Abstract
As we seek to develop and evaluate new vaccines against tuberculosis, it is desirable that we understand the mechanisms of protective immunity in our models. Adoptive transfer of protection with hsp65-specific T-cell clones from infected or vaccinated mice into naïve mice had indicated that cytotoxic T cells can make a major contribution to protection. We characterized 28 CD4+ CD8− and 28 CD4− CD8+ hsp65-specific T-cell clones derived from infected or vaccinated mice. Half of the CD4+ CD8− and 64% of the CD4− CD8+ clones were cytotoxic. Cytotoxicity was associated with high expression of CD44 and gamma interferon production. Most (86%) of the cytotoxic CD4+ CD8− clones lysed target cells via the Fas-FasL pathway, and most (83%) of the cytotoxic CD4− CD8+ clones lysed target cells via cytotoxic granules. Only the clones using the granule-mediated pathway caused substantial loss of viability of virulent Mycobacterium tuberculosis during lysis of infected macrophages, and the degree of killing closely correlated with the availability of granule marker enzyme activity. Granule-mediated cytotoxicity thus may have a key role in protection against tuberculosis by delivering mycobactericidal granule contents.
New vaccines are needed in the fight against tuberculosis (1), but the designing and testing of new vaccines is hampered by our poor understanding of the mechanisms of acquired protective immunity. It is not simply that the key protective antigens have yet to be identified (15); we do not know with certainty what kinds of responses are needed. This knowledge would help both to design vaccines for the best balance of responses and to design clinical tests to monitor or predict vaccine efficacy in the field.
Traditionally, protection against tuberculosis has been regarded as due to phagocytosis and killing of Mycobacterium tuberculosis by immunologically activated macrophages and monocytes (12). This is a result of a type 1 cellular response in which gamma interferon (IFN-γ) is produced by antigen-specific T lymphocytes, as distinct from a type 2 response, in which the cells produce interleukin (IL-4) (19). IFN-γ is the main macrophage-activating factor, and it has been shown to be essential for protection (5, 8). However, substantial killing by the activated macrophages or monocytes has been difficult to demonstrate in vitro (4, 16, 18, 24), and there is increasing evidence for a protective role for antigen-specific cytotoxic T lymphocytes, in both murine and human tuberculoses (2, 17, 22). It is not clear how these cells are protective. One possibility is that the cytotoxic T cells are needed to release bacteria from safe havens inside ineffective macrophages so that they can be phagocytosed by fresh, fully activated monocytes or macrophages (7). Alternatively, studies with human peripheral blood cells in vitro have indicated that lysis of infected macrophages by antigen-specific T cells can directly result in death of the bacteria (17, 22). Mycobacterial death can be due to toxic enzymes discharged from lymphocyte granules during perforin-mediated lysis of infected macrophages (22). Degranulation of the lymphocytes by Sr2+ treatment in advance prevents killing (22). However, there might be alternative bactericidal mechanisms when the lymphocytes do not contain these granules and lyse targets using the Fas-FasL-dependent pathway of apoptosis (17). It is not known whether either of these lytic mechanisms can kill M. tuberculosis in murine macrophages.
We previously studied adoptive transfer of protective immunity with T-cell clones specific for 65-kDa heat shock protein antigen (hsp65) in a murine model of tuberculosis (2, 21). The most protective T cells were CD4− CD8+ and were cytotoxic for infected macrophages in addition to producing IFN-γ and expressing high levels of the activation marker CD44 (CD44hi) (2). Some evidence of antimycobacterial activity during lysis of infected macrophages in vitro was also obtained (21). Here, we further characterize 28 CD4+ CD8− and 28 CD4− CD8+ hsp65-specific clones in vitro and test whether lysis of M. tuberculosis-infected target macrophages by these clones can cause death of the bacteria by either the perforin- or Fas-FasL-dependent pathway.
MATERIALS AND METHODS
T-cell clones.
hsp65-specific T-cell clones were obtained from BALB/c mice 30 days after infection with M. tuberculosis H37Rv or 2 weeks after completion of immunization by four intramuscular injections of naked plasmid DNA pHMG-65 or four intraperitoneal injections of transfected monocytelike cell line J774 expressing hsp65 (J774-hsp65), as previously described (2, 21). In brief, cells with CD4+ CD8− and CD4− CD8+ phenotypes were separated from whole splenocyte populations by negative selection with antibody and complement and cultured for 2 weeks on irradiated J774-hsp65 feeder cells with IL-2 before being cloned. Twenty-eight strongly growing CD4+ CD8− clones (12 from J774-hsp65-immunized mice, 8 from DNA-immunized mice, and 8 from infected mice) and 28 CD4− CD8+ clones (12 from J774-hsp65-immunized mice, 8 from DNA-immunized mice, and 8 from infected mice) were selected for characterization. All were CD3+ and T-cell receptor αβ+ by FACScan analysis (21). Antigen-specific cell proliferation on J774 antigen-presenting cells showed highly consistent dependency on the antigen-processing and presentation pathways expected of the CD4+ and CD8+ phenotypes: chloroquine and anti-L3T4 and anti-I-Ad monoclonal antibodies (MAbs) inhibited all CD4+ clones and not the CD8+ clones; brefeldin A, anti-Lyt-2, and anti-H-2Kd inhibited all CD8+ clones and not CD4+ clones (2, 21).
