Abstract
RALY is a multifunctional RNA-binding protein involved in cancer metastasis, prognosis, and chemotherapy resistance in various cancers. However, the molecular mechanism of which is still unclear. We have established RALY overexpression cell lines and studied the effect of RALY on proliferation and apoptosis in HeLa cells. Then we used RNA-seq to analyze the transcriptomes data. Lastly, RT-qPCR experiments had performed to confirm the RNA-seq results. We found that the overexpression of RALY in HeLa cells inhibited proliferation. Moreover, the overexpression of RALY changed the gene expression profile, and the significant upregulation of genes involved immune/inflammatory response related biological process by NOD-like receptor signaling pathway cytokine-cytokine receptor interaction. The significant downregulation genes involved innate immune response by the Primary immunodeficiency pathway. Notably, IFIT1, IFIT2, IFTI3, IFI44, HERC4, and OASL expression had inhibited by the overexpression of RALY. Furthermore, RALY negatively regulates the expression of transcription factors FOS and FOSB. Notably, we found that 645 alternative splicing events had regulated by overexpression of RALY, which is highly enriched in transcription regulation, RNA splicing, and cell proliferation biological process by the metabolic pathway. We show that RALY regulates the expression of immune/inflammatory response-related genes via alternative splicing of FOS in HeLa cells. The novel role of RALY in regulating immune/inflammatory gene expression may explain its function in regulating chemotherapy resistance and provides novel insights into further exploring the molecular mechanism of RALY in regulating cancer immunity and chemo/immune therapies.
Subject terms: Gene expression, Immunology
Background
RALY, known as a member of the Heterogeneous nuclear ribonucleoproteins (hnRNP) family belonging to RNA binding proteins. It was previously identified as a gene relevant to the embryonic lethality of homozygous lethal yellow mice [1]. The gene encodes an autoantigen that cross-reacts with EBNA-1 of the Epstein Barr virus is highly homologous to RALY, suggesting a potential function of RALY in viral response [2]. Importantly, transcript of RALY is overexpressed in various cancer tissues, furthermore, overexpression of RALY involve poor outcome in ovarian, lung, bladder, brain, and breast cancers [3]. The similar correlation between RALY overexpression and poor survival in hepatocellular carcinoma has also been reported. Meanwhile, a previous study reported that decreased of RALY expression is relevant with poor survival in clear cell renal cell carcinoma [4]. Another study showed that decreasing the expression of RALY inhibits cell proliferation and against aggressive biological behavior in hepatocellular carcinoma cells (HCC) [5]. Oxaliplatin is a third-generation platinum analog that kills cells by forming adducts on DNA, which inhibits the cancer cell replication and transcription [6]. Interestingly, down-regulation of RALY expression sensitizes cell lines of colorectal cancer treated with oxaliplatin without effect on the rate of cell growth, indicating the chemotherapeutic role of RALY [7]. These studies together support a general functional role of RALY in cancer development and progression.
Mechanistically, RALY was initially identified in spliceosomal complexes, indicating that RNA splicing could be involved [8]. RALY, together with the hnRNPs including hnRNPH/F, were also found to interact with RBFOX1/2 [9–11]. RALY, along with NONO/p54nrb16, was identified as an interactor of YB-1, an RNA-binding protein involving translational regulation of specific mRNAs [7]. These evidences strongly indicated the role of RALY in alternative splicing regulation. A proteomic study of the RALY-interacted proteins further suggested its role in regulation of mRNA metabolism and translational control [12]. Several recent studies indicate that RALY may bind poly-U stretches in vitro and in vivo [13, 14], which may associate with the differential expression of the target genes at the mRNA and protein levels [15]. RALY localization in the cytoplasmic compartment is found to co-sediment with ribosomes and polysomes, which may be related to its regulated mRNA and protein levels [16]. In fact, RALY primarily localizes in the nucleus and regulating transcription of many genes which involved in cell cycle and transcription regulation [16], as well as the alternative splicing of the pre-mRNA of PRMT1 and the metastasis of breast cancer cells [17]. RALY has been shown to be a direct target of miR-193a-3p, and a long non-coding RNA zinc finger antisense 1 may exert its oncogenic role as an sponge of miR-193a-3p and thusly releasing RALY for its action [18]. Nevertheless, the amplitude of RALY regulation of alternative splicing as an hnRNP protein and gene expression directly or indirectly has not yet been characterized.
This study set up a RALY overexpression HeLa cell model and obtained the RALY-regulated transcriptome from the control and RALY overexpression cells by an Illumina sequencing approach. We then analyzed RALY-regulated alternative splicing and gene expression in HeLa cells at the whole-transcriptome level. The results revealed 910 RALY-regulated AS events in genes that were strongly enriched in transcription regulation and predicted a mechanism of RALY to deregulate the cancer cell transcriptome. The expression levels of 910 genes were significantly changed in the RALY overexpression cell line, consistent with an indirect transcriptional regulation instead of mRNA stability change. It is noteworthy that RALY selectively increased the expression of genes functioning in virus response and inflammatory and innate immune response and repressed the expression of FOS and FOSB. Our results support a crucial and general role of RALY in regulating alternative splicing and gene expression, which explains some oncogenic and chemotherapeutic functions and predicts its role in defending against viral infection and regulating the immune response.
Methods
Cloning and plasmid construction
We designed primer pairs used for Hot Fusion by the CE Design (V1.04). For each of the primer, unique genomic sequences of RALY and the pIRES-hrGFP-1a vector sequence (17–30 nt) were included. The following sequences were the primer.
