Abstract
Background
The objective was to examine the association of blood pressure variability (BPV) during the first 24 h after intensive care unit admission with the likelihood of delirium and depressed alertness without delirium (“depressed alertness”).
Methods
This retrospective, observational, cohort study included all consecutive adult patients admitted to an intensive care unit at Mayo Clinic, Rochester, Minnesota, from July 1, 2004, through October 31, 2015. The primary outcomes were delirium and delirium-free days, and the secondary outcomes included depressed alertness and depressed alertness-free days. Logistic regression was performed to determine the association of BPV with delirium and depressed alertness. Proportional odds regression was used to assess the association of BPV with delirium-free days and depressed alertness-free days.
Results
Among 66,549 intensive care unit admissions, delirium was documented in 20.2% and depressed alertness was documented in 24.4%. Preserved cognition was documented in 55.4% of intensive care unit admissions. Increased systolic and diastolic BPV was associated with an increased odds of delirium and depressed alertness. The magnitude of the association per 5-mm Hg increase in systolic average real variability (the average of absolute value of changes between consecutive systolic blood pressure readings) was greater for delirium (odds ratio 1.34; 95% confidence interval 1.29–1.40; P < 0.001) than for depressed alertness (odds ratio 1.06; 95% confidence interval 1.02–1.10; P = 0.004). Increased systolic and diastolic BPV was associated with fewer delirium-free days but not with depressed alertness-free days.
Conclusions
BPV in the first 24 h after intensive care unit admission is associated with an increased likelihood of delirium and fewer delirium-free days.
Keywords: Blood pressure, Cognitive orientation, Critical illness, Delirium, Glasgow Coma Scale, Intensive care unit
Introduction
Delirium is an acute, fluctuating, neurocognitive condition characterized by inattention, depressed awareness, and impaired cognition. Patients admitted to an intensive care unit (ICU) often have delirium, with its incidence ranging from 20% in patients who are not intubated to 87% in patients on mechanical ventilation [1, 2]. Delirium is associated with increased ICU and hospital mortality rates, prolonged mechanical ventilation duration, prolonged ICU and hospital length of stay, and an increased risk of emergency department visits, hospital readmissions, and death after hospital discharge [3, 4]. Additionally, some consequences of delirium, such as posttraumatic stress disorder and cognitive impairment, may have a long-term effect on patients [5, 6]. The underlying cause of delirium is most likely multifactorial, including neuroinflammation, oxidative stress, neuroendocrine dysregulation, and impaired cerebrovascular regulation [7].
Although cardiovascular complications related to hypertension are primarily based on absolute blood pressure (BP), evidence is accumulating that these complications are also associated with BP variability (BPV). BPV is a complex phenomenon defined as the magnitude and pattern of BP fluctuations during a certain period of time. BPV can be short term (e.g., hour-to-hour, day-to-day, day-to-night, and 24-h BP fluctuations) or long term (e.g., clinic visit-to-visit BP fluctuations occurring days, weeks, months, or years apart) [8]. Increased short-term and long-term BPV is associated with the development and progression of cardiac, vascular, neurologic, and kidney disease, as well as with an increased risk of cardiovascular events and death [9–14].
Intraoperative BPV has been identified as a risk factor for postoperative delirium [15–18]. The presumed mechanisms of BPV-related complications include microvascular damage with high BP and ischemic changes with low BP in patients with a right shift of the autoregulation curve [10, 12, 19]. Therefore, BPV may increase the risk of delirium because disrupted cerebral autoregulation during critical illness may increase the susceptibility of the brain to potentially injurious effects of BPV [20, 21]. Indeed, we previously reported an association between BPV and acute (delirium and decreased alertness without delirium) and chronic (accelerated decline in global cognitive z scores) cognitive impairment in a small cohort of participants admitted to an ICU [22].
In this study, we aimed to analyze the association between BPV during the first 24 h after ICU admission and the likelihood of acute delirium and depressed alertness without delirium (“depressed alertness”) during ICU admissions in a large patient cohort. We hypothesized that increased BPV is associated with an increased likelihood and duration of delirium and depressed alertness.
Methods
The reporting of this study conformed to the Strengthening The Reporting of OBservational studies in Epidemiology statement and was approved by the Mayo Clinic Institutional Review Board (14-001118); Date: 2/11/2022, Study Title: Acute Brain Injury (Abi) During Critical Illness (Alejandro A. Rabinstein, MD). Declaration of Helsinki (1975) rules do not apply to our retrospective study.
Study Design
The aim of the study was hypothesis-raising: to assess the association of BPV with acute delirium and depressed alertness without delirium in a retrospective cohort analysis. We retrospectively searched our ICU-specific electronic health record, termed the ICU Data Mart [23], for all consecutive adults (≥ 18 years) who were admitted to ICUs at Mayo Clinic in Rochester, Minnesota, from July 1, 2004, through October 31, 2015. The end date of the study period was specifically chosen to ensure data consistency because changes in the internal format of our electronic health records occurred after this date. The ICUs in which patients were admitted included a medical ICU, a coronary care unit, two mixed medical-surgical ICUs, and a cardiosurgical ICU. Patients admitted to our neuroscience ICU were excluded because primary neurologic diseases (e.g., stroke or head trauma) affect the reliability of delirium evaluation methods [24] and provide an alternative explanation for the cause of acute cognitive dysfunction. Patients with ICU admissions shorter than 24 h or health records lacking sufficient BP data (< 10 measurements in the first 24 h after admission) to calculate BPV or missing comorbid condition data were also excluded. We abstracted patient demographic, cognitive/alertness status, and BPV variables of interest for all included patients.