CD44 expression and cytokine secretion.
Cells were stained with fluorescein isothiocyanate-labeled anti-CD44 and Lyt-2 or L3T4 MAb and analyzed by FACScan, and the results were expressed as the median intensity of fluorescence (2). Secretion of cytokines IFN-γ and IL-4 was stimulated with phorbol myristate acetate and anti-CD3 MAb YCD3-1 to achieve maximal stimulation and measured by enzyme-linked immunosorbent assay (21).
Cytotoxicity.
The antigen-specific cytotoxicities of the T-cell clones were determined as previously described (21). J774-hsp65 cells, J774-vector cells that had been preloaded with recombinant hsp65 protein antigen using positively charged liposomes, or J774-vector cells that had been pulsed with antigen (25 μg/ml) for 40 to 48 h were used as targets. The target cells were labeled with 51Cr (100 μCi/ml) and washed, and then 5 × 103 target cells were incubated with 2.5 × 105 freshly cultured effector clones (effector/target [E/T] ratio, 50:1) in 200 μl of test medium in 96-well round-bottom plates. The target cells were also incubated with medium alone and with 0.5% Triton X-100 to determine the spontaneous and maximal 51Cr release, respectively. After 4 h at 37°C, the cell supernatants were collected and assayed for radioactivity. The effects of putative inhibitors of cytotoxicity were tested using J774-hsp65 targets. Blocking antibodies against CD95 or CD95L (anti-Fas or anti-FasL; Pharmingen) were added before the effector and target cells were mixed. Degranulation of the cytotoxic granules was induced by initial treatment of T-cell clones with 25 mM Sr2+ (Aldrich, Milwaukee, Wis.) for 18 h. 51Cr release was determined after a 4-h incubation.
To measure cytotoxicity towards infected macrophages, monolayers of bone marrow macrophages from BALB/c mice were used (21). They were infected on day 5 of culture with live M. tuberculosis H37Rv for 4 h at a bacterium-to-cell ratio of 5:1. Comparison of microscope counts of mycobacteria and their growth on Middlebrook 7H11 agar plates revealed a viability of bacteria in the inoculum of >90% and an absence of clumps. Infected monolayers were rinsed five times with medium to remove nonassociated bacteria. The cells were then detached with 1 mM EDTA and resuspended in fresh medium. The efficiency of infection was determined microscopically after staining the cells with auramine-rhodamine. Typically, there were 3.9 ± 0.3 (standard deviation [SD]; n = 3) bacteria per cell, and 87 ± 6% of the cells were infected. Infected cell suspensions were immediately labeled with 51Cr for 1 h as described above and incubated in 96-well V-bottom plates with 5 × 103 targets per well and serial dilutions of freshly cultured effector clones in a total volume of 200 μl of test medium. The target cells were also incubated with medium alone and with 0.5% Triton X-100 to determine the spontaneous and maximal 51Cr release, respectively, as previously described (21).
Antimycobacterial effect of cytotoxicity against infected macrophages.
Bone marrow macrophages were infected with M. tuberculosis H37Rv, rinsed, and suspended as described above, and then 5 × 103 infected cells were coincubated with cytotoxic-T-cell clones at an E/T ratio of 50:1 in 200 μl for 24 h. No antibiotics were present in the medium. The cells were then lysed with 0.1% saponin to release intracellular bacteria, serial fivefold dilutions were plated in triplicate on 7H11 agar plates, and the CFU were counted after 3 weeks at 37°C. Prolonged incubation of agar plates did not increase the CFU. The involvement of Fas and IFN-γ in antimycobacterial activity was tested by adding MAb against Fas, FasL, or IFN-γ (1.0 μg/ml) before cell mixing. Fluorescence-activated cell sorter analysis of macrophages 24, 48, and 96 h after infection with M. tuberculosis showed that expression of Fas on the target cells was not affected by infection. Involvement of cytotoxic granules was tested by degranulating the T-cell clones by an initial incubation with 25 mM Sr2+ (Aldrich) for 24 h, and degranulation was monitored by measuring the release of granule enzyme Nα-benzyloxycarbonyl-l-lysine thiobenzyl (BLT) esterase (23). Supernatants were collected after 10 h, and 20-μl aliquots were incubated with 35 μl of 1 mM BLT (Sigma), 35 μl of 1 mM 5,5′-dithio-bis-(2-nitrobenzoic acid) (Sigma), and 10 μl of 0.1% Triton X-100. After 30 min at 37°C, the absorbance at 405 nm was determined. Degranulation did not significantly impair subsequent capacity to release IFN-γ.
