Abstract
Background
The opioid-related overdose epidemic remains a persistent public health problem in the United States and has been accelerated by the 2019 coronavirus disease pandemic. Existing, evidence-based treatment options for opioid use disorder (OUD) are broadly underutilized, particularly by people experiencing homelessness (PEH). PEH are also more likely to misuse and overdose on opioids. To better understand current gaps and disparities in OUD treatment experienced by PEH and efforts to address them, we synthesized the literature reporting on the intersection of housing status and OUD treatment.
Methods
We conducted a scoping review of the literature from the electronic databases MEDLINE, Embase, PsycINFO, and Web of Science Core Collection. We included studies describing treatment-related outcomes specific to PEH and articles assessing OUD treatment interventions tailored to this population. Relevant findings were compiled via thematic analysis and narratively synthesized.
Results
60 articles met our inclusion criteria, including 43 descriptive and 17 intervention-focused studies. These studies demonstrated that PEH experience more barriers to OUD treatment than their housed counterparts and access inpatient and detoxification treatment more commonly than pharmacotherapy. However, the reviewed literature indicated that PEH have similar outcomes once engaged in pharmacotherapy. Efficacious interventions for PEH were low-barrier and targeted, with housing interventions also demonstrating benefit.
Conclusions
PEH have diminished access to evidence-based OUD treatment, particularly medications, and require targeted approaches to improve engagement and retention. To mitigate the disproportionate opioid-related morbidity and mortality PEH experience, innovative, flexible, and interdisciplinary OUD treatment models are necessary, with housing support playing an important role.
Keywords: Opioid use disorder, Homelessness, Substance use treatment, MOUD, Healthcare for the homeless
1. Introduction
Drug overdose mortality in the United States has increased over the past decades, with recent surges driven primarily by overdose deaths involving synthetic opioids (Wilson et al., 2020). People experiencing homelessness (PEH) are disproportionately impacted by this epidemic: PEH are more likely to misuse opioids (Doran et al., 2018; Marshall et al., 2019) and overdose compared to their housed counterparts (Yamamoto et al., 2019). Moreover, the emergence of severe acute respiratory syndrome coronavirus 2 (SARS-Cov-2) and the associated restrictions imposed to mitigate the 2019 novel coronavirus disease (COVID-19) pandemic has accelerated this overdose crisis (Centers for Disease Control and Prevention, 2020), especially among PEH. An alarming two-fold increase in percentages of overdose deaths involving fentanyl was observed among PEH in Los Angeles (LA) County from 2019 to mid-2020 (Los Angeles County Department of Public Health and Center for Health Impact Evaluation, 2021). Relatedly, even with the excess mortality fueled by the COVID-19 pandemic and several documented SARS-Cov-2 outbreaks that occurred early on in homeless shelters throughout the United States (Baggett et al., 2020; Tobolowsky et al., 2020), drug-related overdose remained the leading cause of death among PEH in New York City (New York Department of Health and Mental Hygiene, 2021) and LA (Los Angeles County Department of Public Health and Center for Health Impact Evaluation, 2021) in fiscal year 2019 and in the first six months of 2020, respectively.
Beyond overdose, substance use disorders (SUDs) like opioid use disorder (OUD) increase risk for chronic health conditions, including HIV and Hepatitis C infection, and other psychosocial concerns, such as depression, unemployment, and incarceration (Haider et al., 2020; Hser et al., 2015). Homelessness similarly conveys increased risk for physical and mental health morbidities (Fazel et al., 2014). However, PEH encounter multi-level barriers that commonly impede their obtaining of effective care. Personal barriers such as competing priorities, trauma, and medical comorbidities, practical barriers such as transportation and medication security, and structural barriers such as stigma, mistrust of medical institutions, and lack of health insurance coverage (Davies and Wood, 2018) collectively contribute to poor access to ambulatory care by PEH and the persistence of unmet health needs (Baggett et al., 2010; Kushel et al., 2006). Consequently, PEH may tend to delay seeking care and overutilize acute health care systems (Davies and Wood, 2018; Kushel et al., 2006; Lin et al., 2015).
Due to these biopsychosocial issues and patterns of healthcare utilization, effectively engaging and retaining PEH with OUD in evidence-based treatment is conceivably challenging. Medications for opioid use disorder (MOUD) improve outcomes for individuals with OUD; (Hser et al., 2016; Sordo et al., 2017) specifically, opioid agonist therapy (OAT, i.e. buprenorphine or methadone) is associated with a lower risk of overdose and opioid-related acute care compared to most other types of treatment (Wakeman et al., 2020). However, these medications are commonly prescribed in outpatient settings which often have strict induction and program requirements, can be stigmatized and difficult to locate, and suffer from deficient clinical support (Kissin et al., 2006; Tofighi et al., 2019; Walley et al., 2008). Naltrexone is another approved MOUD option but studies regarding its efficacy are mixed (Jarvis et al., 2018; Wakeman et al., 2020), in part because it is a non-reinforcing receptor antagonist that requires abstinence before initiation. Though available as an extended-release injectable (XR-NTX) that is theoretically easier to adhere to than its oral formulation and daily OAT dosing, a systematic review found that many patients who intend to start on the medication never do and that most discontinue prematurely (Jarvis et al., 2018).
Considering the intersecting barriers that steer PEH away from outpatient medical services in conjunction with the difficulties and limitations inherent to these evidence-based options, there is a demonstrable need to improve acceptability and availability of MOUD among PEH. Several innovative treatment models in the United States, including the recent relaxation of methadone rules with take-home dosing during the COVID-19 pandemic (Figgatt et al., 2021), respond to barriers contributing to insufficient uptake and diminished efficacy of OUD treatment experienced by marginalized populations (Bachhuber et al., 2018; Labelle et al., 2016; Stancliff et al., 2012). Existing literature suggests some of these initiatives have been specifically tailored for PEH. We conducted a scoping review of the literature reporting on OUD treatment-associated findings unique to PEH and literature assessing treatment programs designed for PEH. Our objective was to elucidate treatment models and settings within which PEH with OUD may reap the most benefit.
2. Methods
This review is reported in accordance with the Preferred Reporting Items of Systematic Reviews and Meta-Analyses for Scoping Review (PRISMA-ScR) guidelines (Tricco et al., 2018).
2.1. Search strategy and eligibility criteria
We identified studies examining OUD treatment in the context of homelessness by searching electronic databases MEDLINE (Ovid), Embase (Elsevier), PsycINFO (EBSCO), and Web of Science Core Collection (Clarivate). The search was designed to retrieve studies including any treatment modality by combining terms for OUD with terms for housing status (Supplementary Material Appendix A). We searched on April 17, 2020 without date or language restrictions. Controlled vocabulary terms were included when available.
2.1.1. Inclusion criteria
We used Covidence to manage and organize citations (Kellermeyer et al., 2018). Two reviewers independently screened titles and abstracts of the records retrieved in the search. Researchers selected records for full-text review if their abstract referenced drug use, unless specifically use of drugs other than opioids, and homelessness or vulnerable housing. Of those selected for full-text analysis, we initially included (1) quantitative and qualitative articles and brief reports that contained findings related to the intersection of opioid use and housing status. However, due to the large number of resulting articles, we narrowed our review to include only the subset of those that reported on (2) the intersection of OUD treatment and housing status.
2.1.2. Exclusion criteria
Throughout the record screening process, we excluded all (1) review types, (2) opinion articles lacking primary data, (3) theoretical articles, (4) case reports, (5) abstracts and dissertations, and (6) studies conducted outside of the United States. We also excluded (7) studies published before 2000, when the Drug Abuse Treatment Act of 2000 allowed outpatient prescription of buprenorphine, substantially changing the landscape of OUD treatment (Wesson and Smith, 2010).
2.2. Data extraction and synthesis
We developed a standardized template for data extraction. Two reviewers independently extracted data from selected articles, including study details (e.g. author, year of publication, and setting), methods, inclusion/exclusion criteria, and findings and outcomes pertaining to the intersection of housing status and OUD treatment. We also recorded related details such as varying operationalizations of housing status. Discrepancies in extraction were resolved by consensus after discussion with a third author.
We utilized a narrative synthesis approach to organize and present relevant findings. Narrative synthesis is indicated for systematic reviews when a specialized form of synthesis (e.g. meta-analysis) is not feasible due to the necessary inclusion of heterogenous studies in answering the research question (Petticrew et al., 2015; Popay et al., 2006). This approach is characterized by textual summaries and explanations of findings, which are first synthesized by thematic analysis to allow for the exploration of relationships among studies. Thematic analysis involves iteratively identifying, classifying, and sorting the most important themes and concepts across studies (Popay et al., 2006). We employed a combined inductive and deductive analytical approach (Roberts et al., 2019) due to an awareness of the distinction between studies assessing housing status in relation to conventional OUD treatment (i.e. descriptive studies) and studies reporting on tailored treatment models for PEH (i.e. intervention-focused studies) following our systematic review of the literature.
The preliminary thematic analysis was conducted by MM, which involved open coding of relevant findings from individual studies with short, descriptive phrases (e.g. “OAT and overdose risk”). After coding, 13 unique descriptive themes, stratified by our two deduced categories, were initially generated to classify and capture all relevant findings. With analytical input from RL, NDC, and AC, these themes were iteratively refined and consolidated prior to and during manuscript production. Emergent patterns were synthesized and narratively reported.
3. Results
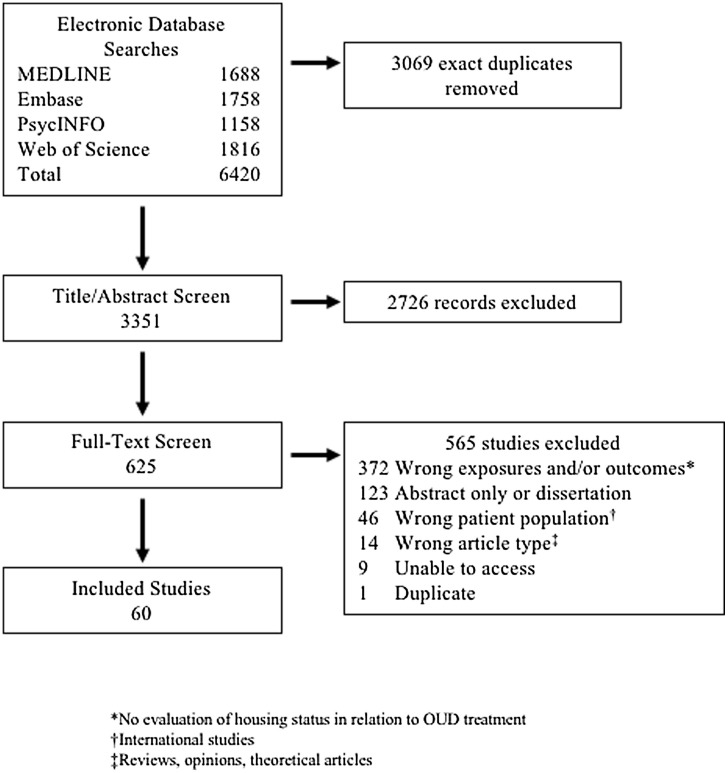
Our search strategy returned 6420 references. After removing 3069 exact duplicates, we screened 3351 titles and abstracts and excluded 2726 citations. We screened 625 full-text citations and included 60 based on our revised inclusion criteria (Fig. 1 ). Forty-three included studies were descriptive and reported on specific ways PEH accessed or engaged with OUD treatment as well unique outcomes they faced compared to populations not experiencing homelessness (Table 1 ). These studies were heterogeneous with respect to setting and reported outcomes. Seventeen described or assessed novel opioid treatment models or interventions tailored to PEH (Table 2 ).
Fig. 1.
Record Screening Flowchart.
Table 1.
