Abstract
Background and Objectives
It is important to identify at what age brain atrophy rates in genetic frontotemporal dementia (FTD) start to accelerate and deviate from normal aging effects to find the optimal starting point for treatment. We investigated longitudinal brain atrophy rates in the presymptomatic stage of genetic FTD using normative brain volumetry software.
Methods
Presymptomatic GRN, MAPT, and C9orf72 pathogenic variant carriers underwent longitudinal volumetric T1-weighted magnetic resonance imaging of the brain as part of a prospective cohort study. Images were automatically analyzed with Quantib® ND, which consisted of volume measurements (CSF and sum of gray and white matter) of lobes, cerebellum, and hippocampus. All volumes were compared with reference centile curves based on a large population-derived sample of nondemented individuals (n = 4,951). Mixed-effects models were fitted to analyze atrophy rates of the different gene groups as a function of age.
Results
Thirty-four GRN, 8 MAPT, and 14 C9orf72 pathogenic variant carriers were included (mean age = 52.1, standard deviation = 7.2; 66% female). The mean follow-up duration of the study was 64 ± 33 months (median = 52; range 13–108). GRN pathogenic variant carriers showed a faster decline than the reference centile curves for all brain areas, though relative volumes remained between the 5th and 75th percentiles between the ages of 45 and 70 years. In MAPT pathogenic variant carriers, frontal lobe volume was already at the 5th percentile at age 45 years and showed a further decline between the ages 50 and 60 years. Temporal lobe volume started in the 50th percentile at age 45 years but showed fastest decline over time compared with other brain structures. Frontal, temporal, parietal, and cerebellar volume already started below the 5th percentile compared with the reference centile curves at age 45 years for C9orf72 pathogenic variant carriers, but there was minimal decline over time until the age of 60 years.
Discussion
We provide evidence for longitudinal brain atrophy in the presymptomatic stage of genetic FTD. The affected brain areas and the age after which atrophy rates start to accelerate and diverge from normal aging slopes differed between gene groups. These results highlight the value of normative volumetry software for disease tracking and staging biomarkers in genetic FTD. These techniques could help in identifying the optimal time window for starting treatment and monitoring treatment response.
Frontotemporal dementia (FTD) is the second most common form of young-onset dementia, typically demonstrating atrophy of the frontal and/or temporal lobes.1 It is characterized by a heterogeneous profile with deterioration of behavioral (behavioral variant FTD, bvFTD), language (primary progressive aphasia, PPA), or motor skills.1-3 FTD has an autosomal dominant inheritance pattern in up to 30% of cases, with pathogenic variants in the GRN, MAPT, and C9orf72 genes being the most common.4 Cohort studies investigating the presymptomatic stage of FTD have demonstrated early changes in neuroimaging, cognition, blood, and CSF.5-13 In particular, brain atrophy, measured by structural MRI, is of interest as a biomarker of neurodegeneration and outcome measure in upcoming clinical trials.5,7,8,10,13
GRN pathogenic variants often lead to an asymmetrical pattern of atrophy in the frontal, temporal, and parietal lobes in later disease stages,14,15 and additional lower gray matter (GM) volume in the insula has been demonstrated in the presymptomatic stage.8,14 In symptomatic pathogenic variant carriers (PVCs), this typically results in a clinical diagnosis of bvFTD or nonfluent variant PPA (nfvPPA) and is often accompanied by parkinsonism.15 Pathogenic variants in the MAPT gene typically lead to focal anterior temporal lobe degradation, including the hippocampus,14 with additional presymptomatic changes in the amygdala and insula.7,8,10 bvFTD is the main phenotype in the symptomatic stage but can be accompanied by atypical parkinsonism such as corticobasal syndrome or progressive supranuclear palsy.14 Last, the atrophy associated with the C9orf72 repeat expansion is rather diffuse, with widespread GM volume loss, including not only the frontal and temporal cortices but also subcortical and cerebellar regions.16 In presymptomatic C9orf72 PVCs, lower GM volume of the thalamus, cerebellum, and frontal, temporal, parietal, and insular cortices has been found.7,8,10 In the symptomatic stage, this is usually accompanied not only by a clinical diagnosis of bvFTD, motor neuron disease, or a combination of both, but also notable psychiatric features.16
Longitudinal studies in presymptomatic FTD provide a unique opportunity to determine the age at which atrophy rates start to deviate from normal.17 Identifying a potential change point at which the atrophy rate accelerates compared with the normal aging process is essential for upcoming clinical trials because it can provide the best time window to start disease-modifying treatment. Yet, most studies investigating brain atrophy in presymptomatic FTD have been cross-sectional in nature and/or plotted using estimated years to onset as a proxy for actual symptom onset,7,8,11,13,18 which has been shown to be unreliable in C9orf72 and GRN because there is large variation between and within families.19 Only a few studies so far have investigated atrophy rates longitudinally.5,10 For example, One of these studies showed that the first changes occurred approximately 2 years before actual symptom onset in 8 FTD converters.5 However, it remains unclear at what age atrophy starts to accelerate compared with normal aging effects.
Software packages that apply automated normative quantitative assessment of brain MRI data are now emerging for clinical use. They provide the user with quantification of brain atrophy by segmentation of brain tissues and structures and compare it with a group of age-matched and sex-matched cognitively healthy individuals.20,21 These approaches are interesting for presymptomatic cohorts because there is evidence that they could lead to an earlier identification of atrophy than visual rating scales and can improve the accuracy of dementia diagnosis.21-23 However, to date, no study has investigated the application of such normative volumetry software in analyzing longitudinal presymptomatic genetic FTD data. The aim of this study was to investigate longitudinal atrophy rates in presymptomatic GRN, MAPT, and C9orf72 PVCs, using the FDA-cleared normative volumetry software package Quantib® Neurodegenerative (ND), to estimate the change points relative to age.
