Abstract
Home infusion therapy is a rapidly growing field in healthcare, allowing patients to receive postacute care at home at a fraction of the cost of an inpatient stay. Understanding the various drug delivery systems can facilitate a more seamless discharge to home with improved patient outcomes. Herein we review several home infusion methods of administration utilized to transition patients from hospital to home care for a variety of therapies.
Keywords: administration, devices, home infusion, infusion pumps, intravenous
One barrier to home antibiotic use may be lack of knowledge surrounding the modalities available to patients and providers.
According to the National Home Infusion Foundation Trends 2020 Report, home and specialty infusion is a $19 billion industry serving >3.2 million patients each year [1]. Traditionally, the elderly population was responsible for the demand in home care services. More recently, younger populations have driven demand by opting for elective surgeries in outpatient settings, which often require follow-up home care.
During the coronavirus disease 2019 pandemic, elective surgeries halted, resulting in a decreased need for follow-up home care [2]. Despite the short-term negative impact of the pandemic on hospital inpatient census, the home infusion market remained versatile and is projected to have an annual growth rate of 7.5% through 2028 [3]. By lowering the cost of healthcare by reducing inpatient stay, and increased options for infusion devices, this trend will likely continue for the foreseeable future.
Home infusion therapy (HIT) provides efficient and effective administration methods directly in a patient's home, seen also in nursing homes, hospitals, and outpatient clinics. Therapies include, but are not limited to, intravenous (IV) administration of various antimicrobials, total parenteral nutrition (TPN), patient-controlled analgesia (PCA), inotropes, home hydration, immunoglobulins, and other immunomodulating agents through various methods of administration, whose versatility provides patients with further autonomy over their own care.
With a variety of administration methods to choose from, there often exists a knowledge gap between the home infusion company and clinicians, potentially resulting in suboptimal therapeutic outcomes. The purpose of this article is to minimize that gap, summarizing various administration methods utilized in HIT with further emphasis on the pros and cons of each (Table 1).
Table 1.
Summary of Delivery Systems
| Pros | Cons |
|---|---|
| IV push | |
|
|
| Elastomeric | |
|
|
| Dial-regulated gravity infusion | |
|
|
| Ambulatory pumps (CADD) | |
|
|
| Stationary pump | |
|
|
Abbreviations: ADLs, activities of daily living; CADD, continuous ambulatory delivery device; IV, intravenous; USP, United States Pharmacopeial Convention; VNA, Visiting Nurse Association.
PICC YOUR ACCESS
To qualify for home infusion, patients must be discharged home with a vascular access device (VAD), which allows for the administration of IV medications. These devices include peripheral venous catheters (PVCs), midlines, and central venous catheters (CVCs), such as peripherally inserted central catheters (PICCs) and ports. Of note, certain medications do not require IV access and may be administered subcutaneously or intramuscularly, which home infusion companies may also accommodate.
A PVC is a short catheter, typically up to 3 inches in length, inserted on either the back of the hand or the lower arm. Unlike the other VADs, a PVC stays local to the insertion point. A midline is slightly longer than a PVC, typically 3–8 inches in length, with the tip ending in the mid-upper arm [4]. A PICC is a central catheter 45–50 cm in length [5]. It is inserted peripherally, usually guided by ultrasound, with the tip of the PICC angled downward toward the right heart border, within the superior vena cava [4]. Ports are placed under the skin and attached to a catheter that is guided into the superior vena cava.
When choosing which VAD to discharge the patient home with, a clinician must consider several factors, such as duration of therapy. A PVC might be considered if the clinician is sending the patient home on 1–5 days of therapy, as a PVC may dwell for 3–5 days before being removed or replaced [6]. A midline may dwell for up to 4 weeks and CVCs may dwell for months to years per patency [6].
There are, however, other factors to consider prior to choosing a specific VAD. For example, the osmolarity and/or the pH of the medication may dictate whether that medication may be infused over a peripheral line versus a central line. Additionally, if the medication is likely to cause extravasation, otherwise known as a vesicant drug, it must be administered through a CVC. Other medications are known to be irritants, such as vancomycin, which is typically infused through a CVC; however, it may be infused through a midline if compounded at a more dilute concentration.
Additionally, the type of VAD may depend on which administration method is chosen. For example, if a patient requires an ambulatory pump for their infusions, most nursing agencies will not accept a patient with a PVC due to the risk of mechanical damage to the vasculature.
Another important consideration is showering, for which disconnecting and reconnecting the IV tubing is strongly discouraged due to risk of contamination. Patients are instructed to shower in between bag changes. If a patient is on a continuous infusion, they should limit their time off the pump to no more than 30 minutes. To prevent contamination of the IV line during showering, patients can place gauze over PICC dressing, wrap with plastic cling wrap, and secure with medical tape at both ends of the wrap. Additionally, there are commercial products available such as disposable arm sleeves or PICC line shower covers. These products are designed for 1-time use, and effectively act as a water-resistant sleeve over the VAD for use during showering. With any shower covering, it is advisable to avoid direct spray to minimize chance of contamination.
AN ABRIDGED SUMMARY OF COMPOUNDED STERILE PRODUCT REGULATIONS
USP <797> is a collection of enforceable standards that govern the compounding of all compounded sterile products (CSPs). Per USP <797>, CSPs must be compounded in an International Organization for Standardization (ISO) 5 environment or better [7]. The ISO classifies these environments with respect to acceptable particles per cubic measure of air. ISO 1 is the highest (or cleanest) standard while ISO 9 is the lowest (or dirtiest) standard. An ISO 5 environment requires the environment to contain no more than 29 particles 5 µm in size, 832 particles 1 µm in size, and 352 particles 0.5 µm in size [8].
