Abstract
Introduction
This post hoc analysis evaluated influenza adverse events (AEs) across rheumatoid arthritis (RA), ulcerative colitis (UC), and psoriatic arthritis (PsA) tofacitinib clinical programs.
Methods
Available data from phase 1, randomized phase 2/3/3b/4 clinical trials (completed by 2018), and long-term extension (LTE) studies (up to May 2019) in patients with RA, UC, and PsA were included [randomized or Overall (phase 1–3b/4 and LTE studies) tofacitinib cohorts]. Incidence rates (IRs; events per 100 patient-years) of combined influenza AEs (seasons 2004/2005 to 2018/2019) were analyzed, including by tofacitinib dose [5 or 10 mg twice daily (BID)] and age (< 65 versus ≥ 65 years). Logistic regression models evaluated risk factors for influenza AEs in the RA Overall tofacitinib cohort.
Results
In randomized cohorts, combined influenza AE IRs were generally similar across tofacitinib, adalimumab, methotrexate, and placebo groups, across indications. Among Overall tofacitinib cohorts, combined influenza AE IRs with tofacitinib 5/10 mg BID, respectively, were higher in the UC (3.66/5.09) versus RA (2.38/2.19) and PsA (1.74/1.29) cohorts. IRs were generally similar across tofacitinib dose and age groups. Most influenza AEs were nonserious and did not require changes to tofacitinib treatment. Significant risk factors for influenza AEs in patients with RA were geographic region, baseline oral corticosteroid and methotrexate use, and tofacitinib dose.
Conclusions
In the RA, UC, and PsA clinical programs, combined influenza AE IRs were highest in UC, while in each indication they were generally similar across tofacitinib, placebo, and comparator groups. Influenza AEs were predominantly nonserious and not associated with changes to tofacitinib treatment.
Trial Registration Numbers
NCT01262118, NCT01484561, NCT00147498, NCT00413660, NCT00550446, NCT00603512, NCT00687193, NCT01164579, NCT00976599, NCT01059864, NCT01359150, NCT02147587, NCT00960440, NCT00847613, NCT00814307, NCT00856544, NCT00853385, NCT01039688, NCT02281552, NCT02187055, NCT02831855, NCT00413699, NCT00661661, NCT00787202, NCT01465763, NCT01458951, NCT01458574, NCT01470612, NCT01877668, NCT01882439, NCT01976364.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40744-022-00507-z.
Keywords: Ulcerative colitis, Rheumatoid arthritis, Psoriatic arthritis, JAK inhibitor, Safety, Tofacitinib, Viral infection, Influenza
Key Summary Points
| Why carry out this study? |
| Influenza is a major cause of global morbidity and mortality. Patients with immune-mediated inflammatory diseases, such as rheumatoid arthritis (RA), ulcerative colitis (UC), and psoriatic arthritis (PsA), who often require long-term immunosuppressive treatment, may be at a higher risk of influenza and its complications. |
| Tofacitinib is a Janus kinase inhibitor for the treatment of RA, UC, and PsA. The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic has further heightened the need to better understand the overall risk of acute respiratory viral infections, including influenza, in patients with immune-mediated inflammatory diseases receiving tofacitinib. |
| This post hoc analysis summarized influenza adverse events (AEs) in patients with RA, UC, and PsA in the tofacitinib clinical program over influenza seasons 2004/2005 to 2018/2019. |
| What was learned from the study? |
| Combined influenza AE incidence rates (IRs; including all reported influenza AEs, influenza complication AEs, and influenza-like illness) were highest in patients with UC versus patients with RA and PsA who received ≥ 1 dose of tofacitinib in the clinical trial program, and generally similar across age (< 65 versus ≥ 65 years) and tofacitinib dose (5 or 10 mg twice daily). Within indications, IRs were generally similar between tofacitinib, placebo, and active comparator treatment groups. Influenza AEs were predominantly nonserious and not associated with changes in tofacitinib treatment. |
| In tofacitinib-treated patients, risk of influenza and its associated complications is similar to that observed in the general population. |
Introduction
The influenza virus is a major global cause of morbidity and mortality, estimated to infect one billion people annually [1, 2]. Approximately 3–5 million people worldwide develop severe influenza, resulting in 290,000–650,000 deaths per annum [3]. Patients with immune-mediated inflammatory diseases, such as rheumatoid arthritis (RA) [4], ulcerative colitis (UC) [5], and psoriatic arthritis (PsA) [6], often require long-term immunosuppressive treatment, which may increase their risk of influenza and its complications [7]. Indeed, compared with controls, patients with RA have an approximately 1.3- and 2.8-fold increase in influenza and influenza-related complications, respectively [8], while patients with UC and Crohn’s disease have a 1.5-fold increased influenza risk and significantly higher hospitalization rates [9].
Tofacitinib is a Janus kinase inhibitor for the treatment of RA, UC, and PsA. In the context of the SARS-CoV-2 pandemic, a known acute respiratory RNA viral infection, there is a need to better understand the overall risk of these infections, including influenza, in patients with immune-mediated inflammatory diseases receiving tofacitinib.
The objective of this post hoc analysis was to summarize influenza adverse events (AEs) in the tofacitinib RA, UC, and PsA clinical programs.
Methods
Study Design and Patient Cohorts
Table S1 (electronic supplementary material) presents a list of studies included in this analysis. Studies were conducted in accordance with the Declaration of Helsinki, Good Clinical Practice Guidelines, and applicable local country regulations and laws. Study protocols were approved by the institutional review boards and/or independent ethics committee at each center. All patients provided written, informed consent. No further ethical approval was required for this post hoc analysis in accordance with the policies of our institutions.
We analyzed RA clinical program data in two cohorts: (1) phase 2–3b/4 cohort, comprising 6690 patients who [as monotherapy or with background conventional synthetic disease-modifying antirheumatic drugs (csDMARDs)] received tofacitinib 5 (N = 2664) or 10 mg twice daily (BID; N = 2024), adalimumab (N = 643), methotrexate (N = 223), or placebo (N = 1136) in phase 2–3b/4 studies; and (2) Overall tofacitinib cohort, comprising 7964 patients who received ≥ 1 tofacitinib dose across the phase 1–3b/4 and open-label long-term extension (LTE) studies (average tofacitinib 5 mg BID, N = 3969; average tofacitinib 10 mg BID, N = 3995).
The UC clinical program was analyzed as three cohorts: (1) phase 2/3 induction cohort, comprising 1220 patients who received tofacitinib 10 mg BID (N = 938) or placebo (N = 282) in 8-week phase 2 and phase 3 induction studies; (2) phase 3 maintenance cohort, comprising 592 patients who completed the phase 3 induction studies with a clinical response and received tofacitinib 5 (N = 198) or 10 mg BID (N = 196), or placebo (N = 198) in a 52-week phase 3 maintenance study; and (3) Overall tofacitinib cohort, comprising 1157 patients who received ≥ 1 tofacitinib dose (average tofacitinib 5 mg BID, N = 198; average tofacitinib 10 mg BID, N = 959) across the phase 2/3 and LTE studies.