Methods
Participants
We included longitudinal data of 56 participants from the FTD Risk Cohort (FTD-RisC) of the Erasmus MC University Medical Center (Rotterdam, the Netherlands). FTD-RisC is an ongoing, longitudinal cohort study in which first-degree family members of C9orf72, GRN, or MAPT PVCs are followed up on a 1-year or 2-year basis.13 Participants for this study were recruited between December 2009 and October 2019.13 DNA genotyping at study entry assigned participants to either the PVC group or the noncarrier group. Inclusion criteria of the current study were as follows: 1) participants were C9orf72, GRN, or MAPT PVCs, 2) at study entry, all participants were presymptomatic (i.e., did not fulfill clinical diagnostic criteria for bvFTD,2 PPA,3 and/or ALS24), and had a Clinical Dementia Rating scale plus National Alzheimer Coordinating Center Frontotemporal Lobar Degeneration (CDR plus NACC FTLD) of 0, and 3) had undergone at least 1 MRI scan.25 Exclusion criteria for this study were as follows: 1) other neurologic that can affect brain volumetry and/or primary psychiatric disorders, and 2) presymptomatic PVCs younger than 45 years because the reference population in Quantib ND consists of cognitively healthy individuals aged between 45 and 95 years26 This resulted in the inclusion of 34 GRN, 8 MAPT, and 14 C9orf72 PVCs at baseline and 33 GRN, 5 MAPT, and 11 C9orf72 PVCs underwent 2 or more MRI scans (Table 1). Six PVCs developed clinical symptoms during the follow-up (C9orf72: one ALS and one FTD-ALS; GRN: one bvFTD and 2 nfvPPA; MAPT: one bvFTD) and progressed on the CDR plus NACC FTLD global score (eFigure 1, links.lww.com/WNL/C369). Participants' characteristics are summarized in Table 2.
Table 1.
Available Longitudinal Data
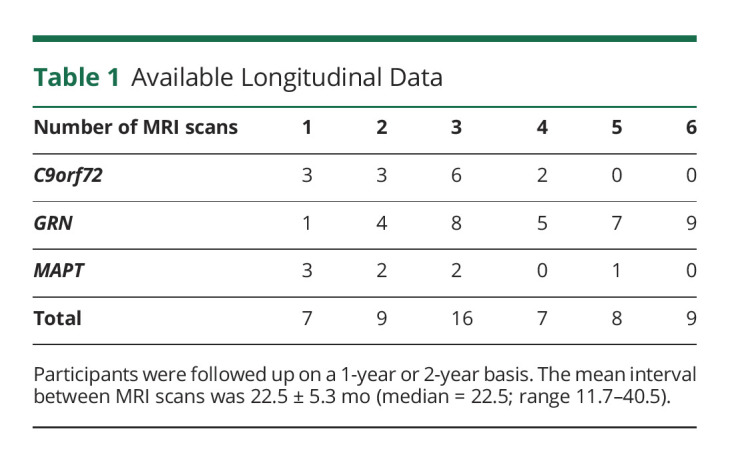
Table 2.
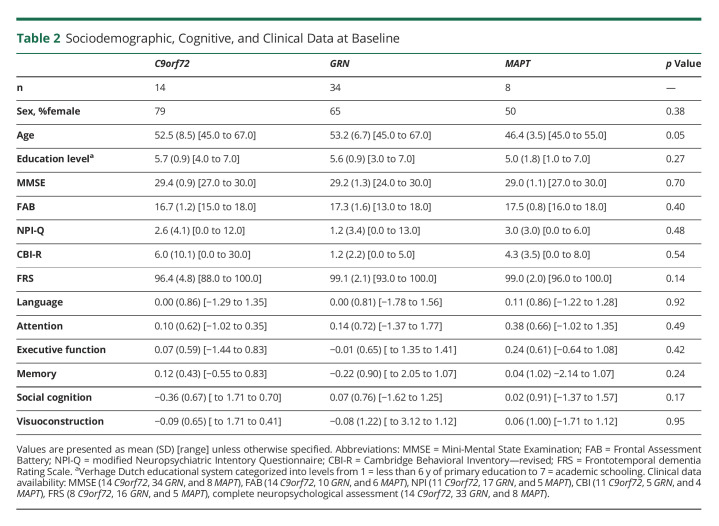
Sociodemographic, Cognitive, and Clinical Data at Baseline
Procedure
At every study visit, participants underwent a standardized clinical assessment consisting of a medical history, family history, neurologic examination, neuropsychological assessment, and brain MRI. Clinical status was based on these assessments and a structured clinical interview with the participant and a knowledgeable informant, including the CDR plus NACC FTLD, modified Neuropsychiatric Inventory Questionnaire (NPI-Q27), Cambridge Behavioral Inventory—Revised (CBI-R28), and Frontotemporal dementia Rating Scale (FRS29). The Mini-Mental State Examination (MMSE) and Frontal Assessment Battery (FAB) were used as global cognitive and frontal executive screeners, respectively.30,31 Neuropsychological assessment consisted of tests assessing language (60-item version Boston Naming Test, categorical fluency), attention and mental processing speed (Trail Making Test (TMT)—part A, Stroop Color-Word Interference Test (SCWIT) word and color naming card, Wechsler Adult Intelligence Scale (WAIS)-III, and digit span forward), executive functioning (TMT—part B, SCWIT ink naming card, modified Wisconsin Card Sorting Test, letter fluency, and WAIS-III digit span backward), memory (Dutch Rey Auditory Verbal Learning Test, Visual Association Test), social cognition (Ekman Faces, Happé Cartoons), and visuoconstruction (clock drawing).
Image Acquisition
Participants underwent volumetric T1-weighted MRI on a Philips 3T Achieva MRI scanner (Philips, Best, the Netherlands). We used an 8-channel SENSE head coil between December 2009 and 2016 and a 32-channel SENSE head coil from 2016 onward. Owing to ongoing recruitment, not all participants have the same number of scans made with either the 8-channel or 32-channel head coil (for numbers per visit, see eTable 1, links.lww.com/WNL/C369). A scanner software update was performed in May 2016. The following scan parameters were used: repetition time = 9.7 ms, echo time = 4.6 ms, field of view = 224 × 177 × 168 mm, flip angle = 8, slices = 140, voxel size = 0.88x0.88x1.20 mm, SENSE = none, total acquisition time = 4.56 minutes. All scans underwent extensive visual quality check. Images were analyzed using Quantib ND software.