Patient and Care Characteristics
Abstracted patient demographic data included age, sex, and comorbid conditions (history of hypertension, stroke, hemiplegia/paraplegia, dementia, diabetes and diabetes with end-organ damage, myocardial infarction, congestive heart failure, chronic obstructive pulmonary disease, asthma, moderate/severe liver disease, cirrhosis, peptic ulcer, kidney failure, peripheral vascular disease, and malignant neoplasm). Other abstracted variables included the source of ICU admission (postsurgical or other) and documentation of invasive or noninvasive mechanical ventilation. Sequential Organ Failure Assessment (SOFA) and Acute Physiology Assessment and Chronic Health Evaluation III scores were calculated on the day of ICU admission by using a previously validated automated system at our institution [23]. Hospital length of stay and 28-day and 1-year mortality rates were also assessed.
Assessments of BPV
Systolic and diastolic BP was measured in 15-min to 1-h intervals during the first 24 h after ICU admission [23]. Systolic BP measurements less than 40 mm Hg or greater than 300 mm Hg and diastolic BP measurements less than 20 mm Hg or greater than 195 mm Hg were set to missing and excluded from the analyses. We used several measures of BPV because the clinical and prognostic implications of BPV may depend on assessment technique, sampling frequency, and BP trends [8]. Our primary BPV measure was average real variability (ARV), which is calculated as the mean absolute difference among consecutive measurements obtained during a specific time period [25]. ARV yields better predictive power for hemodynamic oscillations than other BPV measures because ARV also accounts for the number of consecutive measurements and the order in which they were obtained [25]. Secondary BPV measures included range, which is a measure of BPV independent from the mean; standard deviation (SD), which does not reflect the steepness or rapidity of BPV but reflects the dispersion of hemodynamic measurements around a single value (i.e., mean) and thereby does not account for the order in which the measurements were obtained; and maximum Δ, which is the maximum absolute difference between consecutive measures during an observed time period. BPV measures were calculated separately for systolic and diastolic BP.
Assessments of Acute Cognitive/Alertness Status
Delirium was defined by a positive Confusion Assessment Method of the Intensive Care Unit (CAM-ICU) score (with a Richmond Agitation-Sedation Score greater than − 4). In patients with delirium, delirium-free days were defined as the number of days alive and without delirium in the ICU for up to 28 days. Depressed alertness was defined by two consecutive Glasgow Coma Scale (GCS) scores less than 15 and/or a Full Outline of UnResponsiveness (FOUR) score less than 16 in the absence of ongoing sedation and without a positive CAM-ICU score. In patients who were intubated, a GCS score less than 11 or a FOUR score less than 13 was indicative of depressed alertness [26, 27]. In patients with depressed alertness, depressed alertness-free days were calculated as the number of days alive and without depressed alertness in the ICU for up to 28 days. CAM-ICU was evaluated at least every 8 to 12 h, GCS/FOUR scores every 4 h, and Richmond Agitation-Sedation Score hourly during sedation.
Statistical Analyses
Patient demographics, ICU admission characteristics, and BPV measurements were summarized overall and according to the following cognitive outcome groups: delirium, depressed alertness, and preserved cognition (control). Continuous and ordinal variables were summarized as median (interquartile range [IQR]), and categorical variables were summarized as frequency (percent).
Before fitting the outcome models, BPV measurements were winsorized, in which values below the 2.5th percentile and values above the 97.5th percentile were set to the 2.5th and 97.5th percentiles, respectively. Therefore, our models assume no additional effects of increased or decreased BPV beyond the given range. The functional form of the association between each BPV measure and outcome was assessed visually, and BPV measures were included in the models as linear terms when the linearity assumption was satisfied. For BPV measures that did not satisfy the linearity assumption, the nonlinear association was assessed with graphical methods, with truncation used, when appropriate, if the linearity assumption was valid for a restricted range of the BPV measurement distribution.
Multivariable logistic regression models were fit to the data separately for the binary (any vs. none) delirium and depressed alertness end points to estimate the association between the BPV measures and the given outcome. In each case, the preserved cognition control group comprised patients who had a negative CAM-ICU score and a GCS score of at least 15 and/or a FOUR score of at least 16. Multiplicative increases in the odds of an event (odds ratio [OR]) per 5-mm Hg increase in a BPV measure were reported with 95% confidence intervals (CIs) and P values.
To assess the association between the BPV measures and delirium-free days and depressed alertness-free days in patients who had a given end point, multivariable proportional odds models were fit to the data, modeling the odds of a greater number of end point-free days. Estimated increases in the odds of greater end point-free days per 5-mm Hg increase in a BPV measure were reported as OR with 95% CIs and P values.
All models were adjusted for patient age (spline with knots at the 5th, 50th, and 95th percentiles) and sex, ICU length of stay (log transformed), use of invasive mechanical ventilation during the index ICU admission, ICU admission from surgical procedure or anesthesia, SOFA score components (kidney, coagulation, liver, and respiratory), comorbid conditions (diabetes, liver disease, kidney disease, chronic obstructive pulmonary disease, cerebrovascular accidents, dementia, and hypertension), and emergent surgical procedures. To account for multiple observations per patient, all models were fit by using robust covariance estimates based on the method of generalized estimating equations. All models were fit with SAS v9.4 (SAS Institute Inc). P values less than.05 were considered statistically significant.