The PsA clinical program was analyzed as three cohorts: (1) phase 3 placebo-controlled cohort, comprising 710 patients who received (with a background csDMARD) tofacitinib 5 (N = 238) or 10 mg BID (N = 236), or placebo (N = 236) up to month 3, in phase 3 studies; (2) phase 3 active-controlled cohort, comprising 797 patients who received (with a background csDMARD) tofacitinib 5 (N = 347) or 10 mg BID (N = 344), or adalimumab (N = 106) up to month 12 in phase 3 studies; and (3) Overall tofacitinib cohort including 783 patients who received ≥ 1 tofacitinib dose in the phase 3 and LTE studies (average tofacitinib 5 mg BID, N = 458; average tofacitinib 10 mg BID, N = 325).
Definition and Analysis of Influenza AEs
Combined influenza AEs [identified by investigator-reported Medical Dictionary for Regulatory Activities (MedDRA) terms] were assessed across influenza seasons 2004/2005 to 2018/2019. It is important to note distinctions between classification of influenza AEs in clinical practice versus clinical trials that may impact the interpretation of these data. In clinical practice and public health, the term influenza-like illness (ILI) is used to denote both laboratory-confirmed influenza cases plus unconfirmed cases that clinically appear like influenza [10]. In this analysis, MedDRA version 22.0 terms are intended to distinguish between confirmed cases of influenza and ILI. Combined influenza AEs included all reported influenza AEs, influenza complication AEs, and ILI (each a composite of MedDRA version 22.0 preferred and verbatim terms; Table S2). Nonserious and serious influenza AEs, non-influenza serious AEs (SAEs) occurring within 28 days after combined influenza AE onset, and time to resolution of combined influenza AEs by action taken to tofacitinib treatment were summarized descriptively. Patients with serious infections discontinued the trials per protocol. Patient-reported influenza vaccination status (RA and UC cohorts only) and antiviral use during combined influenza AEs were assessed in the Overall tofacitinib cohorts.
Incidence rates (IRs) of respiratory infections that may overlap with influenza in clinical presentation (based on MedDRA version 22.0 preferred and verbatim terms relating to viral and nonviral respiratory infections; Supplementary Methods, Table S3) were analyzed in the RA phase 2–3b/4 cohort and UC phase 2/3 induction and phase 3 maintenance cohorts (PsA cohorts were not included owing to few events).
Seasonal Influenza Vaccination
In the RA and UC cohorts, seasonal influenza vaccinations from the study start and within 12 months prior to combined influenza AEs were identified from a database and stratified by status of combined influenza AE (yes/no). For patients without combined influenza AEs, any influenza vaccine received during the study period was recorded. Data on vaccination status were not collected systematically.
Statistical Analyses
IRs for combined influenza AEs (encompassing all reported influenza AEs, influenza complication AEs, and ILI) were defined as the number of unique patients (per 100 patient-years of exposure) with any of these events during the time between first and last dose plus 28 days, divided by the patient time accrued between first and last dose plus 28 days, or up to first event, data cutoff, or progression to next study, whichever occurred earlier. IR 95% confidence intervals (CIs) were based on the Exact Poisson method, adjusted for exposure time. For patients who died before/at data cutoff, exposure time was up to the minimum of the date of the last dose plus 28 days, date of first event, or date of death. For time to resolution of combined influenza AEs by action taken in patients with multiple influenza overall AEs, an event was selected according to the order of action taken (permanent discontinuation, temporary discontinuation, dose reduction, no change); for events with the same action taken, the first event was selected. For descriptive analyses of AE rates, the most serious (“serious” > “nonserious”) or severe event (“severe” > “moderate” > “mild”) was chosen in patients with recurrent influenza AEs.
IRs were calculated by randomized tofacitinib dose (randomized phase 2/3/3b/4 cohorts; Table S1) or by average tofacitinib dose (Overall tofacitinib cohorts; average tofacitinib 5 or 10 mg BID based on the average total daily dose of < 15 or ≥ 15 mg, respectively). IRs for influenza AEs were also stratified by patient age (< 65 versus ≥ 65 years; RA and UC Overall cohorts only owing to low numbers of events in the PsA cohort).
Univariate and multivariable regression analyses were used to explore risk factors for combined influenza AEs and recurrent influenza AEs in the RA Overall tofacitinib cohort (Supplementary Methods). Other cohorts could not be analyzed owing to low numbers of events.
Results
Baseline Demographics and Disease Characteristics in the Overall Tofacitinib Cohorts
Baseline demographics and disease characteristics for the Overall tofacitinib cohorts are presented in Table 1 and Table S4. On average, patients from the RA and PsA cohorts were older [mean (standard deviation) age 52.6 (12.1) and 48.7 (12.0) years, respectively] than the UC cohort [41.3 (13.9) years]. The prevalence of obesity and diabetes was also higher in the RA (26.8% and 8.2%, respectively) and PsA (42.5% and 13.7%) cohorts versus the UC cohort (13.8% and 4.1%). Patient-reported influenza vaccination rates (based on data recorded throughout the study period) in the RA and UC cohorts were low (9.1% and 5.4%, respectively), suggesting incomplete vaccination data.
Table 1.
Baseline demographics and clinical characteristics of all tofacitinib patients in the RA, UC, and PsA Overall tofacitinib cohorts
| RA | UC | PsA | |
|---|---|---|---|
| All tofacitinib (N = 7964) | All tofacitinib (N = 1157) | All tofacitinib (N = 783) | |
| Age, years, mean (SD) | 52.6 (12.1) | 41.3 (13.9) | 48.7 (12.0) |
| ≥ 65 years, n (%) | 1270 (15.9) | 77 (6.7) | 72 (9.2) |
| Female, n (%) | 6522 (81.9) | 478 (41.3) | 428 (54.7) |
| BMI, kg/m2, mean (SD) | 27.1 (6.4) | 24.8 (5.0)a | 29.6 (6.0) |
| Obesity (BMI ≥ 30 kg/m2), n (%) | 2138 (26.8)b | 159 (13.8)a | 333 (42.5) |
| Smoking status, n (%) | |||
| Never smoked | 4996 (62.7) | 740 (64.0) | 485 (61.9) |
| Current smoker | 1366 (17.2) | 59 (5.1) | 140 (17.9) |
| Ex-smoker | 1388 (17.4) | 357 (30.9) | 158 (20.2) |
| Missing/unknown | 214 (2.7) | 1 (0.1) | 0 (0.0) |
| Disease duration, years, mean (SD) | 8.1 (8.2) | 8.2 (7.0) | 7.7 (7.2) |
| Concomitant oral corticosteroid use, n (%)c | 4254 (53.4) | 523 (45.2) | 171 (21.8) |
| Diabetes, n (%)d | 651 (8.2) | 48 (4.1) | 107 (13.7) |
| Hypertension, n (%)d | 2818 (35.4) | 161 (13.9) | 299 (38.2) |
| Coronary heart disease, n (%)d | 30 (< 1.0) | 22 (1.9) | 39 (5.0) |
| Influenza vaccination, n (%)e | 722 (9.1) | 62 (5.4) | N/A |
| RF-positive, n (%) | 5146 (71.1) | N/A | 36 (4.6) |
| ACPA-positive, n (%) | 3723 (46.7) | N/A | 36 (4.6) |
| Prior TNFi treatment, n (%) | 1245 (15.6) | 612 (54.4) | 377 (48.1) |
ACPA anti-citrullinated protein antibody, AE adverse event, BID twice daily, BMI body mass index, n number of patients with the specified characteristic, N number of evaluable patients, NA not applicable, PsA psoriatic arthritis, RA rheumatoid arthritis, RF rheumatoid factor, SD standard deviation, TNFi tumor necrosis factor inhibitor, UC ulcerative colitis
aN = 1156
bData were missing for 10 patients
cData based on day 1 of active tofacitinib treatment in the RA/UC/PsA clinical development program
dData based on patient medical history
ePatient-reported data on influenza vaccines received within 12 months prior to combined influenza AEs, or if without combined influenza AEs, any influenza vaccine received during the study period
IRs of Combined Influenza AEs in the Randomized Clinical Trial Cohorts
In the RA phase 2–3b/4 cohort, IRs of combined influenza AEs with tofacitinib 10 mg BID and placebo were generally numerically higher versus tofacitinib 5 mg BID, adalimumab, and methotrexate (Fig. 1a); IRs were generally similar with tofacitinib 5 mg BID, adalimumab, and methotrexate.
