Abstract
Background
Anti-TNF drugs, such as infliximab, are associated with attenuated antibody responses after SARS-CoV-2 vaccination. We aimed to determine how the anti-TNF drug infliximab and the anti-integrin drug vedolizumab affect vaccine-induced neutralising antibodies against highly transmissible omicron (B.1.1.529) BA.1, and BA.4 and BA.5 (hereafter BA.4/5) SARS-CoV-2 variants, which possess the ability to evade host immunity and, together with emerging sublineages, are now the dominating variants causing current waves of infection.
Methods
CLARITY IBD is a prospective, multicentre, observational cohort study investigating the effect of infliximab and vedolizumab on SARS-CoV-2 infection and vaccination in patients with inflammatory bowel disease (IBD). Patients aged 5 years and older with a diagnosis of IBD and being treated with infliximab or vedolizumab for 6 weeks or longer were recruited from infusion units at 92 hospitals in the UK. In this analysis, we included participants who had received uninterrupted biological therapy since recruitment and without a previous SARS-CoV-2 infection. The primary outcome was neutralising antibody responses against SARS-CoV-2 wild-type and omicron subvariants BA.1 and BA.4/5 after three doses of SARS-CoV-2 vaccine. We constructed Cox proportional hazards models to investigate the risk of breakthrough infection in relation to neutralising antibody titres. The study is registered with the ISRCTN registry, ISRCTN45176516, and is closed to accrual.
Findings
Between Sept 22 and Dec 23, 2020, 7224 patients with IBD were recruited to the CLARITY IBD study, of whom 1288 had no previous SARS-CoV-2 infection after three doses of SARS-CoV-2 vaccine and were established on either infliximab (n=871) or vedolizumab (n=417) and included in this study (median age was 46·1 years [IQR 33·6–58·2], 610 [47·4%] were female, 671 [52·1%] were male, 1209 [93·9%] were White, and 46 [3·6%] were Asian). After three doses of SARS-CoV-2 vaccine, 50% neutralising titres (NT50s) were significantly lower in patients treated with infliximab than in those treated with vedolizumab, against wild-type (geometric mean 2062 [95% CI 1720–2473] vs 3440 [2939–4026]; p<0·0001), BA.1 (107·3 [86·40–133·2] vs 648·9 [523·5–804·5]; p<0·0001), and BA.4/5 (40·63 [31·99–51·60] vs 223·0 [183·1–271·4]; p<0·0001) variants. Breakthrough infection was significantly more frequent in patients treated with infliximab (119 [13·7%; 95% CI 11·5–16·2] of 871) than in those treated with vedolizumab (29 [7·0% [4·8–10·0] of 417; p=0·00040). Cox proportional hazards models of time to breakthrough infection after the third dose of vaccine showed infliximab treatment to be associated with a higher hazard risk than treatment with vedolizumab (hazard ratio [HR] 1·71 [95% CI 1·08–2·71]; p=0·022). Among participants who had a breakthrough infection, we found that higher neutralising antibody titres against BA.4/5 were associated with a lower hazard risk and, hence, a longer time to breakthrough infection (HR 0·87 [0·79–0·95]; p=0·0028).
Interpretation
Our findings underline the importance of continued SARS-CoV-2 vaccination programmes, including second-generation bivalent vaccines, especially in patient subgroups where vaccine immunogenicity and efficacy might be reduced, such as those on anti-TNF therapies.
Funding
Royal Devon University Healthcare NHS Foundation Trust; Hull University Teaching Hospital NHS Trust; NIHR Imperial Biomedical Research Centre; Crohn's and Colitis UK; Guts UK; National Core Studies Immunity Programme, UK Research and Innovation; and unrestricted educational grants from F Hoffmann-La Roche, Biogen, Celltrion Healthcare, Takeda, and Galapagos.
Introduction
The COVID-19 pandemic remains a global health crisis, perpetuated by the ongoing evolution of new variants. Since the onset of the first confirmed case in November, 2021, the SARS-CoV-2 omicron (B.1.1.529) variant has spread worldwide and evolved into different subvariants.1, 2 With over 25 mutations in the spike (S1)protein, omicron variants have increased transmissibility and risk of reinfection and reduced vaccine protection compared with previous variants of concern.3 As of September, 2022, omicron BA.4 and BA.5 subvariants (hereafter referred to as BA.4/5) are predominant in the world, and reported to be 4·2-times more resistant to serum samples from vaccinated and boosted individuals than the previous variant BA.2, and more likely to lead to breakthrough infections in vaccinated individuals.4 Current SARS-CoV-2 vaccines continue to effectively protect against severe illness, hospitalisations, and death.5 However, the high rate of breakthrough infections means that COVID-19 remains a considerable burden on society and health-care services, bringing an ongoing risk of mortality, especially in those with impaired immunity.6
We have previously found that treatment with anti-TNF therapy impairs antibody responses after SARS-CoV-2 infection.7, 8 Furthermore, we have previously shown that vaccine-induced antibody responses are attenuated and less durable in patients with inflammatory bowel disease (IBD) treated with the anti-TNF drug infliximab, which was associated with increased risk of breakthrough SARS-CoV-2 infection, than in those treated with the anti-integrin drug vedolizumab, which is gut-specific in the mode of action and does not lead to systemic immunosuppression.5, 9 For patients with IBD treated with immunosuppressive therapies, these findings, along with data from other immunosuppressed patient cohorts,10, 11 have provided evidence supporting the prioritisation of third primary SARS-CoV-2 vaccine doses.
Previous studies in patients with IBD measured S1 receptor binding domain (RBD) binding antibody concentrations. However, binding antibodies do not necessarily mirror functional antibody responses against infection.12 With the fast spread of the more penetrative BA.4/5 variant, it is vital to accurately assess functional neutralising antibody responses to determine cross-reactive protection against SARS-CoV-2 infection provided by SARS-CoV-2 vaccination. Here, we assessed neutralising responses against the SARS-CoV-2 wild-type strain and omicron BA.1 and BA.4/5 subvariants after three doses of vaccine and the association between neutralising antibody potency and subsequent breakthrough infection.
Methods
Study design and population
CLARITY IBD is a nationwide, multicentre, observational cohort study, based in the UK, investigating the effect of the biological therapies infliximab and vedolizumab and concomitant immunomodulators (methotrexate or thiopurines), on SARS-CoV-2 acquisition, illness, and immunity in patients with IBD. The study aims, design, and sample size calculations of the CLARITY IBD study have been described previously.7, 8, 9 In this subgroup analysis, we aimed to assess the neutralising responses against the SARS-CoV-2 variants after three doses of vaccine in patients without previous SARS-CoV-2 infection and the association between neutralising titres and subsequent breakthrough infection. Briefly, patients were recruited at the time of attendance at infusion units for biological therapies across 92 UK hospitals (listed in the appendix [pp 23–38]) between September and December, 2020. Patients older than 5 years with a diagnosis of IBD and treated with infliximab or vedolizumab for 6 weeks or longer (with at least one dose of drug received in the past 16 weeks) were eligible for inclusion in the study. Patients were excluded if they had participated in a SARS-CoV-2 vaccine trial.
Research in context.
Evidence before this study
We searched PubMed and Embase, without language restrictions, for studies published between Jan 1, 2000, and Aug 31, 2022, investigating humoral responses to vaccination in immunosuppressed individuals. We used the search terms (“neutralisation” OR “neutralization” OR “antibody” OR “humoral” OR “immune response”) AND (“vaccine” OR “vaccination”) AND (“immunosuppression” OR “immunosuppressive” OR “immunomodulator” OR “thiopurine” OR “azathioprine” OR “methotrexate” OR “biologic” OR “tumour necrosis factor” OR “infliximab” OR “vedolizumab”). We identified 2775 studies through our search. We have previously shown that infliximab was associated with attenuated antibody responses and increased risk of SARS-CoV-2 breakthrough infection and reinfection following three vaccine doses of SARS-CoV-2 vaccine. However, the magnitude of anti-spike receptor binding domain antibody concentration did not predict breakthrough infection. Multiple studies have shown that anti-TNF treatment is associated with reduced antibody responses, but these studies have generally measured binding antibody concentrations rather than functional neutralising antibodies.
Added value of this study
To our knowledge, this is the first study to assess functional neutralising antibody responses after three doses of SARS-CoV-2 vaccine in a large cohort of individuals with inflammatory bowel disease (IBD). We found that neutralising antibodies against SARS-CoV-2 wild-type strain and omicron (B.1.1.529) subvariants BA.1 and BA.4 and BA.5 (hereafter BA.4/5) were lower in patients treated with infliximab than in those treated with vedolizumab, irrespective of the primary vaccination schedule. We also found that a higher proportion of patients treated with infliximab than with vedolizumab had SARS-CoV-2 breakthrough infection, and that those treated with infliximab had lower neutralising antibody titres against BA.4/5 than did those treated with vedolizumab.
Implications of all the available evidence
In the context of emerging omicron variants and the predominance of BA.4/5, the association between SARS-CoV-2 breakthrough infection in patients with IBD being treated with infliximab and reduced neutralising antibody titres against BA.4/5 supports the prioritisation of SARS-CoV-2 booster vaccination using second-generation bivalent vaccines in these patients.
The UK Government began the rollout of the SARS-CoV-2 vaccination programme for the most clinically vulnerable people, including those with IBD treated with anti-TNF therapies and vedolizumab, in December, 2020, and most people received either the adenovirus vector vaccine ChAdOx1 nCoV-19 (AstraZeneca-Oxford) or the mRNA-based vaccine BNT162b2 (Pfizer-BioNTech) for the first two doses. A third primary vaccine dose (either BNT162b2 or mRNA-1273 [Moderna]) was offered at least 8 weeks after the second dose, from September, 2021, onwards. Thus, individuals received either a homologous vaccine schedule (three doses of mRNA vaccine) or a heterologous vaccine schedule (two doses of adenovirus vector vaccine and one dose of mRNA vaccine).
