methods for detecting necroptosis in vivo described in this review, namely sA5-YFP mouse model, 99mTc labeled necroptosis tracers, Gallium-68-labeled IRDye800CW contrast agent, HMGB1-Lucia™ construct/cells, and SMART. Besides 99mTc probes and Gallium-68-labeled IRDye800CW, data from the cancer imaging field suggests more radiolabeled substrates could be developed. Probes specific for executioner caspases, including caspase-3 and caspase-8 can be used to identify apoptosis; cellular respiration labels can be used to measure mitochondrial membrane potential; MLKL phosphorylation and oligomerization imaging can be used for relatively specific necroptosis detection. These tools are feasible and will need to be developed in the future. Furthermore, more real-time imaging approaches, such as ultrasonography, MRI, and CT, are needed to assess necroptosis under healthy and pathological settings.
7.3. Development of more specific biomarkers
The biggest paradox in necroptosis detection is the lack of specific biomarkers. RIPK1 and RIPK3 could be involved in inflammatory processes and induction66 of NLR family pyrin domain containing 3 (NLRP3) inflammasome,56,67, 68, 69 necroptosis-independent apoptosis induction,70,71 and metabolic regulation.72 MLKL is known as the terminal executor of necroptosis. However, MLKL was found to promote acute inflammation73 in cardiovascular system. MLKL also contributes to endosomal trafficking and extracellular vesicle formation in the absence of RIPK3.74 And MLKL was identified with an unknown role in regulating endosomal trafficking to facilitate lipid handling in macrophages during atherogenesis.75 These non-necroptotic functions of the core necroptotic markers make the identification of necroptosis harder than expected. Therefore, identifying more specific biomarkers and posttranslational modifications of the existing markers are required to achieve better detection.
Declaration of competing interest
The authors declare that they have no conflict of interests.
Acknowledgements
The authors’ research is supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research. Figures were created with BioRender (BioRender.com).
Author contributions
P.W and J.Y. prepared all the figures and drafted the manuscript. Z.L. proofread and edited the manuscript.
References
- 1.Yan J., Wan P., Choksi S., et al. Necroptosis and tumor progression. Trends Cancer. 2022;8(1):21–27. doi: 10.1016/j.trecan.2021.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dhuriya Y.K., Sharma D. Necroptosis: a regulated inflammatory mode of cell death. J Neuroinflammation. 2018;15(1):199. doi: 10.1186/s12974-018-1235-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu W., Liu P., Li J. Necroptosis: an emerging form of programmed cell death. Crit Rev Oncol Hematol. 2012;82(3):249–258. doi: 10.1016/j.critrevonc.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 4.Vanden Berghe T., Grootjans S., Goossens V., et al. Determination of apoptotic and necrotic cell death in vitro and in vivo. Methods. 2013;61(2):117–129. doi: 10.1016/j.ymeth.2013.02.011. [DOI] [PubMed] [Google Scholar]
- 5.Klein R., Nagy O., Tóthová C., et al. Clinical and diagnostic significance of lactate dehydrogenase and its isoenzymes in animals. Vet Med Int. 2020;2020 doi: 10.1155/2020/5346483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hagenhofer M., Germaier H., Hohenadl C., et al. UV-B irradiated cell lines execute programmed cell death in various forms. Apoptosis. 1998;3(2):123–132. doi: 10.1023/a:1009601109509. [DOI] [PubMed] [Google Scholar]
- 7.Munoz L.E., Maueröder C., Chaurio R., et al. Colourful death: six-parameter classification of cell death by flow cytometry-dead cells tell tales. Autoimmunity. 2013;46(5):336–341. doi: 10.3109/08916934.2012.755960. [DOI] [PubMed] [Google Scholar]
- 8.Lee H.L., Pike R., Chong M.H.A., et al. Simultaneous flow cytometric immunophenotyping of necroptosis, apoptosis and RIP1-dependent apoptosis. Methods. 2018;134-135:56–66. doi: 10.1016/j.ymeth.2017.10.013. [DOI] [PubMed] [Google Scholar]
