Abstract
Background and Objectives
The glymphatic system is a whole-brain perivascular network, which promotes CSF/interstitial fluid exchange. Alterations to this system may play a pivotal role in amyloid β (Aβ) accumulation. However, its involvement in Alzheimer disease (AD) pathogenesis is not fully understood. Here, we investigated the changes in noninvasive MRI measurements related to the perivascular network in patients with mild cognitive impairment (MCI) and AD. Additionally, we explored the associations of MRI measures with neuropsychological score, PET standardized uptake value ratio (SUVR), and Aβ deposition.
Methods
MRI measures, including perivascular space (PVS) volume fraction (PVSVF), fractional volume of free water in white matter (FW-WM), and index of diffusivity along the perivascular space (ALPS index) of patients with MCI, those with AD, and healthy controls from the Alzheimer's Disease Neuroimaging Initiative database were compared. MRI measures were also correlated with the levels of CSF biomarkers, PET SUVR, and cognitive score in the combined subcohort of patients with MCI and AD. Statistical analyses were performed with age, sex, years of education, and APOE status as confounding factors.
Results
In total, 36 patients with AD, 44 patients with MCI, and 31 healthy controls were analyzed. Patients with AD had significantly higher total, WM, and basal ganglia PVSVF (Cohen d = 1.15–1.48; p < 0.001) and FW-WM (Cohen d = 0.73; p < 0.05) and a lower ALPS index (Cohen d = 0.63; p < 0.05) than healthy controls. Meanwhile, the MCI group only showed significantly higher total (Cohen d = 0.99; p < 0.05) and WM (Cohen d = 0.91; p < 0.05) PVSVF. Low ALPS index was associated with lower CSF Aβ42 (rs = 0.41, pfdr = 0.026), FDG-PET uptake (rs = 0.54, pfdr < 0.001), and worse multiple cognitive domain deficits. High FW-WM was also associated with lower CSF Aβ42 (rs = −0.47, pfdr = 0.021) and worse cognitive performances.
Discussion
Our study indicates that changes in PVS-related MRI parameters occur in MCI and AD, possibly due to impairment of the glymphatic system. We also report the associations between MRI parameters and Aβ deposition, neuronal change, and cognitive impairment in AD.
The accumulation of extracellular amyloid β (Aβ), a pathologic hallmark of Alzheimer's disease, is initiated >15 years before the onset of dementia.1 This deposition results from multiple impaired brain clearance mechanisms.2 The glymphatic system has been suggested to be an essential component of Aβ clearance in the brain of rodents.3,4 Glymphatic dysfunction has been found to be correlated with Aβ accumulation; however, its involvement in the pathogenesis of Alzheimer disease in humans is yet to be fully understood. The elucidation of its mechanism may promote new diagnostic and therapeutic methods that delay or prevent the neurodegeneration in Alzheimer disease.
According to the glymphatic hypothesis,5 subarachnoid CSF enters the brain's interstitial space from the periarterial space through the AQP4 channel expressed in the astrocyte end-feet and then mixes with the interstitial fluid (ISF) and waste solutes in the brain. The resulting CSF/ISF exchange and waste products, such as Aβ, are then drained out of the brain by the perivenous efflux pathway. Recent studies have demonstrated the possibility of measuring glymphatic functions using MRI, with most of them being MRI tracer–based studies using a gadolinium-based contrast agent (GBCA).6 However, both intrathecal and intravenous GBCA administration are relatively invasive. Promising MRI-based noninvasive methods that do not require MRI tracers, namely, perivascular space (PVS) volumetry,7 calculation of the fractional volume of free water (FW) in brain parenchyma (i.e., brain ISF) from a bitensor diffusion tensor imaging (DTI) model,8 and calculation of the diffusivity along the PVS (ALPS) index,9 were recently introduced for the indirect evaluation of perivascular network activity.
Although the subject remains controversial, MRI-visible PVSs are considered to reflect periarterial spaces instead of perivenous spaces. A study using 7-T MRI reported that the PVS was spatially consistent with the trajectory of small arterioles, but not venules.10 Thus, an MRI-visible PVS corresponds to the CSF influx to the brain parenchyma of the glymphatic system. The dilatation of PVS, which is typically located in white matter (WM) at the centrum semiovale level, basal ganglia (BG), and hippocampus (Hipp), has been hypothesized to be secondary to ISF drainage blockage.11 Neuropathologic and MRI studies have reported that patients with Alzheimer disease have a higher frequency of PVS dilatation compared with cognitively healthy participants,12-15 a finding that was correlated with cortical Aβ deposits.15 Most studies to date have used conventional visual scoring scales to evaluate PVSs based on the number of PVSs counted on predefined brain scan slices.16,17 Although a visual scoring system is relatively easy and reproducible,18 it does not allow for mapping the entire brain. Consequently, the methods are less sensitive and suffer from floor and ceiling effects. Automatic whole-brain PVS volume measurement methods based on isotropic three-dimensional MRI acquisition and computational imaging analyses have been proposed19,20 and demonstrated to correlate well with the visual rating scale using neuroradiologic assessment.19 Nevertheless, changes in the PVS volume in each anatomical predilection site in patients with Alzheimer disease have not been fully investigated.
Estimation of the interstitial water content in the brain parenchyma, calculated as a volume fraction of FW in a voxel using a regularized bitensor model of DTI, has become possible.8 Elevated WM FW has been reported in patients with Alzheimer disease,21-23 suggesting the stagnation of fluid drainage caused by glymphatic dysfunction. Using DTI, Taoka et al.9 proposed the ALPS index, which is calculated from the diffusivity along the deep medullary vein at the level of the lateral ventricle body, as a measure of perivascular clearance activity in the human brain. They previously reported a significant association between the Mini-Mental State Examination (MMSE) score and ALPS index in patients with Alzheimer disease and mild cognitive impairment (MCI). The ALPS index has also been used in the evaluation of various neurologic disorders.24-26 Furthermore, a recent study validated the ALPS index as a measure of glymphatic function.25 Specifically, the ALPS index was found to be significantly correlated with glymphatic function measures calculated on MRI after intrathecal GBCA administration.25
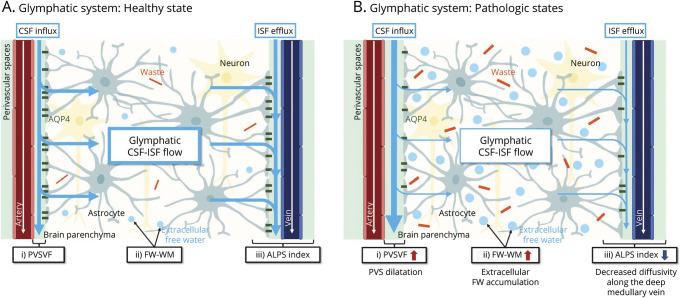
The PVS volume is an indicator of enlargement in the periarterial space; fractional volume of FW in WM (FW-WM), a measure of brain ISF; and ALPS index, a measure of the diffusivity along the perivenular space around the deep medullary vein. Together, they enable indirect evaluation of the glymphatic system (Figure 1). We aimed to determine changes that occur in these MRI parameters in patients with MCI and Alzheimer disease. In addition, we examined the associations of the MRI measurements with Aβ deposition, which is thought to be caused by glymphatic dysfunction, neuropsychological scores, and PET standardized uptake value ratios (SUVRs).