F-primer: TTCTGTGCACAAGGGCTATG
R-primer: ATGGCAGATGCTGCTCTCTT
EcoRI and XhoI (NEB) were used to digest the pIRES-hrGFP-1a vector at 37 °C for 2–3 h. The digested vector was obtained via 1.0% agarose gel and then purified by Qiagen column kit. And we isolated total RNAs by using Trizol, then purified RNA was reverse transcribed to cDNA by oligo-dT primer. And then the insert fragment was synthesized and amplified by PCR. Finally, we added the linearized vector constructed by EcoRI and XhoI (NEB) and the insert fragment to a PCR microtube for ligation with Clon Express® II One Step Cloning Kit (Vazyme). The constructed plasmid was transfected into E. coli by chemical transformation. The cloned RALY sequence was verified by Sanger sequencing.
Cell culture and plasmid transfection
The HeLa cells were cultured with 5% CO2 at 37 °C in Dulbecco’s Modified Eagle’s Medium, in which containing 10% fetal bovine serum (Hyclone), penicillin (100 U/ mL), and streptomycin (100 g/mL). We then transfected the constructed RALY-overexpressed plasmid to HeLa cells by using Lipofectamine 2000 (Invitrogen, Carls-bad, CA, USA). The transfected HeLa cells were harvested after 48 h. The relative expression level of RALY was calculated and normalized to GAPDH mRNA level using 2− ΔΔCT method [19].
Cell proliferation and apoptosis experiments
We then performed MTT assay and Annexin-V/PI to assess the cell proliferation rate regulated by RALY overexpression.
Western blot experiment
Western blot was also used to assess changes in protein levels. RALY overexpression and negative control HeLa cells were lysed in Ripa buffer. The sample was centrifuged, the supernatant was further treated on 10% sds-page gel, and then transferred to PVDF membrane (micropore). A monoclonal Flag antibody (SIG-MA-ALDRICH) was diluted in TBST (1:2000) and the RALY protein level was detected with Abclonal (1:2000) as load control.
RNA-seq experiment
For RNA-seq library construction, total RNA was extracted from HeLa cells with TRIZOL (Ambion). Then removed the DNA by RQ1 DNase (Promega, Madison, WI, USA) and purified the RNA with two phenol-chloroform treatments. For each sample, the VAHTS Stranded mRNA-seq Library Prep Kit (Vazyme) was used to prepare library with 1 μg of the total RNA as input. Only polyadenylated RNAs were purified and fragmented for library. We then used Illumina HiSeq X Ten platform to obtain the sequences with 150 nt paired-end fastq format.
RNA-seq filtering and alignment
After being obtained the raw reads, we removed the reads containing more than 2-N bases. Then adapter sequences and low-quality bases were trimmed from the remaining reads using FASTX-Toolkit (Version 0.0.13), quality filtered reads were aligned to the human GRCH38 genome by TopHat2 [20] allowing no more than 4 mismatches. Uniquely mapped reads were used for gene reads number counting and FPKM calculation [21].
Differentially expressed genes (DEG) analysis
The R Bioconductor package edge R [22] was utilized to screen out the differentially expressed genes (DEGs). A false discovery rate <0.05 and fold change >2 or <0.5 were set as the cut-off criteria to screen out DEGs.
Alternative splicing analysis
The alternative splicing events (ASEs) and regulated alternative splicing events (RASEs) between RALY overexpression and control samples were defined and quantified using the ABL as pipeline as described antecedently [23, 24]. Based on the splice junction reads, ten types of ASEs were detected, including ES, A5SS, A3SS, IR, MXE, 5pMXE, 3pMXE, cassette exon, A3SS&ES, and A5SS&ES.
Reverse transcription qPCR validation of DEGs and AS events
To confirm the validity of the RNA-seq results, qRT-PCR was performed for some of the randomly selected DEGs. We used GAPDH as the internal control normalized the RNA expression levels of all the genes. Meanwhile, qRT-PCR assay was performed for ASE validation. We designed specific primers to detect alternative splicing events which were detailly described in ABL as method. The information of primers is presented in Additional file 1.
Functional enrichment analysis
To explore the functions of DEGs and RASGs, using KOBAS 2.0 server [25] to identify the enriched Gene Ontology (GO) terms and KEGG pathways.
Results
RALY overexpression inhibits cell proliferation and which correlated with prognosis in cancer
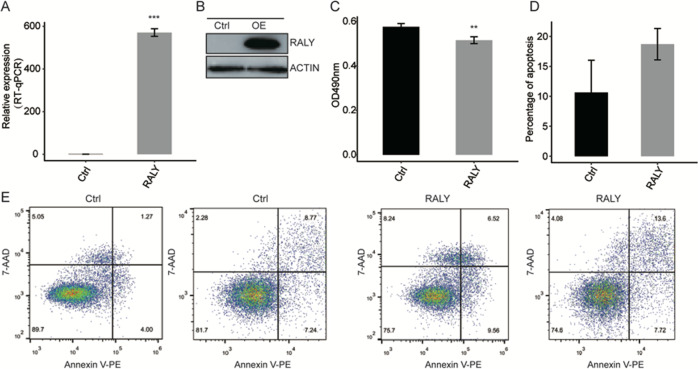
To explore the function of RALY at the cell level, we construct a functional cell model. RALY had overexpressed in the HeLa cell line. And overexpression of RALY was assessed through the qPCR and Western blot, which revealed that the expression level of RALY had upregulated in HeLa cells (Fig. 1A, B). The result of proliferation analysis showed that RALY overexpression inhibits cellular proliferation (Fig. 1C) and promoted the cell apoptosis (Fig. 1D, E). Analysis of RALY expression and survival in a number of cancers showed that the levels of RALY expression were dramatically decreased in cancer tissues compared with corresponding normal tissues (Fig. 2A, B) RALY expression is related with prognosis in cancer patients (Fig. 2C). These data suggest that overexpression of RALY involving the cellular proliferation, the mechanism of RALY in the cellular process still unclear, needs further investigation.
