ABSTRACT
Background:
Leishmaniasis is a vector-borne disease caused by a parasite protozoon from the genus Leishmania. Among the molecular techniques applied for detecting these parasites, real-time PCR with High Resolution Melting (PCR-HRM) proved advantageous since it simultaneously determines both the presence and species of the pathogen in one step, through amplification and later analysis of curves generated by melting temperature.
Methods:
Based on this molecular technique, the goal of this study was to estimate the PCR-HRM sensitivity for Leishmania spp. detection in different canine tissues by evaluating biological samples obtained from popliteal, submandibular, and pre-scapular lymph nodes, from bone marrow and ear pinnae of 28 stray dogs captured in the metropolitan area of Asunción (Paraguay).
Results:
The rk39 immunochromatographic test showed that 25/28 tested dogs (89%) presented antibodies against L. infantum. In 20/25 dogs that tested positive for rk39 (80%), it was possible to detect Leishmania spp. by PCR-HRM and determine that the species corresponded entirely to L. infantum. Regarding the analysis of different tissues, the parasite was detected in all popliteal lymph node samples, followed by high detection in submandibular (at 95%) and pre-scapular lymph nodes (at 90%), bone marrow (at 85%), and ear pinnae (at 85%).
Conclusions:
This study demonstrated that the use of real-time PCR-HRM using the molecular marker hsp70 was a highly sensitive method for simultaneously detecting and identifying Leishmania species in different tissues taken from infected dogs. In addition, the usefulness of ear pinnae as easily accessible tissue for molecular diagnosis was emphasized.
Keywords: Hsp70, Stray dogs, rk39, PCR-HRM, Paraguay
INTRODUCTION
According to World Health Organization (WHO), leishmaniasis is considered a neglected tropical disease (NTD) which is widely distributed in tropical and sub-tropical areas, primarily affecting less developed and developing countries 1 , 2 . It is caused by unicellular flagellate parasites of the genus Leishmania spp. that circulate between the vector and the hosts to carry out their life cycle, multiplying as free promastigotes in the intestinal lumen of the vector while proliferating as obligatory intracellular amastigotes in the macrophages of the hosts 3 .
The disease is prevalent in 92 countries around the world. Its main clinical manifestations in humans include epithelial injuries, characteristic of Cutaneous Leishmaniasis (CL), and lesions in the liver and spleen 2 , 4 in the case of Visceral Leishmaniasis (VL). In addition, there is Mucocutaneous Leishmaniasis (ML) infection with primarily oral and nasal injuries that incapacitate the carrier. Although the presence of the parasite is global, most cases are reported in the continents of Africa, Asia, and South America 4 , 5 . In Paraguay, 48 cases of leishmaniasis in humans were diagnosed between the years 2018 and 2021, of which 20 were VL and 28 of the remaining cases included CL and ML 6 .
In the context of the presence of leishmaniasis in urban areas, domestic dogs (Canis familiaris) are of great epidemiological importance since they constitute the most important reservoir of the parasite. Moreover, dogs may normally present a high parasitic load which naturally constitutes a health threat to the humans that live with them 7 , 8 , 9 . In this sense, an increase in the frequency of cases of Canine Visceral Leishmaniasis (CVL) has been observed as a phenomenon that preceded an increase in human cases 10 , 11 . Studies aimed at investigating the prevalence of antibodies against Leishmania in dogs signal that in high endemic areas, the presence of antibodies could reach between 50 and 75% 12 . Previous serological studies of stray dogs in the metropolitan area of Asunción, captured within a 500-meter radius area around a human case detection point (n=42,000), showed a prevalence of the VL case, with antibody count at 69% according to the rk39 test results 13 .
In relation to the diagnosis of CL, the sensitivity of the technique used to detect the presence of the parasite in a sample depends on the parasitic load and the resolution of the applied technique. It has been observed that any one of them could lead to divergent results in some cases 14 . Furthermore, diverse studies suggest the necessity to adopt one or a variety of criteria to decrease the occurrence of false negative or false positive results in the analyzed samples 15 .
Conventionally, the presence of Leishmania spp. genus parasites can be determined by the parallel application of various techniques, such as the direct observation of the parasite in blood smear, culture, and isolation of samples of different tissues; use of serological assays, such as the Indirect Fluorescent Antibody Technique (IFAT); use of direct agglutination; application of rk39 rapid test; and others 16 . It is noteworthy that although these are routinely applied techniques, they have limitations and, in most cases, these procedures do not detect the presence of the parasite in asymptomatic individuals 17 .
In recent years, a rapid evolution of the molecular diagnostic techniques by Polymerase Chain Reaction (PCR) and its different variants, as well as the utilization of different molecular markers, contributed considerably to the detection of the parasite in an easy and sensitive manner 18 . Among the molecular detection methods, High Resolution Melting Analysis (HRM), post-PCR, highlights its capacity to detect the presence of the parasite and identify the species, all in one step through curves generated by the temperature of fusion, which are related to the G+C content of the amplified fragment 19 , 20 .
Thus, in this manner, the aim of this study was to compare the detection sensitivity of Leishmania spp. in different tissues while simultaneously determining the species of the infectious parasite by using the PCR-HRM technique for samples collected from stray dogs that were captured in the city of Asunción and previously subjected to the rapid immunological rk39 test by Ministry of Public Health and Social Welfare protocol.
METHODS
Stray Dogs
The study population consisted of 28 stray dogs--14 females and 14 males of different breed and ages--captured in the metropolitan area of Asunción (Table 1). The stray dogs were sampled between April and June of 2013 and maintained in the premises of the National Anti-rabies Center (CAN, in its Spanish acronym), an agency dependent of the Ministry of Public Health and Social Welfare (MSPyBS, in its Spanish acronym) and which is part of the National Program for Zoonotic Diseases, in Paraguay. The study was conducted on stray dogs captured within a 500-meter radius circle around a point of human case occurrence of VL and subsequently tested by rk39 strip by MSPyBS guidelines.
