Abstract
Internalization of Listeria monocytogenes into human brain microvascular endothelial cells (HBMEC) has recently been demonstrated to be dependent upon the inlB gene. In the present scanning electron microscopic study we show that L. monocytogenes efficiently interacts with the surface of HBMEC in an inlB-independent manner which is also different from invasion. The inlB-dependent invasion of HBMEC by L. monocytogenes is accompanied by intracellular multiplication, movement, and production of bacterium-containing protrusions. These protrusions extend from the cell surface without perturbation of any adjacent cellular membrane.
Interaction of meningeal pathogens with endothelial cells has been demonstrated for a growing number of bacteria in recent years, including Escherichia coli, group B streptococci, and Citrobacter spp. (1, 14, 16), which use brain endothelial cell invasion as a prerequisite for central nervous system (CNS) penetration leading to meningitis and encephalitis. Listeria monocytogenes, a gram-positive facultative intracellular bacterium, is also known to cause meningitis, encephalitis, and brain abscesses, mainly in immunocompromised individuals (4, 18). CNS penetration of L. monocytogenes suggests that invasion of brain microvascular endothelial cells is an important way of crossing the blood-brain barrier. Recent data have shown that L. monocytogenes is indeed able to invade and replicate within different types of human endothelial cells (3, 7, 8, 15, 21). We have previously shown that invasion of human brain microvascular cells (HBMEC) by L. monocytogenes is strictly dependent on the presence of the inlB gene (7). The gene product of the inlB gene encodes InlB, a 630-amino-acid surface protein of the internalin family of leucine-rich repeat proteins in L. monocytogenes (5, 12). Besides triggering HBMEC (7) and human umbilical vein endothelial cell (15) invasion, InlB was shown to be involved in uptake by other cell types, including hepatocytes (2) and Vero cells (9). The receptor for InlB is not known, but it was shown that it triggers phosphatidylinositol 3-kinase activation during invasion of Vero cells (9). Once inside the HBMEC, L. monocytogenes passes through the intracellular life cycle as described for epithelial and macrophage-like cells (11).
In the present study we examined by scanning electron microscopy (SEM) the interaction of L. monocytogenes with HBMEC. We demonstrate that (i) inlB is not required for initial association with HBMEC, (ii) L. innocua also adheres to HBMEC, (iii) L. monocytogenes adheres to HBMEC through microvilli, and (iv) adhesion is a separate phenomenon from invasion. Furthermore, the very efficient formation of bacterium-containing protrusions on the surface of infected HBMEC is documented following invasion.
MATERIALS AND METHODS
The wild-type L. monocytogenes EGD strain, the L. monocytogenes inlB in-frame deletion mutant WL-111, and the L. innocua Sv6a strain have been described recently (7, 8). The bacteria were cultured aerobically in brain heart infusion broth (BHI) (Difco) at 37°C until they reached the mid-log phase of growth. They were then washed twice with phosphate-buffered saline (PBS) and stored in aliquots in PBS with 20% (vol/vol) glycerol at −80°C until being used for the infection experiments. HBMEC were isolated from a brain biopsy of an adult