Fig. 1. Overexpression of RALY impact on proliferation and apoptosis of HeLa cells.
A RALY expression quantified by qRT-PCR. Error bars represent mean ± SEM. ***p < 0.001. B RALY was overexpressed and validated by Western blotting. C Overexpression of RALY suppressed the proliferation of HeLa cells. *P < 0.05, ***P < 0.001. D Overexpression of RALY promoted the cell apoptosis.
Fig. 2. Analysis of RALY expression and survival in a number of cancers.
A Levels of RALY expression were dramatically decreased in cancer tissues compared with corresponding normal tissues. B RALY expression is related with prognosis in cancer patients.
RALY overexpression changes the genes expression profiles of HeLa cells
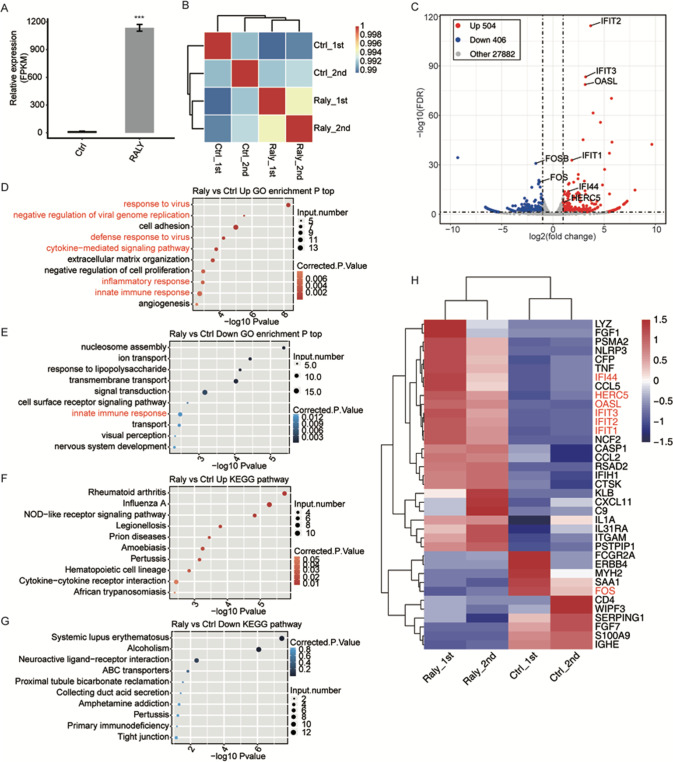
To further explore the role of RALY on genes expressional and transcriptional, we constructed a total of four cDNA libraries prepared from control and RALY-OE cells (two biological replicated). The effective overexpression of RALY had confirmed with RNA‐seq data, which aligned with the RT‐qPCR results, FPKM values had used to generate a Pearson’s distance correlation matrix to contrast the transcriptomes from each sample. The FPKM values of RALY expression significantly upregulated in HeLa cells (Fig. 3A, B). The heatmap analysis of the DEG demonstrated that RALY-OE and the control were not significant distinguished (Fig. 3B). Based on the high-quality RNA-seq data from the RALY overexpression cells and control, the 504 upregulated and 406 downregulated significant differential expression genes were identified in RALY-OE compared to control (Fig. 3C). These results showed that RALY upregulation significantly changed the gene transcription.
Fig. 3. Analysis of the change in gene expression in response to RALY-OE.
A RALY expression quantified by RNA-seq data. FPKM values were calculated as that has been explained in Materials and Methods. B Heat map shows the hierarchically clustered Person’s correlation matrix resulting from comparing the transcript expression values for control and RALY-OE values for control and RALY-OE samples. C Identification of RALY-regulated genes. Up-regulated genes are labeled in red, whereas down-regulated are labeled in blue in the volcano plot. D The top 10 representative GO biological processes of upregulated genes. E The top 10 representative GO biological processes of down-regulated genes. F The top 10 representative KEGG pathways of up-regulated genes. G The top 10 representative KEGG pathways of up-regulated genes. H Hierarchical clustering of DEGs in control and RALY overexpression samples. FPKM values are log2-transformed and then median-centered by each gene. Error bars represent mean ± SEM. ***p < 0.001.
RALY overexpression selectively upregulates the transcription of viral response, inflammatory/innate immune response genes in HeLa cells
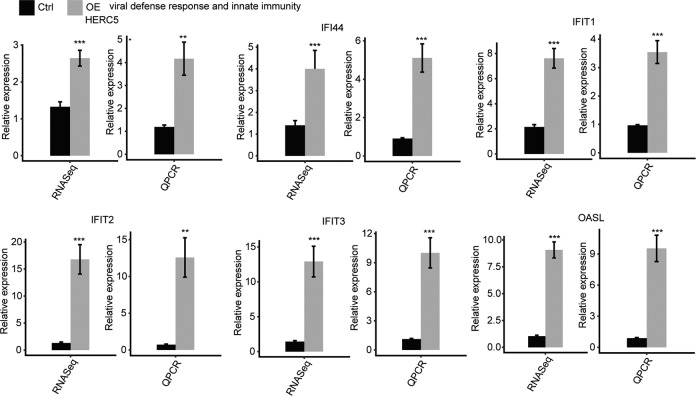
To comprehensively explore the potential roles of 910 DEGs, we performed the GO and KEGG enrichment analysis. In total, 504 upregulated and 406 downregulated DEGs had identified. And the upregulated genes are mainly enriched in “cytokine-mediated signaling pathway,” “negative regulation of cell proliferation,” “inflammatory response,” “innate immune response” in GO enrichment (Fig. 3D). The KEGG analysis showed that the upregulated genes enriched in the “NOD-like receptor signaling pathway,” “cytokine-cytokine receptor interaction” (Fig. 3F). In contrast, the down-regulated genes enriched in the “innate immune response” in GO enrichment (Fig. 3E). And the KEGG pathways were most enriched in those associated with “primary immunodeficiency” (Fig. 3G). The heatmap analysis of the DEG demonstrated that RALY-OE and the control were not significant distinguished (Fig. 3H). The RNA-seq had performed to quantify the changes in mRNA levels in RALY-OE and Control, and we found that the six upregulated genes, including IFIT1, IFIT2, IFTI3, IFI44 HERC4, and OASL, were significantly upregulated in the RALY overexpression cell line (Fig. 4). These data indicated that RALY overexpression changed the transcription of proliferation and immune/inflammatory response-related genes.