Figure 1. Basic Concepts of the Proposed MRI Measures for Indirect Noninvasive Evaluation of Glymphatic System Compartments in Healthy and Pathologic States.
(A) Based on the glymphatic hypothesis, subarachnoid CSF normally enters the brain's interstitial space from the periarterial space [as indexed by the PVSVF (i)] through the AQP4 channel expressed in the astrocyte end-feet and then mixes with the ISF and waste solutes in the brain. The resulting CSF/ISF exchange [as indexed by the FW-WM (ii)] and waste products, such as Aβ, are then drained out of the brain by the perivenous efflux pathway [as indexed by the ALPS index (iii)]. (B) In a pathologic state, such as in Alzheimer disease, glymphatic dysfunction due to brain waste (i.e., Aβ) accumulation might cause enlargement of the PVS, increased brain extracellular FW, and decreased perivenous efflux, as reflected by the higher PVSVF (iii), higher FW-WM (ii), and lower ALPS index (iii), respectively.
Methods
Study Participants
The data used in this study were obtained from the ADNI-2 database (eMethod 1, links.lww.com/WNL/C352 in the supplement; adni.loni.usc.edu). The details of the inclusion criteria, neuropsychological scores, and other biomarkers assessed in this study are available in eMethods 1–3, links.lww.com/WNL/C352, respectively.
MRI Data Acquisition
Diffusion-weighted (DW), T1-weighted (T1w), and fluid-attenuated inversion recovery (FLAIR) imaging data were acquired for each participant using a 3-T scanner. More imaging details can be found in eMethod 4, links.lww.com/WNL/C352 in the supplement. Furthermore, MRI data processing steps are available in eMethod 5.
PVS Segmentation
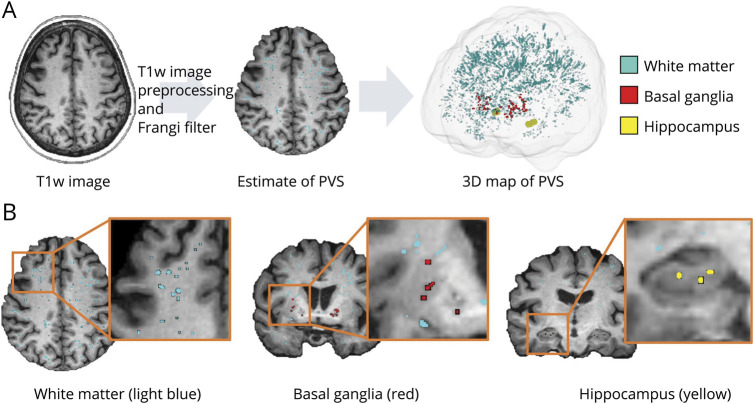
PVSs were mapped from T1w images using an automated and highly reliable quantification method, following the pipeline of previous studies.7,27 WM lesions (WMLs), which lead to a mis-segmented PVS mask, were excluded from the PVS mask to eliminate PVS mis-segmentation caused by WM hyperintensity. The PVS volume was measured in the WM, BG, Hipp, and the sum of these structures (ALL) (Figure 2; eMethod 5 in the supplement, links.lww.com/WNL/C352). The PVS volume fraction (PVSVF; PVSVF = PVS volume/intracranial volume) was then obtained to eliminate interindividual variability in brain size. The details of the pipeline can be found in eMethod 6.
Figure 2. PVS Mapping.
(A) PVS segmentation was performed with preprocessed T1w images and by applying the Frangi filter. (B) PVSs were then extracted from the WM (light blue), BG (red), and Hipp (yellow).
FW Calculation
Maps of the fractional volume of FW were constructed from DW images using a regularized bitensor model with the open-source software package Diffusion Imaging in Python (also known as Dipy) algorithm (dipy.org/).28 Mean cerebral FW-WM was extracted using the T1w imaging–based WM mask calculated at the MRI data processing stage (eFigure 1 in the supplement, links.lww.com/WNL/C352). The effects of WML and PVS were then excluded using the WML and PVS masks calculated at the MRI data processing stage. In the process, the WML and PVS masks were registered to the DW image space by applying the FLAIR-to-T1w and T1w-to-DW image transformation matrices.
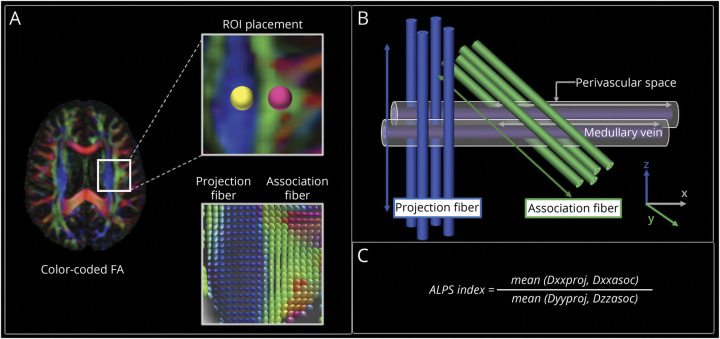
ALPS Index Calculation
ALPS index was calculated from DW images using a semiautomated and highly reliable pipeline developed and validated by Taoka et al.,29 (Figure 3). The details can be found in eMethod 7 in the supplement, links.lww.com/WNL/C352. The average of the left and right ALPS indices (mean ALPS index) was then calculated. An ALPS index close to 1.0 reflects minimal diffusivity, whereas a higher value indicates greater diffusivity.
Figure 3. ALPS Index Calculation.
(A) Color-coded FA map showing the distribution of the projection fibers (z-axis, blue) and association fibers (y-axis, green) at the level of the lateral ventricle of the body. Spherical ROIs measuring 5 mm in diameter were placed in the projection and association areas. Diffusion tensor is shown as an ellipsoid in the lower row. (B) The schematic of the geometric positional relationship of the principal fiber and PVS runs parallel to the medullary vein. The PVS runs orthogonal to the projection and association fibers. (C) The ALPS index was calculated as the ratio of the mean of the x-axis diffusivity in the projection area (Dxx,proj) and x-axis diffusivity in the association area (Dxx,assoc) to the mean of the y-axis diffusivity in the projection area (Dyy,proj) and the z-axis diffusivity in the association area (Dzz,assoc).
