Abstract
Cell culture and organ explant systems have traditionally been used by scientists in the reproductive biology and perinatal medicine area to address various research questions. Although most are unrelated to human pregnancy, animal models were also extensively used to study various mechanisms associated with pregnancy and parturition. However, limitations of traditional approaches have shifted the attention to the use of organ on a chip (OOC) technology. OOC platform simulates an organ using cells, and OOCs are biomimetic microfluidic systems comprising multiple cell types from an organ that mimic the environment of a physiological organ. OOC maintains intercellular interactions and helps to recreate organ physiology as expected for utero in perinatal medicine research. This short review introduces some basic concepts of OOC, and its utility based on some published reports
Keywords: pregnancy, preterm birth, fetal membranes, microfluidic, organ system
Human pregnancy and parturition-associated studies are fascinating for reproductive biologists. Pregnancy involves two independent fetomaternal units that co-exist for ten months (about 40 weeks) and synchronously coordinate events leading to parturition.[1] During pregnancy, the harmonious state established by various factors (e.g., endocrine, immune, mechanical, and paracrine) is compromised as term approaches. Compromise to different homeostatic systems leads to the transition of quiescent fetomaternal uterine tissues that maintain a pregnancy to an active state of labor. Disruption to these processes is associated with adverse pregnancy events. Preterm labor and delivery (preterm birth [PTB], < 37 weeks) contributing to 1 million neonatal deaths around the world/year is one of the major complications impacting about 12% of all pregnancies.[2–5] PTB is not just an early initiation of labor resulting in delivery, but a syndrome initiated by failures in any of the fetomaternal uterine systems that maintain pregnancy.[6,7] Currently, it is thought that the mechanisms of delivery in normal parturition and PTB are different, but the actual answer remains unclear. Reducing PTB risk remains a challenge as this condition may arise with a fetomaternal medical indication for early delivery or could be spontaneous with no known etiology.[7,8] Advances in reproductive biology research have improved our knowledge of the fetomaternal uterine organ system and their contribution to pregnancy and parturition at term and preterm.[9–12] However, several challenges persist, which prevent us from making major impacts in reducing PTB.
The communication between the fetus and the mother throughout gestation that maintains the pregnancy clock and determines labor timing is difficult to study. Many theories and models exist; however, a systematic approach to understanding disease development across various uterine tissues and signaling between different systems is needed. Modeling interindividual and intercellular interactions using current methods is difficult. To overcome these limitations of current in vitro cell culture techniques (both 2D as well as 3D organoid models) or in vivo animal models that do not mimic human pregnancy and complex human organ systems, scientists in the area of reproductive biology and medicine are adopting innovative approaches like organ-on-a-chip (OOCs) for intercellular interaction studies. A biomimetic “organ-on-a-chip” is to recapitulate the multi-cellular organ system as seen in vivo as much as possible using in vitro cell culture systems., These models can better mimic the structure, functions, and responses of organ systems, though do not mean maintaining or growing actual organs on a chip. As stated by Bhatia and Ingber in their review, the goal of OOC is not to build a whole living organ but rather to synthesize minimal functional units that recapitulate tissue- and organ-level functions. The combination of microfabrication, microfluidics, and induced pluripotent stem cell (iPSC) technologies has provided many physiological models that better mimic human anatomy, functions, and responses more accurately, as seen in vivo. These microphysiological systems (MPS) platforms can provide compartmentalized chambers that enable culturing and organizing cellular, extracellular matrices (ECMs), and other microenvironmental layers within these compartments while still providing avenues for cellular signals, and sometimes even cells themselves, to propagate between the compartments through interconnected fluid paths.[13,14] These systems allow live imaging of cell and extracellular vesicle trafficking, high-resolution imaging, and analysis of elutes and cells using high throughput technologies14. Recreating the dynamic flow of materials through various cell layers allows recapitulation of blood flow and other fluid flows.