Fig. 4. RALY regulates expression of genes associated with the viral defense response and innate immunity.
Gene expression quantified by RNA sequencing data and qRT-PCR. Error bars represent mean ± SEM. ***p < 0.001.
RALY overexpression downregulates the transcription of FOS and FOSB
We performed qPCR had quantified the expression transcription factors (TFs) of FOS and FOSB in RALY-OE and control cells (Fig. 5). These two TFs were significantly suppressed under the RALY overexpression condition, demonstrating that RALY negatively regulates the expression of FOS transcription factors in HeLa cells. FOS will affect the immune/inflammatory response via changing the protein binding activity and ubiquitin-specific protease activity [26]. FOSB is 70% homology with Fos, which together with the Jun family members form the group of AP-1 proteins which modulated the gene expression in response to cytokines, growth factors, bacterial and viral infections [27]. Our results showed that RALY regulates immune/inflammatory response-related genes via the impact the transcription factor of FOS and FOSB expression.
Fig. 5. RALY regulates expression of FOS transcription factor.
Gene expression quantified by RNA sequencing data and qRT-PCR. Error bars represent mean ± SEM. ***p < 0.001.
RALY overexpression selectively change the alternative splicing of transcription factor in HeLa cells
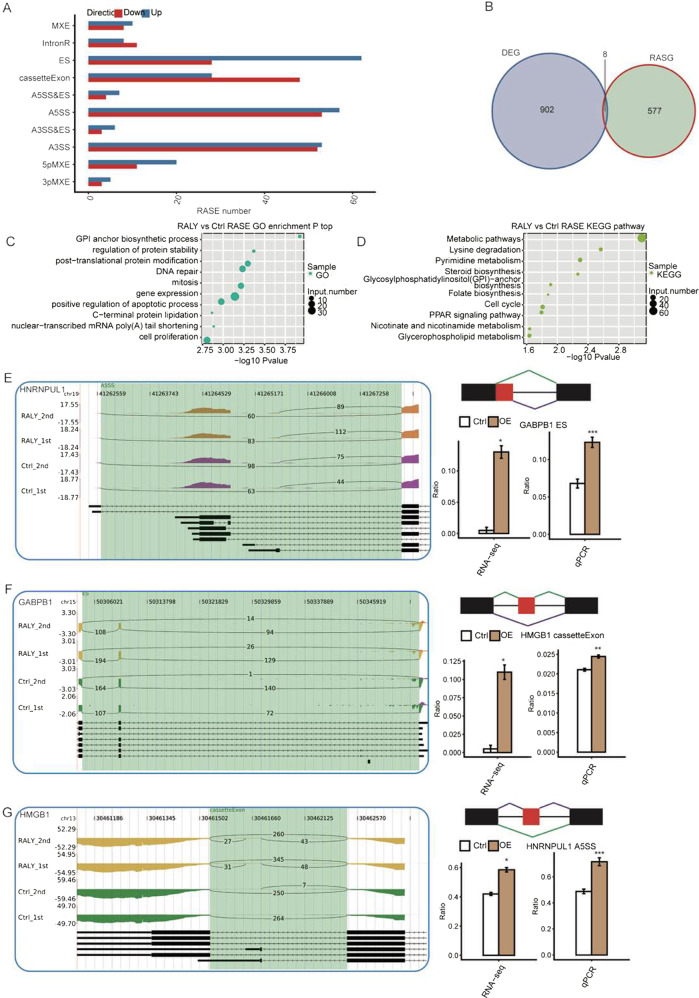
In order to deeply investigate the molecular function of RALY overexpression in HeLa cells, we analyzed the regulated AS events by RALY. The splicing reads from RALY overexpression and control HeLa cells were mapped to the reference genome, and 367,321 annotated exons (60.72% of total annotated ones) were observed. And a total of 162,055 known and 182,549 novel splice junctions, Meanwhile 20,446 known ASEs and 58,427 novel ASEs were detected.
To further validate the high-confidence RALY-regulated alternative splicing events, AS ratio change between RALY overexpression and control cells contrasted by a custom pipeline, a total of 645 RASEs were identified, including 187 IR and 485 NIR RASEs. Among these RASEs, the number of A3SS, A5SS, IntronR, and ES was comparatively high (Fig. 6A). In the aggregate, 637 genes attributed to these RASEs had been identified as RALY-regulated alternative splicing genes after mapping and counting. These results showed that RALY regulated the alternative splicing events in HeLa cells. Eight genes were overlapping between DEGs and RASEs (Fig. 6B). And identified eight such genes: CCDC17, RP11-610P16.1, RP11-91A18.4, MYH16, CTD-2587H24.5, IFIT1, LAMC2, PAK6. The GO biological process analysis showed that the overlapped gene mainly enriched in response to the virus, defense response to the virus, cytokine-mediated signaling pathway, negative regulation of protein binding, regulation of transcription, DNA-dependent, apoptotic process, mitotic cell cycle. Enriched KEGG pathway included those involved in Focal adhesion, T cell receptor signaling pathway, Axon guidance. These data indicated that upregulation of RALY expression level had affected the alternative splicing of DEGs annotated with the immune/inflammatory response.