Statistical Analysis
Between-group differences in PVSVF-WM, PVSVF-BG, PVSVF-Hipp, PVSVF-ALL, FW-WM, mean ALPS index, and diffusivities in the association (Dxx,assoc, Dyy,assoc, and Dzz,assoc) and projection (Dxx,proj, Dyy,proj, and Dzz,proj) areas were evaluated using a general linear model univariate while controlling for age, sex, years of education, scanning site, and APOE ε4 gene carrier status (Model 1). To determine the influence of WMLs on the measurements of the glymphatic system, we also included WML volume fraction (WMLVF; WML volume/ICV) as a confounding factor in Model 2, which included the covariates of age, sex, years of education, scanning site, APOE ε4 gene carrier status, and WMLVF, for the evaluation of PVSVF, FW, and mean ALPS index. Furthermore, to examine the influence of changes in WM integrity on ALPS index assessment, we also included the mean FA and mean diffusivity (MD) values measured in the regions of interest (ROIs) of the association and projections fibers (eMethod 7) as a covariate for the evaluation of the ALPS index in Model 3, which included the covariates of age, sex, years of education, scanning site, APOE ε4 gene carrier status, WMLVF, FA, and MD. The ALPS index has been associated with gray matter (GM) atrophy in older adults with sleep disorder.30 Considering the high incidence of GM atrophy in Alzheimer disease, we also included GM volume fraction (GMVF; GM volume/ICV) as a confounding factor in Models 2 and 3 to create Models 4 and 5, respectively. Statistical significance was set at a p value of <0.05.
Finally, partial Spearman rank correlation tests were used to evaluate the associations of MRI measurements with neuropsychological scores, CSF biomarker values, PET SUVRs, and Hipp volume adjusted for age, sex, years of education, scanning site, and APOE ε4 gene carrier status in the MCI and Alzheimer disease groups combined. Notably, correlation analyses were only performed on patients with available data (Table 1). The false discovery rate was used to correct for multiple correlation tests.
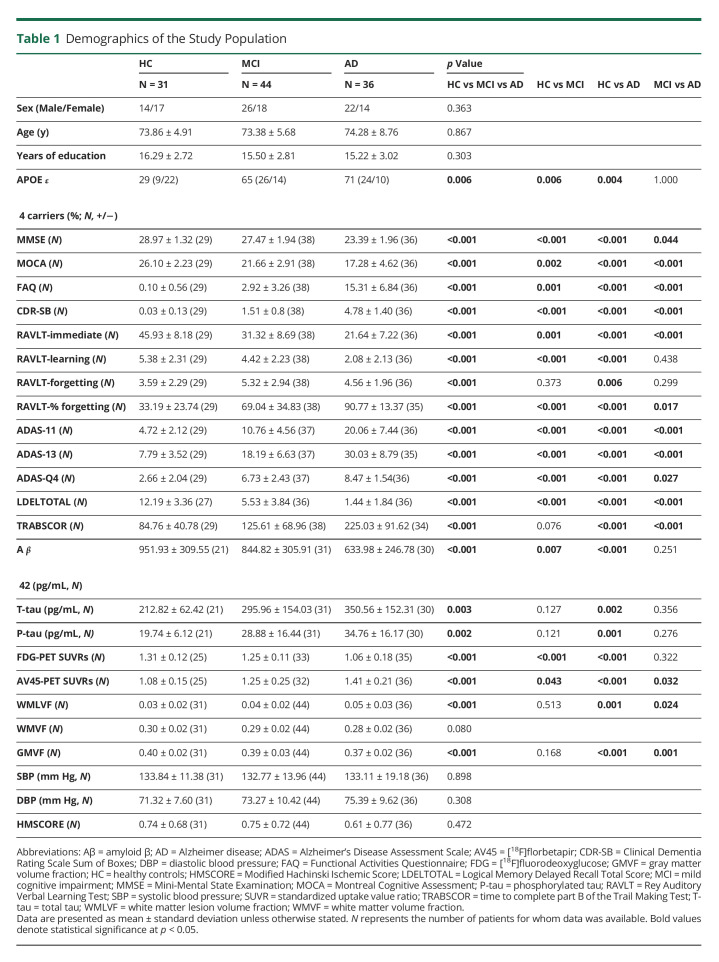
Table 1.
Demographics of the Study Population
Data Availability
The data used in this study are publicly available in the ADNI-2 database (adni.loni.usc.edu). A list of the participants included in this study is available on request to the corresponding author.
Results
Demographic and Clinical Characteristics of the Study Population
This study included 31 cognitively healthy controls (14 men and 17 women; mean age, 73.86 ± 4.91 years), 44 patients with MCI (26 men and 18 women; mean age, 73.38 ± 5.68 years), and 36 patients with Alzheimer disease (22 men and 14 women; mean age, 74.28 ± 8.76 years) (Table 1). Age, sex, years of education, systolic and diastolic blood pressures, modified Hachinski ischemic score, and WM volume fraction (WM volume/ICV) did not vary significantly among the control, MCI, and Alzheimer disease groups. All patients with MCI and Alzheimer disease had a modified Hachinski ischemic score of <4, indicating primary degenerative dementia. The number of APOE ε4 gene carriers in the patients with MCI and Alzheimer disease was significantly higher than controls. By contrast, no significant difference was found between the patients with MCI and those with Alzheimer disease. Although data on neuropsychological scores, blood pressure, PET, and CSF biomarkers were not available for all participants, at least 68% of the controls, 70% of the patients with MCI, and 83% of the patients with Alzheimer disease had complete data.