Fig. 1.
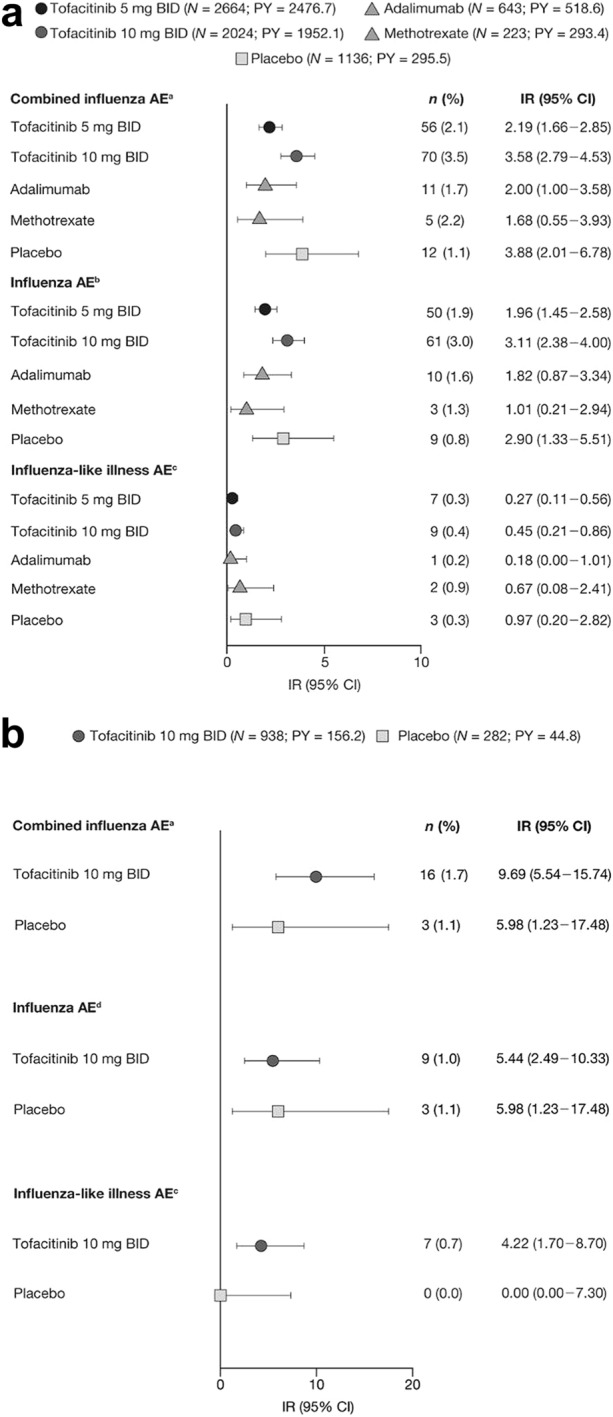

IRs (95% CI) of combined influenza AEs, influenza AEs, and influenza-like illness in the a RA phase 2–3b/4 cohort, b UC phase 2/3 induction cohort, c UC phase 3 maintenance cohort, d PsA placebo-controlled cohort, and e PsA active-controlled cohort. aNo events of influenza complication AEs were reported. bIncludes H1N1, reported in three (0.1%) patients receiving tofacitinib 5 mg BID [IR (95% CI) 0.12 (0.02–0.34)] and two (0.1%) patients receiving tofacitinib 10 mg BID [IR (95% CI) 0.10 (0.01–0.36)]. cNo events of VTs for influenza-like illness were reported. dNo events of avian, encephalitis, H1N1, H2N2, H3N2, pneumonia influenza, or VTs for influenza AEs were reported. PY are total patient-years of study-drug exposure. AE adverse event, BID twice daily, CI confidence interval, IR incidence rate (number of unique patients with events per 100 PY exposure), n number of unique patients with event, N number of patients assessed, PsA psoriatic arthritis, PY patient-years, RA rheumatoid arthritis, UC ulcerative colitis, VT verbatim term
The IR of combined influenza AEs was numerically higher with tofacitinib 10 mg BID versus placebo in the UC phase 2/3 induction cohort (Fig. 1b). IRs of combined influenza AEs were generally similar with both tofacitinib doses and placebo in the UC phase 3 maintenance cohort (Fig. 1c). Across both UC cohorts, 95% CIs were wide and overlapping.
In the PsA phase 3 placebo-controlled cohort, IRs of combined influenza AEs with to facitinib 5 and 10 mg BID were similar to that with placebo (Fig. 1d), although the small sample size precluded comparisons. In the PsA active-controlled cohort, IRs of combined influenza AEs were numerically higher with tofacitinib 5 and 10 mg BID versus adalimumab (Fig. 1e).
IRs of Combined Influenza AEs in the Overall Tofacitinib Cohorts
IRs of combined influenza AEs in the Overall tofacitinib cohorts were higher in the UC cohort than in the RA and PsA cohorts (Fig. 2a–c). IRs were similar across tofacitinib dose groups in the RA and PsA cohorts, while in the UC cohort, the IR of the average tofacitinib 10 mg BID group was numerically higher than that of the average tofacitinib 5 mg BID group (Fig. 2a–c).
Fig. 2.