Studies on access, engagement, and outcomes related to traditional OUD treatment for PEH.
| Author(s) (Year) Location |
Study Design | Population | Relevant Outcomes |
|---|---|---|---|
|
Alford et al. (2007) Boston, MA |
Cohort study | Homeless and housed individuals dependent on opioids | The number of patients who left treatment and the reasons for leaving treatment before 12 months, including the treatment failure and successful program departure groups, appeared similar for both homeless and housed patients. Over the 12-month period, no significant associations were found between housing status and use of opioids or other drugs. |
|
Amodeo et al. (2004) Massachusetts, USA |
Cohort study | Female individuals who inject drugs and reported heroin as primary drug | Homeless females who injected drugs were about 80 % more likely than non-homeless females who injected drugs to use detoxification only (Odds Ratio [OR] = 1.804, 95 % CI 1.538, 2.116). |
|
Bachhuber et al. (2015) USA |
Cohort study | Veterans initiating methadone or buprenorphine treatment | The prevalence of homelessness was 10.2 %, and 5.3 % were at risk for homelessness. Male (vs. female) veterans (10.3 % vs. 8.9 %) and veterans ages 18−34 and 45−54 (12.0 % and 11.7 %, respectively) more frequently screened positive for homelessness. |
|
Bauer et al. (2016) Boston, MA |
Cohort study | Homeless adults who died of drug overdose | Fewer than half of decedents with documented opioid use had received pharmacological treatment for opioid dependence. In particular, treatment with buprenorphine (4.3 %) and methadone (19.6 %) rarely occurred. |
|
Chang (2017) San Francisco, CA |
Qualitative study | Formerly homeless women living in supportive housing | Participants described being tethered to the Tenderloin because they needed to access substances or treatment, including substitution therapy. One participant was on methadone maintenance treatment for OUD and felt geographically tied to the neighborhood due to requirements that she obtain methadone treatment on-site at the clinic daily. |
|
Corsi et al. (2007) Denver, CO |
Cohort study | People who inject opiates | Not considering oneself to be homeless was significantly associated with treatment entry (i.e. methadone maintenance, outpatient drug-free, or residential treatment). Not being homeless independently predicted treatment entry (OR = 1.67, P < 0.05). |
|
Cousins et al. (2016) Los Angeles, CA |
Cohort study | Patients who obtained XR-NTX for opioid dependence | Significantly more patients in XR-NTX treatment who used heroin identified as homeless compared to those in XR-NTX treatment who used non-heroin opioids (P < 0.05). Identifying as homeless was associated with a lower odds of returning for 2 or more doses of XR-NTX; however, this association was not significant (OR = 0.74, 95 % CI 0.34, 1.61). |
|
Damian et al. (2017) Baltimore, MD |
Cohort study | Individuals dependent on opioids | Clients who reported unstable housing, including being homeless, residing in a treatment house, or in a transitional house, had a 41% decreased odds of remaining at least 90 days (i.e. higher odds of treatment failure) compared to clients who lived independently at intake (OR = 0.59, 95 % CI 0.37, 0.96). |
|
Daniulaityte et al. (2019) Dayton, OH |
Cross-sectional study | Adults with OUD who used non-prescribed buprenorphine | Past 6-month homelessness significantly differed between classes. The "Intense Non-Prescribed Buprenorphine Use" class had the lowest prevalence of homelessness (27.3 %), while the "Heavy Heroin/Fentanyl Use" class had the highest prevalence of homelessness (58.9 %). The "More Formal Treatment Use" class had a 55.4 % prevalence of homelessness. |
|
Deck and Carlson (2004) Oregon and Washington, USA |
Cohort study | Medicaid-eligible adults entering treatment for opiate use | Homeless individuals (vs. other) were significantly less likely to obtain access to MMT in both Oregon (OR = 0.29, P < 0.01) and Washington (OR = 0.55, P < 0.01). |
|
Dunn et al. (2019) USA |
Cohort study | Patients initiating detoxification and outpatient OUD treatment | Patients in detoxification had lower odds of receiving planned OAT if they were homeless, as compared to having a dependent/independent housing status (Adjusted Odds Ratio [AOR] = 0.53, P < 0.001). |
|
Englander et al. (2020) Portland, OR |
Cohort study | Inpatients receiving addiction consultation services | Current homelessness was significantly associated with initiation of MOUD or medications for alcohol use disorder (AOR = 2.63, 95 % CI 1.52, 4.53). |
|
Eyrich-Garg et al. (2008) Urban Areas in USA |
Cohort study | Individuals seeking treatment for substance misuse | Inpatient/residential treatment clients were more likely to be literally homeless and marginally housed while outpatient and methadone maintenance clients were more likely to be housed (P < 0.0001). |
|
Fine et al. (2020) Boston, MA |
Cohort study | Adults who engaged in buprenorphine treatment | Homelessness was independently associated with an increased hazard of all-cause mortality (Adjusted Hazard Ratio [AHR] = 1.39, 95 % CI 1.09, 1.78) and an increased hazard of opioid overdose-related mortality (AHR = 1.77, 95 % CI 1.25, 2.50). |
|
Havens et al. (2009) Baltimore, MD |
Randomized controlled trial | People who inject drugs requesting OAT referrals | Being homeless and not living in one's own apartment/home were not significantly associated with number of days in OAT. |
|
Hoffman et al. (2019) Six Sites in USA |
Qualitative study | Research staff and providers for an XR-NTX clinical trial | Homelessness impeded prospective participants' ability to engage in the study. Turbulent living conditions were considered to be a universal barrier to recruitment. One clinician explained that in her clinic, recruiters "might have hooked in with somebody but then the housing falls through and then we lose them." |
|
Jones et al. (2017) USA |
Cohort study | Patients admitted for prescription opioid misuse | Compared to living independently, being homeless was associated with increased relative risk of injection (Relative Risk Ratio [RRR] = 1.73, 95 % CI 1.69, 1.77) and smoking or inhalation (RRR = 1.14, 95 % CI 1.12, 1.17) as the usual route of opioid misuse compared to oral misuse. |
|
Kelly et al. (2018) USA |
Cohort study | Veterans with OUD at Veterans Health Administration facilities that prescribed XR-NTX | Veterans who received XR-NTX were more likely to have been homeless in the past year compared to those who received OAT (AOR = 1.52, 95 % CI 1.07, 2.16). Veterans with a prescription for XR-NTX were more likely to have been homeless in the past year compared to those who received no OUD medication (AOR = 1.58, 95 % CI 1.24, 2.19). |
|
Kertesz et al. (2003) Boston, MA |
Cohort study | Patients admitted to inpatient detoxification | Compared to homeless persons not using stabilization programs, homeless stabilization program users were less likely to report heroin as their substance of choice (11 % vs. 29 %, P = 0.05). Compared to those without a recent experience of literal homelessness, homelessness was associated with later first use post-detoxification among subjects with heroin as their substance of choice (HR = 0.47, 95 % CI 0.23, 0.95). |
|
Krawczyk et al. (2020) Maryland, USA |
Cohort study | Adults admitted to outpatient treatment programs for OUD | Homeless individuals were significantly less likely to ever have received methadone or buprenorphine treatment (AOR = 0.46, 95 % CI 0.43, 0.50). |
|
Krull et al. (2011) USA |
Cross-sectional study | Addiction treatment program directors | Program directors that had less positive attitudes toward buprenorphine as an effective treatment for opiate dependence when they worked in organizations that served a higher percentage of people who were homeless (P < 0.05). |
|
Li et al. (2019) Boston, MA |
Case-control study | Veterans admitted for inpatient opioid detoxification | 36.8 % were homeless at the time of index admission. 26.5 % of the patients alive at time of follow-up were homeless at the time of index admission compared with 47.1% of the deceased patients. Being homeless at index was trending and associated with a higher all-cause mortality but not significantly (P = 0.09). |
|
Lundgren et al. (2003) Massachusetts, USA |
Cohort study | People who inject drugs | Homelessness was positively associated with solely using detoxification (OR = 1.36, 95 % CI 1.27, 1.46) and with using residential treatment (OR = 2.75, 95 % CI 2.55, 2.97) but negatively associated with enrolling in MMT (OR = 0.49, 95 % CI 0.46, 0.53). |
|
Marienfeld and Rosenheck (2015) USA |
Cohort study | Veterans using MMT and/or living with serious mental illness | Patients enrolled in MMT who had at least 1 serious mental illness were more likely to have experienced homelessness than just those in MMT (RR = 1.52, P < 0.001) and much more likely to have experienced homelessness than just those with at least 1 serious mental illness (RR = 3.95, P < 0.001). |
|
Masson et al. (2002) San Francisco, CA |
Cohort study | Patients who used medical services and had a diagnosis associated with complications of opioid use | Compared to those who were not homeless, patients experiencing homelessness had greater mean emergency department visits (P < 0.001) and inpatient service (P < 0.0001) use. Patients experiencing homelessness also had higher mean service charges associated with their emergency department (P < 0.02) and inpatient service (P < 0.01) use. |
|
Midboe et al. (2019) USA |
Cohort study | Veterans who accessed homeless programs in the Veterans Health Administration | 38 % of veterans with OUD received MOUD from the Veterans Health Administration within a year following program entry. Receiving MOUD after program entry was significantly more likely for veterans ages 35 and younger (P < 0.001) and for those without high-dose opioid (P < 0.001) or concomitant opioid-benzodiazepine prescriptions (P = 0.016). |
|
Nyamathi et al. (2004) Los Angeles, CA |
Cohort study | Women experiencing homelessness | Recent substance use treatment was not significantly associated with a motivation to quit for people who used heroin. |
|
Patel et al. (2020) Nashville, TN |
Descriptive report | Discharged patients with SUDs | Of the recovery houses that accepted discharged patients from Vanderbilt Medical Center in 2018, 12.5 % allowed patients to remain on buprenorphine/naloxone, 0 % allowed patients to remain on MMT, and 19 % completely allowed naltrexone maintenance therapy. |
|
Reynoso-Vallejo et al. (2008) Massachusetts, USA |
Cohort study | Latino men who inject drugs | Homelessness was associated with a decreased likelihood of entering methadone maintenance (OR = 0.41, 95 % CI 0.36, 0.47). |
|
Rivers et al. (2006) USA |
Cohort study | Individuals admitted for substance use treatment | Among both users of heroin and non-users respectively, those who reported dependent living (AOR = 1.90, 95 % CI 1.70, 2.13; OR = 2.23, 95 % CI 1.50, 3.31) and independent living (AOR = 2.11, 95 % CI 1.90, 2.34; OR = 2.73, 95 % CI 1.88, 3.98) were more likely to be planned for MMT than those who were homeless. |
|
Riggins et al. (2017) Ten Sites in USA |
Cohort study | HIV-infected patients receiving buprenorphine treatment | Homelessness was significantly associated with a decreased odds of self-reported opioid use at any follow-up visit (OR = 0.57, 95 % CI 0.34,0.96). |
|
Robbins et al. (2010) San Francisco, CA |
Cohort study | People experiencing homelessness who inject drugs | Needing health care 6+ times (OR = 2.12, 95 % CI 1.23, 3.68) and seeking health care (OR = 3.39, 95 % CI 1.77, 6.52) were associated with higher odds of methadone treatment. Methadone treatment was independently associated with increased odds of seeking care (AOR = 2.29; 95 % CI 1.24, 4.24). |
|
Rose-Jacobs et al. (2019) Boston, MA |
Cohort study | Pregnant women being treated for OUD | 61 % reported housing instability. Compared to those with neither food nor housing instability, those reporting both food and housing instability had greater depressive scores (P = 0.02) and clinically but not statistically significant higher intimate partner vulnerability scores. |
|
Royse et al. (2000) Five Sites in USA |
Cohort study | Out-of-treatment people who use substances | Non-homeless individuals were more likely to have participated in methadone detoxification (OR = 1.55, P = 0.006) and methadone maintenance (OR = 1.86, P = 0.000) than those who reported homelessness. |
|
Shah et al. (2000) Baltimore, MD |
Cohort study | People who inject drugs | Regardless of HIV status, not being homeless in the past 6 months was associated with enrollment in MMT (P < 0.05). Homelessness in the past six months was only negatively independently correlated with decreased odds of MMT among HIV-negative subjects (OR = 0.72, 95 % CI 0.55, 0.95). Among those who seroconverted during follow-up, those who reported prior 6-month homelessness were less likely to subsequently enroll in MMT (AOR = 0.05, 95 % CI 0.01, 0.28). |
|
Simon et al. (2017) Seattle, WA |
Cohort study | Patients seeking treatment at an office-based opioid treatment program | Recent homelessness was associated with a decreased odds of reaching induction (AOR = 0.32, 95 % CI 0.10, 1.02). |
|
Stein et al. (2017) Fall River, MA |
Cohort study | Patients seeking inpatient opioid detoxification | Patients who preferred residential treatment after detoxification were more likely to be homeless (AOR = 5.71, 95 % CI 1.13, 28.95) than those who preferred no treatment. |
|
Stein et al. (2015) Fall River, MA |
Cohort study | Patients seeking inpatient opioid detoxification | Patients who preferred residential treatment after detoxification were more likely to be homeless than those who preferred OAT or those who preferred outpatient treatment. There was no evidence that the relationship between homelessness and treatment preference varied by season. |
|
Stein et al. (2014) Fall River, MA |
Cohort study | Patients seeking inpatient opioid detoxification | Homelessness was not significantly correlated with being uninsured. |
|
Timko et al. (2016) USA |
Cohort study | Veterans Health Administration patients with alcohol or opiate dependence | Homelessness was associated with receiving detoxification services (AOR = 3.09, 95 % CI 3.00, 3.19), a detoxification follow-up appointment (AOR = 1.62, 95 % CI 1.51, 1.73), and addiction treatment within 30 days (AOR = 1.69, 95 % CI 1.57, 1.81) and within 60 days (AOR = 1.93, 95 % CI 1.80, 2.07) of detoxification. |
|
Upshur et al. (2018) Eleven Sites in USA |
Cohort study | Homeless women with SUDs | Using heroin was associated with more reported use of drug-related services (OR = 1.89, 95 % CI 1.04, 3.47) and with attending Narcotics Anonymous/Cannabis Anonymous (OR = 1.91, 95 % CI 2.45, 67.59). |
|
Van Ness et al. (2004) Central Harlem, NYC |
Cohort study | People using crack or powder cocaine and/or heroin | Homelessness was not significantly associated with odds of using a methadone clinic. |
|
Velasquez et al. (2019) New York City, NY |
Qualitative study | Adults with OUD recently released from jail | Recent or chronic homelessness was described as a primary barrier to adhering to prescribed treatment (i.e. XR-NTX, methadone, and/or buprenorphine). |
Table 2.