USP <797> also governs the beyond use date (BUD) of all sterile compounds. Depending on the number of manipulations that occur during compounding, a CSP is considered low, medium, or high risk with respect to possible microbial contamination. Low-risk compounds receive a BUD of 14 days if refrigerated, medium-risk compounds receive a BUD of 9 days, and high-risk compounds receive a BUD of only 3 days [7].
The remainder of the article will provide an in-depth discussion of the various administration methods utilized in home infusion.
INTRAVENOUS PUSH
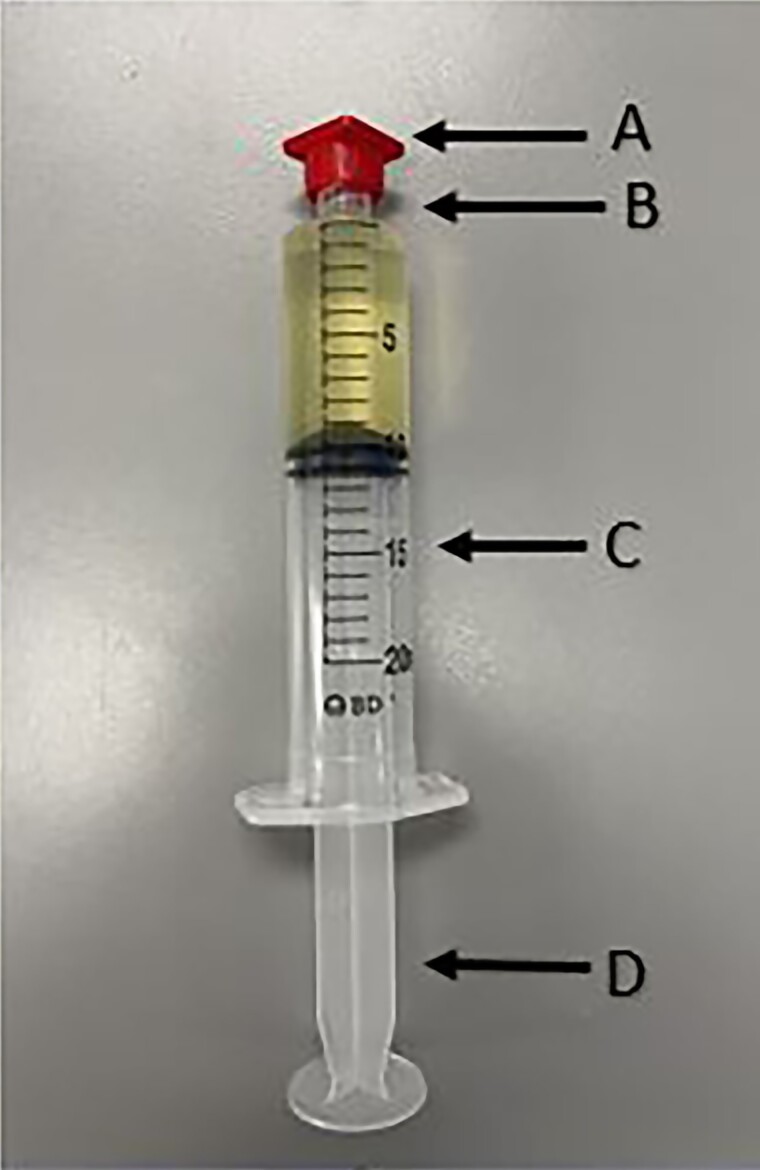
Intravenous push (IVP) is by and large the preferred method of administration in terms of patient preference due to ease of use. This method is characterized by the rapid administration of a small volume of medication into a patient's vein via an IV catheter, usually over a matter of minutes. From a compounding standpoint, this method requires very few steps and manipulations in a USP <797> ISO 5 sterile environment. Most medications come in a powder formulation that require reconstitution with an appropriate diluent such as sterile water or normal saline. Upon reconstitution, the compounding technician will draw up the medication into a syringe and cover the Luer-Lok tip with a sterile red cap (Figure 1A). Reconstituted IVP syringes are required to be refrigerated, as they are compounded in a sterile environment and subject to USP <797> beyond use dating.
Figure 1.
Intravenous push—syringe. A, Sterile red cap. B, Luer-Lok tip. C, Barrel. D, Plunger.
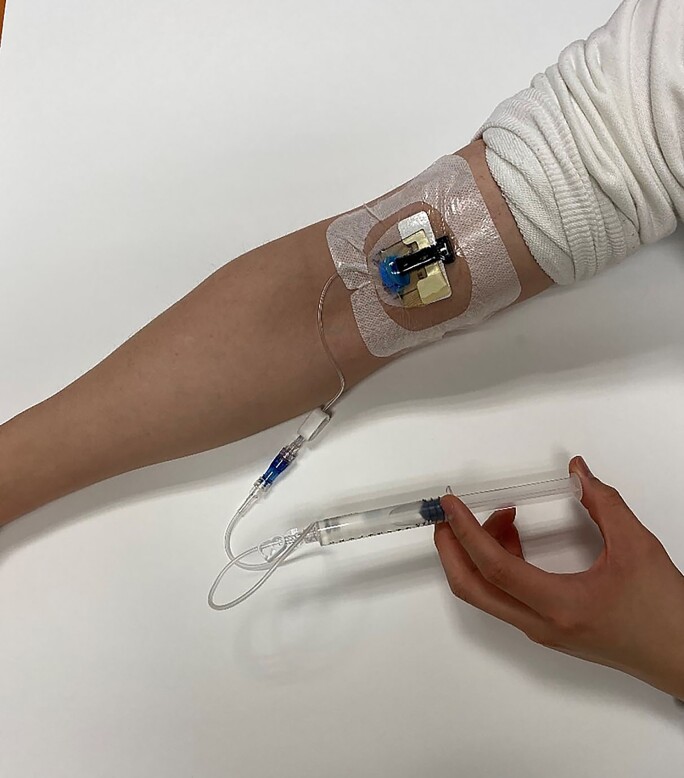
Like other drug delivery systems, patients are encouraged to remove syringes from the refrigerator 1 hour prior to their scheduled dose, with a goal of reaching room temperature to make the administration more comfortable. Additionally, prior to discharge, the patient is educated by the nurse liaison to scrutinize the syringes for cloudiness or discoloration and to remove any air bubbles prior to the administration. Each syringe has a Luer-Lok tip that connects to the hub of the IV line through a “push and twist” motion. Once connected, patients may push the plunger over a specific period according to the label instructions (Figure 2), typically ranging from 2 to 10 minutes, depending on the medication. Once complete, the empty syringe may be discarded into the trash.