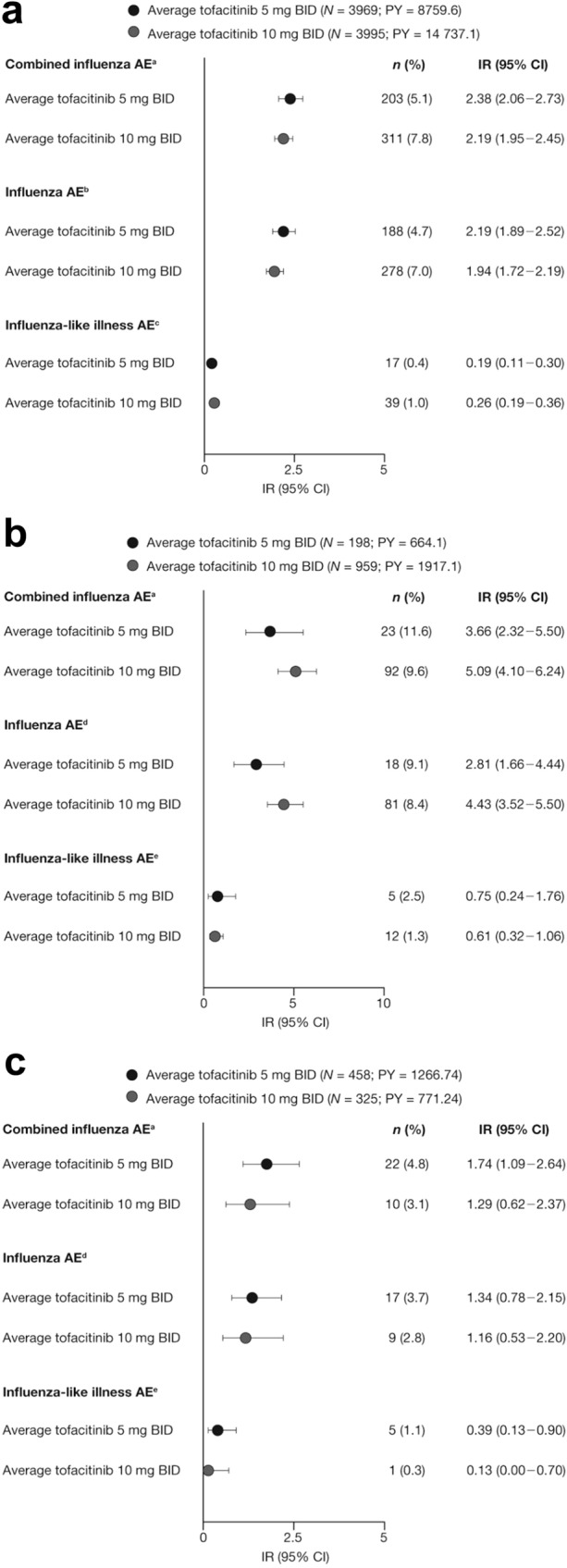
IRs of combined influenza AEs, influenza-like illness, and influenza AEs in the a RA, b UC, and c PsA Overall tofacitinib cohorts. aNo events of influenza complication AEs were reported. bIncludes H1N1, reported in two (0.1%) patients receiving average tofacitinib 5 mg BID [IR (95% CI) 0.02 (0.00–0.08)] and seven (0.2%) patients receiving average tofacitinib 10 mg BID [IR (95% CI) 0.05 (0.02–0.10)]. No events of avian, encephalitis, H2N2, H3N2, pneumonia influenza, or VTs for influenza AEs were reported. cIncludes two (0.1%) patients receiving average tofacitinib 10 mg BID who reported VTs for influenza-like illness. dNo events of avian, encephalitis, H1N1, H2N2, H3N2, pneumonia influenza, or VTs for influenza AEs were reported. eNo events of VTs for influenza-like illness were reported. PY are total patient-years of study-drug exposure. AE adverse event, BID twice daily, CI confidence interval, IR incidence rate (number of unique patients with events per 100 PY exposure), n number of unique patients with event during the risk period, N number of patients assessed, PsA psoriatic arthritis, PY patient-years, RA rheumatoid arthritis, UC ulcerative colitis, VT verbatim term
In the Overall RA tofacitinib cohort, H1N1 influenza was the only influenza subtype reported [IR 0.04 (95% CI, 0.02–0.07)]. In the tofacitinib UC and PsA clinical programs, no events of avian, encephalitis, H1N1, H2N2, H3N2, pneumonia influenza, or other influenza complication AEs were reported.
IRs of Combined Influenza AEs in RA and UC Overall Tofacitinib Cohorts, Stratified by Age
In the RA Overall tofacitinib cohort, when stratified by age (< 65 versus ≥ 65 years), IRs for combined influenza AEs were similar across average tofacitinib doses within and between age groups (Fig. 3a). In the UC Overall tofacitinib cohort, the IR of the average tofacitinib 10 mg BID group was numerically higher than that of the average tofacitinib 5 mg BID group in the < 65 years age group, while IRs were similar across average tofacitinib doses in the ≥ 65 years age group (Fig. 3b). Across both cohorts, the number of events in the ≥ 65 years age group was low.
Fig. 3.
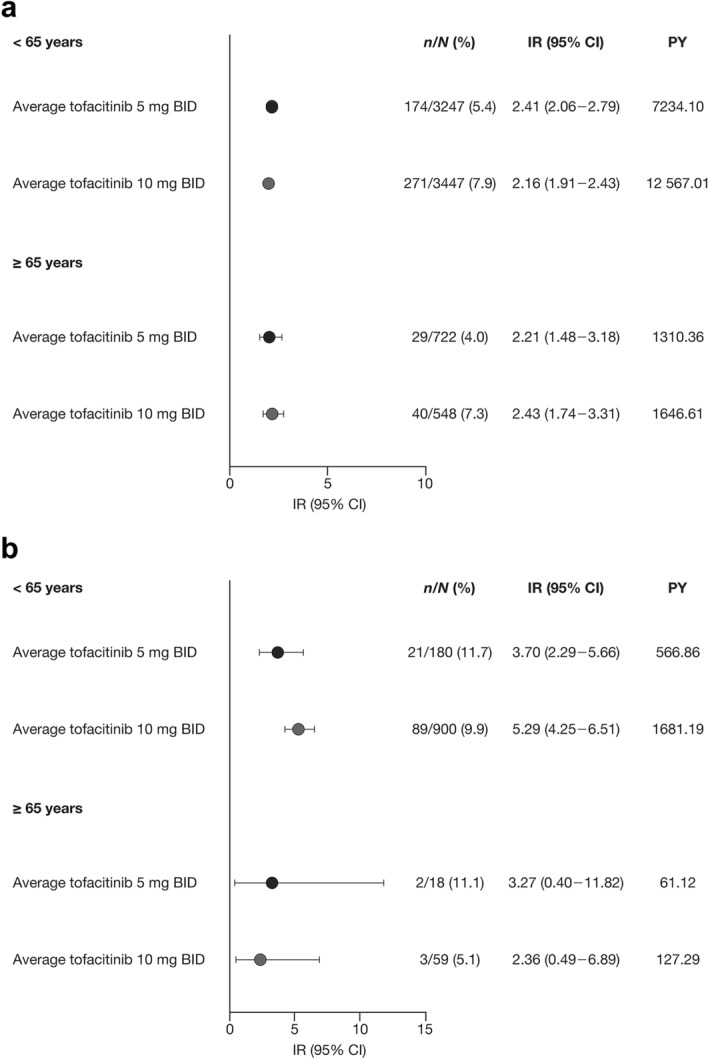
IRs of combined influenza AEs in the a RA and b UC Overall tofacitinib cohorts, stratified by age (< 65 years, ≥ 65 years). PY are total patient-years of study-drug exposure. AE adverse event, BID twice daily, CI confidence interval, IR incidence rate (number of unique patients with events per 100 PY), n number of unique patients with event, N number of patients analyzed, PY patient-years, RA rheumatoid arthritis, UC ulcerative colitis
Descriptive Analyses of Nonserious and Serious Combined Influenza AEs in the Overall Tofacitinib Cohorts
Of the 7964 patients in the RA Overall tofacitinib cohort, 517 patients (6.5%) reported combined influenza AEs, of which 9 (1.7%) were serious (Table 2). Of patients with serious influenza AEs, eight were hospitalized. Two hospitalized patients died; both had H1N1 infection. In total, 82/517 (15.9%) patients had ≥ 2 discrete influenza AEs [average tofacitinib 5 mg BID, n = 27/204 (13.2%); average tofacitinib 10 mg BID, n = 55/313 (17.6%)]. Overall, 12/517 patients (2.3%) had an SAE within 28 days of influenza event onset; the most common SAEs were acute respiratory distress syndrome (n = 2) and pneumonia (n = 2) (Table S5). In patients with combined influenza AEs, no change to tofacitinib treatment was made in 363/517 (70.2%) patients, and tofacitinib was stopped temporarily in 146/517 (28.2%), with a median time to resolution of 8.0 and 9.0 days, respectively. Median times to resolution of influenza AEs were generally similar irrespective of action taken with tofacitinib (Table 3).
