Abstract
Purpose
To identify rates of uveitis reactivation both before and after the coronavirus disease (COVID) 2019 vaccine in subjects with a previous diagnosis of uveitis.
Design
Retrospective study.
Participants
Subjects were identified from the Inflammatory Eye Disease Registry at Auckland District Health Board diagnosed with uveitis between January 1, 2010, and December 31, 2020.
Methods
Date of COVID vaccination was determined from the patient clinical record. Rate of flare was calculated for 3 months before vaccination and 3 months after each vaccination.
Main Outcome Measure
Uveitis flare was defined as the presence of new uveitis activity or increased activity that required a change in uveitis treatment.
Results
A total of 4184 eyes of 3008 patients were included in the study with a total of 8474 vaccinations given during the study period. Median age was 54.8 years, and 1474 (49.0%) were female. Noninfectious etiology was most common, occurring in 2296 patients (76.3%) and infectious etiology occurring in 712 patients (23.7%). Rate of uveitis flare was 12.3 per 1000 patient-months at baseline, 20.7 after the first dose, 15.0 after the second dose, 12.8 after the third dose, and 23.9 after the fourth dose. The median period of quiescence before flare was 3.9 years. An increase in uveitis flare was seen in both infectious uveitis (baseline 13.1 compared with 20.2 after first dose, 154% increase) and noninfectious uveitis (baseline 12.4 compared with 20.9 after first dose, 169% increase). Risk factors for uveitis flare were identified to be recurrent uveitis, chronic uveitis, shorter period of quiescence, and first dose of vaccine. Median time to uveitis flare was 0.53 months after the first vaccination, 1.74 months after the second vaccination, and 1.35 months after the third vaccination.
Conclusions
The current study demonstrates an increased risk of uveitis flare after the first dose of COVID vaccination. This risk was highest in those with previous recurrences, chronic uveitis, and shorter period of quiescence.
Financial Disclosure(s)
Proprietary or commercial disclosure may be found after the references.
Keywords: COVID-19, Uveitis, Vaccination
Severe acute respiratory syndrome coronavirus 2 was first reported in China in late 2019 and manifested as the respiratory illness termed coronavirus disease 2019 (COVID-19). The World Health Organization declared the COVID-19 outbreak a pandemic in 2020. To date, COVID-19 has been implicated in > 6 million deaths worldwide, with the disease sweeping around the globe with dramatic implications for both the health of populations and their everyday functions.
The pandemic prompted a collaborative global effort to develop a vaccine to COVID-19 within an unprecedented timeframe. This has resulted in 4 main types of vaccine. These include mRNA vaccines (BNT162b2, Pfizer-BioNTech; mRNA-1273, Moderna), vector vaccines (Ad26.COV2, Janssen Johnson & Johnson; ChAdOx1 nCoV-19/AZD1222, Oxford-AstraZeneca), protein subunit vaccines (NVX-CoV2373, Novavax), and whole virus vaccines (PiCoVacc, Sinovac; BBIBP-CorV, Sinopharm).1, 2, 3
In 2020, the Food and Drug Administration approved the BNT162b2 mRNA COVID-19 (Pfizer-BioNTech) vaccine, which has significantly decreased the mortality and morbidity related to the disease.1 , 2 In late 2020, the first COVID vaccines were administered in the United Kingdom, later to be rolled out worldwide. It has now been estimated that > 12 billion doses of vaccine have been given across the globe.
After the introduction of the COVID vaccine, cases of possible vaccine-related uveitis have been increasingly reported; however, the true magnitude of the response has not yet been clearly identified. The majority of reports have investigated de novo uveitis or recurrence of uveitis within the 21 to 30 days after the vaccination.4, 5, 6, 7, 8 Because most of the reports have been individual cases or small case series, it is unclear whether this is a genuine association or a chance finding.
The current study aimed to investigate a large population of patients within Auckland, New Zealand, with known uveitis and identify rates of reactivation both before and after the COVID-19 vaccine and endeavor to identify those most at risk.
Methods
Subject Selection
This retrospective study was performed at a major tertiary ophthalmology department in New Zealand (Department of Ophthalmology, Auckland District Health Board, Auckland). Institutional review board/ethics committee approval was obtained from the Auckland Health Research Ethics Committee (AH1339) and the Research Development Office, Auckland District Health Board (A+ 8414). All research adhered to the tenets of the Declaration of Helsinki. The requirement for informed consent was waived because of the retrospective nature of the study.
Subjects were identified from the Inflammatory Eye Disease Registry at Auckland District Health Board, which records the details of patients diagnosed with uveitis. Subjects were included if they were reviewed between January 1, 2010, and December 31, 2020 (before COVID vaccination rollout in New Zealand). Subjects were excluded if they had no record of vaccination, if they were deceased before vaccination, if they transferred their care out of Auckland District Health Board, or if the affected eye was enucleated or eviscerated.
Coronavirus Disease Vaccination
Coronavirus disease vaccinations commenced in early 2021 in the New Zealand population, and it has been estimated that 90% of the population aged > 12 years has received at least 2 doses of the vaccine. The New Zealand government initially had a single vaccine on offer (BNT162b2 mRNA COVID-19, Pfizer-BioNTech); however, subsequently, a small volume of other vaccines have been approved and made available in New Zealand for those who have a negative reaction to the vaccine or who object to mRNA vaccine options. Date and type of COVID vaccination were determined from the patient clinical record, accessed via the Regional Clinical Portal.