The [18F] florbetapir-PET SUVRs and the scores for MMSE, Montreal Cognitive Assessment, Rey Auditory Verbal Learning Test (RAVLT; immediate), and logical memory total delayed recall in the patients with MCI and Alzheimer disease were significantly lower than controls; the same parameters in the patients with MCI were significantly lower than those in the patients with Alzheimer disease. The [18F] fluorodeoxyglucose (FDG)-PET SUVRs and RAVLT (learning and percentage of forgetting) scores in the patients with MCI and Alzheimer disease were significantly lower than controls. The patients with Alzheimer disease had significantly lower CSF Aβ42 and CSF total tau and phosphorylated tau levels compared with controls, whereas the patients with MCI only showed a significantly lower CSF Aβ42 level compared with controls. The WMLVF and GMVF were significantly lower in patients with Alzheimer disease than controls and patients with MCI. Functional Activities Questionnaire (FAQ), Clinical Dementia Rating Scale Sum of Boxes (CDR-SB), Alzheimer's Disease Assessment Scale (ADAS)-11, ADAS-13, and ADAS-Q4 scores in the patients with MCI and Alzheimer disease were significantly higher than controls; the same parameters in the patients with Alzheimer disease were significantly higher than those in the patients with MCI. The patients with Alzheimer disease had significantly higher scores for the time to complete part B of the Trail Making Test compared with controls and patients with MCI. WMLVF, GMVF, CSF total tau and phosphorylated tau levels, RAVLT percentage of forgetting, and the score for the time to complete part B of the Trail Making Test were not significantly different between patients with MCI and controls. Moreover, FDG-PET SUVRs, RAVLT (learning and percentage of forgetting) scores, and the CSF biomarker values were not significantly different between the patients with MCI and those with Alzheimer disease.
Between-Group Differences in MRI Measurements
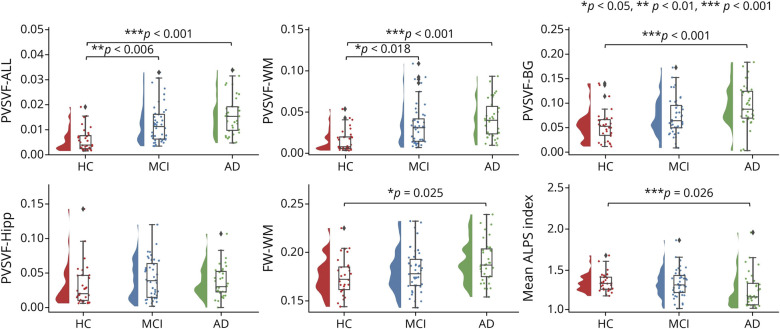
After adjusting for age, sex, years of education, scanning site, and APOE ε4 gene carrier status (Model 1), mean ALPS index (p = 0.026) was significantly lower and Dzz,assoc (p = 0.006), Dyy,proj (p = 0.003), PVSVF-WM (p < 0.001), PVSVF-BG (p < 0.001), PVSVF-ALL (p < 0.001), and cerebral FW-WM (p = 0.025) were significantly higher in patients with Alzheimer disease than controls (Figure 4; eFigure 2; eTable 1 in the supplement, links.lww.com/WNL/C352). PVSVF-ALL (p = 0.006) and PVSVF-WM (p = 0.018) were significantly higher in the patients with MCI than controls. There were no significant differences in Dxx,assoc, Dyy,assoc, Dxx,proj, Dzz,proj, and PVSVF-Hipp among the 3 groups; in Dzz,assoc, Dyy,proj, mean ALPS index, FW-WM, and PVSVF-BG between the patients with MCI and control participants; and in Dzz,assoc, Dyy,proj, mean ALPS index, PVSVF-ALL, PVSVF-WM, and FW-WM between the patients with MCI and those with Alzheimer disease.
Figure 4. Between-Group Differences in MRI Measurements.
Shown are violin and box plots of the mean ALPS index, PVSVF-ALL, PVSVF-WM, PVSVF-BG, PVSVF-Hipp, and FW-WM among the healthy control (HC) participants, patients with MCI, and patients with Alzheimer disease. The p values correspond to the general linear model analysis. Statistical significance was set at p < 0.05.
In Model 2, which included WMLVF as a covariate in addition to those of Model 1, PVSVF-WM (p < 0.001), PVSVF-ALL (p < 0.001), and PVSVF-BG (p = 0.009) remained significantly higher in the patients with Alzheimer disease than controls. Significantly higher PVSVF-ALL (p = 0.004) and PVSVF-WM (p = 0.007) were also consistently observed in patients with MCI than in the healthy control participants. In addition, significantly higher PVSVF-ALL (p < 0.001) and PVSVF-WM (p < 0.001) were observed in patients with Alzheimer disease than in those with MCI. By contrast, significant differences in PVSVF-BG observed between patients with Alzheimer disease and those with MCI observed in Model 1 and that in FW-WM and mean ALPS index observed between patients with Alzheimer disease and the controls were no longer present in Model 2 including the additional covariate of WMLVF.
In Model 3, which included FA, MD, and WMLVF as covariates in addition to those of Model 1, mean ALPS index (p = 0.040) remained significantly lower in patients with Alzheimer disease than that among controls. Consistent with Model 1, a significant difference in mean ALPS index was not observed between patients with MCI and controls or between patients with Alzheimer disease and those with MCI.
In Model 4, which included GMVF as a new covariate added to the covariates of Model 2, the results obtained using Model 2 were relatively preserved. PVSVF-WM (p < 0.001) and PVSVF-ALL (p < 0.001) were significantly higher in patients with Alzheimer disease than the controls and patients with MCI, whereas PVSVF-BG (p = 0.020) were significantly higher in patients with Alzheimer disease than the controls. PVSVF-ALL (p = 0.003) and PVSVF-WM (p = 0.004) remained significantly higher in patients with MCI than the controls. No significant differences in PVSVF-BG, PVSVF-Hipp, FW-WM, and mean ALPS index were found among the 3 groups.
In Model 5, with GMVF included as a covariate in addition to those of Model 3, the significant difference in mean ALPS index observed among the groups in Model 1 was no longer present.
Correlation Analyses
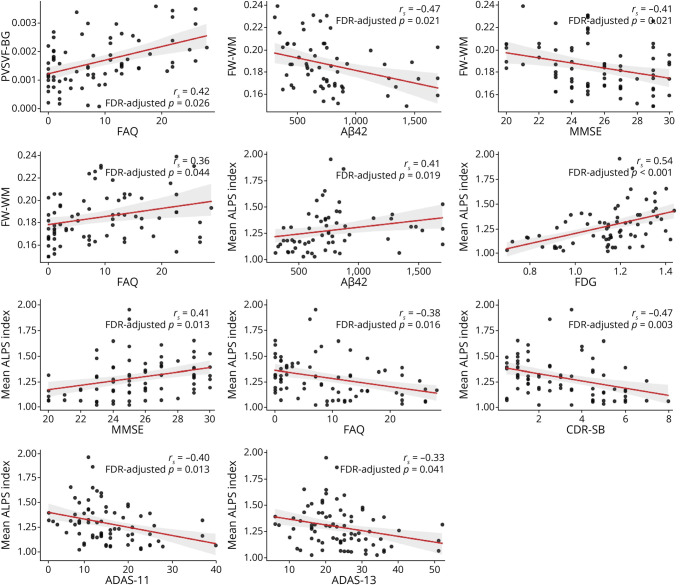
Higher PVSVF-BG was associated with worse FAQ score (rs = 0.42, pfdr = 0.026) (Figure 5; eTable 2 in the supplement, links.lww.com/WNL/C352). Higher FW-WM was also associated with lower CSF Aβ42 (rs = −0.47, pfdr = 0.021), worse MMSE score (rs = −0.41, pfdr = 0.021), and worse FAQ score (rs = 0.36, pfdr = 0.044). Lower mean ALPS index was associated with lower CSF Aβ42 (rs = 0.41, pfdr = 0.026), FDG-PET SUVr (rs = 0.54, pfdr < 0.001), and worse MMSE (rs = 0.41, pfdr = 0.026), FAQ (rs = −0.38, pfdr = 0.016), CDR-SB (rs = −0.47, pfdr = 0.003), ADAS-11 (rs = −0.40, pfdr = 0.013), and ADAS-13 scores (rs = −0.33, pfdr = 0.041). The correlations of PVSVF-ALL, PVSVF-WM, and PVSVF-Hipp with neuropsychological scores, CSF biomarker values, PET SUVRs, and Hipp volume were not significant.