TABLE 1: Clinical characteristics, and immunological and molecular diagnosis of leishmaniasis in dogs studied in the metropolitan area of Asunción.
| Specimen | Breed | Age (Years) | Gender | Clinical signs | Results of rk39 | Results of qPCR hsp70 by tissue | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| BM | EP | PN | PSN | SMN | ||||||
| 1 | Mixed Terrier | 3 | M | Conjunctivitis, hepato and splenomegaly | + | + | + | + | + | + |
| 2 | Mixed | 3 | F | Dermatitis, ganglionar hypertrophy, long nails, pinna injury, hepato and splenomegaly | + | + | + | + | + | + |
| 3 | Mixed | 10 | M | Long nails, hepato and splenomegaly | + | - | - | ** | - | - |
| 4 | Mixed | 6 | F | Dry hair, node hypertrophy, long nails, seborrhea, weight loss, hepato and splenomegaly | + | + | + | + | + | + |
| 5 | Mixed | 5 | F | Seborrhea, conjunctivitis, scabs on the skin, hypertropia ganglionar, hepato and splenomegaly | + | + | + | + | + | + |
| 6 | Mixed | 5 | F | Long nails, pinna injury, node hypertrophy, eye ulcer, anemia, hepato and splenomegaly | + | + | + | + | + | + |
| 7 | Poodle | 3 | M | Cachexia, long nails, anemia, hepato and splenomegaly | + | + | + | + | + | + |
| 8 | Mixed | 7 | M | Cachexia, long nails, dry hair, hair loss, cutaneous ulcers, node hypertrophy, seborrhea, hepato and splenomegaly | + | + | + | + | + | ** |
| 9 | Mixed | 10 | F | Long nails, dry hair, hair loss, cutaneous ulcers, node hypertrophy, seborrhea, conjunctivitis, plantar hyperqueratosis, weight loss, hepato and splenomegaly | + | - | - | - | - | - |
| 10 | Mixed | 3 | M | Long nails, dry hair, nasal and plantar hyperqueratosis, seborrhea, conjunctivitis, nasal ulcer, hepato and splenomegaly | + | - | - | - | - | - |
| 11 | Poodle | >1 | F | Alopecia, weight loss, cutaneous ulcers, seborrhea, hepato and splenomegaly | + | + | - | + | + | + |
| 12 | Mixed | 7 | F | Weight loss, long nails, hair loss, conjunctivitis, plantar hyperkeratosis, hepato and splenomegaly | + | - | - | - | - | - |
| 13 | Poodle | 3 | M | Weight loss, long nails, seborrhea, ear ulcer, hepato and splenomegaly | + | + | + | + | + | + |
| 14 | Poodle | 6 | F | Weight loss, long nails, conjunctivitis, node hypertrophy, seborrhea, hepato and splenomegaly | + | + | + | + | + | + |
| 15 | Mixed | 5 | M | Asymptomatic | + | + | + | + | + | + |
| 16 | Mixed | 4 | F | Long nails, cachexia, dry hair, anemia, hepato and splenomegaly | + | + | + | + | + | + |
| 17 | Mixed | 2 | M | Long nails, blepharitis, conjunctivitis, plantar hyperkeratosis, dry hair, node hypertrophy, seborrhea | + | + | + | + | + | + |
| 18 | Mixed | 1 | M | Plantar hyperkeratosis, hepato and splenomegaly | + | - | - | + | - | + |
| 19 | Mixed | 5 | M | Long nails, cutaneous ulcers, conjunctivitis, anemia, seborrhea, weight loss, node hypertrophy, conjunctivitis | + | + | + | + | + | + |
| 20 | Mixed | 2 | M | Seborrhea, conjunctivitis, weight loss, long nails, lagana, scites, hepato and splenomegaly | + | - | - | - | - | - |
| 21 | Mixed | 7 | F | Cachexia, hyperkeratosis, dry hair, ear ulcer, alopecia | + | + | + | + | + | + |
| 22 | Mixed | 7 | F | Hyperkeratosis, cutaneous ulcers, seborrhea, weight loss, long nails, conjunctivitis, node hypertrophy | + | - | + | + | + | + |
| 23 | Mixed | 7 | F | Dry hair, long nails, alopecia, dermatitis | + | - | + | - | - | - |
| 24 | Pitbull | >1 | F | Eczema, weight loss, cutaneous ulcers, node hypertrophy, edema de members, plantar and nasal hyperkeratosis | + | + | + | + | + | + |
| 25 | Pitbull | 2 | M | Cachexia, plantar hyperkeratosis, seborrhea, dry hair, cutaneous ulcers | + | + | - | + | + | + |
| 26 | Mixed | 3 | M | Asymptomatic | - | - | - | - | - | - |
| 27 | Mixed | 4 | F | Asymptomatic | - | - | - | - | - | - |
| 28 | Mixed | 2 | M | Asymptomatic | - | - | - | - | - | - |
BM: bone marrow; EP: ear pinna; PN: popliteal lymph node; PSN: pre-scapular lymph node; SMN: submandibular lymph node. (**) Sample not obtained. F: female; M: male.
Tissue samples and clinical signs
Five different tissue samples were taken from each animal: from the popliteal node (PN), submandibular node (SMN) and pre-scapular lymph nodes (PSN), the bone marrow (BM), and the ear pinnae (EP). The PN, SMN, PSN, and BM samples were taken by puncture and aspiration with a 1 mL syringe, while the EP samples were obtained by deep scraping with a scalpel blade.
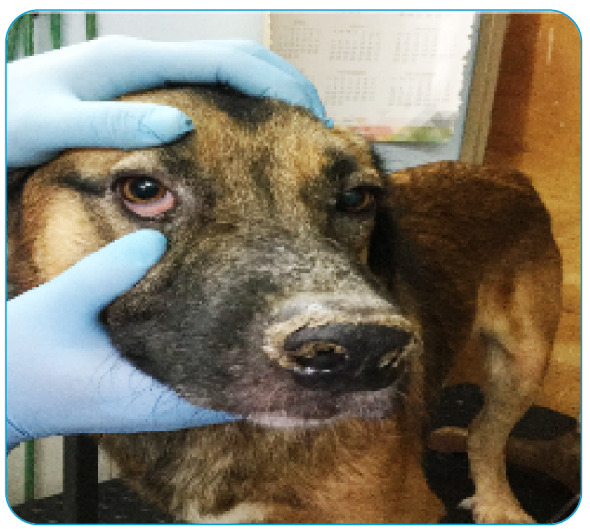
The gender, breed, characteristics, and clinical signs of the disease were observed and registered by professional veterinarians from CAN and the details are presented in Table 1 and Figure 1.