Serum samples were collected between 14 and 70 days after the third vaccine dose. Neutralising antibody responses against wild-type SARS-CoV-2 and omicron variants BA.1 and BA.4/5 after a third primary vaccine were assessed in patients receiving uninterrupted biological therapy since recruitment and without a previous infection before the serum sample collection. Previous infection was defined as a positive SARS-CoV-2 PCR test or an anti-SARS-CoV-2 nucleocapsid (N) concentration, measured using a Roche Elecsys anti-SARS-CoV-2 N immunoassay, above the cutoff index of 0·11.8
The Surrey Borders Research Ethics committee approved the study (REC reference: REC 20/HRA/3114) in September, 2020. Patients were included after providing written informed consent. The study protocol is available online.
Procedures and definitions
Participants attended follow-up visits that coincided with infusions of biological therapies and typically occurred every 8 weeks. Serum samples for this study were taken at one timepoint between 14 and 70 days after the third vaccine dose.
Laboratory analyses were done centrally at Imperial College London (London, UK). SARS-CoV-2 neutralisation assays were done using pseudo-typed viruses. Pseudo-typed SARS-CoV-2 lentiviruses were produced in HEK293T cells using a SARS-CoV-2 spike (S1 and S2) plasmid (wild-type strain, BA.1, or BA.4/5), HIV-1 gag-pol plasmid, and a firefly luciferase reporter.13, 14 Participant serum samples were serially diluted and incubated with pseudo-typed virus viral supernatant for 1 h. HEK293T-ACE2 cells were then co-incubated with the serum sample and pseudo-typed viruses for 72 h before measurement of the luciferase activity using the Bright-Glo Luciferase assay system (Promega, Madison, WI). 50% neutralising titres (NT50) were calculated as the dilution at which relative luminescence was reduced by 50% compared with a control. We used the Reed–Muench method to calculate the NT50.
A breakthrough infection was defined as a SARS-CoV-2 infection diagnosed with a positive PCR test more than 14 days after the third dose of SARS-CoV-2 vaccine. Data were linked by National Health Service (NHS) number or Community Health Index to NHS England, Public Health England, Scotland, and Wales databases, which hold dates of vaccine uptake and dates and results of SARS-CoV-2 PCR tests done in the UK. The breakthrough infections included in this study occurred after November, 2021, when the omicron variants were spread worldwide, and so were assumed to be caused by the omicron variant.
Variables recorded by participants were demographics (age, sex, race, comorbidities, height and bodyweight, smoking status, and postcode) and SARS-CoV-2 symptoms that aligned with the COVID Symptom Study (symptoms, previous testing, and hospital admissions for COVID-19).15 Study sites collected data relating to IBD history (age at diagnosis, disease duration, and phenotype according to the Montreal classifications), IBD disease activity (patient-reported outcomes [PRO2]),16, 17 previous surgeries, current biological and immunomodulator therapy duration, vaccine uptake (type and date of each vaccination), and date of blood collection. Data were entered electronically into a purpose-designed Research Electronic Data Capture database hosted at the Royal Devon University Healthcare NHS Foundation Trust.18 Participants without access to the internet or an electronic device completed their questionnaires on paper case record forms that were subsequently entered by local research teams.
Outcomes
The primary outcome of this analysis of the CLARITY IBD study was anti-SARS-CoV-2 neutralising antibody response against wild-type SARS-CoV-2 and omicron BA.1 and BA.4/5 subvariants after three doses of SARS-CoV-2 vaccine in patients with IBD treated with infliximab or vedolizumab. The secondary outcome was risk of breakthrough infection in relation to neutralising antibody potency.
Statistical analysis
We report continuous data as median (IQR) and discrete data as numbers and percentages. For comparison of the demographics data between infliximab and vedolizumab recipients, we used the Mann-Whitney U test for continuous data and Fisher's exact test for discrete data. We included patients with missing clinical variables in analyses for which they had data and specified the denominator for each variable.
We report neutralising antibody titres as geometric means and 95% CIs of the half inhibitory titre (ie, NT50). Because immunomodulators have been reported to reduce antibody levels and ChAdOx1 nCoV-19 has been reported to induce lower antibody levels than BNT162b2 after two doses,5, 7 we stratified the comparison of antibody titres using these two variables. We used the Kruskal-Wallis test with Benjamini-Hochberg multiple test correction to compare antibody responses between patients treated with infliximab and vedolizumab. We used the Friedman test to compare NT50 against SARS-CoV-2 wild-type strain and omicron BA.1 and BA.4/5 variants.
We constructed multivariable linear regression models to identify factors independently associated with log-transformed NT50. On the basis of our previous findings,5 a priori we included the following variables: age (per 10 years), race (White race vs not White race), sex (male vs female), BMI (continuous), biological medication (infliximab vs vedolizumab), immunomodulator use (thiopurine [yes vs no], methotrexate [yes vs no]), steroid treatment (yes vs no), IBD type (Crohn's disease vs ulcerative colitis or unclassified IBD), IBD PRO2 active status (active vs inactive), third vaccine dose schedule (heterologous vs homologous), number of interval weeks between vaccine doses 2 and 3 (continuous), and number of interval weeks between vaccine dose 3 and blood sampling (continuous). We present results after exponentiation so that the coefficients of the model correspond to the geometric mean ratio (GMR) associated with each covariate. Because there were significant differences in baseline demographics (age, diagnosis, duration of IBD, concomitant use of immunomodulators or steroids, and comorbidities) between patients treated with infliximab or vedolizumab, we did inverse probability of treatment weighting to reduce the bias of unweighted estimators. We calculated propensity scores using the differential covariates, and we weighted stabilised average treatment effects for the regression analysis. The linearity, homogeneity of variance, collinearity, influential observations, and normality of residuals of each multivariable model were assessed and summarised in the appendix (pp 1–4).
We visualised the durability of antibody responses by calculating the 15-day rolling geometric mean NT50. We calculated 95% CIs using likelihood ratios. We used multivariable Cox proportional hazard regression models to identify treatment-related and vaccine-related factors associated with the time from the third vaccine dose to breakthrough infection; these factors were the same as for the multivariable linear regression model investigating factors associated with log-transformed NT50, and included the variables of log-transformed antibody NT50s against wild-type strain, BA.1, and BA.4/5 when investigating factors associated with breakthrough infection among participants who had a breakthrough infection. We tested the proportional hazards assumption using the corresponding set of scaled Schoenfeld residuals against time, to determine the independence between residuals and time. We tested the linearity of continuous variables in the Cox proportional hazard regression models using the function ggcoxfunctional() in the survminer R package.
To determine how IBD subtypes affected the neutralising titres, we stratified our analysis by patients with Crohn's disease or with ulcerative colitis or unclassified IBD. We also did sensitivity analyses on our Cox regression models in subgroups of patients with Crohn's disease or with ulcerative colitis or unclassified IBD, patients with or without active disease, those using immunomodulators and those using steroids, and accounting for the differing time intervals between third dose and sampling timepoint.
We did all statistical analyses using R (version 4.2.1). All tests were two-tailed, and p values of less than 0·05 were considered to be significant. This study is registered with the ISRCTN registry, ISRCTN45176516.
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, writing of the report, or the decision to submit for publication.
Results
Between Sept 22 and Dec 23, 2020, 7224 consecutive patients were recruited at the time of attendance at infusion units into the CLARITY IBD study. For this analysis, we included 1288 participants who had been treated with infliximab (n=871) or vedolizumab (n=417), without previous SARS-CoV-2 infection, and who had an antibody test between 14 and 70 days after the third dose of SARS-CoV-2 vaccine. The median age of included participants was 46·1 years (IQR 33·6–58·2) and 610 (47·4%) of 1288 participants were female, 671 (52·1%) were male, 1209 (93·9%) were White, 46 (3·6%) were Asian, seven were Black (0·6%), and 15 were Mixed race (1·2%; table 1 ). 537 (61·7%) of 871 participants treated with infliximab and 90 (21·6%) of 417 treated with vedolizumab had combination treatment with immunomodulators (thiopurine or methotrexate). 501 (57·5%) participants treated with infliximab and 239 (57·3%) treated with vedolizumab received a heterologous SARS-CoV-2 vaccine schedule and 369 (42·4%) treated with infliximab and 176 (42·2%) treated with vedolizumab received a homologous schedule.
Table 1.