Studies on novel OUD treatment models or interventions tailored to PEH.
| Author(s) (Year) Location |
Study Design | Population | Relevant Outcomes |
|---|---|---|---|
|
Appel et al. (2012) New York, USA |
Cohort study | Methadone patients with serious mental illness | Methadone treatment retention for 31 patients placed in supportive housing vs. 30 comparison participants was 51.6 % vs. 20 % (P < 0.02). Apartment/independent housing retention for patients placed in supportive housing vs. comparison patients was 67.7 % vs. 3 % or 13 % (both P's < 0.01). |
|
Buzza et al. (2019) Oakland, CA |
Descriptive report | Homeless individuals with OUD | 21 homeless patients with OUD had been prescribed buprenorphine through this mobile program. Clinicians regularly visited homeless encampments and maintained availability during follow up. The authors are in the process of formally evaluating the program's preliminary reach and efficacy. |
|
Carter et al. (2019) San Francisco, CA |
Cohort study | Homeless individuals with OUD | The percentages of patients retained in care at 1, 3, 6, 9, and 12 months were 63 %, 53 %, 44 %, 38 %, and 26 %, respectively. The percentages of patients retained on buprenorphine at 1, 3, 6, 9, and 12 months were 37 %, 27 %, 27 %, 26 %, and 18 %, respectively. 23 % of patients had at least one opioid-negative, buprenorphine-positive toxicology test. |
|
Chatterjee et al. (2018) Boston, MA |
Qualitative study | Adults with OUD in a family shelter | Many patients changed OUD treatment plans and locations for various reasons including medication side effects and lack of compassion/support. Barriers to care included logistical ones such as transportation, child care needs, and discharge from clinics as well as stigma and triggers. Ideal treatment was described as helping pain management and comorbidities and involving overdose prevention and the convenience of shelter-based treatment. |
|
Chatterjee et al. (2017) Boston, MA |
Cohort study | Adults with OUD in a family shelter | The mean treatment duration was 7.4 months. No overdoses were documented during the study period. Initial urine drug tests indicated that two had used opioids, and one patient had an opioid-positive urine drug test by the third month. Unprescribed controlled substances were detected in 77 % of 44 tests in the first month and 51 % of 34 tests in the third month (P < 0.01). In the final month of treatment, 3 patients were employed as compared to 1 at treatment initiation. Of the 4 who moved from the shelter system to an office-based program, all relapsed and lost custody of their children. |
|
Davidson et al. (2014) New York City, NY |
Cohort study | Individuals experiencing homelessness who misused substances | Clients of programs with high consumer participation fidelity were less likely to report using stimulants or opiates at follow-up (OR = 0.17, 95 % CI 0.07, 0.57). There were no significant differences in stimulant or opiate use between baseline and follow up among clients of programs with high supportive housing fidelity. |
|
Doorley et al. (2017) San Jose, CA |
Cohort study | Patients referred for a buprenorphine shared medical appointment in a homeless shelter clinic | Of the 77 patients referred to buprenorphine shared medical appointments, 95 % attended at least 1. The median and mean attendance were 10 and 18 shared appointments, respectively. The majority (61 %) were currently homeless. At 12 and 24 weeks, 86 % and 70 % of patients were respectively retained in treatment. |
|
Godersky et al. (2019) Seattle, WA |
Qualitative study | Office-based opioid treatment patients and providers | Homelessness was identified as a major barrier to buprenorphine adherence, both in the context of keeping appointments in order to have prescriptions filled and also taking medications regularly as instructed. Video directly observed therapy was described as a potential solution by mitigating logistical barriers and enhancing access to providers. |
|
Hall et al. (2020) New York, USA |
Cohort study | Applicants to New York III supportive housing programs | OAT was significantly associated with a lower likelihood of housing discharge (AHR = 0.57, 95 % CI 0.46, 0.70). |
|
Hall et al. (2014) New Jersey, USA |
Cohort study | Patients enrolled in the New Jersey Medication Assisted Treatment Initiative (NJ-MATI) | NJ-MATI (i.e. mobile medication unit) clients had a greater likelihood of being homeless compared to traditional methadone clients (OR = 2.8, 95 % CI 2.3, 3.6). However, NJ-MATI clients were less likely than non-MAT clients to be homeless (OR = 0.4, 95 % CI 0.4, 0.5). |
|
Hersh et al. (2011) San Francisco, CA |
Cohort study | Vulnerable patients with OUD | No significant differences in one-year retention were found based on housing status: 54 % of the patients who were homeless at admission were retained in treatment for at least one year, compared to the 67 % housed at admission (P = 0.3). However, only 15 % of housed patients left treatment prior to three months vs. 37 % of homeless patients (P = 0.05), resulting in a mean length of stay of 7 months for homeless patients vs. 10.2 months for those with housing. |
|
Hood et al. (2020) Seattle, WA |
Cohort study | Patients enrolled in low-barrier buprenorphine treatment (i.e. "Bupe Pathways") | The majority (82 %) of Bupe Pathways patients were experiencing homelessness or unstable housing in the 12 months preceding enrollment. Neither retention in Bupe Pathways nor transfer to another clinic for ongoing buprenorphine maintenance varied significantly by housing status. |
|
Krawczyk et al. (2019) Baltimore, MD |
Cohort study | Patients with OUD who were recently incarcerated or exiting jail | The majority (70.8 %) of those who initiated buprenorphine through a mobile van outside the Baltimore City Jail were unstably housed. Housing status was not significantly associated with remaining in care after 30 days. |
|
Lashley (2019) Baltimore, MD |
Descriptive report | Homeless men in addiction recovery | 70 % of clients successfully completed the OAT program (i.e. were successfully titrated from the medication) and remained in the recovery program. Of these, 79 % completed the OAT program in 90 days or less. While treatment extended beyond 90 days for some clients, the majority did not require extended treatment. |
|
Nyamathi et al. (2017) Los Angeles, CA |
Randomized controlled trial | Homeless gay and bisexual men who use stimulants | As determined by urinalysis, there was a significant reduction in opiate use only in the SE + CM group from 4- to 8-month follow-up visits. However, baseline opiate use in both groups was low. |
|
Nyamathi et al. (2012) Los Angeles, CA |
Randomized pilot study | Homeless youth who use drugs | Reductions in heroin use at 6-month follow up in both the health promotion and art messaging groups were not significant. |
|
Tringale et al. (2015) Los Angeles, CA |
Descriptive report | People enrolled at a needle exchange who use heroin | 9 needle exchange patients who refused standard OUD treatment modalities were enrolled in the Stepped Treatment Engagement Program (STEP), which emphasized a nonjudgmental philosophy, acceptance of relapse, goal setting and problem solving. 78 % completed the program, and 33 % transitioned to long-term opioid maintenance therapy. |
3.1. Descriptive studies
3.1.1. Treatment access/engagement
Of the sixty included studies, twenty-seven reported on opioid use treatment access and engagement among PEH (Amodeo et al., 2004; Bachhuber et al., 2015; Bauer et al., 2016; Corsi et al., 2007; Daniulaityte et al., 2019; Deck and Carlson, 2004; Dunn et al., 2019; Englander et al., 2020; Eyrich-Garg et al., 2008; Hoffman et al., 2019; Kelly et al., 2018; Krawczyk et al., 2020; Krull et al., 2011; Lundgren et al., 2003; Masson et al., 2002; Midboe et al., 2019; Nyamathi et al., 2004; Patel et al., 2020; Reynoso-Vallejo et al., 2008; Rivers et al., 2006; Robbins et al., 2010; Royse et al., 2000; Shah et al., 2000; Simon et al., 2017; Timko et al., 2016; Upshur et al., 2018; Van Ness et al., 2004). While most were conducted among general populations of people who use opioids, five evaluated characteristics of treatment engagement exclusively among homeless-experienced populations (Bauer et al., 2016; Midboe et al., 2019; Nyamathi et al., 2004; Robbins et al., 2010; Upshur et al., 2018).
3.1.1.1. Studies with PEH only
Among homeless-experienced women in Los Angeles, those who preferred heroin, as compared to other substances, accessed substance use treatment programs more frequently (Upshur et al., 2018). However, in a comparable population, recent engagement with treatment was not associated with motivation to quit heroin (Nyamathi et al., 2004).
Being specifically in MOUD treatment was more likely for veterans experiencing homelessness who were under 35 years old, veterans with fewer opioid and benzodiazepine prescriptions (Midboe et al., 2019), and for PEH who exhibited care-seeking behavior in a San Francisco population (Robbins et al., 2010). Still, treatment with buprenorphine or methadone rarely occurred among a cohort of PEH who died from overdose in Boston (Bauer et al., 2016).
3.1.1.2. Studies including PEH
3.1.1.2.1. General treatment access/engagement
PEH accessed inpatient treatment more often than outpatient treatment for the use of opioids and other substances (Corsi et al., 2007; Eyrich-Garg et al., 2008; Masson et al., 2002). Masson et al. (2002) found that individuals at a public hospital who were homeless and used opioids had higher mean emergency department visits and inpatient service use than their housed counterparts. Two studies similarly found that methadone maintenance therapy (MMT) and other outpatient treatment services were more likely to be accessed by housed individuals (Corsi et al., 2007; Eyrich-Garg et al., 2008), though findings from these studies were not necessarily specific to individuals who used opioids.