FIGURE 1: Canis familiaris specimen with dermal injuries and ulcers typical of leishmaniasis.
Serological test rk39
Serological tests were performed by CAN using the lateral flow chromatography using the rk39 strip, following the instructions by the manufacturer (https://diagnostics.be/). The samples of serum previously separated by centrifugation were placed on top of the test strip and incubated with a buffer. Visual readings for identification were performed after 10 min; those readings that showed a definite band pattern for this assay were considered as a positive result 21 .
DNA extraction and quantification of L. (L.) infantum strain
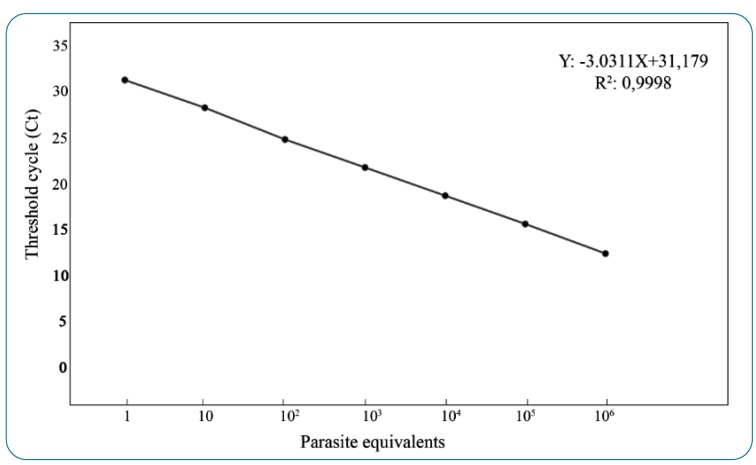
L. (L.) infantum promastigotes (MCAN/ES/92/BCN83) were maintained in culture at 26 °C using Schneider's Insect Medium (Sigma-Aldrich®) supplemented with 10% inactivated fetal bovine serum (FBS; Sigma-Aldrich®). Parasite DNA extraction was performed in its stationary phase equivalent to 1×106 parasites/mL using the commercial GeneJET Genomic DNA Purification Kit (#K0722 Thermo Scientific®), following the manufacturer's instructions. Subsequently, successive dilutions with a 1:10 factor (26 ng/µL L. (L.) infantum DNA) were performed to evaluate Ct values by absolute quantitative qPCR.
DNA extraction and purification of tissue samples
From 26 dogs, the five tissue samples required for this study were obtained; in 2 dogs, only four of the five tissue samples were collected. In total, 138 samples were gathered. DNA of each tissue was extracted and purified in CEDIC using the Gene JET Genomic DNA Purification Kit® (#K0722; Thermo Scientific, Waltham, MA), following the instructions of the manufacturer. At the end of each extraction, the degree of purity of the genetic material was evaluated using a spectrophotometer (DS-11FX + DeNovix®, Wilmington, DE). Good laboratory practices were used to avoid DNA cross contamination.
DNA amplification and quantification of Leishmania spp.
To confirm the presence and absolute quantification Leishmania spp., a fragment of 144bp of the heat shock protein 70 gen (hsp70) was amplified with real-time PCR, using the primers Fhsp70F2 5ʹ-GGAGAACTACGCGTACTCGATGAAG-3ʹ and Rhsp70C 5ʹ-TCCTTCGACGCCTCCTGGTTG-3ʹ, as described by Zampieri et al. 22 . The qPCR was performed in a final volume of 20 μL, using 10 μL of the HRM PCR MasterMix® (QIAGEN, Germantown, MD), with a DNA final concentration ~30 ng/μL ,and both primers with a final concentration of 0.5 µM.
The qPCR was executed in the Rotor-Gene 6000® (QIAGEN) thermocycler, and the cycling conditions were: initial denaturation at 95 ºC for 10 min, followed by 40 cycles of denaturation at 95 ºC for 10 s, annealing at 60 ºC for 30 s, and extension at 72 ºC for 10 s.
For quantitative assays, a standard curve was performed in three successive and independent qPCR assays, using 3 μL of L (L.) infantum DNA (MCAN/ES/92/BCN83). Using the dilutions and PCR conditions mentioned above, in each qPCR, water was used as reaction control (NCT). The efficiency of all assays was analyzed using the LinRegPCR software (version 2021.2) and Excel (Office® 2016).
Finally, a comparison of the parasite load was carried out to determine significant differences in the DNA concentration of L. infantum in the different tissues. Data were submitted to analysis of variance (ANOVA) followed by the Tukey post-test with a significance level of p<0.05. Statistical analyses were performed using Graph Pad Prism version 6.0 software.
Identification of Leishmania spp.
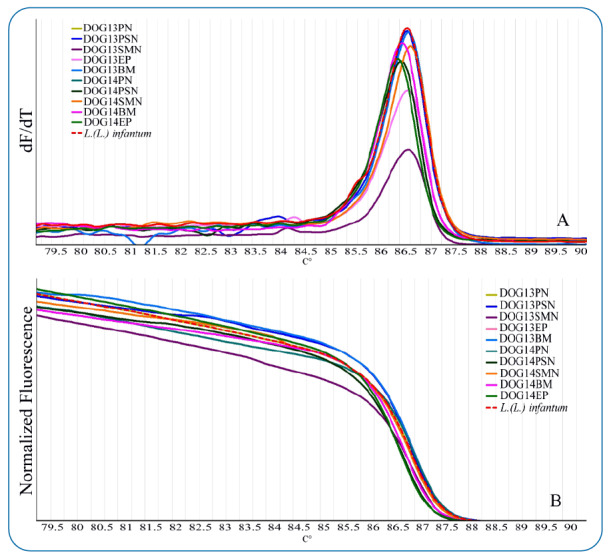
To determine the Leishmania species, the HRM analysis of the amplicon dissociation was performed immediately after the conclusion of real-time PCR. The melting range was established between 80 ºC and 90 ºC, with a slope of 0.1 °C/s. The HRM curve analysis was carried out with the Rotor Gene 6000 software version 2.1.0 (QIAGEN). DNA from the reference strain of L. (L.) infantum (MCAN/ES/92/BCN83) was used as a positive control and the Leishmania species present in the analyzed tissues were identified by comparing the melting profiles with that of the reference strain.
