Graphical abstract
Abbreviations: AdV, Adenoviruses; ATPS, Aqueous two-phase systems; HEK293, human embryonic kidney 293
Keywords: Adenovirus type 5, Aqueous two-phase partitioning system, Caesium chloride, Vaccine, Viral vector purification
Abstract
Adenoviruses (AdVs) are used as gene therapy vectors to treat human diseases and as vaccines against COVID-19. AdVs are produced by transfecting human embryonic kidney 239 (HEK293) or PER.C6 virus producer cells with AdV plasmid vectors or infecting these cells withcell lysates containing replication-defective AdV. Cell lysates can be purified further by caesium chloride or chromatographic protocols to research virus seed stocks (RVSS) for characterisation to high quality master virus seed stocks (MVSS) and working virus seed stocks (WVSS) before downstream production of pure, high titre AdV. Lysates are poorly infectious, block filtration columns and have limited storage capability. Aqueous two-phase systems (ATPS) are an alternative method for AdV purification that rapidly generates cleaner RVSS for characterisation to MVSS. After testing multiple ATPS formulations, an aqueous mixture of 20 % PEG 600 and 20 % (NH4)2SO4 (w/w) was found most effective for AdV partitioning, producing up to 97+3% yield of high-titre virus that was devoid of aggregates both effective in vitro and in vivo with no observable cytotoxicity. Importantly, AdV preparations stored at −20 °C or 4 °C show negligible loss of titre and are suitable for downstream processing to clinical grade to support the need for AdV vaccines.
1. Introduction
Adenoviruses (AdVs) are highly suited to effective gene transfer and commonly used in vaccine production to target both dividing and non-dividing cells and their biology is well understood (Cortin, Thibault et al. 2004; Ura, Okuda et al. 2014) The vectors replicate and are assembled within the nucleus of infected cells before undergoing lysis for the progeny virus to infect adjacent cells. AdV particles have a diameter of 90–110 nm containing capsid proteins and fibres making up their structure surrounding a linear double-stranded DNA genome (Russell, 2009). Seven AdV species (A–G) are known, which have been subdivided into 57 serotypes. Ad serotype 5 (AdV5) is the most commonly used for gene therapy with fibre proteins that bind coxsackievirus and adenovirus (CAR) receptors on permissive cells (Bergelson, Cunningham et al. 1997; Roelvink, Lizonova et al. 1998; Kirby, Lord et al. 2001,) and an RGD motif that binds to the cell surface integrins for cell entry (Huang, Kamata et al. 1996; Davison, Diaz et al. 1997). AdV5 usually elicits a significant host immune response due to recognition of virus proteins by T lymphocytes and neutralising antibodies (Gilgenkrantz, Duboc et al. 1995; Yang, Su et al. 1996).
AdV vectors are designed to carry a large transgene payload and particles are usually produced at high titre from human embryonic kidney 293 (HEK293) or PER.C6 producer cells (Xie, Pilbrough et al. 2002). For therapeutic applications, replication-defective vectors, originally based on AdV5, have been generated. First generation vectors have early genes E1 and/or E3 regions removed allowing ∼8 kb of transgenic sequences to be carried. These early genes that are necessary for virus replication have been inserted into the producer cells to compliment transfection or infection of defective vector genomes into these cells to enable the generation of attenuated progeny virus particles (Fallaux, Kranenburg et al. 1996; Graham, Smiley et al. 1977; Fallaux, Bout et al. 1998). Due to the immune response caused by 1st generation AdV5, second generation vectors have been developed with further deletions of E2A, E2B and E4 genes with larger transgene capacity (Bischoff, Kirn et al. 1996; Fallaux, Kranenburg et al. 1996,). Third generation vectors have all internal components removed, except replication origins and packaging signals leaving the 3′ and 5′ inverted terminal repeats (ITR) remaining and therefore require complementation with helper viruses. AdVs have also been engineered as conditionally replicative carrying mutated E genes that only allow replication in cells not expressing P53 or Rb proteins and so are suited to cancer gene therapy (Bischoff, Kirn et al. 1996).
Human and chimpanzee AdV based vaccines of various sorts have entered human trials against HIV, influenza, Ebola, tuberculosis, and malaria diseases. Natural wild type infection by AdV is common in humans and thus hAdV5 vaccines are recognised by pre-existing anti-hAdV5 antibodies in humans (Xiang, Gao et al. 2003; Liebowitz, Lindbloom et al. 2015). As a result of this, alternative serotypes have been used or AdV from alternative species such as the chimpanzee and gorilla (Cheng, Wang et al. 2015; Baden, Liu et al. 2015; Bos, Rutten et al. 2020; Capone, Raggioli et al. 2020; Folegatti, Ewer et al. 2020; Zhu, Li et al. 2020). Currently, these AdVs are in clinical trials to combat the COVID-19 pandemic.
To obtain large-scale good manufacture practice-certified (GMP) vector preparations at high titre, fed-batch cultures of anchorage independent cells are grown in serum-free suspension bioreactors with improved culture conditions for optimal growth density (Lesch, Heikkila et al. 2015).
AdV production to clinical grade by adherent or suspension culture producer cells requires both up and downstream manufacturing processes that are time consuming, costly and difficult to scale up, thereby limiting AdV manufacture. AdV production requires producer cell plasmid transfection or infection with crude cell lysates containing AdV particles. This produces research virus seed stocks (RVSS) that are characterised to become master and working virus seed stocks (MVSS and WVSS). After infection and cell lysis, AdV particles can be subjected to CsCl density separation to remove producer cell genomes and proteins to generate pure AdV. Both the use of cell lysates and CsCl purification have limitations. Cell lysates have reduced infectivity and can block purification columns used in downstream processing, thereby reducing particle yield. CsCl Ad production avoids this but requires two rounds of ultracentrifugation, which not only significantly contributes to the lengthy time required for AdV preparation but also results in loss of infectious particles during the extraction process. Although automated continuous flow centrifugation can be easily scaled up (∼2 kl) for laboratory use, the need for dialysis to remove this toxic salt adds further cost, time (10 h) and reduces yield. It can also result in particle aggregation that reduces viable infectious titre (Boychyn, Yim et al. 2001; Farid, 2007; McNally, Darling et al. 2014). The use of chromatography to purify lysed cell samples over packed columns also limits supernatant volumes that can be used, and columns can become clogged resulting in a low AdV yield (McNally, Darling et al. 2014). Importantly, robust, high-quality, characterised MVSS and WVSS are required for downstream processing to clinical grade virus. At the upstream stage, a method that could cost effectively improve the purity of the Ad starting material from crude cell lysates and match that of CsCl purification rapidly whilst avoiding early stage chromatography to provide RVSS would be highly beneficial to Ad production.
