Graphical abstract
Keywords: COVID-19, Thrombosis, Enoxaparin, Anticoagulation
SARS-CoV-2 disease (COVID-19) is an emerging disease of global concern due to clinical, social, economic and public health impact. As the pandemic spread, it was seen that patients who presented with critical disease had a higher incidence of thrombotic complications, associated with a poor prognosis [1]. Different mechanisms are involved including endothelial inflammation, hypoxia, immunity, anticoagulant antibodies, immobilization, viral activation of the coagulation system and disseminated intravascular coagulation (DIC), among others [2,3]. These findings lead to the creation of the new term COVID-19 associated coagulopathy (CAC) [4]. Currently prophylactic anticoagulation is recommended for all patients with COVID-19 who require hospitalization. Because of a higher incidence of thrombosis despite prophylaxis [5], a new consideration of increasing to intermediate or therapeutic dosing has been recommended, with uncertainty regarding the adverse effects.
We conducted a study to determine if intermediate and formal anticoagulation were associated with a lower risk of death. From March 12th to July 15th, 2020 we collected information on clinical, biochemical and imaging variables from patients admitted at the ABC Medical Center, a private hospital in Mexico City, as part of the ARMII cohort. We included patients who were 18 years or older and had a diagnosis of COVID-19, defined as a positive PCR for SARS-CoV2 and/or a chest CT scan with characteristic findings and who received thromboprophylaxis with enoxaparin since admission. We excluded patients receiving anticoagulation prior to admission and those who received other anticoagulants. The study was approved by local scientific and ethics committees.
Samples for SARS-COV-2 testing were obtained according to the CDC guidelines. Chest CT scans were defined as positive when findings characteristic of COVID-19 were considered by a consensus achieved by several radiologists.
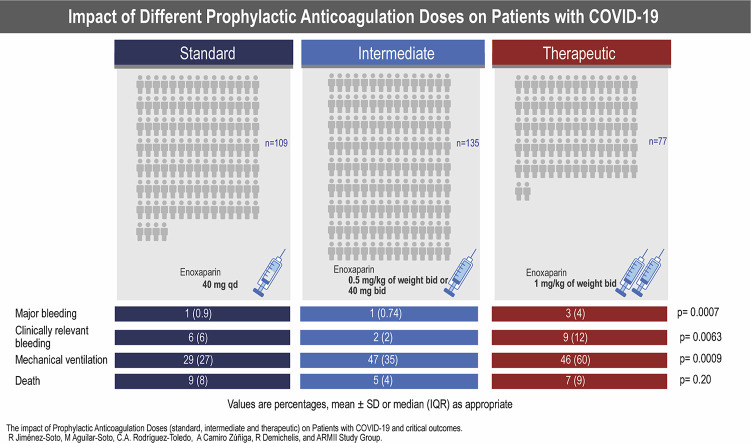
Thromboprophylaxis was part of the standard of care in all patients. The decision of the dose for each patient was done according to existing evidence and to the criteria of the treating physician. We classified patients according to the average dose of enoxaparin based on the ISTH recommendations of low molecular weight heparin (LMWH). Patients were classified as receiving prophylactic standard dose when given 40 mg of enoxaparin qd, intermediate when given 0.5 mg/kg of weight bid or 40 mg bid, or therapeutic anticoagulation when given 1 mg/kg of weight bid.
We defined pulmonary embolism (PE) as patients who had a positive CT pulmonary angiography determined by an experienced radiologist. Major bleeding was defined as symptomatic bleeding in a critical area or organ, fatal bleeding, bleeding requiring the transfusion of two or more units of whole blood or bleeding that causes a fall in hemoglobin level of 2 g/dl or more, as consistent with the ISTH definition. Clinically relevant bleeding was defined as any sign or symptom of bleeding that is not classified as major but that meets one of the following criteria: requiring hospitalization or an increase in care level, requires a prompt face to face evaluation or that requires intervention by a healthcare professional.
Baseline characteristics between the three groups were compared with ANOVA. We conducted univariate, age and sex adjusted and multivariable logistic regressions for death. Covariates used for adjustment included age (continuous), sex, C reactive protein (CRP) levels on admission, D-dimer values on admission (continuous), history of hypertension, and requiring invasive mechanical ventilation (IMV). An analysis restricted only to patients who required IMV was also conducted since it is one of the most important risk factors in hospitalization for death. All statistical tests were 2-sided using a p-value of <0.05. We performed all analyses using SAS University Edition version 9.4 (SAS Institute, Cary, NC).