Baseline characteristics
| Overall population (N=1288) | Patients taking infliximab (n=871) | Patients taking vedolizumab (n=417) | p value | ||
|---|---|---|---|---|---|
| Vaccination schedule | .. | .. | .. | >0·99 | |
| Homologous | 545 (42·3%) | 369 (42·4%) | 176 (42·2%) | .. | |
| Heterologous | 740 (57·5%) | 501 (57·5%) | 239 (57·3%) | .. | |
| Missing | 3 (0·2%) | 1 (0·1%) | 2 (0·5%) | .. | |
| Age, years | 46·1 (33·6–58·2) | 43·6 (32·5–55·5) | 51·30 (37·20–63·30) | <0·0001 | |
| Sex | .. | .. | .. | 0·42 | |
| Female | 610 (47·4%) | 401 (46·0%) | 209 (50·1%) | .. | |
| Male | 671 (52·1%) | 465 (53·4%) | 206 (49·4%) | .. | |
| Other | 2 (0·2%) | 1 (0·1%) | 1 (0·2%) | .. | |
| Missing | 5 (0·4%) | 4 (0·5%) | 1 (0·2%) | ||
| BMI, kg/m2 | 25·9 (22·9–30·2) | 25·9 (22·8–30·4) | 25·92 (23·09–29·52) | 0·96 | |
| Race | .. | .. | .. | 0·25 | |
| Asian | 46 (3·6%) | 24 (2·8%) | 22 (5·3%) | .. | |
| Black | 7 (0·6%) | 5 (0·6%) | 2 (0·5%) | .. | |
| Mixed | 15 (1·2%) | 10 (1·2%) | 5 (1·2%) | .. | |
| Other | 6 (0·5%) | 4 (0·5%) | 2 (0·5%) | .. | |
| White | 1209 (93·9%) | 824 (94·6%) | 385 (92·3%) | .. | |
| Missing | 5 (0·4%) | 4 (0·5%) | 1 (0·2%) | .. | |
| Smoking | .. | .. | .. | 0·14 | |
| Never | 726 (56·3%) | 499 (57·3%) | 227 (54·4%) | .. | |
| Not currently | 450 (34·9%) | 290 (33·3%) | 160 (38·4%) | .. | |
| Current | 104 (8·1%) | 76 (8·7%) | 28 (6·7%) | .. | |
| Missing | 8 (0·6%) | 6 (0·7%) | 2 (0·5%) | .. | |
| Diagnosis | .. | .. | .. | <0·0001 | |
| Crohn's disease | 767 (59·6%) | 614 (70·5%) | 153 (36·7%) | .. | |
| Ulcerative colitis or unclassified IBD | 521 (40·5%) | 257 (29·5%) | 264 (63·3%) | .. | |
| Duration of IBD, years | 11 (6–20) | 11 (5–19) | 12 (7–21) | 0·00050 | |
| PRO2 active disease | 264 (20·5%) | 178 (20·4%) | 86 (20·6%) | >0·99 | |
| Immunomodulators | 627 (48·7%) | 537 (61·7%) | 90 (21·6%) | <0·0001 | |
| Steroids | 64 (5·0%) | 31 (3·6%) | 33 (7·9%) | <0·0001 | |
| Mesalazine | 330 (25·6%) | 185 (21·2%) | 145 (34·8%) | <0·0001 | |
| Heart disease | 53 (4·1%) | 24 (2·8%) | 29 (7·0%) | <0·0001 | |
| Lung disease | 167 (13·0%) | 104 (12·0%) | 63 (15·2%) | 0·12 | |
| Kidney disease | 19 (1·5%) | 11 (1·3%) | 8 (1·9%) | 0·36 | |
| Cancer | 7 (0·5%) | 2 (0·2%) | 5 (1·2%) | 0·040 | |
| Any diabetes | 73 (5·7%) | 40 (4·6%) | 33 (7·9%) | 0·016 | |
| Timing intervals, days | .. | .. | .. | .. | |
| Between third vaccine dose and blood sampling | 40 (26–53) | 40 (26–53) | 39 (26–53) | 0·89 | |
| Between dose 1 and 2 | 77 (70–78) | 77 (70–78) | 76 (70–78) | 0·51 | |
| Between dose 2 and 3 | 189 (173–201) | 188 (171–200) | 191 (179–202) | 0·00050 | |
Data are median (IQR) or n (%). p values were calculated using the Mann-Whitney U test or Fisher's exact test, comparing infliximab vs vedolizumab recipients. Homologous schedule comprises three doses of mRNA vaccine, heterologous schedule comprises two doses of adenovirus vector vaccine plus one dose of mRNA vaccine. IBD=inflammatory bowel disease. PRO2=patient reported outcomes
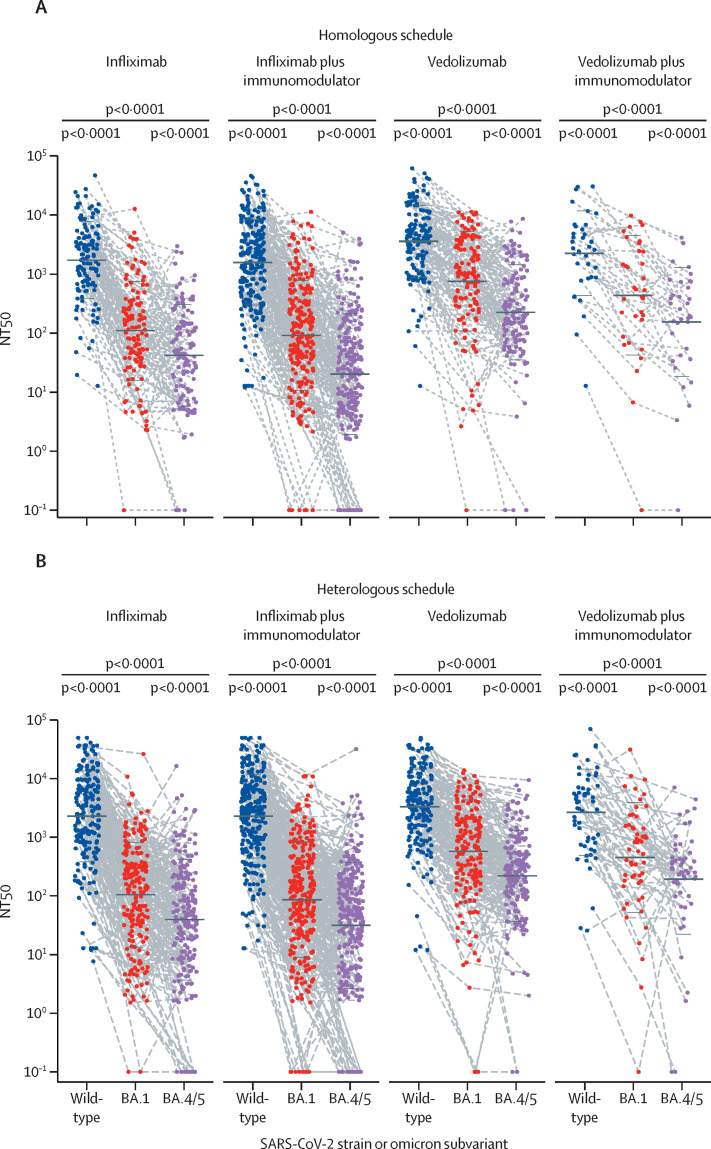
After the third dose of SARS-CoV-2 vaccine, NT50s against omicron BA.1 or BA.4/5 were significantly lower than titres against wild-type SARS-CoV-2 in all IBD treatment groups, irrespective of vaccination schedule (figure 1 , table 2 ). NT50s were also significantly lower in patients treated with infliximab than in those treated with vedolizumab against wild-type (geometric mean 2062 [95% CI 1720–2473] vs 3440 [2939–4026]; p<0·0001), BA.1 (107·3 [86·40–133·2] vs 648·9 [523·5–804·5]; p<0·0001), and BA.4/5 (40·63 [31·99–51·60] vs 223·0 [183·1–271·4]; p<0·0001) variants (table 2). NT50s and 95% CIs by SARS-CoV-2 vaccination schedule are shown in table 2. For each biological treatment group, the difference between NT50s for each variant was significant (p<0·0001). Although the reduction in neutralising titres against omicron BA.4/5, compared with NT50 against the wild-type strain, was observed across all IBD treatment regimens, the size of the reduction was significantly greater in infliximab recipients (64·74 [95% CI 55·94–74·88]) than in vedolizumab recipients (15·13 [12·79–17·91]; p<0·0001; appendix p 5).
Figure 1.
Neutralising antibody titres against SARS-CoV-2 wild-type strain and omicron (B.1.1.529) variants BA.1 and BA.4/5 in patients with IBD, stratified by biological therapy with infliximab or vedolizumab, and after homologous (A) or heterologous (B) SARS-CoV-2 vaccine schedule
Homologous schedule comprises three doses of mRNA vaccine, heterologous schedule comprises two doses of adenovirus vector vaccine plus one dose of mRNA vaccine. The horizontal black bars show the geometric mean and SD. Datapoints are the NT50s from each participant and dotted lines connect data from the same participant. p values were calculated post hoc, using the Friedman test. Samples unable to inhibit half of the virus infection were plotted with the NT50 equal to 0·1. BA.4/5=BA.4 and BA.5. IBD=inflammatory bowel disease. NT50=50% neutralisation titre.
Table 2.
50% neutralising titres against SARS-CoV-2 wild-type strain and omicron BA.1 and BA.4/5, by SARS-CoV-2 vaccination schedule and biological therapy
| Homologous SARS-CoV-2 vaccination | Heterologous SARS-CoV-2 vaccination | Overall | |
|---|---|---|---|
| Infliximab | |||
| Wild type | 1722 (1325–2238) | 2306 (1804–2949) | 2062 (1720–2473) |
| BA.1 | 110·8 (79·02–155·3) | 105·2 (79·20–139·7) | 107·3 (86·40–133·2) |
| BA.4/5 | 42·21 (29·87–59·64) | 39·67 (28·67–54·90) | 40·63 (31·99–51·60) |
| Infliximab plus immunomodulator | |||
| Wild type | 1581 (1280–1951) | 2310 (1914–2786) | 1946 (1691–2239) |
| BA.1 | 91·94 (70·03–120·7) | 86·32 (66·54–112·0) | 88·77 (73·59–107·1) |
| BA.4/5 | 20·32 (15·08–27·39) | 31·47 (23·66–41·86) | 25·84 (21·02–31·75) |
| Vedolizumab | |||
| Wild type | 3574 (2830–4514) | 3350 (2698–4158) | 3440 (2939–4026) |
| BA.1 | 754·4 (546·9–1040) | 581·1 (433·4–779·1) | 648·9 (523·5–804·5) |
| BA.4/5 | 226·1 (166·0–307·9) | 221·3 (170·7–286·8) | 223·0 (183·1–271·4) |
| Vedolizumab plus immunomodulator | |||
| Wild type | 2248 (1278–3953) | 2683 (1696–4244) | 2504 (1766–3552) |
| BA.1 | 438·2 (197·0–974·5) | 454·4 (252·6–817·4) | 448·0 (281·4–713·3) |
| BA.4/5 | 155·0 (74·79–321·3) | 193·5 (107·8–347·3) | 177·5 (113·4–277·8) |
NT50s are presented to four significant figures, with 95% CIs shown in parentheses. BA.4/5=BA.4 and BA.5. NT50=50% neutralising titre.
Next, we compared antibody titres from each treatment group. Patients treated with infliximab had lower NT50s against BA.1 or BA.4/5 than did patients treated with vedolizumab regardless of vaccination schedule (all comparisons were p<0·01; table 2; appendix pp 6–7). Combination treatment with immunomodulators did not significantly reduce antibody titres compared with infliximab or vedolizumab monotherapy (table 2; appendix pp 6–7). For the NT50 against the wild-type strain, patients who received homologous vaccination and treated with infliximab or infliximab plus immunomodulators had lower antibody titres than did those treated with vedolizumab (both comparisons p<0·001 [table 2; appendix p 6]). We found no significant differences in NT50s against the wild-type strain among treatment groups with a heterologous vaccine schedule (table 2). Notably, 71 (5·5% [95% CI 4·4–7·0]) of 1288 patients in the cohort could not generate an NT50 against BA.4/5, of whom 62 (87% [77–94]) were treated with infliximab (figure 1).