Aqueous two-phase systems (ATPS) have been shown to be successful in purifying rapidly several types of biomolecules, including proteins, using immiscible liquids as phases for separation (Albertsson, Andersson et al. 1982; Chao, Shum, 2020). ATPS offers a simple, rapid and promising alternative methodology for the rapid scale-up of vector preparations, whilst retaining particle infectivity and titre. This can streamline the production of RVSS further for downstream applications. Polyethylene glycol (PEG) is especially useful in separating biomolecules and its hydrophobicity is directly correlated to its molecular weight. When PEG is combined with a kosmotropic salt, such as in the PEG300-phosphate ATPS, AdV can be purified in the upper aqueous phase, whilst remaining highly infectious (Negrete, Ling et al. 2007a, b). Dextran and methylcellulose/polyethylene glycol polymers can also be used to generate viable virus particles that are concentrated (up to 1000-fold) using low speed short centrifugation times. ATPS purification has also been applied successfully to adeno-associated virus (AAV) vector production using 10 % PEG8000/13.2 % (NH4)2SO4 solutions that gently remove impurities and avoids dialysis to a level acceptable for in vivo gene transfer (Guo, Xiao et al. 2013; Rogers, Bond et al. 1996). Importantly, ATPS is cost effective and can be easily performed in any laboratory without the need for expensive equipment (Molino, Lopes et al. 2018). These demonstrate advantages of the ATPS for purification of vectors compared to CsCl or chromatographic protocols.
This work applies ATPS technology to generate RVSS of AdV vectors at reduced cost and time with high particle yield, purity and infectivity. Several immiscible liquid compound formulations will be screened for partitioning AdV particles from infected HEK293 T producer cell lysates, their infectivity and potential toxicity in vitro. The ATPS formulation that shows the highest vector recovery will also be applied in vivo to compare CsCl and ATPS purified AdV to ensure comparable levels of gene transfer and potential toxicity in vivo. Because ATPS technology is amenable to scale-up, we propose this technology ideal for the rapid manufacture of large amounts of RVSS for characterisation to MVSS under GMP conditions to support clinical grade therapeutic AdV and AdV-based vaccines.
2. Material and methods
2.1. Cell culture
Phosphate buffered saline (PBS) solution (Fisher Scientific, Loughborough) was prepared in 500 mL batches with sterile dH2O and autoclaved. Cells were cultured in complete medium prepared using Dulbecco’s Modified Eagle Medium (DMEM) GlutaMAX™ supplemented with 1% penicillin streptomycin and 10 % fetal bovine serum (all reagents are purchased Fisher Scientific). 0.05 % trypsin (Fisher Scientific) diluted in PBS was used to detach cells from their substratum. All reagents used showed low levels of endotoxins present and are cGMP compatible. IMRS2, HEK293 T and HepG2 cells were obtained from ATCC (Middlesex, UK). These were thawed and centrifuged at 1500 rpm for 5 min to pellet cells that were resuspended in 10 mL complete medium. Cells were incubated at 37 °C in the presence of 5% CO2 and the medium changed every 2 days. Cells were passaged when reaching 70 %–80 % confluency.
2.2. Generation of adenovirus vectors
For this study, 1st generation human AdVRSVβgal or AdVCMVLuc (E1 and E3 regions deleted), which drive the β-galactosidase or luciferase genes by the RSV and CMV promoters respectively, were generated using HEK293 T cells. 1.5 × 10 7 cells grown in T175 culture flasks were infected with AdV (MOI of 10) for 72 h. Cells were mechanically detached from the flasks and pelleted at 1500 rpm for 10 min, followed by resuspension in 10 mL complete medium. Cells were subjected to 3 freeze thaw cycles between 37 °C and−80 °C and cell debris pelleted by centrifugation at 1500 rpm for 15 min. AdV was purified using high-speed CsCl density gradient ultracentrifugation and AdV particle concentration determined via OD260nm equivalent to AdV titre, for vector genomes and plaque assays on HEK293 T cells respectively, as previously described (Fallaux, Bout et al. 1998). AdV was also recovered via ATPS partitioning from freeze thawed HEK293 T cell batches (see below). AdRSVβgal and AdCMVLuc recovered by CsCl partitioning were tested on HeLa cells for infectivity via Xgal staining for β-galactosidase or luciferase gene expression via d-luciferin light emission, respectively.
2.3. Analysis of AdV vectors for infectivity and replication incompetence
To investigate for the presence or absence of replication competent adenovirus (RCA), infected Hela cells were cultured for a further 2 weeks after which time conditioned supernatants were filtered and then applied to fresh HeLa and HEK293 T cells. These cells were stained using X-gal for β-galactosidase gene expression or tested for luciferase gene expression after 48 h to indicate the presence of positively or negatively infected cells, as previously described (Clifton, 2012). No cells were stained positive (i.e. blue or emitting light) indicating replication incompetence of AdV.
2.4. Generation of ATPS phases and analysis of yield
Polymers and salts were weighed and dissolved in deionised water at 37 °C, 1.0MΩcm-1 (Purite, Thames). Each system was vortexed, then left to separate at room temperature for 15 min. (Table 1 ). ATPS created were: 14 % -14 % w/w PEG 1000 - K2HPO4, 14 % - 14 % w/w (C6H10O6)n - PEG 8000, 14 % - 14 % w/w PEG8000 - HNa2O4P, 14 % - 14 % w/w PEG 8000 - K2HPO4, 14 % - 14 % w/w PEG 600 - (NH4)2SO4, 20 % - 20 % w/w PEG 600 - (NH4)2SO4, 14 % - 14 % w/w UCON 3900 - Na2HPO4, 14 % - 14 % w/w UCON 970 - Na2HPO4, 14 % - 14 % w/w UCON 970 - C6H5Na3O7∙2H2O (Fisher Scientific & Sigma- Aldrich). ATPS consisting of 14 % - 14 % w/w PEG8000 - HNa2O4P, 14 % - 14 % w/w PEG 600 - (NH4)2SO4, 14 % - 14 % w/w UCON 3900 - Na2HPO4, 14 % - 14 % w/w UCON 970 - Na2HPO4 were not used due to the formation of crystal salts.
Table 1.
ATPS solutions tested for the purification of AdVRSVβgal particles. Successful (+) and unsuccessful (-) ATPS solutions for AdV purification are shown for each ATPS tested. All solutions are at 14 % w/w unless otherwise stated. Initial Ad input for purification was 3.5 × 10 12 particles/mL. To detect for the presence or absence of viral nucleic acids and proteins, Abs260nm readings of the upper phase of each ATPS were measured after purification using NanoDrop™2000C. Negligible concentrations of AdV was present in the lower phases (n = 3).
| ATPS | Positive/Negative |
|---|---|
| PEG 1000/K2HPO4 | + |
| (C6H10O6)n/PEG 8000 | + |
| PEG 8000/HNa2O4P | – |
| PEG 8000/K2HPO4 | + |
| PEG 600/(NH4)2SO4 | – |
| 20 % - 20 % w/w PEG 600/(NH4)2SO4 | + |
| UCON 3900/Na2HPO4 | – |
| UCON 970/Na2HPO4 | – |
| UCON 970/C6H5Na3O7∙2H2O | + |
To assess the yield of vector recovery after ATPS, 10 μL containing 3.5 × 10 12 IU of AdVRSVβgal purified using CsCl gradient centrifugation was added to a 1:1 ratio of mixed upper and lower ATPS phases (2 mL total). After gentle mixing, the phases were centrifuged at 1500 rpm for partitioning for 15 min. Each layer was then quantified for AdVRSVβgal concentration by spectrophotometry at an optical density reading of 260 nm for vector genomes (vg) equating to vector particles per ml (vp/mL) to calculate recovery yield using NanoDrop™2000C (Mittereder, March et al. 1996).Recovery was also measured by infection assays on HepG2 cells.