We included a total of 321 patients, of which 109 (34%) received standard enoxaparin dose, 135 (42%) received intermediate dose and 77 (24%) were on therapeutic doses. The mean age of each group was 55 (22–94 yo), 54 (25–91 yo) and 52 (25–82 yo), for therapeutic dose, intermediate dose and standard dose groups, respectively, without a significant difference between them. The percentage of women across groups was similar and intermediate and therapeutic groups were more obese. Patients with therapeutic anticoagulation had higher respiratory rates and lower pulse oximetry on admission as well as higher leukocyte count, but lower lymphocyte count. Levels of CRP, lactic dehydrogenase, ferritin, IL-6 and D-dimer were significantly higher in intermediate dose and therapeutic dose groups.
Regarding treatments, patients in the intermediate and therapeutic group received tocilizumab with a higher frequency.
Patients who received therapeutic dose had a higher frequency of major and clinically relevant bleeding. There was no difference across groups in the incidence of PE. Another argument in favor of the use of this drug is that it has been shown to have anti-inflammatory effects and decreased thrombin generation, thus modifying the natural course of CAC which also explains the lower mortality in the therapeutic dose group [6].
Notably, there were no patients diagnosed with deep venous thrombosis (DVT), related to CAC being a primary pulmonary thrombotic origin, rather than embolism.
Severity outcomes were higher in the therapeutic group but only requiring IMV was statistically significant, with 27% patients in the standard dose group, 35% in the intermediate dose and 60% in the therapeutic anticoagulation group (p = 0.0009).
Patients in the therapeutic group required IMV more frequently when compared to standard dose when adjusting for age and sex, but not after multivariable adjustment.
Baseline characteristics and specific outcomes are summarized in Table 1 .
Table 1.
Baseline characteristics and outcomes divided by prophylactic doses of enoxaparin of 321 patients included in the analyses.
| Dosing categories |
||||
|---|---|---|---|---|
| Standard dose (n = 109) | Intermediate dose (n = 135) | Therapeutic dose (n = 77) | p value | |
| Age, years % | 52 (17) | 54 (15) | 55 (14) | 0.56 |
| Women, % | 39 (36) | 45 (33) | 23 (30) | 0.70 |
| BMI | 0.05 | |||
| Normal | 29 (27) | 27 (20) | 13 (17) | |
| Overweight | 49 (45) | 61 (45) | 37 (48) | |
| Obese | 31 (30) | 47 (35) | 29 (38) | |
| Diabetes, % | 22 (20) | 17 (13) | 15 (19) | 0.20 |
| Hypertension, % | 32 (29) | 36 (27) | 23 (30) | 0.83 |
| Heart rate on admission | 82 (13) | 81 (13) | 85 (14) | 0.21 |
| Respiratory rate on admission | 20 (18–22) | 20 (18–25) | 22 (19–28) | 0.05 |
| Oxymetry on admission | 92 (87–95) | 89 (84–94) | 88 (83–94) | 0.009 |
| Severity scales | ||||
| NEWS | 5 (2) | 6 (2) | 7 (2) | 0.008 |
| MULBSTA | 7 (5–9) | 8 (5–11) | 9 (5–12) | 0.12 |
| CALL-SCORE | 7 (5–10) | 7 (6–10) | 8 (6–10) | 0.10 |
| Laboratory values | ||||
| Leucocytes | 6 (4–9) | 6 (5–9) | 8 (6–11) | 0.02 |
| Lymphocytes | 1190 (820–1660) | 940 (710–1320) | 920 (640–1230) | 0.001 |
| Platelets | 214 (167–261) | 205 (162–275) | 232 (182–310) | 0.06 |
| CRP | 7 (4–15) | 12 (5–20) | 17 (8–30) | <0.0001 |
| LDH | 264 (198–326) | 282 (231–260) | 358 (266–446) | <0.0001 |
| Ferritin | 670 (288–1440) | 925 (450–1597) | 1263 (704–1829) | 0.005 |
| IL-6 | 23 (15–44) | 40 (15–63) | 51 (20–81) | 0.025 |
