Abstract
The neuropeptide, corticotropin releasing factor (CRF), has been an enigmatic target for the development of medications aimed at treating stress-related disorders. Despite a large body of evidence from preclinical studies in rodents demonstrating that CRF receptor antagonists prevent stressor-induced drug seeking, medications targeting the CRF-R1 have failed in clinical trials. Here, we provide an overview of the abundant findings from preclinical rodent studies suggesting that CRF signaling is involved in stressor-induced relapse. The scientific literature that has defined the receptors, mechanisms and neurocircuits through which CRF contributes to stressor-induced reinstatement of drug seeking following self-administration and conditioned place preference in rodents is reviewed. Evidence that CRF signaling is recruited with repeated drug use in a manner that heightens susceptibility to stressor-induced drug seeking in rodents is presented. Factors that may determine the influence of CRF signaling in substance use disorders, including developmental windows, biological sex, and genetics are examined. Finally, we discuss the translational failure of medications targeting CRF signaling as interventions for substance use disorders and other stress-related conditions. We conclude that new perspectives and research directions are needed to unravel the mysterious role of CRF in substance use disorders.
Keywords: CRF, Stress, Relapse, Review, Addiction, Substance use disorder
Introduction
The ability to avoid and escape threats in our environment is critical for survival. Moreover, when faced with duress, selection of the most economically viable pattern of behavior is essential for coping and adaptation. To ensure effective responses to threatening stimuli, the influence of stress on the brain is pervasive and includes brain systems that underlie learning, motivation, and affect, thus shaping behavior through a complex coordination of neurotransmission and glial function that influences the brain at the network level. To understand the impact of stress on the brain it is necessary to move beyond basic constructs (e.g., reward and aversion) and consider its influence from the perspective of more complex processes that guide behavior to promote survival and adaptation. Notably, many of these processes also contribute to drug seeking and misuse in those with substance use disorders (SUDs), thus establishing a close link between stress and drug addiction.
Neuropeptides are well-suited to mediate the influence of stress on the brain (see [1] for review). They are co-released with neurotransmitters/monoamines, but under only conditions of higher frequency stimulation. Their release pattern is slower, and their duration of action is prolonged due to reliance on diffusion and proteolysis for clearance. For the same reason, the field of influence of neuropeptides is relatively large and can include extrasynaptic sites as well as adjacent synapses and cells. This, along with the complexity of signaling related to receptor distribution on neurons an astrocytes, receptor/signaling diversity, and peptide processing, positions neuropeptides as effective regional coordinators of network activity. Finally, via transcriptional/translational control, neuropeptide signaling can be scaled via genomic effects of stress hormones independently from the neurotransmitters/modulators with which they are co-released. While a number of neuropeptides are involved in stress/stress-related responses, the neuropeptide corticotropin releasing factor (CRF) has been implicated in many of the behavioral responses to stressors.
CRF, also known as corticotropin releasing hormone (CRH), is a 41 amino acid neuropeptide encoded by the CRH gene. CRF is conserved across species with a seven amino acid difference in sequence between rodents and human. CRF produces acts via two receptors, both of which are primarily Gs G-protein coupled but also signal via other pathways (see [2] and [3] for review): 1) the CRF-R1 receptor, encoded by the CRHR1 gene, which has a higher affinity for CRF and has a relatively widespread expression pattern in the brain and 2) the CRF-R2 receptor, encoded by the CRHR2 gene, which has a lower affinity for CRF and a more restricted pattern of expression. There are two splice variants of CRF-R2: CRF-R2 α and β. The CRF-R2 receptor binds preferentially to a family of CRF-related peptides, the urocortins. CRF also binds to a binding protein with subnanomolar affinity. The 322 amino acid CRF binding protein is a glycoprotein encoded by the CRHBP gene that is widely expressed in the brain and is colocalized with CRF (see [4] for review). Depolarization-dependent release of CRF binding protein from neurons has been reported [5]. In addition to binding to CRF to limit its binding to CRF receptors, there is evidence that CRF binding protein may interact with CRF receptors, specifically CRF-R2, to create a unique signaling complex [6,7].
Beyond parvocellular neurons in the PVN of the hypothalamus which release CRF into the adenohypophyseal microcirculation via the median eminence to regulate anterior pituitary corticotrope adrenocorticotropic hormone (ACTH) secretion, CRF is released into a number of brain structures from both interneurons and projecting neurons that reside in the hypothalamus and non-hypothalamic structures. Subpopulations of neurons co-release CRF with GABA or glutamate (see e.g., [8]). Like other neuropeptides, CRF is released via dense core vesicles under high-frequency stimulation conditions that are distinct from those that release co-neurotransmitters via clear vesicles, thus enabling independent regulation of CRF and co-transmitter release. Moreover, CRF levels can be scaled via changes in transcription/translation and processing. CRF receptors are also expressed by astrocytes (see e.g., [9]). CRF mRNA is expressed in multiple brain regions in addition to the hypothalamus, most notably the central nucleus of the amygdala (CeA) and the bed nucleus of the stria terminalis – brain regions that have been implicated in emotional processing and interface with mesocorticolimbic system [10–12]. Additionally, CRF is evident throughout the neocortex, particularly the prefrontal cortex. Cortical CRF-expressing cells are primarily GABAergic interneurons [13]. CRF production in the ventral tegmental area (VTA) [14] and hindbrain regions, such as locus coeruleus [15] has also been reported. By contrast, urocortin expression is largely confined to the Edinger-Westphal nucleus in the midbrain [16]. Although expression varies by region, CRF receptor binding [17,18] and mRNA [19–21] can be found throughout the brain, including in regions that comprise the extended amygdala and mesocorticolimbic system (e.g., VTA, nucleus accumbens), and, overall, aligns well with the distribution of CRF-immunoreactive axon terminals. The positioning of the CRF system at the interface between brain networks that process negative emotional stimuli and those involved in reward processing and motivation establishes it as an important mechanism through which aversive stimuli in our environment can guide behavior and contribute to SUDs.
CRF contributions to SUDs
While the contribution of CRF to SUDs is complex and involves the regulation/dysregulation of a variety of constructs and corresponding neurocircuits, its influence can be roughly attributed to two domains. First, at least in rodent models, CRF appears to be a critically important mediator of the effects of stressors on drug seeking. Second, it has been proposed that, along with other “anti-reward “ systems (e.g., dynorphin/kappa opioid), CRF signaling in the brain is recruited with repeated drug use, thus establishing an emergent dysphoric state and allostatic dysregulation of hedonic processing that promotes drug seeking and escalates drug intake through negative reinforcement. This intake-dependent recruitment of CRF signaling may also amplify stressor-induced CRF responses, thus augmenting the impact of stress in SUDs, including its effects on relapse susceptibility. The current review examines the contribution of CRF signaling to stressor-induced relapse. First, brain circuitry and CRF mechanisms that contribute to stressor-induced drug seeking are reviewed. Second, evidence that the recruitment of CRF signaling with excessive drug use heightens risk for stressor-induced drug seeking is presented. Third, factors that may influence the impact of CRF in SUDs, including development, biological sex, and genetics are examined. Finally, clinical studies that raise significant questions about whether or not the CRF system is a viable target for pharmacotherapeutic interventions in human populations with SUDs are summarized.
Contribution of CRF signaling to stressor-induce drug seeking
Stressor-induced relapse
While there is evidence that stress can facilitate the acquisition of drug self-administration and promote escalating patterns of drug use, much research has focused on the mechanisms through which stressors induce relapse to drug use in those with SUDs (see [22] for review). Indeed, the persistently heightened risk for relapse even after protracted periods of abstinence is perhaps the most significant obstacle to the effective management of SUDs. While the relationship between stress and drug use is complex [23–25], measures of stress during the preceding day can predict drug use [26], and high stress levels upon the initiation of treatment predict poor outcomes (i.e., early dropout; [27,28]). Laboratory studies have consistently demonstrated that personalized stress imagery can induce craving in those with SUDS (see e.g., [29–31]), and the magnitude of stress-induced craving in inpatient abstinent users is predictive of subsequent risk for relapse to drug use [32,33].
Stressor-induced reinstatement in rodents
In rodents, stress-induced drug seeking can be evaluated by assessing the ability of stressors to reinstate extinguished nose poking or lever pressing following self-administration or their capacity to re-establish place preference following cocaine conditioning and extinction (see [22] for review). A variety of stressors have been demonstrated to reinstate drug seeking following self-administration in rats, including intermittent footshock, food restriction, forced swim, intraoral quinine, and social defeat predicting cues. Likewise, various stressors have been found to reinstate place preference following conditioned place preference in mice, including intermittent footshock, social defeat, restraint, forced swim, yohimbine, and conditioned fear-predictive cues. Despite differences in the species typically used, the contingency of drug delivery, the patterns/amounts of drug exposure, and the learning constructs involved, there is quite a bit of overlap between the two approaches in terms of the contributing neurobiological mechanisms. While these approaches have their limitations (e.g., reliance on extinction, lack of alternative reinforcers, etc…), they have been used effectively to examine the contribution of CRF to stress induced drug seeking, despite a general lack of findings that have translated well to SUDs in in humans.
CRF and stressor-induced drug seeking
It is well-established that CRF contributes to stressor-induced drug seeking in animal models of craving/relapse (see Table 1). Following intravenous cocaine self-administration and extinction, systemic or intra-cerebroventricular (icv) delivery of CRF receptor antagonists prevents footshock-induced reinstatement of cocaine [34–36], heroin [34,37], alcohol [38,39], methamphetamine [40] and nicotine [41,42] seeking. Similar effects can be observed with other stressors, including yohimbine administration [43] (but see [44]) and food deprivation [45], following self-administration in rats and forced swim stress following conditioned place preference in mice [46,47] or footshock following conditioned place preference in rats [48]. Moreover, central (icv) administration of CRF is sufficient to induce alcohol [49], heroin [37], and cocaine [50–52] seeking following self-administration and extinction. The contribution of CRF to stressor-induced drug seeking is largely independent of its hypothalamic–pituitary–adrenal (HPA) axis effects. When the corticosterone response is eliminated by surgical adrenalectomy along with physiological corticosterone replacement or by the inhibition of synthesis via the 11 β-hydroxylase inhibitor metyrapone, footshock-induced reinstatement of heroin [37,38], alcohol [38] and cocaine [35,36] seeking persists. Similarly, surgical adrenalectomy/corticosterone replacement fails to prevent the reinstatement of cocaine seeking by icv CRF [36]. Although results have been mixed (see e.g., [39]), it has been reported that reinstatement by drug-associated cues is also CRF-dependent [53]. Notably, the presentation of drug cues also produces anxiety/stress responses in rodents and humans that likely contribute to craving and use [54,55]. In rats, these responses have been reported to be CRF-dependent [56]. Results with drug-primed reinstatement have been mixed with full blockade ([53], methamphetamine), partial blockade ([35], cocaine; [37], heroin), or no effects ([36], cocaine) reported.
Table 1.
Studies demonstrating decreases in stressor-induced reinstatement of drug seeking following CRF receptor antagonism/knockdown.
| Drug | Stressor | Species | Sex | Procedure | CRF Ant/Rec | Route | Ref |
|---|---|---|---|---|---|---|---|
| Alcohol | Footshock | Rat | M | SA/ext | d-Phe-CRF (R1/R2) | icv | [38] |
| Alcohol | Footshock | Rat | M | SA/ext | CP-154,526 (R1) | ip | [38] |
| Alcohol | Footshock | Rat | M (msP or dep) | SA/ext | MTIP (R1) | ip | [200] |
| Alcohol | Footshock | mSP rats | M | SA/ext | Antalarmin (R1) | ip | [239, 336] |
| Alcohol | Footshock | Rats | M | SA/ext | d-Phe-CRF (R1/R2) | icv | [38, 39] |
| Alcohol | Footshock | Rat | M | SA/ext | d-Phe-CRF (R1/R2) | intra-MRN | [49] |
| Alcohol | Yohimbine | Rat | M | SA/ext | Antalarmin (R1) | ip | [43] |
| Alcohol | Yohimbine | Rat | M | SA/ext | d-Phe-CRF (R1/R2) | intra-MRN | [140] |
| Alcohol | Yohimbine | iP rats | M | SA/ext | CP376395 (R1) | intra-NI | [145] |
| Alcohol | U50,488 (KOR agonist) | Rat | M | SA/ext | Antalarmin (R1) | ip | [361] |
| Cocaine | Footshock | Rat | M | SA/ext | CP-154,526 (R1) | sc | [34] |
| Cocaine | Footshock | Rat | M | SA/ext | d-Phe-CRF (R1/R2) | icv | [35] |
| Cocaine | Footshock | Rat | M | SA/ext | α-helical CRF (R1/R2) | icv | [36] |
| Cocaine | Footshock | Rat | M | SA/ext | α-helical CRF (R1/R2) | intra-VTA | [75] |
| Cocaine | Footshock | Rat | M | SA/ext | Antalarmin (R1) | Intra-VTA | [70, 76] |
| Cocaine | Footshock | Rat | M | SA/ext | Antisauvagine-30 (R2) | intra-VTA | [102] |
| Cocaine | Footshock | Rat | M | SA/ext | d-Phe-CRF (R1/R2) | intra-BNST | [62] |
| Cocaine | Footshock | Rat | M | SA/ext | d-Phe-CRF (R1/R2) | Disconnection: intra-BNST d-Phe-CRF with intra-CeA TTX | [63] |
| Cocaine | Footshock | Rat | M | SA/ext | Antalarmin (R1) | Disconnection: intra-VTA antalarmin with intra-BNST ICI118,551 | [70] |
| Cocaine | Footshock | Rat | M | SA/ext | Antalarmin (R1) | Disconnection: intra-VTA antalarmin with intra-PFC SCH23390 | [129] |
| Cocaine | Food deprivation | Mouse | M | SA/ext | shRNA knockdown (R1) | VTA | [103] |
| Cocaine | icv NE | Rat | M | SA/ext | d-Phe-CRF (R1/R2) | icv | [44] |
| Cocaine | Intraoral quinine | Rat | M | SA/ext | CP-376395 (R1) | Intra-VTA | [104] |
| Cocaine | Forced swim | Mouse | M | CPP/ext | Antalarmin (R1) | ip | [47] |
| Heroin | Footshock | Rat | M | SA/ext | CP-154,526 | sc | [34] |
| Heroin | Footshock | Rat | M | SA/ext | α-helical CRF (R1/R2) | icv | [37] |
| Heroin | Food deprivation | Rat | M | SA/ext | α-helical CRF (R1/R2) | icv | [45] |
| Methamphetamine | Footshock | Rat | M | SA/ext | α-helical CRF (R1/R2) | icv | [40] |
| Morphine | Forced swim | Rat | M | CPP/ext | NBI 35965 (R1) | Intra-DRN | [139] |
| Morphine | Footshock | Rat | M | CPP/ext | CP-154,526 (R1) | Intra-BNST | [48] |
| Nicotine | Footshock | Rat | M | SA/ext | d-Phe-CRF (R1/R2) | icv | [41] |
| Nicotine | Footshock | Rat | M | SA/ext | R278995/CRA0450 (R1) | icv | [42] |
| Nicotine | Footshock | Mouse | M | SA/ext | Antalarmin (R1) | sc | [362] |
Abbreviations: SA: self-administration; CPP: conditioned place preference; ext: extinction; R1: CRF-R1 receptor; R2: CRF-R2 receptor; MRN: medial raphe nucleus; NI: nucleus incertus; VTA: ventral tegmental area; BNST: bed nucleus of the stria terminalis; PFC: prefrontal cortex; DRN: dorsal raphe nucleus; iP: inbred alcohol preferring; mSP: Marchigian Sardinian alcohol-preferring.
The contribution of CRF signaling to SUDs, most frequently alcohol use disorder, has also been studied using transgenic mice. Although effects on reinstatement have not been reported, studies have examined the effects of CRF, CRFR1, CRFR2, and CRF binding protein knockout, as well as CRF overexpression on alcohol consumption in dependent, non-dependent and stressor exposed mice. CRF knockout mice consume more alcohol, apparently due to reduced sensitivity to alcohol rewarding effects [57], while CRF overexpressing mice consume less alcohol, an effect associated with enhanced alcohol-induced sedation [58]. Although there have been some reports that global CRFR1 knockout reduces high-concentration alcohol consumption ([59] and prevents increased consumption in dependent [60] and sensitized [61] mice, others have found that global CRFR1 knockout increases alcohol consumption following repeated stress [62] and in dependent mice [63]. This unexpected observation is likely attributable to opposing effects of brain and peripheral (e.g., pituitary) CRFR1 receptors as brain-specific deletion of CRFR1 prevents increases in alcohol consumption following stress and in dependent mice [63]. CRFR1 knockout also reduces anxiety-related behaviors during alcohol withdrawal [64]. By contrast, CRFR2 [65] or CRF binding protein [66] knockout alone has little effect on alcohol consumption in dependent or non-dependent mice, although combined CRFR1 and R2 knockout has been reported to prolong the effects of chronic stress on consumption [59]. Fewer studies have used transgenic mice to investigate the contribution of CRF signaling to the consumption of other drugs. CRFR1 knockout mice are more sensitive to cocaine-induced conditioned place preference [67] and less sensitive to opiate withdrawal [68,69].
Pathways and mechanisms that underlie the contribution of CRF to stressor-induced drug seeking
Considering the role of CRF in coordinating adaptive responses to stressors, it is not surprising that the sites at which CRF influences drug seeking involve those that serve as interfaces between brain systems that are implicated in stress and negative affect and those involved in motivation. These sites include structures that comprise the extended amygdala (i.e., BNST, CeA, NAc shell) and midbrain regions that contain cell bodies for monaminergic projections into the corticolimbic system, most notably the VTA and dorsal raphe nuclei. Through actions in these brain regions, CRF coordinates healthy adaptive behaviors across a range of functional domains to promote survival under duress. In the context of SUDs many of these actions are maladaptive and promote drug seeking. The brain regions and CRF mechanisms that have been implicated in stressor-induced drug seeking are summarized briefly below and depicted in Fig. 1.
Fig. 1. Brain regions in which CRF signaling contributes to stressor-induced cocaine seeking in rodents.