AdV batches were also screened for aggregation as previously described (Lefesvre, van Bekkum 2003). Briefly, spectrophotometric ratios from 320/260 and 340/260 readings were assessed and no ratios above 0.3 were found indicating no virus aggregation.
2.5. Infection and cytotoxicity assays of HepG2 and IMRS2 cell lines
HepG2 (liver) and IMRS2 (neuronal) cells were cultured in 12 well dishes at 2 × 10 5 cells/well and incubated at 37 °C with 5% CO2 for 24 h. HepG2 cells have been shown to be highly permissive to AdV infection (Pan, Wu et al. 2012). 10 μL of the upper and lower phases of each phase system containing purified AdVRSVβgal were then added to each well for 24 h before replacing medium. 48 h post infection, cells were stained for LacZ expression to determine the most effective ATPS for AdV purification, as previously described (Clifton, 2012). Cell viability was analysed via trypan blue exclusion 48 h post AdVRSVβgal infection, where live cells exclude dye as opposed to dead cells that do not.
2.6. Animal procedures
Outbred male and female CD1 mice were used in this study (Charles River, Oxford). All animal experiments conducted within this study were in agreement with the United Kingdom Home Office guidelines, approved by the ethical review committee and following the institutional guidelines at University College London. To test hAdV gene transfer in vivo, AdVCMVLuc vector that drives luciferase gene expression from the CMV promoter was generated by infection of HEK293 T cells followed by purification via 20 % - 20 % w/w PEG 600 - (NH4)2SO4 ATPS. Purified vector from supernatant was titrated at 2.9 × 10 13 vector genomes/mL via spectrophotometry. Healthy CD1 mice were anaesthetised with isoflurane with 100 % oxygen (Abbott Laboratories, London, UK) in a heated chamber at 37 °C. 100 μL containing 1 × 10 9 vector genomes/mL AdVCMVLuc particles was administered to adult mice via tail vein intravenous injection. After the injections, mice were allowed to recover fully before being returned to their cages.
2.7. Whole-body bioluminescence imaging
Mice were anaesthetised with isoflurane with 100 % oxygen and given an intraperitoneal injection of 15 mg/mL of d-luciferin (Gold Biotechnologies, ST Louis, USA). Mice were imaged after 5 min using a cooled charged-coupled device camera, (IVIS machine, Perkin Elmer, Coventry, UK) for between 1 s and 5 min. The regions of interest (ROI) were measured using Living Image Software (Perkin Elmer, Beaconsfield) and expressed as photons per second per centimetre squared per steradian (photons/second/cm2/sr).
2.8. Statistical analysis
Significant differences between data were derived using a Man Whitney U statistical test, with a P value cut off of 0.05 for all data sets.
3. Results
3.1. hAdV genome recovery and infectivity following ATPS partitioning
Of the 9 ATPS screened, 5 formulations provided virus recovery, of which the 3 most optimal yields were 75 ± 8.55 % for 14 % - 14 % w/w PEG 1000/K2HPO4, 47 ± 5.94 % for 14 % - 14 % w/w PEG 8000/K2HPO4 and 83 ± 5.99 % for 20 % - 20 % w/w PEG 600/(NH4)2SO4 ATPS. Vector partitioning was mainly restricted to upper phases. The vector titre reached for these ATPS ranged from 1.6 × 10 12 to 2.9 × 10 12 vg/mL (Table 2 ).
Table 2.
AdVRSVβgal genome recovery after separation by ATPS. An initial input of 3.5 × 10 12 Ad vg/mL were separated in PEG1000/K2PO4, PEG8000/K2PO4 and PEG600/(NH4)2SO4. Abs260nm readings were averaged and corrected over a range of dilutions. AdV genome titre (vg/mL) was calculated by multiplying the average Abs260nm value by 1.1 and the product by 1012 (n = 3) (Fallaux, Bout et al. 1998).
| ATPS | Titre (vg/mL) | Yield/% |
|---|---|---|
| PEG 1000 /K2PO4 | 2.6 × 10 12 | 75 ± 8.55 |
| PEG 8000/K2PO4 | 1.6 × 10 12 | 47 ± 5.94 |
| PEG 600/ (NH4)2SO4 | 2.9 × 10 12 | 83 ± 5.99 |
To examine the infectivity of AdVRSVβgal partitioning to each phase, samples from both upper and lower phases were used to infect HepG2 cells, (Pan, Wu et al. 2012). Negligible HepG2 gene transfer was detected after exposing cells to any ATPS lower phases. Quantification of percentage infection by AdVRSVβgal, recovered through upper phase, was 55.2 ± 2.7 % for PEG1000/K2HPO4, 59.9 ± 9.8 % for PEG8000/K2HPO4 and 97 ± 3% with PEG600/(NH4)2SO4 ATPS is shown in (Fig. 1 b).
Fig. 1.
Infection of HepG2 cells by AdVRSVβgal. (a). HepG2 infection by CsCl prepared vector after partitioning by 3 ATPS formulations. Positive staining for β-galactosidase activity was identified in each ATPS upper phase, with highest infection by PEG 600/(NH4)2SO4. Uninfected HepG2 cells appear negative for enzyme activity. (b). Quantification of cell infection: PEG1000/K2HPO4; 55.15 ± 2.74 %, PEG8000/K2HPO4; 59.85 ± 9.84 %, PEG600/(NH4)2SO4; 97.00 ± 3.0 %, (n = 5).
The IMRS2 neuronal cell line, which is of non-liver origin was also used as an alternative cell line to determine the efficacy of AdV infection. To measure IMRS2 permissiveness to AdV infectivity, cells were exposed to 10 11–10 6 CsCl purified AdV (Fig. 2 a and b). Cell infection was observed as 100 ± 0% (10 11), 80 ± 3.84 % (10 10), 60 ± 1.15 % (10 9), 46 ± 2.19 % (10 8), 15 ± 1.76 % (10 7) and 1 ± 0.88 % (10 6) using serial dilutions of AdV. IMRS2 cells appeared highly permissive to AdVRSVβgal at each concentration.
Fig. 2.
Analysis of IMRS2 cell permissiveness to AdVRSVβgal infection. (a). Infected IMRS2 cells at various dilutions of AdV particles (vg/mL). Infection was detected by analysis of β- galactosidase activity demonstrating IMRS2 cells are highly permissive to infection. (b). Quantification of positive cells after exposure to serial dilutions of CsCl prepared AdRSVβgal. IMRS2 infection was: 10 11:100 ± 0%, 10 10: 80 ± 3.84 %, 10 9: 60 ± 1.15 %, 10 8: 46 ± 2.19 %, 10 7: 15 ± 1.76 % and 10 6: 1 ± 0.88 % (n = 3).