Several of the existing OOC models developed for the placenta, amnion membrane, and fetal membrane-maternal interface[15–23] are basic two-cell-type co-culture models. However, a few that incorporate more than two from fetal and maternal cells are emergining.[21,23] Studies using these systems range from very simple drug perfusion studies to more complex ascending infection (from mother to fetus) studies.[21,24,25] There is one very simplistic model of cervix,[26] while none exists for myometrium. A major shortcoming of most of these models is that they are missing essential cell types seen in utero (e.g., extravillous trophoblast, transformation zone, and cervical stroma; immune cells in decidua). Importantly, in utero, these different fetomaternal tissues work cohesively to maintain homeostasis, and interconnected OOC models are required to study intercellular interactions.
Nevertheless, there have been no such efforts thus far. This contrasts with other areas of OOC model development, where interconnected multi-organ OOC models are now emerging (e.g., liver/bone marrow, neurovascular unit, gut/brain/liver, liver/heart/lung).[27–32] Such interconnected models have the potential to decipher mechanisms where multiple organ systems play vital roles together, which is the case for uterine tissues and pregnancy.
Currently, in collaboration with Dr. Arum Han (Texas A&M University, College Station, TX, USA), my laboratory has developed variations of OOCs to study human fetal membranes. We have also developed a cervix and a placenta on a chip. The rest of the review will discuss the application of fetal membranes on a chip and various studies we have conducted to understand different biological mechanisms of fetal membrane functions. A summary of the utility of these devices is provided below. Please refer to specific references under each subheading for experimental details and other outcomes.
The human amnion membrane on a chip (AM-OOC) is a planar two-chamber device connected through an array of 24 microchannels (Figure 1). Microchannels were coated with type IV collagen to mimic the amnion basement membrane. Amnion epithelial cells and mesenchymal cells were used in this device. This device was developed to study the trafficking of cells, their transitions, and cell fate in response to oxidative stress.[33] Recently, we used the same model to test immune cell migration between the choriodecidual interface. We used chorion trophoblast cells from the fetal membranes in the outer chamber and decidua parietalis cells along with CD45+ immune cells (resident isolated from the decidua). In this pilot project, we determined immune cell migration (with live imaging capabilities), their status (active vs. inactive), and their impact on cells that lack them.
Figure 1.
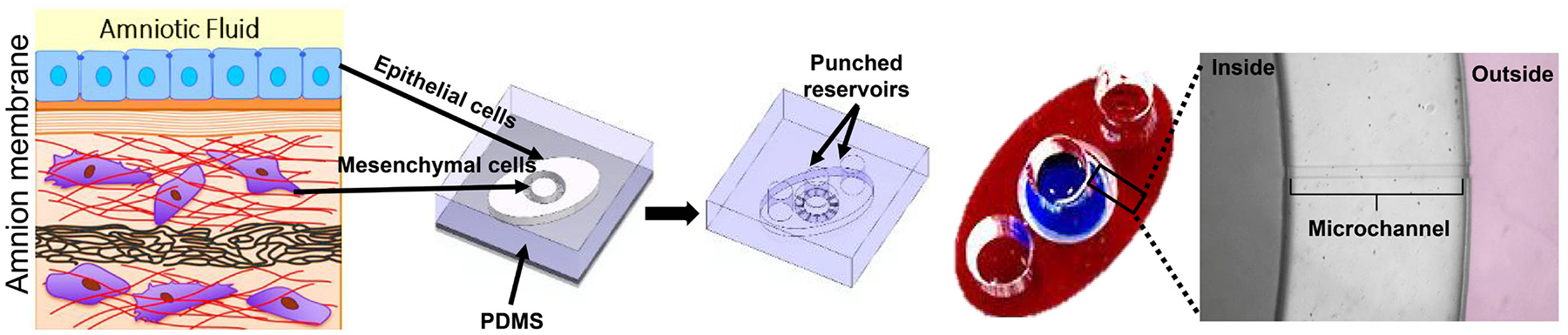
Amnion membrane on a chip (AM-OOC). In this two-chamber device connected through 24 microchannels coated with Type IV collagen to mimic basement membrane region, properties of the cells in the amnion layer (epithelial and mesenchymal cells) were tested. PDMS: polydimethylsiloxane.