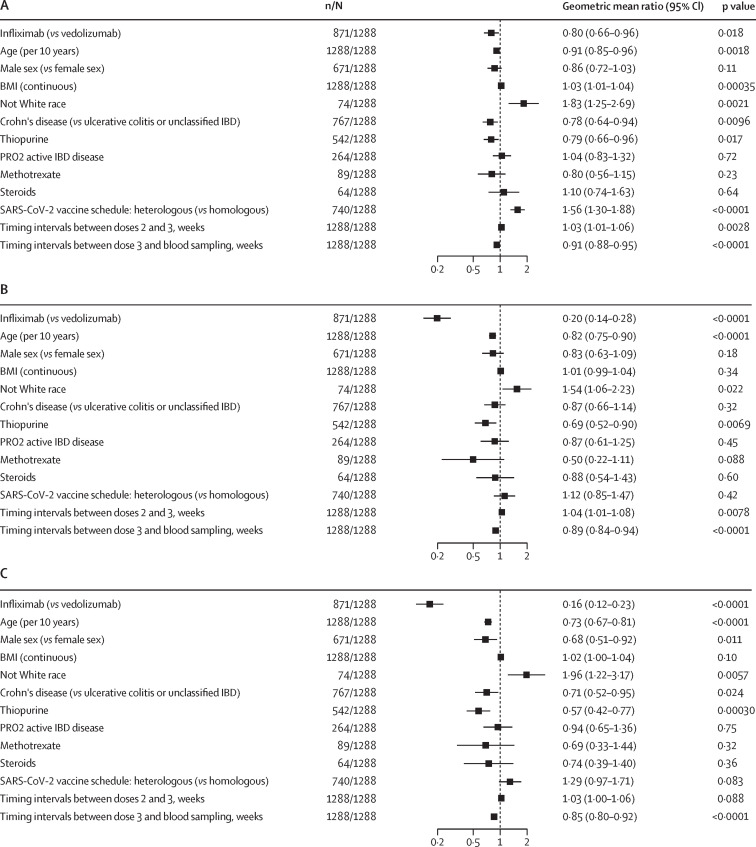
We did multivariable linear regression analyses to determine whether different variables affected neutralising titres (figure 2 ). Patients with Crohn's disease had lower antibody NT50s than did those with ulcerative colitis or unclassified IBD against wild-type strain and BA.4/5, but not BA.1. Older age was associated with lower NT50s against wild-type strain, BA.1, and BA.4/5. Not White race was independently associated with higher NT50s than White race against wild-type strain, BA.1, and BA.4/5. A heterologous third vaccine dose induced higher antibody NT50s than did a homologous schedule against wild-type strain, but not BA.1 or BA.4/5. Thiopurine treatment was associated with lower NT50s against wild-type strain, BA.1, and BA.4/5. Higher BMI was associated with higher antibody NT50s against wild-type strain, but not BA.1 or BA.4/5. A longer interval between vaccine doses 2 and 3 was associated with higher antibody NT50s against wild-type strain and BA.1, but not BA.4/5. A longer interval between the third vaccine dose and blood sampling was associated with lower antibody NT50s against wild-type strain, BA.1, and BA.4/5. Antibody NT50s against BA.4/5 were lower in male than in female participants, but no difference was seen between male and female participants for wild-type strain or BA.1. To further determine if our results were influenced by IBD subtype, we stratified our analysis into patients with ulcerative colitis and Crohn's disease (appendix pp 8–11). We found significant reductions in NT50s against SARS-CoV-2 BA.1 and BA.4/5, both in patients with Crohn's disease and ulcerative colitis treated with infliximab compared with in those treated with vedolizumab; for wild-type strain, a reduction was seen for those with ulcerative colitis, but not for those with Crohn's disease.
Figure 2.
Multivariable linear regression models of log NT50 against SARS-CoV-2 wild-type strain (A), BA.1 (B), and BA.4/5 (C) after the third dose of SARS-CoV-2 vaccine
Data are geometric mean ratios, with 95% CIs, of antibody titres associated with each variable. BA.4/5=BA.4 and BA.5. IBD=inflammatory bowel disease. NT50=50% neutralisation titre.
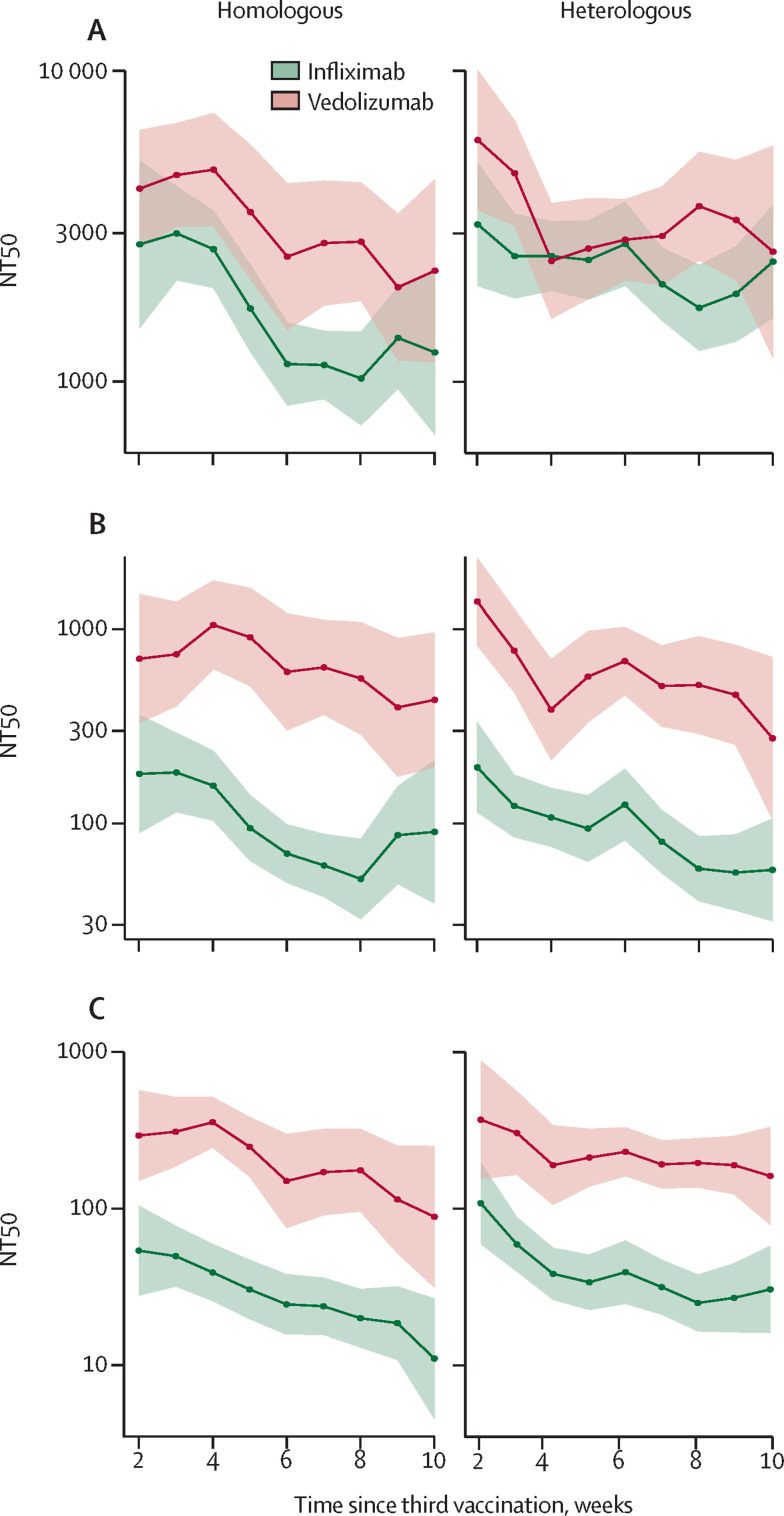
To visualise antibody decay after the third vaccine dose, we plotted rolling geometric means using a rolling 15-day window (7 days on either side of the day indicated; figure 3 ). Antibody titres in patients treated with infliximab were significantly lower than in those treated with vedolizumab for BA.4/5, with some overlap of 95% CIs for the wild-type strain and BA.1. The biggest difference between the two groups was noted in antibody NT50s against BA.4/5. Responses against the wild-type strain, BA.1, and BA.4/5 all decayed over the 10-week study period. A degree of fluctuation in neutralising responses was noted against both wild-type and BA.1 (probably secondary to natural variation in participants sampled at the different timepoints). The downward trend in responses was clearest against BA.4/5 and was most notable in the infliximab-treated group who received a homologous vaccination schedule.
Figure 3.
Rolling geometric mean of NT50 against SARS-CoV-2 wild-type strain (A) and omicron BA.1 (B) and BA.4/5 (C) subvariants, in patients with IBD treated with infliximab or vedolizumab, over time from the third dose of SARS-CoV-2 vaccine
Geometric mean was calculated using a rolling 15-day window (ie, 7 days on either side of the day indicated). The shaded areas show the 95% CIs of the geometric mean. BA.4/5=BA.4 and BA.5. IBD=inflammatory bowel disease. NT50=50% neutralisation titre.
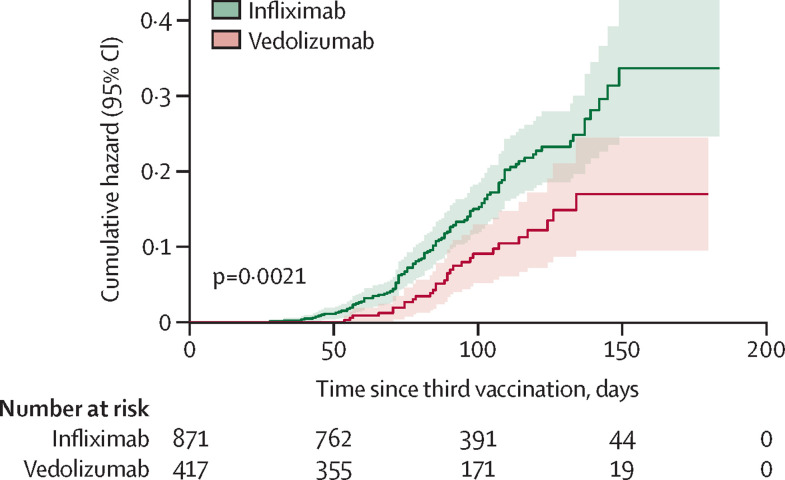
We then investigated the breakthrough infection rate in our cohort. Significantly higher breakthrough infection rates were observed in patients treated with infliximab (119 [13·7%; 95% CI 11·5–16·2] of 871) than those treated with vedolizumab (29 [7·0%; 4·8–10·0] of 417; p=0·00040). The infliximab group had a significantly shorter time to breakthrough infection after third vaccine dose than did the vedolizumab group (median 80·79 days [IQR 76·22–85·63] vs 87·30 [79·71–95·60]; p=0·0021; figure 4 ).