RESULTS
Of the 28 dogs selected for this study, 25 (89%) were determined as positive for Leishmania spp. according to the rk39 strip test. There were also three asymptomatic and negative dogs for this immunological test which were used as negative control for the molecular assays (Table 1).
The clinical evaluation of the 25 positive animals by rk39 revealed signs of the disease, such as hepatosplenomegaly (68%), long nails (56%), ulcers and seborrhea (44%), conjunctivitis and weight loss (40%), dry hair and hyperkeratosis (32%), cachexia (20%), anemia (16%), hair loss (0,12%), and dermatitis and scabs on the skin (0,04%); meanwhile, the remaining 4% did not present any signs of the disease (Table 1).
The results of the standard curve provide the Ct (Threshold Cycle) values for each equivalent parasite concentration which were subsequently analyzed with the linear regression formula ( Supplementary Figure 1). Molecular results obtained by real-time qPCR of the tissues of the 28 dogs included in this study confirmed that 20 of the 25 positive dogs, according to rk39 results (80% of the population), were also positive for Leishmania spp. in one or more than one tissues (Table 1), while the results were negative in the three dogs that were included as negative control (Table 1). The HRM analysis revealed the presence of L. infantum in all positive tissues (Figure 2).
FIGURE 2: (A) Tm values obtained with the high-resolution melt (HRM) assay for positive control (L. (L.) infantum) and canine tissue samples. (B) Normalized curve of the heat shock protein (hsp70) amplicon obtained by HRM.
In 15/20 molecularly positive dogs (75%), it was possible to detect the presence of the parasite from the five analyzed tissues including the estimated parasite load by absolute quantification (Table 1, Supplementary Table 1).
Evaluating the sensitivity of the molecular technique in the positive specimens, divided by the type of analyzed tissues, the parasites were detected in 100% of the samples of the PN, followed by high detection in the SMN (95%), PSN (90%), BM (85%), and EP (85%) (Table 2). In total, the presence of L. infantum was detected in 89/98 molecular positive tissues analyzed (91% of the population) (Table 2).
TABLE 2: Percentage of detection sensitivity of L. infantum by PCR-HRM in different tissues obtained from 20 positive dogs.
| PCR-HRM for L. infantum detection | ||
|---|---|---|
| Tissue | Positive tissue / Analyzed tissue (N) | Sensitivity (%) |
| Popliteal lymph node (PN) | 19/19* | 100 |
| Sub mandibular lymph node (SMN) | 18/19* | 95 |
| Pre-scapular lymph node (PSN) | 18/20 | 90 |
| Bone marrow (BM) | 17/20 | 85 |
| Ear pinna (EP) | 17/20 | 85 |
| Total | 89/98 | 91% |
*One sample of this tissue not obtained.
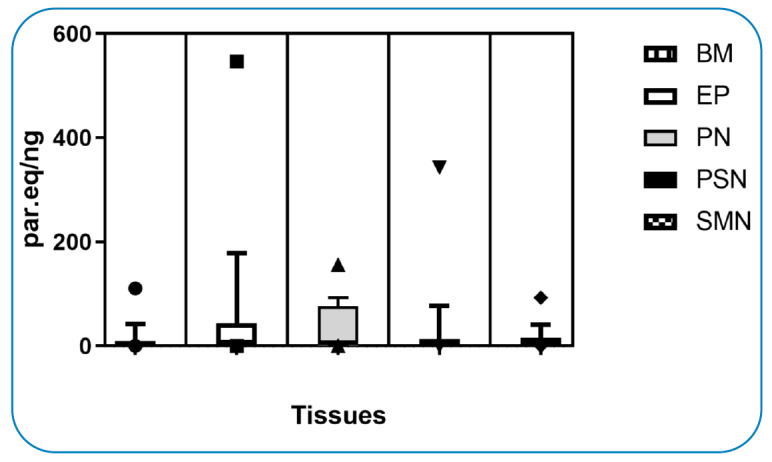
In addition to analyzing the parasitic load by qPCR in each tissue evaluated, there was no significant difference (p<0.05) when comparing the different tissues (Figure 3). However, when comparing parasitic loads between individuals, the animal that presented the highest parasitic load in the ear pinna corresponded to number 15, which did not present symptoms compatible with the illness (Table 1, Supplementary Table 1).
FIGURE 3: Comparative equivalent parasite DNA in tissues using ANOVA.
DISCUSSION
The genotypic detection and identification of parasites originating from vectors as well as vertebrate hosts fulfil an important part in the monitoring of zoonotic diseases. In this sense, domestic dogs are the main hosts of L. (L.) infantum, and accordingly have a fundamental part in the transmission cycle of leishmaniasis 23 . Thus, as a rapid diagnostic is fundamental to direct the treatment in human cases, the identification of reservoirs is just as essential 24 .
This study aimed to evaluate the applicability of the real-time PCR-HRM technique to simultaneously determine the presence and species of Leishmania spp., as well as the technique`s sensitivity in the different tissues obtained from stray dogs that had been naturally infected and captured in the metropolitan area of Asunción. Diverse methods exist that are can be used to detect Leishmania spp. in dogs, from a general method based on the detection of antibodies, often used with a limited efficiency, to the most sensitive and specific method such as the PCR based on samples obtained from distinct types of tissues 25 . Although the detection by direct observation of the parasites in tissues is still considered as the “golden standard” method, its applicability diminishes in cases where the parasitical load in the analyzed tissues is low. For this exact reason, in the last few years detection of the parasite by molecular methods was routinely put to the test, which allowed the development of different techniques 18 . Among these procedures, the one established by Zampieri and collaborators 22 stands out, who, in 2016, reported the use of the PCR-HRM technique to differentiate Leishmania species. The fundamental principle of this technique is the detection of variations in the nucleotide sequence, which generates a pattern of characteristic curves that can be observed in real time 26 .