Figure 2.
Intravenous push administration.
ELASTOMERIC DEVICES OR “THE INFUSION BALL”
Elastomeric infusion pumps are made from polyvinyl chloride with a silicone membrane. They utilize a patented “Sliding Core” that expands the membrane of the elastomeric as fluid is injected [9]. Together, with the positive pressure applied against the membrane and the “Sliding Core,” drug delivery is consistent and uniform throughout the infusion. The elastomeric tubing has a Luer-Lok tip that connects to the patient's IV catheter and, once the line is unclamped, the infusion starts automatically. After the infusion, the empty elastomeric can be safely discarded into the trash.
Elastomeric infusion pumps come in many forms. Two examples with well-established stability data are the Easypump (B. Braun) and the Homepump Eclipse (Baxter), with various infusion rates and sizes depending on the necessary volume needed to infuse. Elastomeric balls come in a variety of sizes from 50 mL to 500 mL with a very large variety of flow rates ranging from 5 mL/hour to 250 mL/hour. This allows for medications to be infused over the following times: 30 minutes, 60 minutes, 90 minutes, 2 hours, or 24 hours [9]. As each device is made of different synthetic material, the individual stability of medications can differ between the 2 devices [10]. Elastomeric devices are primarily used to infuse IV antimicrobials but also have additional utility in other therapies such as chelation, hydration, and even chemotherapy. With no batteries needed and tubing already attached, the patient has the luxury of carrying the elastomeric pump with them during the infusion. Often, patients are instructed to place the elastomeric pump in a large pocket (such as a house coat or sweatshirt) while infusing. There is no need for gravity (eg, hanging the pump at a higher position) for the pump to infuse. This allows for increased adherence and decreased mobility burden to the patient. Elastomeric devices are required to be refrigerated as they are compounded in an ISO 5 sterile environment and subject to USP <797> beyond use dating.
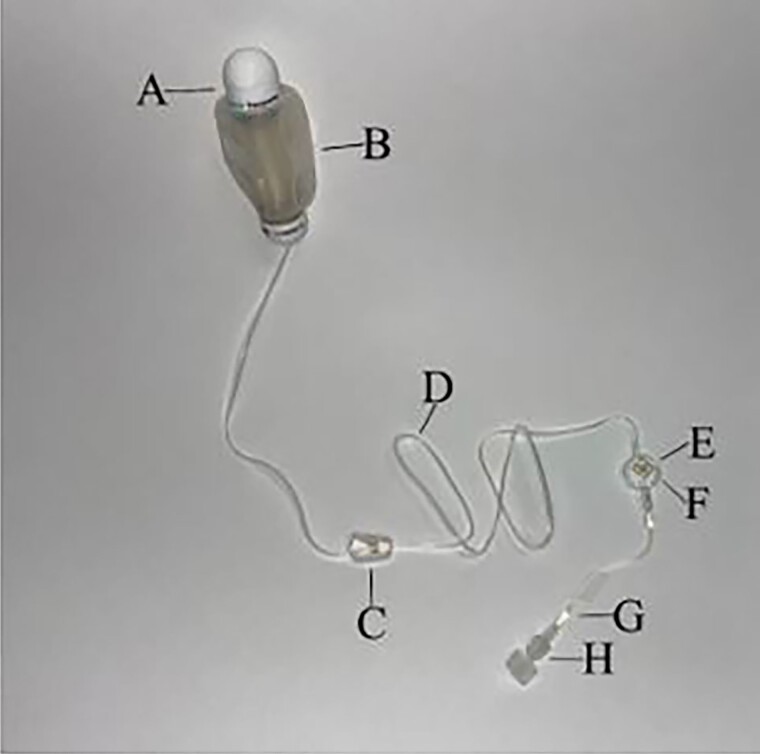
The flow rate of each elastomeric device is predetermined, which eliminates the need for preprogramming. The end of the tubing, proximal to the patient connector, contains a flow restrictor (Figure 3G) that controls the flow rate, and is required to be at room temperature. This can be achieved by taping the flow restrictor onto the patient's skin to maintain optimal temperature, as solution viscosity will change with temperature fluctuations. Elastomeric devices primarily infuse as a single-dose delivery system—so in the case of a patient who requires frequent dosing throughout the day, and with a potential for compliance issues, an alternative administration method may be preferable.
Figure 3.
Elastomeric (Easypump). A, Fill port. B, Balloon (deflated). C, Clamp. D, Tubing. E, Air-eliminating filter. F, Infusion rate indicator. G, Flow restrictor. H, Luer-Lok tip.