The human fetal membrane on a chip (FMi-OOC) is a fourc-hamber device designed to mimic the human fetal membrane (amniochorion) and decidua interface (Figure 2). This chip contained four interconnected chambers with amnion epithelial cells, amnion mesenchymal cells in the extracellular matrix, chorion trophoblast cells, and decidual parietalis cells. Microchannels were coated with type IV collagen to model the amniochorion basement membranes. This is one of the first devices to mimic the human fetomaternal interface at the membrane/decidual region.[21] FMi-OOC was used for various applications as listed below:
Modeling ascending infection: In this study, ascending infection was modeled by treating decidual cells with lipopolysaccharide (LPS). The kinetics of LPS propagation and LPS induced inflammation in maternal and fetal cell layers in a time and dose-dependent manner was reported.[21] This study concluded that ascending infection could cause a fetal inflammatory response to increase an inflammatory load associated with PTB. Validation of this study was performed in animal models using LPS (intraperitoneal injection)[34] and an ascending model of E. coli infection that resulted in PTB.
Testing toxicologic effects of Cadmium on fetomaternal interface cells: Maternal exposure to Cadmium was tested in this study.[35] Cadmium was unable to propagate across the chorion layer of the fetomaternal interface cells resulting in maternal, but not fetal, cell death and inflammation. These results are similar to clinical reports noting Cadmium restricted to the placenta trophoblast layers resulting in minimal cytotoxicity on the fetal side but inducing maternal tissue cytotoxicity. This model suggested that Cadmium-induced PTB is likely mediated by maternal inflammatory response than fetal.[35]
Propagation of senescent fetal exosomes carrying damaged associated molecular pattern marker high mobility group box 1 (HMGB1) across the fetomaternal interface: Our earlier works have shown that human fetal membranes undergo senescence and produce HMGB1 enclosed extracellular vesicles (exosomes 30–160 nm).[36–39] These exosomes can propagate as communication channels between the fetus and the mother to cause uterine activation and labor-associated changes. Using the FMi-OOC, we were able to recapitulate these events.[23] In this study, we were able to show that engineered exosomes containing HMGB1 can propagate from the fetal side to the maternal side and cause inflammatory changes often associated with labor and delivery. Further, we demonstrated the physiologic relevance of this model using mouse model experiments where HMGB1 containing exosomes caused PTB, which was associated with fetomaternal inflammatory changes as observed in our OOC system.[23]
FMi-OOC for studies on drug kinetics: Recently, we have used FMi-OOC to test the kinetics of drugs across the fetomaternal interface at the membrane/decidual parietalis region. We tested the transport of statins (Pravastatin and Rosuvastatin) using the FMi-OOC and determined that drugs can be transported across the fetal membranes. This is novel, as conventionally it was thought that only the placenta transports drugs during pregnancy. In addition, using the FMi-OOC model, we have shown cell and time specific drug metabolites and the differential expressions of the metabolizing enzymes across the fetomaternal interface.
Figure 2.

Amniochorion/decidual interface on a chip (FMi-OOC). This chip contains four concentric circles to hold 4 distinct cell types, all interconnected through microchannels. Reservoirs on the top will function as inlets for media/cells and outlets for elutes. An example of different dye loaded FMi-OOC is shown at bottom right.
In summary, OOC provides a great tool to study intercellular interactions while maintaining the architecture of the tissues. In the perinatal biology field, OOC can be used to determine the mechanisms and pathways of various physiologic and pathologic processes, healthy and disease state modeling, the impact of immune cells, kinetics of materials flow between cells, and preclinical drug and biomarker trials.
Source of Funding
This review is supported by funding from NIH to Ramkumar Menon (R01HD100729).
Footnotes
Conflict of Interest
Ramkumar Menon is an Editorial Board Member of the journal. The article was subject to the journal’s standard procedures, with peer review handled independently of this member and his research group.