Fig. 6. Validation of RALY regulated splicing events.
A classification of different AS types regulated by the RALY. B overlap analysis between RALY regulated genes and splicing genes. C The Top 10 GO biological process analysis. D The Top 10 KEGG functional pathway of splicing gene. E ASEs in HNRNPUL. F ASEs in GABPB1. G ASEs in HMGB1. The IGV-sashimi plots showing the alternative splicing changes that occurred in the control or RALY overexpression HeLa cells. The results for ZNF638, HNRNPUL1, GABPB1, HMGB1, CTNND1, E4F1, ZSCAN32, SPOCD1, VEZF1, and ETV1 are presented. RNA-seq quantification and RT-PCR validation of alternative splicing regulation are shown at the bottom. *P < 0.05, **P < 0.01.
To investigate the function of RASGs, we performed GO analysis, and there were 203 RASGs enriched in protein binding and 67 RASGs with DNA binding. Furthermore, the RASGs enhanced in GO biological process, including transcription DNA dependent, regulation of transcription from RNA polymerase II promoter, regulation of RNA splicing, cell proliferation, gene expression, regulation of translational (Fig. 6C). The enriched pathways include the peroxisome, Nucleotide excision repair, Metabolic pathways (Fig. 6D). To further investigate the function of RALY overexpression on alternative splicing events, 8 RASEs had analyzed by qPCR, and the changes in the ratio of 8 RASEs in qPCR were consistent with the RNA-Seq results (Fig. 6E). These RASEs occurred in ZNF638, HNRNPUL1, GABPB1, HMGB1, CTNND1, E4F1, ZSCAN32, SPOCD1, VEZF1, and ETV1.
Discussion
Alternative splicing is a ubiquitous regulatory mechanism of gene expression; in precursor mRNA, various splice sites had selected to generate functionally distinct mRNA and protein variants [28], which widely expand metazoan genomes’ functional and regulatory ability [15]. The dysregulation of cancer-associated splice variants may play a critical role in cancer cellular behavior, including cellular proliferation and cellular death it is a potential target for therapeutic intervention [29]. As reported, alternative splicing mediated cervical cancer oncogenesis, indicating the novel therapeutic target for the treatment of cervical cancer [30]. We found that the overexpression of RALY inhibited cell proliferation and impacted the expression of immune-inflammatory response-related genes at the transcript level, and involved regulating transcription factors activity. These data indicated that RALY promoted the immune response gene expression through transcriptional regulatory mechanism correlated with RNA binding activity.
RALY has upregulated various types of tumors. Its downregulation reduces cell proliferation [16]. However, another study reported that the increased level of RALY promoted proliferation, migration, and invasion abilities in HCC cells [15]. These results indicated that RALY is a tumorigenesis oncogene in mammalian cells. Notably, our data also revealed that RALY overexpression inhibited cell proliferation in HeLa cells, inconsistent with previous reports. And the transcriptome analysis revealed that RALY changed the expression of proliferation and immune/inflammatory-related genes. Furthermore, the FOS and FOSB, which belong to the Fos family of AP-1 transcription factors, inhabited RALY-OE cell lines. As reported, the FOS and FOSL1 were involved in regulating genes governing progression by the cell cycle and, consequently, are both associated with promoting proliferation [31, 32]. As reported, FOSL is a critical mediator in cancer cell EMT/MET plasticity. These results together indicated that RALY involved the proliferation biological process via regulation of the alternative splicing and transcriptor factors of FOS in HeLa cells.
In this study, we found that RALY promoted the expression of IFIT1, IFIT2, IFTI3, IFI44, HERC4, and OASL. The previous research reported that IFIT1, IFIT2, IFTI3, IFIT3, IFI44, HERC4, and OASL genes are antitumoral effects and modulated the inflammatory response in cancer and infection [5, 18]. OASL can boost innate host defense and improve immunity [33]. IFIT1 and IFITM3 expression were related to several immune checkpoint molecules and tumor-associated macrophage markers [34]. As reported, IFIT1 regulated different stages of the host innate resistant response duration of both viral and bacterial infection and may modulate the inflammatory response in human astrocytes [35]. IFI44 is a type I interferon-inducible gene family [36], participating in microtubule formation, involving an autoimmune response and inhibiting proliferation [37]. These results indicated that overexpression of RALY impacted the expression of inflammatory/immune response and proliferation-related genes in HeLa cells. The immune system has been defined as a crucial component of the immune surveillance of cancer and plays an essential role in cancer progression and tumorigenesis [38]. RALY overexpression inhibited the immune/inflammatory-related genes expression at transcription level might be associated with the chemo treatment resistance in cancer cell biological process.