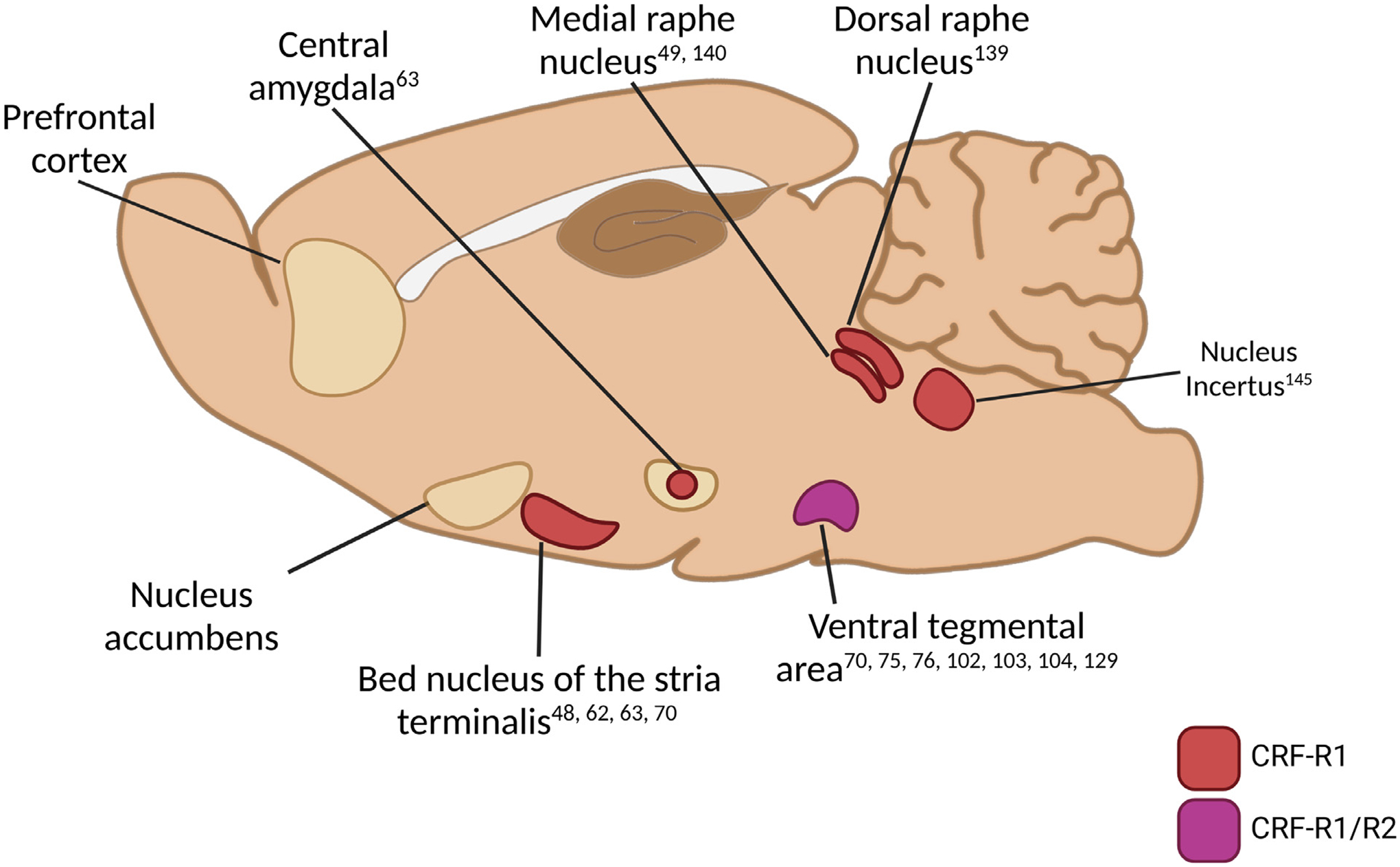
Brain regions into which CRF receptor antagonist micro-infusions prevent stressor-induced drug seeking following self-administration or conditioned place preference and extinction (see text and Table 1 for details). Regions are segregated according to the involvement of CRF-R1 and CRF-R2 in stressor-induced cocaine seeking with supporting references listed.
Extended amygdala
The extended amygdala is a network of structures that includes the bed nucleus of the stria terminalis (BNST), the central amygdala (CeA), and the nucleus accumbens (NAc) shell that is involved in emotional processing and closely interfaces with brain networks that mediate reward and motivation. The extended amygdala is heavily influenced by stressors and is rich in neuropeptides, including CRF, as well as neuropeptide signaling molecules, including CRF receptors and CRF binding protein. The BNST, CeA, and NAc shell have all been implicated in stressor-induced drug seeking. Each of these regions shows increased Fos reactivity following stressor-induced drug seeking and pharmacological inactivation of each region prevents swim-induced cocaine seeking following conditioned place preference in mice and/or shock-induced reinstatement following self-administration in rats [70]. The contributions of CRF signaling in each of these regions to stressor-induced drug seeking are summarized below.
BNST.
The BNST functions as an interface between stress and motivational/reward systems and is particularly important for stressor-induced drug seeking. Inactivation of the ventral BNST prevents stressor-induced reinstatement following cocaine self-administration in rats [70] and conditioned place preference in mice [71]. CRF [10–12], CRF receptors [17–20] and CRF binding protein [72] are all expressed in the BNST, and stressor-induced drug seeking is associated with BNST Fos expression. The source of CRF released into the BNST includes both neurons intrinsic to the region and CRF-releasing afferents, most notably from the medial preoptic area, PVN, and medial and central (CeA) regions of the amygdala [73]. CRF mRNA expression in the BNST is increased following swim stress-induced reinstatement of CPP in mice [47] or shock-induced heroin seeking following self-administration in rats [74]. Moreover, intra-BNST (ventral) micro-infusions of the non-selective CRF receptor antagonist, D-Phe CRF(12–41), prevent footshock-induced reinstatement of cocaine seeking following self-administration in rats [75], while intra-BNST administration of the CRFR1 antagonist, CP-154,526, prevents footshock induced reinstatement of morphine seeking following conditioned place preference in rats [48]. The source of CRF in the BNST that mediates stressor-induced drug seeking likely arises, in part, from the CeA. Disconnection of the CeA-to-vBNST CRF pathway by TTX micro-infusion into the CeA in one hemisphere and D-Phe CRF(12–41) micro-infusion into the contralateral ventral BNST attenuates footshock-induced reinstatement of cocaine seeking following self-administration in rats [76]. Notably, this blockade is partial, suggesting contributions of other CRF or non-CRF mechanisms to reinstatement. Moreover, this observation does not preclude involvement of CRF-releasing cells within the BNST.
CRF release in the BNST is influenced by a variety of neuromodulators (reviewed in [77]). The BNST is heavily innervated by noradrenergic projections. Specifically, projections from the A1 and A2 lateral tegmental medullary cell groups via the ventral noradrenergic bundle to the BNST appear to mediate stressor-induced drug seeking, as 6-OHDA lesions of these cell groups attenuate shock-induced drug seeking while pharmacological inhibition of locus coeruleus noradrenergic neurons has no effect [78]. In particular, noradrenergic signaling via beta-adrenergic receptors appears to be critical for stressor-induced drug seeking [79,80]. Beta adrenergic receptors are expressed in the BNST [81], and prior work has found that bilateral micro-infusions of a beta-1/beta-2 adrenergic receptor antagonist cocktail into the BNST attenuates shock-induced reinstatement following self-administration in rats [82]. We have demonstrated that, similar to what is observed with systemically administered receptor antagonists and swim-induced reinstatement following conditioned place preference in mice [47], shock-induced cocaine seeking following self-administration in rats is prevented by intra-BNST micro-infusions of the beta-2 receptor antagonist, ICI-118,551, but not the beta-1 receptor antagonist, betaxolol, and is reproduced by intra-BNST micro-infusions of the beta-2 adrenergic receptor agonist, clenbuterol, but not the beta-1 receptor agonist, dobutamine [83].
Beta adrenergic receptor activation in the BNST regulates CRF to induce drug seeking. Reinstatement of cocaine seeking by icv norepinephrine following self-administration in rats [44] or by systemic administration of the beta-2 receptor agonist clenbuterol following conditioned place preference in mice [47] is blocked by CRF receptor antagonism. In mice, systemic clenbuterol administration also increases CRF mRNA levels in the BNST [47], while intra-BNST administration of the CRF-R1 receptor antagonist, antalarmin, prevents reinstatement of cocaine seeking in response to intra-BNST administration of clenbuterol [83]. A series of studies by Winder and colleagues have demonstrated that beta adrenergic receptors promote excitatory regulation of key BNST output pathways involved in cocaine seeking via a mechanism that likely requires CRF release from a local population of neurons intrinsic to the BNST and BNST CRF-R1 receptor activation [84,85]. These pathways include CRF-positive neurons that innervate the VTA [85–87], where CRF receptor activation is required for stressor-induced cocaine seeking ([88,89]; see below). Indeed, bath application of the non-selective beta-adrenergic receptor agonist, isoproterenol, promotes excitatory synaptic regulation of retro-labeled VTA-projecting CRF-positive neurons via CRF-R1-dependent glutamate release in the BNST [85]. The BNST-VTA projection is comprised of both GABAergic and glutamatergic neurons [90]. However, it has been reported that CRF-positive neurons that project from the BNST to VTA [83,87] are primarily GABAergic [8]. To confirm that beta-2 adrenergic receptor regulation of a projection from the ventral BNST that releases CRF into the VTA mediates stressor-induced cocaine seeking, we deployed a pharmacological disconnection approach in which we administered the beta-2 adrenergic receptor antagonist, ICI-118,551, into the BNST in one hemisphere and the CRF-R1 antagonist, antalarmin, into the contralateral VTA. Using this approach, we confirmed that the ability of footshock to reinstate cocaine seeking in rats following self-administration requires beta-2 adrenergic receptor activation of a vBNST pathway that releases CRF into the VTA and activates CRF-R1 receptors [83].
CeA.
CRF as well as its receptors and binding protein are expressed in the CeA. Activation of the CeA is required for footshock-induced cocaine seeking [70] and stressor-induced drug seeking are associated with increased CeA Fos expression [91]. CRF mRNA levels also increase with stressor-induced drug seeking [74]. While there are CRF-positive CeA projections to the VTA [87], it has been proposed that a CRF-releasing excitatory pathway from the CeA to the BNST is critical for stressor-induced drug seeking [76]. Consistent with findings that beta-1 adrenergic receptors are expressed on CRF-positive neurons in the CeA and regulate CRF expression [92], it has been found that CeA beta-adrenergic receptor activation is required for shock-induced cocaine seeking [82] and that drug seeking in response to centrally administered norepinephrine [44] or systemic administration of a beta-2 adrenergic receptor agonist [47] is blocked by pretreatment with a CRF-R1 antagonist. CRF-R1 in the CeA has been heavily implicated in SUDs (see e.g., [93] for review). However, while roles for CRF-R1 signaling in withdrawal-related dysphoria (see e.g., [94, 95]) and escalating patterns of drug intake (see e.g., [96–98]) have been identified, the contribution of CRF-R1 receptors to stressor-induced drug seeking has not been well-characterized. Erb et al [75] reported that intra-CeA administration of the CRF receptor antagonist, D-Phe CRF(12–41), failed to affect footshock-induced cocaine seeking following self-administration in rats. However, recently, it has been demonstrated that targeted overexpression of CRFR1 in CaMKII neurons the CeA augments stressor-induced alcohol seeking [99]. Moreover, it has been found that intra-CeA micro-infusions of the CRF-R1 antagonist, CP-154,526, prevent drug-primed but not footshock-induced reinstatement of morphine seeking following conditioned place preference in rats [48].
Nucleus accumbens.
One region in which the contribution of CRF signaling to drug seeking has been surprising understudied is the nucleus accumbens (NAc). The NAc receives CRF-positive inputs from the BNST [100] and, likely, the CeA [101], and NAc expression of CRF [102,103] and its receptors [18] has been reported. NAc CRFR1 and R2 expression is both pre- and post-synaptic [104] and includes localization to cholinergic interneurons [105]. Recently, it has been reported that optical stimulation of CRF neurons in the NAc can increase incentive motivation for a non-drug reward [106], similar to the effects of intra-NAc CRF micro-infusions [107]. Moreover, intra-NAc CRF can influence effortful choice [108]. Further, in naïve rats, CRF micro-infusions into the NAc produce arousal [109] increase dopamine [104], and support place preference [104]. Despite, these observations, the contribution of NAc CRF signaling to stressor-induced cocaine seeking is unclear. One study has reported an enhancement of nicotine self-administration by CRF-R1 overexpression in the NAc core [110], while the CRF projection from the CeA to the NAc core appears to attenuate alcohol drinking [101].
Ventral tegmental area
The VTA is key site for CRF regulation of drug seeking. Both CRF-R1 and CRF-R2 receptors [21,111,112] as well as the CRF binding protein [113] are expressed in the region. The VTA receives CRF-releasing inputs from several brain regions implicated in stress-related hormonal and behavioral responses, most notably the CeA, the paraventricular nucleus of the hypothalamus, and BNST [87]. There is also evidence for local VTA CRF release [14]. During footshock-induced reinstatement of cocaine seeking, extracellular levels of CRF in the VTA are elevated in rats [88]. Moreover, intra-VTA CRF delivery is sufficient to reinstate cocaine seeking [88,89,114].
There is evidence for involvement of both CRF-R1 and CRF-R2 receptors in the VTA in stress-induced drug seeking. We have found that, following self-administration and extinction in rats, VTA CRF-R1 but not CRF-R2 receptors are necessary for CRF- and footshock-induced reinstatement. VTA micro-infusions of the CRF-R1-selective antagonists antalarmin or CP-376395 blocked reinstatement following in intra-VTA CRF or footshock while micro-infusions of the CRF-R2 antagonists astressin2B or antisauvagine-30 had no effect. Moreover, activation of VTA CRF-R1 receptors alone was sufficient to reinstate cocaine seeking [89]. Intra-VTA micro-infusions of the selective CRF-R1 receptor agonist cortagine but not the selective CRF-R2 agonist, rat urocortin 2, reproduced the reinstating effects of stress and CRF on cocaine seeking. Consistent with these reports, it has been found that knockdown of CRF-R1 receptors in the VTA blocks cocaine seeking following acute food deprivation or cocaine cues in mice [115]. We have also found that VTA CRF-R1 antagonism prevents cocaine seeking in response to intraoral delivery of the aversive tastant, quinine [116]. VTA CRF-R2 receptors have also been implicated in stressor-induced drug seeking. Others have reported that footshock-induced reinstatement of cocaine seeking following self-administration and extinction requires CRF-R2 but not - R1 receptors in the VTA [88,114]. In line with findings that CRF can potentiate NMDA receptor mediated synaptic transmission in the VTA via a mechanism that requires interaction with the CRF binding protein [6], it has been reported that binding protein interactions in the VTA are also required for stressor-induced cocaine seeking and associated neurochemical effects [114]. The reasons for the disparate findings regarding CRF mechanisms of action in the VTA are unclear but may be related to differences in the mode and duration of CRF/antagonist delivery, the self-administration history of the rats, and other experimental parameters.
As noted above, the VTA receives CRF-positive projections from several brain regions, most notably the CeA, paraventricular nucleus of the hypothalamus, and the BNST [87]. We have found that that BNST projections to the VTA are critical for stressor-induced drug seeking. Overall, these projections are comprised of both GABAergic and glutamatergic neurons [90,117]. However, the CRF-positive neurons that project from the BNST to the VTA are primarily GABAergic [8]. Consistent with reports that that stressor-induced cocaine seeking requires beta-2 adrenergic [83] and CRF-R1 [75,83] receptor activation in the BNST, it has been found that ex vivo beta adrenergic receptor agonist application to brain slices containing BNST promotes excitatory synaptic regulation of retro-labeled VTA-projecting CRF-positive neurons via CRF-R1-dependent glutamate release [85]. We have used a pharmacological disconnection approach in which we micro-infused the beta-2 adrenergic receptor antagonist, ICI-118,551, into the BNST in one hemisphere and the CRF-R1 antagonist, antalarmin, into the contralateral VTA and demonstrated that a beta-2 receptor regulated CRF-releasing projection from the BNST to the VTA is required for shock-induced reinstatement of cocaine seeking following self-administration in rats [83]. Although a role for CRF release from neurons originating in other brain regions in stressor-induced drug seeking has not been confirmed, it has been demonstrated that CeA inhibition prevents stress-induced cocaine seeking, suggesting that there may be a potential contribution of CeA-VTA projection CRF release [70].
A detailed overview of CRF actions in the VTA is beyond the scope of this review. Consistent with its proposed role as a regional coordinator of adaptive responses and with the heterogeneity of the VTA, CRF regulates a variety of cell types (dopaminergic non-Ddopaminergic, interneurons, glia) at both pre- and post-synaptic sites in a manner that appears to vary across synapses defined according to VTA inputs and outputs, signaling contexts, and pharmacological/environmental histories. CRF has been reported to promote both excitatory [6,112,118,119] and inhibitory [120] transmission in the VTA and alter synaptic integration via intracellular calcium mobilization in dopaminergic cells [121]. Additionally, CRF promotes glutamate release in the VTA [88], a finding that is consistent with reports that CRF also increases the frequency of AMPAR-mediated spontaneous miniature EPSCs in dopaminergic cells in slice preparations [119] and can attenuate GABA release via CRFR2 receptors [122]. The effects of CRF signaling on VTA outputs that lead to drug seeking have been largely assumed to be a function of enhanced excitatory transmission [123]. Accordingly, intra-VTA CRF delivery increases local DA release measured during reinstatement testing [88], and glutamate receptor antagonism in the VTA prevents both increases in local dopamine levels and stressor- and CRF-induced reinstatement [88]. However, stressor effects on neuronal firing in VTA are not uniform and firing patterns and downstream dopamine release can vary according to output pathway. Whereas stressors reliably produce robust activation of VTA neurons that project to the PFC [124–127], effects on NAc dopamine are more complex (see [128] for review) with reports of increases, reductions, or no effect, depending on the NAc subregion, stressor, context, and timeframe [116,129–138].
Shock-induced cocaine seeking is associated with CRF-dependent increases in extracellular dopamine in the VTA [88]. However, CRFR1 expression on VTA neurons varies depending on output pathway [139] while midbrain CRF-R1 deletion selectively reduces dopamine release in the PFC [140]. CRF micro-infusions into the VTA have been reported to reduced NAc dopamine signaling [137]. We reported that VTA CRF-R1 antagonism prevents quinine-induced cocaine seeking and corresponding reductions in NAc shell dopamine [116]. Moreover, we found that reinstatement by intra-VTA CRF administration is prevented by intra-VTA pretreatment with a GABA-B receptor antagonist [141], suggesting that aversive stimuli may produce CRF-R1 dependent drops in NAc dopamine that can promote drug seeking, potentially by removing inhibitory regulation of medium spiny neurons by D2 receptors.
By contrast we have found that CRF-R1 receptor activation of VTA dopamine neurons the project to the prelimbic prefrontal cortex is required for stressor-induced cocaine seeking [142]. Consistent with findings by our lab and others that shock-induced cocaine seeking in rats is blocked by dopamine D1 receptor antagonist micro-infusions into the prelimbic cortex [70,143] and by chemogenetic (Designer Receptor Exclusively Activated by Designer Drug-based) inhibition of prelimbic PFC-projecting VTA neurons [142], we have found that pharmacological disconnection of the VTA-prelimbic cortex pathway by administration of the CRF-R1 antagonist, antalarmin, into the VTA in one hemisphere, and administration of the D1 receptor antagonist SCH 23390 into the prelimbic cortex of the contralateral hemisphere prevents shock-induced cocaine seeking following self-administration in rats [142].