Statistical analysis.
The data are presented as mean and geometric mean values from replicate assays and samples. Student's t test was used to determine statistical significance between groups of data. Linear regression curves were fitted by the least-squares method.
RESULTS
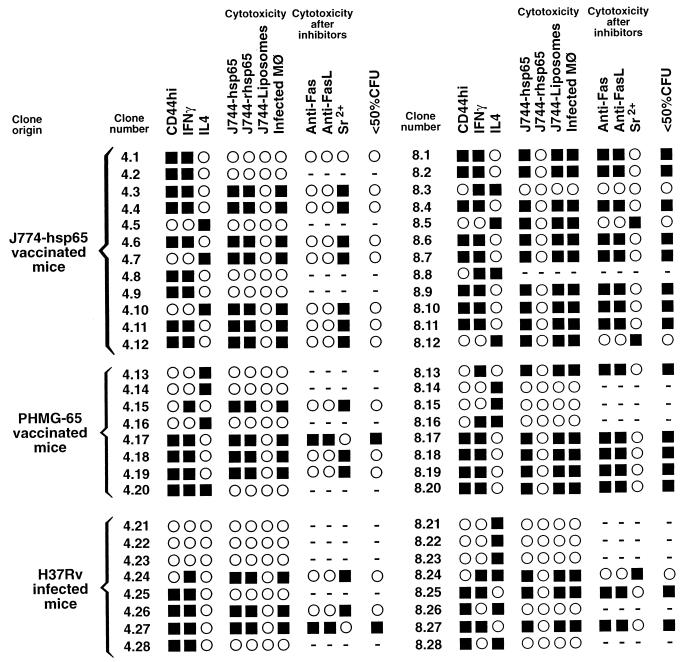
The results of quantitative analyses of properties of the 56 hsp65-specific T-cell clones studied are summarized in Fig. 1.
FIG. 1.
Summary of characteristics of 28 MHC class II-restricted CD4+ CD8− and 28 MHC class I-restricted CD4− CD8+ T-cell clones. The clones were assayed by quantitative methods and scored as positive (■) or negative (○) according to threshold criteria. Thus, positive scores were as follows: CD44, median intensity of fluorescence, >115; IFN-γ secretion, >100 ng/ml; IL-4 secretion, >100 pg/ml; cytotoxicity, specific 51Cr release, >2 SD above that which was induced by a CD4 clone with irrelevant antigenic specificity; antimycobacterial activity against infected macrophages, <50% of the CFU found after incubation with a CD4+ clone with irrelevant antigenic specificity. −, not tested.
Association of CD44hi with IFN-γ.
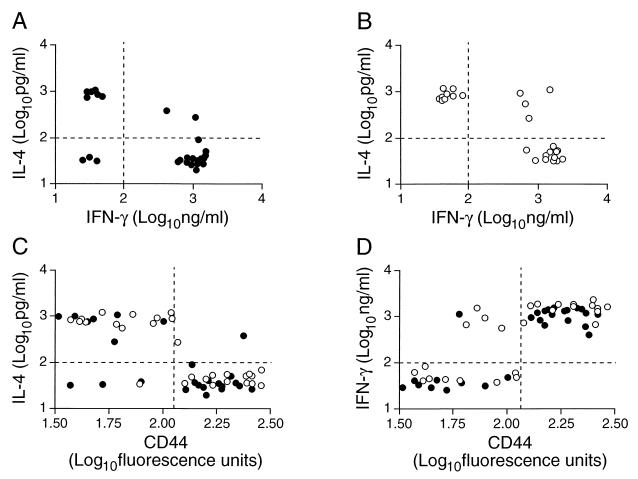
As expected (19), most of the clones produced either IFN-γ or IL-4, although a few produced both cytokines and three produced neither (Fig. 1). IFN-γ production was associated with high levels of expression of CD44, and IL-4 production was associated with low levels of CD44 expression (Fig. 2).
FIG. 2.
Clone phenotype associations. Production of IFN-γ and IL-4 by CD4+ CD8− (A) and CD4−/CD8+ (B) clones under optimal stimulation was measured by enzyme-linked immunosorbent assay. Expression of CD44 was measured by FACScan after the clones were labeled with fluorescent antibody and was plotted against IL-4 (C) and IFN-γ (D) production. Data from individual clones of CD4+ CD8− (●) and CD4− CD8+ (○) phenotypes are shown. The dashed lines indicate arbitrary limits selected to distinguish positive cytokine responses and high CD44 expression.