Data Collection
All data were collected on a standardized proforma. Anatomic location of uveitis was recorded according to the Standardization of Uveitis Nomenclature Working Group. Where available, Standardization of Uveitis Nomenclature classification guides were used for the diagnosis of uveitis etiology. A review of each patient’s clinical documentation was performed. The presence of new uveitis activity was identified via interpretation of clinician documentation on the day of assessment. Uveitis flare was defined as the presence of new uveitis activity or increased activity that required a change in uveitis treatment.9
The rate of flare was calculated for 3 months before vaccination (baseline rate) and for 3 months after each vaccination, reported as rate of flare per 1000 patient-months. Where the time between vaccination or the follow-up time was < 3 months, the rate was calculated for the abbreviated period of time.
Statistical Analysis
All data were entered into an Excel spreadsheet and analyzed in STATA, version 15 (StataCorp 2017). Categorical data are reported as n (%) and continuous as median (interquartile range [IQR]). A mixed effects Poisson model, with rates over time clustered within patients, was used to assess the rate of flare after vaccination. Time to uveitis flare was plotted with a Nelson–Aalen cumulative incidence plot. Hazard function was estimated in STATA with a weighted kernel smooth of the estimated hazard function. A P value of ≤ 0.05 was considered statistically significant.
Results
Notes were reviewed for 3770 patients; 762 were excluded because there was no record of vaccination (n = 436), they died before vaccination (n = 254), they were transferred outside of Auckland District Health Board or to a private clinic provider (n = 66), or they received enucleation or evisceration of the affected eye (n = 6). After exclusions, 4184 eyes of 3008 patients were included in the study with a total of 8474 vaccinations given during the study period (3008 first dose, 2974 second dose, 2315 third dose, and 177 fourth dose). All vaccinations given were the BNT162b2 mRNA COVID-19 except for 1 patient who had a flare after the first dose and received the Novavax (NVX-CoV2373) COVID-19 vaccination for their second dose. Subject demographics are reported in Table 1 . Median age was 54.8 years (IQR, 41.7–67.6), and 1474 (49.0%) were female. The course of disease at time of vaccination was a single acute episode in 1362 patients (45.3%), recurrent acute uveitis in 758 patients (25.2%), and chronic in 888 patients (23.7%). Noninfectious etiology was most common, occurring in 2296 patients (76.3%) and infectious etiology occurring in 712 patients (23.7%). Etiology of uveitis is listed in Table 1. The median time from diagnosis of uveitis to the first vaccine dose was 7.2 years (IQR, 3.4–11.6), and the median period of quiescence was 3.9 years (IQR, 1.6–8.0).
Table 1.
Subject Demographics
| N = 3008 | |
|---|---|
| Age at time of vaccination, yrs | Median, 54.8 (IQR, 41.7–67.6) |
| Female | 1474 (49.0%) |
| Ethnicity | |
| White | 1658 (55.1%) |
| Māori | 200 (6.6%) |
| Pacific peoples | 416 (13.8%) |
| Asian | 651 (21.6%) |
| Other | 56 (1.9%) |
| Unknown | 27 (0.9%) |
| Time course | |
| Acute | 1362 (45.3%) |
| Recurrent | 758 (25.2%) |
| Chronic | 888 (29.5%) |
| Infectious | 712 (23.7%) |
| Etiology | |
| Idiopathic | 951 (31.6%) |
| HLAB27-associated uveitis | 683 (22.7%) |
| Sarcoidosis | 132 (4.4%) |
| Vogt–Koyanagi–Harada disease | 38 (1.3%) |
| Birdshot chorioretinopathy | 24 (0.8%) |
| Multifocal choroiditis | 34 (1.1%) |
| Punctate inner choroiditis | 20 (0.7%) |
| Juvenile idiopathic arthritis | 23 (0.8%) |
| Tubulointerstitial nephritis and uveitis syndrome | 24 (0.8%) |
| Fuchs’ heterochromic iridocyclitis | 62 (2.1%) |
| Other noninfectious etiology | 292 (9.7%) |
| Herpes zoster virus | 273 (9.1%) |
| Herpes simplex virus | 46 (1.5%) |
| Cytomegalovirus | 24 (0.8%) |
| Presumed herpetic uveitis or Posner–Schlossman | 65 (2.2%) |
| Toxoplasmosis | 127 (4.2%) |
| Tuberculosis | 88 (2.9%) |
| Syphilis | 51 (1.7%) |
| Other infectious | 51 (1.7%) |
| Anatomic classification | |
| Anterior | 2218 (73.7%) |
| Intermediate | 118 (3.9%) |
| Posterior | 228 (7.6%) |
| Panuveitis | 444 (14.8%) |
| DMARD | 290 (9.6%) |
| Time from diagnosis to first vaccination | 7.2 yrs (IQR, 3.4–11.6) |
| Time quiescent at first vaccination | 3.9 yrs (IQR, 1.6–8.0) |
DMARD = disease-modifying antirheumatic drug; IQR = interquartile range.
The rate of uveitis flare was 12.3 per 1000 patient-months at baseline, 20.7 after the first dose, 15.0 after the second dose, 12.8 after the third dose, and 23.9 after the fourth dose. The rate of flare is listed by anatomic location of uveitis and clinical course in Table 2 . The fourth dose is excluded in these tables because of small numbers at the time of data collection. A statistically significant increase in anterior uveitis flare was noted, increasing from 11.9 at baseline to 20.5 after the first dose of vaccine (172% increase, P = 0.027). The highest risk of flare was observed in patients with intermediate uveitis, in which the rate increased from 11.3 at baseline to 27.0 after first dose (239% increase, P = 0.397).