In Paraguay, the molecular detection of Leishmania spp. in samples of different sources has been traditionally performed using conventional PCR with restriction enzyme digestion 27 . Nonetheless, many recent local studies were focused on the application of the real-time technique for this same purpose 28 , 29 ; in particular, scientists have started to use an innovative variation of the qPCR-HRM method, using the hsp70 marker for the detection and identification of Leishmania spp. species from human samples 30 .
The high specificity and sensitivity in the molecular detection of Leishmania spp. in samples of BM aspirate, nodes, and blood obtained from infected dogs have been previously reported 31 , 23 . In the context of this study, the obtained results showed that the samples extracted from distinct types of nodes showed higher levels of sensitivity, as previously described 32 , 33 , in comparison to the BM and EP samples. Although the literature mentions that by using the same technique, the bone marrow samples present similar sensitivity values compared to the lymph nodes, the lower sensitivity values observed in different samples in this study could have been due to the low parasitic load in the tissues or associated issues with the obtainment or processing of the samples 34 .
Regarding the sensitivity of the immunochromatography techniques used for diagnosis, the literature mentions that the rapid reveal of the strips is based on the detection of the recombinant rk39 antigen, which can detect the presence of parasites of the L. donovani complex. Additionally, studies showed that the main advantage of this technique is the combination of its efficiency and ease of use, since extensive training or a very sophisticated equipment is not needed for its interpretation 35 . The results of the rapid test compared to the ones of the real-time PCR obtained in this study revealed a confidence of 80%, similar to the values reported by other authors 25 , 36 . Nevertheless, the sensitivity reported by means of this technique in other studies is lesser if compared to the molecular methods, being dependent of the infection time and the parasitic load 37 . In this study the sensitivity of the previously mentioned immunochromatography test is greater when compared to the PCR-HRM results, since five dogs that had been diagnosed as positive by the rk39 test resulted negative in all the tissues according to the molecular analysis, which could have resulted in false positives. In addition, the inability to discriminate between immunity and actual infectiousness suggests that a combination with other non-immunological based tests will be required for highly sensitive/specific diagnosis in order to target control measures in individual reservoirs from a public health perspective, as for individual management from an animal health perspective 38 .
Considering the regions where there is a prevalence of leishmaniasis, combined with the socioeconomic characteristics that lead to it being considered as a neglected disease, the combination of two or more diagnostic methods is an alternative that should be considered. Another point to take into account is that urban dogs could be infected not only by L. infantum but also by L. amazonensis or L. braziliensis, as has already been described in different areas of Brazil 39 , 40 , 41 , 42 ; therefore, the diagnosis of leishmaniasis in dogs should be confirmed using PCR based methods or by the isolation and subsequent characterization of the parasite using reference isoenzymatic methods 43 . As observed in this study, besides the election of the best detection method, the selection of the tissue to be analyzed is a relevant factor to be considered with the purpose of minimizing the stress caused to the animal when taking the sample.
An interesting finding aroused in this study is the high sensitivity of the epithelial tissue area for parasite detection, where a deep cutaneous scraping of the ear atrial margin of the dogs was performed in search of the Leishmania DNA. It should be noted that the detection of the parasite was performed in samples of healthy atrial epithelium, taken from areas without open wounds produced by the infection of Leishmania spp., thereby reproducing results presented by other authors 44 , 45 . The sensitivity of these tissues was 85%, similar to that observed in bone marrow samples, and coinciding with the results obtained by other authors 46 , 47 , 48 . In this way, considering the good sensitivity and less invasiveness of this technique when taking the sample, also taking into account that no significant differences were observed comparing the parasitic load in this tissue in relation to the other tissues, both the ear pinnae and the lymph nodes constitute a viable alternative for use in the diagnosis of Canine Leishmaniasis. However, future studies are necessary to determine the parasitic load in ear pinnae tissue and the detection limit for the molecular technique used in this study, under the objective of guaranteeing the least possible disturbance to the animal.
ACKNOWLEDGMENTS
The authors thank to the National Program of Zoonotic Diseases (MSPyBS - Paraguay) for their collaboration in the study.
SUPPLEMENTARY MATERIAL.
SUPPLEMENTARY TABLE 1: Comparative equivalent parasite DNA value and ct by tissue.
| BM | EP | PN | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Code | ct | *par.eq/ng | Code | ct | *par.eq/ng | Code | ct | *par.eq/ng | ||