- 9.Huang D., Zheng X., Wang Z.A., et al. The MLKL channel in necroptosis is an octamer formed by tetramers in a dyadic process. Mol Cell Biol. 2017;37(5):e00416–e00497. doi: 10.1128/MCB.00497-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martínez-Lagunas K., Yamaguchi Y., Becker C., et al. In vivo detection of programmed cell death during mouse heart development. Cell Death Differ. 2020;27(4):1398–1414. doi: 10.1038/s41418-019-0426-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gong Y.N., Guy C., Olauson H., et al. ESCRT-III acts downstream of MLKL to regulate necroptotic cell death and its consequences. Cell. 2017;169(2):286–300.e16. doi: 10.1016/j.cell.2017.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Delvaeye T., Wyffels L., Deleye S., et al. Noninvasive whole-body imaging of phosphatidylethanolamine as a cell death marker using (99m)Tc-duramycin during TNF-induced SIRS. J Nucl Med. 2018;59(7):1140–1145. doi: 10.2967/jnumed.117.205815. [DOI] [PubMed] [Google Scholar]
- 13.Shekhar A., Heeger P., Reutelingsperger C., et al. Targeted imaging for cell death in cardiovascular disorders. JACC Cardiovasc Imaging. 2018;11(3):476–493. doi: 10.1016/j.jcmg.2017.11.018. [DOI] [PubMed] [Google Scholar]
- 14.Shlomovitz I., Speir M., Gerlic M. Flipping the dogma - phosphatidylserine in non-apoptotic cell death. Cell Commun Signal. 2019;17(1):139. doi: 10.1186/s12964-019-0437-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marino G., Kroemer G. Mechanisms of apoptotic phosphatidylserine exposure. Cell Res. 2013;23(11):1247–1248. doi: 10.1038/cr.2013.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Emoto K., Toyama-Sorimachi N., Karasuyama H., et al. Exposure of phosphatidylethanolamine on the surface of apoptotic cells. Exp Cell Res. 1997;232(2):430–434. doi: 10.1006/excr.1997.3521. [DOI] [PubMed] [Google Scholar]
- 17.Stammes M.A., Knol-Blankevoort V.T., Cruz L.J., et al. Pre-clinical evaluation of a cyanine-based SPECT probe for multimodal tumor necrosis imaging. Mol Imaging Biol. 2016;18(6):905–915. doi: 10.1007/s11307-016-0972-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stroet M.C.M., de Blois E., Haeck J., et al. In vivo evaluation of gallium-68-labeled IRDye800CW as a necrosis avid contrast agent in solid tumors. Contrast Media Mol Imaging. 2021;2021 doi: 10.1155/2021/2853522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao M., Beauregard D.A., Loizou L., et al. Non-invasive detection of apoptosis using magnetic resonance imaging and a targeted contrast agent. Nat Med. 2001;7(11):1241–1244. doi: 10.1038/nm1101-1241. [DOI] [PubMed] [Google Scholar]
- 20.Park S., Lee L.E., Kim H., et al. Detection of intracellular monosodium urate crystals in gout synovial fluid using optical diffraction tomography. Sci Rep. 2021;11(1):10019. doi: 10.1038/s41598-021-89337-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Broz P., Dixit V.M. Inflammasomes: mechanism of assembly, regulation and signalling. Nat Rev Immunol. 2016;16(7):407–420. doi: 10.1038/nri.2016.58. [DOI] [PubMed] [Google Scholar]
- 22.Grootjans S., Vanden Berghe T., Vandenabeele P. Initiation and execution mechanisms of necroptosis: an overview. Cell Death Differ. 2017;24(7):1184–1195. doi: 10.1038/cdd.2017.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hanna-Addams S., Liu S., Liu H., et al. CK1 alpha, CK1 delta, and CK1 epsilon are necrosome components which phosphorylate serine 227 of human RIPK3 to activate necroptosis. Proc Natl Acad Sci U S A. 2020;117(4):1962–1970. doi: 10.1073/pnas.1917112117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun L., Wang H., Wang Z., et al. Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell. 2012;148(1–2):213–227. doi: 10.1016/j.cell.2011.11.031. [DOI] [PubMed] [Google Scholar]