female with epilepsy and cultured by methods previously described (19). HBMEC were subsequently immortalized by transfection with simian virus 40 large-T antigen and maintained their morphologic and functional characteristics for at least 30 passages (20). HBMEC were cultured in gelatin-coated flasks without the addition of antibiotics in RPMI 1640 medium (Gibco), supplemented with fetal calf serum (10%) (Gibco), NuSerum IV (10%) (Becton Dickinson, Bedford, Mass.), nonessential amino acids (1%), vitamins (1%), heparin (5 U/ml), sodium pyruvate (1 mM), l-glutamine (2 mM), and endothelial cell growth supplement (30 mg/ml) (all from Sigma) (these compounds form the complete HBMEC medium) and were incubated at 37°C in a humid atmosphere of 5% CO2. At 48 h prior to infection, HBMEC were split, and the cells seeded into gelatin-treated 24-well tissue culture plates at a density of 105 cells per well with or without cover slides. Immediately prior to the assay each well was found to contain approximately 4 × 105 cells per well. The bacteria were diluted in RPMI 1640 medium, and 500 μl of the suspension was added to each monolayer to reach the desired multiplicity of infection. For live cell counts, the cultures were incubated at 37°C to allow the bacteria to associate with the cells, and then the cells were washed four times with PBS. To measure initial association, the washed cells were lysed and appropriate dilutions were plated on BHI agar. To measure invasion, 1 ml of complete HBMEC medium containing gentamicin (100 μg/ml; Gibco) was added to the washed monolayers to kill extracellular bacteria and the plates were further incubated for 1 h at 37°C. After the cells were washed three times with PBS, they were lysed and plated on BHI agar. All cellular invasion and growth assays were performed in duplicate and repeated three times. The significance of the differences in association and invasion of L. monocytogenes ΔinlB and L. innocua compared with L. monocytogenes EGD was analyzed with a two-tailed, unpaired Student's t test. For SEM, the slides with the infected HBMEC were fixed in cold glutaraldehyde (6.25% in 100 mM phosphate buffer, pH 7.4) overnight at 4°C and then washed five times with phosphate buffer as described previously (10). Cells attached to cover slides were then stepwise dehydrated in acetone and critical point dried with CO2. Specimens were spattered with 30 nm of gold. Photographs were taken with a Zeiss scanning electron microscope (DSM 962; Zeiss, Oberkochen, Germany).
RESULTS AND DISCUSSION
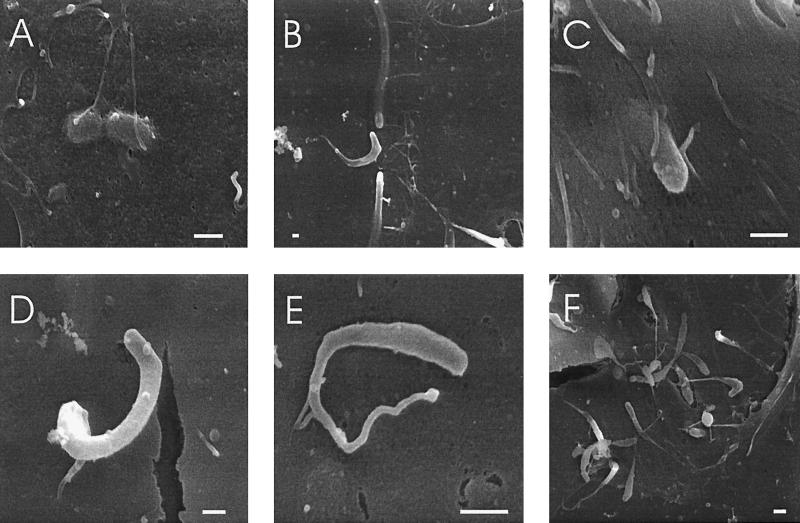
SEM of HBMEC grown on cover slides shows that the cells are thin and flat with short microvilli distributed on most of the surface area (Fig. 1). Since L. monocytogenes was shown to invade Caco-2 epithelial cells with a zipper mechanism from the basolateral side (13) or upon interaction with brush-border microvilli from the apical side (10), we analyzed the mode of interaction of L. monocytogenes with HBMEC. Thirty minutes after addition of the bacteria, the infected cells were fixed, stained, and observed by SEM. As shown in Fig. 2, L. monocytogenes EGD readily associates with the apical surface of the HBMEC, and the bacteria are found in contact with the cell surface in two ways. Some bacteria are attached to the smooth surface of the HBMEC without any sign of specific membrane action (Fig. 2A). More than 90% of the bacteria are, however, found in contact with surface microvilli (Fig. 2B to D), which are sometimes rather long and are even wound around the bacteria (Fig. 2B). The microvilli attaching to the bacteria seem to be in close and intimate contact with the bacterial surface. Figure 2E shows a bacterium obviously just beginning to dive into the host cell. In Fig. 2F a bacterium has already invaded the endothelial cell. This picture also demonstrates the extreme flatness of the HBMEC. L. monocytogenes ΔinlB and L. innocua were recently shown not to invade the type of HBMEC used in this study (7). Here we show that L. monocytogenes ΔinlB (Fig. 3A and B) and L. innocua (Fig. 3C and D) display the capacity to attach to the surface of the HBMEC. L. monocytogenes ΔinlB is mostly (90%) found without obvious contact to microvilli (Fig. 3A), but attaching bacteria being approached or covered with microvilli were also seen rarely (Fig. 3B). L. innocua, however, is often (75%) found in contact with microvilli (Fig. 3D) but also regularly found directly attaching to the smooth surface of the HBMEC (Fig. 3C). This unexpected finding of L. innocua and L. monocytogenes ΔinlB binding to the surface of HBMEC prompted us to investigate quantitatively the association of L. monocytogenes EGD, the inlB mutant, and L. innocua with the endothelial cells. To measure the early association, HBMEC were infected for 35 min, washed, lysed, and plated on BHI agar. Invasion was measured in parallel as a control after 1 h of gentamicin treatment. The results shown in Fig. 4 clearly support our SEM observations: L. innocua and L. monocytogenes ΔinlB associate with HBMEC to the same extent as L. monocytogenes EGD. In contrast, the invasive capacity of the inlB mutant and L. innocua is more than 100-fold lower than that of the wild-type strain, confirming earlier data (7) on the importance of the inlB gene product for HBMEC invasion. From these data we conclude that early association of L. monocytogenes and L. innocua with HBMEC is a process clearly separated from invasion, independent from the inlB gene product, and obviously mediated by structures also present in L. innocua. Hence, association of Listeria with HBMEC is not uniformly followed by uptake. Using HBMEC of different origin, Wilson and Drevets (21) recently presented data showing that adhesion of L. monocytogenes to HBMEC is independent from the presence of the inlB gene, thus confirming our observations. However, they did not find a role for the inlB gene in invasion, a finding which is in strong contradiction to our observation of inlB-dependent HBMEC invasion and recent data on inlB-dependent human umbilical vein endothelial cell invasion (15). It is, however, important to note that the data available from the literature (3, 7, 8, 15, 21) on endothelial cell adhesion and invasion are often conflicting. This may be partially explained by the use of cells from different sources and different culture conditions as well as the use of different infection protocols.
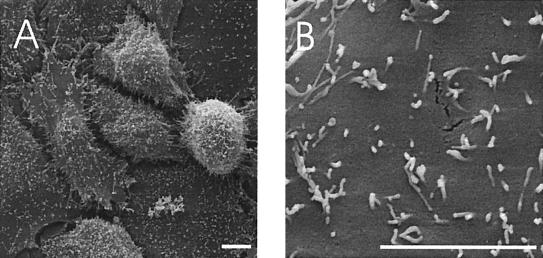
FIG. 1.
SEM of noninfected HBMEC showing the surface of the cells. (A) Several cells at lower magnification; (B) surface of one cell at higher magnification, with numerous microvilli distributed over the surface. Bars, 5 μm.
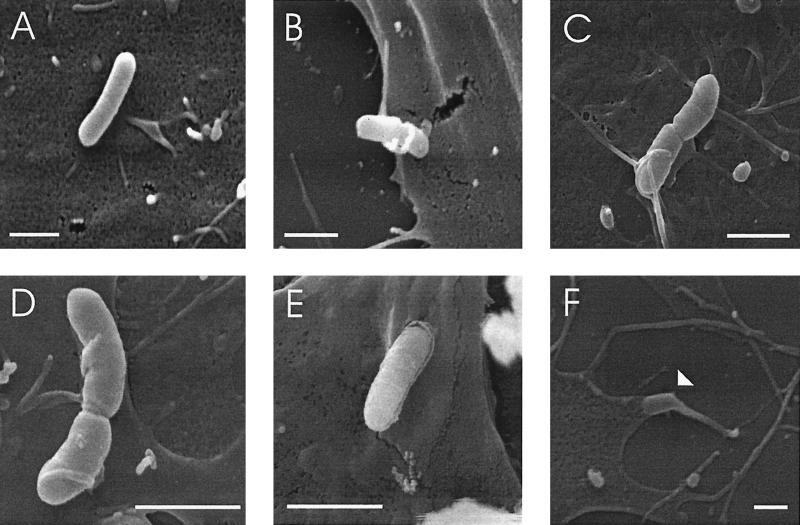
FIG. 2.