In contrast, a latent class analysis in Ohio found the prevalence of homelessness to be meaningfully higher in classes characterized by heavy illicit opioid use and the most reported recent SUD treatment (i.e. MOUD- or abstinence-based), compared to the class characterized by the least reported recent SUD treatment (Daniulaityte et al., 2019).
3.1.1.2.2. MOUD treatment access/engagement
Several articles specifically analyzed access to and engagement with MOUD treatment among PEH. Four reported potential barriers to MOUD uptake for PEH (Hoffman et al., 2019; Krull et al., 2011; Patel et al., 2020; Simon et al., 2017). Krull et al. (2011) found that substance use program directors serving higher percentages of PEH held more negative attitudes towards buprenorphine treatment. Relatedly, a significant majority of recovery homes near Nashville were found to bar discharged inpatients from remaining on any MOUD in 2018 (Patel et al., 2020). Moreover, not only were turbulent living conditions described as a significant barrier to recruitment for an XR-NTX clinical trial (Hoffman et al., 2019), but PEH in Seattle were also disproportionately lost to follow-up for buprenorphine treatment after initial engagement but prior to induction (Simon et al., 2017).
Seven studies consistently found that PEH were significantly less likely to have enrolled in MMT (Deck and Carlson, 2004; Eyrich-Garg et al., 2008; Lundgren et al., 2003; Reynoso-Vallejo et al., 2008; Rivers et al., 2006; Royse et al., 2000; Shah et al., 2000). This was true for PEH regardless of HIV (Shah et al., 2000) or heroin use statuses (Rivers et al., 2006) and among PEH in Oregon and Washington (Deck and Carlson, 2004), and PEH within a population of Latino males in Massachusetts (Reynoso-Vallejo et al., 2008). However, one Central Harlem study found no significant differences between PEH and non-PEH in odds of attending a methadone clinic (Van Ness et al., 2004).
Studies assessing both methadone and other MOUD (i.e. buprenorphine and naltrexone) access reported analogous findings. A retrospective analysis found that PEH were less likely to have received OAT in the context of outpatient treatment admissions for OUD (Krawczyk et al., 2020). Moreover, the prevalence of homelessness among veterans initiating methadone or buprenorphine treatment was only 10.2 % in 2012 (Bachhuber et al., 2015), despite 34.6 % of veterans with OUD being homeless in the following two years (Iheanacho et al., 2018). PEH were also less likely to receive OAT than housed individuals in detoxification settings (Dunn et al., 2019), though they were more likely to receive XR-NTX than OAT and no treatment in a sample of veterans in 2012 (Kelly et al., 2018).
Despite apparently diminished access to MOUD, in one study, homelessness among inpatients receiving addiction consultation predicted higher rates of initiation of medications for OUD or alcohol use disorder (Englander et al., 2020).
3.1.1.2.3. Detoxification access/engagement
While one study found that out-of-treatment individuals not experiencing homelessness were more likely to have participated specifically in methadone detoxification (Royse et al., 2000), two studies consistently found that PEH were more likely to use opioid detoxification programs (Amodeo et al., 2004; Timko et al., 2016).
3.1.2. Treatment outcomes & related findings
Fewer descriptive studies reported on OUD treatment-associated outcomes among PEH, primarily assessing MOUD and detoxification outcomes (Alford et al., 2007; Chang, 2017; Cousins et al., 2016; Damian et al., 2017; Fine et al., 2020; Havens et al., 2009; Jones et al., 2017; Kertesz et al., 2003; Li et al., 2019; Marienfeld and Rosenheck, 2015; Rose-Jacobs et al., 2019; Stein et al., 2017, 2015,2014; Riggins et al., 2017; Velasquez et al., 2019).
3.1.2.1. MOUD treatment outcomes
Several studies examined heterogeneous outcomes, including retention, specific to PEH in relation to MOUD treatment (Alford et al., 2007; Chang, 2017; Cousins et al., 2016; Damian et al., 2017; Fine et al., 2020; Havens et al., 2009; Marienfeld and Rosenheck, 2015; Rose-Jacobs et al., 2019; Riggins et al., 2017; Velasquez et al., 2019). Though PEH experienced similar (Alford et al., 2007) if not better (Riggins et al., 2017) outcomes than housed individuals in opioid use reduction while engaged in two office-based opioid therapy (OBOT) programs, a Boston study observed an increased hazard of all-cause mortality and opioid-overdose mortality among PEH who initiated buprenorphine treatment (Fine et al., 2020).
Findings were mixed on MOUD retention among PEH. While a quantitative and a qualitative study respectively found unstable housing to be related to diminished retention (Damian et al., 2017) and MOUD adherence (Velasquez et al., 2019), three found no significant differences in treatment failure or retention between PEH and non-PEH receiving OAT in Baltimore (Havens et al., 2009), XR-NTX in Los Angeles (Cousins et al., 2016), and buprenorphine in Boston (Alford et al., 2007). However, two of these studies found nonsignificant negative associations between homelessness and days in treatment (Cousins et al., 2016; Havens et al., 2009).
Other heterogeneous MOUD-related findings included greater depressive symptomatology among pregnant women in treatment for OUD experiencing both housing and food instability (Rose-Jacobs et al., 2019) and significantly greater prevalences of homelessness among patients in MMT with serious mental illness, as compared independently to patients only in MMT and patients with only serious mental illness (Marienfeld and Rosenheck, 2015). A qualitative study reported participant feelings of geographical restriction due to on-site methadone dosing in San Francisco’s Tenderloin district (Chang, 2017).
3.1.2.2. Detoxification outcomes
At least five studies described findings pertaining to opioid detoxification among PEH (Kertesz et al., 2003; Li et al., 2019; Stein et al., 2014, 2015, 2017). PEH experienced similar if not better detoxification-associated outcomes compared to housed individuals (Kertesz et al., 2003; Li et al., 2019). A Boston study found that homelessness was associated with a later first use post-detoxification for individuals who preferred heroin (Kertesz et al., 2003), and Li et al. (2019) found no significant differences in all-cause mortality between homeless-experienced and non-homeless-experienced veterans admitted for inpatient opioid detoxification in 2015. Additionally, homelessness was not a significant correlate of being uninsured in a sample of individuals seeking opioid detoxification in Massachusetts (Stein et al., 2014). In terms of detoxification aftercare, PEH were significantly more likely to prefer residential treatment, as compared to OAT or outpatient treatment (Stein et al., 2015) and to no treatment (Stein et al., 2017).
3.1.2.3. Related findings: Patterns of use
One study looked at characteristics of treatment admissions for prescription opioid misuse and found that PEH admitted to federally-funded treatment programs were significantly more likely than housed individuals to report higher-risk routes of consumption, including injection and inhalation, rather than oral use (Jones et al., 2017).
3.2. Intervention studies
3.2.1. Low-barrier OAT
Eight articles described and/or assessed low-threshold OAT models seeking to expand access to PEH through flexible program requirements, intensive outreach, and increased accountability (Buzza et al., 2019; Carter et al., 2019; Godersky et al., 2019; Hall et al., 2014; Hersh et al., 2011; Hood et al., 2020; Krawczyk et al., 2019; Tringale et al., 2015). One such model known as “mobile OAT,” which involves portable OAT induction clinics and street outreach teams, better engaged PEH in care compared to clients receiving fixed-site, outpatient methadone (Hall et al., 2014). Moreover, even though retention rates for mobile programs located in San Francisco (55 % at month 1, 26 % at month 12) (Carter et al., 2019) and outside the Baltimore City Jail (32 % at month 1) (Krawczyk et al., 2019) were lower than those typically observed in OBOT programs, the programs addressed gaps in care by recruiting difficult-to-reach people who were predominantly vulnerably housed. Buzza et al. (2019) reported a similar mobile OAT model in California involving provider visitations to homeless encampments; however, the program has not been evaluated quantitatively.
Three studies assessed integration of OAT programs into existing public health infrastructure (Hersh et al., 2011; Hood et al., 2020; Tringale et al., 2015). For example, Hersh et al. (2011) found significant decreases in opioid use for patients engaged with a buprenorphine program in San Francisco consisting of a centralized induction clinic and pharmacy connected to three treatment sites. One-year treatment retention rates were similar between individuals who were homeless versus housed at admission (54 % vs. 67 %, P = 0.3); however, housed individuals stayed in the program longer on average than PEH (10.2 months vs. 7 months) (Hersh et al., 2011). When buprenorphine treatment was integrated into two syringe exchange programs, neither program retention nor transfer to another clinic for maintenance therapy varied significantly by housing status (Hood et al., 2020), and preliminary success was observed among treatment-resistant PEH (Tringale et al., 2015).
One qualitative study described mixed perspectives regarding video directly observed therapy (VDOT), which involves monitored video recordings of daily medication intake, as a potential, innovative model for OAT adherence (Godersky et al., 2019). While most patients thought VDOT might improve adherence by mitigating logistical barriers and enhancing access to providers, others questioned its practicality due to the unique technological barriers experienced by PEH and other marginalized populations.
3.2.2. Shelter-based OAT
Four intervention-focused articles reported on or evaluated shelter-based opioid therapy (Chatterjee et al., 2017, 2018; Doorley et al., 2017; Lashley, 2019). Specifically, Chatterjee et al. (2017) found decreases in overdose (0 at 3rd month of treatment vs. 4 at baseline) and increases in employment (3 patients at 3rd month of treatment vs. 1 patient at baseline) among a small sample of individuals receiving buprenorphine treatment in Boston family shelters. In a qualitative study within the same setting, PEH who were part of families described shelter-based buprenorphine treatment as convenient and able to offset common barriers to treatment, including transportation, child care needs, and strict clinic requirements (Chatterjee et al., 2018). Another shelter-based program in California offering buprenorphine implemented shared medical appointments which were attended by medical, behavioral, and social specialists (Doorley et al., 2017). A preliminary evaluation found 70 % retention at 24 weeks for this integrated approach. Similarly, a residential, integrated faith-based care model for homeless men in Baltimore found a 70 % completion rate (i.e. successful titration) for individuals receiving OAT (Lashley, 2019).
3.2.3. Housing First
A few studies conducted in New York assessed the efficacy of the Housing First approach (Tsemberis et al., 2003), which aims to connect PEH to permanent housing not contingent on substance use or mental illness treatment, for PEH who use opioids (Appel et al., 2012; Davidson et al., 2014; Hall et al., 2020). One study found significantly higher MMT retention for individuals with severe mental illness placed in supportive housing compared to a group of PEH receiving MMT without housing (Appel et al., 2012). Moreover, this study and one other reported higher rates of housing retention (Appel et al., 2012; Hall et al., 2020), though in the former study, this higher rate was observed among MMT patients placed into supportive housing (vs. not) (Appel et al., 2012), while in the latter, this higher rate was observed among individuals who were on OAT at the time of supportive housing application (vs. untreated at baseline) (Hall et al., 2020). Consistent with these outcomes, one study also found individuals were less likely to use opiates or stimulants at follow-up when they participated in supportive housing programs encouraging harm reduction rather than abstinence (Davidson et al., 2014).
3.2.4. Nurse-led interventions
We found two studies evaluating the efficacy of novel nurse-led interventions aimed at decreasing substance use among youth experiencing homelessness and gay and bisexual PEH (Nyamathi et al., 2012, 2017). The former study compared substance use outcomes between participants randomized to a nurse-led health promotion program and those randomized to develop creative messages pertaining to substance use and health for other youth (Nyamathi et al., 2012). Neither experienced significant reductions in heroin use at follow-up. The latter study compared substance use outcomes between a group of gay and bisexual men receiving nurse care management and contingency management (NCM + CM) and another receiving standard education plus contingency management (SE + CM) (Nyamathi et al., 2017). There was a significant reduction in the number of individuals who used opiates, as determined by urinalysis, only between month four and month eight follow up visits within the SE + CM group; however, baseline opiate positivity was low (Nyamathi et al., 2017).