Table 2.
Summary of combined influenza AEs in the RA, UC, and PsA Overall tofacitinib cohorts
| Average tofacitinib 5 mg BID | Average tofacitinib 10 mg BID | All tofacitinib | |
|---|---|---|---|
| RA | |||
| Any influenza AE (combined), n/N (%) | 204a/3969 (5.1) | 313a/3995 (7.8) | 517a/7964 (6.5) |
| Serious influenza AEs, n/N (%) | 6/204 (2.9) | 3/313 (1.0) | 9/517 (1.7) |
| Hospitalized | 6/6 (100.0) | 2/3 (66.7) | 8/9 (88.9) |
| Deathb | 1c/6 (16.7) | 1c/3 (33.3) | 2c/9 (22.2) |
| UC | |||
| Any influenza AE (combined), n/N (%) | 23/198 (11.6) | 92/959 (9.6) | 115/1157 (9.9) |
| Serious influenza AEs, n/N (%) | 0 (0.0) | 1/92 (1.1) | 1/115 (0.9) |
| Hospitalized | 0 (0.0) | 1/1 (100.0) | 1/1 (100.0) |
| Deathb | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| PsA | |||
| Any influenza AE (combined), n/N (%) | 23d/458 (5.0) | 10/325 (3.1) | 33d/783 (4.2) |
| Serious influenza AEs, n/N (%) | 2/23 (8.7) | 0 (0.0) | 2/33 (6.1) |
| Hospitalized | 2/2 (100) | 0 (0.0) | 2/2 (100) |
| Deathb | 0 (0.0) | 0 (0.0) | 0 (0.0) |
AE adverse event, BID twice daily, n number of unique patients with event, N number of patients assessed, PsA psoriatic arthritis, RA rheumatoid arthritis, UC ulcerative colitis
aOne patient in the average tofacitinib 5 mg BID group and two patients in the average tofacitinib 10 mg BID group reported influenza AEs outside the risk period (28 days beyond last dose)
bWithin 30 days of a combined influenza AE
cPatients had H1N1 infection
dOne patient in the average tofacitinib 5 mg BID group reported influenza AEs outside the risk period (28 days beyond last dose)
Table 3.
Time to resolution of influenza AEs by action taken with tofacitinib in the RA, UC, and PsA Overall tofacitinib cohorts
| Change to tofacitinib treatmenta | Average tofacitinib 5 mg BID | Average tofacitinib 10 mg BID | All tofacitinib | |||
|---|---|---|---|---|---|---|
| n/N (%) | Time to resolution,b days, median (Q1, Q3) | n/N (%) | Time to resolution,b days, median (Q1, Q3) | n/N (%) | Time to resolution,b days, median (Q1, Q3) | |
| RA | ||||||
| No change | 137/204 (67.2) |
8.0 (6.0, 12.0) |
226/313c (72.2) |
8.0 (6.0, 12.0) |
363/517c (70.2) |
8.0 (6.0, 12.0) |
| Dose reduction | 2/204 (1.0) |
5.5 (4.0, 7.0) |
4/313 (1.3) |
11.5 (7.5, 15.0) |
6/517 (1.2) |
7.5 (7.0, 15.0) |
| Temporary discontinuationd | 64/204c (31.4) |
8.0 (6.0, 13.0) |
82/313 (26.2) |
10.0 (6.0, 14.0) |
146/517c (28.2) |
9.0 (6.0, 14.0) |
| Permanent discontinuation | 1/204 (0.5) |
26.0 (26.0, 26.0) |
1/313 (0.3) |
15.0 (15.0, 15.0) |
2/517 (0.4) |
20.5 (15.0, 26.0) |
| UC | ||||||
| No change | 22/23 (95.6) |
10.5 (6.0, 16.0) |
86/92 (93.5) |
8.0 (4.0, 14.0) |
108/115 (93.9) |
8.0 (5.0, 14.0) |
| Dose reduction | 0/23 (0.0) | – | 0/92 (0.0) | – | 0/115 (0.0) | – |
| Temporary discontinuatione | 1/23 (4.3) |
5.0 (5.0, 5.0) |
5/92 (5.4) |
8.0 (7.0, 9.0) |
6/115 (5.2) |
7.5 (5.0, 9.0) |
| Permanent discontinuation | 0/23 (0.0) | – | 1/92 (1.1) | 10.0 (10.0, 10.0) | 1/115 (0.9) |
10.0 (10.0, 10.0) |
| PsA | ||||||
| No change | 18/23 (78.3) |
11.5 (8.0, 22.0) |
8/10 (80.0) |
5.5 (4.5, 11.5) |
26/33 (78.8) |
10.0 (6.0, 16.0) |
| Dose reduction | 0/23 (0.0) | – | 0/10 (0.0) | – | 0/33 (0.0) | – |
| Temporary discontinuation | 4/23 (17.4) |
7.5 (5.0, 15.5) |
2/10 (20.0) |
30.5 (22.0, 39.0) |
6/33 (18.2) |
15.0 (7.0, 23.0) |
| Permanent discontinuation | 1/23 (4.3) |
8.0 (8.0, 8.0) |
0/10 (0.0) | – | 1/33 (3.0) |
8.0 (8.0, 8.0) |
AE adverse event, BID twice daily, n number of unique patients with action taken, N number of patients with an influenza AE, PsA psoriatic arthritis, Q1 25th percentile, Q3 75th percentile, RA rheumatoid arthritis, UC ulcerative colitis
aFor patients with multiple events, the worst event was selected according to the level of action taken. In case of multiple events with the same level of action taken, the first occurred event was selected
bTime to resolution was calculated as the days between the event start date and the resolved date. For resolved events with missing stop date, and for ongoing events or events with unknown outcome, the duration was calculated using the imputed resolved date
cIncludes one fatality due to H1N1 influenza
dTofacitinib treatment was stopped temporarily for a mean duration of 11.6 (average tofacitinib 5 mg BID), 10.6 (average tofacitinib 10 mg BID), and 11.0 (all tofacitinib) days
eTofacitinib treatment was stopped temporarily for a mean duration of 4.0 (average tofacitinib 5 mg BID), 2.0 (average tofacitinib 10 mg BID), and 3.7 (all tofacitinib) days
Of the 1157 patients in the UC Overall tofacitinib cohort, 115 patients (9.9%) reported combined influenza AEs, of which one (0.9%) was serious (Table 2), and one (0.9%) had an SAE within 28 days of the onset of an influenza event (ureter obstruction caused by a blood clot). In total, 24/115 (20.9%) patients with influenza AEs had ≥ 2 discrete episodes of influenza AEs [average tofacitinib 5 mg BID, n = 7/23 (30.4%); average tofacitinib 10 mg BID, n = 17/92 (18.5%)]. In 108/115 (93.9%) patients with combined influenza AEs, no change to tofacitinib treatment was made. The six patients who stopped tofacitinib temporarily had a similar median time to resolution of influenza (7.5 days) to those with no change to tofacitinib treatment (8.0 days) (Table 3).