SEM of HBMEC at 35 min postinfection with L. monocytogenes EGD. The bacteria are either found on the cell surface (A), or (in most cases) in contact with microvilli (B, C, and D). Rarely, bacteria were found in the process of invasion (E), or already taken up by the HBMEC (arrowhead) (F). Bars, 1 μm.
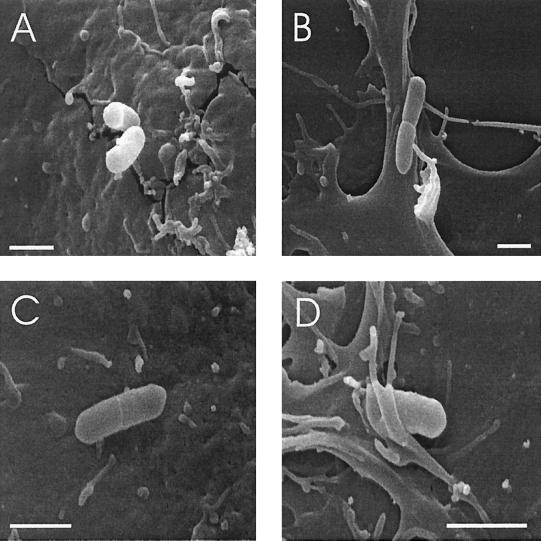
FIG. 3.
SEM of HBMEC at 35 min postinfection with L. monocytogenes ΔinlB (A and B) or L. innocua (C and D). Bars, 1 μm.
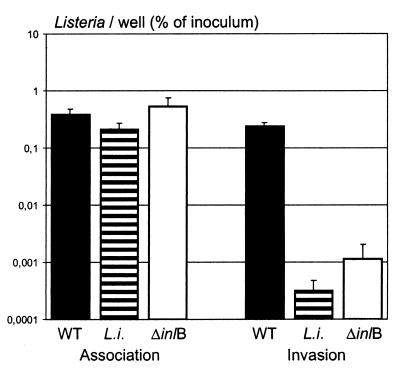
FIG. 4.
Early association with and invasion of HBMEC by L. monocytogenes EGD (WT), L. monocytogenes ΔinlB (ΔinlB), and L. innocua (L.i.). HBMEC were infected (multiplicity of infection = 20), and associated bacteria were counted at 35 min postinfection (left three bars). In parallel, the infected cells were washed and treated further with gentamicin for 1 h, the intracellular bacteria were enumerated (right three bars), and the number of recovered bacteria (as a percentage of the inoculum) is shown. The mean and standard deviations (error bars) of the results of one representative experiment are given. The differences in association with the HBMEC between the three strains are not significant (P > 0.05). The differences in invasion of HBMEC between L. monocytogenes and the two strains L. innocua and L. monocytogenes ΔinlB are highly significant (P < 0.001).