Normative Volumetric Image Data Processing
Quantib® ND (quantib.com) is a postprocessing image analysis tool for T1-weighted images, which quantifies the volume of brain tissues and various structures. The automatic analysis consists of segmentation and volume measurements of brain tissues (CSF and sum of GM and white matter [WM]), intracranial volume (ICV), total brain volume, brain lobes (frontal, temporal, parietal, and occipital), cerebellum, and hippocampus. The algorithms for the segmentation are based on studies conducted by Vrooman et al.20 and Fortunati et al.21 The end outputs for each brain structure are total and lateralized volumes in mm3.The lateralized volumes are expressed as a percentage of the total ICV (%ICV). Percentile scores are calculated by comparing %ICV scores with reference centile curves based on a large population of cognitively healthy individuals.26 The reference population consisted of 4,951 people aged 45–95 years from the Rotterdam study, the largest Dutch prospective cohort study, whose scans were acquired on 1.5T MRI (GE Healthcare, US) between 2005 and 2015. The Rotterdam study is described elsewhere in more detail.26 See, for an example, output file from Quantib® ND eAppendix 1, links.lww.com/WNL/C369.
Statistical Analysis
All statistical analyses were conducted using R v4.0.4. All raw neuropsychological test scores were standardized to z scores, and composite cognitive domain scores were calculated by averaging the z scores of the individual tests (as described in Procedure) per assessment. We compared the continuous sociodemographic and clinical data at baseline between gene groups with one-way ANOVAs and the chi-square tests for dichotomous variables. The significance level was set at p < 0.05 (two-tailed). Clinical and cognitive data were longitudinally assessed with linear mixed-effects models including time since baseline, gene group, and for cognitive data, also age at baseline and education level as fixed effects. We tested the best-fitting model by comparing models with random intercepts and models with additional random slopes (eTable 2, links.lww.com/WNL/C369).
Because this is an ongoing study, participants varied in the number of visits that were completed, and 7 individuals had only 1 available MRI scan (Table 1). All available data were included in the analyses to increase the sample size and match the age range of the control cohort within Quantib® ND software. For 5 converters, all MRI scans up until the clinical diagnosis were included, and for 1 converter, 1 MRI scan 1 year after diagnosis was included. A mixed-effects model with natural cubic splines was fitted for each brain structure to analyze differences in brain atrophy between gene groups as a function of age. This type of model allows for the analysis of longitudinal data with unbalanced time points and missing data (including individuals with 1 MRI scan). In each model, %ICV of the brain structure of interest was used as a dependent variable, and we specified the following fixed effects: age, gene group, age × gene group, sex, and scanner software update. Time interval between MRI scans was not included as a covariate because age controls for the time between MRI scans. Brain structures' %ICV were calculated by dividing the volume of the structure by the total ICV at baseline and corrected for the change in head coil by estimating the beta coefficients of the change in head coil in non-PVCs from FTD-RisC (n = 163)13 and subtracting the beta coefficient from the %ICV. We tested the best-fitting model by comparing models with random intercepts and/or slopes with 2 or 3 splines. An overview of the best-fitting models per brain structure is summarized in eTable 2, links.lww.com/WNL/C369. All assumptions were checked and met. Abnormal brain volume was defined as a %ICV score ≤5th percentile. %ICV scores were plotted against the reference centile curves as outputted by Quantib® ND.
Standard Protocol Approvals, Registrations, and Patient Consents
The study was approved by the Medical and Ethical Review Committee of the Erasmus Medical Center, and participants' written consent was obtained according to the Declaration of Helsinki.
Data Availability
Anonymized data not published within this article will be made available on reasonable request from any qualified investigator.
Results
Sociodemographic, Cognitive, and Clinical Data
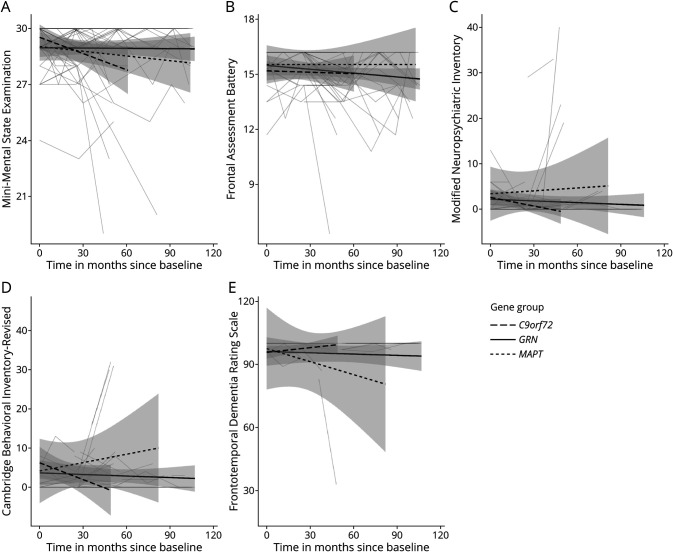
There was a significant difference between gene groups in age (F(2,53) = 3.26, p = 0.05) at baseline (Table 2). At study entry, MAPT PVCs were younger than GRN PVCs (p = 0.04). There were no differences between gene groups in sex (X2(2) = 1.9, p = 0.38), education (F(2,53) = 1.33, p = 0.27), MMSE (F(2, 46) = 0.69, p = 0.51), FAB (F(2, 22) = 1.24, p = 0.31), NPI-Q (F(2,30) = 0.75, p = 0.48), CBI-R (F(2,17) = 0.63, p = 0.54), or FRS (F(2,25) = 2.11, p = 0.14) at baseline. A main effect of time was found on the MMSE (F(1,120) = 5.25, p = 0.02) and FRS (F(1,56) = 5.94, p = 0.02; Figure 1E), but there was no change over time on the FAB (F(1,93) = 3.5, p = 0.07); Figure 1B), NPI-Q (F(1,85) = 0.92, p = 0.34; Figure 1C), and CBI-R (F(1,69) = 0.06, p = 0.81; Figure 1D).