Results
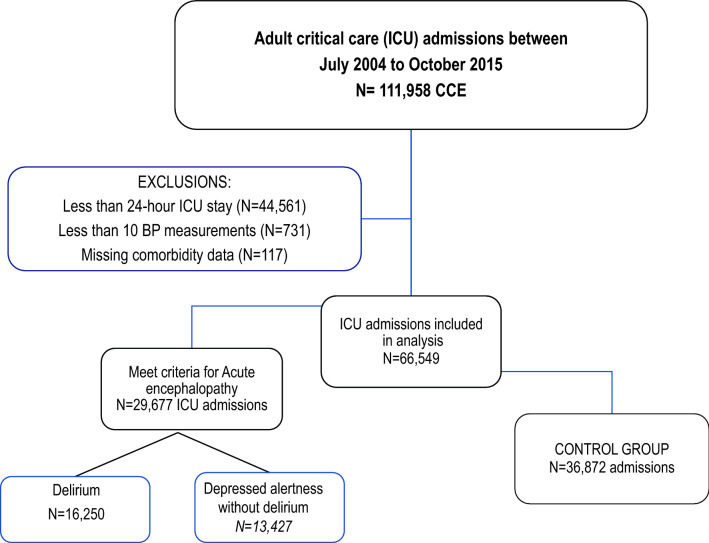
A total of 111,958 hospitalizations that included ICU admission occurred during the study period. Of these ICU admissions, 45,409 were excluded because they did not meet the study criteria (< 24-h ICU stay, < 10 BP measurements in the first 24 h, or missing comorbid condition data; Fig. 1). Therefore, 66,549 ICU admissions of 54,056 unique patients were included in our analysis. Delirium was documented in 13,427 (20.2%) ICU admissions, depressed alertness in 16,250 (24.4%), and preserved cognition in 36,872 (55.4%). The median (IQR) number of delirium-free days was 26 (24–27), and the median (IQR) number of depressed alertness-free days was 26 (25–27). Patient characteristics and ICU outcomes of the total cohort and the delirium, depressed alertness, and preserved cognition groups are summarized in Table 1.
Fig. 1.
Flow diagram of ICU admissions included in the study. This study conformed to the Strengthening The Reporting of OBservational studies in Epidemiology statement. BP, blood pressure, ICU, intensive care unit, CCE, critical care episodes
Table 1.
Patient characteristics and intensive care unit outcomesa
| Variable | Total (N = 66,549) | Deliriumb (n = 13,427) | Depressed alertnessb (n = 16,250) | Preserved cognition (n = 36,872) |
|---|---|---|---|---|
| Age, (yr) | 66 (54–77) | 69 (58–79) | 67 (54–78) | 65 (52–75) |
| Sex | ||||
| Women | 28,234 (42.4) | 5701 (42.5) | 7259 (44.7) | 15,274 (41.4) |
| Men | 38,315 (57.6) | 7726 (57.5) | 8991 (55.3) | 21,598 (58.6) |
| Postsurgical ICU admission | 23,731 (35.7) | 4033 (30.0) | 6826 (42.0) | 12,872 (34.9) |
| Index ICU specialty | ||||
| Cardiac, medical | 10,796 (16.2) | 1136 (8.5) | 1834 (11.3) | 7826 (21.2) |
| Cardiac, surgical | 12,096 (18.2) | 1367 (10.2) | 3937 (24.2) | 6792 (18.4) |
| Medical/surgical/transplant | 8055 (12.1) | 1927 (14.4) | 1542 (9.5) | 4586 (12.4) |
| Medical | 16,573 (24.9) | 4021 (29.9) | 4574 (28.1) | 7878 (21.4) |
| Trauma/general | 10,852 (16.3) | 2503 (18.6) | 2456 (15.1) | 5893 (16.0) |
| Vascular/thoracic | 8177 (12.3) | 2473 (18.4) | 1907 (11.7) | 3797 (10.3) |
| SOFA score |
5 (2–8) (n = 57,923) |
7 (4–9) (n = 12,085) |
6 (4–8) (n = 13,270) |
4 (2–6) (n = 32,568) |
| APACHE III score |
64 (50–80) (n = 61,557) |
76 (61–93) (n = 12,827) |
70 (55–85) (n = 14,586) |
58 (45–72) (n = 34,144) |
| Invasive ventilator use | 29,714 (44.6) | 7868 (58.6) | 10,154 (62.5) | 11,692 (31.7) |
| ICU length of stay, (d) | 2.1 (1.5–3.9) | 4.1 (2.1–8.7) | 2.7 (1.7–4.8) | 1.8 (1.2–2.8) |
| Hospital length of stay, (d) | 7.4 (4.6–12.9) | 12.7 (7.6–22.8) | 8.6 (5.7–14.4) | 6.1 (3.8–9.3) |
| Hospital discharge status | ||||
| Alive | 60,977 (91.6) | 11,355 (84.6) | 14,561 (89.6) | 35,061 (95.1) |
| Deceased | 5572 (8.4) | 2072 (15.4) | 1689 (10.4) | 1811 (4.9) |
APACHE III, Acute Physiology Assessment and Chronic Health Evaluation III, CAM-ICU, Confusion Assessment Method of the Intensive Care Unit; ICU, intensive care unit; IQR, interquartile range, No, number, SOFA, Sequential Organ Failure Assessment
aContinuous variables are summarized as median (IQR), and categorical variables are summarized as No. (%) of ICU admissions
bICU admissions of patients with a positive CAM-ICU score were included in the delirium group. ICU admissions of patients who did not have a positive CAM-ICU score but who had a Glasgow Coma Scale score < 15 and/or Full Outline of UnResponsiveness score < 16 were included in the depressed alertness group
Table 2 summarizes the measures of systolic and diastolic BPV (range, SD, ARV, and maximum Δ) during the first 24 h after ICU admission for the total cohort and each cognitive outcome group. Our primary BPV measure was ARV. The median (IQR) systolic ARV was 8.5 mm Hg (6.7–10.7 mm Hg) for the total cohort, 9.1 mm Hg (7.1–11.4 mm Hg) for the delirium group, 8.4 mm Hg (6.6–10.7 mm Hg) for the depressed alertness group, and 8.3 mm Hg (6.6–10.4 mm Hg) for the preserved cognition group. The median (IQR) diastolic ARV was 5.4 mm Hg (3.8–7.7 mm Hg) for the total cohort, 5.4 mm Hg (3.9–7.9 mm Hg) for the delirium group, 4.9 mm Hg (3.5–7.0 mm Hg) for the depressed alertness group, and 5.6 mm Hg (3.9–7.9 mm Hg) for the preserved cognition group.