GRAVITY INFUSION
Dial-a-flow, gravity infusion, or flow regulator (Figure 4) are terms used interchangeably, characterized by leveraging gravity to infuse medication into the patient. With this approach, patients can spike the IV bags with flow regulator tubing, as it hangs from an IV pole and infuses by gravity. Home hydration is frequently infused via gravity, as the bags do not require refrigeration as it is not subject to USP <797> (eg, no manipulations have been done to them in an ISO 5 environment). The rate of infusion is adjusted by a dial located on the tubing (Figure 4F) that the patient can control, ranging from 5 mL/hour to 250 mL/hour.
Figure 4.
Flow regulator intravenous set, Integrated Medical (IMS033G). A, Spike with rubber cover. B, Vent cap. C, 15-micron filter in drop chamber. D, Drop chamber. E, Roller clamp. F, Flow regulator dial. G, Luer-Lok tip.
Each dial has its own number markings and can be adjusted with rotation. A potential drawback of this lies in the varying numerical increments, which increase by 5, 10, 25, and 50 mL per hour, respectively. From 5 to 10 mL/hour, the dial increases in increments of 5 mL/hour; from 10 to 80 mL/hour, in increments of 10 mL/hour; from 80 to 100 mL/hour, in increments of 20 mL/hour; from 100 to 150 mL/hour, in increments of 25 mL/hour; and from 150 to 250 mL/hour, in increments of 50 mL/hour [11].
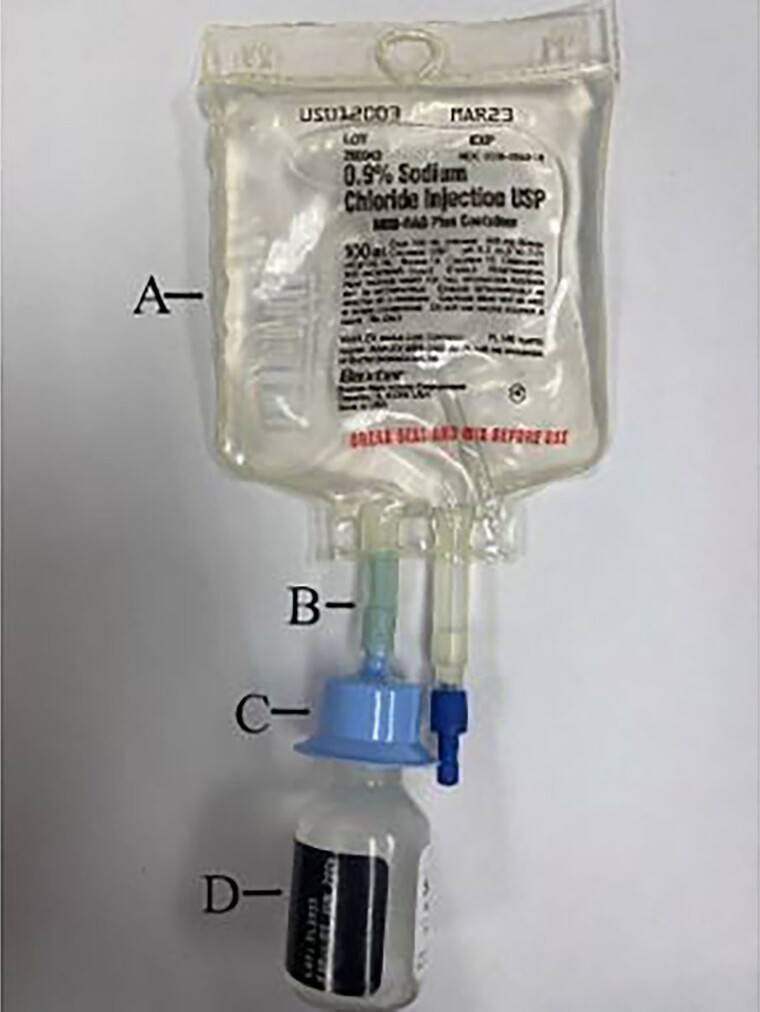
Another administration method that is frequently infused via gravity is an IV bag with adaptor systems such as the Mini-Bag Plus (MBP) system (Figure 5) or the ADD-vantage system. These can both be utilized with a Vista Pump as it does not require air to be removed from the IV bag. A Mini-Bag Plus has 1 port for spiking as well as a drug vial adapter (Figure 5C), while the ADD-vantage system has a separate adaptor piece that allows most vials to attach to an ADD-vantage bag. The compounding technician will connect the medication vial to the adapter via a spiking mechanism, where it will snap into place. At this point in the process, the medication has not been mixed with the diluent and is stable for 28 days. Prior to the administration, the drug may be mixed in the patient's home by snapping the seal above the drug vial adapter (Figure 5B). Reconstitution is achieved by squeezing the normal saline bag, which allows the fluid to flow into the drug vial. After the medication has been reconstituted, the bag is inverted and the positive pressure from the vial allows the drug to flow through the adapter and into the Mini-Bag Plus container. After successful reconstitution of the drug, the patient can spike the second port with the flow regulator tubing, hook it to a pole, and begin the infusion. Neither the MBP nor the ADD-vantage systems require refrigeration as the medication vials have not been reconstituted and are therefore stable. However, it is subject to USP <797> beyond use dating, as manipulation has occurred in an ISO 5 environment.
Figure 5.
Mini-Bag Plus system. A, Mini-Bag Plus Container. B, Breakaway seal. C, Drug vial adapter. D, Drug vial.