Figure 1. Longitudinal Trajectories of the (A) Mini-Mental State Examination, (B) Frontal Assessment Battery, (C) Modified Neuropsychiatric Inventory, (D) Cambridge Behavioral Inventory—Revised, and (E) Frontotemporal Dementia Rating Scale.
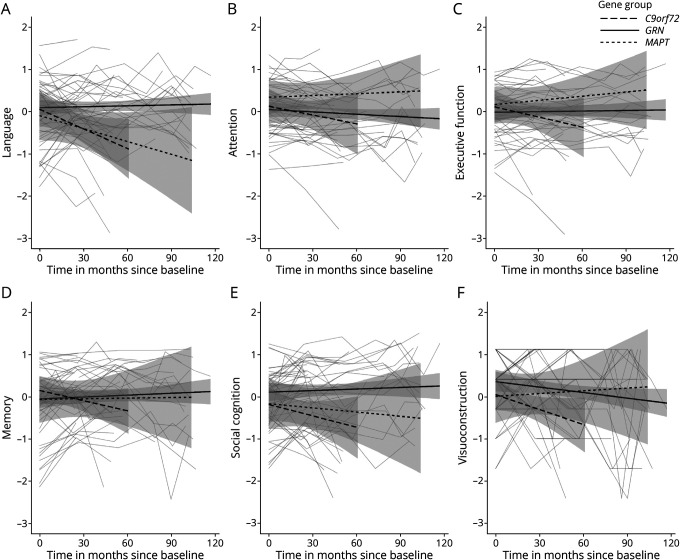
There were no differences between gene groups at baseline on any of the cognitive domains (all p > 0.05; Table 1). There was no main effect of time on any of the cognitive domains (language: F(1,133) = 0.16, p = 0.69, Figure 2A; attention: F(1,132) = 2.16, p = 0.14, Figure 2B; executive function: F(1,130) = 1.19, p = 0.28, Figure 2C; memory: F(1,131) = 0.57, p = 0.45, Figure 2D; social cognition: F(1,131) = 2.01, p = 0.16, Figure 2E; and visuoconstruction: F(1, 133) = 1.32, p = 0.25. Figure 2F), but evidence indicated that C9orf72 (β = −0.02, SE = 0.01, p < 0.01) and MAPT (β = −0.01, SE = 0.00, p = 0.05) PVCs declined on language compared with GRN PVCs (Figure 2A).
Figure 2. Longitudinal Trajectories of the (A) Language, (B) Attention and Mental Processing Speed, (C) Executive Function, (D) Memory, (E) Social Cognition, and (F) Visuoconstruction Domain.
Brain Volume Trajectories
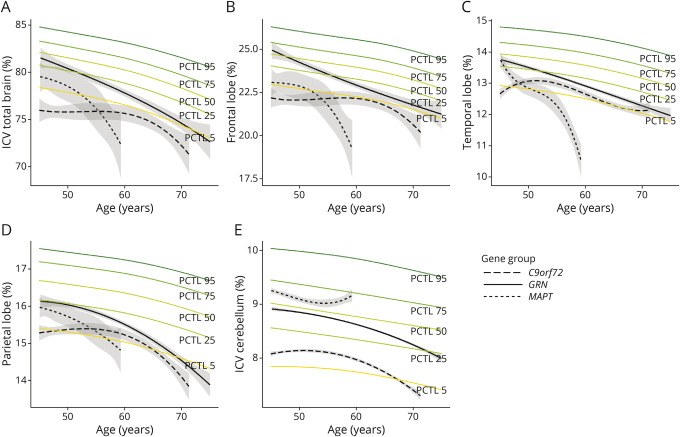
There were interaction effects between age and gene group in the %ICV of the total brain (LR = 17.03, p = 0.002), frontal lobe (LR = 25.00, p < 0.001), and temporal lobe (LR = 48.20, p < 0.001), indicating a different slope per gene group. Model output for lateralized and total brain structures are summarized in eTable 3, links.lww.com/WNL/C369.
In GRN PVCs, a faster decline than the reference centile curves was visible in Figure 3A-3D for total brain volume and frontal, temporal, and parietal lobe volume from age 45 years onward, though all brain structures' percentile scores remained in the normal range between ages 45 and 70 years. For the occipital lobe, cerebellar, and hippocampal volumes, a faster decline than the reference centile curves was visible from age 60 years onward. A steeper decline in brain volume was visible for the left than for the right temporal lobe (eTable 3, links.lww.com/WNL/C369).
Figure 3. Atrophy Rates Compared With Reference Centile Curves for (A) Total Brain, (B) Frontal Lobe, (C) Temporal Lobe, (D) Parietal Lobe, and (E) Cerebellum.
Abbreviations: PCTL = Percentile.
The total brain volume in MAPT PVCs showed the fastest decline compared with the other gene groups because it already crossed the 5th percentile at approximately age 50 years (Figure 3A), whereas at age 45 years, it was still in the normal range. Frontal lobe volume was at the 5th percentile at age 45 years, showing a further decline between ages 50 and 60 years (Figure 3B). The temporal lobe volume was still around the 50th percentile at age 45 years but subsequently showed the fastest decline compared with the other brain structures (Figure 3C). This decline was more pronounced in the left than in the right temporal lobe. Parietal lobe volume declined less steeply than the frontal and temporal structures, albeit still steeper than the reference centile curves, crossing the 5th percentile at approximately age 60 years (Figure 3D). Occipital lobe, cerebellar, and hippocampal volumes remained within the normal range across the entire age range.
C9orf72 PVCs had overall lower total brain volume than the other gene groups, already starting below the 5th percentile compared with reference/normative data at age 45 years, though with minimal decline over time until age 60 years (Figure 3A). Frontal lobe volume was the lowest, being already below the 5th percentile at age 45 years (Figure 3B), and the temporal and parietal lobes and cerebellum largely followed the 5th reference centile curve between ages 45 and 70 years (Figure 3C-3E). Occipital lobe and hippocampal volumes remained in the average range.