Table 2.
Blood pressure variability measures within the first 24 h after intensive care unit admission
| BPV measure, mm Hg | Total (N = 66,549) | Cognitive outcome group, median (IQR) | |||
|---|---|---|---|---|---|
| Median (IQR) | 2.5th, 97.5th percentilea | Deliriumb (n = 13,427) | Depressed alertnessb (n = 16,250) | Preserved cognition (n = 36,872) | |
| Systolic | |||||
| Range | 65 (50–84) | 29, 140 | 73 (57–93) | 69 (53–88) | 61 (47–78) |
| SD | 13.6 (10.7–17.3) | 6.7, 27.3 | 14.9 (11.7–18.9) | 14.0 (11.1–17.8) | 13.0 (10.2–16.4) |
| ARV | 8.5 (6.7–10.7) | 4.2, 16.7 | 9.1 (7.1–11.4) | 8.4 (6.6–10.7) | 8.3 (6.6–10.4) |
| Maximum Δ | 38 (28–52) | 16, 102 | 44 (32–59) | 40 (30–55) | 36 (27–48) |
| Mean | 115 (106–127) | 90, 156 | 115 (106–128) | 114 (106–126) | 116 (106–127) |
| Diastolic | |||||
| Range | 43 (32–60) | 19, 112 | 48 (35–65) | 43 (31–59) | 42 (31–58) |
| SD | 8.5 (6.4–11.2) | 4.0, 19.5 | 9.0 (6.7–11.9) | 8.2 (6.2–10.9) | 8.4 (6.4–11.1) |
| ARV | 5.4 (3.8–7.7) | 2.2, 14.6 | 5.4 (3.9–7.9) | 4.9 (3.5–7.0) | 5.6 (3.9–7.9) |
| Maximum Δ | 28 (19–42) | 10, 92 | 31 (21–47) | 27 (18–42) | 28 (19–41) |
| Mean | 59 (53–67) | 42, 84 | 58 (52–65) | 58 (52–65) | 60 (54–68) |
ARV, average real variability; BPV, blood pressure variability, CAM-ICU, Confusion Assessment Method of the Intensive Care Unit, ICU, intensive care unit, IQR, interquartile range, SD, standard deviation
aPercentiles were used to winsorize variables for modeling purposes
bICU admissions of patients with any positive CAM-ICU scores were included in the delirium group. ICU admissions of patients who did not have a positive CAM-ICU score but who had a Glasgow Coma Scale score < 15 and/or Full Outline of UnResponsiveness score < 16 were included in the depressed alertness group
Table 3 summarizes the covariate-adjusted analyses of the association between BPV and the likelihood of delirium and delirium-free days. For all BPV measures, increased BPV was associated with a higher odd of delirium (P < 0.001). An increased duration of delirium (i.e., fewer delirium-free days) was also associated with an increase in all BPV measures (P ≤ 0.001) except SD (P = 0.22). Table 4 summarizes the covariate-adjusted analyses of the association between BPV and the likelihood of depressed alertness and depressed alertness-free days. Higher systolic ARV was associated with an increased odds of depressed alertness (P = 0.004) but not with depressed alertness-free days (P = 0.65). The magnitude of the association of BPV measures was greater for the delirium group than for the depressed alertness group. Specifically, a 5-mm Hg increase in systolic ARV was associated with a 34% increase in the odds of delirium (OR 1.34; 95% CI 1.29–1.40) and a 6% increase in the odds of depressed alertness (OR 1.06; 95% CI 1.02–1.10) (Tables 3, 4).
Table 3.
Association of blood pressure variability measures with delirium and delirium-free days during the first 24 h after ICU Admissiona
| BPV measure | Deliriumb | Delirium-free daysc | ||
|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |
| Systolic | ||||
| Range | 1.05 (1.04–1.05) | < .001 | 0.99 (0.98–1.00) | .001 |
| SD | 1.25 (1.22–1.28) | < .001 | 0.98 (0.95–1.01) | .22 |
| ARV | 1.34 (1.29–1.40) | < .001 | 0.91 (0.87–0.96) | < .001 |
| Maximum Δ, ≤ 75 mm Hg | 1.07 (1.06–1.07) | < .001 | 0.98 (0.97–0.99) | < .001 |
| Diastolic | ||||
| Range | 1.04 (1.04–1.05) | < .001 | 0.98 (0.98–0.99) | < .001 |
| SD | 1.27 (1.22–1.31) | < .001 | 0.93 (0.89–0.97) | < .001 |
| ARV | 1.23 (1.18–1.29) | < .001 | 0.90 (0.85–0.95) | < .001 |
| Maximum Δ | 1.05 (1.04–1.05) | < .001 | 0.98 (0.97–0.99) | < .001 |
ARV, average real variability; BPV, blood pressure variability; CI, confidence interval; ICU, intensive care unit; OR, odds ratio; SD, standard deviation
aORs and corresponding 95% CI and P values were generated from multivariable logistic and proportional odds regression models accounting for multiple observations per subject patient by using generalized estimating equations with independent correlation structure. Covariates included patient age (spline with three knots) and sex, ICU length of stay (log transformed), use of invasive mechanical ventilation during the index ICU admission, ICU admission from surgical procedure/anesthesia, Sequential Organ Failure Assessment components (kidney, coagulation, liver, and respiratory), diabetes with complications, history of moderate to severe liver disease, kidney disease, chronic obstructive pulmonary disease, cerebrovascular accident, dementia, emergent surgical procedure, and hypertension. All ORs are reported per 5-mm Hg increase in BPV. The preserved cognition control group was used as a reference for comparisons
bData were analyzed with multivariable logistic regression
cData were analyzed with multivariable proportional odds regression. Only ICU admissions of patients with a positive Confusion Assessment Method of the Intensive Care Unit score were included in the delirium-free days analysis
Table 4.