REFERENCES
- 1.Menon R Fetal inflammatory response at the fetomaternal interface: A requirement for labor at term and preterm. Immunol Rev 2022;308:149–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beck S, Wojdyla D, Say L, Betran AP, Merialdi M, Requejo JH, et al. The worldwide incidence of preterm birth: a systematic review of maternal mortality and morbidity. Bull World Health Organ 2010;88:31–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shapiro-Mendoza CK, Lackritz EM. Epidemiology of late and moderate preterm birth. Semin Fetal Neonatal Med 2012;17:120–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simmons LE, Rubens CE, Darmstadt GL, Gravett MG. Preventing preterm birth and neonatal mortality: exploring the epidemiology, causes, and interventions. Semin Perinatol 2010;34:408–415. [DOI] [PubMed] [Google Scholar]
- 5.Blencowe H, Cousens S, Oestergaard MZ, Chou D, Moller AB, Narwal R, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet 2012;379:2162–2172. [DOI] [PubMed] [Google Scholar]
- 6.Villar J, Papageorghiou AT, Knight HE, Gravett MG, Iams J, Waller SA, et al. The preterm birth syndrome: a prototype phenotypic classification. Am J Obstet Gynecol 2012;206:119–123. [DOI] [PubMed] [Google Scholar]
- 7.Romero R, Dey SK, Fisher SJ. Preterm labor: one syndrome, many causes. Science 2014;345:760–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mendelson CR, Montalbano AP, Gao L. Fetal-to-maternal signaling in the timing of birth. J Steroid Biochem Mol Biol 2017;170:19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elovitz MA, Mrinalini C. Animal models of preterm birth. Trends Endocrinol Metab 2004;15:479–487. [DOI] [PubMed] [Google Scholar]
- 10.Chauhan SP, Ananth CV. Periviable births: epidemiology and obstetrical antecedents. Semin Perinatol 2013;37:382–388. [DOI] [PubMed] [Google Scholar]
- 11.Lawn JE, Kinney MV, Belizan JM, Mason EM, McDougall L, Larson J, et al. Born too soon: accelerating actions for prevention and care of 15 million newborns born too soon. Reprod Health 2013;10 Suppl 1:S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pavlicev M, Norwitz ER. Human Parturition: Nothing More Than a Delayed Menstruation. Reprod Sci 2018;25:166–173. [DOI] [PubMed] [Google Scholar]
- 13.Richardson L, Kim S, Menon R, Han A. Organ-On-Chip Technology: The Future of Feto-Maternal Interface Research? Front Physiol 2020;11:715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bhatia SN, Ingber DE. Microfluidic organs-on-chips. Nat Biotechnol 2014;32:760–772. [DOI] [PubMed] [Google Scholar]
- 15.Yin F, Zhu Y, Zhang M, Yu H, Chen W, Qin J. A 3D human placenta-on-a-chip model to probe nanoparticle exposure at the placental barrier. Toxicol in Vitro 2019;54:105–113. [DOI] [PubMed] [Google Scholar]
- 16.Blundell C, Tess ER, Schanzer AS, Coutifaris C, Su EJ, Parry S, et al. A microphysiological model of the human placental barrier. Lab Chip 2016;16:3065–3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blundell C, Yi YS, Ma L, Tess ER, Farrell MJ, Georgescu A, et al. Placental Drug Transport-on-a-Chip: A Microengineered In Vitro Model of Transporter-Mediated Drug Efflux in the Human Placental Barrier. Adv Healthc Mater 2018;7:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee JS, Romero R, Han YM, Kim HC, Kim CJ, Hong JS, et al. Placenta-on-a-chip: a novel platform to study the biology of the human placenta. J Matern Fetal Neonatal Med 2016;29:1046–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miura S, Sato K, Kato-Negishi M, Teshima T, Takeuchi S. Fluid shear triggers microvilli formation via mechanosensitive activation of TRPV6. Nat Commun 2015;6:8871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pemathilaka RL, Caplin JD, Aykar SS, Montazami R, Hashemi NN. Placenta-on-a-Chip: In Vitro Study of Caffeine Transport across Placental Barrier Using Liquid Chromatography Mass Spectrometry. Glob Chall 2019;3:1800112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Richardson LS, Kim S, Han A, Menon R. Modeling ascending infection with a feto-maternal interface organ-on-chip. Lab Chip 2020;20:4486–4501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Richardson