Surprisingly, small increases in occipital lobe volume for C9orf72 and MAPT PVCs, and in hippocampus volume for MAPT PVCs were observed, though not statistically significant (eTable 3, links.lww.com/WNL/C369). Intraindividual trajectories revealed that this increase occurred between time points when the study switched from an 8-channel to a 32-channel head coil.
All analyses were rerun without the scans of the converters (n = 6), and interpretation of the results remained similar. Atrophy rates for the 6 individual converters can be found in eFigures 1–2, links.lww.com/WNL/C369.
Discussion
The aim of this study was to investigate longitudinal atrophy rates in presymptomatic GRN, MAPT, and C9orf72 PVCs older than 45 years, against a background of normal age-related brain changes. To this end, we used Quantib® ND, which enabled comparison with a large normative population-derived reference dataset. We compared atrophy rates over time in lobar volumes, hippocampus, and cerebellum between gene groups and found gene-specific differences for the age and brain areas at which atrophy rates start to diverge from normal. The results from this study confirm more progressive atrophy rates in cognitively healthy FTD PVCs than can be expected from normal aging, indicating the diagnostic value of normative brain volumetry software in genetic FTD. Furthermore, by identifying the pattern and timing of brain changes and the speed of change over time, these results hold important potential for upcoming gene-specific clinical trials because they provide insight into the best time window to start treatment in the different PVCs.
GRN PVCs showed brain structure volumes between the 5th and 75th age-specific and sex-specific normative percentile over an age range of 45–70 years, but showed more progressive decline than the reference centile curves for frontal, temporal, and parietal lobe volume from age 45 years onward, and for occipital lobe, cerebellum, and hippocampus from age 60 years, without evidence of cognitive decline. This means that although on group level, presymptomatic GRN PVCs were never in the absolute “abnormal” range compared with the normative population, they declined faster over time than what would be expected in normal aging. This finding provides an explanation as to why previous cross-sectional studies did not find differences between presymptomatic GRN PVCs and controls8,10 and highlights the value of multitime point data. A study on the temporal ordering of biomarker changes indeed showed that structural MRI biomarkers are relatively late to change in GRN-related FTD, when compared with, for example, language functioning and neurofilament light chain.32 This coincides with other studies on cognitive and fluid biomarkers, which similarly showed no decline compared with controls in the presymptomatic stage, with rapid changes occurring in a short time frame before overt disease onset.6,9,10 A longitudinal imaging study demonstrated a time window of approximately 2 years before symptom onset in which brain volume deteriorated rapidly in 8 GRN PVCs.5 The volume loss in 3 GRN converters from this study similarly showed a steep decline over time from approximately the 80th percentile to below the 5th percentile in a 5-year period. One hypothesis is that additional injury (a second hit) is required to start the neurodegenerative process, which is then followed by rapid brain volume loss and symptom onset.8,33,34 A second hypothesis is the asymmetry often seen in GRN, which can variably affect the right or left hemisphere and thereby mediate effects found on group level.5,14,33 Nonetheless, in our study, a more progressive decline was visible from the fourth decade of life compared with the reference centile curves in the absence of cognitive decline. Moreover, the brain areas that showed faster decline over time overlap with atrophy signatures found in symptomatic GRN PVCs, including atrophy of dorsolateral and ventromedial prefrontal, superolateral temporal, and lateral parietal lobe areas, anterior cingulate, insula, precuneus, and striatum.8,15 This indicates the progression of a neurodegenerative process that is only accompanied by cognitive changes in later stages, most specifically in frontal-mediated executive functions and social cognition.14,15 A possible explanation for why our findings demonstrate a decline over time and previous studies did not could be the longer follow-up time. It could be that although decline is not statistically detectable with a follow-up of 2 or 4 years or on cognitive measures, marginal decline in brain volume is already set in motion. This underlines the importance of continuing current longitudinal cohort studies to further unravel disease processes.
Frontal lobe volume was already below the 5th percentile at age 45 years in MAPT PVCs, with progressive decline over time in the frontal and parietal lobes, but most notably temporal lobe, crossing the 5th reference centile curve at age 55 years. This decline was more pronounced in the left than in the right temporal lobe and was accompanied by a decline in language functioning. These findings are in line with previous studies investigating atrophy in symptomatic MAPT PVCs, showing anteromedial temporal lobe atrophy as the key neuroimaging feature and as a result semantic and/or episodic memory problems.8,14,35 Mild impairments have even been demonstrated as early as the presymptomatic stage.36,37 However, cross-sectional studies investigating presymptomatic MAPT PVCs have not been able to demonstrate differences that survived multiple testing correction compared with controls, while uncorrected results indicated similar temporal areas to be affected first.8,10,35 Rohrer et al.7 found decline in the temporal lobe and hippocampus approximately 10–15 years before estimated symptom onset. Following this, a 4-year follow-up study using actual age at symptom onset showed GM volume changes in the temporal lobe and WM changes in the uncinate fasciculus up to 2 years before symptom onset in MAPT converters.5 The progressive decline that is already seen from the age of 45 years onward in this study is probably because the MAPT PVCs are closer to symptom onset due to the younger average age at symptom onset in this group.19 Most of the MAPT PVCs in our cohort come from families with a P301L variant, and previous studies have shown an average age at symptom onset of 51 years,38 explaining why our results indicate a fast decline in the frontal and temporal lobes in the first 10-year age bracket of our study (i.e. between ages 45 and 55 years).