In addition, Systemic inflammatory response may accelerate the progression of cancer and distant metastasis by a diversity of mechanisms. Based on the above evidence, we may speculate that RALY is involved in cancer immunity. This founding widened our current knowledge of the critical role of RALY in regulating the immune/ inflammatory response in cancer. As reported, RALY is modulated in both alternative splicing and translation [39]. In this study, transcriptome analysis suggested differential expression in genes related to the immune-inflammatory response. Meanwhile, we have detected RALY-dependent alternative splicing of transcription factors and splice factors. ZNF638 is a transcriptional coactivator that operates as an early regulator of adipogenesis in vitro [40]. HNRNPUL 1 and 2 are recruited to sites of DNA damage in an RNA-independent manner and promote adequate DNA resection [41]. GABPB1 is a transcription factor subunit. The higher expression levels of GABPB1 had associated with poor prognostic in renal cancer [42]. HMGB1 is a nuclear protein that acts as an intriguing molecule in inflammatory disorders and promotes inflammation through elucidated signal and molecular transport mechanisms [43]. CTNND1 overexpression in HCC cells induced EMT, invasion, and migration traits in vitro and boosted metastatic capacity in vivo [44]. E4F1 was a central metabolic node that regulated pyruvate oxidation and promoted the tricarboxylic acid cycle to meet energy demand [45]. SPOCD1 encodes a protein that pertains to the transcription factor S-II (TFIIS) family of transcription factors and accelerates the proliferation and metastasis of glioma cells by up-regulating PTX3 [46]. VEZF1 has expressed in the anterior-most mesoderm, and Its expression is later restricted in the vascular endothelium [47]. In addition, Ets-transcription factor ETV1 is a viral oncogene, and stromal expansion in PDAC contributed to the development of larger primary tumors. Furthermore, the KEGG analysis showed several tumorigeneses and immune-response relevant signaling pathways. Notably, most genes in this pathway are involved in the immune-inflammatory response. These results highlight that RALY regulates the inflammatory response through a metabolic pathway with alternative splicing. However, RALY selectively regulates the alternative splicing of transcription factors mainly associated with immune/inflammatory response and tumorigenesis in HeLa cells, which affect the cellular process, including proliferation and apoptosis, and metabolism in cancer cells.
Conclusion
In summary, we concluded that RALY overexpression inhibits proliferation in HeLa cells. Simultaneously, RALY regulates the expression of immunity and inflammatory response genes by modulating the splicing of regulators factors and alternative splicing, which might correlate with tumorigenesis in HeLa cells. Which data provides evidence for further investigation of the function of RALY in cancer immunology and clinical resistance in cancer advance under the given therapy. Nevertheless, there are some limitations to our study. Further studies are required to verify the molecular function of RALY in cancer metabolism. Further investigation of the role of all RALY proteins in carcinogenesis, especially in vivo and in vitro, will be more significant and preferred the new perspectives in cancer treatment.
Availability of data and material
All data generated or analyzed during this study have been included in this published article and its supplementary information files. The datasets supporting the results of this article are available in the NCBI Gene Expression Omnibus and are accessible through GEO series accession number (GSE157125).
Supplementary information
Acknowledgements
The authors would like to thank Dr Dong Chen (ABLife BioBing Data Institute) for his helpful discussions. This work was supported by Natural Science Foundation of Xinjiang Autonomous Region(2019D01C312)
Author contributions
Z.L. and B.A. designed the project, A.R., E.H. and D.C. supervised the Experiments. D.C. performed the experiment. Z.J. and B.A. analyzed the data. B.A. wrote the paper. All authors analyzed the results and approved the final version of the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Zhao Liang, Buzukela Abuduaini.
Supplementary information
The online version contains supplementary material available at 10.1038/s41435-022-00178-4.
References
- 1.Michaud EJ, Bultman SJ, Klebig ML, van Vugt MJ, Stubbs LJ, Russell LB, et al. A molecular model for the genetic and phenotypic characteristics of the mouse lethal yellow (Ay) mutation. Proc Natl Acad Sci USA. 1994;91:2562–6. doi: 10.1073/pnas.91.7.2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rhodes GH, Valbracht JR, Nguyen MD, Vaughan JH. The p542 gene encodes an autoantigen that cross-reacts with EBNA-1 of the Epstein Barr virus and which may be a heterogeneous nuclear ribonucleoprotein. J Autoimmun. 1997;10:447–54. doi: 10.1006/jaut.1997.9996. [DOI] [PubMed] [Google Scholar]
- 3.Song G, Guo G, Du T, Li X, Wang J, Yan Y, et al. RALY may cause an aggressive biological behavior and a dismal prognosis in non-small-cell lung cancer. Exp Cell Res. 2020;389:111884. doi: 10.1016/j.yexcr.2020.111884. [DOI] [PubMed] [Google Scholar]
- 4.Cui ZW, Xia Y, Ye YW, Jiang ZM, Wang YD, Wu JT, et al. RALY RNA binding protein-like reduced expression is associated with poor prognosis in clear cell renal cell carcinoma. Asian Pac J Cancer Prev. 2012;13:3403–8. doi: 10.7314/APJCP.2012.13.7.3403. [DOI] [PubMed] [Google Scholar]