Of the 783 patients in the PsA Overall tofacitinib cohort, 33 patients (4.2%) reported combined influenza AEs, of which two (6.1%) patients had a serious influenza AE and were hospitalized (Table 2). No change to tofacitinib treatment was made in 26 (78.8%) of the 33 patients with combined influenza AEs, and tofacitinib was stopped temporarily in six patients (18.2%; Table 3). Median times to resolution of influenza AEs were generally similar irrespective of the action taken with tofacitinib, although patient numbers were low.
No deaths were reported in the UC or PsA Overall tofacitinib cohorts. Overall, the two deaths recorded in hospitalized patients in the RA Overall tofacitinib cohort account for two deaths in 665 patients with combined influenza AEs, giving a fatality rate of 0.3%.
Antiviral Treatment during Combined Influenza AEs in the Overall Tofacitinib Cohorts
In the Overall RA, UC, and PsA cohorts, 57/517 (11.0%), 15/115 (13.0%), and 5/33 (15.2%) patients with combined influenza AEs, respectively, were treated with antiviral drugs used for influenza (Table S6). The most commonly used antiviral agent was oseltamivir [RA: 49/57 (86.0%); UC: 8/15 (53.3%); PsA: 4/5 (80.0%) patients receiving antiviral treatment].
Risk Factors for Combined Influenza AEs and Recurring Influenza AEs in the RA Overall Tofacitinib Cohort
Risk factors for combined influenza AEs (yes versus no) and recurring influenza AEs (1 versus ≥ 2) in the RA Overall cohort were identified by assessing demographic and baseline characteristics (Tables S7 and S8) using univariate logistic regression analyses (Table S9).
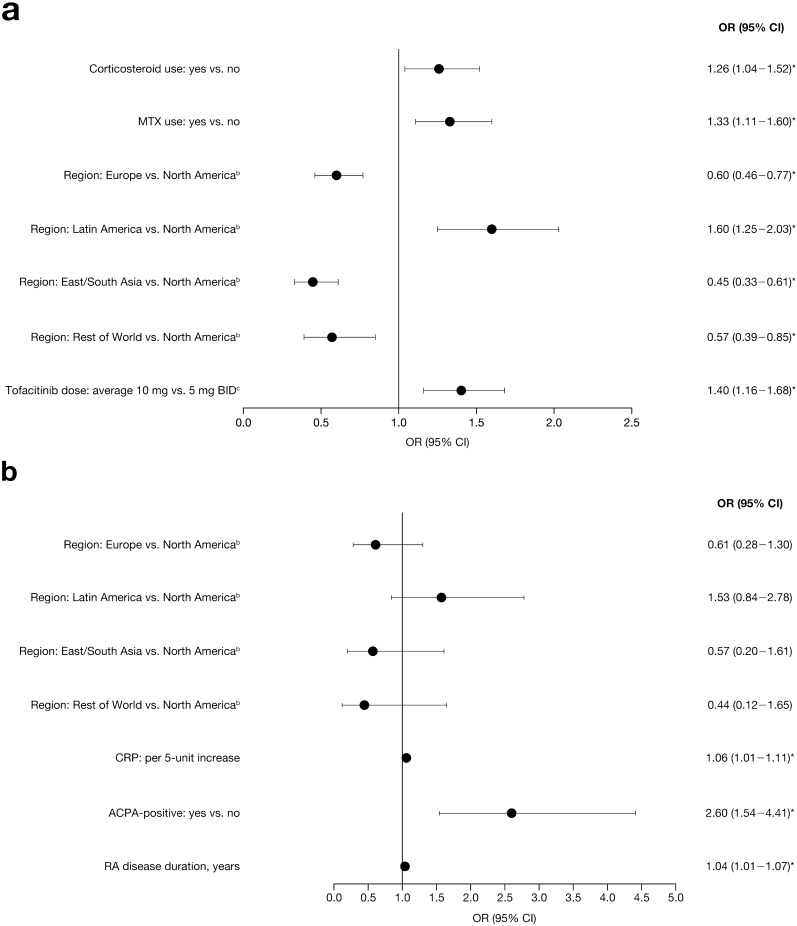
From the multivariable regression analyses (Fig. 4) in the RA Overall tofacitinib cohort, geographic region, tofacitinib average dose (10 versus 5 mg BID; in patients with ≥ 1 event, this was the average tofacitinib dose within 2 weeks prior to the first event, and in patients with no event, this was the average tofacitinib dose during the study period), baseline oral corticosteroid use, and baseline methotrexate use were significant independent predictors of combined influenza AEs. Significant predictors of recurrent influenza AEs were geographic region, higher C-reactive protein (CRP) levels and anti-citrullinated protein antibody (ACPA)-positive status at baseline, and longer RA disease duration.
Fig. 4.
Multivariable model results using a stepwise selection methoda summarizing OR (95% CI) for a combined influenza AEs and b recurrent influenza AEs in the RA Overall tofacitinib cohort. *p < 0.05. For categorical variables with more than two levels, the pairwise comparisons are considered significant if both the overall and pairwise p values are < 0.05. aA stepwise procedure was used to screen significant risk factors (p < 0.20) from the univariate analysis. The full list of potential risk factors included in the univariate analysis is presented in Table S9. The final model includes all selected covariates after the stepwise selection procedure with entry criterion p value of 0.15 and stay criterion p value of 0.05. bGeographic region was significant regardless of the pairwise p values in each comparison. cPatients with at least one event: average tofacitinib dose within 2 weeks prior to first event; patients with no event: average tofacitinib dose during the study period. ACPA anti-citrullinated protein antibody, AE adverse event, BID twice daily, CI confidence interval, CRP C-reactive protein, MTX methotrexate, NA not applicable, OR odds ratio, RA rheumatoid arthritis
Univariate and multivariable regression analyses were not conducted for UC and PsA cohorts owing to the low number of events observed.
Viral Respiratory Infections
As with combined influenza AEs, IRs of viral respiratory infections that may have overlapping clinical presentation with influenza (i.e., preferred terms relating to viral and nonviral respiratory infections) were largely similar across treatment groups in the RA phase 2–3b/4 cohort, and the UC phase 2/3 induction and phase 3 maintenance cohorts (Tables S10–S12).