Figure 5. Correlation Analyses.
Relationships between variables that showed significant correlation in partial Spearman rank correlation tests in the patients with MCI and Alzheimer disease combined. Red lines depict linear regression with 95% confidence interval (shadow).
Discussion
This study used noninvasive MRI measures to assess the whole-brain perivascular network in patients with MCI and Alzheimer disease. The observed higher PVSVFs and FW-WM and lower mean ALPS index in patients with Alzheimer disease as compared with control participants might reflect the dilatation of the PVS, increased extracellular free water in the WM interstitial space, and reduced water diffusivity in the perivenous spaces, possibly due to impairment of the glymphatic system. By contrast, PVSVF-ALL and PVSVF-WM changes in patients with MCI might indicate early impairment in the periarterial space. Of these measures, FW-WM and mean ALPS index were significantly associated with CSF Aβ levels, FDG-PET SUVRs, and multiple cognitive scores.
In patients with Alzheimer disease, after adjusting for age, sex, years of education, scanning site, and APOE ε4 gene carrier status, PVSVF was significantly enlarged in all parts except the hippocampal region compared with the controls. This result is consistent with previous studies showing that perivascular luminal expansion in Alzheimer disease is mainly seen in the WM (particularly in the centrum semiovale)12-14,20 and in some cases in the BG.13,14 Research has also shown that the hippocampal PVS is not associated with cognitive function or the occurrence of dementia.31 Furthermore, in line with previous studies,13,32 patients with MCI only showed higher PVSVF-ALL and PVSVF-WM compared with control participants. Considering MCI as a prodromal phase of Alzheimer disease, our results suggest that enlargement of the periarterial space occurs the earliest. Notably, the significant differences in PVSVF-ALL and PVSVF-WM between patients with Alzheimer disease and those with MCI were only observed after the inclusion of WMLVF as a confounding factor. As expected, WMLVF was significantly higher in patients with Alzheimer disease than in those with MCI, providing support that WMLs might contribute to an increase in the risk of Alzheimer disease. Thus, our findings demonstrate that WMLVF should be included in the model for the evaluation of PVSVF. Although the exact cause of PVS dilation associated with Alzheimer disease remains unknown, its pathology has been proposed to result from blockage of brain drainage pathways due to the accumulation of Aβ.33 Indeed, Aβ deposition has also been shown to occur in the pericapillary and periarteriolar membranes, but not in the perivenular membranes.34 The significant correlations between other MRI measures (mean ALPS index and FW-WM) and CSF Aβ observed in patients with MCI and Alzheimer disease further suggest perivascular accumulation of Aβ as the cause of perivascular clearance impairment.
The significantly higher FW-WM in Alzheimer disease is consistent with previously reported data.21,22 Here, we further showed that increases in FW-WM may reflect the stagnation of ISF drainage caused by perivascular clearance impairment. In line with a previous study,22 our study also observed significant correlations between FW-WM and cognitive functions, such as executive function, visual construction, visuomotor coordination, and verbal performance, highlighting the effect of brain ISF accumulation on cognitive impairment. However, by contrast, the significant difference in FW-WM between patients with MCI and control participants observed by Dumont et al.21 in the same ADNI-2 cohort was not detected in this study, which might be due to the sampling size difference between our studies. In this study, we evaluated a smaller sample (31 controls, 44 patients with MCI, and 36 patients with Alzheimer disease) relative to that examined by Dumont et al.21 (81 controls, 103 patients with MCI, and 42 patients with Alzheimer disease) because we only selected patients imaged on a GE scanner to avoid intervendor variability. Furthermore, unlike in our study, they did not consider age, sex, years of education, scanning site, and APOE ε4 gene carrier as confounding factors. Notably, in contrast to PVSVF, even though the normal-appearing WM without WMLs was included in the measurement of FW-WM, the significant changes in FW-WM were no longer observed after the inclusion of WMLVF as a confounding factor, indicating that the changes in FW-WM were affected by WMLs. In this study, WMLVF was higher in patients with Alzheimer disease than in those with MCI and the healthy controls; therefore, small-vessel disease pathology might have also influenced the elevated FW levels detected in patients with Alzheimer disease in this study. Indeed, FW in normal-appearing white matter has been reported to be increased in patients with small-vessel disease.35
We observed a significantly reduced mean ALPS index in patients with Alzheimer disease as compared with control participants, but we did not detect any significant difference between patients with MCI and control participants. These findings are partially consistent with those of a previous preliminary study by Steward et al.,36 who comparatively evaluated the ALPS index in a small cohort of 16 patients with Alzheimer disease, 10 patients with MCI, and 10 healthy participants using a general linear model adjusted for age, sex, and APOE ε4 gene status. In line with our results, they found a significant decrease in the ALPS index of patients with Alzheimer disease; however, in contrast to our results, they found a significant decrease in the ALPS index of patients with MCI as compared with control participants. Such a discrepancy may be attributed to their small sample size (N = 36 participants) relative to ours (N = 111 participants). In addition, unlike in this study, their study did not adjust for years of education, which is an important risk factor for dementia.37 The fact that the mean ALPS index did not significantly differ between patients with MCI and control participants in this study suggests the relative preservation of water diffusivity in perivenous spaces in MCI. Again, a longitudinal study is essential to support our findings. Of note, the changes in the mean ALPS index were preserved even after the inclusion of FA and MD as confounding factors in multivariate analyses. Furthermore, we observed significant changes only in Dzz,assoc and Dyy,proj among patients with Alzheimer disease compared with controls, reflecting increased water diffusion perpendicular to the association and projection fibers, respectively. These findings demonstrate degeneration of these fibers in Alzheimer disease, in concurrence with the results of previous studies.38-42 The lack of significant changes in Dxxassoc and Dxxproj values suggests that the increased water diffusion perpendicular to the WM tracts is offset by reduction in water diffusion along the PVS. Therefore, the ALPS index is calculated by dividing the average of Dxxproj and Dxxassoc by the average of Dyyproj and Dzzassoc to eliminate the influence of diffusivity related to the WM microstructure and emphasize diffusivity along the PVS; this indicates that the ALPS index is significantly lower in the case of Alzheimer disease. Together, we demonstrated that the change in the mean ALPS index primarily contributed by the changes in water diffusivity in the perivenous space and was not affected by WM integrity. Nevertheless, in contrast with the other parameters included in our models, the significant changes observed in the mean ALPS index was not present after the inclusion of WMLVF and GMVF as covariates, indicating the influence of WMLs and GM atrophy on glymphatic dysfunction in MCI and Alzheimer disease which warrants further investigation. WML pathology is considered to affect the end feet of astrocytes, leading to impairment of the glymphatic system.43 Furthermore consistent with the findings of this study, associations between the ALPS index and regional GM atrophy were previously reported in a study of older adults,30 which also explored the association of the ALPS index with the sleep profile and neuropsychological performance. The demonstration of an association between the ALPS index and the sleep profile and the GM volume was a strength of the study, although patients with dementia were not included. Thus, the findings of this study, including a lower ALPS index in patients with Alzheimer disease and the association of the ALPS index with CSF Aβ levels and multiple neuropsychological scores, complement the findings of that study.