Cytotoxicity against exogenous and endogenous antigen.
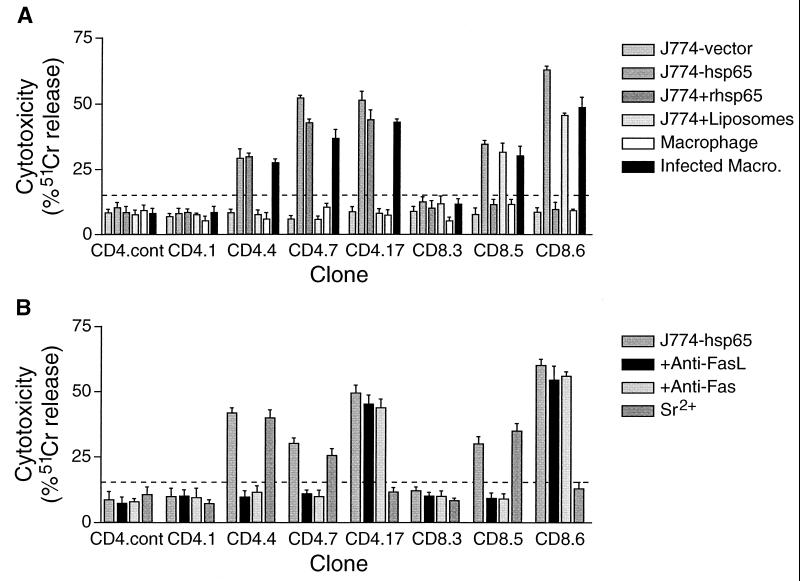
Fourteen of the 28 CD4+ CD8− clones and 18 of 27 tested CD4− CD8+ clones were cytotoxic for macrophages that were infected with M. tuberculosis but not for uninfected macrophages. They also lysed the transfected monocytelike cell line J774-hsp65, which presents endogenous hsp65 on both major histocompatibility complex (MHC) classes I and II (20). All of the cytotoxic CD4+ CD8− clones lysed J774 cells that were supplied with exogenous recombinant hsp65 protein but not when they were given the same protein via a liposome delivery vehicle, consistent with lysis by these clones only when the antigen is presented on MHC class II. Conversely, all of the cytotoxic CD4− CD8+ clones lysed J774 cells that were supplied with hsp65 that was delivered into the cells by liposomes and not when the protein was given without the liposomes, consistent with lysis only of targets presenting antigen on MHC class I (21). Representative results with two cytotoxic CD4+ CD8− and two cytotoxic CD4− CD8+ clones are shown in Fig. 3.
FIG. 3.
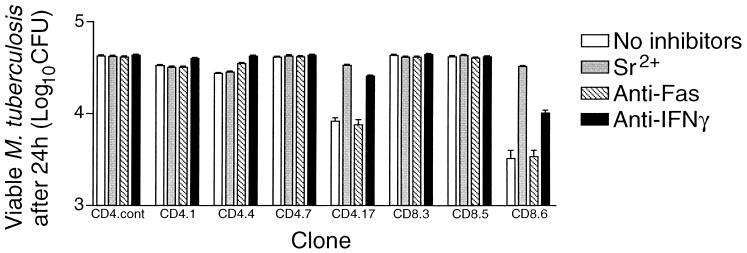
Antigen-dependent cytotoxicity of representative clones and the effects of specific inhibitors. The target cells were either the J774 monocytelike cell line or bone marrow-derived macrophages. (A) Macrophages were either uninfected or infected with an average of about four M. tuberculosis H37Rv organisms per cell, and J774 cells either were expressing endogenous hsp65 after stable transfection (J774-hsp65; known to present the antigen on both MHC class I and MHC class II [20]), were pulsed with antigen as a recombinant protein (J774-rhsp65), or were preloaded with recombinant protein using positively charged liposomes (J774-liposomes). Cells transfected with vector only (J774-vector) served as an antigen-free control. (B) Putative inhibitors of cytotoxicity were tested with J774-hsp65 targets. MAbs against Fas or FasL were added, or the clones were degranulated with Sr2+ (23) before the effectors and targets were mixed. The bars show means ± SD (n = 3) for specific 51Cr release after 4 h at 37°C. The dashed line indicates 2 SD above control targets that were incubated with a CD4+ CD8− clone that had irrelevant antigen specificity (CD4.cont). Clone 4.4 represents the most common phenotype among CD4+ CD8− clones, and clone 8.6 represents the most common phenotype among CD4− CD8+ clones (Fig. 1).