- 5.Zhu Z, Zhang Y, Huang C, Tang Y, Sun C, Ju W, et al. Overexpression of RALY promotes migration and predicts poor prognosis in hepatocellular carcinoma. Cancer Manag Res. 2018;10:5559–72. doi: 10.2147/CMAR.S182996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Desoize B, Madoulet C. Particular aspects of platinum compounds used at present in cancer treatment. Crit Rev Oncol Hematol. 2002;42:317–25. doi: 10.1016/S1040-8428(01)00219-0. [DOI] [PubMed] [Google Scholar]
- 7.Tsofack SP, Garand C, Sereduk C, Chow D, Aziz M, Guay D, et al. NONO and RALY proteins are required for YB-1 oxaliplatin induced resistance in colon adenocarcinoma cell lines. Mol Cancer. 2011;10:145. doi: 10.1186/1476-4598-10-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jurica MS, Licklider LJ, Gygi SR, Grigorieff N, Moore MJ. Purification and characterization of native spliceosomes suitable for three-dimensional structural analysis. RNA. 2002;8:426–39. doi: 10.1017/S1355838202021088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun S, Zhang Z, Fregoso O, Krainer AR. Mechanisms of activation and repression by the alternative splicing factors RBFOX1/2. RNA. 2012;18:274–83. doi: 10.1261/rna.030486.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jin Y, Suzuki H, Maegawa S, Endo H, Sugano S, Hashimoto K, et al. A vertebrate RNA-binding protein Fox-1 regulates tissue-specific splicing via the pentanucleotide GCAUG. EMBO J. 2003;22:905–12. doi: 10.1093/emboj/cdg089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Underwood JG, Boutz PL, Dougherty JD, Stoilov P, Black DL. Homologues of the Caenorhabditis elegans Fox-1 protein are neuronal splicing regulators in mammals. Mol Cell Biol. 2005;25:10005–16. doi: 10.1128/MCB.25.22.10005-10016.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tenzer S, Moro A, Kuharev J, Francis AC, Vidalino L, Provenzani A, et al. Proteome-wide characterization of the RNA-binding protein RALY-interactome using the in vivo-biotinylation-pulldown-quant (iBioPQ) approach. J Proteome Res. 2013;12:2869–84. doi: 10.1021/pr400193j. [DOI] [PubMed] [Google Scholar]
- 13.Ray D, Kazan H, Cook KB, Weirauch MT, Najafabadi HS, Li X, et al. A compendium of RNA-binding motifs for decoding gene regulation. Nature. 2013;499:172–7. doi: 10.1038/nature12311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lambert N, Robertson A, Jangi M, McGeary S, Sharp PA, Burge CB. RNA Bind-n-Seq: quantitative assessment of the sequence and structural binding specificity of RNA binding proteins. Mol Cell. 2014;54:887–900. doi: 10.1016/j.molcel.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rossi A, Moro A, Tebaldi T, Cornella N, Gasperini L, Lunelli L, et al. Identification and dynamic changes of RNAs isolated from RALY-containing ribonucleoprotein complexes. Nucleic Acids Res. 2017;45:6775–92. doi: 10.1093/nar/gkx235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cornella N, Tebaldi T, Gasperini L, Singh J, Padgett RA, Rossi A, et al. The hnRNP RALY regulates transcription and cell proliferation by modulating the expression of specific factors including the proliferation marker E2F1. J Biol Chem. 2017;292:19674–92. doi: 10.1074/jbc.M117.795591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bondy-Chorney E, Baldwin RM, Didillon A, Chabot B, Jasmin BJ, Cote J. RNA binding protein RALY promotes Protein Arginine Methyltransferase 1 alternatively spliced isoform v2 relative expression and metastatic potential in breast cancer cells. Int J Biochem Cell Biol. 2017;91:124–35. doi: 10.1016/j.biocel.2017.07.008. [DOI] [PubMed] [Google Scholar]
- 18.Cui X, Wang Z, Liu L, Liu X, Zhang D, Li J, et al. The long non-coding RNA ZFAS1 sponges miR-193a-3p to modulate hepatoblastoma growth by targeting RALY via HGF/c-Met pathway. Front Cell Dev Biol. 2019;7:271. doi: 10.3389/fcell.2019.00271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 20.Kim D, Pertea G, Trapnell C, Pimentel H, Kelley R, Salzberg SL. TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 2013;14:R36. doi: 10.1186/gb-2013-14-4-r36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26:139–40. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jin L, Li G, Yu D, Huang W, Cheng C, Liao S, et al. Transcriptome analysis reveals the complexity of alternative splicing regulation in the fungus Verticillium dahliae. BMC Genom. 2017;18:130. doi: 10.1186/s12864-017-3507-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trapnell C, Williams BA, Pertea G, Mortazavi A, Kwan G, van Baren MJ, et al. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat Biotechnol. 2010;28:511–5. doi: 10.1038/nbt.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xia H, Chen D, Wu Q, Wu G, Zhou Y, Zhang Y, et al. CELF1 preferentially binds to exon-intron boundary and regulates alternative splicing in HeLa cells. Biochim Biophys Acta Gene Regul Mech. 2017;1860:911–21.. doi: 10.1016/j.bbagrm.2017.07.004. [DOI] [PubMed] [Google Scholar]
- 25.Xie C, Mao X, Huang J, Ding Y, Wu J, Dong S, et al. KOBAS 2.0: a web server for annotation and identification of enriched pathways and diseases. Nucleic Acids Res. 2011;39:W316–22. doi: 10.1093/nar/gkr483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu J, Yao K, Guo J, Shi H, Ma L, Wang Q, et al. miR-181a and miR-150 regulate dendritic cell immune inflammatory responses and cardiomyocyte apoptosis via targeting JAK1-STAT1/c-Fos pathway. J Cell Mol Med. 2017;21:2884–95. doi: 10.1111/jcmm.13201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Janson ND, Jehanathan N, Jung S, Priyathilaka TT, Nam BH, Kim MJ, et al. Insight into the molecular function and transcriptional regulation of activator protein 1 (AP-1) components c-Jun/c-Fos ortholog in red lip mullet (Liza haematocheila) Fish Shellfish Immunol. 2019;93:597–611. doi: 10.1016/j.fsi.2019.08.013. [DOI] [PubMed] [Google Scholar]