3.2. Isolation of Ad directly from 293 T producer cell lysate
The potential of ATPS to partition AdVRSVβgal directly from infected HEK293 T cell lysates was assessed. HEK293 cells have previously been reported to produce high titre recombinant Ad virus (Wei, Fan et al. 2017; Chen, Wu et al. 2018). 293 T producer cells were infected with CsCl purified AdRSVβgal seed stock at a MOI of 10. After producer cell infection, cells were isolated from culture flasks and subjected to freeze thaw cycles for partitioning through each of the 3 ATPS. These products can be RVSS or stocks generated using cGMP grade reagents for characterisation to MVSS. HepG2 and IMRS2 cells were infected and cells positive for β-galactosidase gene expression were counted. HepG2 and IMRS2 infection was 93.3 ± 0.9 % versus 85.3 ± 1.8 % using PEG 1000/K2HPO4, 91.7 ± 2.4 % versus 30 ± 1.2 % using PEG 8000/K2HPO4. 91.3 ± 1.5 % versus 93.7 ± 2.2 % infection was observed with PEG 600/(NH4)2SO4, closely matching that of percentage infection using CsCl purified AdVRSVβgal partitioned through ATPS (Fig. 3 a and b). Contaminating proteins can be detected via spectrophotometry (Glasel, 1995; Goldfarb, Saidel et al. 1951). All Abs260nm/280nm in ATPS purified AdV direct from cell lysates are above 0.6. Further HPLC analysis of ATPS purified AdV showed contaminating proteins present but to a lower extent that in crude lysates (data not shown). This demonstrates successful partitioning of AdV into the upper phase of ATPS, with the highest recovery using the PEG 600/(NH4)2SO4 system.
Fig. 3.
Analysis of AdV recovery direct from 293 T cell lysates (a). Infection of HepG2 and IMRS2 cells using AdVRSVβgal purified directly from 293 T cell lysate using 3 ATPS formulations. Infection of AdV was characterised by staining for β- galactosidase activity. (b). Quantification of HepG2 and IMRS2 cell infection by AdV purified directly from 293 T cell lysate. The upper phase presented the highest recovery of AdVRSVβgal particles and was used for infection. For HepG2 and IMRS2 cells, respectively, 93.3 ± 0.9 % and 85.3 ± 1.8 % cells were infected using PEG 1000/K2HPO4. 91.7 ± 2.4 % and 30.0 ± 1.2 % cells stained positive for AdV infection using PEG 8000/K2HPO4. 91.3 ± 1.5 % and 93.7 ± 2.2 % cells were infected using PEG 600/(NH4)2SO4 (n = 3).
3.3. Cell survival following infection by AdRSVβgal recovered from ATPS formulations
HepG2 cell survival was quantified using AdV after ATPS treatment and AdV infection (Fig. 3a). We found HepG2 viability after AdVRSVβgal infection and gene transfer was similar between vector separated via ATPS from CsCl isolation or direct from HEK293 T cell lysates. Cells showed between 77.33 ± 5.06 % and 93.25 ± 0.63 % viability with PEG 1000/K2HPO4 treatments. PEG 8000/K2HPO4 treatments showed between 87.25 ± 4.40 % and 92.20 ± 2.99 % viability. PEG 600/(NH4)2SO4 treatments showed a viability of between 83.75 ± 4.12 % and 93.50 ± 0.99 % (Fig. 4 ). No significant difference was found in comparison to untreated cells (P < 0.05). This suggests no cytotoxicity due to ATPS treatment or Ad infection, including AdV purified direct from cell lysates.
Fig. 4.
Analysis of cell survival after AdV/ATPS treatments. Percentage cell survival after treatment with upper phase ATPS +/- AdVRSVβgal. HepG2 cells + ATPS only (dark grey), + CsCl AdV (light grey) and + 293 T AdV (hash). PEG 1000/K2HPO4 treatment showed viability of 77.33 ± 5.06 % (+ ATPS), 90.502.88) (+ CsCl AdV ATPS) and 93.25 ± 0.63 % (+293 T AdV). Cells treated with PEG 8000/K2HPO4 upper phase showed a viability of 87.25 ± 4.40 % (+ATPS), 92.20 ± 2.99 % (+CsCl AdV) and 91.00 ± 1.83 % (+293 T AdV). PEG 600/(NH4)2SO4 upper phase treatment showed a viability of 83.75 ± 4.12 % (+ATPS), 93.50 ± 0.99 % (+CsCl AdV) and 90.25 ± 1.49 % (+293 T AdV). No significant difference in viability was observed (P < 0.05). This demonstrates the low toxicity of ATPS subsequent to AdV purification and recovery (n = ≥4).
3.4. In vivo application of AdCMVLuc
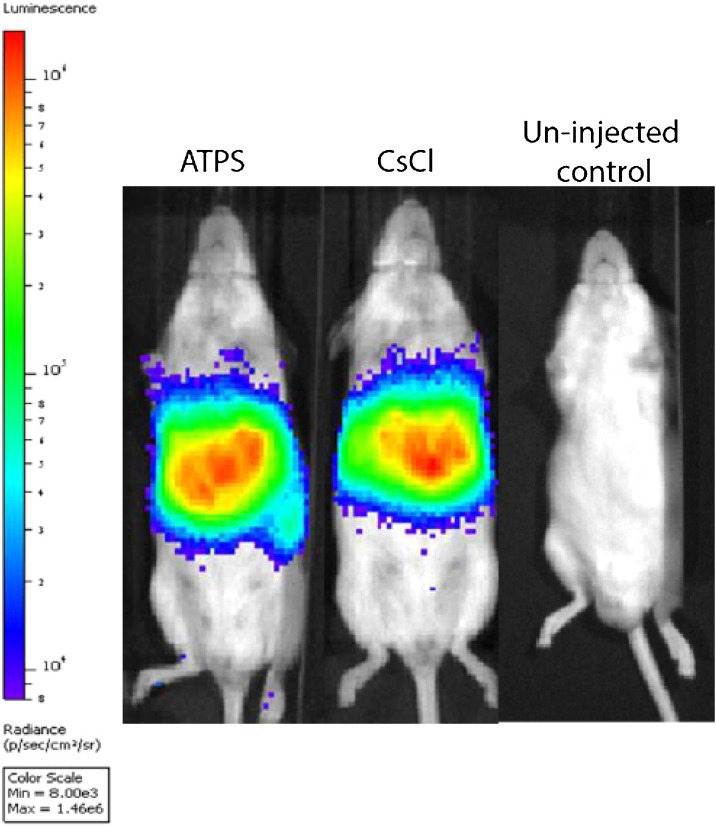
In vivo gene transfer was examined 3 days post injection of ATPS purified AdV (Fig. 5 ). None of the treated mice (n = 3 each) displayed adverse reaction to vector injection, whilst displaying robust luciferase reporter expression. Levels of gene expression using ATPS purified AdV were comparable to CsCl purified AdV, indicating successful in vivo recovery.
Fig. 5.