Gravity infusions also have the most robust published drug stability data of all the administration methods, as this is the most widely used method of administration. When new medications come to market, the stability information is always presented as though the medication is infused via gravity (ie, reconstituted and diluted with commercially available diluent). Newer drugs may not boast as much evidence on the various administration devices to provide adequate stability data on the various materials of the devices. Depending on the recency of medication approval by the US Food and Drug Administration, MBP or ADD-vantage via gravity infusions may be the only option for the patient.
AMBULATORY PUMPS
Ambulatory pumps, or continuous ambulatory delivery devices (CADDs), allow patients to infuse their medications without being restricted to a single location during the infusion. The pumps themselves do not contain medication—rather, a reconstituted or IV medication is attached to the pump to facilitate automatic drug delivery. Manufacturers, such as Smiths Medical and Moog Medical, produce pumps (CADD-Prizm, CADD-Solis [Figure 6], Moog-Curlin) with their own defining features, each designed to achieve the same overall effect. Ambulatory pumps allow for intermittent, continuous, and tapered infusions, which is why this method is commonly used with an array of medications (eg, antimicrobials, TPN, IV hydration, PCA, and chemotherapy).
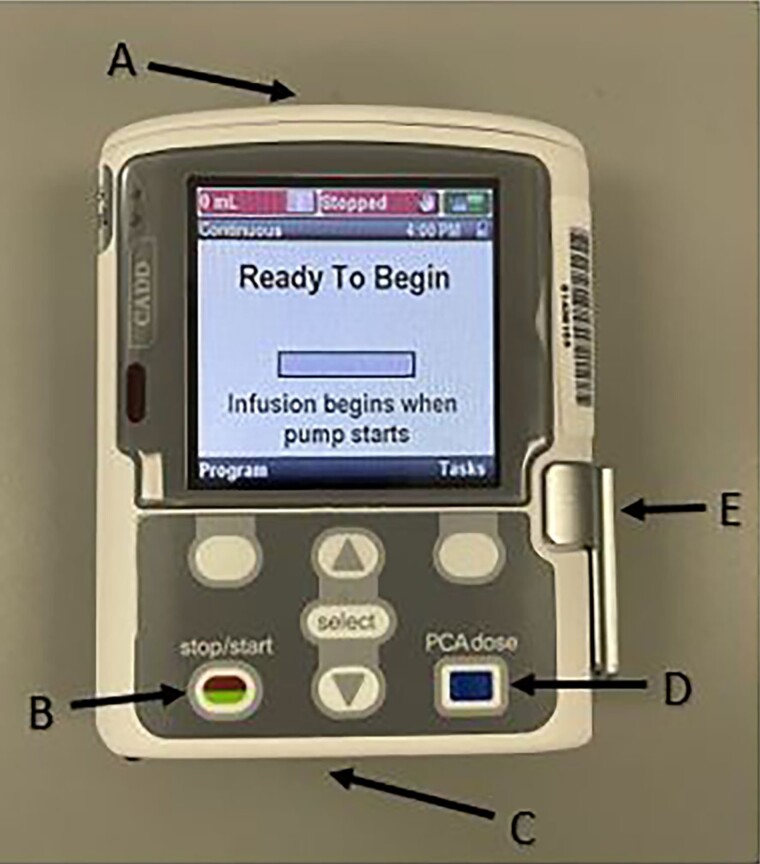
Figure 6.
Ambulatory pump (CADD-Solis). A, Battery placement (4 AA batteries). B, Start/Stop function. C, Area of attachment between cassette and pump. D, Bolus dose button used specifically for patient-controlled analgesia. E, Latch for pump connect and disconnect.
This article will specifically discuss the use of antimicrobials with CADD-Solis pumps. Upon reconstitution of the powdered formulations, medications are injected into a polyvinyl chloride bag containing the appropriate diluent (eg, dextrose 5% in water, normal saline). The compounding technician will then remove the air from the bag and attach the appropriate tubing, which includes a 0.2- or 1.2-µm air-eliminating filter and a cassette (Figure 7B) that connects the tubing to the pump (Figure 8). Last, the compounding technician will prime the tubing and remove any additional air prior to dispensing. Specifically, once the tubing is spiked into the bag, the technician will allow the fluid from the bag to flow through the tubing until all air has been removed. The technician will then clamp the tubing to prevent further flow or leakage and add a sterile red cap to the Luer-Lok tip. Patients are also provided with a pouch (Figure 9) that allows them to store their ambulatory pump, medicine bag, and ice bricks (if the medicine is required to remain cold throughout the infusion), allowing them a certain degree of mobility. While maximum infusion rates differ among the different manufacturers, the CADD-Solis pump may deliver infusions at speeds of up to 500 mL/hour [12]. These pumps require batteries for operation and electronic programming.
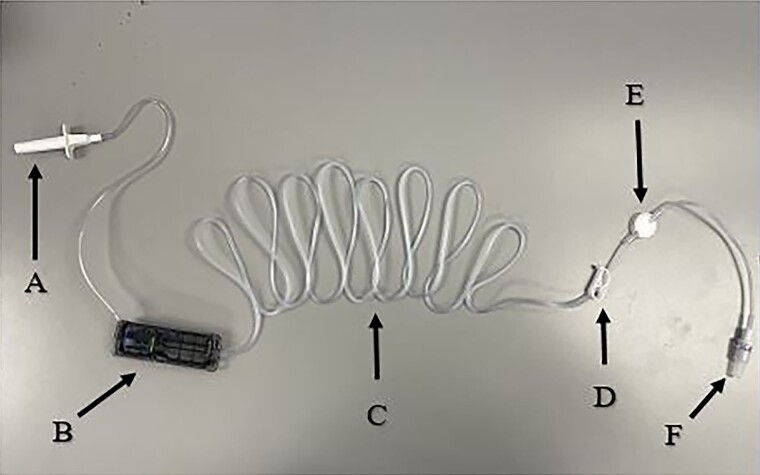
Figure 7.