C9orf72 PVCs had lower volumes of the frontal lobe, temporal lobe, and cerebellum at age 45 years compared with the other 2 gene groups, and these were already below the 5th percentile compared with the age-matched healthy controls. Notably, a decline in language functioning, but not any of the other cognitive domains, was observed compared with that in GRN PVCs. Brain volume remained quite stable below the 5th percentile between ages 45 and 70 years, showing minimal volume decline with increasing age. Previous cross-sectional studies investigating brain changes in presymptomatic C9orf72 PVCs have indeed shown differences in frontal, temporal, and cerebellar structures compared with controls.7,8,10,18 A two-year follow-up study showed cross-sectional differences between presymptomatic C9orf72 PVCs and noncarriers in GM volume of the cerebellum and frontal and planum temporale, but without significant change over time.10 Lee et al.39 showed focal GM and structural and functional connectivity deficits from the fourth decade of life, similar to what the results of this study showed. In addition, this is accompanied by early changes in other modalities, such as reduced WM integrity5,39 and reduced cerebral blood flow.12 These deficits may represent the earliest signs of neurodegeneration that already start before the fourth decade of life. Our data suggest that brain volume loss already occurs before the age of 45 years, but there is relatively minimal decline or cognitive deterioration over time. It could be that the neurodegenerative process associated with the C9orf72 repeat expansion can be very slowly progressive in nature. An alternative hypothesis suggests that early deficits in C9orf72 PVCs might be caused by abnormal brain development. The C9orf72 protein is believed to play an essential role in the development of the central nervous system, and loss of the protein because of altered expression affects brain development.39-41 Thus, it has been hypothesized that C9orf72 PVCs already have lower brain volume from birth, which is superimposed by an additional neurodegenerative process later in life.39,40 It has been suggested that presymptomatic C9orf72 PVCs exert a lifelong neuropsychiatric vulnerability that manifests as personality and behavioral changes early on in life.42 Recent studies investigating the neuroanatomical associations of psychiatric symptoms in C9orf72 PVCs have indeed shown a widespread pattern of cortical (i.c. frontal, temporal, parietal, and occipital lobes and insula) and subcortical (i.c. basal ganglia, thalamus, and cerebellum) GM volume loss similar to the widespread atrophy profile found in this study.43
Over the past decade, automated normative quantification of brain morphology, function, connectivity, and pathology has improved considerably with machine and deep learning techniques.44 Different types of approaches have been investigated in the detection and classification of dementia and proven sensitive, but most often in Alzheimer dementia.21,22,44,45 Studies in FTD are more limited in number and focus on diagnostic accuracy and classification between different types of dementia.46-48 Our study has investigated brain volume loss in presymptomatic genetic FTD with up to 9 years of follow-up, using software for automated normative brain volumetry. Future work could combine this work with machine learning classification techniques, toward an artificial intelligence-based tracking tool for disease onset and progression predicting the change point in atrophy and other biomarkers at an individual level.
Major strengths of this study are the inclusion of all 3 major genetic causes of FTD, the long follow-up time, the use of nonlinear modeling, and automated software for normative volumetry, allowing a direct comparison of the gene-specific model predictions with a very large reference population. Thus far, most studies in genetic FTD have compared with much smaller sample sizes of noncarrying family members with the same number of follow-ups, instead of comparing with normative data from a reference population. The relatively long follow-up time in this study also allowed the use of a mixed-effects model with natural cubic splines so that a nonlinear change of brain volume over time could be modeled without making assumptions about the shape of this change.49 Previous longitudinal neuroimaging studies in genetic FTD have typically used linear models,5,10,11 where a unit change in time is associated with a constant change in the outcome. As is reflected in the current results, this does not necessarily apply to the neurodegenerative process of genetic FTD. Nonlinear models might be more suitable for the analysis of longitudinal atrophy rates, similar to what has been shown in presymptomatic atrophy in genetic Alzheimer dementia.17 A limitation of our study is the relatively small sample size of the MAPT and C9orf72 cohorts and should therefore be carefully interpreted. Replication in larger cohorts, such as GENFI, is therefore warranted. This is partly due to the age range of 45–95 years of the reference population used in Quantib ND. As a result, we had to exclude presymptomatic PVCs younger than 45 years, resulting in smaller sample sizes. This is unfortunate because our data suggest that for some gene groups, the acceleration point lies before that time. Specifically, we cannot corroborate claims about a possible neurodevelopmental component in C9orf72-related FTD. Another drawback of this study is that Quantib® ND provides segmentation only for the (lateralized) lobes, cerebellum, and hippocampus and no other substructures. Furthermore, 6 PVCs developed FTD during study follow-up, and this could have affected the results. However, the analyses were also performed without the scans of the converters, and results remained largely similar, so that the influence is likely negligible. Last, a seemingly paradoxical increase in occipital lobe volume was visible for MAPT and C9orf72 PVCs and to a lesser extent in the hippocampus for MAPT PVCs, between ages 45 and 60 years. This seems to be caused by the change in head coil during study follow-up, which influenced the segmentation process, especially for the occipital lobe. This can be explained by a higher signal-to-noise ratio with the 32ch coil for specifically posterior cortical areas.50 Panman et al.50 compared the use of the 8ch and 32ch coil in FTD-RisC and found higher GM volume in the occipital lobe, cerebellum, and subcortical areas using the 32ch coil, whereas frontal, temporal, and parietal GM volume were smaller using the 32ch coil.50
To conclude, we investigated longitudinal brain volume patterns in cognitively healthy GRN, MAPT, and C9orf72 PVCs using automated software for normative volumetry. We provide evidence for accelerated brain volume loss in the presymptomatic stage of FTD, and, in addition, gene-specific atrophy patterns in the frontal and temporal lobes, in the absence of prominent cognitive decline. These results confirm the value of accelerated brain atrophy as a disease-tracking and staging biomarker in genetic FTD and could inform upcoming clinical trials in characterizing the optimal time window for starting treatment and could help monitoring treatment response.
Acknowledgment
The authors thank all participants and their family members for participating in our study.