4. Discussion
In this scoping review synthesizing literature pertaining to OUD treatment among PEH, we found sixty articles that reported differential OUD treatment-associated findings among PEH and/or evaluated innovative treatment models for this population. Consistent with previous findings of high rates of emergency department utilization and hospitalizations among PEH (Kushel et al., 2001, 2002; Lin et al., 2015), we found that PEH were more likely to utilize the emergency room and inpatient services, including detoxification, for opioid-related care than their housed counterparts (Eyrich-Garg et al., 2008, Masson et al., 2002, Timko et al., 2016). Relatedly, while one study found that PEH were more likely to receive XR-NTX than no treatment (Kelly et al., 2018), PEH were consistently less likely than their housed counterparts to have enrolled in or received outpatient OAT for their opioid use (Bauer et al., 2016; Deck and Carlson, 2004; Dunn et al., 2019; Eyrich-Garg et al., 2008; Kelly et al., 2018; Krawczyk et al., 2020; Lundgren et al., 2003; Reynoso-Vallejo et al., 2008; Rivers et al., 2006; Royse et al., 2000; Shah et al., 2000). Given buprenorphine and methadone are more effective at reducing overdose risk and opioid-related morbidity than naltrexone maintenance therapy, inpatient, residential, or behavioral services (Wakeman et al., 2020), it is unsurprising that opioid-involved overdoses and deaths continue to disproportionately impact PEH compared to stably housed populations (Baggett et al., 2013; Fine et al., 2020; Yamamoto et al., 2019).
However, despite consistent evidence of decreased access to OAT, studies did not clearly demonstrate that homelessness was associated with worse treatment efficacy once PEH became engaged. While studies found diminished retention (Damian et al., 2017) and increased mortality among PEH receiving buprenorphine (Fine et al., 2020), two studies found no significant differences in OAT outcomes and retention between housed and unstably housed populations (Alford et al., 2007; Havens et al., 2009), though PEH did initially require more case management after enrollment (Alford et al., 2007). Additionally, Riggins et al. (2017) found that homelessness independently predicted decreased odds of opioid use at follow-up among a cohort receiving buprenorphine. Because PEH may equivalently benefit once enrolled, there remains a need to explore and mitigate barriers, including structural ones, that contribute to their suboptimal rates of induction into agonist therapy. In addition, while buprenorphine and methadone are comparably effective at treating OUD, addressing PEH-related limitations unique to each treatment modality is urgently needed to offset this disparity in accessibility. For example, a buprenorphine prescription requires a secure storage space and may not be permitted in some shelters or recovery residences (Patel et al., 2020), and MMT entails physically burdensome daily dosing at stationary program sites (Chang, 2017). Extended-release (i.e. implant and injection formulations) buprenorphine may help overcome some of these issues but remains largely unavailable, only being offered at 2% of substance use disorder treatment facilities in the United States in 2018 (Shover, 2021).
Though PEH largely underutilize OUD treatment, especially pharmacotherapy, innovative treatment models that aim to address these limitations and disparities have been established and reported in this review. PEH experience logistical barriers and competing priorities impeding appointment and medication adherence in standard, office-based MOUD programs. Models that lower access thresholds and remove obstacles to treatment, such as mobile and street-outreach MOUD clinics (Buzza et al., 2019; Carter et al., 2019; Hall et al., 2014; Krawczyk et al., 2019) and shelter-based treatment programs (Chatterjee et al., 2018, 2017; Doorley et al., 2017; Lashley, 2019), more successfully reached and comparably retained marginalized populations such as PEH compared to similar office-based programs. These low-barrier, accessible options are extremely important now more than ever, as the COVID-19 pandemic has substantially disrupted the availability of in-person medical appointments and OUD treatment. In fact, though not necessarily tailored to PEH, regulatory changes during the pandemic have allowed for take-home methadone dosing (Figgatt et al., 2021), in some cases occurring at shelters and temporary housing programs, and buprenorphine initiation via telemedicine (Wang et al., 2021), adaptations that have potentially benefitted this population. Nevertheless, it is unclear whether these modifications will remain permanently in place post-pandemic, and as with VDOT for OAT (Godersky et al., 2019), there are concerns that a greater reliance on virtual care may leave some groups with diminished digital access and literacy behind. Still, these changes present a unique opportunity to continue to innovate existing MOUD models to meet the needs of PEH.
Unlike these successful MOUD models, educational and behavioral interventions were largely ineffective at improving opioid use outcomes for PEH. However, they were successful at achieving their intended outcomes of generally reducing substance use among this population (Nyamathi et al., 2012, 2017). Given that psychosocial interventions are indicated for the treatment of OUD when administered in conjunction with MOUD (Dugosh et al., 2016), similar nurse-led interventions designed specifically for PEH who use opioids may be efficacious in the context of concurrent pharmacological treatment. This task-sharing of evidence-based care may aid in addressing existing gaps in OUD treatment provision (Magidson et al., 2019).
We also found that PEH who use opioids benefitted from supportive housing initiatives that were not contingent on substance use treatment or sobriety (Appel et al., 2012; Davidson et al., 2014; Hall et al., 2020). PEH in these supportive housing programs experienced greater MMT retention compared to unhoused counterparts (Appel et al., 2012), and those already receiving OAT upon placement experienced greater housing retention (Hall et al., 2020). Consistent with research finding harm reduction approaches to be more successful at retaining and treating individuals with SUDs (Padgett et al., 2011), opiate and stimulant use decreased among those placed in housing programs prioritizing harm reduction rather than abstinence (Davidson et al., 2014). It is important to note that these three studies were conducted in progressive urban contexts and that abstinence from substances, including substances like opioid agonists that treat addiction, is still a prerequisite for many residential treatment and housing programs (Patel et al., 2020). In fact in some places, PEH who use substances have been barred from accessing temporary isolation and quarantine facilities designed to slow the spread of SARS-Cov-2 during the COVID-19 pandemic (American Society of Addiction Medicine and COVID-19 Task Force, 2020). Moreover, even with policy priorities shifting towards more permanent housing options for PEH, homeless service systems in the United States were only able to offer year-round beds to slightly more than half of individual PEH in 2019 (National Alliance to End Homelessness, 2020). This housing shortage, in addition to the persistence of abstinence-only housing programs, poses a significant threat to the management of OUD and other SUDs among homeless populations in the United States.
Some of the interventions identified in this review have likely demonstrated success because they adhere to well-developed frameworks such as the healthcare for the homeless (HCH) model (Zlotnick et al., 2013). HCH interventions are characterized by outreach and engagement, community collaborations, case management, and respite care. Among our included studies, we found strong concordance with these first three features. Successful opioid treatment programs for PEH are flexible, dynamic, and on-demand, implementing measures which move the point of care out of the clinic in order to meet patients where they are at (Carter et al., 2019; Hall et al., 2014; Krawczyk et al., 2019). These efforts respond to documented transportation and access barriers experienced by PEH (Chatterjee et al., 2018; Godersky et al., 2019) as well as their taking shelter in difficult-to-reach locations. Moreover, many of these programs tolerated continued missed appointments and gaps in care, which are expected among patients with housing instability and multiple morbidities, as well as relapse and polysubstance use (Carter et al., 2019; Hood et al., 2020; Tringale et al., 2015). Efficacious interventions involved collaborations with community clinics, pharmacies (Buzza et al., 2019; Hersh et al., 2011), and syringe exchange programs (Hood et al., 2020; Tringale et al., 2015) and implemented multidisciplinary care approaches, sometimes involving case management, that addressed the complex biopsychosocial factors influencing treatment outcomes for PEH (Alford et al., 2007; Doorley et al., 2017; Lashley, 2019). While we did not find opioid use treatment literature involving respite care, transitional shelter-based and supportive housing programs successfully supported these populations in addressing their opioid use (Appel et al., 2012; Chatterjee et al., 2017, 2018; Davidson et al., 2014; Hall et al., 2020).
Although innovative programs have been developed to address the unique challenges that PEH who use opioids experience, our review reveals there is still limited information with which to evaluate them. Included outreach and street medicine studies were in early stages of evaluation. Studies of shelter-based programs were scarce and limited by small sample sizes and lack of comparison groups. Moreover, while Housing First interventions for PEH with SUDs have been shown to be effective under certain conditions, their heterogeneous implementations limit uniform success (Davidson et al., 2014). Ultimately, more work is needed to rigorously evaluate and progress these interventions in the field. Considering that the results from a pilot study evaluating a mobile buprenorphine treatment program for veterans were published shortly after our search date (Iheanacho et al., 2020), more robust and tailored findings related to treating OUD among PEH are soon expected as this novel body of literature evolves.
We additionally noticed deficits in how the literature assesses OUD treatment and race in relation to homelessness. OUD treatment access in the United States has historically been segregated along racial and ethnic lines, with methadone more common in low-income, Black and Hispanic/Latinx communities and buprenorphine more common in high-income, White communities (Goedel et al., 2020; Hansen et al., 2013). While both effectively treat OUD (Wakeman et al., 2020), MMT status is particularly stigmatized and associated with racial discrimination (Pro and Zaller, 2020), making it even more vulnerable to underutilization and nonadherence. Interestingly, one of our included studies found that a mobile OAT treatment program more successfully recruited Black individuals as well as PEH than a fixed-site methadone clinic (Hall et al., 2014). It follows that OUD treatment programs tailored to reach PEH may benefit other populations, including racial and ethnic minorities. Because people of minority racial identities may suboptimally interact with OUD treatment options related to a legacy of racism in health care and addiction treatment, such programs must work to ameliorate experiences of trauma and sentiments of mistrust among these populations. Offering the option to forgo toxicology screening due to prior traumatic experiences with urine testing (Carter et al., 2019) and providing trauma-informed care (Hood et al., 2020) are two features that may make these models more approachable for populations that have historically been the least engaged in addiction treatment. Immigration status is also an issue that overlaps with homelessness and shapes access to OUD treatment, via insurance coverage, but is not explored in the existing literature.
There are several limitations to our review. We only included studies that assessed the relationship between homelessness and treatment for opioid use. Though several studies examined populations with non-trivial prevalences of homelessness, they were not included if they did not report on the distinct connection between housing or housing status and treatment outcomes or models. As a result, certain studies evaluating novel treatment interventions for opioid use were excluded, even though some may have been beneficial for PEH. Conversely, some of our included findings did not necessarily tease out independent relationships between housing status and treatment specifically for opioid use. For example, Davidson et al. (2014) found decreased use of stimulants or opiates among those in client-oriented supportive housing. However, considering the pervasiveness of polysubstance use (Crummy et al., 2020), we still found these findings relevant despite the lack of opioid specificity. Our synthesis was also limited by variable times and locations of data collection. Outcomes related to OUD treatment may vary by regional and generational differences in substance use trends and epidemiological factors. For example, the primary opioid associated with overdose in the United States has changed over time, with a marked transition from heroin to fentanyl occurring within the last decade (Hedegaard et al., 2018). Moreover, the United States is currently experiencing a “fourth wave” overdose epidemic, driven primarily by increases in methamphetamine in the West and cocaine in the East in combination with opioids (Kariisa et al., 2019; Hedegaard et al., 2019). These differing contexts limit generalizability, particularly of findings pertaining to treatment outcomes, and may hinder direct comparison of study results. Finally, definitions of homelessness and housing statuses varied widely by study, and analyses sometimes included subcategories of vulnerable housing. Merely synthesizing this data oversimplifies the differing contexts of specific living conditions and the assumptions underlying study findings.