Figure 4.
Cumulative hazard of time to breakthrough infection in patients with IBD treated with infliximab or vedolizumab after a third dose of SARS-CoV-2 vaccine
p value was calculated using a log-rank test.
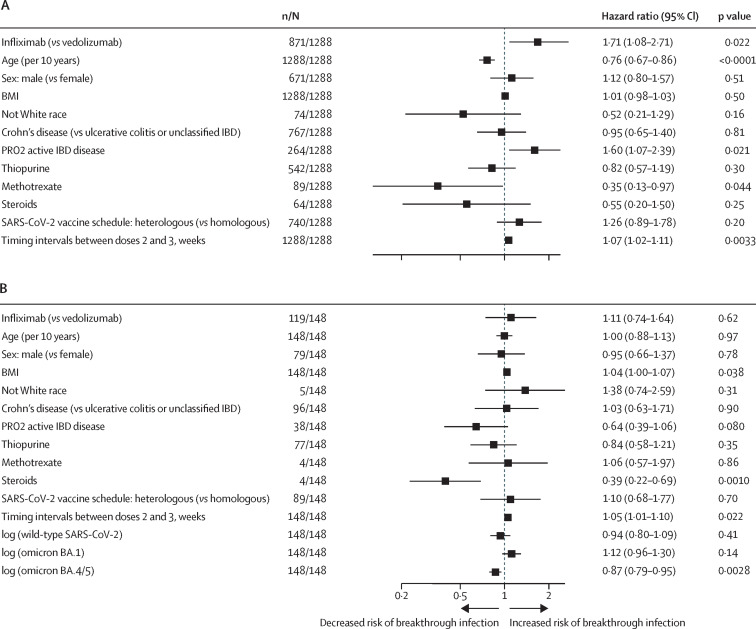
Among the total cohort investigated, infliximab, active IBD disease, and a longer interval between SARS-CoV-2 vaccine doses 2 and 3 were associated with an increased risk of breakthrough infection after a third vaccine dose (figure 5A ). Methotrexate and older age were associated with a decreased risk of breakthrough infection. Steroids were not associated with an increased risk of breakthrough infection.
Figure 5.
Multivariable Cox proportional hazards regression model of factors associated with the risk of SARS-CoV-2 breakthrough infection in all patients included this study (A) and patients who had a breakthrough infection (B)
The dependent variable of the Cox proportional hazards model is the time from third vaccine dose to breakthrough infection and the coefficients represent the hazard ratio of developing SARS-CoV-2 breakthrough infection for each variable. BA.4/5=BA.4 or BA.5. IBD=inflammatory bowel disease. PRO2=patient-reported outcomes.
Among patients who had a breakthrough infection, higher antibody NT50s against BA.4/5 was associated with a lower hazard ratio and, hence, a longer time to breakthrough infection (figure 5B). We found no significant association between antibody NT50s against wild-type strain or BA.1 and time to breakthrough infection.
In sensitivity analyses, we found that among patients who had a breakthrough infection, lower antibody NT50s against BA.4/5 in patients with ulcerative colitis or unclassified IBD, using combination therapy with immunomodulators, not using steroids, or inactive IBD disease were associated with shorter time to breakthrough infection. Antibody NT50s against BA.4/5 were not associated with time to breakthrough infections in subgroups of patients with Crohn's disease, active disease, using steroids, and not using immunomodulators. All sensitivity analyses are shown in the appendix (pp 11–21).
Discussion
After three doses of SARS-CoV-2 vaccine, patients with IBD treated with infliximab had significantly lower neutralising antibody titres against wild-type SARS-CoV-2 and omicron BA.1 and BA.4/5 subvariants than did patients treated with vedolizumab. The concomitant use of thiopurine was associated with lower antibody NT50s against wild-type, BA.1, and BA.4/5 than with monotherapy. 13·7% of patients being treated with infliximab treatment had a breakthrough infection, compared with 7·0% of patients being treated with vedolizumab, and infliximab treatment was associated with a shorter time to breakthrough infection than was vedolizumab treatment. Additionally, lower neutralising NT50s against BA.4/5 were associated with a shorter time to breakthrough infection.
Multiple mutations of the SARS-CoV-2 S1 protein can lead to immune escape,19 which explains the lower neutralising responses we observed here against the omicron variants than against the wild-type virus. The mutation at S1 residue L452 in BA.4/5 facilitates escape from antibodies directed to the RBD's class 2 and 3 regions.4 The F486V mutation compromises the viral receptor's S1 affinity and enables the escape of class 1 and 2 antibodies.4
Although our study focused on neutralising antibody responses against the wild-type strain of SARS-CoV-2 and its omicron variants, other data are emerging regarding vaccine-induced antibody responses directed against the wild-type strain of SARS-CoV-2 in individuals with IBD. Consistent with our findings, the VIP study found that patients treated with infliximab, infliximab plus thiopurine combination therapy, or tofacitinib had significantly lower immune responses than did healthy controls after two and three doses of vaccine.20, 21, 22 Other studies have also reported reduced vaccine-induced antibody responses against the wild-type strain of SARS-CoV-2's S1 protein in patients with IBD treated with anti-TNF therapies after two or three vaccine doses.23, 24, 25 Unlike serological responses, reports on T-cell responses in patients with IBD treated with anti-TNF therapies have been more conflicting. Our data from after two doses of SARS-CoV-2 vaccine suggested no difference in T-cell responses between infliximab-treated and vedolizumab-treated patients,9 and the VIP study showed no significant differences between these groups and healthy controls after three doses.21 Dayam and colleagues26 reported enhanced waning of T-cell immunity in patients with IBD treated with anti-TNF therapies compared with healthy controls 3 months after their second dose of SARS-CoV-2 vaccine. However, Qui and colleagues27 observed augmented antigen-specific T-cell responses in such patients after two doses of SARS-CoV-2 vaccine.
Previously, we found that anti-S1 RBD antibody concentrations did not predict the risk of breakthrough infection with the omicron variant.5 However, in the current study, we found an independent association between lower neutralising NT50s against BA.4/5 and a shorter time to breakthrough infection. This association is probably due to the S1 sequence rapidly evolving from the wild-type strain's sequence to the substantially mutated omicron variant, with functional neutralising antibodies against the omicron variant predicting omicron breakthrough infection. Reassuringly, although treatment with infliximab was associated with increased risk of breakthrough infection, COVID-19 symptoms were mild, and severe disease (eg, hospitalisations and deaths) was still uncommon.5 This finding probably reflects the effectiveness of vaccination and the reduced virulence of the omicron variant compared with the previously dominant variants of concern. Moreover, anti-TNF therapy has been reported to potentially prevent serious illness by inhibiting the systemic inflammatory response associated with severe COVID-19.28 Other studies have also suggested that anti-TNF treatment is associated with a lower risk of COVID-19-associated hospitalisation or death than other immunomodulatory treatment regimens.29, 30
As well as having lower antibody responses against all variants assessed, we observed that the magnitude of the reduction in neutralising titres against omicron BA.4/5, compared with the wild-type strain, was significantly greater in infliximab recipients than in vedolizumab recipients. These data indicate that adopting bivalent or variant-specific vaccination strategies might be especially valuable in infliximab-treated patients.
Our study has several strengths. We adopted a novel approach to assessing functional neutralising antibody responses directed against the wild-type strain of SARS-CoV-2 and omicron variants, which are relevant to the current clinical situation. We have also harnessed robust pseudo-neutralisation assays rather than anti-S1 RBD binding assays, which have been used in most other IBD studies. And we actively recruited a large patient cohort who were established on infliximab and vedolizumab drug regimens across the UK to provide much-needed data on vaccine-induced immunogenicity.
Our study also has several limitations. First, although neutralising antibody responses reflect part of functional protection against infection, we did not measure other immunological effects, and in particular, we do not report anti-viral T-cell immune responses contributing to anti-viral immunity. However, we note the results of the VIP study showing no significant differences in T-cell responses between healthy controls and patients with IBD treated with infliximab and vedolizumab after three doses of SARS-CoV-2 vaccine.21 Second, we did not include healthy controls in this cohort to compare the neutralising antibody potency between patients with IBD and controls after three doses of SARS-CoV-2 vaccine and only two drugs were investigated in this cohort. However, previous data from the VIP study indicate that patients treated with vedolizumab have equivalent SARS-CoV-2 vaccine-induced humoral immune responses to healthy controls, and ustekinumab and thiopurine monotherapy do not impair antibody or T-cell responses.21 Third, we did not explore the immunological mechanisms for reduced vaccine-induced antibody responses observed in patients treated with infliximab. Fourth, we did not sequence the variant involved in each breakthrough infection, and so do not know for certain which variants caused the infections. The infections in the study occurred after November, 2021, so they were probably caused by the omicron variant, which became dominant in the UK around that time. However, we could not infer which omicron subvariant was the cause in each case. Additionally, breakthrough infections might have been missed if patients were asymptomatic and did not take a PCR test. Moreover, some patients treated with infliximab have diminished serological response to infection; therefore, the anti-N assay result might underestimate the infection rate in this group. Finally, the covariates we selected for the analysis were based on an a priori statistical analysis plan and findings derived from previous publications,5 and we did not plot a causal directed acyclic graph. There could be built-in selection bias in the HR analysis, and we cannot exclude the possibility that our results are affected by measurement bias, residual confounding, or unmeasured confounders.