Association of blood pressure variability measures with depressed alertness and depressed alertness-free days during the first 24 h after ICU Admissiona
| BPV measure | Depressed alertnessb | Depressed alertness-free daysc | ||
|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |
| Systolic | ||||
| Range | 1.03 (1.02–1.03) | < .001 | 0.99 (0.98–0.99) | < .001 |
| SD | 1.13 (1.10–1.15) | < .001 | 0.96 (0.93–0.99) | .007 |
| ARV | 1.06 (1.02–1.10) | .004 | 0.99 (0.94–1.04) | .65 |
| Maximum Δ, ≤ 75 mm Hg | 1.04 (1.03–1.04) | < .001 | 0.98 (0.98–0.99) | < .001 |
| Diastolic | ||||
| Range | 1.01 (1.01–1.02) | < .001 | 0.99 (0.98–1.00) | .005 |
| SD | 1.03 (1.00–1.06) | .04 | 0.96 (0.92–1.00) | .07 |
| ARV | 0.92 (0.88–0.95) | < .001 | 0.99 (0.93–1.05) | .74 |
| Maximum Δ | 1.01 (1.01–1.02) | < .001 | 0.99 (0.98–1.00) | .01 |
ARV, average real variability; BPV, blood pressure variability; CI, confidence interval; ICU, intensive care unit; OR, odds ratio; SD, standard deviation
aORs and corresponding 95% CI and P values were generated from multivariable logistic and proportional odds regression models accounting for multiple observations per patient by using generalized estimating equations with exchangeable correlation structure. Covariates are the same as those listed in Table 3. All ORs are reported per 5-mm Hg increase in BPV. The preserved cognition control group was used as a reference for comparisons
bData were analyzed with multivariable logistic regression
cData were analyzed with multivariable proportional odds regressions. ICU admissions of patients who did not have a positive Confusion Assessment Method of the Intensive Care Unit score but had a Glasgow Coma Scale score < 15 and/or Full Outline of UnResponsiveness score < 16 were included in this analysis
Discussion
Increased BPV during the first 24 h after ICU admission was associated with a higher likelihood and duration of delirium. Increased BPV was also associated with a higher likelihood of depressed alertness, but this association was weaker and less consistent. Additional research is needed to understand whether reduced BPV in patients with critical illness lowers the risk of delirium and depressed alertness and subsequently improves long-term patient outcomes.
Delirium in patients admitted to an ICU is associated with higher mortality rates, longer hospitalization, greater risk of post-ICU syndrome, more rapid cognitive impairment, and higher health care costs than in ICU-admitted patients without delirium [3–5, 28, 29]. The increased severity of these complications in patients with delirium led to the launching of various initiatives to detect and eliminate precipitating risk factors for delirium and to implement interventions for modifiable risk factors. To this end, bundle interventions, such as the ICU Liberation initiative [30], have been introduced to prevent delirium in patients admitted to an ICU; however, current bundle interventions do not include BPV as an intervention target [31]. Our findings show an association between BPV and the likelihood and duration of delirium. The association between BPV during the first 24 h after ICU admission and delirium was strong and consistent for all measures of BPV. If our observations are confirmed in future studies, new interventions may be developed and subsequently assessed in clinical trials to examine whether reducing BPV can decrease the burden of delirium (i.e., the occurrence and duration of delirium) in patients with critical illness.
BPV has been repeatedly linked with adverse outcomes independent of absolute BP control [9, 32, 33]. For example, high systolic BPV is associated with poor outcomes of acute stroke [10, 11], hastened progression of cerebral small vessel disease (i.e., white matter hyperintensities, lacunae of presumed vascular origin, cerebral microbleeds, and enlarged perivascular spaces) [34, 35], increased risk of cardiovascular-related disease and death [9, 13, 36, 37], and progression of chronic kidney disease [14, 38]. Long-term BPV (clinic visit-to-visit variability) is also associated with rapid onset and development of mild cognitive impairment and dementia [39], despite excellent BP control [40]. Although the results of studies investigating the link between intraoperative hypotension and postoperative cognitive decline and delirium are inconsistent [41–44], increased short-term intraoperative BPV is associated with postoperative delirium [17, 18, 45]. Disparity between the two types of BP measures (i.e., mean BP vs. BPV) can be attributed to the deleterious effects of BPV, which is independent of mean arterial BP [40]. The deleterious effects of BPV on cerebral function can be explained by microvascular and blood–brain barrier damage caused by enlarged pulsatile loads, which are inadequately buffered by impaired cerebral autoregulation during acute critical illness [46, 47].
Our study did not address long-term cognitive effects of delirium after ICU admission, but earlier studies have shown that delirium is associated with accelerated cognitive decline, including a higher likelihood of mild cognitive impairment and dementia [28, 48, 49]. In our previous study of a small cohort of participants enrolled in the Mayo Clinic Olmsted Study of Aging who were admitted to an ICU, increased BPV during the first 24 h after ICU admission was associated with an increased likelihood of delirium and worsened long-term cognitive outcomes [22]. Another study reported an association between increased BPV and an increased presence of neurofibrillary tangles, an indicator of Alzheimer disease, on autopsy [32, 39]. Therefore, minimizing BPV may reduce not only in-hospital delirium but also progression of long-term cognitive decline.