Application of PEG 600/ (NH4)2SO4 ATPS purified AdVLuc in vivo. AdVLuc virus was purified using PEG 600/(NH4)2SO4 ATPS or CsCl ultracentrifugation. 1 × 10 9 vg/mL AdVluc particles recovered from the ATPS upper phase or CsCl were injected into CD-1 mice via their tail vein mice to determine in vivo gene transfer. Imaging for luciferase activity showed gene expression was comparable between the ATPS and CsCl purified vector thus demonstrating ATPS AdV can be used successfully in vivo without obvious side effects (a single representative mice of n = 3 shown only).
4. Discussion
AdVs are important gene transfer vectors that are under development as licensed bioproducts for therapeutic applications including gene therapy of genetic diseases, vaccination or ‘virotherapy’. They are being developed for human diseases that are hereditary or acquired (Albertsson, Andersson et al. 1982; Oelmeier, Dismer et al. 2011, McNally; Darling et al. 2014) and preclinical studies have been initiated using these potentially therapeutic pharmaceuticals in a large number of laboratories (Rogers, Bond et al. 1996; Nemunaitis, Ganly et al. 2000; Oelmeier, Dismer et al. 2011; Clifton, 2012; Yan, Cao 2012; Guo, Xiao et al. 2013; Tan, Wang et al. 2015; Molino, Lopes et al. 2018). To service intensive clinical studies, manufacture requires cGMP production of large quantities of recombinant virus vectors, often in excess of 10 15 virus particles even for early phase clinical trials. Human AdV (hAdV) based vaccines have entered human trials against various pathogens but as hAd5 vaccines are recognised by pre-existing immune response in humans, novel AdV from different species have been developed (Baden, Liu et al. 2015; Cheng, Wang et al. 2015). hAdV5, hAdV26 and AdVs based on chimpanzee and gorilla adenoviruses are in clinical trials, including to combat the COVID-19 pandemic (Baden, Liu et al. 2015; Cheng, Wang et al. 2015; Bos, Rutten et al. 2020; Capone, Raggioli et al. 2020; Folegatti, Ewer et al. 2020; Zhu, Li et al. 2020). Such a variety of AdVs with differing complex surface topologies will require suitable manufacturing processes for their production at large scale. For example, the enormous challenge to meet the world-wide demand to vaccinate against COVID-19 is estimated to be 1.76 × 10 19 particles (Vellinga, Smith et al. 2014) and therefore rapid and cost effective alternatives to current manufacturing would be highly beneficial.
To generate high titre AdV on a small scale or from large-scale fed-batch cultures of anchorage independent producer cells, cells are transfected with plasmids carrying the cis and trans elements required for particle production or infected with attenuated replication defective particles obtained directly from cell lysates or purified AdV. This production generates AdV RVSS for direct non-clinical use and usually is purified by CsCl buoyancy ultracentifugation. AdV are then characterised and subsequently amplified to produce MVSS to be used in large scale downstream processing.
Before cell lysates can be used, they require filtration. These lysates are often contaminated with small cellular proteins or producer cell DNA. This can reduce producer cell infection and can result in the blockage of columns that are used in the filtration step prior to further purification. For downstream purification, although CsCl buoyancy ultracentrifugation is regarded to be an excellent method, this requires two rounds of ultracentrifugation and dialysis to remove this toxic salt, which is costly and time consuming. It also reduces particle yield and can result in particle aggregation thereby reducing viable infectious titre yield (Berkner, 1988; Baden, Liu et al. 2015; Cheng, Wang et al. 2015). Chromatographic methods over packed columns are also used to purify lysed cell samples, however these limit supernatant volumes and can also become clogged (Berkner, 1988). For clinical production of AdV, purification is achieved via ultrafiltration using tangential flow filtration followed by anion exchange and then further chromatographic steps.
Importantly, if the RVSS AdV particles required are relatively clear of cellular contaminants then downstream processing to MVSS would be more cost effective. To avoid the use of crude contaminated cell lysates or expensive and time consuming CsCl purification, we sought an alternative rapid, cost effective and simple method to generate RVSS AdV stocks for characterisation to MVSS. We also considered that this method should be amenable to provide AdV particles at a scale for downstream processing to high titre and infectious MVSS and WVSS AdV. ATPS technology has been shown successful to rapidly purify several types of biomolecules including proteins using immiscible liquids as phases for partitioning and permanent separation (Albertsson, Andersson et al. 1982). Its system provides an effective purification method, producing a relatively pure product with little to no need for dialysis. Moreover, it is an environmentally friendly, cost effective and easily scalable method that has been previously shown as an alternative methodology for rapid scale-up of AdV preparations, whilst retaining particle infectivity and titre. PEG has been found especially useful in separating biomolecules and its hydrophobicity is directly correlated to its molecular weight. When combined with a kosmotropic salt, such as PEG300-phosphate ATPS, highly infectious AdV partitioning occurs in the upper aqueous phase (Negrete, Ling et al. 2007a, b). Also, several polymers have been shown useful to generate concentrated viable virus particles using low-speed short centrifugation times. Sodium dextran sulphate is particularly useful because it allows salt removal from virus suspensions via precipitation, further concentrating virus stocks that are pure, viable are almost totally devoid of aggregates and dextran can also be precipitated from virus preparations (Negrete, Ling et al. 2007a, b; Oelmeier, Dismer et al. 2012; Yan, Cao 2012; Tan, Wang et al. 2015). ATPS partitioning of adeno-associated vector (AAV) has also been shown using 10 % PEG8000/13.2 % (NH4)2SO4 solutions circumventing dialysis to generate particles pure enough for in vivo gene transfer (Rogers, Bond et al. 1996; Guo, Xiao et al. 2013).
To explore further the suitability of ATPS technology to generate effective AdV RVSS we tested several immiscible liquid compound formulations for their effectiveness in partitioning AdV particles from infected HEK293 T producer cells lysates. To test vector yield from 9 polymer/salt combinations, CsCl purified vector was firstly partitioned between these ATPS immiscible phases. Of these, 3 ATPS; 14 % - 14 % w/w PEG1000 - K2HPO4, and 14 % - 14 % w/w PEG8000 - K2HPO4 and 20 % - 20 % w/w PEG 600 - (NH4)2SO4. provided improved, high yield recovery above those previously described, of between 47–83 %. Particles appeared partitioned mainly in the upper, PEG rich phase and efficiently infected HepG2 cells with no observable cytotoxicity, with the most effective ATPS being PEG 600- (NH4)2SO4. Using this optimal system, the quantities required for early phase clinical trials is estimated at 1 × 10 15 virus AdV particles, which would require a volume of 350 mL. ATPS can be scalable up to 2 L batches, enough to easily achieve this estimate (Negrete, Ling et al. 2007b). Each ATPS was next used to directly partition AdV particles from 293 T cell lysates. While the T antigen is undesirable in GMP manufacture of AdV vectors, this study shows improved proof of concept that these ATPS are useful for processing of high titre AdVs to RVSS. All three ATPS appeared to partition AdV particles, with the majority of high titre preparations in the upper aqueous phases. Infection of HepG2 and IMRS2 reached 93.3(±0.9)% and 85.3(±1.8)% using PEG 1000/K2HPO4, 91.7(±2.4)% and 30(±1.2)% using PEG 8000/K2HPO4 and 91.3(±1.5)% versus 93.7(±2.2)% with PEG 600/(NH4)2SO4 for each cell line respectively, closely matching that of infection with CsCl purified vector partitioned through ATPS.