Glossary
- FAB
frontal assessment Battery
- FTD
frontotemporal dementia
- GM
gray matter
- ICV
intracranial volume
- MMSE
Mini-Mental State Examination
- NPI-Q
Neuropsychiatric Inventory Questionnaire
- PVCs
pathogenic variant carriers
- TMT
Trail Making Test
- WM
white matter
Appendix. Authors
Footnotes
Editorial, page 1077
Study Funding
This work was supported by the Dioraphte Foundation (grant numbers 09-02-00); the Association for Frontotemporal Dementias Research Grant 2009; The Netherlands Organization for Scientific Research (NWO) grant HCMI (grant number 056-13-018); ZonMw Memorabel (Deltaplan Dementie, [project numbers 733 050 103 and 733 050 813]; JPND PreFrontAls consortium project number 733051042; and Alzheimer Nederland and the Bluefield project.
Disclosure
Quantib B.V. is a spin-off company of Erasmus MC. Erasmus MC owns stocks in Quantib B.V. The authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Seelaar H, Rohrer JD, Pijnenburg YA, Fox NC, van Swieten JC. Clinical, genetic and pathological heterogeneity of frontotemporal dementia: a review. J Neurol Neurosurg Psychiatry. 2011;82(5):476-486. [DOI] [PubMed] [Google Scholar]
- 2.Rascovsky K, Hodges JR, Knopman D, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011;134(Pt 9):2456-2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gorno-Tempini ML, Hillis AE, Weintraub S, et al. Classification of primary progressive aphasia and its variants. Neurology. 2011;76(11):1006-1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lashley T, Rohrer JD, Mead S, Revesz T. An update on clinical, genetic and pathological aspects of frontotemporal lobar degenerations. Neuropathol Appl Neurobiol. 2015;41(7):858-881. [DOI] [PubMed] [Google Scholar]
- 5.Jiskoot LC, Panman JL, Meeter LH, et al. Longitudinal multimodal MRI as prognostic and diagnostic biomarker in presymptomatic familial frontotemporal dementia. Brain. 2018;142(1):193-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jiskoot LC, Panman JL, van Asseldonk L, et al. Longitudinal cognitive biomarkers predicting symptom onset in presymptomatic frontotemporal dementia. J Neurol. 2018;265(6):1381-1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rohrer JD, Nicholas JM, Cash DM, et al. Presymptomatic cognitive and neuroanatomical changes in genetic frontotemporal dementia in the Genetic Frontotemporal dementia Initiative (GENFI) study: a cross-sectional analysis. Lancet Neurol. 2015;14(3):253-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cash DM, Bocchetta M, Thomas DL, et al. Patterns of gray matter atrophy in genetic frontotemporal dementia: results from the GENFI study. Neurobiol Aging. 2018;62:191-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van der Ende EL, Meeter LH, Poos JM, et al. Serum neurofilament light chain in genetic frontotemporal dementia: a longitudinal, multicentre cohort study. Lancet Neurol. 2019;18(12):1103-1111. [DOI] [PubMed] [Google Scholar]
- 10.Panman JL, Jiskoot LC, Bouts M, et al. Gray and white matter changes in presymptomatic genetic frontotemporal dementia: a longitudinal MRI study. Neurobiol Aging. 2019;76:115-124. [DOI] [PubMed] [Google Scholar]
- 11.Benussi A, Gazzina S, Premi E, et al. Clinical and biomarker changes in presymptomatic genetic frontotemporal dementia. Neurobiol Aging. 2019;76:133-140. [DOI] [PubMed] [Google Scholar]
- 12.Mutsaerts HJMM, Mirza SS, Petr J, et al. Cerebral perfusion changes in presymptomatic genetic frontotemporal dementia: a GENFI study. Brain. 2019;142(4):1108-1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dopper EGP, Rombouts SARB, Jiskoot LC, et al. Structural and functional brain connectivity in presymptomatic familial frontotemporal dementia. Neurology. 2014;83(2):e19–e26. [DOI] [PubMed] [Google Scholar]
- 14.Rohrer JD, Warren JD. Phenotypic signatures of genetic frontotemporal dementia. Curr Opin Neurol. 2011;24(6):542-549. [DOI] [PubMed] [Google Scholar]
- 15.Le Ber I, Camuzat A, Hannequin D, et al. Phenotype variability in progranulin mutation carriers: a clinical, neuropsychological, imaging and genetic study. Brain. 2008;131(Pt 3):732-746. [DOI] [PubMed] [Google Scholar]
- 16.Mahoney CJ, Downey LE, Ridgway GR, et al. Longitudinal neuroimaging and neuropsychological profiles of frontotemporal dementia with C9ORF72 expansions. Alzheimers Res Ther. 2012;4(5):41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kinnunen KM, Cash DM, Poole T, et al. Presymptomatic atrophy in autosomal dominant Alzheimer's disease: a serial magnetic resonance imaging study. Alzheimer's Demen. 2018;14(1):43-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Papma JM, Jiskoot LC, Panman JL, et al. Cognition and gray and white matter characteristics of presymptomatic C9orf72 repeat expansion. Neurology. 2017;89(12):1256-1264. [DOI] [PubMed] [Google Scholar]
- 19.Moore KM, Nicholas J, Grossman M, et al. Age at symptom onset and death and disease duration in genetic frontotemporal dementia: an international retrospective cohort study. Lancet Neurol. 2020;19(2):145-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vrooman HA, Cocosco CA, van der Lijn F, et al. Multi-spectral brain tissue segmentation using automatically trained k-Nearest-Neighbor classification. Neuroimage. 2007;37(1):71-81. [DOI] [PubMed] [Google Scholar]
- 21.Fortunati V, Verhaart RF, Niessen WJ, Veenland JF, Paulides MM, van Walsum T. Automatic tissue segmentation of head and neck MR images for hyperthermia treatment planning. Phys Med Biol. 2015;60(16):6547. [DOI] [PubMed] [Google Scholar]
- 22.Brewer JB, Magda S, Airriess C, Smith ME. Fully-automated quantification of regional brain volumes for improved detection of focal atrophy in Alzheimer disease. Am J Neuroradiology. 2009;30(3):578-580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vernooij MW, Jasperse B, Steketee R, et al. Automatic normative quantification of brain tissue volume to support the diagnosis of dementia: a clinical evaluation of diagnostic accuracy. NeuroImage: Clin. 2018;20:374-379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brooks BR, Miller RG, Swash M, Munsat TL. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler other Mot Neuron Disord. 2000;1(5):293-299. [DOI] [PubMed] [Google Scholar]