The primary strengths of our study are its large cohort size of patients with critical illness and analysis of both delirium and delirium-free days as cognitive end points. However, our study also has limitations. We measured BPV only within the first 24 h after ICU admission. Additional studies are needed to determine whether BPV continuing after the first day of ICU admission has additional effects on cognitive outcomes. Because the criteria for delirium or depressed alertness were met for many patients on the day of ICU admission, we cannot establish causality. Thus, we could not determine whether BPV contributed to delirium or was simply a marker of its occurrence. Delirium and increased BPV may share a common underlying cause, but BPV may still exacerbate delirium in this case. Despite using composite SOFA and Acute Physiology Assessment and Chronic Health Evaluation III scores to account for comorbidities, as with any critical care observational studies, residual confounding is always possible. Some potential confounders include variable physiological derangement, comorbidities (premorbid cognitive status, atrial fibrillation), and medications administered (γ-aminobutyric acid-ergic (GABAergic), anticholinergics). We also aimed to minimize bias by only including nonneurologic critically ill patients and by using measures obtained consistently on all patients. Furthermore, because cognitive and sedation assessment scores in the Data Mart are entered by ICU nurses, the excellent concordance of these entries with physician evaluation was confirmed in a previous study [26]. The end date of our study period was October 31, 2015, which may not be representative of contemporary ICU practice. We specifically chose this end date because changes in the internal format of our electronic health records after the study end date may have affected the consistency of the data in our analyses. Although we are not aware of any practice changes after our study end date that would have influenced our findings, the COVID-19 pandemic has substantially changed the critical care patient population. How our results would apply to ICU-admitted patients with COVID-19 is unknown.
Conclusions
Our findings indicate that increased early BPV is associated with a higher burden of delirium during hospitalization with ICU admission. If these results are confirmed in future studies, BPV could be incorporated in bundle interventions aimed at preventing delirium in patients with critical illness.
Acknowledgements
We express our great appreciation to our data analyst Andrew J. Jennissen for his statistical contributions to the article.
Author contributions
NZG, AAR: conception and design of the work, acquisition, analysis and interpretation of data, drafting of the work and revisions for important intellectual content, final approval. JS, OG, TNW: conception and design of the work, interpretation of data, critical revisions for important intellectual content, final approval. VH, TDS, DJVM: acquisition and interpretation of data, revisions for important intellectual content, final approval; ACH, DRS: data analysis, statistical work, revisions of the work for important intellectual content, final approval.
Source of support
This study was supported by the Mayo Anesthesiology Small Grant Program (FP00119684, Sprung) and Mayo Critical Care Independent Multidisciplinary Program Research Subcommittee Small Grants Program (Rabinstein).
Conflicts of interest
The authors declare that they have no conflicts of interest.
Ethical approval/informed consent
The reporting of this study conformed to the STROBE statement and was approved by the Mayo Clinic Institutional Review Board (14-001118).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Van Rompaey B, Schuurmans MJ, Shortridge-Baggett LM, Truijen S, Elseviers M, Bossaert L. A comparison of the CAM-ICU and the NEECHAM Confusion Scale in intensive care delirium assessment: an observational study in non-intubated patients. Crit Care. 2008;12:R16. doi: 10.1186/cc6790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ely EW, Margolin R, Francis J, May L, Truman B, Dittus R, et al. Evaluation of delirium in critically ill patients: validation of the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU) Crit Care Med. 2001;29:1370–1379. doi: 10.1097/00003246-200107000-00012. [DOI] [PubMed] [Google Scholar]
- 3.Salluh JI, Wang H, Schneider EB, Nagaraja N, Yenokyan G, Damluji A, et al. Outcome of delirium in critically ill patients: systematic review and meta-analysis. BMJ. 2015;350:h2538. doi: 10.1136/bmj.h2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fiest KM, Soo A, Hee Lee C, Niven DJ, Ely EW, Doig CJ, et al. Long-term outcomes in ICU patients with delirium: a population-based cohort study. Am J Respir Crit Care Med. 2021;204:412–420. doi: 10.1164/rccm.202002-0320OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sakusic A, Gajic O, Singh TD, O'Horo JC, Jenkins G, Wilson GA, et al. Risk factors for persistent cognitive impairment after critical illness, nested case-control study. Crit Care Med. 2018;46:1977–1984. doi: 10.1097/CCM.0000000000003395. [DOI] [PubMed] [Google Scholar]
- 6.Bulic D, Bennett M, Georgousopoulou EN, Shehabi Y, Pham T, Looi JCL, et al. Cognitive and psychosocial outcomes of mechanically ventilated intensive care patients with and without delirium. Ann Intensive Care. 2020;10:104. doi: 10.1186/s13613-020-00723-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maldonado JR. Acute brain failure: pathophysiology, diagnosis, management, and sequelae of delirium. Crit Care Clin. 2017;33:461–519. doi: 10.1016/j.ccc.2017.03.013. [DOI] [PubMed] [Google Scholar]
- 8.Parati G, Ochoa JE, Lombardi C, Bilo G. Assessment and management of blood-pressure variability. Nat Rev Cardiol. 2013;10:143–155. doi: 10.1038/nrcardio.2013.1. [DOI] [PubMed] [Google Scholar]