| D-dimer | 648 (450–1021) | 787 (503–1198) | 1168 (796–2619) | <0.0001 |
| Fibrinogen | 467 (131) | 458 (155) | 416 (178) | 0.257 |
| INR | 0.98 (0.93–1.05) | 1 (0.93–1.07) | 1.04 (0.95–1.09) | 0.200 |
| PT | 11 (10−12) | 10.9 (10.4–11.7) | 11.3 (10.5–11.8) | 0.23 |
| aPTT | 29 (26–33) | 31 (27–35) | 27 (24–32) | 0.04 |
| TT | 17 (16–18) | 17 (16–18) | 18 (17–22) | 0.011 |
| Treatment | ||||
| Lopinavir/ritonavir, % | 60 (55) | 91 (67) | 47 (61) | 0.14 |
| Azithromycin, % | 83 (76) | 109 (81) | 55 (71) | 0.30 |
| Hydroxychloroquine, % | 96 (88) | 93 (69) | 65 (84) | 0.0002 |
| Glucocorticoids, % | 11 (10) | 9 (7) | 4 (5) | 0.50 |
| Tocilizumab, % | 29 (27) | 57 (42) | 43 (56) | 0.0001 |
| Outcomes | ||||
| Major bleeding, % | 1 (0.9) | 1 (0.74) | 3 (4) | 0.0007 |
| Clinically relevant bleeding, % | 6 (6) | 2 (2) | 9 (12) | 0.0063 |
| PE, % | 2 (2) | 1 (0.7) | 2 (3) | 0.55 |
| Admitted ICU, % | 14 (13) | 25 (19) | 17 (22) | 0.16 |
| Required ICU, % | 9 (11) | 30 (18) | 17 (30) | 0.10 |
| Required IMV, % | 29 (27) | 47 (35) | 46 (60) | 0.0009 |
| Death, % | 9 (8) | 5 (4) | 7 (9) | 0.20 |
Values are percentages, mean ± SD or median (IQR) as appropriate.
BMI: body mass index, C reactive protein: CRP, LDH: lactic dehydrogenase, INR: internationalized normalized ratio, PT: prothrombin time, aTTP: activated Partial tromboplastin time, TT: thrombin time, PE: pulmonary embolism, ICU: intensive care unit, IMV: invasive mechanical ventilation.
Regarding the risk of death, both intermediate and therapeutic doses were associated with a lower risk of death after multivariable adjustment, nevertheless, this association was not significant (see Table 2 ).
Table 2.
Multivariate logistic regression for death and standard, intermediate and therapeutic anticoagulation.a
| Odds ratio (95%CI) | Standard dose (n = 109) | Intermediate dose (n = 135) | Therapeutic dose (n = 77) |
|---|---|---|---|
| Unadjusted | Reference | 0.43 (0.14–1.33) | 1.14 (0.41–3.22) |
| Age and sex adjusted | Reference | 0.42 (0.12–1.39) | 1.34 (0.43–4.15) |
| Multivariableb | Reference | 0.30 (0.08–1.16) | 0.63 (0.16–2.46) |
Values are odds ratio (95% CI) unless otherwise specified.
Model is multivariate analysis adjusted for age (continuous), sex (men or women), CRP levels on admission (continuous), D-dimer values on admission (continuous), history of hypertension, and use of mechanical invasive ventilation.
When restricting the analysis of death for patients who required IMV, intermediate and therapeutic anticoagulation doses were also associated with lower risk of death, but this association was not significant.
Since the first COVID-19 reports a high prevalence of thrombosis and abnormal coagulation activation was observed and frequently related to severe disease [5].
Several societies around the world currently recommend that all hospitalized patients with COVID-19 receive thromboprophylaxis with LMWH, unless the risk of bleeding is higher than of thrombosis [6].
The decision of which patients benefit from a standard, new intermediate or therapeutic dosage is not simple and has not yet been standardized. Baseline characteristics in our study are similar to the data reported across the world [7].
We found some clinical and laboratory data of severe disease seem to have influenced whether to start an intermediate or therapeutic dose of LMWH. The mortality rate in our study was of 6.5%, lower than the one reported in several other centers in Mexico City which go as high as 73.7% and even higher for patients who require IMV [8].