The significant correlation between mean ALPS index and brain glucose metabolism is also worth noting. Aβ deposition was shown to cause a decrease in glucose metabolism in neurons and astrocytes that might contribute to neuronal loss.44 Furthermore, in line with previous studies,9,36 we observed significant correlations between the mean ALPS index and multiple cognitive functioning scales, such as the MMSE, CDR-SB, FAQ, ADAS-11, and ADAS-13. Altogether, our findings suggest the association of glymphatic system impairment with neuronal loss, which contributes to the progression of cognitive and daily living dysfunctions in Alzheimer disease. Furthermore, we also showed the use of the mean ALPS index as a disease progression biomarker in Alzheimer disease.
This study has some limitations. First, the ALPS index cannot evaluate whole-brain glymphatic function. It is based on the orthogonal geometric relationship between projection and association fibers and medullary arteries and veins in the lateral ventricle body.9 Therefore, the ALPS can only be evaluated at the level of the lateral ventricle body. Notably, the ALPS index does not exclusively measure the diffusivity of the perivenous space around the deep medullary vein—that is, it is also influenced by the surrounding white matter microstructure included in the ROI. Thus, the ALPS index needs more careful interpretation and further investigation. Second, we did not use GBCAs in measuring glymphatic functions. Although use of CSF tracers is fairly invasive, it can be considered as the current gold standard for measuring glymphatic functions.6 However, as mentioned above, the ALPS index has been reported to highly correlate with the glymphatic function measures calculated on MRI by intrathecal GBCA administration.25 Third, we used only T1w images for PVS segmentation. Sepehrband et al.27 reported a higher number of PVSs when only T1w or T2-weighted (T2w) images were used relative to when enhanced PVS contrast images were obtained by dividing filtered images (i.e., T1w/T2w). However, no evidence suggesting a more superior image in PVS segmentation was found. In addition, all PVS masks in this study were manually checked to identify incorrect and undetected PVSs. We also acknowledge the limitations of the PVS segmentation method. Future studies should use high-resolution and quasi-isotropic structural MRI. Furthermore, the accuracy of PVS segmentation depends on the quality of the image preprocessing step and tubular noise might be recognized as PVS. Fourth, this study lacked histopathologic validation. Although previous studies have suggested that a higher level of FW-WM reflects ISF stasis,45,46 the exact histopathologic process of extracellular water changes indicated by changes in FW is not fully understood yet. The changes in FW can occur through different physiologic mechanisms, such as atrophy, edema, neuroinflammation, a reduction in myelin content, or modulation in the permeability of the blood–brain barrier. Fifth, we only evaluated cross-sectional data from the ADNI-2 database. Future studies should also assess longitudinal ADNI-2 data to validate the usefulness of the noninvasive glymphatic MRI measurements. Sixth, glymphatic dysfunction has been associated with sleep disorders30; however, in the present study, we were unable to assess the association between sleep quality and glymphatic dysfunction in patients with MCI and Alzheimer disease because of the lack of data. Thus, future studies should also assess the potential correlation between glymphatic dysfunction and sleep disorders. Finally, we acknowledge that the evidence supporting the utility of these MRI indicators in assessing glymphatic system function remains insufficient.
In summary, we report that PVSVF and FW-WM are higher, whereas the ALPS index is lower in individuals with Alzheimer disease, which might reflect impairment of the glymphatic system. Furthermore, our findings provide evidence of early periarterial space changes (indexed by the PVSVF), as seen in patients with MCI. Finally, our study also suggested associations between glymphatic dysfunction in Alzheimer disease and Aβ deposition, neuronal damage, and cognitive impairment. However, considering the methodological limitations of this study, with regard to ALPS index in particular, the results should be interpreted with caution.
Acknowledgment
Data collection and sharing for this project was funded by the Alzheimer's Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12-2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie, Alzheimer's Association; Alzheimer's Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol-Myers Squibb Company; CereSpir, Inc.; Cogstate; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer's Therapeutic Research Institute at the University of Southern California. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California.The authors gratefully acknowledge the research support of Wataru Uchida, Mana Owaki, and Kaori Nishiyama.