- 25.McQuade T., Cho Y., Chan F.K. Positive and negative phosphorylation regulates RIP1- and RIP3-induced programmed necrosis. Biochem J. 2013;456(3):409–415. doi: 10.1042/BJ20130860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang H., Sun L., Su L., et al. Mixed lineage kinase domain-like protein MLKL causes necrotic membrane disruption upon phosphorylation by RIP3. Mol Cell. 2014;54(1):133–146. doi: 10.1016/j.molcel.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 27.Chen W., Zhou Z., Li L., et al. Diverse sequence determinants control human and mouse receptor interacting protein 3 (RIP3) and mixed lineage kinase domain-like (MLKL) interaction in necroptotic signaling. J Biol Chem. 2013;288(23):16247–16261. doi: 10.1074/jbc.M112.435545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murphy J.M., Czabotar P.E., Hildebrand J.M., et al. The pseudokinase MLKL mediates necroptosis via a molecular switch mechanism. Immunity. 2013;39(3):443–453. doi: 10.1016/j.immuni.2013.06.018. [DOI] [PubMed] [Google Scholar]
- 29.Baik J.Y., Wan P., Liu Z.G. Examining MLKL phosphorylation to detect necroptosis in murine mammary tumors. STAR Protoc. 2022;3(3) doi: 10.1016/j.xpro.2022.101457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li J., McQuade T., Siemer A.B., et al. The RIP1/RIP3 necrosome forms a functional amyloid signaling complex required for programmed necrosis. Cell. 2012;150(2):339–350. doi: 10.1016/j.cell.2012.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu S., Liu H., Johnston A., et al. Proc Natl Acad Sci U S A. Vol. 14. 2017. MLKL forms disulfide bond-dependent amyloid-like polymers to induce necroptosis; pp. E7450–E7459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Afonso M.B., Rodrigues C.M.P., Rodrigues Necrosome formation and necroptosis in experimental cholestasis. Methods Mol Biol. 2019;1981:149–162. doi: 10.1007/978-1-4939-9420-5_10. [DOI] [PubMed] [Google Scholar]
- 33.Ofengeim D., Ito Y., Najafov A., et al. Activation of necroptosis in multiple sclerosis. Cell Rep. 2015;10(11):1836–1849. doi: 10.1016/j.celrep.2015.02.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Petrie E.J., Sandow J.J., Jacobsen A.V., et al. Conformational switching of the pseudokinase domain promotes human MLKL tetramerization and cell death by necroptosis. Nat Commun. 2018;9(1):2422. doi: 10.1038/s41467-018-04714-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rodriguez D.A., Weinlich R., Brown S., et al. Characterization of RIPK3-mediated phosphorylation of the activation loop of MLKL during necroptosis. Cell Death Differ. 2016;23(1):76–88. doi: 10.1038/cdd.2015.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murai S., Yamaguchi Y., Shirasaki Y., et al. A FRET biosensor for necroptosis uncovers two different modes of the release of DAMPs. Nat Commun. 2018;9(1):4457. doi: 10.1038/s41467-018-06985-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Samson A.L., Fitzgibbon C., Patel K.M., et al. A toolbox for imaging RIPK1, RIPK3, and MLKL in mouse and human cells. Cell Death Differ. 2021;28(7):2126–2144. doi: 10.1038/s41418-021-00742-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Samson A.L., Zhang Y., Geoghegan N.D., et al. MLKL trafficking and accumulation at the plasma membrane control the kinetics and threshold for necroptosis. Nat Commun. 2020;11(1):3151. doi: 10.1038/s41467-020-16887-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang Z., Feng J., Yu J., et al. FKBP12 mediates necroptosis by initiating RIPK1-RIPK3-MLKL signal transduction in response to TNF receptor 1 ligation. J Cell Sci. 2019;132(10) doi: 10.1242/jcs.227777. [DOI] [PubMed] [Google Scholar]
- 40.Jorgensen I., Rayamajhi M., Miao E.A. Programmed cell death as a defence against infection. Nat Rev Immunol. 2017;17(3):151–164. doi: 10.1038/nri.2016.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cai Z., Jitkaew S., Zhao J., et al. Proc Natl Acad Sci U S A. Vol. 109. 2012. Mixed lineage kinase domain-like is a key receptor interacting protein 3 downstream component of TNF-induced necrosis; pp. 5322–5327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Caserta T.M., Smith A.N., Gultice A.D., et al. Q-VD-OPh, a broad spectrum caspase inhibitor with potent antiapoptotic properties. Apoptosis. 2003;8(4):345–352. doi: 10.1023/a:1024116916932. [DOI] [PubMed] [Google Scholar]