Altogether findings from our laboratory suggest that CRF-releasing inputs from the BNST produce CRF-R1 dependent activation of VTA neurons that project to the prelimbic PFC resulting in D1-dependent augmentation of excitatory regulation of pyramidal neuron PFC outputs, most notably those projecting to the NAc core [70] to induce cocaine seeking. At the same time, CRF-R1 mediated inhibition of NAc-projecting neurons, potentially through effects on GABAergic transmission, reduce NAc dopamine levels, thus reducing tone on D2Rs and dis-inhibiting medium spiny neuron outputs.
Raphe nuclei
The medial and dorsal raphe nuclei (MRN and DRN), localized to the midbrain, are among the most rostral nuclei of the raphe cluster and send serotonergic projections throughout the brain, including to structures in the extended amygdala and mesocorticolimbic system. The contribution of serotonin to drug seeking is complex and varies with receptor and context (see e.g., [144,145]). CRF, CRF receptors, and CRF binding protein are expressed in the MRN and DRN and regulate serotonergic signaling, particularly in response to stressors (see [146,147] for review). Overall, CRF application decreases 5-HT neuronal firing [148,149] via CRFR1-dependent reductions in GABA release and CRFR-2-dependent enhancement of post-synaptic GABA sensitivity [150]. Indeed, muscimol or 5-HT1A receptor agonist-induced suppression of DRN neurons is sufficient to reinstate alcohol seeking in rats [49,151]. Accordingly, CRF micro-infusions into the MRN [49] or CRF-R1 agonist micro-infusions into the DRN [152] are sufficient to induce alcohol [49] or opioid [152] seeking. Likewise, CRF receptor antagonism in the MRN blocks footshock- [49] or yohimbine- [153] induced alcohol seeking following self-administration or forced swim-induced reinstatement of morphine seeking following conditioned place preference [152]. Although the downstream targets that are affected by CRF-dependent reductions in serotonergic signaling to promote drug seeking are not well-defined, a likely site of action is the nucleus accumbens where stressor-induced reduction in 5-HT and signaling via 5-HT1B receptors gave been shown to promote cocaine preference [154,155].
Other brain regions
CRF actions that influence drug seeking extend beyond the regions and functions described above and will be briefly summarized here. First, CRF-R1 signaling in the basolateral amygdala has recently been implicated in reconsolidation of cocaine context memories as demonstrated by disruption of context-induced reinstatement of cocaine seeking when CRF-R1 receptors are blocked during earlier reconsolidation [156]. Second, cue induced cocaine seeking is disrupted when CRF-R1 receptors are blocked in the anterior dorsal agranular subregion of the insular cortex [157]. Third, CRF-R1 but not CRF-R2 antagonism in the nucleus incertus prevents yohimbine-induced alcohol seeking in alcohol-preferring rats [158]. Finally, while CRF effects in the prefrontal cortex on alcohol self-administration have been reported [159], the contribution of CRF signaling in subregions of the PFC to stress-induced drug seeking remains unexplored.
CRF signaling mechanisms
A comprehensive overview of all of the signaling mechanisms with which CRF interacts is beyond the scope of this review (see e.g., [140,141,160–170]). As is the case with other neuropeptides, CRF is co-released with neurotransmitters and other neuromodulators (including neuropeptides) and signals interactively with these partners in target cells. Moreover, in many cases the field of influence of CRF extends to adjacent synapses and cell populations, thus allowing CRF to regionally influence signaling via mediators beyond those with which it is co-released. Several areas of particular interest that require additional investigation have emerged related to CRF signaling.
a. CRF receptor-containing heteromers.
GPCRs can interact in heteromers, macromolecular complexes consisting of at least two different receptor types [170]. When part of a heteromeric complex, GPCRs often signal distinctly than then they do in isolation. Thus far, putative heteromeric complexes comprised of the CRF-R2 and dopamine D1 receptors [171,172], the CRFR1 and CRFR2β receptors [173], the CRFR1 and vasopressin V1b receptors [174], the CRFR2 and orexin OX1 receptors [175], and the CRFR1, the orexin OX1, and σ1R receptors [176] have been identified. In several cases, it has been demonstrated that activation of one receptor in these heteromeric complexes alters signaling via the other [175–177]. It has been reported that signaling via CRF receptor-containing heteromers can be influenced by cocaine [176] or amphetamine [175]. In the case of the CRFR1-OX1σ1R complex, cocaine may directly influence CRF signaling via its interaction with σ1R [176]. The contribution of these heteromers to the effects of CRF on drug seeking remains to be determined.
b. CRF-BP:
The CRF-BP is a secreted glycoprotein with no transmembrane domains that binds CRF with an equal or greater affinity than the CRF receptors [178–181]. Its expression is tissue-specific, with human CRF-BP detected in plasma, pituitary, and brain [182]. Rodent CRF-BP is detected only in brain and pituitary [183]. Within the CNS, CRF-BP is expressed in cerebral cortex (including the PFC), hippocampus, the amygdaloid complex, BNST, and VTA [113,182–184]. Rat CRF and CRF-BP co-localize in a number of regions, including BNST and CeA [183], key sites implicated in SUDs and stress. CRF-BP is also detected in a number of CRF target sites (BLA, VTA and anterior pituitary), where CRF-BP and CRF receptors are often co-expressed [113,183,185]. CRF-BP mRNA levels in pituitary and amygdala are increased by stress [185–187]. Multiple functions have been suggested for the CRF-BP. Approximately 40–60% of CRF in human brain is bound by CRF-BP, supporting a role for CRF-BP in limiting CRF bioavailability [188]. In pituitary corticotrope cultures, CRF-BP binds CRF and neutralizes its ACTH-releasing activity via CRF-R1 [178,183]. CRF-BP deficient mice exhibit increased anxiety-like behaviors, consistent with elevated “free” CRF levels [189], supporting an inhibitory role for the CRF-BP, at least for CRF actions at CRF-R1. However, other studies have suggested alternative roles for the CRF-BP, including a facilitative role for CRF-BP in VTA via actions with CRF-R2 in stress-induced cocaine seeking [6,114,123] or CRF receptor independent actions [184]. The possibility that the CRF-BP can modulate CRF signaling, particularly in VTA and other sites within the stress/reward pathways, has important physiological significance and warrants further studies to address its role in stress induced drug seeking.
c. Effects of CRF on astrocytes:
Electron microscopy has revealed CRF-positive axon terminals in close apposition to astrocytic processes [190], and CRF has been found to regulate astrocytic calcium signaling in culture [191] and astrocyte proliferation [192]. Both CRFR1 and CRFR2 receptors [9,193–196] and the CRF binding protein [197] are expressed by astrocytes, and it has been reported that astrocytic CRFR2 expression in the midbrain can be regulated by repeated cocaine or methamphetamine administration [195]. It is likely that, during periods of stress, CRF influences astrocytic glucose utilization and promotes lactate release to fuel neurons in under conditions of high energy demand. However, the relevance of CRF regulation of astrocytes to SUDs is not clear. Astrocytes in the nucleus accumbens have been demonstrated to play a critical role in regulating drug seeking, in part via the management of extrasynaptic glutamate levels and signaling via extrasynaptic metabotropic glutamate and NMDA receptors (see e.g., [198–202]). Astrocytes can also release ATP which can directly influence neuronal function via purinergic receptors or be converted to adenosine which signals through adenosine receptor subtypes [203–205]. Notably, A2A adenosine receptors in the nucleus accumbens [206–208] and prefrontal cortex [209] regulate drug seeking, although their contribution to drug seeking during periods of stress is unknown. Understanding the relevance of CRF effects on astrocytes to drug seeking will require further investigation.
Recruitment of CRF signaling and stressor-induced drug seeking
Drug addiction has been conceptualized as a surfeit disorder wherein “anti-reward “ systems, including those that mediate stress-related signaling, are recruited with repeated cycles of drug use and abstinence/withdrawal, resulting in an allostatic negative emotional state/hyperkatifeia that promotes drug seeking and use through negative reinforcement [210]. CRF systems are among those that contribute to a range of persistent dysphoric effects that emerge with repeated drug use and likely fuel SUDs. Indeed, key dysphoria-related aspects of withdrawal from cocaine (see e.g., [211,212]), opioids (see e.g., [68,213–215]), alcohol (see e.g., [64,215–219]), and other abused drugs (e.g., nicotine: [220,221]; cannabinoids: [222]; benzodiazepines: [223]) are mediated by heightened CRF signaling.
A detailed overview of alterations in CRF signaling that emerge with chronic drug exposure is beyond the scope of this review. However, changes in CRF signaling in a variety of brain regions including the amygdala (see e.g., [97,224–232]), hippocampus (see e.g., [233]), BNST (see e.g., [85,234–236]), septum (see e.g., [237]), prefrontal cortex (see e.g., [238]), nucleus accumbens (see e.g., [239,240]), serotonergic raphe nuclei (see e.g., [241–243]), noradrenergic cell groups (see e.g., [244,245]), and VTA [88,119,120,246–248] have been demonstrated. The functional consequences of altered CRF signaling likely extend beyond dysphoric effects and may include CRF-dependent alterations in decision making [249], reward conditioning [250], drug memory reconsolidation [156] and cognition [251].
CRF and escalating patterns of drug self-administration
One consequence of the recruitment of CRF signaling with repeated drug use may be the emergence of escalating patterns of drug self-administration. Escalation of drug self-administration in rats occurs with high levels of drug exposure resulting from extended or protracted drug access and can be observed across drug classes (alcohol: [252]; cocaine: [253,254]; heroin: [255]; methamphetamine: [256]; nicotine: [257]. It has been demonstrated that CRF receptor antagonism selectively attenuates escalated cocaine [258], ethanol [259–261], or heroin [262] self-administration and corresponding indicators of hyperkatifeia (e.g., mechanical allodynia; [215]) without producing effects under “non-escalated” conditions. Altogether, these findings suggest that drug-induced recruitment of brain CRF systems escalates future self-administration, thus establishing a vicious cycle of drug-taking behavior.
Recruitment of CRF signaling and stressor-induced drug seeking
The recruitment of CRF signaling with excessive drug use also increases susceptibility to CRF-dependent stressor-induced reinstatement of drug seeking (Fig. 2). Although stress has been identified as a contributor to relapse, its relationship with drug seeking is complex. The onset of stress does not always serve as a reliable trigger for cocaine use [23,24]. A key determinant of stress-induced cocaine seeking is the extent/pattern of prior use. High-frequency cocaine users show heightened drug craving, anxiety and associated physiological responses upon exposure to stress imagery compared with lower-frequency users in a laboratory setting [263] and display stronger relationships between daily stress and cocaine/opioid craving as assessed via smartphone monitoring [264]. Consistent with these observations, we find that reliable stress-induced cocaine seeking is observed in male rats with a history of long-access cocaine self-administration (14 × 6–10 h/day) but not rats with a history of short-access self-administration (14 × 1–2 h/day) [51]. Similar findings of heightened stressor-induced reinstatement following long-access self-administration have been reported with heroin [255].
Fig. 2. Glucocorticoid-dependent recruitment of CRF-R1 receptors in the VTA establishes stressor-induced regulation of mesocortical dopamine neurons and cocaine seeking.
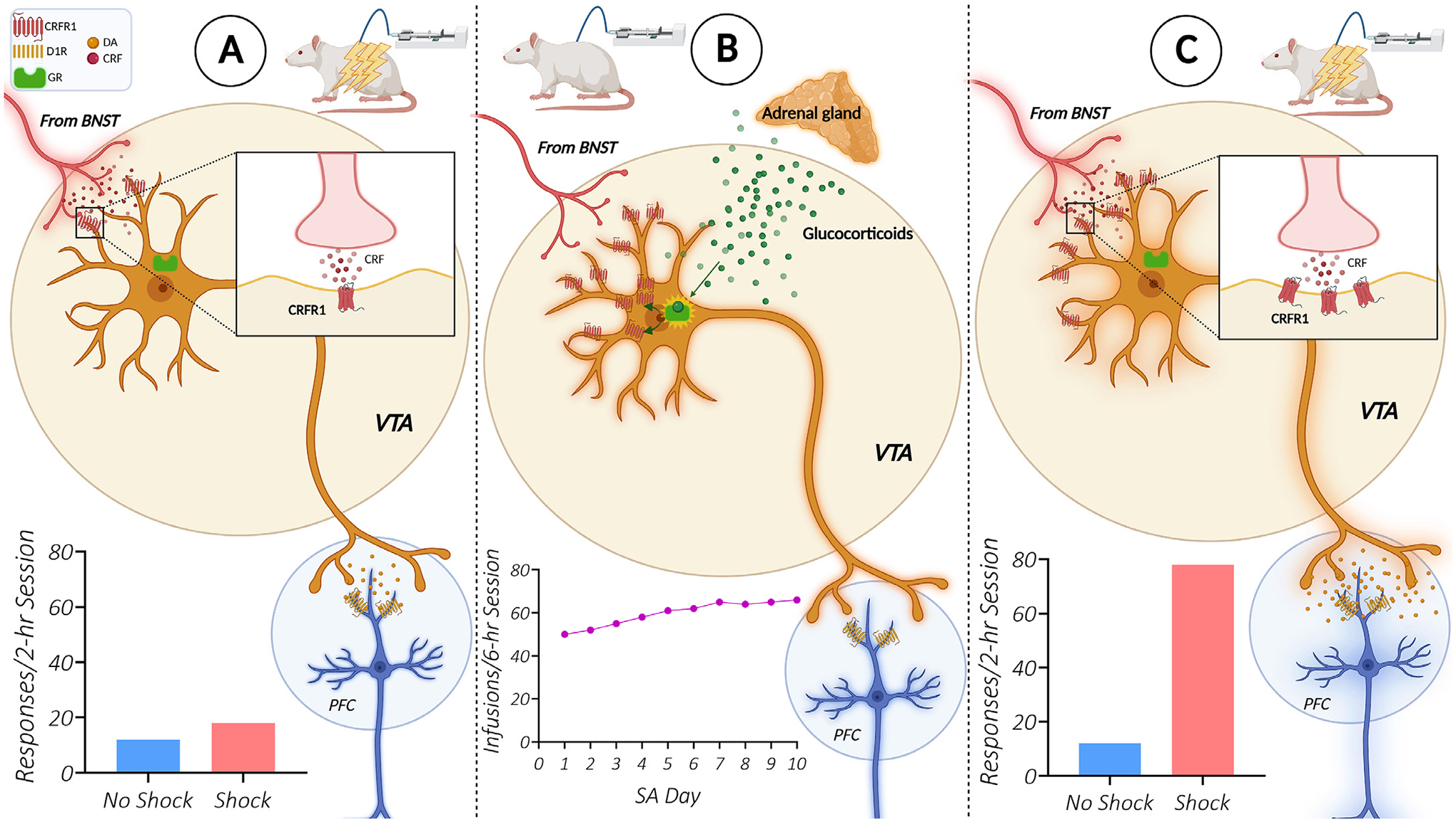
The ability of a stressor, electric footshock, to reinstate extinguished cocaine seeking is intake-dependent [51], requires elevated glucocorticoids during self-administration [36], and involves increased CRF-R1 expression in the VTA and the establishment of CRF regulation of mesocortical dopamine neurons [89,142]. A. In rats with a history of short-access cocaine self-administration (1–2 h/day), footshock does not reinstate cocaine seeking. B. Elevated glucocorticoids during long-access self-administration (6+ hrs/day) [277] likely contribute to an upregulation of VTA CRF-R1 receptors on dopamine neurons that project to the prelimbic (PL) prefrontal cortex [142]. C. As a result, the ability of CRF to regulate PL-projecting dopamine neurons [142] and produce CRF-R1 dependent cocaine seeking during stress [89] in response to activation of CRF-releasing afferents from the BNST [83] is established. Abbreviations: CRF (corticotropin releasing factor), DA (dopamine), D1R (dopamine D1 receptor), GR (glucocorticoid receptor), BNST (bed nucleus of the stria terminalis), VTA (ventral tegmental area), PFC (prefrontal cortex), SA (self-administration).
As is the case with stressor-induced reinstatement, drug seeking in response to icv CRF delivery is also only observed following long-access cocaine self-administration and extinction, indicating that drug-induced adaptations in CRF signaling may establish the ability of stress to trigger relapse [51]. In general, these findings are consistent with clinical reports that subjective and physiological responses to CRF infusions are heightened in cocaine-dependent individuals [265]. We have found that a key region at which CRF signaling is recruited to promote stressor-induced drug seeking is the VTA. In line with findings that repeated cocaine administration increases CRF binding in the VTA [111], enhances CRF-R1 dependent regulation of excitatory transmission in the VTA [119], and establishes CRF control of VTA dopaminergic signaling [88], we have found that self-administration under long-access conditions establishes CRF-dependent regulation of cocaine-seeking behavior in male rats [89]. As is observed with icv CRF, the ability of VTA micro-infusions into the VTA to reinstate extinguished cocaine seeking is only observed when rats have a history of long-access cocaine self-administration [89]. More recently, we have found that the establishment of CRF-dependent stressor-induced cocaine seeking is associated with increased CRF-R1 mRNA levels in the VTA and a heightened CRF-R1 dependent Fos response in the prelimbic cortex [142]. Along with findings that a stressor-induced cocaine seeking requires CRF-R1 receptor regulation of a dopaminergic projection from the VTA to the prelimbic PFC and prelimbic D1 receptor activation [142], these findings suggest that excessive cocaine use produces persistent increases in CRF-R1 expression in VTA dopamine neurons that project to the prelimbic cortex to establish susceptibility to stress triggered relapse (Fig. 2).
Chronic stress effects on CRF signaling and drug self-administration/reinstatement
Chronic stress can also escalate drug use via processes that involve recruitment of CRF signaling. For example, chronic social defeat stress can persistently escalate alcohol [266–268] and cocaine [269–272] self-administration in mice and rats as a result of enhanced CRF actions in the VTA [267,269–272]. Social defeat-escalated cocaine self-administration is associated with increased VTA CRF tone and may involve both CRF-R1 and CRF-R2 receptor signaling in subregions of the VTA [271]. Exposure to predator odor can also escalate alcohol drinking in a manner that can be blocked by CRF-R1 antagonism in the CeA [98]. Although not directly demonstrated, the effects of chronic stress in combination with self-administration are also likely evident as CRF-dependent heightened susceptibility to stressor-induced drug seeking in relapse models. We have reported that daily footshock at the time of drug self-administration escalates cocaine intake [273]. Recently, we have found that footshock escalated cocaine self-administration is also associated with a persistently increased stressor-induced reinstatement following extinction (J. McReynolds and J. Mantsch, unpublished observations).