Dependence of cytotoxicity on either Fas or granule interactions.
The cytotoxicity of all except two of the 14 cytotoxic CD4+ CD8− clones was inhibited by antibody against either Fas or FasL and not by the degranulating agent Sr2+. The remaining two clones were inhibited by Sr2+ and not by the antibodies (Fig. 3). In contrast, most of the CD4− CD8+ clones were inhibited by Sr2+, and only three were inhibited by anti-Fas or anti-FasL.
Antimycobacterial effect of cytotoxicity.
The numbers of live M. tuberculosis cells in control macrophages (incubated with a CD4+ CD8− clone having irrelevant specificity) increased about twofold during a 24-h incubation (increasing from about 2 × 104 to 4 × 104 per well). Therefore, a decrease in CFU counts exceeding 50% relative to the control indicates killing, and a smaller decrease might indicate merely bacteriostatic activity. Although the cytotoxic activities of all 14 cytotoxic CD4+ CD8− clones were similar in degree (not shown), only two clones caused >50% decrease in the viability of M. tuberculosis (relative to viability in the presence of the control clone with irrelevant specificity) within 24 h (Fig. 1). The two clones with this substantial antibacterial activity were the ones that depended on the cytotoxic granule (Sr2+-inhibitable) pathway of target cell lysis. In contrast, 15 of 18 cytotoxic CD4− CD8+ clones decreased bacterial viability by >50%, and again, these were the clones dependent on the cytotoxic-granule pathway of lysis.
Dependence of antimycobacterial effect on T-cell granules.
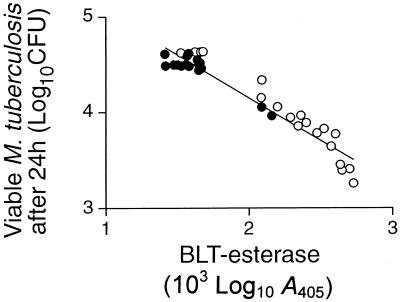
The loss of bacterial viability caused by different clones ranged up to 92%. This was equivalent to a loss of over 80% of the viable bacteria that were initially present at the start of incubation. Furthermore, there was a close correlation (r2 = 0.9218; P < 0.0001) between antibacterial activity and the availability of a granule marker enzyme, BLT esterase (22), for release by Sr2+ (Fig. 4). Evidence that the loss of bacterial viability was mainly due to the clone's cytotoxic granules was obtained from the effects of inhibitors on representative clones (Fig. 5). Clone 8.6 had one of the highest antibacterial activities, reducing viability by 92%; it produced IFN-γ and displayed granule-dependent cytotoxicity. Anti-IFN-γ neutralized some of the antibacterial effect (t test; P < 0.01), decreasing it from 92 to 76%, whereas prior degranulation with Sr2+ almost completely eliminated the antibacterial action. The antibacterial activity of a CD4+ CD8− clone that had granule-dependent cytotoxicity and produced IFN-γ (clone 4.17) was similarly markedly reduced by degranulation and was less effectively reduced by the presence of anti-IFN-γ. The slight antibacterial activities of two CD4+ CD8− clones (21 and 35% reduction in bacterial viability) that either had no cytotoxic activity (clone 4.1) or used the Fas-FasL pathway (clone 4.4) were blocked by anti-IFN-γ (P < 0.001) and not by Sr2+. The slight activity of clone 4.4 was also inhibited by anti-Fas (P < 0.001). Clone 4.7, which did not produce IFN-γ, had no antibacterial activity despite having Fas-dependent cytotoxicity.
FIG. 4.
Correlation of clone antimycobacterial activity in vitro and BLT esterase content. The antimycobacterial activities of all 14 cytotoxic CD4+ CD8− clones (●) and all 18 cytotoxic CD4− CD8+ clones (○) were determined and plotted against BLT esterase activity released by incubating the clone with Sr2+ for 10 h at 37°C. The correlation was highly significant (r2 = 0.922; P < 0.0001). The error bars indicate SD.
FIG. 5.
Antimycobacterial activity of representative clones against infected macrophages and the effects of specific inhibitors. Bone marrow-derived macrophages were infected with M. tuberculosis H37Rv (about four bacteria/macrophage) and then incubated with representative clones at an E/T ratio of 50:1. MAbs against Fas or FasL were added, or the clones were degranulated with Sr2+ (23) before the effectors and targets were mixed. After 24 h at 37°C, the cells were fully lysed by adding saponin, and the CFU were counted. The bars show means ± SD (n = 3) for representative clones and a nonspecific CD4+ CD8− control. Clone 4.4 represents the most common phenotype among CD4+ CD8− clones, and clone 8.6 represents the most common phenotype among CD4− CD8+ clones (Fig. 1).