- 9.Stevens SL, Wood S, Koshiaris C, Law K, Glasziou P, Stevens RJ, et al. Blood pressure variability and cardiovascular disease: systematic review and meta-analysis. BMJ. 2016;354:i4098. doi: 10.1136/bmj.i4098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bennett AE, Wilder MJ, McNally JS, Wold JJ, Stoddard GJ, Majersik JJ, et al. Increased blood pressure variability after endovascular thrombectomy for acute stroke is associated with worse clinical outcome. J Neurointerv Surg. 2018;10:823–827. doi: 10.1136/neurintsurg-2017-013473. [DOI] [PubMed] [Google Scholar]
- 11.Lattanzi S, Cagnetti C, Provinciali L, Silvestrini M. Blood pressure variability and clinical outcome in patients with acute intracerebral hemorrhage. J Stroke Cerebrovasc Dis. 2015;24:1493–1499. doi: 10.1016/j.jstrokecerebrovasdis.2015.03.014. [DOI] [PubMed] [Google Scholar]
- 12.Kirkness CJ, Burr RL, Mitchell PH. Intracranial and blood pressure variability and long-term outcome after aneurysmal sub-arachnoid hemorrhage. Am J Crit Care. 2009;18:241–251. doi: 10.4037/ajcc2009743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rossignol P, Girerd N, Gregory D, Massaro J, Konstam MA, Zannad F. Increased visit-to-visit blood pressure variability is associated with worse cardiovascular outcomes in low ejection fraction heart failure patients: insights from the HEAAL study. Int J Cardiol. 2015;187:183–189. doi: 10.1016/j.ijcard.2015.03.169. [DOI] [PubMed] [Google Scholar]
- 14.Saito K, Saito Y, Kitahara H, Nakayama T, Fujimoto Y, Kobayashi Y. In-hospital blood pressure variability: a novel prognostic marker of renal function decline and cardiovascular events in patients with coronary artery disease. Kidney Blood Press Res. 2020;45:748–757. doi: 10.1159/000509291. [DOI] [PubMed] [Google Scholar]
- 15.Lizano-Diez I, Poteet S, Burniol-Garcia A, Cerezales M. The burden of perioperative hypertension/hypotension: a systematic review. PLoS ONE. 2022;17:e0263737. doi: 10.1371/journal.pone.0263737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Radinovic K, Markovic Denic L, Milan Z, Cirkovic A, Baralic M, Bumbasirevic V. Impact of intraoperative blood pressure, blood pressure fluctuation, and pulse pressure on postoperative delirium in elderly patients with hip fracture: a prospective cohort study. Injury. 2019;50:1558–1564. doi: 10.1016/j.injury.2019.06.026. [DOI] [PubMed] [Google Scholar]
- 17.Wang NY, Hirao A, Sieber F. Association between intraoperative blood pressure and postoperative delirium in elderly hip fracture patients. PLoS ONE. 2015;10:e0123892. doi: 10.1371/journal.pone.0123892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hirsch J, DePalma G, Tsai TT, Sands LP, Leung JM. Impact of intraoperative hypotension and blood pressure fluctuations on early postoperative delirium after non-cardiac surgery. Br J Anaesth. 2015;115:418–426. doi: 10.1093/bja/aeu458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou TL, Kroon AA, van Sloten TT, van Boxtel MPJ, Verhey FRJ, Schram MT, et al. Greater blood pressure variability is associated with lower cognitive performance. Hypertension. 2019;73:803–811. doi: 10.1161/HYPERTENSIONAHA.118.12305. [DOI] [PubMed] [Google Scholar]
- 20.Slessarev M, Mahmoud O, McIntyre CW, Ellis CG. Cerebral blood flow deviations in critically ill patients: potential insult contributing to ischemic and hyperemic injury. Front Med (Lausanne) 2020;7:615318. doi: 10.3389/fmed.2020.615318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Armstead WM. Cerebral blood flow autoregulation and dysautoregulation. Anesthesiol Clin. 2016;34:465–477. doi: 10.1016/j.anclin.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zorko Garbajs N, Singh TD, Valencia Morales DJ, Herasevich V, Warner DO, Martin DP, et al. Association of blood pressure variability with short- and long-term cognitive outcomes in patients with critical illness. J Crit Care. 2022;71:154107. doi: 10.1016/j.jcrc.2022.154107. [DOI] [PubMed] [Google Scholar]
- 23.Herasevich V, Pickering BW, Dong Y, Peters SG, Gajic O. Informatics infrastructure for syndrome surveillance, decision support, reporting, and modeling of critical illness. Mayo Clin Proc. 2010;85:247–254. doi: 10.4065/mcp.2009.0479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.von Hofen-Hohloch J, Awissus C, Fischer MM, Michalski D, Rumpf JJ, Classen J. Delirium screening in neurocritical care and stroke unit patients: a pilot study on the influence of neurological deficits on CAM-ICU and ICDSC outcome. Neurocrit Care. 2020;33:708–717. doi: 10.1007/s12028-020-00938-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mena LJ, Felix VG, Melgarejo JD, Maestre GE. 24-hour blood pressure variability assessed by average real variability: a systematic review and meta-analysis. J Am Heart Assoc. 2017;6:e006895. doi: 10.1161/JAHA.117.006895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reddy DR, Singh TD, Guru PK, Sakusic A, Gajic O, O'Horo JC, et al. Identification of acute brain failure using electronic medical records. J Crit Care. 2016;34:12–16. doi: 10.1016/j.jcrc.2016.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Singh TD, O'Horo JC, Gajic O, Sakusic A, Day CN, Mandrekar J, et al. Risk factors and outcomes of critically ill patients with acute brain failure: a novel end point. J Crit Care. 2018;43:42–47. doi: 10.1016/j.jcrc.2017.08.028. [DOI] [PubMed] [Google Scholar]
- 28.Sprung J, Roberts RO, Weingarten TN, Nunes Cavalcante A, Knopman DS, Petersen RC, et al. Postoperative delirium in elderly patients is associated with subsequent cognitive impairment. Br J Anaesth. 2017;119:316–323. doi: 10.1093/bja/aex130. [DOI] [PubMed] [Google Scholar]
- 29.Kinchin I, Mitchell E, Agar M, Trepel D. The economic cost of delirium: a systematic review and quality assessment. Alzheimers Dement. 2021;17:1026–1041. doi: 10.1002/alz.12262. [DOI] [PubMed] [Google Scholar]