4.1. Conclusion
The opioid epidemic contributes significantly to mortality in the United States, with a disproportionately severe impact on PEH that has worsened during the COVID-19 pandemic. Researchers, policymakers, and healthcare professionals must discover and evaluate ways to respond to the needs of specific communities, with the guidance of existing recommendations for healthcare for PEH. This scoping review surveyed the current body of literature assessing how PEH access and experience OUD treatment, identifying sustained barriers and recognizing preliminarily efficacious, flexible treatment strategies situated within the HCH model. Expanding upon the findings from these recent developments will be crucial to mitigate the hugely disparate burden of opioid-related morbidity and mortality experienced by PEH in the United States.
Role of funding source
Nothing declared.
Contributors
M. McLaughlin, R. Li, and N. Domínguez Carrero screened articles and extracted the data. M. McLaughlin, R. Li, N. Domínguez Carrero, and A. Chatterjee analyzed the data. P. Bain developed the search strategy. All authors contributed to the writing of the manuscript and development and conceptualization of the scoping review.
Declaration of competing interest
No conflict declared.
Acknowledgements
We acknowledge the patients and staff at the Boston Healthcare for the Homeless Program.
Footnotes
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.drugalcdep.2021.108717.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- Alford D.P., LaBelle C.T., Richardson J.M., O’Connell J., Hohl C.A., Cheng D.M., Samet J.H. Treating homeless opioid dependent patients with buprenorphine in an office-based setting. J. Gen. Intern. Med. 2007;22:171–176. doi: 10.1007/s11606-006-0023-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Society of Addiction Medicine, COVID-19 Task Force . 2020. Treating Unhoused People with Addiction during COVID-19.https://www.asam.org/docs/default-source/covid-19/1-tf_treating-unhoused-people-with-addiction-during-covid-19_final.pdf?sfvrsn=31ba58c2_2 Accessed February 16, 2021. [Google Scholar]
- Amodeo M., Chassler D., Ferguson F., Fitzgerald T., Lundgren L. Use of mental health and substance abuse treatment services by female injection drug users. Am. J. Drug Alcohol Abuse. 2004;30:101–120. doi: 10.1081/ada-120029868. [DOI] [PubMed] [Google Scholar]
- Appel P.W., Tsemberis S., Joseph H., Stefancic A., Lambert-Wacey D. Housing First for severely mentally ill homeless methadone patients. J. Addict. Dis. 2012;31:270–277. doi: 10.1080/10550887.2012.694602. [DOI] [PubMed] [Google Scholar]
- Bachhuber M.A., Roberts C.B., Metraux S., Montgomery A.E. Screening for homelessness among individuals initiating medication-assisted treatment for opioid use disorder in the Veterans Health Administration. J. Opioid Manag. 2015;11:459–462. doi: 10.5055/jom.2015.0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachhuber M.A., Thompson C., Prybylowski A., Benitez J., Mazzella S., Barclay D. Description and outcomes of a buprenorphine maintenance treatment program integrated within Prevention Point Philadelphia, an urban syringe exchange program. Subst. Abuse. 2018;39:167–172. doi: 10.1080/08897077.2018.1443541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baggett T.P., O’Connell J.J., Singer D.E., Rigotti N.A. The unmet health care needs of homeless adults: a national study. Am. J. Public Health. 2010;100:1326–1333. doi: 10.2105/AJPH.2009.180109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baggett T.P., Hwang S.W., O’Connell J.J., Porneala B.C., Stringfellow E.J., Orav E.J., Singer D.E., Rigotti N.A. Mortality among homeless adults in Boston: shifts in causes of death over a 15-year period. JAMA Intern. Med. 2013;173:189–195. doi: 10.1001/jamainternmed.2013.1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baggett T.P., Keyes H., Sporn N., Gaeta J.M. Prevalence of SARS-CoV-2 infection in residents of a large homeless shelter in Boston. JAMA. 2020;323:2191–2192. doi: 10.1001/jama.2020.6887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer L.K., Brody J.K., León C., Baggett T.P. Characteristics of homeless adults who died of drug overdose: a retrospective record review. J. Health Care Poor Underserved. 2016;27:846–859. doi: 10.1353/hpu.2016.0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzza C., Elser A., Seal J. A mobile buprenorphine treatment program for homeless patients with opioid use disorder. Psychiatr. Serv. 2019;70:635–636. doi: 10.1176/appi.ps.70701. [DOI] [PubMed] [Google Scholar]
- Carter J., Zevin B., Lum P.J. Low barrier buprenorphine treatment for persons experiencing homelessness and injecting heroin in San Francisco. Addict. Sci. Clin. Pract. 2019;14:20. doi: 10.1186/s13722-019-0149-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention . 2020. Overdose Deaths Accelerating During COVID-19 Pandemic: Expanded Prevention Efforts Needed.https://www.cdc.gov/media/releases/2020/p1218-overdose-deaths-covid-19.html Accessed February 8, 2021. [Google Scholar]
- Chang J.S. Health in the Tenderloin: a resident-guided study of substance use, treatment, and housing. Soc. Sci. Med. 2017;176:166–174. doi: 10.1016/j.socscimed.2017.01.014. [DOI] [PubMed] [Google Scholar]
- Chatterjee A., Obando A., Strickland E., Nestler A., Harrington-Levey R., Williams T., LaCoursiere-Zucchero T. Shelter-based opioid treatment: increasing access to addiction treatment in a family shelter. Am. J. Public Health. 2017;107:1092–1094. doi: 10.2105/AJPH.2017.303786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee A., Yu E.J., Tishberg L. Exploring opioid use disorder, its impact, and treatment among individuals experiencing homelessness as part of a family. Drug Alcohol Depend. 2018;188:161–168. doi: 10.1016/j.drugalcdep.2018.04.012. [DOI] [PubMed] [Google Scholar]
- Corsi K.F., Kwiatkowski C.F., Booth R.E. Treatment entry and predictors among opiate-using injection drug users. Am. J. Drug Alcohol Abuse. 2007;33:121–127. doi: 10.1080/00952990601091093. [DOI] [PubMed] [Google Scholar]
- Cousins S.J., Radfar S.R., Crèvecoeur-MacPhail D., Ang A., Darfler K., Rawson R.A. Predictors of continued use of extended-released naltrexone (XR-NTX) for opioid-dependence: an analysis of heroin and non-heroin opioid users in Los Angeles County. J. Subst. Abuse Treat. 2016;63:66–71. doi: 10.1016/j.jsat.2015.12.004. [DOI] [PubMed] [Google Scholar]
- Crummy E.A., O’Neal T.J., Baskin B.M., Ferguson S.M. One is not enough: understanding and modeling polysubstance use. Front. Neurosci. 2020;14:569. doi: 10.3389/fnins.2020.00569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damian A.J., Mendelson T., Agus D. Predictors of buprenorphine treatment success of opioid dependence in two Baltimore City grassroots recovery programs. Addict. Behav. 2017;73:129–132. doi: 10.1016/j.addbeh.2017.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniulaityte R., Nahhas R.W., Silverstein S., Martins S., Zaragoza A., Moeller A., Carlson R.G. Patterns of non-prescribed buprenorphine and other opioid use among individuals with opioid use disorder: a latent class analysis. Drug Alcohol Depend. 2019;204 doi: 10.1016/j.drugalcdep.2019.107574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson C., Neighbors C., Hall G., Hogue A., Cho R., Kutner B., Morgenstern J. Association of housing first implementation and key outcomes among homeless persons with problematic substance use. Psychiatr. Serv. 2014;65:1318–1324. doi: 10.1176/appi.ps.201300195. [DOI] [PubMed] [Google Scholar]
- Davies A., Wood L.J. Homeless health care: meeting the challenges of providing primary care. Med. J. Aust. 2018;209:230–234. doi: 10.5694/mja17.01264. [DOI] [PubMed] [Google Scholar]
- Deck D., Carlson M.J. Access to publicly funded methadone maintenance treatment in two western states. J. Behav. Health Serv. Res. 2004;31:164–177. doi: 10.1007/BF02287379. [DOI] [PubMed] [Google Scholar]
- Doorley S.L., Ho C.J., Echeverria E., Preston C., Ngo H., Kamal A., Cunningham C.O. Buprenorphine shared medical appointments for the treatment of opioid dependence in a homeless clinic. Subst. Abus. 2017;38:26–30. doi: 10.1080/08897077.2016.1264535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doran K.M., Rahai N., McCormack R.P., Milian J., Shelley D., Rotrosen J., Gelberg L. Substance use and homelessness among emergency department patients. Drug Alcohol Depend. 2018;188:328–333. doi: 10.1016/j.drugalcdep.2018.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugosh K., Abraham A., Seymour B., McLoyd K., Chalk M., Festinger D. A systematic review on the use of psychosocial interventions in conjunction with medications for the treatment of opioid addiction. J. Addict. Med. 2016;10:93–103. doi: 10.1097/ADM.0000000000000193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn K.E., Huhn A.S., Strain E.C. Differential adoption of opioid agonist treatments in detoxification and outpatient settings. J. Subst. Abuse Treat. 2019;107:24–28. doi: 10.1016/j.jsat.2019.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englander H., King C., Nicolaidis C., Collins D., Patten A., Gregg J., Korthuis P.T. Predictors of opioid and alcohol pharmacotherapy initiation at hospital discharge among patients seen by an inpatient addiction consult service. J. Addict. Med. 2020;14:415–422. doi: 10.1097/ADM.0000000000000611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyrich-Garg K.M., Cacciola J.S., Carise D., Lynch K.G., McLellan A.T. Individual characteristics of the literally homeless, marginally housed, and impoverished in a US substance abuse treatment-seeking sample. Soc. Psychiatry Psychiatr. Epidemiol. 2008;43:831–842. doi: 10.1007/s00127-008-0371-8. [DOI] [PubMed] [Google Scholar]
- Fazel S., Geddes J.R., Kushel M. The health of homeless people in high-income countries: descriptive epidemiology, health consequences, and clinical and policy recommendations. Lancet. 2014;384:1529–1540. doi: 10.1016/S0140-6736(14)61132-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figgatt M.C., Salazar Z., Day E., Vincent L., Dasgupta N. Take-home dosing experiences among persons receiving methadone maintenance treatment during COVID-19. J. Subst. Abuse Treat. 2021;123 doi: 10.1016/j.jsat.2021.108276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine D.R., Yu L., Triant V.A., Baggett T.P., Metlay J.P. Baseline factors associated with mortality in patients who engaged in buprenorphine treatment for opioid use disorder: a cohort study. J. Gen. Intern. Med. 2020;35:2375–2382. doi: 10.1007/s11606-020-05779-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godersky M.E., Saxon A.J., Merrill J.O., Samet J.H., Simoni J.M., Tsui J.I. Provider and patient perspectives on barriers to buprenorphine adherence and the acceptability of video directly observed therapy to enhance adherence. Addict. Sci. Clin. Pract. 2019;14:11. doi: 10.1186/s13722-019-0139-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goedel W.C., Shapiro A., Cerdá M., Tsai J.W., Hadland S.E., Marshall B.D.L. Association of racial/ethnic segregation with treatment capacity for opioid use disorder in counties in the United States. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.3711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haider M.R., Brown M.J., Gupta R.D., Karim S., Olatosi B., Li X. Psycho-social correlates of opioid use disorder among the US adult population: evidence from the National Survey on Drug Use and Health, 2015-2018. Subst. Use Misuse. 2020;55:2002–2010. doi: 10.1080/10826084.2020.1788086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall G., Neighbors C.J., Iheoma J., Dauber S., Adams M., Culleton R., Muench F., Borys S., McDonald R., Morgenstern J. Mobile opioid agonist treatment and public funding expands treatment for disenfranchised opioid-dependent individuals. J. Subst. Abuse Treat. 2014;46:511–515. doi: 10.1016/j.jsat.2013.11.002. [DOI] [PubMed] [Google Scholar]