Table 2.
Rate of Flare by Anatomic Location and Time Course
| Number | Baseline | First Dose | Second Dose | Third Dose | P Value | P Value (First Dose) | |
|---|---|---|---|---|---|---|---|
| Anatomic location | |||||||
| Anterior | 2,218 | 11.9 | 20.5 | 16.3 | 11.5 | 0.150 | 0.027 |
| Intermediate | 118 | 11.3 | 27.0 | 14.4 | 17.1 | 0.560 | 0.397 |
| Posterior | 228 | 10.2 | 19.4 | 4.8 | 6.1 | 0.940 | 0.429 |
| Panuveitis | 444 | 18.0 | 21.2 | 13.7 | 21.3 | 0.782 | 0.729 |
| Time course | |||||||
| Acute | 1,362 | 8.7 | 17.5 | 8.4 | 9.4 | 0.411 | 0.088 |
| Recurrent | 758 | 17.2 | 23.7 | 23.8 | 17.5 | 0.460 | 0.374 |
| Chronic | 888 | 14.6 | 24.2 | 18.0 | 14.4 | 0.309 | 0.153 |
P value compares the baseline rate with after vaccination. P value (first dose) compares the baseline rate with after the first dose of vaccination.
Rate of uveitis flare divided by etiology of uveitis is reported in Table 3 for those diagnoses with > 100 affected patients. An increase in uveitis flare was seen in both infectious uveitis (baseline 13.1 vs. 20.2 after first dose, 154% increase, P = 0.303) and noninfectious uveitis (baseline 12.4 vs. 20.9 after first dose, 169% increase P = 0.026). The rate of flare after the first dose increased 131% for idiopathic uveitis (P = 0.508), 240% for HLAB27 uveitis (P = 0.030), and 353% for sarcoid uveitis (P = 0.264). For infectious etiology, the rate of flare increased 129% for herpes zoster uveitis (P = 0.702). There was no increase observed after the first dose for toxoplasmosis (67% decrease, P = 0.583), but the numbers were small, and a peak was observed after the third dose of vaccination.
Table 3.
Rate of Flare by Uveitis Etiology
| Number | Baseline | First Dose | Second Dose | Third Dose | P Value | P Value (First Dose) | |
|---|---|---|---|---|---|---|---|
| Not infectious | 2296 | 12.4 | 20.9 | 15.5 | 11.1 | 0.178 | 0.026 |
| Idiopathic | 951 | 11.9 | 15.6 | 11.4 | 9.3 | 0.948 | 0.508 |
| HLAB27 uveitis | 683 | 12.7 | 30.5 | 23.2 | 14.1 | 0.109 | 0.030 |
| Sarcoid | 132 | 7.6 | 26.8 | 17.9 | 12.4 | 0.386 | 0.264 |
| Infectious | 712 | 13.1 | 20.2 | 13.4 | 18.0 | 0.471 | 0.303 |
| Herpes zoster | 273 | 14.7 | 18.9 | 10.1 | 6.0 | 0.767 | 0.702 |
| Toxoplasmosis | 127 | 7.9 | 2.6 | 5.3 | 40.7 | 0.627 | 0.583 |
Rate of flare per 1000 patient-months reported in etiology with > 100 patients. P value compares the baseline rate with after vaccination. P value (first dose) compares the baseline rate with after the first dose of vaccination.
Some infections may be expected to be a single event in the absence of reinfection (e.g., syphilis). Other infections are usually (but not always) monophasic, such as acute retinal necrosis. Tuberculosis may resolve with adequate treatment, although up to one third may experience paradoxical flare, and many will have chronic inflammation. Therefore, we repeated the analysis excluding all infections other than viral etiology (herpes zoster virus, herpes simplex virus, cytomegalovirus, and presumed herpetic) or toxoplasmosis because these are more prone to recurrent inflammation. Repeating the analysis showed impact of the first vaccine had an incidence rate ratio (IRR) of 1.600 (P = 0.035) on univariate analysis and 1.500 (P = 0.093 on multivariate analysis.
Risk factors for uveitis flare are reported in Table 4 . On univariate analysis, recurrent uveitis (IRR, 1.831; P = 0.003), chronic uveitis (IRR, 1.690; P = 0.009), shorter period of quiescence (IRR, 0.994; P < 0.001), and first dose of vaccine (IRR, 1.642; P = 0.016) were associated with uveitis flare. On multivariate analysis, recurrent uveitis (IRR, 1.872; P = 0.006), chronic uveitis (IRR, 1.768; P = 0.012), quiescence (IRR, 0.993; P < 0.001), and first vaccination (IRR, 1.675; P = 0.017) were associated with risk of flare.
Table 4.