Vector purity is most important to avoid cytotoxicity, so to investigate whether any of the three ATPS or ATPS partitioned AdV cause cytotoxic side effects, each cell line was infected, and cell viability measured. Each ATPS alone or AdV particles purified by CsCl or direct from 293 T cell lysates when used to infect each cell line showed no toxicity, viabilities between approximately 90–94%, with PEG 600/(NH4)2SO4 partitioned vector being the highest. When using PEG 600/(NH4)2SO4 partitioned AdV carrying the luciferase gene to inject CD-1 mice via the tail vein, no adverse effects were observed when compared with animals infected by CsCl purified AdV.
PEG600 is commonly used in a clinical setting as an emulsifier, in ointments and toothpastes. The concentration of PEG600 used throughout this study remained within IGD limits and can be readily cleared by the kidneys or oxidised to form acidic metabolites (D’souza and Shegokar, 2016).
We propose that ATPS methodology partitions AdV particles highly efficiently. It also offers a simple, scalable procure and long-term storage with low toxicity and high yield. Importantly, it avoids the need for expensive equipment and costly reagents for the production of RVSS Ad or stocks for characterisation to MVSS/WVSS and clinical grade AdV. AdV particles purified to high yields through the upper phase of PEG 600/(NH4)2SO4 ATPS can be used readily for in vitro and in vivo gene transfer. These products may be used to enter downstream processing for clinical production. ATPS is suitable to economies hoping to generate large amounts of AdV stocks for processing to generate Ad based vaccines against COVID-19.
5. Conclusion
The results of this study show that 20 % - 20 % w/w PEG 600 - (NH4)2SO4 provided up to 97 ± 3% recovery yields of Ad particles by high-level partitioning of quality vector direct from producer cell lysates for in vitro and in vivo gene transfer. AdV can be produced in this way at high titre, devoid of aggregates and can be frozen for long-term storage without loss of viability. Viable particles are effective for gene transfer both in vitro and in vivo without obvious cytotoxic effects. As ATPS technology is readily scalable, without the need for expensive high-end equipment, it will be highly useful to generate RVSS of AdV for MVSS/WVSS characterisation and transport to centres throughout the world hoping to generate these particles as vaccines to counteract the coronavirus pandemic.
Author contributions
Saqlain Suleman: Validation, Investigation, Writing- original draft, review and editing. Kuteiba Schrubaji: Validation, Investigation. Chrysovalanto Filippou: Validation, Investigation. Svetlana Ignatova: Conceptualisation, Methodology, Investigation, Supervision. Peter Hewitson: Conceptualisation, Investigation, Methodology, Supervision. Jonathan Huddleston: Conceptualisation, Validation, Methodology, Investigation. Rajvinder Karda: Validation, Formal analysis. Simon Waddington: Validation, Formal analysis, Supervision. Michael Themis: Conceptualisation, Methodology, Writing- Review and editing, Supervision.
Funding source
This research was partly funded by a Brunel University London Brief award. No other public, commercial or not-for-profit sectors supported this work.
Declaration of Competing Interest
The authors declare no conflict of interest.
Acknowledgments
We would like to thank Generon, France for providing both AdRSVβgal and AdCMVLuc 1st generation vectors and their expertise in producing these vectors.
References
- Albertsson P.A., Andersson B., Larsson C., Akerlund H.E. Phase partition--A method for purification and analysis of cell organelles and membrane vesicles. Methods Biochem. Anal. 1982;28:115–150. doi: 10.1002/9780470110485.ch2. [DOI] [PubMed] [Google Scholar]
- Baden L.R., Liu J., Li H., Johnson J.A., Walsh S.R., Kleinjan J.A., Engelson B.A., Peter L., Abbink P., Milner D.A., Golden K.L., Viani K.L., Stachler M.D., Chen B.J., Pau M.G., Weijtens M., Carey B.R., Miller C.A., Swann E.M., Wolff M., Loblein H., Seaman M.S., Dolin R., Barouch D.H. Induction of HIV-1-Specific mucosal immune responses following intramuscular recombinant adenovirus serotype 26 HIV-1 vaccination of humans. J. Infect. Dis. 2015;211(4):518–528. doi: 10.1093/infdis/jiu485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergelson J.M., Cunningham J.A., Droguett G., Kurt-Jones E.A., Krithivas A., Hong J.S., Horwitz M.S., Crowell R.L., Finberg R.W. Isolation of a common receptor for coxsackie B viruses and adenoviruses 2 and 5. Science. 1997;275(5304):1320–1323. doi: 10.1126/science.275.5304.1320. [DOI] [PubMed] [Google Scholar]
- Berkner K.L. Development of adenovirus vectors for the expression of heterologous genes. Biotechniques. 1988;6(7):616–629. [PubMed] [Google Scholar]
- Bischoff J.R., Kirn D.H., Williams A., Heise C., Horn S., Muna M., Ng L., Nye J.A., Sampson-Johannes A., Fattaey A., Mccormick F. An adenovirus mutant that replicates selectively in p53-deficient human tumour cells. Science (New York, N.Y.) 1996;274(5286):373–376. doi: 10.1126/science.274.5286.373. [DOI] [PubMed] [Google Scholar]
- Bos R., Rutten L., Van Der Lubbe J.E.M., Bakkers M.J.G., Hardenberg G., Wegmann F., Zuijdgeest D., De Wilde A.H., Koornneef A., Verwilligen A., Van Manen D., Kwaks T., Vogels R., Dalebout T.J., Myeni S.K., Kikkert M., Snijder E.J., Li Z., Barouch D.H., Vellinga J., Langedijk J.P.M., Zahn R.C., Custers J., Schuitemaker H. Ad26 vector-based COVID-19 vaccine encoding a prefusion-stabilized SARS-CoV-2 spike immunogen induces potent humoral and cellular immune responses. NPJ Vaccines. 2020;5(91):s41541–00243. doi: 10.1038/s41541-020-00243-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boychyn M., Yim S.S.S., Shamlou P.A., Bulmer M., More J., Hoare M. Characterization of flow intensity in continuous centrifuges for the development of laboratory mimics. Chem. Eng. Sci. 2001;56(16):4759–4770. [Google Scholar]