| 1BM | 33.227 | 0.211 | 1EP | 28.939 | 5.483 | 2PN | 27.381 | 17.902 | ||
| 2BM | 26.974 | 24.393 | 2EP | 29.853 | 2.739 | 4PN | 32.055 | 0.514 | ||
| 4BM | 36.394 | 0.019 | 4EP | 30.421 | 1.778 | 5PN | 31.524 | 0.769 | ||
| 5BM | 35.972 | 1.891 | 5EP | 29.663 | 3.163 | 6PN | 24.535 | 155.615 | ||
| 6BM | 35.262 | 0.045 | 6EP | 25.970 | 52.306 | 7PN | 27.162 | 21.140 | ||
| 7BM | 32.293 | 0.429 | 7EP | 28.224 | 9.442 | 8PN | 25.325 | 85.348 | ||
| 8BM | 34.189 | 0.102 | 8EP | 25.301 | 86.965 | 11PN | 25.543 | 72.324 | ||
| 11BM | 30.770 | 1.364 | 13EP | 26.518 | 34.504 | 13PN | 29.888 | 2.666 | ||
| 13BM | 34.422 | 0.085 | 14EP | 28.564 | 7.289 | 14PN | 28.528 | 7.494 | ||
| 14BM | 27.756 | 13.470 | 15EP | 22.881 | 546.598 | 15PN | 29.657 | 3.178 | ||
| 15BM | 28.532 | 7.468 | 16EP | 25.858 | 56.945 | 16PN | 25.426 | 79.087 | ||
| 16BM | 24.982 | 110.749 | 17EP | 30.961 | 1.180 | 17PN | 34.217 | 0.099 | ||
| 17BM | 30.885 | 1.250 | 19EP | 27.677 | 14.299 | 19PN | 28.385 | 8.352 | ||
| 19BM | 33.495 | 0.172 | 21EP | 34.642 | 0.072 | 21PN | 25.479 | 75.956 | ||
| 21BM | 31.497 | 0.785 | 22EP | 29.935 | 2.572 | 22PN | 30.496 | 1.680 | ||
| 24BM | 34.711 | 0.068 | 23EP | 32.051 | 0.516 | 24PN | 30.478 | 1.704 | ||
| 25BM | 33.137 | 0.226 | 24EP | 29.089 | 4.892 | 25PN | 34.033 | 0.114 | ||
| 18PN | 25.378 | 81.991 | ||||||||
| PSN | SMN | |||||||||
| Code | ct | *par.eq/ng | Code | ct | *par.eq/ng | |||||
| 1PSN | 33.167 | 0.221 | 1SMN | 33.302 | 0.199 | |||||
| 2PSN | 27.763 | 13.393 | 2SMN | 28.466 | 7.851 | |||||
| 4PSN | 30.406 | 1.799 | 4SMN | 30.062 | 2.337 | |||||
| 5PSN | 32.870 | 0.277 | 5SMN | 32.614 | 0.336 | |||||
| 6PSN | 28.836 | 5.928 | 6SMN | 27.560 | 15.631 | |||||
| 7PSN | 30.234 | 2.051 | 7SMN | 28.695 | 6.600 | |||||
| 8PSN | 26.108 | 47.104 | 11SMN | 33.448 | 0.178 | |||||
| 11PSN | 28.669 | 6.732 | 13SMN | 36.551 | 0.017 | |||||
| 13PSN | 27.121 | 21.823 | 14SMN | 29.971 | 2.503 | |||||
| 14PSN | 32.095 | 0.499 | 15SMN | 29.410 | 3.833 | |||||
| 15PSN | 27.669 | 14.387 | 16SMN | 25.215 | 92.827 | |||||
| 16PSN | 23.494 | 343.183 | 17SMN | 37.165 | 0.011 | |||||
| 17PSN | 36.113 | 0.024 | 19SMN | 27.581 | 15.388 | |||||
| 19PSN | 27.855 | 12.494 | 21SMN | 30.765 | 1.370 | |||||
| 21PSN | 29.390 | 3.894 | 22SMN | 31.711 | 0.667 | |||||
| 22PSN | 33.212 | 0.213 | 24SMN | 28.704 | 6.554 | |||||
| 24PSN | 32.515 | 0.362 | 25SMN | 37.274 | 34.711 | |||||
| 25PSN | 33.232 | 0.210 | 18SMN | 26.677 | 30.564 | |||||
SUPPLEMENTARY FIGURE 1: Standard curve target hsp70: threshold cycle values and equivalent parasite DNA by reaction.
Footnotes
Financial Support: This study was supported by the Organization for the Structural Convergence in the Mercosur Region (FOCEM, in its Spanish acronym. Grant number: FOCEM/MERCOSUR COF N°03/11), National Research Incentive Program of the National Council for Science and Technology (PRONII-CONACYT) and the Repatriation and Settlement Program for foreign researchers in Paraguay (CONACYT).
REFERENCES
- 1.World Health Organization . Control of Neglected Tropical Disease. WHO: Geneva Switzerland; 2020. [2021 June 1]. Available from: https://www.who.int/teams/control-of-neglected-tropical-diseases/neglected-zoonotic-diseases . [Google Scholar]
- 2.World Health Organization . Health Topics, Leishmaniasis. WHO: Geneva Switzerland; 2020. [2021 June 1]. Available from: https://www.who.int/health-topics/leishmaniasis#tab=tab_1 . [Google Scholar]
- 3.Handman E, Bullen VR. Interaction of Leishmania with the host macrophage. Trends Parasitol. 2002;18(1):332–334. doi: 10.1016/s1471-4922(02)02352-8. [DOI] [PubMed] [Google Scholar]
- 4.Panamerican Health Organization . Informe Epidemiológico das Américas. PAHO; Washington USA: 2020. [2021 May 23]. Available from: https://iris.paho.org/handle/10665.2/53091 . [Google Scholar]
- 5.World Health Organization . Leishmaniasis. Key Fact. WHO: Geneva Switzerland; 2020. [2021 June 1]. Available from: https://www.who.int/news-room/fact-sheets/detail/leishmaniasis . [Google Scholar]
- 6.Ministerio de Salud Pública y Bienestar Social . Boletín epidemiológico semanal. Boletín Epidemiológico. MSPyBS DGVS; Asunción Paraguay: 2019. [2021 August 23]. Available from: http://dgvs.mspbs.gov.py/files/boletines/SE10_2021_Boletin.pdf . [Google Scholar]
- 7.Giunchetti RC, Mayrinkz W, Genaro O, Carneiro CM, Corrêa-Oliveira R, Martins-Filho AO, et al. Relationship between canine visceral leishmaniosis and the Leishmania (Leishmania) chagasi burden in dermal inflammatory foci. J Comp Path. 2006;135(2-3):100–107. doi: 10.1016/j.jcpa.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 8.Coura-Vital W, Marques MJ, Giunchetti RC, Teixeira-Carvalho A, Moreira ND, Vitoriano-Souza J, et al. Humoral and cellular immune responses in dogs with inapparent natural Leishmania infantum infection. Vet J. 2011;190(2):e43-e47. doi: 10.1016/j.tvjl.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 9.Gramiccia M, Gradoni L. The current status of zoonotic leishmaniases and approaches to disease control. Int J Parasitol. 2005;35(11-12):1169–1180. doi: 10.1016/j.ijpara.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 10.Fraga DBM, Solcà MS, Silva VMG, Borja LS, Nascimento EG, Oliveira GGS. Temporal distribution of positive results of tests for detecting Leishmania infection in stray dogs of an endemic area of visceral leishmaniasis in the Brazilian tropics: A 13 years survey and association with human disease. Vet Parasitol. 2012;191:591–594. doi: 10.1016/j.vetpar.2012.06.025. [DOI] [PubMed] [Google Scholar]