Glossary
- Aβ
amyloid β
- ADAS
Alzheimer's Disease Assessment Scale
- ADNI
Alzheimer's Disease Neuroimaging Initiative
- ALPS
diffusivity along the perivascular space
- BG
basal ganglia
- CDR-SB
Clinical Dementia Rating Scale Sum of Boxes
- DBP
diastolic blood pressure
- DTI
diffusion tensor imaging
- DW
diffusion weighted
- FA
fractional anisotropy
- FAQ
Functional Activities Questionnaire
- FDG
[18F] fluorodeoxyglucose
- FLAIR
fluid-attenuated inversion recovery
- FSL
FMRIB Software Library 6.0.3
- FW
free water
- GBCA
gadolinium-based contrast agent
- GMVF
gray matter volume fraction
- Hipp
hippocampus
- HMSCORE
Modified Hachinski Ischemic Score
- ISF
interstitial fluid
- MCI
mild cognitive impairment
- MMSE
Mini-Mental State Examination
- PVS
perivascular space
- PVSVF
PVS volume fraction
- RAVLT
Rey Auditory Verbal Learning Test
- ROI
region of interest
- SBP
systolic blood pressure
- SUVR
standardized uptake value ratio
- T1w
T1 weighted
- WM
white matter
- WML
white matter lesion
- WMLVF
white matter lesion volume fraction
- WMVF
white matter volume fraction
Appendix. Authors
Study Funding
Data collection and sharing for this project were funded by the ADNI (National Institutes of Health grant no. U01 AG024904) and Department of Defense ADNI (Department of Defense award number W81XWH-12-2-0012). This study was partially supported by the Juntendo Research Branding Project, JSPS KAKENHI (grant nos. JP16H06280, JP18H02772, and 19K17244), a Grant-in-Aid for Special Research in Subsidies for ordinary expenses of private schools from The Promotion and Mutual Aid Corporation for Private Schools of Japan, the Brain/MINDS Beyond program (grant no. JP19dm0307101) of the Japan Agency for Medical Research and Development (AMED), and AMED under grant number JP21wm0425006. The Department of Innovative Biomedical Visualization (iBMV), Nagoya University Graduate School of Medicine, is financially supported by Canon Medical Systems Corporation.
Disclosure
K. Kamagata receives JSPS KAKENHI Grant Number 19K17244 and a grant from Juntendo Research Branding Project and the Brain/MINDS Beyond program (grant no. JP19dm0307101) of the Japan Agency for Medical Research and Development and is an editorial board member of BMC Neurology. C. Andica reports no disclosures relevant to the manuscripts. K. Takabayashi reports no disclosures relevant to the manuscripts. Y. Saito reports no disclosures relevant to the manuscripts. T. Taoka receives JSPS KAKENHI Grant Number 21K07563 and the Department of Innovative Biomedical Visualization (iBMV), Nagoya University Graduate School of Medicine, is financially supported by Canon Medical Systems Corporation. H. Nozaki reports no disclosures relevant to the manuscripts. J. Kikuta receives JSPS KAKENHI Grant Number 20K16737. S. Fujita reports no disclosures relevant to the manuscripts. A. Hagiwara reports no disclosures relevant to the manuscripts. K. Kamiya receives JSPS KAKENHI Grant Number 21K07629. A. Wada receives JSPS KAKENHI Grant Number 18K07730. T. Akashi reports no disclosures relevant to the manuscripts. K. Sano reports no disclosures relevant to the manuscripts. M. Nishizawa reports no disclosures relevant to the manuscripts. M. Hori receives JSPS KAKENHI Grant Number 19K08161 and is an deputy editor-in-chief of Magnetic Resonance in Medical Sciences, an editor of Neuroradiology and Japanese Journal of Radiology. S. Naganawa reports no disclosures relevant to the manuscripts. S. Aoki receives JSPS KAKENHI Grant Number 16H06280, 18H02772 and the Juntendo Research Branding Project and a Grant-in-Aid for Special Research in Subsidies for ordinary expenses of private schools from The Promotion and Mutual Aid Corporation for Private Schools of Japan, and the Brain/MINDS Beyond program (grant no. JP19dm0307101) of the Japan Agency for Medical Research and Development (AMED), and AMED under grant number JP21wm0425006. Go to Neurology.org/N for full disclosures.
References
- 1.Sperling RA, Karlawish J, Johnson KA. Preclinical Alzheimer disease-the challenges ahead. Nat Rev Neurol. 2013;9:54-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hampel H, Hardy J, Blennow K, et al. The amyloid-beta pathway in Alzheimer's disease. Mol Psychiatry. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iliff JJ, Wang M, Liao Y, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Sci Transl Med. 2012;4:147ra111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kress BT, Iliff JJ, Xia M, et al. Impairment of paravascular clearance pathways in the aging brain. Ann Neurol. 2014;76:845-861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xie L, Kang H, Xu Q, et al. Sleep drives metabolite clearance from the adult brain. Science. 2013;342:373-377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taoka T, Naganawa S. Glymphatic imaging using MRI. J Magn Reson Imaging. 2020;51:11-24. [DOI] [PubMed] [Google Scholar]
- 7.Donahue EK, Murdos A, Jakowec MW, et al. Global and regional changes in perivascular space in idiopathic and familial Parkinson's disease. Movement disorders. official J Mov Disord Soc. 2021;36:1126-1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pasternak O, Sochen N, Gur Y, Intrator N, Assaf Y. Free water elimination and mapping from diffusion MRI. Magn Reson Med. 2009;62:717-730. [DOI] [PubMed] [Google Scholar]
- 9.Taoka T, Masutani Y, Kawai H, et al. Evaluation of glymphatic system activity with the diffusion MR technique: diffusion tensor image analysis along the perivascular space (DTI-ALPS) in Alzheimer's disease cases. Jpn J Radiol. 2017;35:172-178. [DOI] [PubMed] [Google Scholar]
- 10.Bouvy WH, Biessels GJ, Kuijf HJ, Kappelle LJ, Luijten PR, Zwanenburg JJ. Visualization of perivascular spaces and perforating arteries with 7 T magnetic resonance imaging. Invest Radiol. 2014;49:307-313. [DOI] [PubMed] [Google Scholar]
- 11.Wardlaw JM, Smith EE, Biessels GJ, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013;12:822-838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Banerjee G, Kim HJ, Fox Z, et al. MRI-visible perivascular space location is associated with Alzheimer's disease independently of amyloid burden. Brain. 2017;140:1107-1116. [DOI] [PubMed] [Google Scholar]
- 13.Chen W, Song X, Zhang Y. Alzheimer's Disease Neuroimaging I. Assessment of the Virchow-Robin Spaces in Alzheimer disease, mild cognitive impairment, and normal aging, using high-field MR imaging. AJNR Am J Neuroradiol. 2011;32:1490-1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hansen TP, Cain J, Thomas O, Jackson A. Dilated perivascular spaces in the Basal Ganglia are a biomarker of small-vessel disease in a very elderly population with dementia. AJNR Am J Neuroradiol. 2015;36:893-898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roher AE, Kuo YM, Esh C, et al. Cortical and leptomeningeal cerebrovascular amyloid and white matter pathology in Alzheimer's disease. Mol Med. 2003;9:112-122. [PMC free article] [PubMed] [Google Scholar]
- 16.Wuerfel J, Haertle M, Waiczies H, et al. Perivascular spaces--MRI marker of inflammatory activity in the brain? Brain. 2008;131:2332-2340. [DOI] [PubMed] [Google Scholar]