CADD tubing. A, Spike w/ rubber cover. B, Cassette. C, Tubing. D, Clamp. E, Air-eliminating filter. F, Luer-Lok tip.
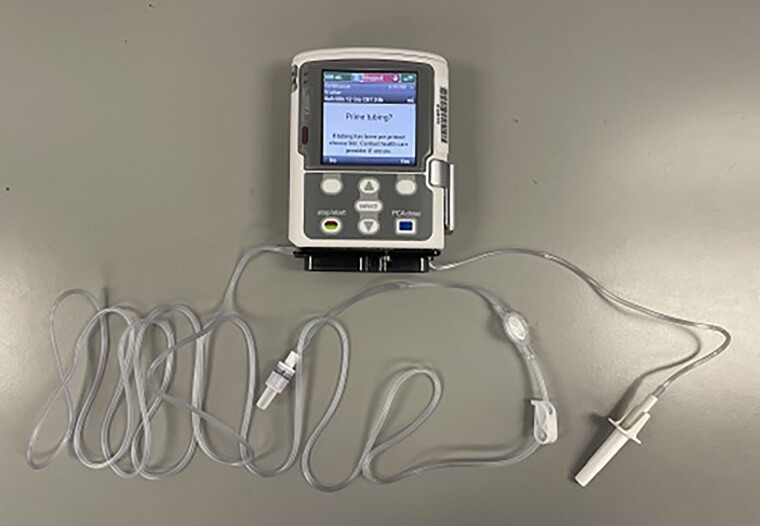
Figure 8.
Tubing connected to CADD-Solis Pump.
Figure 9.
CADD Pouch (shoulder strap).
To use the ambulatory pump, the patient will have to open the cassette latch (Figure 6E) and insert the cassette hooks into the hinge pins on the bottom of the pump (Figure 7B). Once connected (Figure 8), the patient will close the cassette latch, which should close easily if attached correctly. The patient will then sanitize their hands, scrub the IV hub with an alcohol pad, remove the red sterile cap from the medication tubing, then push and twist the medication tubing into the IV hub. The patient will unlock the clamps on both the catheter and the tubing. They will then follow the prompts on the pump to start the infusion. One important aspect to remember is the potential overfill in the bag. When the infusion is complete, there will be a minimal amount of additional fluid left over (usually 50–100 mL). Once complete, the patient may discard the remaining fluid in coffee grounds, kitty litter, or a pile of paper towels inside a plastic bag, prior to disposing of the empty medication bag into the trash.
One of the major benefits of this delivery system is that these bags can infuse throughout a 24-hour period, which may ensure better compliance than single-dosing methods (eg, elastomeric, IV push) that require multiple doses throughout the day. For example, if a patient is ordered for piperacillin/tazobactam 3.375 g IV every 8 hours, the bag will be compounded to contain the total daily dose. Therefore, the bag will contain piperacillin/tazobactam 10.125 g and is given a BUD of 9 days from the date of compounding. This means that the patient can attach only 1 bag per day to their ambulatory pump. Subsequently, this allows the patient the added flexibility to ambulate, leave the home, and increase independence and thus compliance (Figure 10). As a prescriber, this gives flexibility to either administer the medication as a continuous infusion (ie, infuse the medication at a steady rate over a period of 24 hours) or program the pump to dispense 3.375 g at 8-hour intervals (eg, as either a 30-minute bolus or as a prolonged infusion over 3–4 hours). Generally, for intermittent dosing, the pump is programmed to deliver fluid at a rate of 1 mL/hour in between doses to maintain line patency, a concept often referred to as keep-vein-open. The pharmacist will also be able to program the pump so that each dose is delivered every 8 hours over a specific amount of time, which is ideal for extended-interval dosing (Figure 11), or continuously over 24 hours if desired by the provider, requiring no additional patient education.
Figure 10.

CADD-Solis pump parameters (continuous 24-hour dosing).
Figure 11.
Stationary pump: VISTA basic pump. A, Open door button. B, Function keys. C, On/Off button. D, Silence alarm button. E, Start/Stop button.
While ambulatory pumps are widely preferred by clinicians for specific medications, most patients do not enjoy the idea of carrying around a pump for their long-term antimicrobial infusions. Additionally, patients will sometimes run into pump alarm issues that may require nursing intervention, such as upstream and downstream occlusions. Upstream occlusions are blockages that occur in the tubing before fluid has passed through the pump, usually due to a clamp that has been left closed or a kink in the tubing between the medication bag and the pump. Downstream occlusions are blockages that occur in the tubing between the pump and the patient's catheter, also usually due to a closed clamp or a kink in the tubing. Occasionally, some pump issues may not be resolved via nursing intervention and will warrant a replacement pump, which may result in an interruption in therapy.
Ambulatory pumps are usually loaned out via the home infusion company. Once the need for IV medications is complete, the ambulatory pump must be returned to the home infusion company—otherwise, the patient or their insurance may be responsible for the cost of the device. Any medication bags used with the ambulatory pumps are disposed of in the trash.
STATIONARY PUMPS
Stationary pumps are larger, heavier, and less-mobile versions of the ambulatory pumps. These pumps also do not contain medication but can be connected to infusion bags to facilitate infusion. Stationary pumps accurately deliver IV solutions to a patient with a maximum rate of 1000 mL/hour [13]. If a visiting nurse must remain in the home for the entirety of the infusion, a stationary pump will likely be preferred over gravity infusions as stationary pumps provide a more reliable infusion rate. While stationary pumps tout the fastest rate out of all the administration methods, they are extremely heavy and cumbersome to move about, even when attached to a pole. The pump is not battery powered and must be plugged into an electrical outlet. This restricts patients to a singular location during the infusion and introduces the requirement for functioning electricity.