- 11.Grimaldi G, Teva A, Santos CB, Ferreira AL, Falqueto A. The effect of removing potentially infectious dogs on the numbers of canine Leishmania infantum infections in an endemic area with high transmission rates. Am J Trop Med Hyg. 2012;86(6):966–971. doi: 10.4269/ajtmh.2012.12-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dantas-Torres F. Canine leishmaniosis in South America. Parasit Vectors. 2009;2(1) doi: 10.1186/1756-3305-2-S1-S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miret J, Medina M, Velázquez AL, Sosa L, Castagnino M. Leishmaniosis visceral en caninos errantes en la ciudad de Asunción Paraguay. Rev Parag Epidemiol. 2011;2(2):13–22. [Google Scholar]
- 14.Pessoa R, Vaitkevicius-Antão V, Santos TA, Oliveira A, Oliveira G, Mendonça LA. The diagnosis of canine visceral leishmaniasis in Brazil: Confrontin old problems. Exp Parasitol. 2019;199:9–16. doi: 10.1016/j.exppara.2019.02.012. [DOI] [PubMed] [Google Scholar]
- 15.Maia C, Ramada J, Cristovão J, Gonçalves L, Campino L. Diagnosis of canine leishmaniasis: Conventional and molecular techniques using different tissues. Vet J. 2009;179:142–144. doi: 10.1016/j.tvjl.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 16.Montalvo AM, Fraga J, Monzote CL, García M, Fonseca L. Diagnóstico de la leishmaniasis: de la observación microscópica del parásito a la detección del ADN. Rev Cub Med Trop. 2012;64(2):108–131. [PubMed] [Google Scholar]
- 17.Mohammadihaa A, Haghighi A, Mohebali M, Mahdiand R, Abadi AR, Zarei Z, et al. Canine visceral leishmaniasis: A comparative study of real-time PCR, conventional PCR, and direct agglutination on sera for the detection of Leishmania infantum infection. Vet Parasitol. 2013;192:83–90. doi: 10.1016/j.vetpar.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 18.Sundar S, Singh OP. Molecular Diagnosis of Visceral Leishmaniasis. Mol Diagn Ther. 2018;22:443–457. doi: 10.1007/s40291-018-0343-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tamburro M, Ripabelli G. High resolution melting as rapid, reliable, accurate and cost-effective emerging tool for genotypic pathogenic bacteria and enhancing molecular epidemiological surveillance: a comprehensive review of the literature. Ann Ig. 2017;29:293–316. doi: 10.7416/ai.2017.2153. [DOI] [PubMed] [Google Scholar]
- 20.Afshar A, Rassi Y, Sharifi I, Vatandoost H, Mollaie HR, Oshaghi MA, et al. First report on natural Leishmania infection of Phlebotomus sergenti due Leishmania tropica by high resolution melting curve method in Southeastern Iran. Asian Pac J Trop Med. 2014;1:93–96. doi: 10.1016/S1995-7645(14)60002-X. [DOI] [PubMed] [Google Scholar]
- 21.Quinnell R, Carson C, Reithinger R, Garcez L, Courtenay O. Evaluation of rK39 rapid diagnostic tests for canine visceral leishmaniasis: longitudinal study and meta-analysis. PLoS Negl Trop Dis. 2013;7(1):e1992. doi: 10.1371/journal.pntd.0001992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zampieri RA, Laranjeira-Silva MF, Muxel SM, Stocco AC, Shaw JJ, Floeter-Winter LM. High resolution melting analysis targeting hsp70 as a fast and efficient method for the discrimination of Leishmania species. PLoS Negl Trop Dis. 2016;10(2):1–18. doi: 10.1371/journal.pntd.0004485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Travi B, Cordeiro A, Filipe Dantas-Torres F, Miro G. Canine visceral leishmaniasis: Diagnosis and management of the reservoir living among us. PLoS Negl Trop Dis. 2018;12(1):1–13. doi: 10.1371/journal.pntd.0006082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Romero-Peñuela MH, Sánchez-Valencia JA. El diagnóstico de la leishmaniasis visceral canina (Leishmania infantum) Vet Zootec. 2007;1(1):1–59. [Google Scholar]
- 25.Reithinger R, Quinnell RJ, Alexander B, Davies CL. Rapid detection of Leishmania infantum infection in dogs: Comparative study using an immunochromatographic dipstick test, enzyme-linked immunosorbent assay, and PCR. J Clin Microbiol. 2002;40(7):2352–2356. doi: 10.1128/JCM.40.7.2352-2356.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wittwer CT. High-resolution DNA melting analysis: advancements and limitations. Hum Mut. 2008;30(6):857–859. doi: 10.1002/humu.20951. [DOI] [PubMed] [Google Scholar]
- 27.Chena L, Nara E, Canese A, Oddone R, Russomando G. Aplicación de la PCR para la detección de género y complejos de Leishmania en diferentes tipos de muestras biológicas. Mem Inst Investig Cienc Salud. 2013;11(1):45–51. [Google Scholar]
- 28.Salvioni OD, Pereira J, González M, Vega MC. Molecular detection of Leishmania infantum in atypical cutaneous lesions from paraguayan patients. J Dermatol Clin Res. 2017;5(3):1104–1106. [Google Scholar]
- 29.Salvioni OD, González N, Giménez A, Vega C, González M, Ferreira M, et al. First DNA report of Leishmania infantum in Evandromyia (complex) cortelezzii and Lutzomyia longipalpis in Alto Paraná. Int J Curr Res. 2017;9(8):559931–555934. [Google Scholar]
- 30.Salvioni OD, Pereira J, Rolon MS, Rojas A, Aldama O, Vega MC. First molecular report of Leishmania (Leishmania) amazonensis and Leishmania (Viannia) guyanensis in paraguayan inhabitants using high-resolution melt-PCR. Am J Trop Med Hyg. 2019;101(4):780–788. doi: 10.4269/ajtmh.18-0880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Monteiro F, Machado A, Rocha-Silva F, Barbosa C, Graciele-Melo C, Costa L, et al. Canine visceral leishmaniasis: Detection of Leishmania spp. genome in peripheral blood of seropositive dogs by real-time polymerase chain reaction (rt-PCR) Microbiol Pathogen. 2018;126:263–268. doi: 10.1016/j.micpath.2018.10.036. [DOI] [PubMed] [Google Scholar]