Risk Factors for Uveitis Flare
| Univariate |
Multivariate |
|||
|---|---|---|---|---|
| IRR | P Value | IRR | P Value | |
| Age at vaccine | 0.992 | 0.056 | 0.999 | 0.920 |
| Female | 1.034 | 0.826 | 1.221 | 0.224 |
| Ethnicity | ||||
| Māori | 0.956 | 0.893 | 1.012 | 0.974 |
| Pacific peoples | 1.098 | 0.690 | 1.104 | 0.701 |
| Asian | 1.377 | 0.081 | 1.231 | 0.316 |
| Other | 1.452 | 0.460 | 1.636 | 0.336 |
| Infectious | 1.052 | 0.774 | 1.017 | 0.935 |
| Time course | ||||
| Recurrent | 1.831 | 0.003 | 1.872 | 0.006 |
| Chronic | 1.690 | 0.009 | 1.768 | 0.012 |
| Anatomic location | ||||
| Intermediate | 1.156 | 0.702 | 1.366 | 0.435 |
| Posterior | 0.708 | 0.319 | 0.894 | 0.776 |
| Panuveitis | 1.296 | 0.193 | 1.246 | 0.366 |
| DMARD | 1.453 | 0.094 | 0.980 | 0.943 |
| Quiescence (mos) | 0.994 | < 0.001 | 0.995 | <0.001 |
| Vaccine | ||||
| First dose | 1.642 | 0.016 | 1.675 | 0.017 |
| Second dose | 1.174 | 0.469 | 1.234 | 0.362 |
| Third dose | 1.003 | 0.987 | 1.064 | 0.809 |
| Fourth dose | 1.858 | 0.302 | 2.038 | 0.242 |
DMARD = disease-modifying antirheumatic drug; IRR = incidence rate ratio.
Ethnicity is compared with White; time course is compared with acute, and anatomic location is compared with anterior. Mixed effects Poisson model. Values in bold were statistically significant on multivariate analysis.
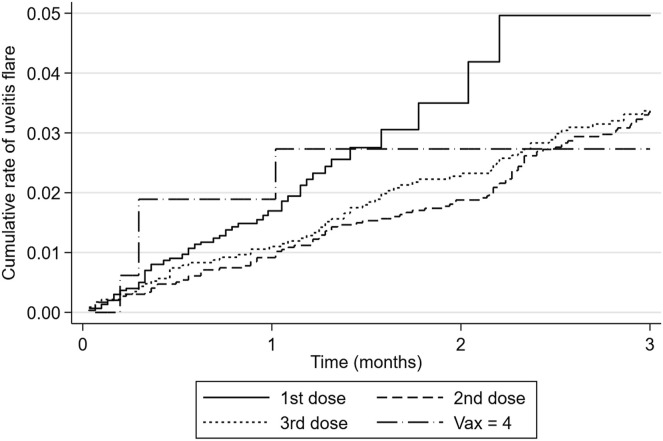
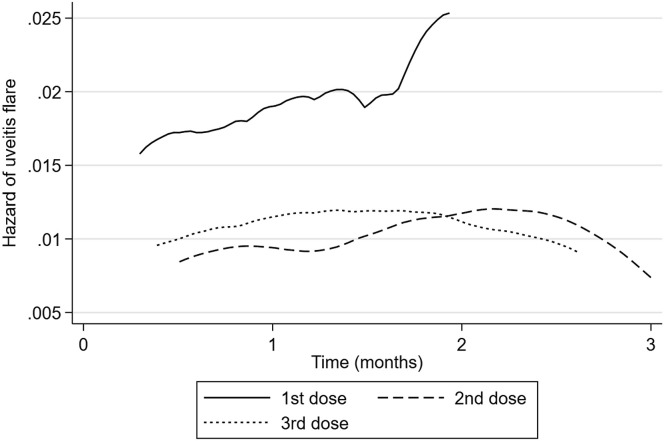
Cumulative rate of uveitis flare after vaccination is plotted in Figure 1 , and hazard of uveitis flare is plotted in Figure 2 . If there was no association between vaccination and uveitis flare, one would expect the hazard plot to be a flat line. Instead, a peak is observed of increased risk after vaccination. Hazard of uveitis flare was highest at 2 months after vaccination for the first and second doses and at 6 weeks after the third dose of vaccination. Median time to uveitis flare was 0.53 months (IQR, 0.30–0.95) after the first vaccination, 1.74 months (IQR 0.89–2.34) after the second vaccination, and 1.35 months (IQR, 0.49–2.17) after the third vaccination.
Figure 1.
Cumulative rate of uveitis flare after vaccination.
Figure 2.
Hazard of uveitis flare after vaccination. Fourth dose excluded because of small numbers.
Discussion
Vaccinations for COVID-19 have been shown to reduce the risk of developing COVID-19 and to reduce mortality and morbidity after COVID-19 illness.2 After the introduction of the mRNA-based vaccines for COVID-19, initial safety studies did not identify any increased risk of uveitis after vaccination.10 However, since this time, there have been increasing reports of de novo uveitis or uveitis recurrence with a temporal association to the COVID-19 vaccine,8 underlining the importance of postmarketing surveillance and clinician vigilance in detecting rare complications of treatments. This possible association creates a dilemma for clinicians aiming to prevent potential morbidity associated with recurrent uveitis. Identifying those most at risk provides an essential tool for clinicians to both counsel and manage patients effectively. Up until now, comparative rates of recurrence before and after any vaccination and risk factors for reactivation have not been identified. With the global vaccine rollout of the COVID-19 vaccination occurring simultaneously and over a short period of time, this has allowed for more thorough investigation into rates and identify risk factors for associated recurrence. The current study highlights the increase in risk of uveitis flare occurring post-BNT162b2 mRNA COVID-19 vaccination in subjects with preexisting uveitis. Those with a shorter period of quiescence and those with a history of recurrent or chronic disease were at increased risk.