Discussion
This post hoc analysis evaluated the risk of reported influenza within the RA, UC, and PsA tofacitinib clinical programs over influenza seasons 2004/2005 to 2018/2019. We found the incidence of reported influenza events to be highest in the UC cohort, and within programs, incidences were generally similar between tofacitinib, placebo, and active comparator treatment groups. Across indications, almost all influenza events were nonserious and not associated with complications leading to hospitalization, and the observed mortality of these infections was low. The risk of influenza varied across region, and within the RA Overall tofacitinib cohort, tofacitinib average dose (10 versus 5 mg BID), baseline oral corticosteroid use, and baseline methotrexate use were each significant independent risk factors for reported influenza infection.
Our data suggest that the risk of influenza or influenza-associated complications in tofacitinib-treated patients is similar to that observed in the general population. Influenza AEs occurred in 4.2–9.9% of patients in the Overall tofacitinib cohorts, consistent with an earlier analysis of US influenza data spanning multiple influenza seasons that found that 3.0–11.3% of the general population developed symptomatic influenza across the seasons [11]. In this analysis, 0.9–6.1% of patients (all tofacitinib) in the RA, UC, and PsA Overall tofacitinib cohorts reported serious influenza AEs, and few hospitalized cases of influenza or pneumonia within 28 days of the onset of influenza AEs were observed. Estimations of the annual burden of influenza in the USA by the Centers for Disease Control and Prevention (CDC) indicate that 1.1–2.0% of influenza cases lead to hospitalization [12], consistent with the 1.7% hospitalization rate observed in our analysis across the RA, UC, and PsA cohorts. Further, the mortality rate among those with reported events was similar to that reported in the general public within the USA (0.3% versus 0.1–0.2%, respectively) [12]. The IR of combined influenza AEs for UC was higher than for RA and PsA, even in the placebo group, which may indicate a higher baseline risk of influenza in UC, although further studies are required to confirm this.
The World Health Organization [13] and CDC [14] consider adults aged ≥ 65 years to be at higher risk of influenza and of developing severe disease or complications than younger patients. In our analysis, IRs for combined influenza AEs in older (≥ 65 years) and younger (< 65 years) patients were generally similar, and categorical age was not identified as a risk factor in the univariate and multivariable analyses. In an analysis of influenza vaccination rates among patients with RA and PsA, fewer patients aged < 65 years were vaccinated than older patients [15]. The data on influenza vaccination status for patients in our analysis were not collected systematically and are thus likely incomplete. Indeed, the vaccination rate observed for RA patients in our analysis (9.1%) was markedly lower than vaccination coverage reported in previous studies [16–18]. However, as vaccination rates in patients with RA have been shown to vary widely between countries [17], our data may be skewed by the inclusion of patients from countries where vaccination uptake is particularly low. Therefore, a potentially unrecognized high vaccination rate in patients aged ≥ 65 years could have introduced a downward bias to the IR of influenza AEs in this group. Risk factors that were identified in the RA Overall tofacitinib cohort included geographic region, baseline oral corticosteroid and methotrexate use, and tofacitinib dose (10 versus 5 mg BID). For the univariate and multivariable analyses, the average tofacitinib dose for patients with ≥ 1 influenza event was based on average tofacitinib dose within 2 weeks prior to the first event. In contrast, IRs for influenza AEs were calculated by average tofacitinib dose across the study period. This difference in dosing calculations is likely to be why IRs of combined influenza AEs in the RA Overall tofacitinib cohort were similar between average tofacitinib dose groups, despite dosage being identified as a risk factor. Our model identified both oral corticosteroid and methotrexate use at baseline as risk factors for influenza, although it is unclear whether these drugs do increase the risk of influenza, as there are few published data suggesting an association. Oral steroids have previously been shown as a risk factor in patients with inflammatory disease [9], and methotrexate has been associated with decreased influenza vaccine response [19]. However, it is unclear whether the associations in our analysis are simply due to residual confounding related to disease activity, although disease activity measures were included in our model. For recurrent influenza AEs in RA, significant risk factors included higher CRP levels, ACPA-positivity, and longer disease duration, which are not unexpected given that higher disease activity is known to be associated with a higher risk of infection in patients with RA [20].
This analysis has some limitations. In the RA, UC, and PsA tofacitinib clinical programs, influenza was diagnosed at the discretion of the investigator, without a confirmatory laboratory test, using investigator-reported MedDRA terms that distinguish between confirmed cases of influenza and ILI. However, in a real-world setting, ILI denotes both confirmed influenza cases based on laboratory testing and unconfirmed cases. Antigens in seasonal influenza vaccinations can vary from year to year and may have influenced the AEs reported each year; however, the data on vaccination status in our analysis were not collected systematically. Additionally, as patients in the Overall tofacitinib cohorts may have changed tofacitinib dose throughout the open-label LTE studies, analyses were based on average tofacitinib doses during the trials, which may be a further confounding factor when assessing the impact of dose on risk of an event. Further limitations are the varying exposure across tofacitinib treatment arms and relatively low number of patients in the UC and PsA cohorts.
Conclusions
This post hoc analysis of influenza AEs across the tofacitinib RA, UC, and PsA clinical programs showed the highest IRs in the UC cohort; in each indication, IRs were generally low and similar between tofacitinib, adalimumab, methotrexate, and placebo groups, and between tofacitinib dose and patient age groups. The vast majority of influenza AEs were nonserious and not associated with changes in tofacitinib treatment.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to thank the participants of the studies.
Funding
This study was sponsored by Pfizer. Medical writing support was funded by Pfizer. The journal’s Rapid Service Fee for this article was also funded by Pfizer.
Medical Writing and Editorial Assistance
Medical writing support, under the direction of the authors, was provided by Kirsten Woollcott, MSc, CMC Connect, a division of IPG Health Medical Communications, and Karleen Nicholson, PhD, on behalf of CMC Connect, and was funded by Pfizer, New York, NY, USA, in accordance with Good Publication Practice (GPP 2022) guidelines (Ann Intern Med. 2022;175:1298–304).
Authorship
All named authors meet the International Committee of Medical Journal Editors criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval of the version to be submitted.
Author Contributions
A.Y., D.H., J.C.W., K.K., and T.V.J. contributed to the study conception/design and data analysis. H.J. was involved in the data analysis. J.P. contributed to data acquisition. All authors were involved in interpretation of data and reviewed and approved the manuscript’s content before submission.