- 32.Almeida A, Sousa V, Gasparetto N, Da Silva G, Figueiredo F, Dutra V, et al. Canine visceral leishmaniasis: diagnostic approaches based on polymerase chain reaction employing different biological samples. Diagn Microbiol Infect Dis. 2013;76:321–324. doi: 10.1016/j.diagmicrobio.2013.03.017. [DOI] [PubMed] [Google Scholar]
- 33.Aschar M, Braga E, Laurenti M, Marcondes M, Tolezano J, Mitsuyoshi R, et al. Value of the oral swab for the molecular diagnosis of dogs in different stages of infection with Leishmania infantum. Vet Parasitol. 2016;225:108–113. doi: 10.1016/j.vetpar.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 34.Marcelino A, Alves J, Valgas C, Rodrigues S, Cardoso F, Afonso I, et al. Comparative PCR-based diagnosis for the detection of Leishmania infantum in naturally infected dogs. Acta Trop. 2020;207:1–6. doi: 10.1016/j.actatropica.2020.105495. [DOI] [PubMed] [Google Scholar]
- 35.Deborggraeve S, Boelaert M, Rijal S, De Doncker S, Dujardin J, Herdewijn P, et al. Diagnostic accuracy of a new Leishmania PCR for clinical visceral leishmaniasis in Nepal and its role in diagnosis of disease. Trop Med Int Health. 2008;13(11):1378–1383. doi: 10.1111/j.1365-3156.2008.02154.x. [DOI] [PubMed] [Google Scholar]
- 36.Ozerdem D, Eroglu F, Genc A, Demirkazik M, Koltas IS. Comparison of microscopic examination, rK39, and PCR for visceral leishmaniasis diagnosis in Turkey. Parasitol Res. 2009;106:197–200. doi: 10.1007/s00436-009-1650-3. [DOI] [PubMed] [Google Scholar]
- 37.Maia Z, Monique L, Mistro S, Mendes C, Mehta S, Badaro R. Comparative study of rk39 Leishmania antigen for serodiagnosis of visceral leishmaniasis: systematic review with meta-analysis. PLoS Negl Trop Dis. 2012;6(1):1–8. doi: 10.1371/journal.pntd.0001484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Salomón O, Pérez A, Riarte A, Casas N, Fragueiro-Frías V, Negri V, et al. Performance of rapid tests for canine visceral leishmaniasis diagnosis in Argentina. Med. 2020;80(2):103–110. [PubMed] [Google Scholar]
- 39.Pires M, Madeira M, Bittencourt V, Pacheco R. Cutaneous and visceral leishmaniasis co-infection in dogs from Rio de Janeiro, Brazil: evaluation by specific PCR and RFLP-PCR assays. Rev Soc Bras Med. 2014;47(2):243–246. doi: 10.1590/0037-8682-0007-2013. [DOI] [PubMed] [Google Scholar]
- 40.Quaresma P, Murta S, Ferreira E, Rocha-Lima A, Xavier A, Gontijo C. Molecular diagnosis of canine visceral leishmaniasis: identification of Leishmania species by PCR-RFLP and quantification of parasite DNA by real-time PCR. Acta Trop. 2009;111(3):289–294. doi: 10.1016/j.actatropica.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 41.Dias E, Regina-Silva S, França-Silva J, Paz G, Michalsky E, Araújo S, et al. Eco-epidemiology of visceral leishmaniasis in the urban area of Paracatu, state of Minas Gerais, Brazil. Vet Parasitol. 2010;176(2-3):101–111. doi: 10.1016/j.vetpar.2010.11.014. [DOI] [PubMed] [Google Scholar]
- 42.Tolezano J, Uliana S, Taniguchi H, Araújo M, Barbosa J, Barbosa J, et al. The first records of Leishmania (Leishmania) amazonensis in dogs (Canis familiaris) diagnosed clinically as having canine visceral leishmaniasis from Aracatuba County, São Paulo State Brazil. Vet Parasitol. 2007;149(3-4):280–284. doi: 10.1016/j.vetpar.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 43.Paz GJ, Rugani M, Marcelino A, Gontijo C. Implications of the use of serological and molecular methods to detect infection by Leishmania spp. in urban pet dogs. Acta Trop. 2018;182:198–201. doi: 10.1016/j.actatropica.2018.03.018. [DOI] [PubMed] [Google Scholar]
- 44.Mohebali M, Taran M, Zarei Z. Rapid detection of Leishmania infantum infection in dogs: comparative study using an immunochromatographic dipstick rk39 test and direct agglutination. Vet Parasitol. 2004;121(3-4):239–245. doi: 10.1016/j.vetpar.2004.02.014. [DOI] [PubMed] [Google Scholar]
- 45.De Queiroz N, De Assis J, Oliveira T, Machado R, Nunes C, Starke-Buzetti W. Diagnóstico da leishmaniose visceral canina pelas técnicas de imunoistoquímica e PCR em tecidos cutâneos em associação com a RIFI e ELISA-teste. Rev Bras Parasitol Vet. 2010;19(1):32–38. doi: 10.4322/rbpv.01901006. [DOI] [PubMed] [Google Scholar]
- 46.De Queiroz GP, Da Silveira R, De Noronha A, Oliveira T, Machado R, Starke-Buzetti W. Detection of Leishmania (L.) chagasi in canine skin. Vet Parasitol. 2011;178:1–8. doi: 10.1016/j.vetpar.2010.12.033. [DOI] [PubMed] [Google Scholar]
- 47.Ferreira S, Souza R, Trindade L, Almeida G, Souza D, Fujiwara R, et al. Canine skin and conjunctival swab samples for the detection and quantification of Leishmania infantum DNA in an endemic urban area in Brazil. PLoS Negl Trop Dis. 2012;6(4):1–9. doi: 10.1371/journal.pntd.0001596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Almeida A, Sousa V, Gasparetto N, Da Silva G, Figueiredo F, Dutra V, et al. Canine visceral leishmaniasis: diagnostic approaches based on polymerase chain reaction employing different biological samples. Diagn Microbiol Infect Dis. 2013;76:321–324. doi: 10.1016/j.diagmicrobio.2013.03.017. [DOI] [PubMed] [Google Scholar]