In order to monitor the fate of Listeria-infected HBMEC, we performed additional SEM at 4 h postinfection. Infection with L. monocytogenes ΔinlB or L. innocua did not result in any visible bacteria associated with the monolayer after 4 h of treatment with gentamicin since the attaching bacteria were obviously killed by the gentamicin and washed away. In contrast, L. monocytogenes EGD invaded the HBMEC as expected, and numerous bacteria were seen associated with the HBMEC in different stages of intracellular multiplication, movement, and spread. Since the HBMEC are flat, occasionally even intracellular bacteria could be seen, as shown in Fig. 5A, where Listeria cells were fixed in the process of division. Even the actin tail behind moving bacteria is visible through the cell membrane (Fig. 5B). Figure 5C to E shows different stages of the formation of finger-like protrusions on the cellular surface which presumably allow cell-to-cell spread. While Fig. 5C shows a bacterium just inducing protrusion formation, Fig. 5D and E show typical protrusions, with a bacterium at the tip and the actin tail behind. The pictures clearly show that the diameter of the protrusions is shrinking toward the cell and the “stalks” are very thin at the cell surface. The protrusions start at the host cell surface without any sign of additional membrane perturbations. As shown in the general view (Fig. 5F) the formation of protrusions is a very common event, probably because the host cell is thin and the bacteria reach the inner side of the cell membrane quite often. The morphology of the protrusions induced by L. monocytogenes in different cell types was not investigated in detail. However, in the case of Shigella flexneri it was shown that around the site of exit of the protrusions at the cell surface, major rearrangements of the cytoskeleton occur with the formation of many tiny villosities (6, 17). The absence of such membrane structures at the base of the L. monocytogenes-induced protrusions strongly implies that the formation of the protrusions during cell-to-cell spread is an ordered event. Protrusion formation is obviously induced and controlled by the bacteria by totally unknown mechanisms and hence differs between bacterial species using identical mechanisms of cell-to-cell spread.
FIG. 5.
SEM of HBMEC at 4 h postinfection with L. monocytogenes EGD. Intracellular dividing (A) and moving (B) bacteria are seen through the cell membrane. (C to E) Different stages of protrusion formation are shown. (F) A general view of an infected cell at lower magnification is shown. Bars, 1 μm.
ACKNOWLEDGMENTS
This work was supported by the Deutsche Forschungsgemeinschaft through grant SFB 479-B1; by the European Union through the BIOMED 2 Project “Listeria Eurolab,” grant CT950659; and by U.S. Public Health Service grant RO1-NS 26310.
Many thanks to G. Krohne for helpful discussions and technical support.
REFERENCES
- 1.Badger J L, Stins M S, Kim K S. Citrobacter freundii invades and replicates in human brain microvascular endothelial cells. Infect Immun. 1999;67:4208–4215. doi: 10.1128/iai.67.8.4208-4215.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dramsi S, Biswas I, Maguin E, Braun L, Mastroeni P, Cossart P. Entry of Listeria monocytogenes into hepatocytes requires expression of inlB, a surface protein of the internalin multigene family. Mol Microbiol. 1995;16:251–261. doi: 10.1111/j.1365-2958.1995.tb02297.x. [DOI] [PubMed] [Google Scholar]
- 3.Drevets D A, Sawyer R T, Potter T A, Campbell P A. Listeria monocytogenes infects human endothelial cells by two distinct mechanisms. Infect Immun. 1995;63:4268–4276. doi: 10.1128/iai.63.11.4268-4276.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Farber J M, Peterkin P I. Listeria monocytogenes, a food-borne pathogen. Microbiol Rev. 1991;55:476–511. doi: 10.1128/mr.55.3.476-511.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gaillard J L, Berche P, Frehel C, Gouin E, Cossart P. Entry of Listeria monocytogenes into cells is mediated by internalin, a repeat protein reminiscent of surface antigens from gram-positive cocci. Cell. 1991;65:1127–1141. doi: 10.1016/0092-8674(91)90009-n. [DOI] [PubMed] [Google Scholar]