Corroboration that granule-mediated cytotoxicity plays a part in protective immunity was obtained by comparing granule content with the protective effect that was previously observed when some of the clones were tested for adoptive transfer of immunity to naïve recipient mice (2). There was a significant positive correlation with protection (Fig. 6).
FIG. 6.
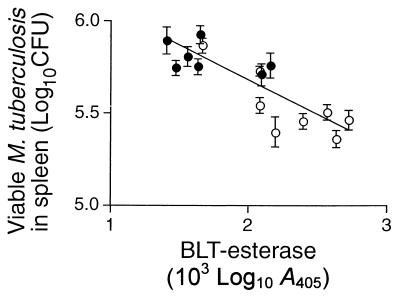
Correlation of clone adoptive transfer of protection and BLT esterase content. The seven cytotoxic CD4+ CD8− clones (●) and the eight cytotoxic CD4− CD8+ clones (○) that were derived from DNA-immunized and infected mice had all been tested previously for the ability to protect recipient animals from challenge infection (2). The mean numbers of live M. tuberculosis H37Rv organisms in spleens 4 weeks after challenge are shown as means ± SD for groups of five animals, plotted against clone BLT esterase released by Sr2+. The correlation was highly significant (r2 = 0.673; P < 0.0001).
DISCUSSION
The hsp65-specific T-cell clones tested here were conventionally MHC restricted; they recognized antigen that was either processed endogenously and presented on MHC class I if they were CD4− CD8+ cells or processed exogenously and presented on MHC class II if they were CD4+ CD8− cells, as previously described (2, 21). A remarkably high proportion of the clones were cytotoxic, whether they originated from infected mice or from DNA- or J774-hsp65-vaccinated mice, and this reflects their high frequency in vivo (13). Cytotoxicity was particularly associated with the CD44hi phenotype; most of the CD44hi clones from J774-hsp65-vaccinated or DNA-vaccinated mice were cytotoxic (13 of 16 and 7 of 8, respectively). Although only half of the CD44hi clones from M. tuberculosis-infected mice were cytotoxic (4 of 8), implying that the activated cells may be less likely to attain cytotoxic function during the disease process than after these vaccinations, the differences did not attain statistical significance (χ2 > 0.05).
The importance of IFN-γ as a macrophage-activating factor for antimycobacterial action is well established (3). However, our previous studies of these hsp65-specific clones in adoptive transfer of protection against tuberculosis had shown an association between the protective activity and the ability to lyse infected macrophages in addition to the production of IFN-γ (2, 21). We have confirmed here that lysis of infected macrophages by cytotoxic-T-cell clones in vitro can cause an apparent loss of M. tuberculosis viability (killing). This can exceed 90% of the bacteria during 24 h and strongly suggests that cytotoxic T cells directly contribute to protection against tuberculosis by killing the bacteria in vivo. In contrast, the IFN-γ produced by the clones imparted only a modest inhibition of the intracellular mycobacteria during this short period, consistent with activation for bacteriostasis.
Strikingly, only the T-cell clones using the granule-dependent pathway showed clear evidence of killing intracellular M. tuberculosis when they lysed infected macrophages. Those clones using the Fas-FasL pathway had small effects, equivalent to bacteriostasis. These might be partly attributable to discharge of the bacteria into the less favorable growth environment provided by the tissue culture medium and partly to activation of nonlysed macrophages by IFN-γ. The close correlation between the degree of killing and the granule content of the clone, and the selective inhibition of killing by prior degranulation, are consistent with killing by the granule contents. The mechanism is likely to be similar to that which was recently revealed in studies of human cells (22). Thus, the granule enzyme perforin may lyse macrophage membranes to allow access of potent microbicidal granule enzymes, such as granulysin, to the target mycobacteria. Attempts to identify the mycobactericidal agent in the mouse cells are under way.
The correlation between the ability of the cytotoxic clones to protect against challenge with M. tuberculosis in vivo and the clones' granule content is further indication that this mycobacterial killing mechanism has a role in protective immunity. It may account for the major component of the protective effect of these clones that was resistant to neutralization in vivo by injection of antibody against IFN-γ (21). Although others found that knockout mice that do not express the perforin gene did not have decreased resistance to the early stages of tuberculosis (6, 11), this defense mechanism may be more important later in infection. Our high-dose intravenous-challenge model probably resembles late-stage rather than early-stage tuberculosis.