Glucocorticoid regulation of CRF signaling
Using experiments examining the effects of long-access cocaine self-administration on reinstatement, our laboratory has begun to explore the neurobiological processes through excessive drug use recruits CRF signaling and stress-triggered cocaine seeking. It is well-established that self-administered cocaine elevates corticosterone levels in plasma [274,275] and in the brain [276]. These elevations are prolonged and exaggerated when rats self-administer cocaine under daily long-access conditions [277]. Prior work has demonstrated that chronically elevated glucocorticoids can promote brain CRF signaling via allostatic alterations that may contribute to neuropathology [278]. Consistent with this, we have demonstrated that when rats undergo surgical adrenalectomy and along with basal (diurnal) corticosterone replacement prior to a 14-day long-access cocaine self-administration period, reinstatement of cocaine seeking in response to footshock or icv CRF (both of which require prior long-access self-administration [51]) is not observed [36]. By contrast, when rats undergo adrenalectomy/corticosterone replacement after long-access self-administration but prior to testing for reinstatement, cocaine seeking in response to icv CRF or footshock is comparable to sham-operated controls. Altogether, these findings suggest that the adrenal response during periods of excessive drug use, but not thereafter, is necessary for establishing adaptations in CRF signaling that underlie susceptibility to stressor-induced drug seeking (Fig. 2). Surprisingly, while the adrenal response during long-access self-administration is required for subsequent stressor-induced cocaine seeking, elevated corticosterone alone is not sufficient to establish susceptibility to reinstatement. We have found that daily corticosterone administration during short-access self-administration, at a dose that reproduces plasma patterns observed during long-access self-administration, fails to alter later reinstatement [279]. We speculate that other adrenal hormones likely contribute to neuroadaptations that establish CRF-dependent stressor-induced cocaine seeking. One hormone of interest is epinephrine, which can stimulate vagal afferents into the brain [280] and has been demonstrated function cooperatively with corticosterone to contribute to cocaine-induced behavioral plasticity [281].
Factors that influence the contribution of CRF to SUDs
Various factors can influence the relationship between CRF signaling and SUDs. In particular, CRF effects have been reported to vary across development and between sexes and are likely defined by genetic differences. Evidence that these factors can determine the contribution of CRF to SUDs is provided below.
Development
Early-life trauma and adversity are key determinants of SUD risk and severity later in life [282]. In humans, genetic variation in CRF receptors is associated with the impact of early-life adversity on adult SUD outcomes (see e.g., [283]). Neonatal and adolescent stress has also been shown to increase susceptibility to adult drug self-administration and seeking and related behaviors in rodents (see [284,285]). Studies have implicated CRF signaling in many of these effects of stress (see e.g., [286,287] for reviews). For example, the ability of social defeat stress during adolescence to promote escalating patterns of cocaine self-administration during adulthood is prevented by administration of a CRF-R1 antagonist into the VTA [288] while the enhancement of adult responses to cocaine by removal of enrichment during adolescence in mice is associated with increases CRF mRNA in the BNST and prevented by the blocking CRF-R1 receptors [289]. Moreover, early post-natal stress disrupts connectivity between stress- and reward/motivation-related networks in a manner that is prevented by attenuating CRF signaling in the amygdala [290,291]. Conversely, drug use during adolescence can alter CRF signaling during adulthood, thereby increasing the risk for stress-related psychopathology in adults (see e.g., [292–295]). Altogether, these findings establish CRF signaling during early life as a determinant of adult SUDs and suggest that early life events may influence CRF signaling in adulthood to promote SUD risk.
Sex differences
Sex differences in stress reactivity and vulnerability have been identified (see [296–299] for reviews) and females have been reported to be more vulnerable to stress-related drug seeking/craving and related responses [300–305]. Indeed, findings of sex differences in the effects of medications on drug craving (see e.g., [306]) have emphasized the need for consideration of sex as a biological variable when developing medications that target the influence of stress in SUDs [307]. Sex differences in CRF signaling have also been well-documented [308–311]. Importantly, CRF delivery differentially influences functional connectivity in networks that include the extended amygdala, prefrontal cortex, and nucleus accumbens in female rats [312,313]. These differences appear to translate to sex differences in the contribution of CRF to drug seeking. Indeed, rodent studies have found that female rats are more susceptible to reinstatement of cocaine seeking following icv CRF delivery [52]. Moreover, alcohol-induced neuroadaptations in CRF signaling in the central amygdala are sex dependent [311,314]. These findings parallel those from clinical studies which have found that cocaine-dependent women display heightened CRF reactivity [265].
Genetic linkages
The risk for and severity of SUDs involves a complex intersection of multiple genes with environmental determinants and drug-effects. There is evidence for genetic links between components of CRF signaling and drug misuse. Variants of or haplotypes involving variants of the CRHR1, CRHR2 or CRFBP genes have been associated with various aspects of SUDs. Variants of CRHR1 have been associated with binge alcohol drinking [315], the overall lifetime prevalence of drinking [315], habitual alcohol intake [316], and adolescent drinking initiation and progression [317]. Associations between CRHR1 variants and nicotine dependence [318] and heroin addiction [319] have also been reported. CRHR2 variants have been associated with heroin use [319,320] while variants in CRHBP have been associated with the use of heroin [321,322] and cocaine [321,323]. Genetic variation in the CRF system appears to be a particularly important determinant of the influence of environmental factors in SUDs, especially the impact of early-life stress. CRHR1 polymorphisms/variant-containing haplotypes are associated with heightened stress-related alcohol consumption in adolescents [283,317] and are predictive of the impact of childhood sexual abuse [324] and early-life trauma [325] on adult drinking/AUD. Strong associations with the effects of stress in SUDs are also observed with genetic variation related to the CRHBP gene. CRHBP variants have been associated with heightened stress-related alcohol craving [326] and heroin relapse [327]. These associations may be attributable to altered PFC processing of emotional stimuli, as CRHR1 variants appear to influence negative emotionality and corresponding ventrolateral PFC function [328] while CRHBP variants are associated with alterations in cortical EEG [329]
Genetic associations between the CRF system and SUD-related behaviors can also be observed in rodents. Most notably, differences in CRF signaling are observed in rat lines genetically selected for high alcohol preference (see [330] for review), including Sardinian alcohol preferring (sP) and non-preferring (sNP) rats developed from a Wistar foundation stock at the University of Cagliari, Italy and subsequently bred at Scripps, and the Marchigian subline of Sardinian Preferring (msP) (reviewed in [331]) and the alcohol-preferring (P) and non-preferring (NP) lines developed at the Walter Reed Army Hospital, also derived from a Wistar stock and transferred to the University of Indiana (reviewed in [332]). The msP rats have two G-to-A substitutions in the promoter region of the Crhr1 gene that leads to increased brain expression and are associated with CRF-R1 associated anxiety-related behaviors [333,334], heightened CRFR1-dependent stressor-induced alcohol seeking [261,335,336], and the establishment of CRFR1-mediated alcohol self-administration under both dependent and non-dependent conditions [261,335,336]. Compared to Wistar controls, these rats also display altered CRFR1 regulation of both inhibitory and excitatory synaptic transmission in the CeA [337,338]. By contrast CRFR1 antagonist effects on alcohol consumption under dependence or non-dependence conditions are not observed in sP rats [339,340], which lack the A alleles in the Crhr1 promoter [341]. Alcohol consumption in P rats is sensitive to CRFR1 antagonism, despite the absence of the alleles, but only in dependent animals [342], an effect that may be attributed to changes in both CRF [343] and its receptors [344,345]. Overall, these observations are consistent with human data suggesting that genetic alterations in CRF signaling can confer susceptibility to SUDs, particularly during periods of stress.
Section IV: therapeutic implications
The effectiveness of CRF receptor antagonists in preclinical rodent models inspired efforts to develop oral, non-peptide brain penetrant CRF antagonists for clinical use in individuals diagnosed with anxiety, depression, PTSD, and SUDs. Unfortunately, after some early indications of potential utility, more recent clinical trials using the newest generation of CRF-R1 antagonists (pexacerfont and verucerfont) have failed to demonstrate efficacy, despite evidence of neuroendocrine effectiveness, CNS penetrance, and functional brain effects (see [346] for commentary). An initial study published in 2015 reported that the CRF-R1 antagonist, pexacerfont, did not attenuate alcohol craving, emotional responses, anxiety or corresponding neural activity [347]. Subsequently, similar findings were reported in women using the CRF-R1 antagonist, verucerfont, which blocked the HPA response to CRF and the amygdala responses to negative affective stimuli [348]. These outcomes are consistent with those from clinical trials investigating the efficacy of CRF receptor antagonists in individuals with generalized anxiety disorder [349], major depression [350]; GlaxoSmithKline, CRS106139), or PTSD [351]. The translational failure of CRF antagonists may be attributable to a number of factors, including lack of sufficient target engagement in the human brain, lack of universal effectiveness in all subpopulations of individuals with SUDs, and the need for co-administration with other medications. However, these studies raise serious questions about the translatability of preclinical finding in rodents to humans and, potentially, the models used to define the neurobiological mechanisms through which stress influences drug seeking. To date, our understanding of the potential contribution of CRF signaling to SUDs has relied heavily on rodent-based approaches in which the ability of stimuli to trigger drug seeking in rodents following drug self-administration and extinction has been used as an indicator of relapse susceptibility. Limitations to these approaches include the use of forced abstinence/extinction-based versus voluntary abstinence protocols, the absence of assessment of drug seeking in the context of other relevant stimuli and alternative reinforcers, and the lack of adequate consideration of subpopulations of users with varying vulnerability based on factors such as co-morbidity, early-life experience (e.g., childhood trauma), biological sex, and genetic background. The application of approaches that better capture the complex role of stress in drug craving and seeking (e.g., the interactive effects of stress with other stimuli [352]) and use of models guided by reverse translation [353–355], including those that incorporate voluntary abstinence [356,357], have the potential to reveal more relevant targets for interventions. Importantly, our ability to effectively leverage mechanisms them will rely on our capacity to diagnosis those with cocaine use disorder whose drug seeking is stress-related.
Conclusions
In conclusion, the contribution of CRF to SUDs in humans remains enigmatic. Despite an abundance of evidence that CRF signaling is fundamental to behaviors in rodent models assumed to be relevant to human addiction, medications designed to target CRF have repeatedly failed in clinical trials. Considering that CRF and its receptors are expressed in the human brain and dysregulated in stress-related disorders [358–360] and SUDs [265], these failures have been puzzling. New perspectives and additional research are needed to unravel the mysterious role of CRF in SUDs.
Acknowledgments
Supported by NIH R01s DA048280 and DA052169 to JRM. Erik Van Newenhizen is acknowledged for his support with figure preparation. No conflict of interest is declared.
Abbreviations:
- SUDs
substance use disorders
- CRF
corticotropin releasing factor
- VTA
ventral tegmental area
- ACTH
adrenocorticotropic hormone
- BNST
bed nucleus of the stria terminalis
- CeA
central nucleus of the amygdala
- NAc
nucleus accumbens
- MRN
medial raphe nucleus
- DRN
dorsal raphe nucleus
- PTSD
post-traumatic stress disorder
- HPA
hypothalamic pituitary adrenal
- CRF-BP
CRF binding protein
Footnotes
Declaration of Competing Interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data Availability
No data were used for the research described in the article.
References
- [1].Nusbaum MP, Blitz DM, Marder E, Functional consequences of neuropeptide and small-molecule co-transmission, Nat. Rev. Neurosci 18 (7) (2017) 389–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Behan DP, et al. , Neurobiology of corticotropin releasing factor (CRF) receptors and CRF-binding protein: implications for the treatment of CNS disorders, Mol. Psychiatry 1 (4) (1996) 265–277. [PubMed] [Google Scholar]
- [3].Bale TL, Vale WW, CRF and CRF receptors: role in stress responsivity and other behaviors, Annu. Rev. Pharmacol. Toxicol 44 (2004) 525–557. [DOI] [PubMed] [Google Scholar]
- [4].Seasholtz AF, Valverde RA, Denver RJ, Corticotropin-releasing hormone-binding protein: biochemistry and function from fishes to mammals, J. Endocrinol 175 (1) (2002) 89–97. [DOI] [PubMed] [Google Scholar]
- [5].Blanco EH, et al. , Corticotropin-releasing factor binding protein enters the regulated secretory pathway in neuroendocrine cells and cortical neurons, Neuropeptides 45 (4) (2011) 273–279. [DOI] [PubMed] [Google Scholar]
- [6].Ungless MA, et al. , Corticotropin-releasing factor requires CRF binding protein to potentiate NMDA receptors via CRF receptor 2 in dopamine neurons, Neuron 39 (3) (2003) 401–407. [DOI] [PubMed] [Google Scholar]
- [7].Slater PG, et al. , CRF binding protein facilitates the presence of CRF type 2α receptor on the cell surface, Proc. Natl. Acad. Sci. U. S. A 113 (15) (2016) 4075–4080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Dabrowska J, et al. , Central CRF neurons are not created equal: phenotypic differences in CRF-containing neurons of the rat paraventricular hypothalamus and the bed nucleus of the stria terminalis, Front. Neurosci 7 (2013) 156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kapcala LP, Dicke JA, Brain corticotropin-releasing hormone receptors on neurons and astrocytes, Brain Res 589 (1) (1992) 143–148. [DOI] [PubMed] [Google Scholar]
- [10].Swanson LW, et al. , Organization of ovine corticotropin-releasing factor immunoreactive cells and fibers in the rat brain: an immunohistochemical study, Neuroendocrinology 36 (3) (1983) 165–186. [DOI] [PubMed] [Google Scholar]
- [11].Cummings S, et al. , Corticotropin-releasing factor immunoreactivity is widely distributed within the central nervous system of the rat: an immunohistochemical study, J. Neurosci 3 (7) (1983) 1355–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Swanson LW, Simmons DM, Differential steroid hormone and neural influences on peptide mRNA levels in CRH cells of the paraventricular nucleus: a hybridization histochemical study in the rat, J. Comp. Neurol 285 (4) (1989) 413–435. [DOI] [PubMed] [Google Scholar]
- [13].Chen P, et al. , Prefrontal cortex corticotropin-releasing factor neurons control behavioral style selection under challenging situations, Neuron 106 (2) (2020) 301–315 e7.. [DOI] [PubMed] [Google Scholar]
- [14].Grieder TE, et al. , VTA CRF neurons mediate the aversive effects of nicotine withdrawal and promote intake escalation, Nat. Neurosci 17 (12) (2014) 1751–1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Valentino RJ, Foote SL, Aston-Jones G, Corticotropin-releasing factor activates noradrenergic neurons of the locus coeruleus, Brain Res 270 (2) (1983) 363–367. [DOI] [PubMed] [Google Scholar]
- [16].Kozicz T, Yanaihara H, Arimura A, Distribution of urocortin-like immunoreactivity in the central nervous system of the rat, J. Comp. Neurol 391 (1) (1998) 1–10. [DOI] [PubMed] [Google Scholar]
- [17].De Souza EB, et al. , Corticotropin-releasing factor receptors in rat forebrain: autoradiographic identification, Science 224 (4656) (1984) 1449–1451. [DOI] [PubMed] [Google Scholar]
- [18].De Souza EB, et al. , Corticotropin-releasing factor receptors are widely distributed within the rat central nervous system: an autoradiographic study, J. Neurosci 5 (12) (1985) 3189–3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Wynn PC, et al. , Brain and pituitary receptors for corticotropin releasing factor: localization and differential regulation after adrenalectomy, Peptides 5 (6) (1984) 1077–1084. [DOI] [PubMed] [Google Scholar]
- [20].Potter E, et al. , Distribution of corticotropin-releasing factor receptor mRNA expression in the rat brain and pituitary, Proc. Natl. Acad. Sci. U. S. A 91 (19) (1994) 8777–8781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Van Pett K, et al. , Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse, J. Comp. Neurol 428 (2) (2000) 191–212. [DOI] [PubMed] [Google Scholar]
- [22].Mantsch JR, et al. , Stress-induced reinstatement of drug seeking: 20 years of progress, Neuropsychopharmacology 41 (1) (2016) 335–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Preston KL, Epstein DH, Stress in the daily lives of cocaine and heroin users: relationship to mood, craving, relapse triggers, and cocaine use, Psychopharmacology (Berl.) 218 (1) (2011) 29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Furnari M, et al. , Some of the people, some of the time: field evidence for associations and dissociations between stress and drug use, Psychopharmacology (Berl.) 232 (19) (2015) 3529–3537. [DOI] [PubMed] [Google Scholar]
- [25].Preston KL, et al. , Before and after: craving, mood, and background stress in the hours surrounding drug use and stressful events in patients with opioid-use disorder, Psychopharmacology (Berl.) 235 (9) (2018) 2713–2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Wemm SE, et al. , A day-by-day prospective analysis of stress, craving and risk of next day alcohol intake during alcohol use disorder treatment, Drug Alcohol Depend 204 (2019) 107569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Panlilio LV, et al. , Stress, craving and mood as predictors of early dropout from opioid agonist therapy, Drug Alcohol Depend 202 (2019) 200–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Martins JS, et al. , Alcohol craving and withdrawal at treatment entry prospectively predict alcohol use outcomes during outpatient treatment, Drug Alcohol Depend 231 (2022) 109253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Sinha R, Catapano D, O’Malley S, Stress-induced craving and stress response in cocaine dependent individuals, Psychopharmacology (Berl.) 142 (4) (1999) 343–351. [DOI] [PubMed] [Google Scholar]