- Capone S., Raggioli A., Gentile M., Battella S., Lahm A., Sommella A., Contino A.M., Urbanowicz R.A., Scala R., Barra F., Leuzzi A., Lilli E., Miselli G., Noto A., Ferraiuolo M., Talotta F., Tsoleridis T., Castilletti C., Matusali G., Colavita F., Lapa D., Meschi S., Capobianchi M., Soriani M., Folgori A., Ball J.K., Colloca S., Vitelli A. Immunogenicity of a new Gorilla adenovirus vaccine candidate for COVID-19. Mol. Ther. 2020 doi: 10.1016/j.ymthe.2021.04.022. S12525-0016(21), online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao Y., Shum H.C. ’Emerging aqueous two-phase systems: from fundamentals of interfaces to biomedical applications’. Chem. Soc. Rev. 2020;49(1):114–142. doi: 10.1039/c9cs00466a. [DOI] [PubMed] [Google Scholar]
- Chen K.D., Wu X.W., Yu D.S., Ou H.L., Li Y.H., Zhou Y.Q., Li L.J. Process optimization for the rapid production of adenoviral vectors for clinical trials in a disposable bioreactor system. Appl. Microbiol. Biotechnol. 2018;102(15):6469–6477. doi: 10.1007/s00253-018-9091-5. [DOI] [PubMed] [Google Scholar]
- Cheng C., Wang L., Ko S.Y., Kong W.P., Schmidt S.D., Gall J.G.D., Colloca S., Seder R.A., Mascola J.R., Nabel G.J. Combination recombinant simian or chimpanzee adenoviral vectors for vaccine development. Vaccine. 2015;33(51):7344–7351. doi: 10.1016/j.vaccine.2015.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifton N.J. Detection of β-Galactosidase activity: X-gal staining. Methods Mol. Biol. 2012;886:241–250. doi: 10.1007/978-1-61779-851-1_21. [DOI] [PubMed] [Google Scholar]
- Cortin V., Thibault J., Jacob D., Garnier A. High-titer adenovirus vector production in 293S cell perfusion culture. Biotechnol. Prog. 2004;20(3):858–863. doi: 10.1021/bp034237l. [DOI] [PubMed] [Google Scholar]
- D’souza A.A., Shegokar R. Polyethylene glycol (PEG): a versatile polymer for pharmaceutical applications. Expert Opin. Drug Deliv. 2016;13(9):1257–1275. doi: 10.1080/17425247.2016.1182485. [DOI] [PubMed] [Google Scholar]
- Davison E., Diaz R.M., Hart I.R., Santis G., Marshall J.F. Integrin Alpha5Beta1-Mediated adenovirus infection is enhanced by the integrin-activating antibody TS2/16. J. Virol. 1997;71(8):6204–6207. doi: 10.1128/jvi.71.8.6204-6207.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallaux F.J., Kranenburg O., Cramer S.J., Houweling A., Van Ormondt H., Hoeben R.C., Van Der Eb A.J. Characterization of 911: a new helper cell line for the titration and propagation of early region 1-Deleted adenoviral vectors. Hum. Gene Ther. 1996;7(2):215–222. doi: 10.1089/hum.1996.7.2-215. [DOI] [PubMed] [Google Scholar]
- Fallaux F.J., Bout A., Van Der Velde I., Van Den Wollenberg D.J., Hehir K.M., Keegan J., Auger C., Cramer S.J., Van Ormondt H., Van Der Eb A.J., Valerio D., Hoeben R.C. New helper cells and matched early region 1-Deleted adenovirus vectors prevent generation of replication-competent adenoviruses. Hum. Gene Ther. 1998;9(13):1909–1917. doi: 10.1089/hum.1998.9.13-1909. [DOI] [PubMed] [Google Scholar]
- Farid S.S. Process economics of industrial monoclonal antibody manufacture. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2007;848(1):8–18. doi: 10.1016/j.jchromb.2006.07.037. [DOI] [PubMed] [Google Scholar]
- Folegatti P.M., Ewer K.J., Aley P.K., Angus B., Becker S., Belij-Rammerstorfer S., Bellamy D., Bibi S., Bittaye M., Clutterbuck E.A., Dold C., Faust S.N., Finn A., Flaxman A.L., Hallis B., Heath P., Jenkin D., Lazarus R., Makinson R., Minassian A.M., Pollock K.M., Ramasamy M., Robinson H., Snape M., Tarrant R., Voysey M., Green C., Douglas A.D., Hill A.V.S., Lambe T., Gilbert S.C., Pollard A.J., Oxford Covid Vaccine Trial Group Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet. 2020;396(10249):467–478. doi: 10.1016/S0140-6736(20)31604-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilgenkrantz H., Duboc D., Juillard V., Couton D., Pavirani A., Guillet J.G., Briand P., Kahn A. Transient expression of genes transferred in vivo into heart using first-generation adenoviral vectors: role of the immune response. Hum. Gene Ther. 1995;6(10):1265–1274. doi: 10.1089/hum.1995.6.10-1265. [DOI] [PubMed] [Google Scholar]
- Glasel J.A. Validity of nucleic acid purities monitored by 260nm/280nm absorbance ratios. Biotechniques. 1995;18(1):62–63. [PubMed] [Google Scholar]
- Goldfarb A.R., Saidel L.J., Mosovich E. The ultraviolet absorption spectra of proteins. J. Biol. Chem. 1951;193(1):397–404. [PubMed] [Google Scholar]
- Graham F.L., Smiley J., Russell W.C., Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J. Gen. Virol. 1977;36(1):59–74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- Guo P., Xiao X., El-Gohary Y., Paredes J., Prasadan K., Shiota C., Wiersch J., Welsh C., Gittes G.K. A simplified purification method for AAV variant by polyethylene glycol aqueous two-phase partitioning. Bioengineered. 2013;4(2):103–106. doi: 10.4161/bioe.22293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S., Kamata T., Takada Y., Ruggeri Z.M., Nemerow G.R. Adenovirus interaction with distinct integrins mediates separate events in cell entry and gene delivery to hematopoietic cells. J. Virol. 1996;70(7):4502–4508. doi: 10.1128/jvi.70.7.4502-4508.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby I., Lord R., Davison E., Wickham T.J., Roelvink P.W., Kovesdi I., Sutton B.J., Santis G. Adenovirus type 9 Fiber knob binds to the coxsackie B virus-adenovirus receptor (CAR) with lower affinity than Fiber knobs of other CAR-Binding adenovirus serotypes. J. Virol. 2001;75(15):7210–7214. doi: 10.1128/JVI.75.15.7210-7214.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefesvre P., Van Bekkum D.W. The effect of adenoviral aggregation on gene transfer and toxicity in vitro and in vivo. Mol. Ther. 2003;7(5):199–200. [Google Scholar]
- Lesch H.P., Heikkila K.M., Lipponen E.M., Valonen P., Muller A., Rasanen E., Tuunanen T., Hassinen M.M., Parker N., Karhinen M., Shaw R., Yla-Herttuala S. Process development of adenoviral vector production in fixed bed bioreactor: from bench to commercial scale. Hum. Gene Ther. 2015;26(8):560–571. doi: 10.1089/hum.2015.081. [DOI] [PubMed] [Google Scholar]
- Liebowitz D., Lindbloom J.D., Brandl J.L., Garg S.J., Tucker S.N. High titre neutralising antibodies to influenza after oral tablet immunisation: a phase 1, randomised, placebo-controlled trial. Lancet Infect. Dis. 2015;15(9):1041–1048. doi: 10.1016/S1473-3099(15)00266-2. [DOI] [PubMed] [Google Scholar]