- Hall G., Walters S., Gould H., Lim S. Housing versus treatment first for supportive housing participants with substance use disorders: a comparison of housing and public service use outcomes. Subst. Abus. 2020;41:70–76. doi: 10.1080/08897077.2018.1449049. [DOI] [PubMed] [Google Scholar]
- Hansen H.B., Siegel C.E., Case B.G., Bertollo D.N., DiRocco D., Galanter M. Variation in use of buprenorphine and methadone treatment by racial, ethnic, and income characteristics of residential social areas in New York City. J. Behav. Health Serv. Res. 2013;40:367–377. doi: 10.1007/s11414-013-9341-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havens J.R., Latkin C.A., Pu M., Cornelius L.J., Bishai D., Huettner S., Rapp C., Ricketts E.P., Lloyd J.J., Strathdee S.A. Predictors of opiate agonist treatment retention among injection drug users referred from a needle exchange program. J. Subst. Abuse Treat. 2009;36:306–312. doi: 10.1016/j.jsat.2008.07.002. [DOI] [PubMed] [Google Scholar]
- Hedegaard H., Bastian B.A., Trinidad J.P., Spencer M., Warner M. Drugs most frequently involved in drug overdose deaths: United States, 2011-2016. Vital Stat. Rep. 2018;67:1–14. [PubMed] [Google Scholar]
- Hedegaard H., Bastian B.A., Trinidad J.P., Spencer M.R., Warner M. Regional differences in the drugs most frequently involved in drug overdose deaths: United States, 2017. Vital Stat. Rep. 2019;68:1–16. [PubMed] [Google Scholar]
- Hersh D., Little S.L., Gleghorn A. Integrating buprenorphine treatment into a public healthcare system: The San Francisco Department of Public Health’s office-based buprenorphine pilot program. J. Psychoactive Drugs. 2011;43:136–145. doi: 10.1080/02791072.2011.587704. [DOI] [PubMed] [Google Scholar]
- Hoffman K.A., Baker R., Kunkel L.E., Needham Waddell E., Lum P.J., McCarty D., Korthuis P.T. Barriers and facilitators to recruitment and enrollment of HIV-infected individuals with opioid use disorder in a clinical trial. BMC Health Serv. Res. 2019;19:862. doi: 10.1186/s12913-019-4721-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood J.E., Banta-Green C.J., Duchin J.S., Breuner J., Dell W., Finegood B., Glick S.N., Hamblin M., Holcomb S., Mosse D., Oliphant-Wells T., Shim M.H.M. Engaging an unstably housed population with low-barrier buprenorphine treatment at a syringe services program: lessons learned from Seattle, Washington. Subst Abus. 2020;41:356–364. doi: 10.1080/08897077.2019.1635557. [DOI] [PubMed] [Google Scholar]
- Hser Y.I., Evans E., Grella C., Ling W., Anglin D. Long-term course of opioid addiction. Harv. Rev. Psychiatry. 2015;23:76–89. doi: 10.1097/HRP.0000000000000052. [DOI] [PubMed] [Google Scholar]
- Hser Y.I., Evans E., Huang D., Weiss R., Saxon A., Carroll K.M., Woody G., Liu D., Wakim P., Matthews A.G., Hatch-Maillette M., Jelstrom E., Wiest K., McLaughlin P., Ling W. Long‐term outcomes after randomization to buprenorphine/naloxone versus methadone in a multi‐site trial. Addiction. 2016;111:695–705. doi: 10.1111/add.13238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iheanacho T., Stefanovics E., Rosenheck R. Opioid use disorder and homelessness in the Veterans Health Administration: the challenge of multimorbidity. J. Opioid Manag. 2018;14:171–182. doi: 10.5055/jom.2018.0447. [DOI] [PubMed] [Google Scholar]
- Iheanacho T., Payne K., Tsai J. Mobile, community-based buprenorphine treatment for veterans experiencing homelessness with opioid use disorder: a pilot, feasibility study. Am. J. Addict. 2020;29:485–491. doi: 10.1111/ajad.13055. [DOI] [PubMed] [Google Scholar]
- Jarvis B.P., Holtyn A.F., Subramaniam S., Tompkins D.A., Oga E.A., Bigelow G.E., Silverman K. Extended-release injectable naltrexone for opioid use disorder: a systematic review. Addiction. 2018;113:1188–1209. doi: 10.1111/add.14180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones C.M., Christensen A., Gladden R.M. Increases in prescription opioid injection abuse among treatment admissions in the United States, 2004-2013. Drug Alcohol Depend. 2017;176:89–95. doi: 10.1016/j.drugalcdep.2017.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kariisa M., Scholl L., Wilson N., Seth P., Hoots B. Drug overdose deaths involving cocaine and psychostimulants with abuse potential - United States, 2003-2017. MMWR Morb. Mortal. Wkly. Rep. 2019;68:388–395. doi: 10.15585/mmwr.mm6817a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellermeyer L., Harnke B., Knight S. Covidence and Rayyan. J. Med. Libr. Assoc. 2018;106:580–583. [Google Scholar]
- Kelly M.M., Reilly E., Quiñones T., Desai N., Rosenheck R. Long-acting intramuscular naltrexone for opioid use disorder: utilization and association with multi-morbidity nationally in the Veterans Health Administration. Drug Alcohol Depend. 2018;183:111–117. doi: 10.1016/j.drugalcdep.2017.10.017. [DOI] [PubMed] [Google Scholar]
- Kertesz S.G., Horton N.J., Friedmann P.D., Saitz R., Samet J.H. Slowing the revolving door: stabilization programs reduce homeless persons’ substance use after detoxification. J. Subst. Abuse Treat. 2003;24:197–207. doi: 10.1016/s0740-5472(03)00026-6. [DOI] [PubMed] [Google Scholar]
- Kissin W., McLeod C., Sonnefeld J., Stanton A. Experiences of a national sample of qualified addiction specialists who have and have not prescribed buprenorphine for opioid dependence. J. Addict. Dis. 2006;25:91–103. doi: 10.1300/J069v25n04_09. [DOI] [PubMed] [Google Scholar]
- Krawczyk N., Buresh M., Gordon M.S., Blue T.R., Fingerhood M.I., Agus D. Expanding low-threshold buprenorphine to justice-involved individuals through mobile treatment: addressing a critical care gap. J. Subst. Abuse Treat. 2019;103:1–8. doi: 10.1016/j.jsat.2019.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krawczyk N., Mojtabai R., Stuart E.A., Fingerhood M., Agus D., Lyons B.C., Weiner J.P., Saloner B. Opioid agonist treatment and fatal overdose risk in a state-wide US population receiving opioid use disorder services. Addiction. 2020;115:1683–1694. doi: 10.1111/add.14991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krull I., Lundgren L., Zerden L.S. Attitudes toward evidence-based pharmacological treatments among community-based addiction treatment programs targeting vulnerable patient groups. J. Addict. Dis. 2011;30:323–333. doi: 10.1080/10550887.2011.609808. [DOI] [PubMed] [Google Scholar]
- Kushel M.B., Vittinghoff E., Haas J.S. Factors associated with the health care utilization of homeless persons. JAMA. 2001;285:200–206. doi: 10.1001/jama.285.2.200. [DOI] [PubMed] [Google Scholar]
- Kushel M.B., Perry S., Bangsberg D., Clark R., Moss A.R. Emergency department use among the homeless and marginally housed: results from a community-based study. Am. J. Public Health. 2002;92:778–784. doi: 10.2105/ajph.92.5.778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushel M.B., Gupta R., Gee L., Haas J.S. Housing instability and food insecurity as barriers to health care among low-income Americans. J. Gen. Intern. Med. 2006;21:71–77. doi: 10.1111/j.1525-1497.2005.00278.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labelle C.T., Han S.C., Bergeron A., Samet J.H. Office-based opioid treatment with buprenorphine (OBOT-B): statewide implementation of the Massachusetts Collaborative Care Model in community health centers. J. Subst. Abuse Treat. 2016;60:6–13. doi: 10.1016/j.jsat.2015.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lashley M. An on-site health home for homeless men in addiction recovery. Public Health Nurs. 2019;36:184–191. doi: 10.1111/phn.12564. [DOI] [PubMed] [Google Scholar]
- Li K.J., Smedberg D.L., DeLisi L.E. A retrospective 4-year outcome study of veterans admitted to an acute inpatient detoxification unit for opioid use disorder. Am. J. Addict. 2019;28:318–323. doi: 10.1111/ajad.12893. [DOI] [PubMed] [Google Scholar]
- Lin W.C., Bharel M., Zhang J., O’Connell E., Clark R.E. Frequent emergency department visits and hospitalizations among homeless people with Medicaid: implications for Medicaid expansion. Am. J. Public Health. 2015;105:S716–S722. doi: 10.2105/AJPH.2015.302693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Los Angeles County Department of Public Health, Center for Health Impact Evaluation . 2021. Recent Trends in Mortality Rates and Causes of Death among People Experiencing Homelessness in Los Angeles County.http://publichealth.lacounty.gov/chie/reports/HomelessMortality2020_CHIEBrief_Final.pdf Accessed February 8, 2021. [Google Scholar]
- Lundgren L.M., Schilling R.F., Ferguson F., Davis K., Amodeo M. Examining drug treatment program entry of injection drug users: human capital and institutional disaffiliation. Eval. Program Plann. 2003;26:123–132. doi: 10.1016/S0149-7189(03)00013-2. [DOI] [PubMed] [Google Scholar]
- Magidson J.F., Jack H.E., Regenauer K.S., Myers B. Applying lessons from task sharing in global mental health to the opioid crisis. J. Consult. Clin. Psychol. 2019;87:962–966. doi: 10.1037/ccp0000434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marienfeld C., Rosenheck R.A. Psychiatric services and prescription fills among veterans with serious mental illness in methadone maintenance treatment. J. Dual Diagn. 2015;11:128–135. doi: 10.1080/15504263.2015.1025024. [DOI] [PubMed] [Google Scholar]
- Marshall J.R., Gassner S.F., Anderson C.L., Cooper R.J., Lotfipour S., Chakravarthy B. Socioeconomic and geographical disparities in prescription and illicit opioid-related overdose deaths in Orange County, California, from 2010-2014. Subst. Abus. 2019;40:80–86. doi: 10.1080/08897077.2018.1442899. [DOI] [PubMed] [Google Scholar]
- Masson C.L., Sorensen J.L., Batki S.L., Okin R., Delucchi K.L., Perlman D.C. Medical service use and financial charges among opioid users at a public hospital. Drug Alcohol Depend. 2002;66:45–50. doi: 10.1016/s0376-8716(01)00182-x. [DOI] [PubMed] [Google Scholar]
- Midboe A.M., Byrne T., Smelson D., Jasuja G., McInnes K., Troszak L.K. The opioid epidemic in veterans who were homeless or unstably housed. Health Aff. (Millwood). 2019;38:1289–1297. doi: 10.1377/hlthaff.2019.00281. [DOI] [PubMed] [Google Scholar]
- National Alliance to End Homelessness . 2020. State of Homelessness: 2020 Edition.https://endhomelessness.org/homelessness-in-america/homelessness-statistics/state-of-homelessness-2020/ Accessed February 15, 2021. [Google Scholar]
- New York Department of Health and Mental Hygiene . 2021. Fifteenth Annual Report on Deaths Among Persons Experiencing Homelessness (July 1, 2019 – June 30, 2020)https://a860-gpp.nyc.gov/concern/nyc_government_publications/pz50gz035 Accessed February 8, 2021. [Google Scholar]