- [30].Sinha R, et al. , Psychological stress, drug-related cues and cocaine craving, Psychopharmacology (Berl.) 152 (2) (2000) 140–148. [DOI] [PubMed] [Google Scholar]
- [31].Kwako LE, et al. , Methods for inducing alcohol craving in individuals with co-morbid alcohol dependence and posttraumatic stress disorder: behavioral and physiological outcomes, Addict. Biol 20 (4) (2015) 733–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Sinha R, et al. , Stress-induced cocaine craving and hypothalamic-pituitary-adrenal responses are predictive of cocaine relapse outcomes, Arch. Gen. Psychiatry 63 (3) (2006) 324–331. [DOI] [PubMed] [Google Scholar]
- [33].Back SE, et al. , Reactivity to laboratory stress provocation predicts relapse to cocaine, Drug Alcohol Depend 106 (1) (2010) 21–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Shaham Y, et al. , CP-154,526, a selective, non-peptide antagonist of the corticotropin-releasing factor1 receptor attenuates stress-induced relapse to drug seeking in cocaine- and heroin-trained rats, Psychopharmacology (Berl.) 137 (2) (1998) 184–190. [DOI] [PubMed] [Google Scholar]
- [35].Erb S, Shaham Y, Stewart J, The role of corticotropin-releasing factor and corticosterone in stress- and cocaine-induced relapse to cocaine seeking in rats, J. Neurosci 18 (14) (1998) 5529–5536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Graf EN, et al. , Adrenal activity during repeated long-access cocaine self-administration is required for later CRF-Induced and CRF-dependent stressor-induced reinstatement in rats, Neuropsychopharmacology 36 (7) (2011) 1444–1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Shaham Y, et al. , Corticotropin-releasing factor, but not corticosterone, is involved in stress-induced relapse to heroin-seeking in rats, J. Neurosci 17 (7) (1997) 2605–2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Lê AD, et al. , The role of corticotrophin-releasing factor in stress-induced relapse to alcohol-seeking behavior in rats, Psychopharmacology (Berl.) 150 (3) (2000) 317–324. [DOI] [PubMed] [Google Scholar]
- [39].Liu X, Weiss F, Additive effect of stress and drug cues on reinstatement of ethanol seeking: exacerbation by history of dependence and role of concurrent activation of corticotropin-releasing factor and opioid mechanisms, J. Neurosci 22 (18) (2002) 7856–7861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Nawata Y, Kitaichi K, Yamamoto T, Increases of CRF in the amygdala are responsible for reinstatement of methamphetamine-seeking behavior induced by footshock, Pharmacol. Biochem. Behav 101 (2) (2012) 297–302. [DOI] [PubMed] [Google Scholar]
- [41].Zislis G, et al. , Effects of the CRF receptor antagonist D-Phe CRF(12–41) and the alpha2-adrenergic receptor agonist clonidine on stress-induced reinstatement of nicotine-seeking behavior in rats, Neuropharmacology 53 (8) (2007) 958–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Bruijnzeel AW, Prado M, Isaac S, Corticotropin-releasing factor-1 receptor activation mediates nicotine withdrawal-induced deficit in brain reward function and stress-induced relapse, Biol. Psychiatry 66 (2) (2009) 110–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Marinelli PW, et al. , The CRF1 receptor antagonist antalarmin attenuates yohimbine-induced increases in operant alcohol self-administration and reinstatement of alcohol seeking in rats, Psychopharmacology (Berl.) 195 (3) (2007) 345–355. [DOI] [PubMed] [Google Scholar]
- [44].Brown ZJ, et al. , Interaction between noradrenaline and corticotrophin-releasing factor in the reinstatement of cocaine seeking in the rat, Psychopharmacology (Berl.) 203 (1) (2009) 121–130. [DOI] [PubMed] [Google Scholar]
- [45].Shalev U, et al. , A role for corticotropin-releasing factor, but not corticosterone, in acute food-deprivation-induced reinstatement of heroin seeking in rats, Psychopharmacology (Berl.) 187 (3) (2006) 376–384. [DOI] [PubMed] [Google Scholar]
- [46].Kreibich AS, et al. , Stress-induced potentiation of cocaine reward: a role for CRF R1 and CREB, Neuropsychopharmacology 34 (12) (2009) 2609–2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].McReynolds JR, et al. , Beta-2 adrenergic receptors mediate stress-evoked reinstatement of cocaine-induced conditioned place preference and increases in CRF mRNA in the bed nucleus of the stria terminalis in mice, Psychopharmacology (Berl.) 231 (20) (2014) 3953–3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Wang J, et al. , Region-specific effects of brain corticotropin-releasing factor receptor type 1 blockade on footshock-stress- or drug-priming-induced reinstatement of morphine conditioned place preference in rats, Psychopharmacology (Berl.) 185 (1) (2006) 19–28. [DOI] [PubMed] [Google Scholar]
- [49].Lê AD, et al. , The role of corticotropin-releasing factor in the median raphe nucleus in relapse to alcohol, J. Neurosci 22 (18) (2002) 7844–7849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Erb S, et al. , Central injections of CRF reinstate cocaine seeking in rats after postinjection delays of up to 3 h: an influence of time and environmental context, Psychopharmacology (Berl.) 187 (1) (2006) 112–120. [DOI] [PubMed] [Google Scholar]
- [51].Mantsch JR, et al. , Stressor- and corticotropin releasing factor-induced reinstatement and active stress-related behavioral responses are augmented following long-access cocaine self-administration by rats, Psychopharmacology (Berl.) 195 (4) (2008) 591–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Buffalari DM, et al. , Corticotrophin releasing factor (CRF) induced reinstatement of cocaine seeking in male and female rats, Physiol. Behav 105 (2) (2012) 209–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Moffett MC, Goeders NE, CP-154,526, a CRF type-1 receptor antagonist, attenuates the cue-and methamphetamine-induced reinstatement of extinguished methamphetamine-seeking behavior in rats, Psychopharmacology (Berl.) 190 (2) (2007) 171–180. [DOI] [PubMed] [Google Scholar]
- [54].Berger SP, et al. , Haloperidol antagonism of cue-elicited cocaine craving, Lancet 347 (9000) (1996) 504–508. [DOI] [PubMed] [Google Scholar]
- [55].Sinha R, et al. , Hypothalamic-pituitary-adrenal axis and sympatho-adreno-medullary responses during stress-induced and drug cue-induced cocaine craving states, Psychopharmacology (Berl.) 170 (1) (2003) 62–72. [DOI] [PubMed] [Google Scholar]
- [56].DeVries AC, et al. , Conditioned release of corticosterone by contextual stimuli associated with cocaine is mediated by corticotropin-releasing factor, Brain Res 786 (1–2) (1998) 39–46. [DOI] [PubMed] [Google Scholar]
- [57].Olive MF, et al. , A role for corticotropin releasing factor (CRF) in ethanol consumption, sensitivity, and reward as revealed by CRF-deficient mice, Psychopharmacology (Berl.) 165 (2) (2003) 181–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Palmer AA, et al. , Corticotropin-releasing factor overexpression decreases ethanol drinking and increases sensitivity to the sedative effects of ethanol, Psychopharmacology (Berl.) 176 (3–4) (2004) 386–397. [DOI] [PubMed] [Google Scholar]
- [59].Pastor R, et al. , Ethanol concentration-dependent effects and the role of stress on ethanol drinking in corticotropin-releasing factor type 1 and double type 1 and 2 receptor knockout mice, Psychopharmacology (Berl.) 218 (1) (2011) 169–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Chu K, et al. , Dependence-induced increases in ethanol self-administration in mice are blocked by the CRF1 receptor antagonist antalarmin and by CRF1 receptor knockout, Pharmacol. Biochem. Behav 86 (4) (2007) 813–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Pastor R, et al. , Corticotropin-releasing factor-1 receptor involvement in behavioral neuroadaptation to ethanol: a urocortin1-independent mechanism, Proc. Natl Acad. Sci. U. S. A 105 (26) (2008) 9070–9075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Sillaber I, et al. , Enhanced and delayed stress-induced alcohol drinking in mice lacking functional CRH1 receptors, Science 296 (5569) (2002) 931–933. [DOI] [PubMed] [Google Scholar]
- [63].Molander A, et al. , Brain-specific inactivation of the Crhr1 gene inhibits post-dependent and stress-induced alcohol intake, but does not affect relapse-like drinking, Neuropsychopharmacology 37 (4) (2012) 1047–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Timpl P, et al. , Impaired stress response and reduced anxiety in mice lacking a functional corticotropin-releasing hormone receptor 1, Nat. Genet 19 (2) (1998) 162–166. [DOI] [PubMed] [Google Scholar]
- [65].Sharpe AL, et al. , Mice deficient in corticotropin-releasing factor receptor type 2 exhibit normal ethanol-associated behaviors, Alcohol Clin. Exp. Res 29 (9) (2005) 1601–1609. [DOI] [PubMed] [Google Scholar]
- [66].Ketchesin KD, Stinnett GS, Seasholtz AF, Binge drinking decreases corticotropin-releasing factor-binding protein expression in the medial prefrontal cortex of mice, Alcohol Clin. Exp. Res 40 (8) (2016) 1641–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Contarino A, et al. , CRF(1) receptor-deficiency increases cocaine reward, Neuropharmacology 117 (2017) 41–48. [DOI] [PubMed] [Google Scholar]
- [68].Contarino A, Papaleo F, The corticotropin-releasing factor receptor-1 pathway mediates the negative affective states of opiate withdrawal, Proc. Natl. Acad. Sci. U. S. A 102 (51) (2005) 18649–18654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Papaleo F, Kitchener P, Contarino A, Disruption of the CRF/CRF1 receptor stress system exacerbates the somatic signs of opiate withdrawal, Neuron 53 (4) (2007) 577–589. [DOI] [PubMed] [Google Scholar]
- [70].McFarland K, et al. , Limbic and motor circuitry underlying footshock-induced reinstatement of cocaine-seeking behavior, J. Neurosci 24 (7) (2004) 1551–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Briand LA, et al. , Ventral tegmental afferents in stress-induced reinstatement: the role of cAMP response element-binding protein, J. Neurosci 30 (48) (2010) 16149–16159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Erb S, et al. , Effects of chronic cocaine exposure on corticotropin-releasing hormone binding protein in the central nucleus of the amygdala and bed nucleus of the stria terminalis, Neuroscience 123 (4) (2004) 1003–1009. [DOI] [PubMed] [Google Scholar]
- [73].Giardino WJ, et al. , Parallel circuits from the bed nuclei of stria terminalis to the lateral hypothalamus drive opposing emotional states, Nat. Neurosci 21 (8) (2018) 1084–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Shalev U, et al. , Time-dependent changes in extinction behavior and stress-induced reinstatement of drug seeking following withdrawal from heroin in rats, Psychopharmacology (Berl.) 156 (1) (2001) 98–107. [DOI] [PubMed] [Google Scholar]
- [75].Erb S, Stewart J, A role for the bed nucleus of the stria terminalis, but not the amygdala, in the effects of corticotropin-releasing factor on stress-induced reinstatement of cocaine seeking, J. Neurosci 19 (20) (1999) Rc35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Erb S, et al. , A role for the CRF-containing pathway from central nucleus of the amygdala to bed nucleus of the stria terminalis in the stress-induced reinstatement of cocaine seeking in rats, Psychopharmacology (Berl.) 158 (4) (2001) 360–365. [DOI] [PubMed] [Google Scholar]
- [77].Snyder AE, Silberman Y, Corticotropin releasing factor and norepinephrine related circuitry changes in the bed nucleus of the stria terminalis in stress and alcohol and substance use disorders, Neuropharmacology 201 (2021) 108814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Shaham Y, et al. , Clonidine blocks stress-induced reinstatement of heroin seeking in rats: an effect independent of locus coeruleus noradrenergic neurons, Eur. J. Neurosci 12 (1) (2000) 292–302. [DOI] [PubMed] [Google Scholar]
- [79].Mantsch JR, et al. , Involvement of noradrenergic neurotransmission in the stress-but not cocaine-induced reinstatement of extinguished cocaine-induced conditioned place preference in mice: role for β-2 adrenergic receptors, Neuropsychopharmacology 35 (11) (2010) 2165–2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Vranjkovic O, et al. , β-adrenergic receptor mediation of stress-induced reinstatement of extinguished cocaine-induced conditioned place preference in mice: roles for β1 and β2 adrenergic receptors, J. Pharmacol. Exp. Ther 342 (2) (2012) 541–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Cecchi M, et al. , Beta1 adrenergic receptors in the bed nucleus of stria terminalis mediate differential responses to opiate withdrawal, Neuropsychopharmacology 32 (3) (2007) 589–599. [DOI] [PubMed] [Google Scholar]
- [82].Leri F, et al. , Blockade of stress-induced but not cocaine-induced reinstatement by infusion of noradrenergic antagonists into the bed nucleus of the stria terminalis or the central nucleus of the amygdala, J. Neurosci 22 (13) (2002) 5713–5718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Vranjkovic O, et al. , Stress-induced cocaine seeking requires a beta-2 adrenergic receptor-regulated pathway from the ventral bed nucleus of the stria terminalis that regulates CRF actions in the ventral tegmental area, J. Neurosci 34 (37) (2014) 12504–12514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Nobis WP, et al. , β-Adrenergic receptors enhance excitatory transmission in the bed nucleus of the stria terminalis through a corticotrophin-releasing factor receptor-dependent and cocaine-regulated mechanism, Biol. Psychiatry 69 (11) (2011) 1083–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Silberman Y, Matthews RT, Winder DG, A corticotropin releasing factor pathway for ethanol regulation of the ventral tegmental area in the bed nucleus of the stria terminalis, J. Neurosci 33 (3) (2013) 950–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Dumont EC, Williams JT, Noradrenaline triggers GABAA inhibition of bed nucleus of the stria terminalis neurons projecting to the ventral tegmental area, J. Neurosci 24 (38) (2004) 8198–8204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Rodaros D, et al. , Corticotropin-releasing factor projections from limbic forebrain and paraventricular nucleus of the hypothalamus to the region of the ventral tegmental area, Neuroscience 150 (1) (2007) 8–13. [DOI] [PubMed] [Google Scholar]
- [88].Wang B, et al. , Cocaine experience establishes control of midbrain glutamate and dopamine by corticotropin-releasing factor: a role in stress-induced relapse to drug seeking, J. Neurosci 25 (22) (2005) 5389–5396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Blacktop JM, et al. , Augmented cocaine seeking in response to stress or CRF delivered into the ventral tegmental area following long-access self-administration is mediated by CRF receptor type 1 but not CRF receptor type 2, J. Neurosci 31 (31) (2011) 11396–11403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Kaufling J, et al. , Species-specific diversity in the anatomical and physiological organisation of the BNST-VTA pathway, Eur. J. Neurosci 45 (9) (2017) 1230–1240. [DOI] [PubMed] [Google Scholar]
- [91].Erb S, Lopak V, Smith C, Cocaine pre-exposure produces a sensitized and context-specific c-fos mRNA response to footshock stress in the central nucleus of the AMYGDALA, Neuroscience 129 (3) (2004) 719–725. [DOI] [PubMed] [Google Scholar]
- [92].Rudoy CA, Reyes AR, Van Bockstaele EJ, Evidence for beta1-adrenergic receptor involvement in amygdalar corticotropin-releasing factor gene expression: implications for cocaine withdrawal, Neuropsychopharmacology 34 (5) (2009) 1135–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Agoglia AE, Herman MA, The center of the emotional universe: alcohol, stress, and CRF1 amygdala circuitry, Alcohol 72 (2018) 61–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Huang MM, et al. , Corticotropin-releasing factor (CRF) sensitization of ethanol withdrawal-induced anxiety-like behavior is brain site specific and mediated by CRF-1 receptors: relation to stress-induced sensitization, J. Pharmacol. Exp. Ther 332 (1) (2010) 298–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Bruijnzeel AW, et al. , Blockade of CRF1 receptors in the central nucleus of the amygdala attenuates the dysphoria associated with nicotine withdrawal in rats, Pharmacol. Biochem. Behav 101 (1) (2012) 62–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Funk CK, et al. , Corticotropin-releasing factor within the central nucleus of the amygdala mediates enhanced ethanol self-administration in withdrawn, ethanol-dependent rats, J. Neurosci 26 (44) (2006) 11324–11332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Sommer WH, et al. , Upregulation of voluntary alcohol intake, behavioral sensitivity to stress, and amygdala crhr1 expression following a history of dependence, Biol. Psychiatry 63 (2) (2008) 139–145. [DOI] [PubMed] [Google Scholar]
- [98].Weera MM, et al. , The role of central amygdala corticotropin-releasing factor in predator odor stress-induced avoidance behavior and escalated alcohol drinking in rats, Neuropharmacology 166 (2020) 107979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Broccoli L, et al. , Targeted overexpression of CRH receptor subtype 1 in central amygdala neurons: effect on alcohol-seeking behavior, Psychopharmacology (Berl.) 235 (6) (2018) 1821–1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Dabrowska J, et al. , Targeting corticotropin-releasing factor projections from the oval nucleus of the bed nucleus of the stria terminalis using cell-type specific neuronal tracing studies in mouse and rat brain, J. Neuroendocrinol (12) (2016) 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Borrego MB, et al. , Central nucleus of the amygdala projections onto the nucleus accumbens core regulate binge-like alcohol drinking in a CRF-dependent manner, Neuropharmacology 203 (2022) 108874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Merchenthaler I, et al. , Immunocytochemical localization of corticotropin-releasing factor (CRF) in the rat brain, Am. J. Anat 165 (4) (1982) 385–396. [DOI] [PubMed] [Google Scholar]
- [103].Olschowka JA, et al. , The distribution of corticotropin releasing factor-like immunoreactive neurons in rat brain, Peptides 3 (6) (1982) 995–1015. [DOI] [PubMed] [Google Scholar]
- [104].Lemos JC, et al. , Severe stress switches CRF action in the nucleus accumbens from appetitive to aversive, Nature 490 (7420) (2012) 402–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Lemos JC, Shin JH, Alvarez VA, Striatal cholinergic interneurons are a novel target of corticotropin releasing factor, J. Neurosci 39 (29) (2019) 5647–5661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Baumgartner HM, Schulkin J, Berridge KC, Activating corticotropin-releasing factor systems in the nucleus accumbens, amygdala, and bed nucleus of stria terminalis: incentive motivation or aversive motivation? Biol. Psychiatry 89 (12) (2021) 1162–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Peciña S, Schulkin J, Berridge KC, Nucleus accumbens corticotropin-releasing factor increases cue-triggered motivation for sucrose reward: paradoxical positive incentive effects in stress? BMC Biol 4 (2006) 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Bryce CA, Floresco SB, Alterations in effort-related decision-making induced by stimulation of dopamine D(1), D(2), D(3), and corticotropin-releasing factor receptors in nucleus accumbens subregions, Psychopharmacology (Berl.) 236 (9) (2019) 2699–2712. [DOI] [PubMed] [Google Scholar]