- 17.Zhu YC, Tzourio C, Soumare A, Mazoyer B, Dufouil C, Chabriat H. Severity of dilated Virchow-Robin spaces is associated with age, blood pressure, and MRI markers of small vessel disease: a population-based study. Stroke. 2010;41:2483-2490. [DOI] [PubMed] [Google Scholar]
- 18.Potter GM, Chappell FM, Morris Z, Wardlaw JM. Cerebral perivascular spaces visible on magnetic resonance imaging: development of a qualitative rating scale and its observer reliability. Cerebrovasc Dis. 2015;39:224-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ballerini L, Lovreglio R, Valdes Hernandez MDC, et al. Perivascular spaces segmentation in brain MRI using optimal 3D filtering. Scientific Rep. 2018;8:2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ramirez J, Berezuk C, McNeely AA, Scott CJ, Gao F, Black SE. Visible Virchow-Robin spaces on magnetic resonance imaging of Alzheimer's disease patients and normal elderly from the Sunnybrook Dementia Study. J Alzheimer's Dis. 2015;43:415-424. [DOI] [PubMed] [Google Scholar]
- 21.Dumont M, Roy M, Jodoin PM, et al. Free water in white matter differentiates MCI and AD from control subjects. Front Aging Neurosci. 2019;11:270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ji F, Pasternak O, Liu S, et al. Distinct white matter microstructural abnormalities and extracellular water increases relate to cognitive impairment in Alzheimer's disease with and without cerebrovascular disease. Alzheimers Res Ther. 2017;9:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maier-Hein KH, Westin CF, Shenton ME, et al. Widespread white matter degeneration preceding the onset of dementia. Alzheimers Dement. 2015;11:485-493 e482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McKnight CD, Trujillo P, Lopez AM, et al. Diffusion along perivascular spaces reveals evidence supportive of glymphatic function impairment in Parkinson disease. Parkinsonism Relat Disord. 2021;89:98-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang W, Zhou Y, Wang J, et al. Glymphatic clearance function in patients with cerebral small vessel disease. Neuroimage. 2021;238:118257. [DOI] [PubMed] [Google Scholar]
- 26.Kikuta J, Kamagata K, Takabayashi K, et al. An investigation of water diffusivity changes along the perivascular space in elderly subjects with hypertension. AJNR Am J Neuroradiol. 2022;43:48-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sepehrband F, Barisano G, Sheikh-Bahaei N, et al. Image processing approaches to enhance perivascular space visibility and quantification using MRI. Sci Rep. 2019;9:12351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garyfallidis E, Brett M, Amirbekian B, et al. Dipy, a library for the analysis of diffusion MRI data. Front Neuroinform 2014;8:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taoka T, Ito R, Nakamichi R, et al. Reproducibility of diffusion tensor image analysis along the perivascular space (DTI-ALPS) for evaluating interstitial fluid diffusivity and glymphatic function: CHanges in Alps index on Multiple conditiON acquIsition eXperiment (CHAMONIX) study. Jpn J Radiol. 2022;40:147-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Siow TY, Toh CH, Hsu JL, et al. Association of sleep, neuropsychological performance, and gray matter volume with glymphatic function in community-dwelling older adults. Neurology. 2022;98:e829–e838. [DOI] [PubMed] [Google Scholar]
- 31.Yao M, Zhu YC, Soumare A, et al. Hippocampal perivascular spaces are related to aging and blood pressure but not to cognition. Neurobiol Aging. 2014;35:2118-2125. [DOI] [PubMed] [Google Scholar]
- 32.Sepehrband F, Barisano G, Sheikh-Bahaei N, et al. Volumetric distribution of perivascular space in relation to mild cognitive impairment. Neurobiol Aging. 2021;99:28-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Keable A, Fenna K, Yuen HM, et al. Deposition of amyloid beta in the walls of human leptomeningeal arteries in relation to perivascular drainage pathways in cerebral amyloid angiopathy. Biochim Biophys Acta. 2016;1862:1037-1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tarasoff-Conway JM, Carare RO, Osorio RS, et al. Clearance systems in the brain-implications for Alzheimer disease. Nat Rev Neurol. 2015;11:457-470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Duering M, Finsterwalder S, Baykara E, et al. Free water determines diffusion alterations and clinical status in cerebral small vessel disease. Alzheimers Dement. 2018;14:764-774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Steward CE, Venkatraman VK, Lui E, et al. Assessment of the DTI-ALPS parameter along the perivascular space in older adults at risk of dementia. J Neuroimaging. 2021;31:569-578. [DOI] [PubMed] [Google Scholar]
- 37.Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396:413-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Acosta-Cabronero J, Williams GB, Pengas G, Nestor PJ. Absolute diffusivities define the landscape of white matter degeneration in Alzheimer's disease. Brain. 2010;133:529-539. [DOI] [PubMed] [Google Scholar]
- 39.Pievani M, Agosta F, Pagani E, et al. Assessment of white matter tract damage in mild cognitive impairment and Alzheimer's disease. Hum Brain Mapp. 2010;31:1862-1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alves GS, O'Dwyer L, Jurcoane A, et al. Different patterns of white matter degeneration using multiple diffusion indices and volumetric data in mild cognitive impairment and Alzheimer patients. PLoS One. 2012;7:e52859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li Y, Feng F, Lin P, et al. Cognition-related white matter integrity dysfunction in Alzheimer's disease with diffusion tensor image. Brain Res Bull. 2018;143:207-216. [DOI] [PubMed] [Google Scholar]
- 42.Mayo CD, Garcia-Barrera MA, Mazerolle EL, et al. Relationship between DTI metrics and cognitive function in Alzheimer's disease. Front Aging Neurosci. 2018;10:436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carotenuto A, Cacciaguerra L, Pagani E, Preziosa P, Filippi M, Rocca MA. Glymphatic system impairment in multiple sclerosis: relation with brain damage and disability. Brain. 2021. [DOI] [PubMed] [Google Scholar]
- 44.Tarczyluk MA, Nagel DA, Rhein Parri H, et al. Amyloid beta 1-42 induces hypometabolism in human stem cell-derived neuron and astrocyte networks. J Cereb Blood Flow Metab. 2015;35:1348-1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chad JA, Pasternak O, Salat DH, Chen JJ. Re-examining age-related differences in white matter microstructure with free-water corrected diffusion tensor imaging. Neurobiol Aging. 2018;71:161-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maillard P, Fletcher E, Singh B, et al. Cerebral white matter free water: a sensitive biomarker of cognition and function. Neurology. 2019;92:e2221–e2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used in this study are publicly available in the ADNI-2 database (adni.loni.usc.edu). A list of the participants included in this study is available on request to the corresponding author.