Patients are required to prime their tubing (Figure 12) prior to their infusion. Before beginning the infusion, the stationary pump will prompt a question, asking if priming is needed and what the desired priming amount is (determined by the length specific to each tubing). The patient's IV hub is not attached to the tubing during the priming process, as it would introduce air into the patient's IV line. The pumps also have numerous safety features, such as the ability to detect upstream and downstream occlusions, air-in-line, and so forth. The pump will sound an immediate alarm if there is a problem during fluid delivery. Stationary pumps allow for a wide range of infusion rates and may be preferable to those who are indifferent to being nonambulatory during their infusion.
Figure 12.
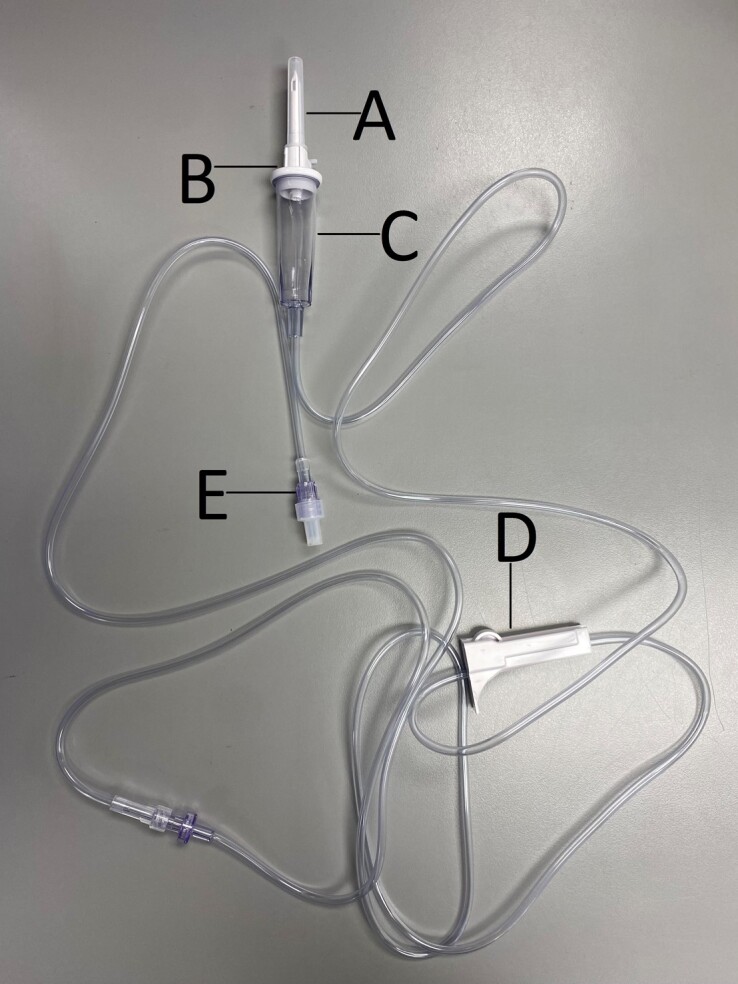
VISTA basic pump intravenous set (B. Braun, V9905). A, Spike with rubber cover. B, Vent cap. C, Drop chamber. D, Roller clamp. E, Luer-Lok tip.
Stationary pumps are also usually loaned out via the home infusion company and must be returned upon completion of therapy. Any medication bags and tubing used with the stationary pumps are disposed of in the trash. The pumps are brought back to the home infusion company where they are cleaned and inspected.
SELECTION OF INFUSION DEVICE
The decision on which method of administration would best benefit the patient is typically a discussion had between the patient and the home infusion company's liaison with some input from the home infusion company's pharmacy department. The process of selecting which infusion device or modality is used revolves around 4 factors: medication stability, dosing frequency and duration, cost, and patient preference. Medication stability affects how many doses the home infusion company can send in 1 shipment. For example, ceftriaxone syringes have a BUD of 9 days, so a week's worth can be sent at one time. Ampicillin elastomeric balls, however, are only stable for a short amount of time and must be used within 3 days of compounding. This becomes logistically difficult to manage for both the home infusion company and the patient. The patient will need to be available for a courier delivery multiple times a week and have different shipments with different BUDs on hand at one time. This will increase the chance of either missing a dose due to a missed delivery or infusing an expired dose with lower efficacy.
Dosing duration and frequency can quickly change a patient's preference of one method of administration to another. For example, the elastomeric ball is almost always preferred for meropenem 1 g every 8 hours infused over 1 hour. However, if meropenem 1 g every 6 hours is ordered, most patients tend to select an ambulatory pump to avoid waking up at night. Though there is not a high degree of variability in cost with respect to the different modalities, some methods of administration are less expensive than others. The variance in cost appears in the per diem charge the patient receives from the infusion company. This charge is designed to cover the cost of ancillary supplies for that patient, while they are on service. While these charges vary between companies, IV push and gravity tend to be less expensive (eg, $20 per day), while infusion pumps and elastomeric devices tend to be more expensive (eg, $40 per day). Depending on the length of therapy and what percentage of these charges are covered by a patient's insurance plan, this could be a significant factor. Patient preference plays an important role in success rate of home therapy. Patients who are comfortable with the infusion method they choose are more likely to succeed and experience less frustration during their transition to home care. To that end, patients typically prefer an IV push or an elastomeric device whenever possible. The IV push method is quick yet requires manual manipulation of a syringe, while an elastomeric infusion is longer, yet requires little manipulation. Table 2 provides a quick reference of common offerings to assist providers and liaisons in making decisions about drug therapy and preferred methods of administration for common antibiotics seen in home infusion. This table is meant to serve as a discussion starter rather than a firm guideline as the 4 factors detailed above can all impact the decision on which method of administration the patient could go home on.