- [109].Holahan MR, Kalin NH, Kelley AE, Microinfusion of corticotropin-releasing factor into the nucleus accumbens shell results in increased behavioral arousal and oral motor activity, Psychopharmacology (Berl.) 130 (2) (1997) 189–196. [DOI] [PubMed] [Google Scholar]
- [110].Uribe KP, et al. , Overexpression of corticotropin-releasing factor in the nucleus accumbens enhances the reinforcing effects of nicotine in intact female versus male and ovariectomized female rats, Neuropsychopharmacology 45 (2) (2020) 394–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Goeders NE, Bienvenu OJ, De Souza EB, Chronic cocaine administration alters corticotropin-releasing factor receptors in the rat brain, Brain Res 531 (1–2) (1990) 322–328. [DOI] [PubMed] [Google Scholar]
- [112].Korotkova TM, et al. , Effects of arousaland feeding-related neuropeptides on dopaminergic and GABAergic neurons in the ventral tegmental area of the rat, Eur. J. Neurosci 23 (10) (2006) 2677–2685. [DOI] [PubMed] [Google Scholar]
- [113].Wang HL, Morales M, Corticotropin-releasing factor binding protein within the ventral tegmental area is expressed in a subset of dopaminergic neurons, J. Comp. Neurol 509 (3) (2008) 302–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Wang B, et al. , Stress-induced relapse to cocaine seeking: roles for the CRF(2) receptor and CRF-binding protein in the ventral tegmental area of the rat, Psychopharmacology (Berl.) 193 (2) (2007) 283–294. [DOI] [PubMed] [Google Scholar]
- [115].Chen NA, et al. , Knockdown of CRF1 receptors in the ventral tegmental area attenuates cue- and acute food deprivation stress-induced cocaine seeking in mice, J. Neurosci 34 (35) (2014) 11560–11570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Twining RC, et al. , Aversive stimuli drive drug seeking in a state of low dopamine tone, Biol. Psychiatry 77 (10) (2015) 895–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Tagliaferro P, Morales M, Synapses between corticotropin-releasing factor-containing axon terminals and dopaminergic neurons in the ventral tegmental area are predominantly glutamatergic, J. Comp. Neurol 506 (4) (2008) 616–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Wanat MJ, et al. , Corticotropin-releasing factor increases mouse ventral tegmental area dopamine neuron firing through a protein kinase C-dependent enhancement of Ih, J. Physiol 586 (8) (2008) 2157–2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Hahn J, Hopf FW, Bonci A, Chronic cocaine enhances corticotropin-releasing factor-dependent potentiation of excitatory transmission in ventral tegmental area dopamine neurons, J. Neurosci 29 (20) (2009) 6535–6544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Beckstead MJ, et al. , CRF enhancement of GIRK channel-mediated transmission in dopamine neurons, Neuropsychopharmacology 34 (8) (2009) 1926–1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Riegel AC, Williams JT, CRF facilitates calcium release from intracellular stores in midbrain dopamine neurons, Neuron 57 (4) (2008) 559–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Williams CL, Buchta WC, Riegel AC, CRF-R2 and the heterosynaptic regulation of VTA glutamate during reinstatement of cocaine seeking, J. Neurosci 34 (31) (2014) 10402–10414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Wise RA, Morales M, A ventral tegmental CRF-glutamate-dopamine interaction in addiction, Brain Res 1314 (2010) 38–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Thierry AM, et al. , Selective activation of mesocortical DA system by stress, Nature 263 (5574) (1976) 242–244. [DOI] [PubMed] [Google Scholar]
- [125].Reinhard JF Jr., Bannon MJ, Roth RH, Acceleration by stress of dopamine synthesis and metabolism in prefrontal cortex: antagonism by diazepam, Naunyn Schmiedebergs Arch. Pharmacol 318 (4) (1982) 374–377. [DOI] [PubMed] [Google Scholar]
- [126].Deutch AY, Tam SY, Roth RH, Footshock and conditioned stress increase 3,4-dihydroxyphenylacetic acid (DOPAC) in the ventral tegmental area but not substantia nigra, Brain Res 333 (1) (1985) 143–146. [DOI] [PubMed] [Google Scholar]
- [127].Speciale SG, et al. , Activation of specific central dopamine pathways: locomotion and footshock, Brain Res. Bull 16 (1) (1986) 33–38. [DOI] [PubMed] [Google Scholar]
- [128].Kelly EA, Fudge JL, The neuroanatomic complexity of the CRF and DA systems and their interface: what we still don’t know, Neurosci. Biobehav. Rev 90 (2018) 247–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Abercrombie ED, et al. , Differential effect of stress on in vivo dopamine release in striatum, nucleus accumbens, and medial frontal cortex, J. Neurochem 52 (5) (1989) 1655–1658. [DOI] [PubMed] [Google Scholar]
- [130].Puglisi-Allegra S, et al. , Acute stress induces time-dependent responses in dopamine mesolimbic system, Brain Res 554 (1–2) (1991) 217–222. [DOI] [PubMed] [Google Scholar]
- [131].Kalivas PW, Duffy P, Selective activation of dopamine transmission in the shell of the nucleus accumbens by stress, Brain Res 675 (1–2) (1995) 325–328. [DOI] [PubMed] [Google Scholar]
- [132].Tidey JW, Miczek KA, Social defeat stress selectively alters mesocorticolimbic dopamine release: an in vivo microdialysis study, Brain Res 721 (1–2) (1996) 140–149. [DOI] [PubMed] [Google Scholar]
- [133].Lammel S, et al. , Projection-specific modulation of dopamine neuron synapses by aversive and rewarding stimuli, Neuron 70 (5) (2011) 855–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Badrinarayan A, et al. , Aversive stimuli differentially modulate real-time dopamine transmission dynamics within the nucleus accumbens core and shell, J. Neurosci 32 (45) (2012) 15779–15790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Roitman MF, et al. , Real-time chemical responses in the nucleus accumbens differentiate rewarding and aversive stimuli, Nat. Neurosci 11 (12) (2008) 1376–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Oleson EB, et al. , Subsecond dopamine release in the nucleus accumbens predicts conditioned punishment and its successful avoidance, J. Neurosci 32 (42) (2012) 14804–14808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Wanat MJ, Bonci A, Phillips PE, CRF acts in the midbrain to attenuate accumbens dopamine release to rewards but not their predictors, Nat. Neurosci 16 (4) (2013) 383–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Chang S, et al. , Unpleasant sound elicits negative emotion and reinstates drug seeking, Mol. Neurobiol 56 (11) (2019) 7594–7607. [DOI] [PubMed] [Google Scholar]
- [139].Heymann G, et al. , Synergy of distinct dopamine projection populations in behavioral reinforcement, Neuron 105 (5) (2020) 909–920 e5.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Refojo D, et al. , Glutamatergic and dopaminergic neurons mediate anxiogenic and anxiolytic effects of CRHR1, Science 333 (6051) (2011) 1903–1907. [DOI] [PubMed] [Google Scholar]
- [141].Blacktop JM, et al. , Antagonism of GABA-B but not GABA-A receptors in the VTA prevents stress- and intra-VTA CRF-induced reinstatement of extinguished cocaine seeking in rats, Neuropharmacology 102 (2016) 197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Vranjkovic O, et al. , Enhanced CRFR1-dependent regulation of a ventral tegmental area to prelimbic cortex projection establishes susceptibility to stress-induced cocaine seeking, J. Neurosci 38 (50) (2018) 10657–10671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Capriles N, et al. , A role for the prefrontal cortex in stress- and cocaine-induced reinstatement of cocaine seeking in rats, Psychopharmacology (Berl.) 168 (1–2) (2003) 66–74. [DOI] [PubMed] [Google Scholar]
- [144].Burmeister JJ, et al. , Differential roles of 5-HT receptor subtypes in cue and cocaine reinstatement of cocaine-seeking behavior in rats, Neuropsychopharmacology 29 (4) (2004) 660–668. [DOI] [PubMed] [Google Scholar]
- [145].Cunningham KA, Anastasio NC, Serotonin at the nexus of impulsivity and cue reactivity in cocaine addiction, Neuropharmacology 76 (2014) 460–478 Pt B 0 0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Valentino RJ, Lucki I, Van Bockstaele E, Corticotropin-releasing factor in the dorsal raphe nucleus: Linking stress coping and addiction, Brain Res 1314 (2010) 29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Fox JH, Lowry CA, Corticotropin-releasing factor-related peptides, serotonergic systems, and emotional behavior, Front. Neurosci 7 (2013) 169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Price ML, et al. , Effects of corticotropin-releasing factor on brain serotonergic activity, Neuropsychopharmacology 18 (6) (1998) 492–502. [DOI] [PubMed] [Google Scholar]
- [149].Kirby LG, Rice KC, Valentino RJ, Effects of corticotropin-releasing factor on neuronal activity in the serotonergic dorsal raphe nucleus, Neuropsychopharmacology 22 (2) (2000) 148–162. [DOI] [PubMed] [Google Scholar]
- [150].Kirby LG, et al. , Corticotropin-releasing factor increases GABA synaptic activity and induces inward current in 5-hydroxytryptamine dorsal raphe neurons, J. Neurosci 28 (48) (2008) 12927–12937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Lê AD, et al. , Intra-median raphe nucleus (MRN) infusions of muscimol, a GABA-A receptor agonist, reinstate alcohol seeking in rats: role of impulsivity and reward, Psychopharmacology (Berl.) 195 (4) (2008) 605–615. [DOI] [PubMed] [Google Scholar]
- [152].Li C, et al. , CRF-5-HT interactions in the dorsal raphe nucleus and motivation for stress-induced opioid reinstatement, Psychopharmacology (Berl.) 238 (1) (2021) 29–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Lê AD, et al. , Role of corticotropin-releasing factor in the median raphe nucleus in yohimbine-induced reinstatement of alcohol seeking in rats, Addict. Biol 18 (3) (2013) 448–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Schindler AG, et al. , Stress produces aversion and potentiates cocaine reward by releasing endogenous dynorphins in the ventral striatum to locally stimulate serotonin reuptake, J. Neurosci 32 (49) (2012) 17582–17596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Fontaine HM, et al. , Stress decreases serotonin tone in the nucleus accumbens in male mice to promote aversion and potentiate cocaine preference via decreased stimulation of 5-HT(1B) receptors, Neuropsychopharmacology (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Ritchie JL, et al. , Basolateral amygdala corticotropin-releasing factor receptor type 1 regulates context-cocaine memory strength during reconsolidation in a sex-dependent manner, Neuropharmacology 200 (2021) 108819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Cosme CV, Gutman AL, LaLumiere RT, The dorsal agranular insular cortex regulates the cued reinstatement of cocaine-seeking, but not food-seeking, behavior in rats, Neuropsychopharmacology 40 (10) (2015) 2425–2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Walker LC, et al. , Nucleus incertus corticotrophin-releasing factor 1 receptor signalling regulates alcohol seeking in rats, Addict. Biol 22 (6) (2017) 1641–1654. [DOI] [PubMed] [Google Scholar]
- [159].Robinson SL, Perez-Heydrich CA, Thiele TE, Corticotropin releasing factor type 1 and 2 receptor signaling in the medial prefrontal cortex modulates binge-like ethanol consumption in C57BL/6J mice, Brain. Sci 9 (7) (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Bernardi RE, et al. , Dissociable role of corticotropin releasing hormone receptor subtype 1 on dopaminergic and D1 dopaminoceptive neurons in cocaine seeking behavior, Front. Behav. Neurosci 11 (2017) 221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Dedic N, et al. , Chronic CRH depletion from GABAergic, long-range projection neurons in the extended amygdala reduces dopamine release and increases anxiety, Nat. Neurosci 21 (6) (2018) 803–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Kash TL, et al. , Dopamine enhances fast excitatory synaptic transmission in the extended amygdala by a CRF-R1-dependent process, J. Neurosci 28 (51) (2008) 13856–13865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Forster GL, et al. , Corticotropin-releasing factor in the dorsal raphe nucleus increases medial prefrontal cortical serotonin via type 2 receptors and median raphe nucleus activity, Eur. J. Neurosci 28 (2) (2008) 299–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Roberto M, et al. , Corticotropin releasing factor-induced amygdala gamma-aminobutyric Acid release plays a key role in alcohol dependence, Biol. Psychiatry 67 (9) (2010) 831–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Varodayan FP, Logrip ML, Roberto M, P/Q-type voltage-gated calcium channels mediate the ethanol and CRF sensitivity of central amygdala GABAergic synapses, Neuropharmacology 125 (2017) 197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Ciccocioppo R, et al. , Restraint stress alters nociceptin/orphanin FQ and CRF systems in the rat central amygdala: significance for anxiety-like behaviors, J. Neurosci 34 (2) (2014) 363–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Kang-Park M, et al. , Interaction of CRF and kappa opioid systems on GABAergic neurotransmission in the mouse central amygdala, J. Pharmacol. Exp. Ther 355 (2) (2015) 206–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Gehlert DR, et al. , Stress and central Urocortin increase anxiety-like behavior in the social interaction test via the CRF1 receptor, Eur. J. Pharmacol 509 (2–3) (2005) 145–153. [DOI] [PubMed] [Google Scholar]
- [169].Flores-Ramirez FJ, et al. , Blockade of corticotropin-releasing factor-1 receptors in the infralimbic cortex prevents stress-induced reinstatement of alcohol seeking in male Wistar rats: evidence of interaction between CRF(1) and orexin receptor signaling, Neuropharmacology 210 (2022) 109046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Ferré S, Hormones and neuropeptide receptor heteromers in the ventral tegmental area. targets for the treatment of loss of control of food intake and substance use disorders, Curr. Treat. Options Psychiatry 4 (2) (2017) 167–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Fuenzalida J, et al. , Dopamine D1 and corticotrophin-releasing hormone type-2α receptors assemble into functionally interacting complexes in living cells, Br. J. Pharmacol 171 (24) (2014) 5650–5664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Yarur HE, Andrés ME, Gysling K, Type 2β corticotrophin releasing factor receptor forms a heteromeric complex with dopamine D1 receptor in living cells, Front. Pharmacol 10 (2019) 1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Hasdemir B, et al. , Actin cytoskeleton-dependent regulation of corticotropin-releasing factor receptor heteromers, Mol. Biol. Cell 28 (18) (2017) 2386–2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Murat B, et al. , V1b and CRHR1 receptor heterodimerization mediates synergistic biological actions of vasopressin and CRH, Mol. Endocrinol 26 (3) (2012) 502–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Navarro G, et al. , Differential effect of amphetamine over the corticotropin-releasing factor CRF(2) receptor, the orexin OX(1) receptor and the CRF(2)-OX(1) heteroreceptor complex, Neuropharmacology 152 (2019) 102–111. [DOI] [PubMed] [Google Scholar]
- [176].Navarro G, et al. , Orexin-corticotropin-releasing factor receptor heteromers in the ventral tegmental area as targets for cocaine, J. Neurosci 35 (17) (2015) 6639–6653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Yarur HE, et al. , Cross-talk between dopamine D1 and corticotropin releasing factor type 2 receptors leads to occlusion of their ERK1/2 signaling, J. Neurochem 155 (3) (2020) 264–273. [DOI] [PubMed] [Google Scholar]
- [178].Cortright DN, Nicoletti A, Seasholtz AF, Molecular and biochemical characterization of the mouse brain corticotropin-releasing hormone-binding protein, Mol. Cell. Endocrinol 111 (2) (1995) 147–157. [DOI] [PubMed] [Google Scholar]
- [179].Behan DP, Linton EA, Lowry PJ, Isolation of the human plasma corticotrophin-releasing factor-binding protein, J. Endocrinol 122 (1) (1989) 23–31. [DOI] [PubMed] [Google Scholar]
- [180].Orth DN, Mount CD, Specific high-affinity binding protein for human corticotropin-releasing hormone in normal human plasma, Biochem. Biophys. Res. Commun 143 (2) (1987) 411–417. [DOI] [PubMed] [Google Scholar]
- [181].Potter E, et al. , Cloning and characterization of the cDNAs for human and rat corticotropin releasing factor-binding proteins, Nature 349 (6308) (1991) 423–426. [DOI] [PubMed] [Google Scholar]
- [182].Westphal NJ, Seasholtz AF, CRH-BP: the regulation and function of a phylogenetically conserved binding protein, Front. Biosci 11 (2006) 1878–1891. [DOI] [PubMed] [Google Scholar]
- [183].Potter E, et al. , The central distribution of a corticotropin-releasing factor (CRF)-binding protein predicts multiple sites and modes of interaction with CRF, Proc. Natl. Acad. Sci. U. S. A 89 (9) (1992) 4192–4196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Chan RK, Vale WW, Sawchenko PE, Paradoxical activational effects of a corticotropin-releasing factor-binding protein “ligand inhibitor” in rat brain, Neuroscience 101 (1) (2000) 115–129. [DOI] [PubMed] [Google Scholar]
- [185].Speert DB, SJ MC, Seasholtz AF, Sexually dimorphic expression of corticotropin-releasing hormone-binding protein in the mouse pituitary, Endocrinology 143 (12) (2002) 4730–4741. [DOI] [PubMed] [Google Scholar]
- [186].Lombardo KA, et al. , Effects of acute and repeated restraint stress on corticotropin-releasing hormone binding protein mRNA in rat amygdala and dorsal hippocampus, Neurosci. Lett 302 (2–3) (2001) 81–84. [DOI] [PubMed] [Google Scholar]
- [187].McClennen SJ, Cortright DN, Seasholtz AF, Regulation of pituitary corticotropin-releasing hormone-binding protein messenger ribonucleic acid levels by restraint stress and adrenalectomy, Endocrinology 139 (11) (1998) 4435–4441. [DOI] [PubMed] [Google Scholar]
- [188].Behan DP, et al. , Corticotropin-releasing factor (CRF), CRF-binding protein (CRF-BP), and CRF/CRF-BP complex in Alzheimer’s disease and control postmortem human brain, J. Neurochem 68 (5) (1997) 2053–2060. [DOI] [PubMed] [Google Scholar]
- [189].Karolyi IJ, et al. , Altered anxiety and weight gain in corticotropin-releasing hormone-binding protein-deficient mice, Proc. Natl. Acad. Sci. U. S. A 96 (20) (1999) 11595–11600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Milner TA, et al. , Ultrastructural localization and afferent sources of corticotropin-releasing factor in the rat rostral ventrolateral medulla: implications for central cardiovascular regulation, J. Comp. Neurol 333 (2) (1993) 151–167. [DOI] [PubMed] [Google Scholar]
- [191].Takuma K, et al. , Corticotropin-releasing factor stimulates Ca2+ influx in cultured rat astrocytes, Biochem. Biophys. Res. Commun 199 (3) (1994) 1103–1107. [DOI] [PubMed] [Google Scholar]
- [192].Ha BK, et al. , Corticotropin releasing factor induces proliferation of cerebellar astrocytes, J. Neurosci. Res 62 (6) (2000) 789–798. [DOI] [PubMed] [Google Scholar]
- [193].Hamke M, et al. , Substance P induces expression of the corticotropin-releasing factor receptor 1 by activation of the neurokinin-1 receptor, Brain Res 1102 (1) (2006) 135–144. [DOI] [PubMed] [Google Scholar]
- [194].Bishop GA, Seelandt CM, King JS, Cellular localization of corticotropin releasing factor receptors in the adult mouse cerebellum, Neuroscience 101 (4) (2000) 1083–1092. [DOI] [PubMed] [Google Scholar]