- Nyamathi A., Longshore D., Galaif E.R., Leake B. Motivation to stop substance use and psychological and environmental characteristics of homeless women. Addict. Behav. 2004;29:1839–1843. doi: 10.1016/j.addbeh.2004.03.032. [DOI] [PubMed] [Google Scholar]
- Nyamathi A., Branson C., Kennedy B., Salem B., Khalilifard F., Marfisee M., Getoff D., Leake B. Impact of nursing intervention on decreasing substances among homeless youth. Am. J. Addict. 2012;21:558–565. doi: 10.1111/j.1521-0391.2012.00288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyamathi A., Reback C.J., Shoptaw S., Salem B.E., Zhang S., Yadav K. Impact of tailored interventions to reduce drug use and sexual risk behaviors among homeless gay and bisexual men. Am. J. Mens Health. 2017;11:208–220. doi: 10.1177/1557988315590837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padgett D.K., Stanhope V., Henwood B.F., Stefancic A. Substance use outcomes among homeless clients with serious mental illness: comparing Housing First with Treatment First programs. Community Ment. Health J. 2011;47:227–232. doi: 10.1007/s10597-009-9283-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel P., Ramaswamy R., Mardam Bey R. Persisting gaps in M-OUD coverage at post-discharge recovery houses necessitate our continued advocacy. Subst. Abus. 2020;41:11–13. doi: 10.1080/08897077.2019.1695038. [DOI] [PubMed] [Google Scholar]
- Petticrew M., Anderson L., Elder R., Grimshaw J., Hopkins D., Hahn R., Krause L., Kristjansson E., Mercer S., Sipe T., Tugwell P., Ueffing E., Waters E., Welch V. Complex interventions and their implications for systematic review: a pragmatic approach. Int. J. Nurs. Stud. 2015;52:1211–1216. doi: 10.1016/j.ijnurstu.2015.01.004. [DOI] [PubMed] [Google Scholar]
- Popay J., Roberts H., Sowden A., Petticrew M., Arai L., Rodgers M., Britten N., Roen K., Duffy S. 2006. Guidance on the Conduct of Narrative Synthesis in Systematic Reviews. A Product From the ESRC Methods Programme. Version 1.https://www.lancaster.ac.uk/media/lancaster-university/content-assets/documents/fhm/dhr/chir/NSsynthesisguidanceVersion1-April2006.pdf Accessed February 13, 2021. [Google Scholar]
- Pro G., Zaller N. Interaction effects in the association between methadone maintenance therapy and experiences of racial discrimination in U.S. Healthcare settings. PLoS One. 2020;15 doi: 10.1371/journal.pone.0228755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynoso-Vallejo H., Chassler D., Witas J., Lundgren L.M. Patterns of drug treatment entry by Latino male injection drug users from different national/geographical backgrounds. Eval. Program Plann. 2008;31:92–101. doi: 10.1016/j.evalprogplan.2007.05.011. [DOI] [PubMed] [Google Scholar]
- Riggins D.P., Cunningham C.O., Ning Y., Fox A.D. Recent incarceration and buprenorphine maintenance treatment outcomes among human immunodeficiency virus-positive patients. Subst. Abus. 2017;38:297–302. doi: 10.1080/08897077.2016.1220443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivers P.A., Dobalian A., Oyana T.J., Bae S. Socioeconomic determinants of planned methadone treatment. Am. J. Health Behav. 2006;30:451–459. doi: 10.5555/ajhb.2006.30.5.451. [DOI] [PubMed] [Google Scholar]
- Robbins J.L., Wenger L., Lorvick J., Shiboski C., Kral A.H. Health and oral health care needs and health care-seeking behavior among homeless injection drug users in San Francisco. J. Urban Health. 2010;87:920–930. doi: 10.1007/s11524-010-9498-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts K., Dowell A., Nie J.B. Attempting rigour and replicability in thematic analysis of qualitative research data; a case study of codebook development. BMC Med. Res. Methodol. 2019;19:66. doi: 10.1186/s12874-019-0707-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose-Jacobs R., Trevino-Talbot M., Vibbert M., Lloyd-Travaglini C., Cabral H.J. Pregnant women in treatment for opioid use disorder: material hardships and psychosocial factors. Addict. Behav. 2019;98 doi: 10.1016/j.addbeh.2019.106030. [DOI] [PubMed] [Google Scholar]
- Royse D., Leukefeld C., Logan T.K., Dennis M., Wechsberg W., Hoffman J., Cottler L., Inciardi J. Homelessness and gender in out-of-treatment drug users. Am. J. Drug Alcohol Abuse. 2000;26:283–296. doi: 10.1081/ada-100100605. [DOI] [PubMed] [Google Scholar]
- Shah N.G., Celentano D.D., Vlahov D., Stambolis V., Johnson L., Nelson K.E., Strathdee S.A. Correlates of enrollment in methadone maintenance treatment programs differ by HIV-serostatus. AIDS. 2000;14:2035–2043. doi: 10.1097/00002030-200009080-00020. [DOI] [PubMed] [Google Scholar]
- Shover C. Availability of extended-release buprenorphine to treat opioid use disorders among Medicaid-covered patients. Psychiatr. Serv. 2021;72:225–226. doi: 10.1176/appi.ps.202000165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon C.B., Tsui J.I., Merrill J.O., Adwell A., Tamru E., Klein J.W. Linking patients with buprenorphine treatment in primary care: predictors of engagement. Drug Alcohol Depend. 2017;181:58–62. doi: 10.1016/j.drugalcdep.2017.09.017. [DOI] [PubMed] [Google Scholar]
- Sordo L., Barrio G., Bravo M.J., Indave B.I., Degenhardt L., Wiessing L., Ferri M., Pastor-Barriuso R. Mortality risk during and after opioid substitution treatment: systematic review and meta-analysis of cohort studies. BMJ. 2017;357:j1550. doi: 10.1136/bmj.j1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stancliff S., Joseph H., Fong C., Furst T., Comer S.D., Roux P. Opioid maintenance treatment as a harm reduction tool for opioid-dependent individuals in New York City: the need to expand access to buprenorphine/naloxone in marginalized populations. J. Addict. Dis. 2012;31:278–287. doi: 10.1080/10550887.2012.694603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein M.D., Bailey G.L., Thurmond P., Paull N. Looking for the uninsured in Massachusetts? Check opioid dependent persons seeking detoxification. Drug Alcohol Depend. 2014;136:166–169. doi: 10.1016/j.drugalcdep.2013.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein M.D., Anderson B.J., Bailey G.L. Preferences for aftercare among persons seeking short-term opioid detoxification. J. Subst. Abuse Treat. 2015;59:99–103. doi: 10.1016/j.jsat.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein M.D., Flori J.N., Risi M.M., Conti M.T., Anderson B.J., Bailey G.L. Overdose history is associated with postdetoxification treatment preference for persons with opioid use disorder. Subst. Abus. 2017;38:389–393. doi: 10.1080/08897077.2017.1353570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timko C., Gupta S., Schultz N., Harris A.H.S. Veterans’ service utilization patterns after alcohol and opioid detoxification in VHA Care. Psychiatr. Serv. 2016;67:460–464. doi: 10.1176/appi.ps.201400579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobolowsky F.A., Gonzales E., Self J.L., Rao C.Y., Keating R., Marx G.E., McMichael T.M., Lukoff M.D., Duchin J.S., Huster K., Rauch J., McLendon H., Hanson M., Nichols D., Pogosjans S., Fagalde M., Lenahan J., Maier E., Whitney H., Sugg N., Chu H., Rogers J., Mosites E., Kay M. COVID-19 outbreak among three affiliated homeless service sites – King County, Washington, 2020. MMWR Morb. Mortal. Wkly. Rep. 2020;69:523–526. doi: 10.15585/mmwr.mm6917e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tofighi B., Williams A.R., Chemi C., Suhail-Sindhu S., Dickson V., Lee J.D. Patient barriers and facilitators to medications for opioid use disorder in primary care. Subst. Use Misuse. 2019;54:2409–2419. doi: 10.1080/10826084.2019.1653324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tricco A.C., Lillie E., Zarin W., O’Brien K.K., Colquohoun H., Levac D., Moher D., Peters M.D.J., Horsley T., Weeks L., Hempel S., Akl E.A., Chang C., McGowan J., Stewart L., Hartling L., Aldcroft A., Wilson M.G., Garritty C., Lewin S., Godfrey C.M., Macdonald M.T., Langlois E.V., Soares-Weiser K., Moriarty J., Clifford T., Tunçalp Ö., Straus S.E. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann. Intern. Med. 2018;169:467–473. doi: 10.7326/M18-0850. [DOI] [PubMed] [Google Scholar]
- Tringale R., Subica A.M., Danielian A., Kaplan C. The stepped treatment engagement protocol for homeless, needle exchange heroin-dependent patients. J. Addict. Med. 2015;9:163–164. doi: 10.1097/ADM.0000000000000096. [DOI] [PubMed] [Google Scholar]
- Tsemberis S.J., Moran L., Shinn M., Asmussen S.M., Shern D.L. Consumer preference programs for individuals who are homeless and have psychiatric disabilities: a drop-in center and a supported housing program. Am. J. Community Psychol. 2003;32:305–317. doi: 10.1023/b:ajcp.0000004750.66957.bf. [DOI] [PubMed] [Google Scholar]
- Upshur C.C., Jenkins D., Weinreb L., Gelberg L., Orvek E.A. Homeless women’s service use, barriers, and motivation for participating in substance use treatment. Am. J. Drug Alcohol Abuse. 2018;44:252–262. doi: 10.1080/00952990.2017.1357183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Ness P.H., Davis W.R., Johnson B.D. Socioeconomic marginality and health services utilization among Central Harlem substance users. Subst. Use Misuse. 2004;39:61–85. doi: 10.1081/ja-120027766. [DOI] [PubMed] [Google Scholar]
- Velasquez M., Flannery M., Badolato R., Vittitow A., McDonald R.D., Tofighi B., Garment A.R., Giftos J., Lee J.D. Perceptions of extended-release naltrexone, methadone, and buprenorphine treatments following release from jail. Addict. Sci. Clin. Pract. 2019;14:37. doi: 10.1186/s13722-019-0166-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakeman S.E., Larochelle M.R., Ameli O., Chaisson C.E., McPheeters J.T., Crown W.H., Azocar F., Sanghavi D.M. Comparative effectiveness of different treatment pathways for opioid use disorder. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2019.20622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walley A.Y., Alperen J.K., Cheng D.M., Botticelli M., Castro-Donlan C., Samet J.H., Alford D.P. Office-based management of opioid dependence with buprenorphine: clinical practices and barriers. J. Gen. Intern. Med. 2008;23:1393–1398. doi: 10.1007/s11606-008-0686-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Weiss J., Bogel Ryan E., Waldman J., Rubin S., Griffin J.L. Telemedicine increases access to buprenorphine initiation during the COVID-19 pandemic. J. Subst. Abuse Treat. 2021;124 doi: 10.1016/j.jsat.2020.108272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesson D.R., Smith D.E. Buprenorphine in the treatment of opiate dependence. J. Psychoactive Drugs. 2010;42:161–175. doi: 10.1080/02791072.2010.10400689. [DOI] [PubMed] [Google Scholar]
- Wilson N., Kariisa M., Seth P., Smith H., Davis N.L. Drug and opioid-involved overdose deaths – United States, 2017-2018. MMWR Morb. Mortal. Wkly. Rep. 2020;69:290–297. doi: 10.15585/mmwr.mm6911a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto A., Needleman J., Gelberg L., Kominski G., Shoptaw S., Tsugawa Y. Association between homelessness and opioid overdose and opioid-related hospital admissions/emergency department visits. Soc. Sci. Med. 2019;242 doi: 10.1016/j.socscimed.2019.112585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlotnick C., Zerger S., Wolfe P.B. Health care for the homeless: what we have learned in the past 30 years and what’s next. Am. J. Public Health. 2013;103:S199–S205. doi: 10.2105/AJPH.2013.301586. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.