It was also striking that most of the cells lysing targets using the cytotoxic granule pathway were CD4− CD8+ cells, whereas most of cells that lysed targets using the Fas-FasL pathway were CD4+ CD8− cells. This suggests that cytotoxicity may generally serve different purposes in tuberculosis depending on whether it is triggered by endogenous or exogenous antigen. Thus, the cytotoxic CD4− CD8+ T cells may have a predominantly antimicrobial function against this intracellular pathogen, whereas the cytotoxic CD4+ CD8− T cells may have a predominantly immunomodulatory role in removing the cells that sustain immune “help” by presenting antigen on MHC class II (23). However, this division of labor is not absolute, since a minority of cytotoxic CD4+ CD8− clones used the granule pathway and a minority of the CD4− CD8+ clones used the Fas-FasL pathway. Furthermore, at least in studies of human T cells, lysis of infected target macrophages by non-granule-dependent pathways may sometimes have direct antimycobacterial effects (10, 14, 17) and non-MHC-restricted cells may contribute significantly to granule-dependent killing (22). These diverse and somewhat contradictory findings may reflect the plasticity of the target cells. Cells of the mononuclear phagocyte lineage can proceed from being undifferentiated immature monocytes to widely diverse forms, including dendritic cells (9, 25), in vivo and in vitro, depending on the environmental stimuli encountered. Adherence of the cells to different surfaces and exposure to different cytokines prior to apoptosis may have resulted in biochemically and functionally different cells in the different studies. Hence, we hypothesize that in some differentiated states the cells have a mycobactericidal potential that is mobilized during apoptosis, perhaps through autolytic generation of toxic products. In other states they do not, and bacterial killing during cytolysis then depends on delivery of the toxic agent by the lymphocyte.
ACKNOWLEDGMENTS
This study was supported in part by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
REFERENCES
- 1.Bloom B R, Fine P E M. The BCG experience: implications for future vaccines against tuberculosis. In: Bloom B R, editor. Tuberculosis: pathogenesis, protection, and control. Washington, D.C.: American Society for Microbiology; 1994. pp. 531–557. [Google Scholar]
- 2.Bonato V L D, Lima V M F, Tascon R E, Lowrie D B, Silva C L. Identification and characterization of protective T cells in hsp65 DNA-vaccinated and Mycobacterium tuberculosis-infected mice. Infect Immun. 1998;66:169–175. doi: 10.1128/iai.66.1.169-175.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chan J, Kaufmann S H E. Immune mechanisms of protection. In: Bloom B R, editor. Tuberculosis: pathogenesis, protection, and control. Washington, D.C.: American Society for Microbiology; 1994. pp. 389–415. [Google Scholar]
- 4.Chan J, Xing Y, Magliozzo R S, Bloom B R. Killing of virulent Mycobacterium tuberculosis by reactive nitrogen intermediates produced by activated murine macrophages. J Exp Med. 1992;175:1111–1122. doi: 10.1084/jem.175.4.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cooper A M, Dalton D K, Stewart T A, Griffin J P, Russell D G, Orme I M. Disseminated tuberculosis in interferon gamma gene-disrupted mice. J Exp Med. 1993;178:2243–2247. doi: 10.1084/jem.178.6.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cooper A M, D'Souza C, Frank A A, Orme I M. The course of Mycobacterium tuberculosis infection in the lungs of mice lacking expression of either perforin- or granzyme-mediated cytolytic mechanisms. Infect Immun. 1997;65:1317–1320. doi: 10.1128/iai.65.4.1317-1320.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Libero G, Flesch I, Kaufmann S H E. Mycobacteria-reactive Lyt-2+ T cell lines. Eur J Immunol. 1988;18:59–66. doi: 10.1002/eji.1830180110. [DOI] [PubMed] [Google Scholar]
- 8.Flynn J L, Chan J, Triebold K J, Dalton D K, Stewart T A, Bloom B R. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J Exp Med. 1993;178:2249–2254. doi: 10.1084/jem.178.6.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hausser G, Ludewig B, Gelderblom H R, Tsunetsugu-Yokota Y, Akagawa K, Meyerhans A. Monocyte-derived dendritic cells represent a transient stage of differentiation in the myeloid lineage. Immunobiology. 1997;197:534–542. doi: 10.1016/S0171-2985(97)80085-X. [DOI] [PubMed] [Google Scholar]