The incidence of both PE and DVT were lower in our cohort than in other reports, which we may infer from a lack of routine ultrasound of lower extremities. Similar to worldwide reports, we identified a higher risk for PE than DVT, that could be explained as CAC being a primary pulmonary thrombotic origin, rather than embolism. The diagnosis of PE is also challenging in COVID-19 patients since the disease by itself might present with a similar clinical picture.
Patients who received anticoagulation were at an increased risk of presenting major or clinically significant bleeding, while coagulopathy itself in COVID-19 may also contribute to a higher risk of bleeding.
When comparing the risk of death between patients with different doses, we saw a lower risk among patients with intermediate and therapeutic anticoagulation, but the risk was not significant. However, since the incidence of death was relatively low in our hospital, this might account for the low significance. In the analysis restricted for patients who required IMV the risk of death was low, but then again this was also non significant.
To our knowledge, this is the first study in Mexican population that evaluates this intervention and its outcomes [8]. Our findings are consistent with other observational studies who concluded that in hospitalized patients with COVID-19, anticoagulation was associated with lower risk of mortality and with low rates of major bleeding [9].
Our study has some limitations it is a single-center retrospective study, we are unable to evaluate anticoagulation at different times. We did not have data on specific hemostatic tests and coagulation factors levels.
In conclusion, our study suggests that therapeutic anticoagulation in COVID-19 critical disease, might be beneficial by decreasing the mortality and need for IMV, but at the expense of higher bleeding risk. Randomized-controlled trials comparing the doses are needed to understand the role of higher doses of prophylactic anticoagulation in COVID-19 to confirm our findings, currently the INSPIRATION/INSPIRATION-S studies are being conducted to compare intermediate versus standard-dose anticoagulation [10].
CRediT authorship contribution statement
M.A.S. and A.C.Z. analyzed the data, performed statistical analysis and wrote results; R.J.S. organized this project, designed the study and wrote the manuscript; C.A.R.T. wrote the manuscript; R.D. reviewed and analyzed the data, manuscript and contributed to the writing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors thank the ARMII Study Group investigators who collected patients for this study and to Dr. Eduardo Fernández-Campuzano for the support.
References
- 1.Klok F.A., Kruip M.J.H.A., van der Meer N.J.M., et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb. Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bikdeli B., Madhavan M.V., Jimenez D., et al. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up: JACC state-of-the-art review. J. Am. Coll. Cardiol. 2020;75(23):2950–2973. doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marieta M, Coluccio V. Handling the complexity: the case of COVID-19 coagulopathy and the risk of venous thromboembolism. Italian Society on Thrombosis and Haemostasis (SISET). Available at: http://www.siset.org/news-e-comunicazioni/siset-online/321.
- 4.Connors J.M., Levy J.H. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135(23):2033–2040. doi: 10.1182/blood.2020006000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Middeldorp S., Coppens M., van Haaps T.F., et al. Incidence of venous thromboembolism in hospitalized patients with COVID-19. J. Thromb. Haemost. 2020;18(8):1995–2002. doi: 10.1111/jth.14888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thachil J., Tang N., Gando S., et al. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J. Thromb. Haemost. 2020;18(5):1023–1026. doi: 10.1111/jth.14810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guan W.J., Ni Z.Y., Hu Y., et al. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Namendys Silva S.A., Alvarado Ávila P.E., Domínguez Cherit G., et al. Outcomes of patients with COVID-19 in the intensive care unit in Mexico: a multicenter observational study. Heart Lung. 2020:1–5. doi: 10.1016/j.hrtlng.2020.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nadkarni G.N., Lala A., Bagiella E., et al. Anticoagulation, bleeding, mortality, and pathology in hospitalized patients with COVID-19. J. Am. Coll. Cardiol. 2020;76(16):1815–1826. doi: 10.1016/j.jacc.2020.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bikdeli B., Talasaz A.H., Rashidi F., et al. Intermediate versus standard-dose prophylactic anticoagulation and statin therapy versus placebo in critically-ill patients with COVID-19: rationale and design of the INSPIRATION/INSPIRATION-S studies. Thromb. Res. 2020;196:382–394. doi: 10.1016/j.thromres.2020.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]