- 43.Rodríguez-Enfedaque A., Delmas E., Guillaume A. zVAD-fmk upregulates caspase-9 cleavage and activity in etoposide-induced cell death of mouse embryonic fibroblasts. Biochim Biophys Acta. 2012;1823(8):1343–1352. doi: 10.1016/j.bbamcr.2012.05.013. [DOI] [PubMed] [Google Scholar]
- 44.Someda M., Kuroki S., Miyachi H., et al. Caspase-8, receptor-interacting protein kinase 1 (RIPK1), and RIPK3 regulate retinoic acid-induced cell differentiation and necroptosis. Cell Death Differ. 2020;27(5):1539–1553. doi: 10.1038/s41418-019-0434-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Plemel J.R., Caprariello A.V., Keough M.B., et al. Unique spectral signatures of the nucleic acid dye acridine orange can distinguish cell death by apoptosis and necroptosis. J Cell Biol. 2017;216(4):1163–1181. doi: 10.1083/jcb.201602028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Degterev A., Hitomi J., Germscheid M., et al. Identification of RIP1 kinase as a specific cellular target of necrostatins. Nat Chem Biol. 2008;4(5):313–321. doi: 10.1038/nchembio.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Takahashi N., Duprez L., Grootjans S., et al. Necrostatin-1 analogues: critical issues on the specificity, activity and in vivo use in experimental disease models. Cell Death Dis. 2012;3(11):e437. doi: 10.1038/cddis.2012.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mandal P., Berger S.B., Pillay S., et al. RIP3 induces apoptosis independent of pronecrotic kinase activity. Mol Cell. 2014;56(4):481–495. doi: 10.1016/j.molcel.2014.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kaiser W.J., Sridharan H., Huang C., et al. Toll-like receptor 3-mediated necrosis via TRIF, RIP3, and MLKL. J Biol Chem. 2013;288(43):31268–31279. doi: 10.1074/jbc.M113.462341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang X.S., Yi T.L., Zhang S., et al. Hypoxia-inducible factor-1 alpha is involved in RIP-induced necroptosis caused by in vitro and in vivo ischemic brain injury. Sci Rep. 2017;7(1):5818. doi: 10.1038/s41598-017-06088-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jouan-Lanhouet S., Riquet F., Duprez L., et al. Necroptosis, in vivo detection in experimental disease models. Semin Cell Dev Biol. 2014;35:2–13. doi: 10.1016/j.semcdb.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 52.Polykratis A., Hermance N., Zelic M., et al. Cutting edge: RIPK1 Kinase inactive mice are viable and protected from TNF-induced necroptosis in vivo. J Immunol. 2014;193(4):1539–1543. doi: 10.4049/jimmunol.1400590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kaiser W.J., Daley-Bauer L.P., Thapa R.J., et al. Proc Natl Acad Sci U S A. Vol. 111. 2014. RIP1 suppresses innate immune necrotic as well as apoptotic cell death during mammalian parturition; pp. 7753–7758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rickard J.A., O'Donnell J.A., Evans J.M., et al. RIPK1 regulates RIPK3-MLKL-driven systemic inflammation and emergency hematopoiesis. Cell. 2014;157(5):1175–1188. doi: 10.1016/j.cell.2014.04.019. [DOI] [PubMed] [Google Scholar]
- 55.Dillon C.P., Weinlich R., Rodriguez D.A., et al. RIPK1 blocks early postnatal lethality mediated by caspase-8 and RIPK3. Cell. 2014;157(5):1189–1202. doi: 10.1016/j.cell.2014.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Khan N., Lawlor K.E., Murphy J.M., et al. More to life than death: molecular determinants of necroptotic and non-necroptotic RIP3 kinase signaling. Curr Opin Immunol. 2014;26:76–89. doi: 10.1016/j.coi.2013.10.017. [DOI] [PubMed] [Google Scholar]
- 57.He S., Wang L., Miao L., et al. Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-alpha. Cell. 2009;137(6):1100–1111. doi: 10.1016/j.cell.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 58.Duprez L., Takahashi N., Van Hauwermeiren F., et al. RIP kinase-dependent necrosis drives lethal systemic inflammatory response syndrome. Immunity. 2011;35(6):908–918. doi: 10.1016/j.immuni.2011.09.020. [DOI] [PubMed] [Google Scholar]