- 6.Goldberg M B, Sansonetti P J. Shigella subversion of the cellular cytoskeleton: a strategy for epithelial colonization. Infect Immun. 1993;61:4941–4946. doi: 10.1128/iai.61.12.4941-4946.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Greiffenberg L, Goebel W, Kim K S, Weiglein I, Bubert A, Engelbrecht F, Stins M, Kuhn M. Interaction of Listeria monocytogenes with human brain microvascular endothelial cells: InlB-dependent invasion, long-term intracellular growth, and spread from macrophages to endothelial cells. Infect Immun. 1998;66:5260–5267. doi: 10.1128/iai.66.11.5260-5267.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Greiffenberg L, Sokolovic Z, Schnittler H-J, Spory A, Böckmann R, Goebel W, Kuhn M. Listeria monocytogenes-infected human umbilical vein endothelial cells: internalin-independent invasion, intracellular growth, movement, and host cell responses. FEMS Microbiol Lett. 1997;157:163–170. doi: 10.1111/j.1574-6968.1997.tb12768.x. [DOI] [PubMed] [Google Scholar]
- 9.Ireton K, Payrastre B, Chap H, Ogawa W, Sakaue H, Kasuga M, Cossart P. A role for phosphoinositide 3-kinase in bacterial invasion. Science. 1996;274:780–782. doi: 10.1126/science.274.5288.780. [DOI] [PubMed] [Google Scholar]
- 10.Karunasagar I, Senghaas B, Krohne G, Goebel W. Ultrastructural study of Listeria monocytogenes entry into cultured human colonic epithelial cells. Infect Immun. 1994;62:3554–3558. doi: 10.1128/iai.62.8.3554-3558.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuhn M, Goebel W. Molecular studies on the virulence of Listeria monocytogenes. Genet Eng. 1995;17:31–51. [PubMed] [Google Scholar]
- 12.Kuhn M, Goebel W. Internalization of Listeria monocytogenes by nonprofessional and professional phagocytes. Subcell Biochem, 2000;33:411–436. doi: 10.1007/978-1-4757-4580-1_16. [DOI] [PubMed] [Google Scholar]
- 13.Mengaud J, Ohayon H, Gounon P, Mege R-M, Cossart P. E-cadherin is the receptor for internalin, a surface protein required for entry of Listeria monocytogenes into epithelial cells. Cell. 1996;84:923–932. doi: 10.1016/s0092-8674(00)81070-3. [DOI] [PubMed] [Google Scholar]
- 14.Nizet V, Kim K S, Stins M, Jonas M, Chi E Y, Nguyen D, Rubens C E. Invasion of brain microvascular endothelial cells by group B streptococci. Infect Immun. 1997;65:5074–5081. doi: 10.1128/iai.65.12.5074-5081.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parida S K, Domann E, Rohde M, Müller S, Darji A, Hain T, Wehland J, Chakraborty T. Internalin B is essential for adhesion and mediates the invasion of Listeria monocytogenes into human endothelial cells. Mol Microbiol. 1998;28:81–93. doi: 10.1046/j.1365-2958.1998.00776.x. [DOI] [PubMed] [Google Scholar]
- 16.Prasadarao N V, Wass C A, Kim K S. Endothelial cell GlcNAc β1-4GlcNAc epitopes for outer membrane protein A enhance traversal of Escherichia coli across the blood-brain barrier. Infect Immun. 1996;64:154–160. doi: 10.1128/iai.64.1.154-160.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prevost M-C, Lesourd M, Arpin M, Vernel F, Mounier J, Hellio R, Sansonetti P J. Unipolar reorganization of F-actin layer at bacterial division and bundling of actin filaments by plastin correlate with movement of Shigella flexneri within HeLa cells. Infect Immun. 1992;60:4088–4099. doi: 10.1128/iai.60.10.4088-4099.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schuchat A, Swaminathan B, Broome C V. Epidemiology of human listeriosis. Clin Microbiol Rev. 1991;4:169–183. doi: 10.1128/cmr.4.2.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stins M F, Prasadarao N V, Ibric L, Wass C A, Luckett P, Kim K S. Binding characteristics of S fimbriated Escherichia coli to isolated brain microvascular endothelial cells. Am J Pathol. 1994;145:1228–1236. [PMC free article] [PubMed] [Google Scholar]
- 20.Stins M F, Prasadarao N V, Zhou J, Arditi M, Kim K S. Bovine brain microvascular endothelial cells transfected with SV40-large T antigen: development of an immortalized cell line to study pathophysiology of CNS disease. In Vitro Cell Dev Biol Anim. 1997;33:243–247. doi: 10.1007/s11626-997-0042-1. [DOI] [PubMed] [Google Scholar]
- 21.Wilson S L, Drevets D A. Listeria monocytogenes infection and activation of human brain microvascular endothelial cells. J Infect Dis. 1998;178:1658–1666. doi: 10.1086/314490. [DOI] [PubMed] [Google Scholar]