- Mcnally D.J., Darling D., Farzaneh F., Levison P.R., Slater N.K. Optimised concentration and purification of retroviruses using membrane chromatography. J. Chromatogr. A. 2014;1340:24–32. doi: 10.1016/j.chroma.2014.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittereder N., March K.L., Trapnell B.C. Evaluation of the concentration and bioactivity of adenovirus vectors for gene therapy. J. Virol. 1996;70(11):7498–7509. doi: 10.1128/jvi.70.11.7498-7509.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molino J.V.D., Lopes A.M., Viana Marques D.A., Mazzola P.G., Da Silva J.L., Hirata M.H., Hirata R.D.C., Gatti M.S.V., Pessoa A. Application of aqueous two-phase micellar system to improve extraction of adenoviral particles from cell lysate. Biotechnol. Appl. Biochem. 2018;65(3):381–389. doi: 10.1002/bab.1627. [DOI] [PubMed] [Google Scholar]
- Negrete A., Ling T.C., Lyddiatt A. Aqueous two-phase recovery of bio-nanoparticles: a miniaturization study for the recovery of bacteriophage T4. J. Chromatogr. B. 2007;854(1, 2):13–19. doi: 10.1016/j.jchromb.2007.03.041. [DOI] [PubMed] [Google Scholar]
- Negrete A., Ling T.C., Lyddiatt A. Production of adenoviral vectors and its recovery. Process. Biochem. 2007;42(7):1107–1113. [Google Scholar]
- Nemunaitis J., Ganly I., Khuri F., Arseneau J., Kuhn J., Mccarty T., Landers S., Maples P., Romel L., Randlev B., Reid T., Kaye S., Kirn D. Selective replication and oncolysis in p53 mutant tumors with ONYX-015, an E1B-55kD gene-deleted adenovirus, in patients with advanced head and neck Cancer: a phase II trial. Cancer Res. 2000;60(22):6359–6366. [PubMed] [Google Scholar]
- Oelmeier S.A., Dismer F., Hubbuch J. Application of an aqueous two-phase systems high-throughput screening method to evaluate mAb HCP separation. Biotechnol. Bioeng. 2011;108(1):69–81. doi: 10.1002/bit.22900. [DOI] [PubMed] [Google Scholar]
- Oelmeier S.A., Dismer F., Hubbuch J. Molecular dynamics simulations on aqueous two-phase systems - single PEG-Molecules in solution. BMC Biophys. 2012;5(14):5–14. doi: 10.1186/2046-1682-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan X., Wu L., Cao J., Guo W., Wang Z., Han B., Hu W. Recombinant adenovirus vector-mediated human MDA-7 gene transfection suppresses hepatocellular carcinoma growth in a mouse xenograft model. J. Biomed. Res. 2012;26(1):53–58. doi: 10.1016/S1674-8301(12)60007-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roelvink P.W., Lizonova A., Lee J.G.M., Li Y., Bergelson J.M., Finberg R.W., Brough D.E., Kovesdi I., Wickham T.J. The coxsackievirus-adenovirus receptor protein can function as a cellular attachment protein for adenovirus serotypes from subgroups A, C, D, E, and F. J. Virol. 1998;72(10):7909–7915. doi: 10.1128/jvi.72.10.7909-7915.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers R.D., Bond A.H., Bauer C.B., Zhang J., Griffin S.T. Metal ion separations in polyethylene glycol-based aqueous biphasic systems: correlation of partitioning behaviour with available thermodynamic hydration data. J. Chromatogr. B, Biomed. Appl. 1996;680(1–2):221–229. doi: 10.1016/0378-4347(95)00447-5. [DOI] [PubMed] [Google Scholar]
- Russell W.C. Adenoviruses: update on structure and function. J. Gen. Virol. 2009;90(Pt 1):1–20. doi: 10.1099/vir.0.003087-0. [DOI] [PubMed] [Google Scholar]
- Tan Z.J., Wang C.Y., Yang Z.Z., Yi Y.J., Wang H.Y., Zhou W.L., Li F.F. Ionic liquid-based ultrasonic-assisted extraction of secoisolariciresinol diglucoside from flaxseed (Linum usitatissimum L.) with further purification by an aqueous two-phase system. Molecules (Basel, Switzerland) 2015;20(10):17929–17943. doi: 10.3390/molecules201017929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ura T., Okuda K., Shimada M. Developments in viral vector-based vaccines. Vaccines. 2014;2(3):624–641. doi: 10.3390/vaccines2030624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vellinga J., Smith J.P., Lipiec A., Majhen D., Lemckert A., Van Ooij M., Ives P., Yallop C., Custers J., Havenga M. Challenges in manufacturing adenoviral vectors for global vaccine product deployment. Hum. Gene Ther. 2014;25(4):318–327. doi: 10.1089/hum.2014.007. [DOI] [PubMed] [Google Scholar]
- Wei Q., Fan J., Liao J., Zou Y., Song D., Liu J., Cui J., Liu F., Ma C., Hu X., Li L., Yu Y., Qu X., Chen L., Yu X., Zhang Z., Zhao C., Zeng Z., Zhang R., Yan S., Wu X., Shu Y., Reid R.R., Lee M.J., Wolf J.M., He T.C. Engineering the rapid adenovirus production and amplification (RAPA) cell line to expedite the generation of recombinant adenoviruses. Cell. Hysiol. Biochem. Int. J. Experimental Cell. Physiol. Biochem. Pharmacol. 2017;41(6):2383–2398. doi: 10.1159/000475909. [DOI] [PubMed] [Google Scholar]
- Xiang Z.Q., Gao G.P., Reyes-Sandoval A., Li Y., Wilson J.M., Ertl H.C.J. Oral vaccination of mice with adenoviral vectors is not impaired by preexisting immunity to the vaccine carrier. J. Virol. 2003;77(20):10780–10789. doi: 10.1128/JVI.77.20.10780-10789.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie L., Pilbrough W., Metallo C., Zhong T., Pikus L., Leung J., Auniņš J.G., Zhou W. Serum-free suspension cultivation of PER.C6(R) cells and recombinant adenovirus production under different pH conditions. Biotechnol. Bioeng. 2002;80(5):569–579. doi: 10.1002/bit.10443. [DOI] [PubMed] [Google Scholar]
- Yan B., Cao X. Preparation of aqueous two-phase systems composed of two pH-response polymers and liquid-Liquid extraction of demeclocycline. J. Chromatogr. A. 2012;1245:39–45. doi: 10.1016/j.chroma.2012.05.045. [DOI] [PubMed] [Google Scholar]
- Yang Y., Su Q., Wilson J.M. Role of viral antigens in destructive cellular immune responses to adenovirus vector-transduced cells in mouse lungs. J. Virol. 1996;70(10):7209–7212. doi: 10.1128/jvi.70.10.7209-7212.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu F.C., Li Y.A., Guan X.H., Hou L.H., Wang W.J., Li J.X., Wu S.P., Wang B.S., Wang Z., Wang L., Jia S.Y., Jiang H.D., Wang L., Jiang T., Hu Y., Gou J.B., Xu S.B., Xu J.J., Wang X.W., Wang W., Chen W. Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: a dose-escalation, open-label, non-randomised, First-In-Human trial. Lancet. 2020;395(10240):1845–1854. doi: 10.1016/S0140-6736(20)31208-3. [DOI] [PMC free article] [PubMed] [Google Scholar]