In summary, infliximab treatment attenuated neutralising antibody responses against omicron variants and was associated with increased rates of breakthrough SARS-CoV-2 infection after three doses of SARS-CoV-2 vaccine compared with treatment with the anti-integrin drug vedolizumab. Breakthrough infection was associated with lower magnitude of neutralising antibody NT50 against BA.4/5. These data support policies prioritising patients treated with infliximab for newly available bivalent vaccines to enhance neutralising antibody induction against divergent variants. We hope that our findings are generalisable to other anti-TNF drugs, including adalimumab, golimumab, certolizumab, and etanercept.5, 31 Because anti-TNF medications are used to treat many immune-mediated inflammatory diseases, the implications of our findings could be relevant for millions of patients across the world.
Data sharing
The data of this study are available under a transfer agreement from the corresponding author based on a reasonable request. There will be limitations on how data can be used or how long data will be available for.
Declaration of interests
SL reports non-financial support from Pfizer and Ferring. SS reports grants from Takeda, Tillots Pharma, Janssen, and Amgen; speaker honoraria from Janssen, Tillotts Pharma, Amgen, AbbVie, and Ferring; personal fees from Takeda, Janssen, and Tillots Pharma; and serving as an advisory board member for Takeda. CAL declares research support or fees for development and delivery of non-promotional education from Janssen, Takeda, AbbVie, AstraZeneca, Eli Lilly, Orion, Pfizer, Roche, Sanofi Aventis, Ferring, Dr Falk, UCB, Biogen, and Genentech. ALH has served as consultant, advisory board member, or speaker for AbbVie, Arena, Atlantic, Bristol-Myers Squibb, Celgene, Celltrion, Falk, Ferring, Galapagos, Janssen, MSD, Napp Pharmaceuticals, Pfizer, Pharmacosmos, Shire, and Takeda and serves on the global steering committee for Genentech. KK reports payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from Janssen and Ferring; support for attending meetings or travel from Janssen and Takeda; and participation on a data safety monitoring board or advisory board for Janssen and PredictImmune. CWL reports personal consulting fees from Galapagos, AbbVie, Takeda, Pfizer, Janssen, and Iterative Scopes; institutional consulting fees from Trellus Health; and personal fees and support for attending meetings from Galapagos, AbbVie, Takeda, Pfizer, Janssen, GSK, Gilead, Fresenius Kabi, Ferring, and Dr Falk. JRG reports grants from F Hoffmann-La Roche, Biogen, Celltrion Healthcare, Takeda, and Galapagos during the study. KMP is chief, principal, or co-investigator for vaccine clinical trials and experimental medicine studies (NCT05007275, NCT04753892, EudraCT 2020-001646-20, NCT04400838, NCT04324606, EudraCT 2017-004610-26, NCT03970993, and NCT03816137), is a member of the data safety monitoring board for two vaccine trials (NCT05249829 and NCT05575492), has received a fee for speaking from Seqirus and Sanofi Pasteur, and has research funding from the Chan Zuckerberg Initiative, the UK Medical research Council/UK Research and Innovation, the Vaccine Task Force, and National Institute for Health Research (NIHR) Imperial Biomedical Research Centre (BRC), outside the submitted work. NAK reports grants from F Hoffmann-La Roche AG, Biogen, Celltrion Healthcare, Takeda, and Galapagos during the conduct of the study; grants and non-financial support from AbbVie, MSD, Napp, Pfizer, Celgene, and Pharmacosmos; grants and personal fees from Celltrion; personal fees and non-financial support from Janssen, Tillots Pharma and Dr Falk; and personal fees from Allergan, Ferring, Pharmacosmos, Takeda, Amgen, Bristol-Myers Squibb, and Mylan, outside the submitted work. TA reports grants from F Hoffmann-La Roche AG, Biogen, Celltrion Healthcare, Takeda, Galapagos, and Crohn's and Colitis UK during the conduct of the study; payment for educational events for Takeda; and serving as medical advisor to Crohn's and Colitis UK, outside of the submitted work. NP reports grants from F Hoffmann-La Roche AG, Biogen, Celltrion Healthcare, Takeda, Galapagos, and Crohn's and Colitis UK during the conduct of the study; research grants from Bristol Myers Squibb and Pfizer; and personal fees from Takeda, Janssen, Pfizer, Galapagos, Bristol-Myers Squibb, AbbVie, Roche, Lilly, Allergan, Celgene, and Astra Zeneca outside the submitted work; and has served as a speaker or advisory board member for AbbVie, Allergan, Bristol-Myers Squibb, Celgene, Dr Falk, Galapagos, AstraZeneca, and Vifor Pharma. SL is supported by a Wellcome GW4-CAT fellowship. NC acknowledges support from Crohn's and Colitis UK. CAL acknowledges support from the NIHR Newcastle BRC and the support of the Programmed Investigation Unit at Royal Victoria Infirmary, Newcastle upon Tyne. CWL is funded by a UKRI Future Leaders Fellowship. NP is supported by the NIHR Imperial BRC. All other authors declare no competing interests.
Acknowledgments
Acknowledgments
CLARITY IBD is an investigator-led UK NIHR COVID-19 urgent public health study. This study was funded by Royal Devon University Healthcare NHS Foundation Trust, Hull University Teaching Hospital NHS Trust, NIHR Imperial BRC, Crohn's and Colitis UK (M2021/1), Guts UK, National Core Studies Immunity programme UKRI (awarded to RJB, DMA, NP, and TA; MR/W020610/1), and unrestricted educational grants from F Hoffmann-La Roche (Switzerland), Biogen (USA), Celltrion Healthcare (South Korea), Takeda (UK), and Galapagos (Belgium). The NIHR Clinical Research Network supported study set-up, site identification, and delivery of this study. We acknowledge the contribution of our patient advisory group who helped shape the trial design around patient priorities. Our partners, Crohn's and Colitis UK, continue to support this group and participate in Study Management Team meetings. The Exeter NIHR Clinical Research Facility coordinated sample storage and management. We acknowledge the study co-ordinators of the Exeter Inflammatory Bowel Disease Research Group: Marian Parkinson and Helen Gardner-Thorpe for their ongoing administrative support to the study. This research used data assets made available by National Safe Haven as part of the Data and Connectivity National Core Study, led by Health Data Research UK in partnership with the Office for National Statistics and funded by UK Research and Innovation (research which commenced between Oct 1, 2020, and March 31, 2021 [grant reference MC_PC_20029], and April 1, 2021, and Sept 30, 2022 [MC_PC_20058]). The sponsor of the study was the Royal Devon University Healthcare NHS Foundation Trust. The SARS-CoV-2 BA.4/5 spike protein was a gift from Marceline Côté from University of Ottawa (Addgene plasmid # 186031).
Contributors
TA, NP, and ZL participated in the conception and design of this study. CB was the project manager and coordinated patient recruitment. ZL, KL, XZ, JLA, SL, CB, NC, RN, TJM, CAL, SS, KK, CWL, DMA, ALH, RJB, KMP, RCP, JRG, NAK, TA, and NP were involved in the acquisition, analysis, or interpretation of data. ZL and NP drafted the manuscript. All the authors contributed to the critical review and final approval of the manuscript. ZL, NAK, JRG, TA, and NP accessed and verified the underlying study data. All authors were responsible for the decision to submit the manuscript.
Contributor Information
CLARITY study investigators:
Madiha Islam, Nick Croft, Bessie Cipriano, Caroline Francia, Nosheen Khalid, Ashley Kingston, Irish Lee, Anouk Lehmann, Kinnari Naik, Kevin Samuels, Nicolene Plaatjies, Hafiza Khatun, Farjana Bokth, Elise Pabriaga, Rebecca Saich, Hayley Cousins, Wendy Fraser, Rachel Thomas, Matthew Brown, Benjamin White, Nikolaos Kirkineziadis, Bernadette Tilley, Pennie Porter, Rachel Bryant, Natalia Robaczewska, Rafeeq Muhammed, Rehana Bi, Catherine Cotter, Jayne Grove, Kate Hong, Ruth Howman, Monica Mitchell, Sophie Clayton, Louise Rogers, Sugrah Sultan, Melanie Rooney, Charlotte Cottrill, Salil Singh, Chris Dawe, Robert Hull, Natalie Silva, Julie Chadwick, Laura Robertson, Jonathan Manning, Lauren Finlayson, Allison Roebuck, Joy Dawson, Sunil Sonwalkar, Naomi Chambers, Matthew Robinson, Andrew Haigh, Lear Matapure, Tim Raine, Varun George, Christina Kapizioni, Konstantina Strongili, Tina Thompson, Mohamed Ahmed, Christos Kontos, Claire Dawson, Christophe Bourges, Isabella Barbutti, Megan E Gozzard, Philip Hendy, Rhian Bull, Patricia Costa, Lisa Davey, Hayley Hannington, Kribashnie Nundlall, Catarina Martins, Laura Avanzi, Jaime Carungcong, Sabrina Barr, Richard Appleby, Emma Johnson, Eathar Shakweh, Kath Phillis, Rachel Gascoyne, Amanda Crowder, Amanda Whileman, Ian London, Jenny Grounds, Emmeline Martin, Susie Pajak, Jude Price, Kathryn Cawley, Sandra Powell, Nichola