- 59.Wu J., Huang Z., Ren J., et al. Mlkl knockout mice demonstrate the indispensable role of Mlkl in necroptosis. Cell Res. 2013;23(8):994–1006. doi: 10.1038/cr.2013.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shkarina K., Hasel de Carvalho E., et al. Optogenetic activators of apoptosis, necroptosis, and pyroptosis. J Cell Biol. 2022;221(6) doi: 10.1083/jcb.202109038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Broza Y.Y., Zhou X., Yuan M., et al. Disease detection with molecular biomarkers: from chemistry of body fluids to nature-inspired chemical sensors. Chem Rev. 2019;119(22):11761–11817. doi: 10.1021/acs.chemrev.9b00437. [DOI] [PubMed] [Google Scholar]
- 62.Plante G.E., Chakir M., Lehoux S., et al. Disorders of body fluid balance: a new look into the mechanisms of disease. Can J Cardiol. 1995;11(9):788–802. [PubMed] [Google Scholar]
- 63.Ulz P., Auer M., Heitzer E. Detection of circulating tumor DNA in the blood of cancer patients: an important tool in cancer chemoprevention. Methods Mol Biol. 2016;1379:45–68. doi: 10.1007/978-1-4939-3191-0_5. [DOI] [PubMed] [Google Scholar]
- 64.Rieger-Christ K.M., Mourtzinos A., Lee P.J., et al. Identification of fibroblast growth factor receptor 3 mutations in urine sediment DNA samples complements cytology in bladder tumor detection. Cancer. 2003;98(4):737–744. doi: 10.1002/cncr.11536. [DOI] [PubMed] [Google Scholar]
- 65.Wong L.J., Lueth M., Li X.N., et al. Detection of mitochondrial DNA mutations in the tumor and cerebrospinal fluid of medulloblastoma patients. Cancer Res. 2003;63(14):3866–3871. [PubMed] [Google Scholar]
- 66.Moriwaki K., Chan F.K. Necrosis-dependent and independent signaling of the RIP kinases in inflammation. Cytokine Growth Factor Rev. 2014;25(2):167–174. doi: 10.1016/j.cytogfr.2013.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vince J.E., Wong W.W., Gentle I., et al. Inhibitor of apoptosis proteins limit RIP3 kinase-dependent interleukin-1 activation. Immunity. 2012;36(2):215–227. doi: 10.1016/j.immuni.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 68.Kang T.B., Yang S.H., Toth B., et al. Caspase-8 blocks kinase RIPK3-mediated activation of the NLRP3 inflammasome. Immunity. 2013;38(1):27–40. doi: 10.1016/j.immuni.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 69.Kang T.B., Yang S.H., Toth B., et al. Activation of the NLRP3 inflammasome by proteins that signal for necroptosis. Methods Enzymol. 2014;545:67–81. doi: 10.1016/B978-0-12-801430-1.00003-2. [DOI] [PubMed] [Google Scholar]
- 70.Newton K., Dugger D.L., Wickliffe K.E., et al. Activity of protein kinase RIPK3 determines whether cells die by necroptosis or apoptosis. Science. 2014;343(6177):1357–1360. doi: 10.1126/science.1249361. [DOI] [PubMed] [Google Scholar]
- 71.Dondelinger Y., Aguileta M.A., Goossens V., et al. RIPK3 contributes to TNFR1-mediated RIPK1 kinase-dependent apoptosis in conditions of cIAP1/2 depletion or TAK1 kinase inhibition. Cell Death Differ. 2013;20(10):1381–1392. doi: 10.1038/cdd.2013.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang D.W., Shao J., Lin J., et al. RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science. 2009;325(5938):332–336. doi: 10.1126/science.1172308. [DOI] [PubMed] [Google Scholar]
- 73.Dai J., Zhang C., Guo L., et al. A necroptotic-independent function of MLKL in regulating endothelial cell adhesion molecule expression. Cell Death Dis. 2020;11(4):282. doi: 10.1038/s41419-020-2483-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yoon S., Kovalenko A., Bogdanov K., et al. MLKL, the protein that mediates necroptosis, also regulates endosomal trafficking and extracellular vesicle generation. Immunity. 2017;47(1):51–65.e7. doi: 10.1016/j.immuni.2017.06.001. [DOI] [PubMed] [Google Scholar]
- 75.Rasheed A., Robichaud S., Nguyen M.A., et al. Loss of MLKL (mixed lineage kinase domain-like protein) decreases necrotic core but increases macrophage lipid accumulation in atherosclerosis. Arterioscler Thromb Vasc Biol. 2020;40(5):1155–1167. doi: 10.1161/ATVBAHA.119.313640. [DOI] [PubMed] [Google Scholar]