Reactivation of uveitis after vaccination has been reported after a wide range of vaccinations, including bacilli Calmette–Guérin, diphtheria-pertussis-tetanus; hepatitis A; hepatitis B; human papillomavirus; influenza; measles; measles-mumps-rubella; pneumococcal; smallpox; and varicella zoster.11 , 12 This recurrence of uveitis after vaccination has been postulated to be due to autoimmunity triggered by the vaccines and may occur by various mechanisms, including cytokine production, expression of HLAs, modification of surface antigens, induction of novel antigens, molecular mimicry, bystander activation, epitope spreading, polyclonal activation of B cells, and an immune reaction to vaccination adjuvants.11 , 12
Reports of possible ocular complications after the COVID vaccine include facial nerve palsy, anterior uveitis, herpetic keratitis, corneal graft rejection, anterior scleritis, posterior uveitis, panuveitis,13 occlusive vasculitis, bilateral multifocal choroiditis, toxoplasma retinochoroiditis, Vogt-Koyanagi-Harada reactivations or Vogt-Koyanagi-Harada–like disease, Behçet’s disease, multiple evanescent white dot syndromes, acute macular neuroretinopathy, retinal vein occlusions, nonarteritic ischemic optic neuropathy, activation of quiescent choroidal neovascularization secondary to myopia or uveitis, and central serous chorioretinopathy.6, 7, 8 , 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26 Uveitis, in particular, may occur de novo or as a recurrence in a subject with a known history of uveitis.
Systemic conditions with an association to uveitis also have been reported to have higher rates of flare after the COVID vaccination. These include rheumatoid arthritis, systemic lupus erythematous, and ankylosing spondylitis. It has also been reported that higher rates of flare of these conditions were seen in individuals with higher levels of disease activity and shorter periods of disease quiescence before vaccination.27
A recent retrospective cohort study investigated the rates of noninfectious uveitis in a large population of individuals in the 21 days after the first 2 doses of the BNT162b2 mRNA COVID-19 vaccine, including a population with previous uveitis events.6 Tomkins-Netzer et al6 noted a 21-day overall risk of uveitis flare of 3.85 and 3.61 per 100 000 vaccinated individuals after the first and second vaccine doses, respectively. This risk was observed to be higher in those with a history of uveitis, with the authors noting an attributable risk of 116.94 per 100 000 and 31.27 per 100 000 vaccinees after the first and second doses, respectively, in those individuals with a history of uveitis.6
In the current study, rate of uveitis flare was 12.3 per 1000 patient-months at baseline, 20.7 after the first dose, 15.0 after the second dose, and 12.8 after the third dose. This suggests that the highest risk period is after the first dose of vaccine.4 , 6 The numbers for fourth vaccination are too small to draw inferences from; however, the increase in uveitis flares observed in this small sample highlights the importance of ongoing monitoring. The observation that the first vaccine is associated with the highest risk of flare confirms the findings of the population-based study of Tomkins-Netzer et al.6 Our current study strengthens these findings by including both infectious and noninfectious uveitis, and by providing more granularity, to examine different types of uveitis, diagnoses, period of quiescence, and baseline immunosuppression.
As reported by Tomkins-Netzer et al,6 flares occurred more frequently after vaccination in those with noninfectious uveitis. However, the current study also observed a similar increase in flares in those with infectious uveitis. Vaccination is associated with a transient period of lymphopenia and may result in a temporary period of relative immunosuppression in the subject.10 Recent reports also have suggested that vaccines can cause epigenetic changes that affect the immune system’s reactivity, causing a period of vaccine-induced immune suppression limiting the body’s ability to keep previously latent infections under control.27
De novo herpes zoster has been reported to occur at a higher rate after vaccination.23 Several authors have noted isolated cases or small series of flares in herpes zoster after vaccination, and this current study is the first to confirm that, in subjects with known herpes zoster ophthalmicus, rate of flare after vaccination was 129% higher than baseline. Reports of recurrent toxoplasmosis chorioretinitis have been presented.5 , 28 It has previously been reported that the risk of toxoplasmosis recurrence should decline with time after the initial diagnosis,29 but the current study observed a spike after the third dose. Numbers were too small to draw significant inference from this, and further investigation is needed to determine whether vaccination does truly increase the risk of flare in those with a history of ocular toxoplasmosis.
The current study demonstrated that risk factors for recurrence of uveitis after vaccination were known previous recurrences, chronic uveitis, and shorter period of quiescence before vaccination. The first dose of vaccine was associated with the highest risk of flare. This may be due to a stronger immunogenic reaction after the initial dose of a de novo vaccine. It is also possible that people with a history of flare with previous vaccine doses may have already recommenced prophylactic therapy before subsequent doses or their baseline treatment increased. It must be noted that 1 patient with flare swapped vaccine type for a subsequent dose with no further flare observed.