Kearsley, Anjan Dhar, Ellen Brown, Amanda Cowton, Kimberley Stamp, Ben Warner, Carmel Stuart, Louise Lacey, Shanika de Silva, Clare Allcock, Philip Harvey, Lesley Jones, Elise Cooke, Jayne Slater, Dominic King, Johanne Brooks, Pearl Baker, Hannah Beadle, Carina Cruz, Debbie Potter, Joe Collum, Farzana Masters, Aashish Kumar, Samantha Coetzee, Mihaela Peiu, Becky Icke, Jill Williams, Meena Raj, Edward Gaynor, Sibongile Chadokufa, Bonita Huggett, Hamza Meghari, Sara El-Khouly, Fevronia Kiparissi, Waffa Girshab, Lynda Russell-Walker, Christopher Jackson, Sara Sidler, Andrew Claridge, Emily Fowler, Laura McCafferty, Lesley Haxton, Peter Irving, Karolina Christodoulides, Angela Clifford, Patrick Dawson, Sailish Honap, Samuel Lim, Raphael Luber, Karina Mahiouz, Susanna Meade, Parizade Raymode, Rebecca Reynolds, Anna Stanton, Sherill Tripoli, Naomi Hare, Sopuluchukwu Odukwe, Senthuran Balachandran, Emma North, Jessica North, Bria Browne, Jessica Cordle, Ella Jameson, Yih Harn Siaw, Lane Manzano, Jonathan Segal, Ibrahim Al-Bakir, Imran Khakoo, Sofiya Portukhay, Nora Thoua, Katherine Davidson, Jagrul Miah, Lisa Canclini, Alex Hall, Hassina Furreed, Christine Mitchell-Inwang, Melony Hayes, Sally Myers, Alison Talbot, Jack Turnbull, Emma Whitehead, Katie Stamp, Alison Pattinson, Verghese Mathew, Leanne Sherris, Julie Wilcox, Sankaranarayanan Ramachandran, Hayley Robertson, Angela Harvey, Lucy Hicks, Tara-Marie Byrne, Leilani Cabreros, Hannah Downing-Wood, Sophie Hunter, Mohammad Aamir Saifuddin, Hemanth Prabhudev, Sharmili Balarajah, Jan Krucznski, Kalliopi Driva, Andrea D'Mello, Parith Shah, Rocio Castro-Seoane, Hajir Ibraheim, Laura E Constable, Jonathan W Lo, Melissa Torkizadeh, Sherine K Hermangild, Helen Sutherland, Elva Wilhelmsen, Katherine Mackintosh, Ajay M Verma, Juliemol Sebastian, Mohammad Farhad Peerally, Parizade Raymode, Anne-marie Guerdette, Susan Coburn, Ching Yee Novem lam, Donna Durrant, Belinda Schaefer, Solange Serna, Muhammad Shahzad, Alexandra Kent, Lee Meng Choong, Benedetta Pantaloni, Pantelis Ravdas, Babu Vadamalayan, Stephen Foley, Becky Arnold, Cheryl Heeley, Wayne Lovegrove, Donna Sowton, Lynne Allsop, Heidi Gregory, Mandy Gill, Megan Holmes, Valeria Balan, Susan Smith, Sarah Turner, Philip J Smith, Alan Steel, Giovanna Bretland, Sarah King, Martina Lofthouse, Lindsey Rigby, Sreedhar Subramanian, David Tyrer, Kate Martin, Christopher Probert, Nikolaos Kamperidis, Temi Adedoyin, Manisha Baden, Jeannette Brown, Feba Chacko, Lisa Young, Michela Cicchetti, Mohammad Saifuddin, Priya Yesupatham, Rohit Gowda, Maureen Williams, Karen Kemp, Rima Akhand, Glaxy Gray, Anu John, Maya John, Tasnim Mohammed, Diamond Sathe, Natasha Jones, Jennifer Soren, Michael Sprakes, Julie Burton, Patricia Kane, Stephanie Lupton, Jacqueline Bartholomew, Elizabeth Denis, Alison Daniels, George MacFaul, Diane Scaletta, Loria Siamia, Felicity Williams, Chloe Green, Zeljka Ver, Chris Lamb, Mary Doona, Ashleigh Hogg, Lesley Jeffrey, Andrew King, R Alexander Speight, Jennifer Doyle, Ruth Owen, Jenny Haworth, Linda Patterson, Vithusa Varnaulasingam, Craig Mowat, Debbie Rice, Susan MacFarlane, Anne MacLeod, Samera Mohammed, Shona Murray, Anne Elliott, Mary Anne Morris, Louise Coke, Grace Hindle, Eirini Kolokouri, Catherine Wright, Claire Lee, Nicola Ward, Adele Dann, Melanie Lockett, Charlotte Cranfield, Louise Jennings, Ankur Srivastava, Lana Ward, Nouf Jeynes, Poonam Ranga, Praveen Rajasekhar, Lisa Gallagher, Jill Ward, Rae Basnett, Judy Murphy, Lauren Parking, Emma Lawson, Stacey Short, David Devadason, Gordon Moran, Neelam Khan, Lauren Tarr, Charmaine Olivia, Samantha Warbarton, Sian Kelly, Jimmy Limdi, Kay Goulden, Asad Javed, Lauren McKenzie, Julie Melville, Eleanor Liu, Joseph Sabine, Patricia Jacob, Denise McSorland, Nick Schofield, Lisa Cornwall, James Quirke, Emma Crook, Anne Turner, Pradeep Bhandari, Michelle Baker-Moffatt, Joanne Dash, Alison Le Poidevin, Hayley Downe, Lucille Bombeo, Helen Blackman, Rebecca Smith, Alan Wiles, Hannah Bloxham, Jose Dias, Evelyn Nadar, Hollie Curgenven, Ellie Gilham, Jonathan Macdonald, Shona Finan, Faye McMeeken, Misbah Mahmood, Stephanie Shields, John Paul Seenan, Des DeSilva, Susanna Malkakorpi, Rachel Carson, Holly Lawrence, Ofori Boateng, Felix Kpodo, Simon Whiteoak, Kelli Edger-Earley, Luke Vamplew, Joanna Samways, Sue Roffe, Sarah Ingram, Joel James, Sharon Botfield, Fiona Hammonds, Clare James, Zoe Berry, Gemma Aspinall, Sarah Hawkins, Marian Parkinson, Helen Gardner-Thorpe, Suzie Marriott, Clare Redstone, Halina Windak, Ana-Marie Adam, Hannah Mabb, Emma Stevenson, Jessica Record, Charles Murray, Cynthia Diaba, Fexy Joseph, Glykeria Pakou, Yvonne Gleeson, Annalyn Nunag, James Berrill, Natalie Stroud, Carla Pothecary, Lisa Roche, Keri Turner, Lisa Deering, Lynda Israel, Evelyn Baker, Maxine Nash, Andrew Fagbemi, Felicia Jennings, Imelda Mayor, Jill Wilson, Alice Wheeler, Nicola Phillips, John Gordon, Emma Levell, Silvia Zagalo, Ina Hoad, Bindu Anil, Richard Russell, Paul Henderson, Margaret Millar, Christopher Alexakis, Natalia Michalak, Cheryl Marriott, Sarah Stone, Veronika Pristopan, John Saunders, Helen Burton, Vanessa Cambridge, Tonia Clark, Charlotte Ekblad, Sarah Hierons, Joyce Katebe, Emma Saunsbury, Rachel Perry, Matthew Brookes, Kathryn Davies, Marie Green, Ann Plumbe, Clare Ormerod, Helen Christensen, Hannah Howlett, Anne Keen, Jonathan Ogor, Marie Greenhaigh, Karen Knowles, Shanzi Yin, Maria Poulaka, Alpha Anthony, Emily Newitt, Fiona Trim, Ruth Casey, Katherine Seymour, Catherine Reed, Lijo Joy, Edward Fogden, Kalisha Russell, Samia Hussain, Anne Phillips, Muaad Abdulla, Jeff Butterworth, Colene Adams, Mandy Carnahan, Elizabeth Buckingham, Danielle Childs, Alison Magness, Jo Stickley, Nichola Motherwell, Louise Tonks, Hannah Gibson, Kate Wistance, Caradog Thomas, Elaine Brinkworth, Lynda Connor, Amanda Cook, Tabitha Rees, Rachel Harford, Sean Farley, Marie Jones, Emma Wesley, Alison Moss, Jacob Lucas, Claire Lorimer, Maria Oleary, Maxine Dixon, Fiona Goodchild, Rebecca Twenlow, Corinne Pawley, Arvind Ramadas, Julie Tregonning, Olaku Okeke, Wendy Jackson, Ioannis Koumoutsos, Viji George, Swapna Kunhunny, Sophie Laverick, Isla Anderson, Sophie Smith, Joan Joyce, Sarala Janarthan, Kamal Patel, Mariam Ali, Hilda Mhandu, Aleem Rana, Katherine Spears, Joana Teixeira, Mark Mencias, Abigail Seaward, Jessica Sousa, Nooria Said, Mark Soomaroo, Valentina Raspa, Asha Tacouri, Nicholas Reps, Rebecca Martin, Tinashe Samakomva, Christian Selinger, Jenelyn Carbonell, Felicia Onovira, Doris Quartey, Alice L'Anson, Andrew Ashworth, Jessica Bailey, Angie Dunn, Gjuzel Bespaloi, Harold Rasalan, Zahid Mahmood, Racheal Campbell, Liane Marsh, Tricia Coughlan, Wisam Jafar, Janet Marrs, Christopher McPheat, Monira Rahman, Sarah Davies, Ruth Habibi, Ellen Jessup-Dunton, Teishel Joefield, Reina Layug, Vinod Patel, Joanne Vere, Victoria Turner, Susan Kilroy, Martina Coulding, Martyn Clark, Jacqueline McCormick, Attiya Nisar, Gareth Walker, Stacey Atkins, Jasmine Growdon, Becky George, Charlotte McNeill, Bryony Reed, Angela Foulds, Catherine Marshall, Michele Allison, Briony Dillon, Rachel Cooney, Lillie Bennett, Louise Bowlas, Sharafaath Shariff, Aileen Fraser, Dwayne Punnette, Rebecca Lambert, Charlotte Bishop-Hurman, Elizabeth Undrell, Katherine Belfield, Said Din, Catherine Addleton, Marie Appleby, Johanna Brown, Kathleen Holding, Catherine Fraser, Janice Birkenshaw, Jodie Williams, Kamille Maulion, Meg Lane, Arita Kravale, Claud Smith, Patricia Hooper, John deCaestecker, Olivia Watchorn, Ellie Clarke, Chris Hayward, Susan Inniss, Lucy Pritchard, Karen Rudge, Amanda Carney, Sarah Griffee, Jervoise Andreyev, Sathish Babu, Caroline Hayhurst, Carol Lockwood, Lynn Osborne, Amanda Roper, Karen Warner, Julia Hindle, Tara Lawrence, Kimberley Netherton, Caroline Watt, Kinga Szymiczek, Shameer Mehta, James Bell, William Blad, Lisa Whitley, Roman Jastrub, Dhamaraj Durai, Mark Baker, Elizabeth John Sivamurugan, Mim Evans, Fraser Cummings, Clare Harris, Amy Jones, Liga Krauze, Sohail Rahmany, Michelle Earl, Jenny Vowles, Audrey Torokwa, Mirela Petrova, Andrew Procter, Jo Stanley, Claudia Silvamoniz, Marion Bettey, Amar Wahid, Zoe Morrison, Rhian Thomas-Turner, Louise Yendle, Jennifer Muller, Marcus Mitchell, John Kirkwood, Anna Barnes, Rakesh Chaudhary, Melanie Claridge, Chiara Ellis, Cheryl Kemp, Ogwa Tobi, Jentus Milton, Emma Johnston, Metod Oblak, Carmen Winpenny, Marie-Louise Svensson, Jo Godden, Marium Ashhar, Debbie Alexander, Kate Covil, Lauranne Derikx, Sryros Siakavellas, Helen Baxter, Scott Robertson, Linda Smith, Beena Poulose, Anne Colemam, Margareta Balint, Gareth Rhys-Jones, Helen Watters, Susan Begg, Beatriz Grosalcalde, Judy Coyle, Kerrie Johns, Rachel Hughes, Janet Phipps, Abigail Taylor, Catherine MacPhee, Suzanne Brooks, Jolene John, Michelle Edwards, Katie Smith, Linda Howard, Dianne Wood, Ajay Muddu, Laura Barman, Janine Mallinson, Tania Neale, Diana Ionita, Kerry Elliot, Alison Turnball, Iola Thomas, Alice Thomas, Kelly Andrews, Jonathon Sutton, Caroline Mulvaney Jones, Julia Roberts, and Jeannie Bishop