Table 2.
Commonly Dispensed Antibiotics by New England Life Care for Home Infusion With Device Preferencesa
| Drug | Preferred Method of Administration | Alternative Method of Administration 1 | Alternative Method of Administration 2 |
|---|---|---|---|
| Ceftriaxone | IV push | Elastomeric | Gravity |
| Cefazolin | IV push | Elastomeric | Ambulatory pump |
| Vancomycin | Elastomeric | Gravity | Ambulatory pump |
| Ertapenem | Elastomeric | Gravity | Ambulatory pump |
| Daptomycin | Elastomeric | Gravity | Ambulatory pump |
| Cefepime | Elastomeric | Gravity | Ambulatory pump |
| Meropenemb | Elastomeric | Gravity | Ambulatory pump |
| Piperacillin/tazobactamb | Elastomeric | Gravity | Ambulatory pump |
| Penicillin | Ambulatory pump | None available | None available |
| Ampicillinc | Ambulatory pump | Elastomeric | None available |
Abbreviations: IV, intravenous.
This table represents some common offerings. Slight discrepancies may exist between various home infusion providers.
If extended dosing is required, ambulatory pump defaults as the preferred MOA.
If dosed every 8 hours or longer, elastomeric may be preferred.
An IV medication may potentially be delivered in several different ways (eg, IV push or continuous infusion). Decisions are not made in a vacuum, and clinicians should engage in discussions with the patient and the home infusion company if certain infusion methods are preferred.
CONCLUSIONS
With a variety of administration methods available for HIT, clinicians are often unaware of what can and cannot be done through the home infusion company to accommodate the needs of their patients. In most cases, home infusion companies can fulfill those needs and offer patients a smoother transition from hospital to home. Ideally, this article will help minimize the knowledge gap that exists between clinicians and the home infusion company by providing a better understanding of the various administration methods used with HIT.
Contributor Information
Alain Loriaux, New England Life Care, Canton, Massachusetts, USA.
Michael Desmond, New England Life Care, Canton, Massachusetts, USA.
Peng Cheng Li, New England Life Care, Canton, Massachusetts, USA.
Notes
Author contributions. All authors had equal share in the conceptualization, writing, and reviewing of the manuscript.
Patient consent. This manuscript does not include factors necessitating patient consent. All photo images were taken by and are property of the authors. No patient data were used in the preparation of this manuscript, including the photo images.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. National Home Infusion Association . NHIF infusion industry trends report.https://nhia.org/nhif/nhif_infusion_industry_trends_report. Accessed 5 May 2022.
- 2. Mills S. How COVID-19 reinforced the important role of home health care. HomeCare Magazine. https://www.homecaremag.com/january-2022/how-covid-19-reinforced-role-home-health. Accessed 5 May 2022.
- 3. Fortune Business Insights . U.S. home infusion therapy market size, share and COVID-19 impact analysis, by product (devices, drugs, and services), by indication (anti-infective, chemotherapy, hydration therapy, enteral nutrition, total parenteral nutrition, immunoglobulins, and others), and forecast, 2021–2028. https://www.fortunebusinessinsights.com/u-s-home-infusion-therapy-market-106327. Accessed 11 July 2022.
- 4. Gorski LA, Hadaway L, Hagle ME, et al. Infusion therapy standards of practice. J Infus Nurs 2016; 39:S1–159. [DOI] [PubMed] [Google Scholar]
- 5. Joshi S, Kulkarni A, Bhargava AK. Evaluation of length of central venous catheter inserted via cubital route in Indian patients. Indian J Crit Care Med 2010; 14:180–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Smith B. Peripheral intravenous catheter dwell times: a comparison of 3 securement methods for implementation of a 96-hour scheduled change protocol. J Infus Nurs 2006; 29:14–7. [DOI] [PubMed] [Google Scholar]
- 7. United States Pharmacopeial Convention . USP <797>: guidebook to pharmaceutical compounding: sterile preparations. Rockville, MD: United States Pharmacopeial Convention; 2008. [Google Scholar]
- 8. International Organization for Standardization . ISO 14644-1:2015. Cleanrooms and associated controlled environments—part 1: classification of air cleanliness by particle concentration. Geneva, Switzerland: International Organization for Standardization; 2015.
- 9. B. Braun Medical Inc . Easypump elastomeric infusion pump system for short-term and long-term infusions. https://www.bbraunusa.com/en/products/b4/easypump-st-lt.html. Accessed 4 May 2022.
- 10. Bing CM, Nowobilski-Vasilios A, et al. Extended stability for parenteral drugs. 6th ed. Bethesda, MD: American Society of Health-System Pharmacists; 2017. [Google Scholar]
- 11. McKesson . Primary IV administration set MedStream 20 drops/mL drip rate 92 inch tubing 1 port. https://mms.mckesson.com/product/1145010/McKesson-Brand-MS750E. Accessed 4 May 2022.
- 12. Smiths Medical . https://www.smiths-medical.com/en-us/products/infusion/ambulatory-infusion/ambulatory-infusion-pumps/caddsolis-ambulatory-infusion-pump. Accessed 4 May 2022.
- 13. B. Braun Medical Inc . Vista basic. https://www.bbraunusa.com/en/products/b3/vista-basic-largevolumeinfusionpump.html. Accessed 4 May 2022.