The highest risk of flare was observed in patients with intermediate uveitis, in which the rate increased from 11.3 at baseline to 27.0 after the first dose (239% increase). Previous reports of vaccine uveitis (before COVID vaccination) have suggested that any case of recurrent uveitis after vaccination is more likely to recur in intermediate, pan, or posterior uveitis.30 This may be related to the fact that intermediate and posterior uveitis are harder to treat, require longer treatment periods, and are more likely to be chronic, to require disease-modifying antirheumatic drugs (DMARDs), and to require longer periods of time to be considered quiescent.31 , 32
Previous reports of uveitis flares after vaccination have noted average periods of quiescence before reactivation of 12 months to 3.3 years.4 , 5 , 33 , 34 The current study noted a longer median time of quiescence before vaccine-related recurrence of 3.9 years. Shorter periods of quiescence were identified to be a significant risk factor for recurrence of uveitis after the vaccine. Duration of uveitis quiescence has been previously identified as a predictive factor for recurrence with longer periods of inactivity related to lower rates of reactivation.35 , 36
In a previous review of non-COVID vaccine–related uveitis (published before the pandemic), the authors noted the median time from vaccination to onset of uveitis was 16 days.11 , 16 After COVID-19 vaccination, previous studies have identified median times to diagnosis of recurrent uveitis of 8.5 to 12 days.4 , 5 , 34 However, a report has been presented of reactivation of Vogt–Koyanagi–Harada disease 6 weeks after a second dose of the BNT162b2 mRNA COVID-19 vaccine.37
Tomkins-Netzer et al6 noted that the median time between vaccine and uveitis recurrence in the population with previous uveitis was 8.5 days and 10 days after the first vaccine dose and the second vaccine dose, respectively. However, they only studied the population to 21 days, so those with a longer latency will have been missed. The majority of case reports and studies have identified recurrences within the 30 days after vaccine doses. We specifically chose a longer time period to enable us to capture the full risk of flare and to determine the optimal time period for studying vaccine-associated uveitis flare. We observed that the highest hazard of flare was at 2 months postvaccination and that the median time to diagnosis recurrence was 0.53 months (IQR, 0.30–0.95) after the first dose, 1.74 months (IQR, 0.89–2.34) after the second dose, and 1.35 months (IQR, 0.49–2.17) after the third dose. Therefore, we recommend that future studies of vaccine flare include a time period of 10 to 12 weeks after vaccination to ensure cases are not missed.
Where the time between vaccination or the follow-up time was < 3 months, the rate was calculated for the abbreviated period of time. The median time from first to second vaccine was 42 days (1.38 months). The authors acknowledge that that some delayed vaccine-associated uveitis flares after the first dose may have occurred in the time interval after the second dose. Conversely, assuming a higher rate of flare early postvaccine, the shorter observation period may result in less dilution of the data.
Patient use of DMARDs did not appear to confer significant risk of uveitis flare or to provide significant protection against uveitis flare after vaccination. At the time of initiation of COVID vaccination within the New Zealand population, the recommendation was to skip the dose of DMARD that week if the disease was considered stable enough to do so. Therefore, there was a risk that uveitis flares may occur because of recommended changes in medication dosing, rather than because of vaccination per se. The lack of any association with DMARD use and vaccine-associated uveitis flare in our study means that this is less likely to have made a strong contribution to the numbers observed.
Although vaccination for COVID-19 was associated with increased risk of flare of uveitis, COVID-19 infection itself may also pose significant risk to a patient’s eye health. Reported ophthalmic manifestations of COVID-19 include conjunctivitis, keratitis, de novo and recurrent uveitis, episcleritis, scleritis, posterior uveitis, retinal artery and vein occlusions, acute macular neuroretinopathy, paracentral acute middle maculopathy, optic neuropathy, and cranial nerve palsies.25 , 38 Further study will be required to determine how the risk of flare of uveitis compares between COVID vaccination and COVID-19 disease.
Study Limitations
The authors of this study recognize the limitations of this retrospective observational study. Data were collected from clinical notes that may not be complete and were taken from a single tertiary center. The clinicians were not blinded to the vaccination status of the patient, and interpretation of disease activity may have been influenced by the knowledge of the patient having received a recent vaccination. During the observational time period, COVID-related “lockdowns” may have limited patients’ access to tertiary level care, and disease may have been self-managed, especially in patients with known chronic disease. Data regarding prophylactic therapy before subsequent vaccine doses or increased baseline treatment were not collected and may have influenced rates of flare and would be an interesting direction for further studies.
The current study focused on subjects with previous diagnoses of uveitis; however, there have been reports of initial uveitis diagnosis after the COVID vaccine.6 , 39 Further work will be required to examine de novo uveitis after COVID vaccination and to explore the long-term sequelae of this. We also recognize that the data presented are limited to BNT162b2 mRNA COVID-19 vaccine–related uveitis recurrence because of the vaccine schedule available to the New Zealand population at the time of observation. Data were on concurrent use if prophylactic antimicrobial or antiviral therapy was not collected, and the impact of this on flare rate in infectious uveitis would be an important further direction for study.
Conclusions
Coronavirus disease vaccination has effectively and rapidly reduced morbidity and mortality from COVID-19 around the globe. As with any treatment, this vaccination comes with associated risk. Multiple small case series have been published highlighting the potential role of COVID vaccination in uveitis flare.4 , 6, 7, 8 , 11 , 13 , 15, 16, 17 , 20 , 22 , 23 , 25 , 28 , 33 , 34 This study of 3008 patients confirms that, for both infectious and noninfectious uveitis, vaccination is associated with an increased risk of uveitis flare. This increased risk is small on a real scale, increasing from 13.1 compared with 20.2 flares per 1000 patient-months in infectious uveitis (154% increase) and 12.4 compared with 20.9 per 1000 patient-months (169%) for noninfectious uveitis after the first dose. However, this may be particularly important in those with poorly controlled disease, severe uveitis, or shorter period of quiescence or in only eyes. In these individuals, this study allows more nuanced conversations around the associated risk and allows clinicians to weigh the pros and cons of pretreating before vaccination.
Further study will be required to examine the effect of pretreatment before vaccination, the effects of prophylactic antimicrobial or antiviral therapy, the risk of flare of uveitis with other vaccine subtypes, and the implications for new vaccine formulae to manage the ever-evolving strains of COVID-19 that continue to emerge.
Manuscript no. OPHTHA-D-22-01669
Footnotes
Disclosure(s): All authors have completed and submitted the ICMJE disclosures form.