Supplementary Material
References
- 1.Torjesen I. Covid-19: Omicron may be more transmissible than other variants and partly resistant to existing vaccines, scientists fear. BMJ. 2021;375:n2943. doi: 10.1136/bmj.n2943. [DOI] [PubMed] [Google Scholar]
- 2.Mahase E. Covid-19: What we know about the BA.4 and BA.5 omicron variants. BMJ. 2022;378:o1969. doi: 10.1136/bmj.o1969. [DOI] [PubMed] [Google Scholar]
- 3.Andrews N, Stowe J, Kirsebom F, et al. COVID-19 vaccine effectiveness against the omicron (B.1.1.529) variant. N Engl J Med. 2022;386:1532–1546. doi: 10.1056/NEJMoa2119451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang Q, Guo Y, Iketani S, et al. Antibody evasion by SARS-CoV-2 omicron subvariants BA.2.12.1, BA.4 and BA.5. Nature. 2022;608:603–608. doi: 10.1038/s41586-022-05053-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kennedy NA, Janjua M, Chanchlani N, et al. Vaccine escape, increased breakthrough and reinfection in infliximab-treated patients with IBD during the omicron wave of the SARS-CoV-2 pandemic. Gut. 2022 doi: 10.1136/gutjnl-2022-327570. published online July 28. [DOI] [PubMed] [Google Scholar]
- 6.Sun J, Zheng Q, Madhira V, et al. Association between immune dysfunction and COVID-19 breakthrough infection after SARS-CoV-2 vaccination in the US. JAMA Intern Med. 2022;182:153–162. doi: 10.1001/jamainternmed.2021.7024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kennedy NA, Goodhand JR, Bewshea C, et al. Anti-SARS-CoV-2 antibody responses are attenuated in patients with IBD treated with infliximab. Gut. 2021;70:865–875. doi: 10.1136/gutjnl-2021-324388. [DOI] [PubMed] [Google Scholar]
- 8.Kennedy NA, Lin S, Goodhand JR, et al. Infliximab is associated with attenuated immunogenicity to BNT162b2 and ChAdOx1 nCoV-19 SARS-CoV-2 vaccines in patients with IBD. Gut. 2021;70:1884–1893. doi: 10.1136/gutjnl-2021-324789. [DOI] [PubMed] [Google Scholar]
- 9.Lin S, Kennedy NA, Saifuddin A, et al. Antibody decay, T cell immunity and breakthrough infections following two SARS-CoV-2 vaccine doses in inflammatory bowel disease patients treated with infliximab and vedolizumab. Nat Commun. 2022;13:1379. doi: 10.1038/s41467-022-28517-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mahil SK, Bechman K, Raharja A, et al. The effect of methotrexate and targeted immunosuppression on humoral and cellular immune responses to the COVID-19 vaccine BNT162b2: a cohort study. Lancet Rheumatol. 2021;3:e627–e637. doi: 10.1016/S2665-9913(21)00212-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prendecki M, Clarke C, Edwards H, et al. Humoral and T-cell responses to SARS-CoV-2 vaccination in patients receiving immunosuppression. Ann Rheum Dis. 2021;80:1322–1329. doi: 10.1136/annrheumdis-2021-220626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perkmann T, Perkmann-Nagele N, Koller T, et al. Anti-spike protein assays to determine SARS-CoV-2 antibody levels: a head-to-head comparison of five quantitative assays. Microbiol Spectr. 2021;9:e0024721. doi: 10.1128/spectrum.00247-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pollock KM, Cheeseman HM, Szubert AJ, et al. Safety and immunogenicity of a self-amplifying RNA vaccine against COVID-19: COVAC1, a phase I, dose-ranging trial. EClinicalMedicine. 2022;44:101262. doi: 10.1016/j.eclinm.2021.101262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tauzin A, Chatterjee D, Dionne K, et al. SARS-CoV-2 BA.4/5 Spike recognition and neutralization elicited after the third dose of mRNA vaccine. medRxiv. 2022 doi: 10.1101/2022.08.03.22278386. published online Aug 4. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Menni C, Valdes AM, Freidin MB, et al. Real-time tracking of self-reported symptoms to predict potential COVID-19. Nat Med. 2020;26:1037–1040. doi: 10.1038/s41591-020-0916-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khanna R, Zou G, D'Haens G, et al. A retrospective analysis: the development of patient reported outcome measures for the assessment of Crohn's disease activity. Aliment Pharmacol Ther. 2015;41:77–86. doi: 10.1111/apt.13001. [DOI] [PubMed] [Google Scholar]
- 17.Jairath V, Khanna R, Zou GY, et al. Development of interim patient-reported outcome measures for the assessment of ulcerative colitis disease activity in clinical trials. Aliment Pharmacol Ther. 2015;42:1200–1210. doi: 10.1111/apt.13408. [DOI] [PubMed] [Google Scholar]
- 18.Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95:103208. doi: 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hachmann NP, Miller J, Collier AY, et al. Neutralization escape by SARS-CoV-2 omicron subvariants BA.2.12.1, BA.4, and BA.5. N Engl J Med. 2022;387:86–88. doi: 10.1056/NEJMc2206576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alexander JL, Kennedy NA, Ibraheim H, et al. COVID-19 vaccine-induced antibody responses in immunosuppressed patients with inflammatory bowel disease (VIP): a multicentre, prospective, case-control study. Lancet Gastroenterol Hepatol. 2022;7:342–352. doi: 10.1016/S2468-1253(22)00005-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alexander JL, Liu Z, Muñoz Sandoval D, et al. COVID-19 vaccine-induced antibody and T-cell responses in immunosuppressed patients with inflammatory bowel disease after the third vaccine dose (VIP): a multicentre, prospective, case-control study. Lancet Gastroenterol Hepatol. 2022;7:1005–1015. doi: 10.1016/S2468-1253(22)00274-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu Z, Alexander JL, Lin KW, VIP study investigators Infliximab and tofacitinib attenuate neutralizing antibody responses against SARS-CoV-2 ancestral and omicron variants in IBD patients following 3 doses of COVID-19 vaccine. Gastroenterology. 2022 doi: 10.1053/j.gastro.2022.10.010. published online Oct 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Caldera F, Knutson KL, Saha S, et al. Humoral immunogenicity of mRNA COVID-19 vaccines among patients with inflammatory bowel disease and healthy controls. Am J Gastroenterol. 2022;117:176–179. doi: 10.14309/ajg.0000000000001570. [DOI] [PubMed] [Google Scholar]
- 24.Long MD, Weaver KN, Zhang X, et al. Strong response to SARS-CoV-2 vaccine additional doses among patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol. 2022;20:1881–1883. doi: 10.1016/j.cgh.2022.01.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weaver KN, Zhang X, Dai XF, et al. Impact of SARS-CoV-2 vaccination on inflammatory bowel disease activity and development of vaccine-related adverse events: results from PREVENT-COVID. Inflamm Bowel Dis. 2022;28:1497–1505. doi: 10.1093/ibd/izab302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dayam RM, Law JC, Goetgebuer RL, et al. Accelerated waning of immunity to SARS-CoV-2 mRNA vaccines in patients with immune-mediated inflammatory diseases. JCI Insight. 2022;7:e159721. doi: 10.1172/jci.insight.159721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qui M, Le Bert N, Chan WPW, et al. Favorable vaccine-induced SARS-CoV-2-specific T cell response profile in patients undergoing immune-modifying therapies. J Clin Invest. 2022;132:e159500. doi: 10.1172/JCI159500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Akiyama S, Hamdeh S, Micic D, Sakuraba A. Prevalence and clinical outcomes of COVID-19 in patients with autoimmune diseases: a systematic review and meta-analysis. Ann Rheum Dis. 2021;80:384–391. doi: 10.1136/annrheumdis-2020-218946. [DOI] [PubMed] [Google Scholar]
- 29.Gianfrancesco M, Hyrich KL, Al-Adely S, et al. Characteristics associated with hospitalisation for COVID-19 in people with rheumatic disease: data from the COVID-19 Global Rheumatology Alliance physician-reported registry. Ann Rheum Dis. 2020;79:859–866. doi: 10.1136/annrheumdis-2020-217871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Izadi Z, Brenner EJ, Mahil SK, et al. Association between tumor necrosis factor inhibitors and the risk of hospitalization or death among patients with immune-mediated inflammatory disease and COVID-19. JAMA Netw Open. 2021;4:e2129639. doi: 10.1001/jamanetworkopen.2021.29639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chanchlani N, Lin S, Chee D, et al. Adalimumab and infliximab impair SARS-CoV-2 antibody responses: results from a therapeutic drug monitoring study in 11 422 biologic-treated patients. J Crohn's Colitis. 2022;16:389–397. doi: 10.1093/ecco-jcc/jjab153. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data of this study are available under a transfer agreement from the corresponding author based on a reasonable request. There will be limitations on how data can be used or how long data will be available for.