- 28.Urbanski LM, Leclair N, Anczukow O. Alternative-splicing defects in cancer: Splicing regulators and their downstream targets, guiding the way to novel cancer therapeutics. Wiley Interdiscip Rev RNA. 2018;9:e1476. doi: 10.1002/wrna.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Angiolini F, Belloni E, Giordano M, Campioni M, Forneris F, Paronetto MP, et al. A novel L1CAM isoform with angiogenic activity generated by NOVA2-mediated alternative splicing. Elife. 2019;8:e44305. doi: 10.7554/eLife.44305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Song Q, Yi F, Zhang Y, Jun Li DK, Wei Y, Yu H, et al. CRKL regulates alternative splicing of cancer-related genes in cervical cancer samples and HeLa cell. BMC Cancer. 2019;19:499. doi: 10.1186/s12885-019-5671-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang H, Lu B, Ren S, Wu F, Wang X, Yan C, et al. Long Noncoding RNA LINC01116 contributes to gefitinib resistance in non-small cell lung cancer through regulating IFI44. Mol Ther Nucleic Acids. 2020;19:218–27. doi: 10.1016/j.omtn.2019.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kovacs LA, Berta G, Csernus V, Ujvari B, Furedi N, Gaszner B. Corticotropin-releasing factor-producing cells in the paraventricular nucleus of the hypothalamus and extended amygdala show age-dependent FOS and FOSB/deltaFOSB immunoreactivity in acute and chronic stress models in the rat. Front Aging Neurosci. 2019;11:274. doi: 10.3389/fnagi.2019.00274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhu J, Ghosh A, Sarkar SN. OASL-a new player in controlling antiviral innate immunity. Curr Opin Virol. 2015;12:15–9. doi: 10.1016/j.coviro.2015.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu Y, Ji K, Wu M, Hao B, Yao KT, Xu Y. A miRNA-HERC4 pathway promotes breast tumorigenesis by inactivating tumor suppressor LATS1. Protein Cell. 2019;10:595–605. doi: 10.1007/s13238-019-0607-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ghosh A, Shao L, Sampath P, Zhao B, Patel NV, Zhu J, et al. Oligoadenylate-synthetase-family protein OASL inhibits activity of the DNA sensor cGAS during DNA virus infection to limit interferon production. Immunity. 2019;50:51–63. doi: 10.1016/j.immuni.2018.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leisching G, Wiid I, Baker B. The association of OASL and type I interferons in the pathogenesis and survival of intracellular replicating bacterial species. Front Cell Infect Microbiol. 2017;7:196. doi: 10.3389/fcimb.2017.00196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fleith RC, Mears HV, Leong XY, Sanford TJ, Emmott E, Graham SC, et al. IFIT3 and IFIT2/3 promote IFIT1-mediated translation inhibition by enhancing binding to non-self RNA. Nucleic Acids Res. 2018;46:5269–85. doi: 10.1093/nar/gky191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Andrejeva G, Rathmell JC. Similarities and distinctions of cancer and immune metabolism in inflammation and tumors. Cell Metab. 2017;26:49–70. doi: 10.1016/j.cmet.2017.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Perie L, Verma N, Xu L, Ma X, Mueller E. Transcriptional regulation of ZNF638 in thermogenic cells by the cAMP response element binding protein in male mice. J Endocr Soc. 2019;3:2326–40. doi: 10.1210/js.2019-00238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Polo SE, Blackford AN, Chapman JR, Baskcomb L, Gravel S, Rusch A, et al. Regulation of DNA-end resection by hnRNPU-like proteins promotes DNA double-strand break signaling and repair. Mol Cell. 2012;45:505–16. doi: 10.1016/j.molcel.2011.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen SC, Yen MC, Chen FW, Wu LY, Yang SJ, Kuo PL, et al. Knockdown of GA-binding protein subunit beta1 inhibits cell proliferation via p21 induction in renal cell carcinoma. Int J Oncol. 2018;53:886–94. doi: 10.3892/ijo.2018.4411. [DOI] [PubMed] [Google Scholar]
- 42.Andersson U, Yang H, Harris H. Extracellular HMGB1 as a therapeutic target in inflammatory diseases. Expert Opin Ther Targets. 2018;22:263–77. doi: 10.1080/14728222.2018.1439924. [DOI] [PubMed] [Google Scholar]
- 43.Tang B, Tang F, Wang Z, Qi G, Liang X, Li B, et al. Overexpression of CTNND1 in hepatocellular carcinoma promotes carcinous characters through activation of Wnt/beta-catenin signaling. J Exp Clin Cancer Res. 2016;35:82. doi: 10.1186/s13046-016-0344-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lacroix M, Rodier G, Kirsh O, Houles T, Delpech H, Seyran B, et al. E4F1 controls a transcriptional program essential for pyruvate dehydrogenase activity. Proc Natl Acad Sci USA. 2016;113:10998–1003. doi: 10.1073/pnas.1602754113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu Q, Wang XY, Qin YY, Yan XL, Chen HM, Huang QD, et al. SPOCD1 promotes the proliferation and metastasis of glioma cells by up-regulating PTX3. Am J Cancer Res. 2018;8:624–35. [PMC free article] [PubMed] [Google Scholar]
- 46.AlAbdi L, He M, Yang Q, Norvil AB, Gowher H. The transcription factor Vezf1 represses the expression of the antiangiogenic factor Cited2 in endothelial cells. J Biol Chem. 2018;293:11109–18. doi: 10.1074/jbc.RA118.002911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Heeg S, Das KK, Reichert M, Bakir B, Takano S, Caspers J, et al. ETS-transcription factor ETV1 regulates stromal expansion and metastasis in pancreatic cancer. Gastroenterology. 2016;151:540–53. doi: 10.1053/j.gastro.2016.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study have been included in this published article and its supplementary information files. The datasets supporting the results of this article are available in the NCBI Gene Expression Omnibus and are accessible through GEO series accession number (GSE157125).