The author(s) have made the following disclosure(s): R.N.: Speaker payments – AbbVie and GSK.
Presented at: the Annual RANZCO Scientific Conference, Brisbane, Queensland, Australia, October 28 to November 1, 2022. The authors confirm it has not previously been presented or submitted for publication.
HUMAN SUBJECTS: Human subjects data were included in this study. This retrospective study was performed at a major tertiary ophthalmology department in New Zealand (Department of Ophthalmology, Auckland District Health Board, Auckland). Institutional Review Board (IRB)/Ethics Committee approval was obtained from the Auckland Health Research Ethics Committee (AH1339) and the Research Development Office, Auckland District Health Board (A+ 8414). All research adhered to the tenets of the Declaration of Helsinki. The requirement for informed consent was waived because of the retrospective nature of the study.
No animal subjects were used in this study.
Author Contributions:
Conception and design: Jordan, Sims, McGhee, Niederer
Data collection: Jordan, Townend, Allen, Niederer
Analysis and interpretation: Jordan, Sims, McGhee, Niederer
Obtained funding: N/A; Study was performed as part of regular employment duties at The University of Auckland and Auckland District Health Board. No additional funding was provided.
Overall responsibility: Jordan, Townend, Allen, Sims, McGhee
References
- 1.Baden L.R., El Sahly H.M., Essink B., et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Creech C.B., Walker S.C., Samuels R.J. SARS-CoV-2 vaccines. JAMA. 2021;325:1318–1320. doi: 10.1001/jama.2021.3199. [DOI] [PubMed] [Google Scholar]
- 3.Self W.H., Tenforde M.W., Rhoads J.P., et al. Comparative effectiveness of Moderna, Pfizer-BioNTech, and Janssen (Johnson & Johnson) vaccines in preventing COVID-19 hospitalizations among adults without immunocompromising conditions - united states, march-august 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1337–1343. doi: 10.15585/mmwr.mm7038e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferrand N., Accorinti M., Agarwal M., et al. COVID-19 vaccination and uveitis: epidemiology, clinical features and visual prognosis. Ocul Immunol Inflamm. 2022:1–9. doi: 10.1080/09273948.2022.2058964. [DOI] [PubMed] [Google Scholar]
- 5.Chew M.C., Wiryasaputra S., Wu M., et al. Incidence of COVID-19 vaccination-related uveitis and effects of booster dose in a tertiary uveitis referral center. Front Med (Lausanne) 2022;9 doi: 10.3389/fmed.2022.925683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tomkins-Netzer O., Sar S., Barnett-Griness O., et al. Association between vaccination with the BNT162b2 mRNA Coronavirus Disease 2019 vaccine and noninfectious uveitis: a population-based study. Ophthalmology. 2022;129:1087–1095. doi: 10.1016/j.ophtha.2022.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singh R.B., Singh Parmar U.P., Kahale F., et al. Vaccine-associated uveitis following SARS-CoV-2 vaccination: a CDC-VAERS database analysis. Ophthalmology. 2022;S0161-6420(22):00672–00678. [Google Scholar]
- 8.Wang M.T.M., Niederer R.L., McGhee C.N.J., Danesh-Meyer H.V. COVID-19 vaccination and the eye. Am J Ophthalmol. 2022;240:79–98. doi: 10.1016/j.ajo.2022.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jabs D.A. Improving the reporting of clinical case series. Am J Ophthalmol. 2005;139:900–905. doi: 10.1016/j.ajo.2004.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barda N., Dagan N., Ben-Shlomo Y., et al. Safety of the BNT162b2 mRNA covid-19 vaccine in a nationwide setting. N Engl J Med. 2021;385:1078–1090. doi: 10.1056/NEJMoa2110475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benage M., Fraunfelder F.W. Vaccine-associated uveitis. Mo Med. 2016;113:48–52. [PMC free article] [PubMed] [Google Scholar]
- 12.Cunningham E.T., Moorthy R.S., Fraunfelder F.W., Zierhut M. Vaccine-associated uveitis. Ocul Immunol Inflamm. 2019;27:517–520. doi: 10.1080/09273948.2019.1626188. [DOI] [PubMed] [Google Scholar]
- 13.Mudie L.I., Zick J.D., Dacey M.S., Palestine A.G. Panuveitis following vaccination for COVID-19. Ocul Immunol Inflamm. 2021;29:741–742. doi: 10.1080/09273948.2021.1949478. [DOI] [PubMed] [Google Scholar]
- 14.Haseeb A.A., Solyman O., Abushanab M.M., et al. Ocular complications following vaccination for COVID-19: a one-year retrospective. Vaccines (Basel) 2022;10:342. doi: 10.3390/vaccines10020342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.ElSheikh R.H., Haseeb A., Eleiwa T.K., Elhusseiny A.M. Acute uveitis following COVID-19 vaccination. Ocul Immunol Inflamm. 2021;29:1207–1209. doi: 10.1080/09273948.2021.1962917. [DOI] [PubMed] [Google Scholar]
- 16.Goyal M., Murthy S.I., Annum S. Bilateral multifocal choroiditis following COVID-19 vaccination. Ocul Immunol Inflamm. 2021;29:753–757. doi: 10.1080/09273948.2021.1957123. [DOI] [PubMed] [Google Scholar]