- 30.Rose L, Burry L, Agar M, Campbell NL, Clarke M, Lee J, et al. A Core outcome set for research evaluating interventions to prevent and/or treat delirium in critically ill adults: an international consensus study (Del-COrS) Crit Care Med. 2021;49(9):1535–1546. doi: 10.1097/CCM.0000000000005028. [DOI] [PubMed] [Google Scholar]
- 31.Ely EW. The ABCDEF bundle: science and philosophy of how ICU liberation serves patients and families. Crit Care Med. 2017;45:321–330. doi: 10.1097/CCM.0000000000002175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ma Y, Tully PJ, Hofman A, Tzourio C. Blood pressure variability and dementia: a state-of-the-art review. Am J Hypertens. 2020;33:1059–1066. doi: 10.1093/ajh/hpaa119. [DOI] [PubMed] [Google Scholar]
- 33.Muntner P, Whittle J, Lynch AI, Colantonio LD, Simpson LM, Einhorn PT, et al. Visit-to-visit variability of blood pressure and coronary heart disease, stroke, heart failure, and mortality: a cohort study. Ann Intern Med. 2015;163:329–338. doi: 10.7326/M14-2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tully PJ, Yano Y, Launer LJ, Kario K, Nagai M, Mooijaart SP, et al. Association between blood pressure variability and cerebral small-vessel disease: a systematic review and meta-analysis. J Am Heart Assoc. 2020;9:e013841. doi: 10.1161/JAHA.119.013841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Castello JP, Teo KC, Abramson JR, Keins S, Takahashi CE, Leung IYH, et al. Long-term blood pressure variability and major adverse cardiovascular and cerebrovascular events after intracerebral hemorrhage. J Am Heart Assoc. 2022;11:e024158. doi: 10.1161/JAHA.121.024158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mehlum MH, Liestol K, Kjeldsen SE, Julius S, Hua TA, Rothwell PM, et al. Blood pressure variability and risk of cardiovascular events and death in patients with hypertension and different baseline risks. Eur Heart J. 2018;39:2243–2251. doi: 10.1093/eurheartj/ehx760. [DOI] [PubMed] [Google Scholar]
- 37.Mancia G, Bombelli M, Facchetti R, Madotto F, Corrao G, Trevano FQ, et al. Long-term prognostic value of blood pressure variability in the general population: results of the Pressioni Arteriose Monitorate e Loro Associazioni Study. Hypertension. 2007;49:1265–1270. doi: 10.1161/HYPERTENSIONAHA.107.088708. [DOI] [PubMed] [Google Scholar]
- 38.Wang Q, Wang Y, Wang J, Zhang L, Zhao MH, Dagger CS. Short-term systolic blood pressure variability and kidney disease progression in patients with chronic kidney disease: results from C-STRIDE. J Am Heart Assoc. 2020;9:e015359. doi: 10.1161/JAHA.120.015359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ma Y, Song A, Viswanathan A, Blacker D, Vernooij MW, Hofman A, et al. Blood pressure variability and cerebral small vessel disease: a systematic review and meta-analysis of population-based cohorts. Stroke. 2020;51:82–89. doi: 10.1161/STROKEAHA.119.026739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.de Havenon A, Anadani M, Prabhakaran S, Wong KH, Yaghi S, Rost N. Increased blood pressure variability and the risk of probable dementia or mild cognitive impairment: a post hoc analysis of the SPRINT MIND Trial. J Am Heart Assoc. 2021;10:e022206. doi: 10.1161/JAHA.121.022206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wachtendorf LJ, Azimaraghi O, Santer P, Linhardt FC, Blank M, Suleiman A, et al. Association between intraoperative arterial hypotension and postoperative delirium after noncardiac surgery: a retrospective multicenter cohort study. Anesth Analg. 2022;134:822–833. doi: 10.1213/ANE.0000000000005739. [DOI] [PubMed] [Google Scholar]
- 42.Moller JT, Cluitmans P, Rasmussen LS, Houx P, Rasmussen H, Canet J, et al. Long-term postoperative cognitive dysfunction in the elderly ISPOCD1 study. ISPOCD investigators. International Study of Post-Operative Cognitive Dysfunction. Lancet. 1998;351:857–861. doi: 10.1016/S0140-6736(97)07382-0. [DOI] [PubMed] [Google Scholar]
- 43.Williams-Russo P, Sharrock NE, Mattis S, Liguori GA, Mancuso C, Peterson MG, et al. Randomized trial of hypotensive epidural anesthesia in older adults. Anesthesiology. 1999;91:926–935. doi: 10.1097/00000542-199910000-00011. [DOI] [PubMed] [Google Scholar]
- 44.Langer T, Santini A, Zadek F, Chiodi M, Pugni P, Cordolcini V, et al. Intraoperative hypotension is not associated with postoperative cognitive dysfunction in elderly patients undergoing general anesthesia for surgery: results of a randomized controlled pilot trial. J Clin Anesth. 2019;52:111–118. doi: 10.1016/j.jclinane.2018.09.021. [DOI] [PubMed] [Google Scholar]
- 45.Patti R, Saitta M, Cusumano G, Termine G, Di Vita G. Risk factors for postoperative delirium after colorectal surgery for carcinoma. Eur J Oncol Nurs. 2011;15:519–523. doi: 10.1016/j.ejon.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 46.Rickards CA, Tzeng YC. Arterial pressure and cerebral blood flow variability: friend or foe? A review. Front Physiol. 2014;5:120. doi: 10.3389/fphys.2014.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Goodson CM, Rosenblatt K, Rivera-Lara L, Nyquist P, Hogue CW. Cerebral blood flow autoregulation in sepsis for the intensivist: why its monitoring may be the future of individualized care. J Intensive Care Med. 2018;33:63–73. doi: 10.1177/0885066616673973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pandharipande PP, Girard TD, Jackson JC, Morandi A, Thompson JL, Pun BT, et al. Long-term cognitive impairment after critical illness. N Engl J Med. 2013;369:1306–1316. doi: 10.1056/NEJMoa1301372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sakusic A, Rabinstein AA. Cognitive outcomes after critical illness. Curr Opin Crit Care. 2018;24:410–414. doi: 10.1097/MCC.0000000000000527. [DOI] [PubMed] [Google Scholar]