Disclosures
Kevin L. Winthrop has received grants and/or research support from AbbVie, Bristol-Myers Squibb, Eli Lilly, Galapagos, Gilead Sciences, Pfizer Inc, Roche, and UCB Pharma. Arne Yndestad, Dan Henrohn, John C. Woolcott, Kenneth Kwok, Andrea B. Shapiro, Annette Diehl, and Chinyu Su are employees and stockholders of Pfizer Inc. Thomas V. Jones was an employee and stockholder of Pfizer Inc at the time of the analysis. Silvio Danese has received consultancy fees from AbbVie, Allergan, Amgen, AstraZeneca, Biogen, Boehringer Ingelheim, Celgene, Celltrion, Ferring, Gilead Sciences, Hospira, Janssen, Johnson & Johnson, MSD, Mundipharma, Pfizer Inc, Roche, Sandoz, Takeda, TiGenix, UCB, and Vifor. Sara Marsal has received grants and/or research support from AbbVie, Bristol-Myers Squibb, Celgene, Janssen-Cilag, MSD, Novartis, Pfizer Inc, Roche, Sandoz, Sanofi, and UCB Pharma, and consultancy fees from AbbVie, Celgene, Galapagos, Gilead Sciences, Pfizer Inc, Sandoz, and Sanofi, and is a shareholder of IMIDomics. Maria Galindo has received grants and/or research support from AbbVie, Eli Lilly, GSK, and Janssen-Cilag. Hyejin Jo is an employee of Syneos Health, who were paid contractors to Pfizer in the development of this manuscript. Julian Panés has received consulting fees from AbbVie, Arena Pharmaceuticals, Boehringer Ingelheim, Celgene, Celltrion, Ferring, Genentech, GSK, Janssen, Origo, Pandion, Pfizer Inc, Progenity, Robarts, Roche, Takeda, Theravance, and Wassermann; speakers’ fees from AbbVie, Janssen, and Takeda; and research funding from AbbVie and Pfizer Inc. Stanley B. Cohen has received grants and/or research support and consultancy fees from AbbVie, Eli Lilly, Genentech, Gilead Sciences, and Pfizer Inc.
Compliance with Ethics Guidelines
Studies were conducted in accordance with the Declaration of Helsinki, Good Clinical Practice Guidelines, and applicable local country regulations and laws. Study protocols were approved by the institutional review boards and/or independent ethics committee at each center. All patients provided written, informed consent. No further ethical approval was required for this post hoc analysis in accordance with the policies of our institutions.
Data Availability
Upon request, and subject to review, Pfizer will provide the data that support the findings of this study. Subject to certain criteria, conditions, and exceptions, Pfizer may also provide access to the related individual de-identified participant data. See https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information.
References
- 1.GBD 2017 Influenza Collaborators Mortality, morbidity, and hospitalisations due to influenza lower respiratory tract infections, 2017: an analysis for the Global Burden of Disease Study 2017. Lancet Respir Med. 2019;2019(7):69–89. doi: 10.1016/S2213-2600(18)30496-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iuliano AD, Roguski KM, Chang HH, et al. Estimates of global seasonal influenza-associated respiratory mortality: a modelling study. Lancet. 2018;391:1285–1300. doi: 10.1016/S0140-6736(17)33293-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. Global influenza strategy 2019–2030. 2019. https://www.who.int/influenza/Global_Influenza_Strategy_2019_2030_Summary_English.pdf?ua=1. Accessed 1 Aug 2021.
- 4.Fraenkel L, Bathon JM, England BR, et al. 2021 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol. 2021;73:1108–1123. doi: 10.1002/art.41752. [DOI] [PubMed] [Google Scholar]
- 5.Feuerstein JD, Isaacs KL, Schneider Y, et al. AGA clinical practice guidelines on the management of moderate to severe ulcerative colitis. Gastroenterology. 2020;158:1450–1461. doi: 10.1053/j.gastro.2020.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gossec L, Baraliakos X, Kerschbaumer A, et al. EULAR recommendations for the management of psoriatic arthritis with pharmacological therapies: 2019 update. Ann Rheum Dis. 2020;79:700–712. doi: 10.1136/annrheumdis-2020-217159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shale M, Czub M, Kaplan GG, Panaccione R, Ghosh S. Anti-tumor necrosis factor therapy and influenza: keeping it in perspective. Therap Adv Gastroenterol. 2010;3:173–177. doi: 10.1177/1756283X10366368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blumentals WA, Arreglado A, Napalkov P, Toovey S. Rheumatoid arthritis and the incidence of influenza and influenza-related complications: a retrospective cohort study. BMC Musculoskelet Disord. 2012;13:158. doi: 10.1186/1471-2474-13-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tinsley A, Navabi S, Williams ED, et al. Increased risk of influenza and influenza-related complications among 140,480 patients with inflammatory bowel disease. Inflamm Bowel Dis. 2019;25:369–376. doi: 10.1093/ibd/izy243. [DOI] [PubMed] [Google Scholar]
- 10.Domínguez À, Soldevila N, Torner N, et al. Usefulness of clinical definitions of influenza for public health surveillance purposes. Viruses. 2020;12:95. doi: 10.3390/v12010095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tokars JI, Olsen SJ, Reed C. Seasonal incidence of symptomatic influenza in the United States. Clin Infect Dis. 2018;66:1511–1518. doi: 10.1093/cid/cix1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention. Disease burden of influenza. 2021. https://www.cdc.gov/flu/about/burden/index.html. Accessed 1 Aug 2021.
- 13.World Health Organization. Influenza (seasonal). 2018. https://www.who.int/en/news-room/fact-sheets/detail/influenza-(seasonal). Accessed 1 August 2021.
- 14.Centers for Disease Control and Prevention. People at high risk for flu complications. 2021. https://www.cdc.gov/flu/highrisk/index.htm. Accessed 1 August 2021.
- 15.Coca A, Dolan J, Ritchlin CT. Rates of influenza vaccination in a cohort of patients with rheumatoid arthritis and psoriatic arthritis [abstract] Arthritis Rheumatol. 2018;70(Suppl 10):231. [Google Scholar]
- 16.Harrison N, Poeppl W, Miksch M, et al. Predictors for influenza vaccine acceptance among patients with inflammatory rheumatic diseases. Vaccine. 2018;36:4875–4879. doi: 10.1016/j.vaccine.2018.06.065. [DOI] [PubMed] [Google Scholar]
- 17.Hmamouchi I, Winthrop K, Launay O, Dougados M. Low rate of influenza and pneumococcal vaccine coverage in rheumatoid arthritis: data from the international COMORA cohort. Vaccine. 2015;33:1446–1452. doi: 10.1016/j.vaccine.2015.01.065. [DOI] [PubMed] [Google Scholar]
- 18.Costello R, Winthrop KL, Pye SR, Brown B, Dixon WG. Influenza and pneumococcal vaccination uptake in patients with rheumatoid arthritis treated with immunosuppressive therapy in the UK: a retrospective cohort study using data from the clinical practice research datalink. PLoS ONE. 2016;11:e0153848. doi: 10.1371/journal.pone.0153848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park JK, Lee YJ, Shin K, et al. Impact of temporary methotrexate discontinuation for 2 weeks on immunogenicity of seasonal influenza vaccination in patients with rheumatoid arthritis: a randomised clinical trial. Ann Rheum Dis. 2018;77:898–904. doi: 10.1136/annrheumdis-2018-213222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Au K, Reed G, Curtis JR, et al. High disease activity is associated with an increased risk of infection in patients with rheumatoid arthritis. Ann Rheum Dis. 2011;70:785–791. doi: 10.1136/ard.2010.128637. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Upon request, and subject to review, Pfizer will provide the data that support the findings of this study. Subject to certain criteria, conditions, and exceptions, Pfizer may also provide access to the related individual de-identified participant data. See https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information.