- [195].Sharpe AL, et al. , Repeated cocaine or methamphetamine treatment alters astrocytic CRF2 and GLAST expression in the ventral midbrain, Addict. Biol 27 (2) (2022) e13120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Lee KH, et al. , Cellular localization of the full-length isoform of the type 2 corticotropin releasing factor receptor in the postnatal mouse cerebellar cortex, J. Neurosci. Res 85 (9) (2007) 1996–2005. [DOI] [PubMed] [Google Scholar]
- [197].Behan DP, et al. , Corticotropin releasing factor binding protein (CRF-BP) is expressed in neuronal and astrocytic cells, Brain Res 698 (1–2) (1995) 259–264. [DOI] [PubMed] [Google Scholar]
- [198].Knackstedt LA, Melendez RI, Kalivas PW, Ceftriaxone restores glutamate homeostasis and prevents relapse to cocaine seeking, Biol. Psychiatry 67 (1) (2010) 81–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [199].Scofield MD, et al. , Gq-DREADD selectively initiates glial glutamate release and inhibits cue-induced cocaine seeking, Biol. Psychiatry 78 (7) (2015) 441–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Scofield MD, et al. , Cocaine self-administration and extinction leads to reduced glial fibrillary acidic protein expression and morphometric features of astrocytes in the nucleus accumbens core, Biol. Psychiatry 80 (3) (2016) 207–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Baker DA, et al. , Neuroadaptations in cystine-glutamate exchange underlie cocaine relapse, Nat. Neurosci 6 (7) (2003) 743–749. [DOI] [PubMed] [Google Scholar]
- [202].Kruyer A, et al. , Heroin cue-evoked astrocytic structural plasticity at nucleus accumbens synapses inhibits heroin seeking, Biol. Psychiatry 86 (11) (2019) 811–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [203].Fellin T, Pascual O, Haydon PG, Astrocytes coordinate synaptic networks: balanced excitation and inhibition, Physiology (Bethesda) 21 (2006) 208–215. [DOI] [PubMed] [Google Scholar]
- [204].Halassa MM, Fellin T, Haydon PG, Tripartite synapses: roles for astrocytic purines in the control of synaptic physiology and behavior, Neuropharmacology 57 (4) (2009) 343–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205].Halassa MM, Haydon PG, Integrated brain circuits: astrocytic networks modulate neuronal activity and behavior, Annu. Rev. Physiol 72 (2010) 335–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [206].Yao L, et al. , Adenosine A2a blockade prevents synergy between mu-opiate and cannabinoid CB1 receptors and eliminates heroin-seeking behavior in addicted rats, Proc. Natl. Acad. Sci. U. S. A 103 (20) (2006) 7877–7882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [207].Bachtell RK, Self DW, Effects of adenosine A2A receptor stimulation on cocaine-seeking behavior in rats, Psychopharmacology (Berl.) 206 (3) (2009) 469–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [208].O’Neill CE, LeTendre ML, Bachtell RK, Adenosine A2A receptors in the nucleus accumbens bi-directionally alter cocaine seeking in rats, Neuropsychopharmacology 37 (5) (2012) 1245–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [209].Wydra K, et al. , Effects of intra-accumbal or intra-prefrontal cortex microinjections of adenosine 2A receptor ligands on responses to cocaine reward and seeking in rats, Psychopharmacology (Berl.) 235 (12) (2018) 3509–3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [210].Koob GF, Neurobiology of Opioid Addiction: Opponent Process, Hyperkatifeia, and Negative Reinforcement, Biol. Psychiatry 87 (1) (2020) 44–53. [DOI] [PubMed] [Google Scholar]
- [211].Sarnyai Z, et al. , Brain corticotropin-releasing factor mediates ‘anxiety-like’ behavior induced by cocaine withdrawal in rats, Brain Res 675 (1–2) (1995) 89–97. [DOI] [PubMed] [Google Scholar]
- [212].Basso AM, et al. , Corticotropin-releasing factor antagonist attenuates the “anxiogenic-like” effect in the defensive burying paradigm but not in the elevated plus–maze following chronic cocaine in rats, Psychopharmacology (Berl.) 145 (1) (1999) 21–30. [DOI] [PubMed] [Google Scholar]
- [213].Stinus L, et al. , Buprenorphine and a CRF1 antagonist block the acquisition of opiate withdrawal-induced conditioned place aversion in rats, Neuropsychopharmacology 30 (1) (2005) 90–98. [DOI] [PubMed] [Google Scholar]
- [214].Navarro-Zaragoza J, et al. , Effects of corticotropin-releasing factor receptor-1 antagonists on the brain stress system responses to morphine withdrawal, Mol. Pharmacol 77 (5) (2010) 864–873. [DOI] [PubMed] [Google Scholar]
- [215].Edwards S, et al. , Development of mechanical hypersensitivity in rats during heroin and ethanol dependence: alleviation by CRF1 receptor antagonism, Neuropharmacology 62 (2) (2012) 1142–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [216].Baldwin HA, et al. , CRF antagonist reverses the “anxiogenic” response to ethanol withdrawal in the rat, Psychopharmacology (Berl.) 103 (2) (1991) 227–232. [DOI] [PubMed] [Google Scholar]
- [217].Rassnick S, et al. , Microinjection of a corticotropin-releasing factor antagonist into the central nucleus of the amygdala reverses anxiogenic-like effects of ethanol withdrawal, Brain Res 605 (1) (1993) 25–32. [DOI] [PubMed] [Google Scholar]
- [218].Valdez GR, et al. , Increased ethanol self-administration and anxiety-like behavior during acute ethanol withdrawal and protracted abstinence: regulation by corticotropin-releasing factor, Alcohol Clin. Exp. Res 26 (10) (2002) 1494–1501. [DOI] [PubMed] [Google Scholar]
- [219].Overstreet DH, Knapp DJ, Breese GR, Modulation of multiple ethanol withdrawal-induced anxiety-like behavior by CRF and CRF1 receptors, Pharmacol. Biochem. Behav 77 (2) (2004) 405–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [220].Bruijnzeel AW, et al. , Antagonism of CRF receptors prevents the deficit in brain reward function associated with precipitated nicotine withdrawal in rats, Neuropsychopharmacology 32 (4) (2007) 955–963. [DOI] [PubMed] [Google Scholar]
- [221].George O, et al. , CRF-CRF1 system activation mediates withdrawal-induced increases in nicotine self-administration in nicotine-dependent rats, Proc. Natl. Acad. Sci. U. S. A 104 (43) (2007) 17198–17203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [222].Rodríguez de Fonseca F, et al. , Activation of corticotropin-releasing factor in the limbic system during cannabinoid withdrawal, Science 276 (5321) (1997) 2050–2054. [DOI] [PubMed] [Google Scholar]
- [223].Skelton KH, et al. , The CRF1 receptor antagonist R121919 attenuates the neuroendocrine and behavioral effects of precipitated lorazepam withdrawal, Psychopharmacology (Berl.) 192 (3) (2007) 385–396. [DOI] [PubMed] [Google Scholar]
- [224].Merlo Pich E, et al. , Increase of extracellular corticotropin-releasing factor-like immunoreactivity levels in the amygdala of awake rats during restraint stress and ethanol withdrawal as measured by microdialysis, J. Neurosci 15 (8) (1995) 5439–5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [225].Richter RM, et al. , Sensitization of cocaine-stimulated increase in extracellular levels of corticotropin-releasing factor from the rat amygdala after repeated administration as determined by intracranial microdialysis, Neurosci. Lett 187 (3) (1995) 169–172. [DOI] [PubMed] [Google Scholar]
- [226].Zhou Y, et al. , Corticotropin-releasing factor and type 1 corticotropin-releasing factor receptor messenger RNAs in rat brain and pituitary during “binge”-pattern cocaine administration and chronic withdrawal, J. Pharmacol. Exp. Ther 279 (1) (1996) 351–358. [PubMed] [Google Scholar]
- [227].Ambrosio E, Sharpe LG, Pilotte NS, Regional binding to corticotropin releasing factor receptors in brain of rats exposed to chronic cocaine and cocaine withdrawal, Synapse 25 (3) (1997) 272–276. [DOI] [PubMed] [Google Scholar]
- [228].Zorrilla EP, Valdez GR, Weiss F, Changes in levels of regional CRF-like-immunoreactivity and plasma corticosterone during protracted drug withdrawal in dependent rats, Psychopharmacology (Berl.) 158 (4) (2001) 374–381. [DOI] [PubMed] [Google Scholar]
- [229].Fu Y, et al. , Long-term potentiation (LTP) in the central amygdala (CeA) is enhanced after prolonged withdrawal from chronic cocaine and requires CRF1 receptors, J. Neurophysiol 97 (1) (2007) 937–941. [DOI] [PubMed] [Google Scholar]
- [230].Orozco-Cabal L, et al. , Dopamine and corticotropin-releasing factor synergistically alter basolateral amygdala-to-medial prefrontal cortex synaptic transmission: functional switch after chronic cocaine administration, J. Neurosci 28 (2) (2008) 529–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [231].Lowery-Gionta EG, et al. , Corticotropin releasing factor signaling in the central amygdala is recruited during binge-like ethanol consumption in C57BL/6J mice, J. Neurosci 32 (10) (2012) 3405–3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [232].Agoglia AE, et al. , Corticotropin-releasing factor receptor-1 neurons in the lateral amygdala display selective sensitivity to acute and chronic ethanol exposure, eNeuro 7 (2) (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [233].Guan X, et al. , Cocaine withdrawal enhances long-term potentiation in rat hippocampus via changing the activity of corticotropin-releasing factor receptor subtype 2, Neuroscience 161 (3) (2009) 665–670. [DOI] [PubMed] [Google Scholar]
- [234].Olive MF, et al. , Elevated extracellular CRF levels in the bed nucleus of the stria terminalis during ethanol withdrawal and reduction by subsequent ethanol intake, Pharmacol. Biochem. Behav 72 (1–2) (2002) 213–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [235].Francesconi W, et al. , Protracted withdrawal from alcohol and drugs of abuse impairs long-term potentiation of intrinsic excitability in the juxtacapsular bed nucleus of the stria terminalis, J. Neurosci 29 (17) (2009) 5389–5401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [236].Snyder AE, et al. , Chronic intermittent ethanol and acute stress similarly modulate BNST CRF neuron activity via noradrenergic signaling, Alcohol Clin. Exp. Res 43 (8) (2019) 1695–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [237].Liu J, et al. , Chronic cocaine administration switches corticotropin-releasing factor2 receptor-mediated depression to facilitation of glutamatergic transmission in the lateral septum, J. Neurosci 25 (3) (2005) 577–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [238].George O, et al. , Recruitment of medial prefrontal cortex neurons during alcohol withdrawal predicts cognitive impairment and excessive alcohol drinking, Proc. Natl. Acad. Sci. U. S. A 109 (44) (2012) 18156–18161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [239].Hansson AC, et al. , Region-specific down-regulation of Crhr1 gene expression in alcohol-preferring msP rats following ad lib access to alcohol, Addict. Biol 12 (1) (2007) 30–34. [DOI] [PubMed] [Google Scholar]
- [240].Torres OV, et al. , Behavioral, Biochemical, and Molecular Indices of Stress are Enhanced in Female Versus Male Rats Experiencing Nicotine Withdrawal, Front. Psychiatry 4 (2013) 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [241].Zorrilla EP, et al. , Extended access cocaine self-administration differentially activates dorsal raphe and amygdala corticotropin-releasing factor systems in rats, Addict. Biol 17 (2) (2012) 300–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [242].Lunden JW, Kirby LG, Opiate exposure and withdrawal dynamically regulate mRNA expression in the serotonergic dorsal raphe nucleus, Neuroscience 254 (2013) 160–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [243].Staub DR, et al. , Morphine history sensitizes postsynaptic GABA receptors on dorsal raphe serotonin neurons in a stress-induced relapse model in rats, Psychoneuroendocrinology 37 (6) (2012) 859–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [244].Valentino RJ, Van Bockstaele E, Opposing regulation of the locus coeruleus by corticotropin-releasing factor and opioids. Potential for reciprocal interactions between stress and opioid sensitivity, Psychopharmacology (Berl.) 158 (4) (2001) 331–342. [DOI] [PubMed] [Google Scholar]
- [245].Xu GP, et al. , Chronic morphine sensitizes the brain norepinephrine system to corticotropin-releasing factor and stress, J. Neurosci 24 (38) (2004) 8193–8197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [246].Sparta DR, et al. , Binge ethanol-drinking potentiates corticotropin releasing factor R1 receptor activity in the ventral tegmental area, Alcohol Clin. Exp. Res 37 (10) (2013) 1680–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [247].Rinker JA, et al. , Extended amygdala to ventral tegmental area corticotropin-releasing factor circuit controls binge ethanol intake, Biol. Psychiatry 81 (11) (2017) 930–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [248].Harlan BA, et al. , Opposing actions of CRF-R1 and CB1 receptors on VTA-GABAergic plasticity following chronic exposure to ethanol, Neuropsychopharmacology 43 (10) (2018) 2064–2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [249].Bryce CA, Floresco SB, Perturbations in effort-related decision-making driven by acute stress and corticotropin-releasing factor, Neuropsychopharmacology 41 (8) (2016) 2147–2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [250].Tovar-Díaz J, et al. , Cooperative CRF and α1 adrenergic signaling in the VTA promotes NMDA plasticity and drives social stress enhancement of cocaine conditioning, Cell Rep 22 (10) (2018) 2756–2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [251].Bangasser DA, Kawasumi Y, Cognitive disruptions in stress-related psychiatric disorders: a role for corticotropin releasing factor (CRF), Horm. Behav 76 (2015) 125–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [252].O’Dell LE, et al. , Enhanced alcohol self-administration after intermittent versus continuous alcohol vapor exposure, Alcohol Clin. Exp. Res 28 (11) (2004) 1676–1682. [DOI] [PubMed] [Google Scholar]
- [253].Ahmed SH, Koob GF, Transition from moderate to excessive drug intake: change in hedonic set point, Science 282 (5387) (1998) 298–300. [DOI] [PubMed] [Google Scholar]
- [254].Deroche V, Le Moal M, Piazza PV, Cocaine self-administration increases the incentive motivational properties of the drug in rats, Eur. J. Neurosci 11 (8) (1999) 2731–2736. [DOI] [PubMed] [Google Scholar]
- [255].Ahmed SH, Walker JR, Koob GF, Persistent increase in the motivation to take heroin in rats with a history of drug escalation, Neuropsychopharmacology 22 (4) (2000) 413–421. [DOI] [PubMed] [Google Scholar]
- [256].Kitamura O, et al. , Escalation of methamphetamine self-administration in rats: a dose-effect function, Psychopharmacology (Berl.) 186 (1) (2006) 48–53. [DOI] [PubMed] [Google Scholar]
- [257].Cohen A, Koob GF, George O, Robust escalation of nicotine intake with extended access to nicotine self-administration and intermittent periods of abstinence, Neuropsychopharmacology 37 (9) (2012) 2153–2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [258].Specio SE, et al. , CRF(1) receptor antagonists attenuate escalated cocaine self-administration in rats, Psychopharmacology (Berl.) 196 (3) (2008) 473–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [259].Funk CK, et al. , Corticotropin-releasing factor 1 antagonists selectively reduce ethanol self-administration in ethanol-dependent rats, Biol. Psychiatry 61 (1) (2007) 78–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [260].Finn DA, et al. , Increased drinking during withdrawal from intermittent ethanol exposure is blocked by the CRF receptor antagonist D-Phe-CRF(12–41), Alcohol Clin. Exp. Res 31 (6) (2007) 939–949. [DOI] [PubMed] [Google Scholar]
- [261].Gehlert DR, et al. , 3-(4-Chloro-2-morpholin-4-yl-thiazol-5-yl)-8-(1-ethylpropyl)-2,6-dimethyl-imidazo[1,2-b]pyridazine: a novel brain-penetrant, orally available corticotropin-releasing factor receptor 1 antagonist with efficacy in animal models of alcoholism, J. Neurosci 27 (10) (2007) 2718–2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [262].Park PE, et al. , Chronic CRF1 receptor blockade reduces heroin intake escalation and dependence-induced hyperalgesia, Addict. Biol 20 (2) (2015) 275–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [263].Fox HC, et al. , Frequency of recent cocaine and alcohol use affects drug craving and associated responses to stress and drug-related cues, Psychoneuroendocrinology 30 (9) (2005) 880–891. [DOI] [PubMed] [Google Scholar]
- [264].Panlilio LV, et al. , Beyond abstinence and relapse II: momentary relationships between stress, craving, and lapse within clusters of patients with similar patterns of drug use, Psychopharmacology (Berl.) 238 (6) (2021) 1513–1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [265].Brady KT, et al. , Response to corticotropin-releasing hormone infusion in cocaine-dependent individuals, Arch. Gen. Psychiatry 66 (4) (2009) 422–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [266].Hwa LS, Debold JF, Miczek KA, Alcohol in excess: CRF1 receptors in the rat and mouse VTA and DRN, Psychopharmacology (Berl.) 225 (2) (2013) 313–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [267].Hwa LS, et al. , Social stress-escalated intermittent alcohol drinking: modulation by CRF-R1 in the ventral tegmental area and accumbal dopamine in mice, Psychopharmacology (Berl.) 233 (4) (2016) 681–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [268].Newman EL, et al. , Persistent escalation of alcohol consumption by mice exposed to brief episodes of social defeat stress: suppression by CRF-R1 antagonism, Psychopharmacology (Berl.) 235 (6) (2018) 1807–1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [269].Boyson CO, et al. , Prevention of social stress-escalated cocaine self-administration by CRF-R1 antagonist in the rat VTA, Psychopharmacology (Berl.) 218 (1) (2011) 257–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [270].Boyson CO, et al. , Social stress and CRF-dopamine interactions in the VTA: role in long-term escalation of cocaine self-administration, J. Neurosci 34 (19) (2014) 6659–6667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [271].Holly EN, et al. , Episodic social stress-escalated cocaine self-administration: role of phasic and tonic corticotropin releasing factor in the anterior and posterior ventral tegmental area, J. Neurosci 36 (14) (2016) 4093–4105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [272].Han X, DeBold JF, Miczek KA, Prevention and reversal of social stress-escalated cocaine self-administration in mice by intra-VTA CRFR1 antagonism, Psychopharmacology (Berl.) 234 (18) (2017) 2813–2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [273].Mantsch JR, Katz ES, Elevation of glucocorticoids is necessary but not sufficient for the escalation of cocaine self-administration by chronic electric footshock stress in rats, Neuropsychopharmacology 32 (2) (2007) 367–376. [DOI] [PubMed] [Google Scholar]
- [274].Galici R, et al. , Comparison of noncontingent versus contingent cocaine administration on plasma corticosterone levels in rats, Eur. J. Pharmacol 387 (1) (2000) 59–62. [DOI] [PubMed] [Google Scholar]
- [275].Mantsch JR, et al. , Effects of cocaine self-administration on plasma corticosterone and prolactin in rats, J. Pharmacol. Exp. Ther 294 (1) (2000) 239–247. [PubMed] [Google Scholar]
- [276].Palamarchouk V, Smagin G, Goeders NE, Self-administered and passive cocaine infusions produce different effects on corticosterone concentrations in the medial prefrontal cortex (MPC) of rats, Pharmacol. Biochem. Behav 94 (1) (2009) 163–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [277].Mantsch JR, et al. , Neuroendocrine alterations in a high-dose, extended-access rat self-administration model of escalating cocaine use, Psychoneuroendocrinology 28 (7) (2003) 836–862. [DOI] [PubMed] [Google Scholar]