- 10.Lammas D A, Stober C, Harvey C J, Kendrick N, Panchalingam S, Kumararatne D S. ATP-induced killing of mycobacteria by human macrophages is mediated by purinergic P2Z(P2X(7)) receptors. Immunity. 1997;7:433–444. doi: 10.1016/s1074-7613(00)80364-7. [DOI] [PubMed] [Google Scholar]
- 11.Laochumroonvorapong P, Wang J, Liu C C, Ye W G, Moreira A L, Elkon K B, Freedman V H, Kaplan G. Perforin, a cytotoxic molecule which mediates cell necrosis, is not required for the early control of mycobacterial infection in mice. Infect Immun. 1997;65:127–132. doi: 10.1128/iai.65.1.127-132.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lowrie D B. Mononuclear phagocyte-mycobacterium interaction. In: Ratledge C, Stanford J, editors. The biology of the mycobacteria. Vol. 2. London, United Kingdom: Academic Press; 1983. pp. 235–278. [Google Scholar]
- 13.Lowrie D B, Colston M J, Tascon R E, Silva C L. DNA encoding individual mycobacterial antigens protects mice against tuberculosis. In: Brown F, Burton D, Doherty P, Mekalanos J, Norrby E, editors. Vaccines 97: molecular approaches to the control of infectious diseases. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1997. pp. 163–166. [Google Scholar]
- 14.Molloy A, Laochumroonvorapong P, Kaplan G. Apoptosis, but not necrosis, of infected monocytes is coupled with killing of intracellular bacillus Calmette-Guérin. J Exp Med. 1994;180:1499–1509. doi: 10.1084/jem.180.4.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mustafa A S, Amoudy H A, Wiker H G, Abal A T, Ravn P, Oftung F, Andersen P. Comparison of antigen-specific T-cell responses of tuberculosis patients using complex or single antigens of Mycobacterium tuberculosis. Scand J Immunol. 1998;48:535–543. doi: 10.1046/j.1365-3083.1998.00419.x. [DOI] [PubMed] [Google Scholar]
- 16.O'Brien S, Jackett P S, Lowrie D B, Andrew P W. Guinea-pig alveolar macrophages kill Mycobacterium tuberculosis in vitro, but killing is independent of susceptibility to hydrogen peroxide or triggering of the respiratory burst. Microb Pathog. 1991;10:199–207. doi: 10.1016/0882-4010(91)90054-e. [DOI] [PubMed] [Google Scholar]
- 17.Oddo M, Renno T, Attinger A, Bakker T, MacDonald H R, Meylan P R A. Fas ligand-induced apoptosis of infected human macrophages reduces the viability of intracellular Mycobacterium tuberculosis. J Immunol. 1998;160:5448–5454. [PubMed] [Google Scholar]
- 18.Rastogi N. Killing intracellular mycobacteria—dogmas and realities—closing comments. Res Microbiol. 1990;141:269–270. doi: 10.1016/0923-2508(90)90029-p. [DOI] [PubMed] [Google Scholar]
- 19.Romagnani S. The Th1/Th2 paradigm. Immunol Today. 1997;18:263. doi: 10.1016/s0167-5699(97)80019-9. [DOI] [PubMed] [Google Scholar]
- 20.Silva C L, Palacios A, Colston M J, Lowrie D B. Mycobacterium leprae 65hsp antigen expressed from a retroviral vector in a macrophage cell line is presented to T cells in association with MHC class II in addition to MHC class I. Microb Pathog. 1992;12:27–38. doi: 10.1016/0882-4010(92)90063-t. [DOI] [PubMed] [Google Scholar]
- 21.Silva C L, Silva M F, Pietro R C L R, Lowrie D B. Characterization of T cells that confer a high degree of protective immunity against tuberculosis in mice after vaccination with tumor cells expressing mycobacterial hsp65. Infect Immun. 1996;64:2400–2407. doi: 10.1128/iai.64.7.2400-2407.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stenger S, Hanson D A, Teitelbaum R, Dewan P, Niazi K R, Froelich C J, Ganz T, Thoma-Uszynski S, Melian A, Bogdan C, Porcelli S A, Bloom B R, Krensky A M, Modlin R L. An antimicrobial activity of cytolytic T cells mediated by granulysin. Science. 1998;282:121–125. doi: 10.1126/science.282.5386.121. [DOI] [PubMed] [Google Scholar]
- 23.Stenger S, Mazzaccaro R J, Uyemura K, Cho S, Barnes P F, Rosat J P, Sette A, Brenner M B, Porcelli S A, Bloom B R, Modlin R L. Differential effects of cytolytic T cell subsets on intracellular infection. Science. 1997;276:1684–1687. doi: 10.1126/science.276.5319.1684. [DOI] [PubMed] [Google Scholar]
- 24.Warwick-Davies J, Dhillon J, O'Brien L, Andrew P W, Lowrie D B. Apparent killing of Mycobacterium tuberculosis by cytokine-activated human monocytes can be an artefact of a cytotoxic effect on the monocytes. Clin Exp Immunol. 1994;96:214–217. doi: 10.1111/j.1365-2249.1994.tb06544.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou L J, Tedder T F. CD14(+) blood monocytes can differentiate into functionally mature CD83(+) dendritic cells. Proc Natl Acad Sci USA. 1996;93:2588–2592. doi: 10.1073/pnas.93.6.2588. [DOI] [PMC free article] [PubMed] [Google Scholar]