L, Jeong S, Kim S, Han A, Menon R. Amnion membrane organ-on-chip: an innovative approach to study cellular interactions. The FASEB Journal 2019;33:8945–8960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Radnaa E, Richardson LS, Sheller-Miller S, Baljinnyam T, de Castro Silva M, et al. Extracellular vesicle mediated feto-maternal HMGB1 signaling induces preterm birth. Lab Chip 2021;21:1956–1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mosavati B, Oleinikov AV, Du E. Development of an Organ-on-a-Chip-Device for Study of Placental Pathologies. Int J Mol Sci 2020;21:8755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arumugasaamy N, Rock KD, Kuo CY, Bale TL, Fisher JP. Microphysiological systems of the placental barrier. Adv Drug Deliv Rev 2020;161–162:161–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tantengco OAG, Richardson LS, Medina PMB, Han A, Menon R. Organ-on-chip of the cervical epithelial layer: A platform to study normal and pathological cellular remodeling of the cervix. FASEB J 2021;35:e21463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wikswo JP, Curtis EL, Eagleton ZE, Evans BC, Kole A, Hofmeister LH, et al. Scaling and systems biology for integrating multiple organs-on-a-chip. Lab on a chip 2013;13:3496–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Skardal A, Murphy SV, Devarasetty M, Mead I, Kang HW, Seol YJ, et al. Multi-tissue interactions in an integrated three-tissue organ-on-a-chip platform. Scientific reports 2017;7:8837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McAleer CW, Long CJ, Elbrecht D, Sasserath T, Bridges LR, et al. Multi-organ system for the evaluation of efficacy and off-target toxicity of anticancer therapeutics. Sci Transl Med 2019;11:eaav1386. [DOI] [PubMed] [Google Scholar]
- 30.Maoz BM, Herland A, FitzGerald EA, Grevesse T, Vidoudez C, Pacheco AR, et al. A linked organ-on-chip model of the human neurovascular unit reveals the metabolic coupling of endothelial and neuronal cells. Nat Biotechnol 2018;36:865–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Edington CD, Chen WLK, Geishecker E, Kassis T, Soenksen LR, Bhushan BM, et al. Interconnected Microphysiological Systems for Quantitative Biology and Pharmacology Studies. Sci Rep 2018;8:4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gold K, Gaharwar AK, Jain A. Emerging trends in multiscale modeling of vascular pathophysiology: Organ-on-a-chip and 3D printing. Biomaterials 2019;196:2–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Richardson L, Jeong S, Kim S, Han A, Menon R. Amnion membrane organ-on-chip: an innovative approach to study cellular interactions. FASEB J 2019;33:8945–8960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spencer NR, Radnaa E, Baljinnyam T, Kechichian T, Tantengco OAG, Bonney E, et al. Development of a mouse model of ascending infection and preterm birth. PLoS One 2021;16:e0260370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim S, Richardson L, Radnaa E, Chen Z, Rusyn I, Menon R, et al. Molecular mechanisms of environmental toxin cadmium at the feto-maternal interface investigated using an organ-on-chip (FMi-OOC) model. J Hazard Mater 2022;422:126759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sheller-Miller S, Urrabaz-Garza R, Saade G, Menon R. Damage-Associated molecular pattern markers HMGB1 and cell-Free fetal telomere fragments in oxidative-Stressed amnion epithelial cell-Derived exosomes. J Reprod Immunol 2017;123:3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Menon R Initiation of human parturition: signaling from senescent fetal tissues via extracellular vesicle mediated paracrine mechanism. Obstet Gynecol Sci 2019;62:199–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Menon R, Taylor BD. Exploring Inflammatory Mediators in Fetal and Maternal Compartments During Human Parturition. Obstet Gynecol 2019;134:765–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Menon R, Mesiano S, Taylor RN. Programmed Fetal Membrane Senescence and Exosome-Mediated Signaling: A Mechanism Associated With Timing of Human Parturition. Front Endocrinol (Lausanne) 2017;8:196. [DOI] [PMC free article] [PubMed] [Google Scholar]