- 25.Miyagawa T, Brushaber D, Syrjanen J, et al. Utility of the global CDR® plus NACC FTLD rating and development of scoring rules: data from the ARTFL/LEFFTDS Consortium. Alzheimer’s Demen. 2020;16(1):106-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ikram MA, Brusselle GGO, Murad SD, et al. The Rotterdam Study: 2018 update on objectives, design and main results. Eur J Epidemiol. 2017;32(9):807-850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaufer DI, Cummings JL, Ketchel P, et al. Validation of the NPI-Q, a brief clinical form of the Neuropsychiatric Inventory. J Neuropsychiatry Clin Neurosciences. 2000;12(2):233-239. [DOI] [PubMed] [Google Scholar]
- 28.Wear HJ, Wedderburn CJ, Mioshi E, et al. The Cambridge behavioural inventory revised. Demen Neuropsychologia. 2008;2(2):102-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mioshi E, Hsieh S, Savage S, Hornberger M, Hodges JR. Clinical staging and disease progression in frontotemporal dementia. Neurology. 2010;74:1591-1597. [DOI] [PubMed] [Google Scholar]
- 30.Folstein MF, Folstein SE, McHugh PR. Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189-198. [DOI] [PubMed] [Google Scholar]
- 31.Dubois B, Slachevsky A, Litvan I, Pillon B. The FAB: a frontal assessment battery at bedside. Neurology. 2000;55(11):1621-1626. [DOI] [PubMed] [Google Scholar]
- 32.Panman JL, Venkatraghavan V, van der Ende EL, et al. Modelling the cascade of biomarker changes in GRN-related frontotemporal dementia. J Neurol Neurosurg Psychiatry. 2021;92(5):494-501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rohrer JD, Ridgway GR, Modat M, et al. Distinct profiles of brain atrophy in frontotemporal lobar degeneration caused by progranulin and tau mutations. Neuroimage. 2010;53(3):1070-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martens LH, Zhang J, Barmada SJ, et al. Progranulin deficiency promotes neuroinflammation and neuron loss following toxin-induced injury. J Clin Invest. 2012;122(11):3955-3959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chu SA, Flagan TM, Staffaroni AM, et al. Brain volumetric deficits in MAPT mutation carriers: a multisite study. Ann Clin translational Neurol. 2021;8(1):95-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Poos JM, Russell LL, Peakman G, et al. Impairment of episodic memory in genetic frontotemporal dementia: a GENFI study. Alzheimer's Demen Diagn Assess Dis Monit. 2021;13(1):e12185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moore K, Convery R, Bocchetta M, et al. A modified Camel and Cactus Test detects presymptomatic semantic impairment in genetic frontotemporal dementia within the GENFI cohort. Appl Neuropsychol Adult. 2020:1-8. [DOI] [PubMed] [Google Scholar]
- 38.Daude N, Kim C, Kang S-G, et al. Diverse, evolving conformer populations drive distinct phenotypes in frontotemporal lobar degeneration caused by the same MAPT-P301L mutation. Acta neuropathologica. 2020;139(6):1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee SE, Sias AC, Mandelli ML, et al. Network degeneration and dysfunction in presymptomatic C9ORF72 expansion carriers. NeuroImage: Clin. 2017;14:286-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lulé DE, Müller H-P, Finsel J, et al. Deficits in verbal fluency in presymptomatic C9orf72 mutation gene carriers—a developmental disorder. J Neurol Neurosurg Psychiatry 2020;91(11):1195-1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miller JA, Ding S-L, Sunkin SM, et al. Transcriptional landscape of the prenatal human brain. Nature. 2014;508(7495):199-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gossink F, Dols A, Stek ML, et al. Early life involvement in C9orf72 repeat expansion carriers. J Neurol Neurosurg Psychiatry. 2022;93(1):93-100. [DOI] [PubMed] [Google Scholar]
- 43.Devenney E, Landin-Romero R, Irish M, et al. The neural correlates and clinical characteristics of psychosis in the frontotemporal dementia continuum and the C9orf72 expansion. NeuroImage: Clin. 2017;13:439-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wen J, Thibeau-Sutre E, Diaz-Melo M, et al. Convolutional neural networks for classification of Alzheimer's disease: overview and reproducible evaluation. Med image Anal. 2020;63:101694. [DOI] [PubMed] [Google Scholar]
- 45.Bron EE, Smits M, Niessen WJ, Klein S. Feature selection based on the SVM weight vector for classification of dementia. IEEE J Biomed Health Inform. 2015;19(5):1617-1626. [DOI] [PubMed] [Google Scholar]
- 46.Möller C, Pijnenburg YAL, van der Flier WM, et al. Alzheimer disease and behavioral variant frontotemporal dementia: automatic classification based on cortical atrophy for single-subject diagnosis. Radiology. 2016;279(3):838-848. [DOI] [PubMed] [Google Scholar]
- 47.Bron EE, Smits M, Papma JM, et al. Multiparametric computer-aided differential diagnosis of Alzheimer's disease and frontotemporal dementia using structural and advanced MRI. Eur Radiol. 2017;27(8):3372-3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bouts MJRJ, Möller C, Hafkemeijer A, et al. Single subject classification of Alzheimer's disease and behavioral variant frontotemporal dementia using anatomical, diffusion tensor, and resting-state functional magnetic resonance imaging. J Alzheimer's Dis. 2018;62(4):1827-1839. [DOI] [PubMed] [Google Scholar]
- 49.Croxford R. Restricted Cubic Spline Regression: A Brief Introduction: Institute for Clinical Evaluative Sciences; 2016:1-5. [Google Scholar]
- 50.Panman JL, To YY, Van Der Ende EL, et al. Bias introduced by multiple head coils in MRI research: an 8 channel and 32 channel coil comparison. Front Neurosci. 2019;13:729. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data not published within this article will be made available on reasonable request from any qualified investigator.