- 17.Bolletta E., Iannetta D., Mastrofilippo V., et al. Uveitis and other ocular complications following COVID-19 vaccination. J Clin Med. 2021;10:5960. doi: 10.3390/jcm10245960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jain A., Kalamkar C. COVID-19 vaccine-associated reactivation of uveitis. Indian J Ophthalmol. 2021;69:2899–2900. doi: 10.4103/ijo.IJO_1435_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cunningham E.T., Moorthy R.S. Vaccine-associated posterior uveitis. Retina. 2020;40:595–598. doi: 10.1097/IAE.0000000000002816. [DOI] [PubMed] [Google Scholar]
- 20.Kim S.Y., Kang M.S., Kwon H.J. Bilateral panuveitis mimicking Vogt-Koyanagi-Harada disease following the first dose of ChAdOx1 nCoV-19 vaccine. Ocul Immunol Inflamm. 2022:1–4. doi: 10.1080/09273948.2022.2026410. [DOI] [PubMed] [Google Scholar]
- 21.De Domingo B., Lopez M., Lopez-Valladares M., et al. Vogt-Koyanagi-Harada disease exacerbation associated with COVID-19 vaccine. Cells. 2022;11:1012. doi: 10.3390/cells11061012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ng X.L., Betzler B.K., Testi I., et al. Ocular adverse events after COVID-19 vaccination. Ocul Immunol Inflamm. 2021;29:1216–1224. doi: 10.1080/09273948.2021.1976221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Agrawal S., Verma K., Verma I., Gandhi J. Reactivation of herpes zoster virus after COVID-19 vaccination: is there any association? Cureus. 2022;14 doi: 10.7759/cureus.25195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hebert M., Couture S., Schmit I. Bilateral panuveitis with occlusive vasculitis following Coronavirus Disease 2019 vaccination. Ocul Immunol Inflamm. 2022:1–5. doi: 10.1080/09273948.2022.2042325. [DOI] [PubMed] [Google Scholar]
- 25.Hu K., Patel J., Swiston C., Patel B.C. StatPearls. Treasure Island. StatPearls Publishing LLC; FL: 2022. Ophthalmic manifestations of coronavirus (COVID-19) [PubMed] [Google Scholar]
- 26.Li Z., Hu F., Li Q., et al. Ocular adverse events after inactivated COVID-19 vaccination. Vaccines (Basel) 2022;10:918. doi: 10.3390/vaccines10060918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cohen S., Olshaker H., Fischer N., et al. Herpetic eye disease following the SARS-CoV-2 vaccinations. Ocul Immunol Inflamm. 2022:1–12. doi: 10.1080/09273948.2022.2103831. [DOI] [PubMed] [Google Scholar]
- 28.Testi I., Brandao-de-Resende C., Agrawal R., et al. Ocular inflammatory events following COVID-19 vaccination: a multinational case series. J Ophthalmic Inflamm Infect. 2022;12:4. doi: 10.1186/s12348-021-00275-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wadhwa H., Sims J.L., Niederer R.L. Rate of recurrence of toxoplasmosis retinochoroiditis at a tertiary eye centre in Auckland. N Z Med J. 2022;135:10–18. [PubMed] [Google Scholar]
- 30.Lazow M., Kim S. Incidence and risk factors for recurrent uveitis after long-term treatment. Invest Ophthalmol Vis Sci. 2014;55:5326. [Google Scholar]
- 31.Kempen J.H., Gewaily D.Y., Newcomb C.W., et al. Remission of intermediate uveitis: Incidence and predictive factors. Am J Ophthalmol. 2016;164:110–117.e2. doi: 10.1016/j.ajo.2015.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Niederer R.L., Sharief L., Bar A., et al. Predictors of long-term visual outcome in intermediate uveitis. Ophthalmology. 2017;124:393–398. doi: 10.1016/j.ophtha.2016.11.013. [DOI] [PubMed] [Google Scholar]
- 33.Bolletta E., Iannetta D., Mastrofilippo V., et al. Uveitis and other ocular complications following COVID-19 vaccination. J Clin Med. 2021;10:5960. doi: 10.3390/jcm10245960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rabinovitch T., Ben-Arie-Weintrob Y., Hareuveni-Blum T., et al. Uveitis after the BNT162b2 mRNA vaccination against SARS-CoV-2 infection: a possible association. Retina. 2021;41:2462–2471. doi: 10.1097/IAE.0000000000003277. [DOI] [PubMed] [Google Scholar]
- 35.Sobrin L., Pistilli M., Dreger K., et al. Factors predictive of remission of chronic anterior uveitis. Ophthalmology. 2020;127:826–834. doi: 10.1016/j.ophtha.2019.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grunwald L., Newcomb C.W., Daniel E., et al. Risk of relapse in primary acute anterior uveitis. Ophthalmology. 2011;118:1911–1915. doi: 10.1016/j.ophtha.2011.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Papasavvas I., Herbort C.P., Jr. Reactivation of Vogt-Koyanagi-Harada disease under control for more than 6 years, following anti-SARS-CoV-2 vaccination. J Ophthalmic Inflamm Infect. 2021;11:21. doi: 10.1186/s12348-021-00251-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nofal A., Fawzy M.M., Sharaf El Deen S.M.S., El-Hawary E.E. Herpes zoster ophthalmicus in COVID-19 patients. Int J Dermatol. 2020;59:1545–1546. doi: 10.1111/ijd.15240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ferrand N., Accorinti M., Agarwal M., et al. COVID-19 vaccination and uveitis: epidemiology, clinical features and visual prognosis. Ocul Immunol Inflamm. 2022:1–9. doi: 10.1080/09273948.2022.2058964. [DOI] [PubMed] [Google Scholar]