- [278].Schulkin J, Gold PW, McEwen BS, Induction of corticotropin-releasing hormone gene expression by glucocorticoids: implication for understanding the states of fear and anxiety and allostatic load, Psychoneuroendocrinology 23 (3) (1998) 219–243. [DOI] [PubMed] [Google Scholar]
- [279].Mantsch JR, et al. , Surgical adrenalectomy with diurnal corticosterone replacement slows escalation and prevents the augmentation of cocaine-induced reinstatement in rats self-administering cocaine under long-access conditions, Neuropsychopharmacology 33 (4) (2008) 814–826. [DOI] [PubMed] [Google Scholar]
- [280].Miyashita T, Williams CL, Epinephrine administration increases neural impulses propagated along the vagus nerve: role of peripheral beta-adrenergic receptors, Neurobiol. Learn. Mem 85 (2) (2006) 116–124. [DOI] [PubMed] [Google Scholar]
- [281].de Jong IE, Steenbergen PJ, de Kloet ER, Behavioral sensitization to cocaine: cooperation between glucocorticoids and epinephrine, Psychopharmacology (Berl.) 204 (4) (2009) 693–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [282].Leza L, et al. , Adverse childhood experiences (ACEs) and substance use disorder (SUD): a scoping review, Drug Alcohol Depend 221 (2021) 108563. [DOI] [PubMed] [Google Scholar]
- [283].Blomeyer D, et al. , Interaction between CRHR1 gene and stressful life events predicts adolescent heavy alcohol use, Biol. Psychiatry 63 (2) (2008) 146–151. [DOI] [PubMed] [Google Scholar]
- [284].Levis SC, Baram TZ, Mahler SV, Neurodevelopmental origins of substance use disorders: evidence from animal models of early-life adversity and addiction, Eur. J. Neurosci (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [285].Noschang C, et al. , Social isolation at adolescence: a systematic review on behaviour related to cocaine, amphetamine and nicotine use in rats and mice, Psychopharmacology (Berl.) 238 (4) (2021) 927–947. [DOI] [PubMed] [Google Scholar]
- [286].Forster GL, et al. , Negative consequences of early-life adversity on substance use as mediated by corticotropin-releasing factor modulation of serotonin activity, Neurobiol. Stress 9 (2018) 29–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [287].Bardo MT, Hammerslag LR, Malone SG, Effect of early life social adversity on drug abuse vulnerability: focus on corticotropin-releasing factor and oxytocin, Neuropharmacology 191 (2021) 108567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [288].Burke AR, DeBold JF, Miczek KA, CRF type 1 receptor antagonism in ventral tegmental area of adolescent rats during social defeat: prevention of escalated cocaine self-administration in adulthood and behavioral adaptations during adolescence, Psychopharmacology (Berl.) 233 (14) (2016) 2727–2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [289].Nader J, et al. , Loss of environmental enrichment increases vulnerability to cocaine addiction, Neuropsychopharmacology 37 (7) (2012) 1579–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [290].Bolton JL, et al. , Anhedonia following early-life adversity involves aberrant interaction of reward and anxiety circuits and is reversed by partial silencing of amygdala corticotropin-releasing hormone gene, Biol. Psychiatry 83 (2) (2018) 137–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [291].Birnie MT, et al. , Plasticity of the reward circuitry after early-life adversity: mechanisms and significance, Biol. Psychiatry 87 (10) (2020) 875–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [292].Boutros N, et al. , Risky choice and brain CRF after adolescent ethanol vapor exposure and social stress in adulthood, Behav. Brain Res 311 (2016) 160–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [293].Boutros N, et al. , Effects of adolescent alcohol exposure on stress-induced reward deficits, brain CRF, monoamines and glutamate in adult rats, Psychopharmacology (Berl.) 235 (3) (2018) 737–747. [DOI] [PubMed] [Google Scholar]
- [294].Gilpin NW, Karanikas CA, Richardson HN, Adolescent binge drinking leads to changes in alcohol drinking, anxiety, and amygdalar corticotropin releasing factor cells in adulthood in male rats, PLoS One 7 (2) (2012) e31466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [295].Karanikas CA, Lu YL, Richardson HN, Adolescent drinking targets corticotropin-releasing factor peptide-labeled cells in the central amygdala of male and female rats, Neuroscience 249 (2013) 98–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [296].Wellman CL, et al. , Sex differences in risk and resilience: stress effects on the neural substrates of emotion and motivation, J. Neurosci 38 (44) (2018) 9423–9432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [297].Bangasser DA, Eck SR, Ordoñes Sanchez E, Sex differences in stress reactivity in arousal and attention systems, Neuropsychopharmacology 44 (1) (2019) 129–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [298].Hodes GE, Epperson CN, Sex differences in vulnerability and resilience to stress across the life span, Biol. Psychiatry 86 (6) (2019) 421–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [299].Rincón-Cortés M, et al. , Stress: Influence of sex, reproductive status and gender, Neurobiol. Stress 10 (2019) 100155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [300].Back SE, et al. , Gender differences in stress reactivity among cocaine-dependent individuals, Psychopharmacology (Berl.) 180 (1) (2005) 169–176. [DOI] [PubMed] [Google Scholar]
- [301].Li CS, Kosten TR, Sinha R, Sex differences in brain activation during stress imagery in abstinent cocaine users: a functional magnetic resonance imaging study, Biol. Psychiatry 57 (5) (2005) 487–494. [DOI] [PubMed] [Google Scholar]
- [302].Waldrop AE, et al. , Community-dwelling cocaine-dependent men and women respond differently to social stressors versus cocaine cues, Psychoneuroendocrinology 35 (6) (2010) 798–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [303].Feltenstein MW, Henderson AR, See RE, Enhancement of cue-induced reinstatement of cocaine-seeking in rats by yohimbine: sex differences and the role of the estrous cycle, Psychopharmacology (Berl.) 216 (1) (2011) 53–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [304].Potenza MN, et al. , Neural correlates of stress-induced and cue-induced drug craving: influences of sex and cocaine dependence, Am. J. Psychiatry 169 (4) (2012) 406–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [305].Doncheck EM, et al. , Sex, stress, and prefrontal cortex: influence of biological sex on stress-promoted cocaine seeking, Neuropsychopharmacology 45 (12) (2020) 1974–1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [306].Fox HC, Morgan PT, Sinha R, Sex differences in guanfacine effects on drug craving and stress arousal in cocaine-dependent individuals, Neuropsychopharmacology 39 (6) (2014) 1527–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [307].Martin EL, et al. , Consideration of sex as a biological variable in the translation of pharmacotherapy for stress-associated drug seeking, Neurobiol. Stress 15 (2021) 100364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [308].Curtis AL, Bethea T, Valentino RJ, Sexually dimorphic responses of the brain norepinephrine system to stress and corticotropin-releasing factor, Neuropsychopharmacology 31 (3) (2006) 544–554. [DOI] [PubMed] [Google Scholar]
- [309].Bangasser DA, et al. , Sex differences in corticotropin-releasing factor receptor signaling and trafficking: potential role in female vulnerability to stress-related psychopathology, Mol. Psychiatry 15 (9) (2010) 877 896–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [310].Valentino RJ, Van Bockstaele E, Bangasser D, Sex-specific cell signaling: the corticotropin-releasing factor receptor model, Trends Pharmacol. Sci 34 (8) (2013) 437–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [311].Agoglia AE, Tella J, Herman MA, Sex differences in corticotropin releasing factor peptide regulation of inhibitory control and excitability in central amygdala corticotropin releasing factor receptor 1-neurons, Neuropharmacology 180 (2020) 108296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [312].Wiersielis KR, et al. , Sex differences in corticotropin releasing factor-evoked behavior and activated networks, Psychoneuroendocrinology 73 (2016) 204–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [313].Salvatore M, et al. , Sex differences in circuits activated by corticotropin releasing factor in rats, Horm. Behav 97 (2018) 145–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [314].Agoglia AE, et al. , Sex-specific plasticity in CRF regulation of inhibitory control in central amygdala CRF1 neurons after chronic voluntary alcohol drinking, Addict. Biol 27 (1) (2022) e13067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [315].Treutlein J, et al. , Genetic association of the human corticotropin releasing hormone receptor 1 (CRHR1) with binge drinking and alcohol intake patterns in two independent samples, Mol. Psychiatry 11 (6) (2006) 594–602. [DOI] [PubMed] [Google Scholar]
- [316].Gelernter J, et al. , Genome-wide association study of maximum habitual alcohol intake in > 140,000 U.S. European and African American veterans yields novel risk loci, Biol. Psychiatry 86 (5) (2019) 365–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [317].Schmid B, et al. , Interacting effects of CRHR1 gene and stressful life events on drinking initiation and progression among 19-year-olds, Int. J. Neuropsychopharmacol 13 (6) (2010) 703–714. [DOI] [PubMed] [Google Scholar]
- [318].Tang X, et al. , Ethnic-specific genetic association of variants in the corticotropin-releasing hormone receptor 1 gene with nicotine dependence, Biomed. Res. Int (2015) 263864 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [319].Levran O, et al. , Stress-related genes and heroin addiction: a role for a functional FKBP5 haplotype, Psychoneuroendocrinology 45 (2014) 67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [320].Levran O, et al. , A non-coding CRHR2 SNP rs255105, a cis-eQTL for a downstream lincRNA AC005154.6, is associated with heroin addiction, PLoS One 13 (6) (2018) e0199951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [321].Levran O, et al. , Genetic variations in genes of the stress response pathway are associated with prolonged abstinence from heroin, Pharmacogenomics 19 (4) (2018) 333–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [322].Levran O, et al. , Drug addiction and stress-response genetic variability: association study in African Americans, Ann. Hum. Genet 78 (4) (2014) 290–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [323].Peles E, et al. , Genetic variant in the CRH-binding protein gene (CRHBP) is associated with cessation of cocaine use in methadone maintenance patients with opioid addiction, J. Addict. Med 13 (6) (2019) 430–435. [DOI] [PubMed] [Google Scholar]
- [324].Nelson EC, et al. , H2 haplotype at chromosome 17q21.31 protects against childhood sexual abuse-associated risk for alcohol consumption and dependence, Addict. Biol 15 (1) (2010) 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [325].Ray LA, et al. , The CRHR1 gene, trauma exposure, and alcoholism risk: a test of G × E effects, Genes Brain Behav 12 (4) (2013) 361–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [326].Ray LA, Stress-induced and cue-induced craving for alcohol in heavy drinkers: preliminary evidence of genetic moderation by the OPRM1 and CRH-BP genes, Alcohol Clin. Exp. Res 35 (1) (2011) 166–174. [DOI] [PubMed] [Google Scholar]
- [327].Su H, Wang Z, Zhao M, Association between stress pathway gene (CRHR1\CRHBP) polymorphisms and heroin dependence, J. Clin. Neurosci 54 (2018) 33–38. [DOI] [PubMed] [Google Scholar]
- [328].Glaser YG, et al. , Indirect effect of corticotropin-releasing hormone receptor 1 gene variation on negative emotionality and alcohol use via right ventrolateral prefrontal cortex, J. Neurosci 34 (11) (2014) 4099–4107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [329].Enoch MA, et al. , Common genetic origins for EEG, alcoholism and anxiety: the role of CRH-BP, PLoS One 3 (10) (2008) e3620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [330].Bell RL, et al. , Rat animal models for screening medications to treat alcohol use disorders, Neuropharmacology 122 (2017) 201–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [331].Ciccocioppo R, et al. , Genetically selected Marchigian Sardinian alcohol-preferring (msP) rats: an animal model to study the neurobiology of alcoholism, Addict. Biol 11 (3–4) (2006) 339–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [332].Bell RL, et al. , The alcohol-preferring P rat and animal models of excessive alcohol drinking, Addict. Biol 11 (3–4) (2006) 270–288. [DOI] [PubMed] [Google Scholar]
- [333].Colombo G, et al. , Sardinian alcohol-preferring rats: a genetic animal model of anxiety, Physiol. Behav 57 (6) (1995) 1181–1185. [DOI] [PubMed] [Google Scholar]
- [334].Cippitelli A, et al. , Polymorphism in the corticotropin-releasing factor receptor 1 (CRF1-R) gene plays a role in shaping the high anxious phenotype of Marchigian Sardinian alcohol-preferring (msP) rats, Psychopharmacology (Berl.) 232 (6) (2015) 1083–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [335].Hansson AC, et al. , Variation at the rat Crhr1 locus and sensitivity to relapse into alcohol seeking induced by environmental stress, Proc. Natl. Acad. Sci. U. S. A 103 (41) (2006) 15236–15241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [336].Ayanwuyi LO, et al. , Role of a genetic polymorphism in the corticotropin-releasing factor receptor 1 gene in alcohol drinking and seeking behaviors of marchigian sardinian alcohol-preferring rats, Front. Psychiatry 4 (2013) 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [337].Herman MA, et al. , Enhanced GABAergic transmission in the central nucleus of the amygdala of genetically selected Marchigian Sardinian rats: alcohol and CRF effects, Neuropharmacology 67 (2013) 337–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [338].Herman MA, et al. , Glutamatergic transmission in the central nucleus of the amygdala is selectively altered in Marchigian Sardinian alcohol-preferring rats: Alcohol and CRF effects, Neuropharmacology 102 (2016) 21–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [339].Sabino V, et al. , Pharmacological characterization of the 20% alcohol intermittent access model in Sardinian alcohol-preferring rats: a model of binge-like drinking, Alcohol Clin. Exp. Res 37 (4) (2013) 635–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [340].Sabino V, et al. , Dissociation between opioid and CRF1 antagonist sensitive drinking in Sardinian alcohol-preferring rats, Psychopharmacology (Berl.) 189 (2) (2006) 175–186. [DOI] [PubMed] [Google Scholar]
- [341].Logrip ML, et al. , Evaluation of alcohol preference and drinking in msP rats bearing a Crhr1 promoter polymorphism, Front. Psychiatry 9 (2018) 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [342].Gilpin NW, Richardson HN, Koob GF, Effects of CRF1-receptor and opioid-receptor antagonists on dependence-induced increases in alcohol drinking by alcohol-preferring (P) rats, Alcohol Clin. Exp. Res 32 (9) (2008) 1535–1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [343].George SR, et al. , Corticotropin-releasing factor is altered in brains of animals with high preference for ethanol, Alcohol Clin. Exp. Res 14 (3) (1990) 425–429. [DOI] [PubMed] [Google Scholar]
- [344].Ehlers CL, et al. , Corticotropin releasing factor (CRF): studies in alcohol preferring and non-preferring rats, Psychopharmacology (Berl.) 106 (3) (1992) 359–364. [DOI] [PubMed] [Google Scholar]
- [345].Knapp DJ, et al. , Effects of a stressor and corticotrophin releasing factor on ethanol deprivation-induced ethanol intake and anxiety-like behavior in alcohol-preferring P rats, Psychopharmacology (Berl.) 218 (1) (2011) 179–189. [DOI] [PubMed] [Google Scholar]
- [346].Shaham Y, de Wit H, Lost in translation: CRF1 receptor antagonists and addiction treatment, Neuropsychopharmacology 41 (12) (2016) 2795–2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [347].Kwako LE, et al. , The corticotropin releasing hormone-1 (CRH1) receptor antagonist pexacerfont in alcohol dependence: a randomized controlled experimental medicine study, Neuropsychopharmacology 40 (5) (2015) 1053–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [348].Schwandt ML, et al. , The CRF1 antagonist verucerfont in anxious alcohol-dependent women: translation of neuroendocrine, but not of anti-craving effects, Neuropsychopharmacology 41 (12) (2016) 2818–2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [349].Coric V, et al. , Multicenter, randomized, double-blind, active comparator and placebo-controlled trial of a corticotropin-releasing factor receptor-1 antagonist in generalized anxiety disorder, Depress. Anxiety 27 (5) (2010) 417–425. [DOI] [PubMed] [Google Scholar]
- [350].Binneman B, et al. , A 6-week randomized, placebo-controlled trial of CP-316,311 (a selective CRH1 antagonist) in the treatment of major depression, Am. J. Psychiatry 165 (5) (2008) 617–620. [DOI] [PubMed] [Google Scholar]
- [351].Dunlop BW, et al. , Corticotropin-releasing factor receptor 1 antagonism is ineffective for women with posttraumatic stress disorder, Biol. Psychiatry 82 (12) (2017) 866–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [352].Caccamise A, Van Newenhizen E, Mantsch JR, Neurochemical mechanisms and neurocircuitry underlying the contribution of stress to cocaine seeking, J. Neurochem 157 (5) (2021) 1697–1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [353].Kuhn BN, Kalivas PW, Bobadilla AC, Understanding addiction using animal models, Front. Behav. Neurosci 13 (2019) 262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [354].Venniro M, et al. , Improving translation of animal models of addiction and relapse by reverse translation, Nat. Rev. Neurosci 21 (11) (2020) 625–643. [DOI] [PubMed] [Google Scholar]
- [355].Epstein DH, et al. , Toward a model of drug relapse: an assessment of the validity of the reinstatement procedure, Psychopharmacology (Berl.) 189 (1) (2006) 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [356].Fredriksson I, et al. , Animal models of drug relapse and craving after voluntary abstinence: a review, Pharmacol. Rev 73 (3) (2021) 1050–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [357].Venniro M, Caprioli D, Shaham Y, Novel models of drug relapse and craving after voluntary abstinence, Neuropsychopharmacology 44 (1) (2019) 234–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [358].Bissette G, et al. , Elevated concentrations of CRF in the locus coeruleus of depressed subjects, Neuropsychopharmacology 28 (7) (2003) 1328–1335. [DOI] [PubMed] [Google Scholar]
- [359].Wang SS, et al. , Gene expression analysis in the human hypothalamus in depression by laser microdissection and real-time PCR: the presence of multiple receptor imbalances, Mol. Psychiatry 13 (8) (2008) 786–799 741. [DOI] [PubMed] [Google Scholar]
- [360].Pandey GN, et al. , Increased protein and mRNA expression of corticotropin-releasing factor (CRF), decreased CRF receptors and CRF binding protein in specific postmortem brain areas of teenage suicide subjects, Psychoneuroendocrinology 106 (2019) 233–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [361].Funk D, Coen K, Lê AD, The role of kappa opioid receptors in stress-induced reinstatement of alcohol seeking in rats, Brain Behav 4 (3) (2014) 356–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [362].Plaza-Zabala A, et al. , Hypocretins regulate the anxiogenic-like effects of nicotine and induce reinstatement of nicotine-seeking behavior, J. Neurosci 30 (6) (2010) 2300–2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data were used for the research described in the article.


