Abstract
Objective
To describe the characteristics of people who experience changes to their menstrual cycle after COVID-19 vaccination.
Design
Longitudinal study.
Patient(s)
We recruited a volunteer sample with and without a history of SARS-CoV-2 infection who enrolled in the Arizona COVID-19 Cohort (CoVHORT) study and participated in a reproductive sub-cohort who were pre-menopausal, not pregnant, and had received a COVID-19 vaccine in 2021 (n = 545).
Exposure(s)
Demographic and reproductive characteristics were collected via self-reports.
Main Outcome Measure(s)
Information on self-reported changes in the menstrual cycle after COVID-19 vaccination was collected from May 2021 to December 2021. We looked at demographic and reproductive characteristics as predictors of menstrual cycle change.
Result(s)
The majority of our vaccinated sample received the Pfizer-BioNTech vaccine (58%), and were 26-35 years old (51%), non-Hispanic (84%), and White (88%). Approximately 25% of vaccinated participants reported a change in their menstrual cycle after vaccination; the majority reported changes after their second dose (56%) as compared with their first (18%) and third (14%) doses. The most commonly reported changes were irregular menstruation (43%), increased premenstrual symptoms (34%), increased menstrual pain or cramps (30%), and abnormally heavy or prolonged bleeding (31%). High self-reported perceived stress levels compared with low perceived stress (OR, 2.22; 95% CI 1.12-4.37) and greater body mass index (OR, 1.04; 95% CI 1.00-1.07) were associated with greater odds of experiencing the menstrual cycle changes after the vaccination. Participants having a history of SARS-CoV-2 infection were less likely to report changes in their menstrual cycle after vaccination compared with the participants with no history of SARS-CoV-2 infection (OR, 0.58; 95% CI 0.32-1.04).
Conclusion(s)
Among vaccinated participants, approximately 25% of them reported predominantly temporary changes in the menstrual cycle, however, we are unable to determine whether these changes are due to normal cycle variability. The COVID-19 vaccines are safe and effective for everyone, including pregnant people and people trying to conceive; hence, these findings should not discourage vaccination.
Key Words: COVID-19 vaccination, menstrual cycle, SARS-CoV-2, menstrual regularity
Abstract
Vacunación COVID-19 y cambios en el ciclo menstrual entre personas vacunadas.
Objetivo
Describir las características de la gente que experimenta cambios en su ciclo menstrual después de la vacunación COVID-19.
Diseño
Estudio longitudinal.
Paciente (s)
Reclutamos una muestra voluntaria con y sin historia de infección de SARS-CoV-2 que se inscribió en el estudio de Cohorte COVID-19 de Arizona y participó en un sub-cohorte reproductivo que eran pre-menopáusicas, no embarazadas y habían recibido una vacuna COVID-19 en 2021 (n=545).
Intervención (es)
Características demográficas y reproductivas fueron recolectadas via autoevaluación.
Principal (es) Medida (s) de Resultado (s)
Información en la autoevaluación de cambios en el ciclo menstrual después de vacunación COVID-19 fue recolectada desde Mayo 2021 a Diciembre 2021. Buscamos características demográficas y reproductivas como predictores de cambios en el ciclo menstrual.
Resultado (s)
La mayoría de nuestra muestra vacunada recibió la vacuna Pfizer-BioNTech (58%), y tenían 26-35 años (51%), no hispanas (84%), y blancas (88%). Aproximadamente 25% de las participantes vacunadas informaron un cambio en su ciclo menstrual después de la vacunación; la mayoría informó cambios después de la segunda dosis (56%) en comparación con la primera (18%) y la tercera (14%) dosis. Los cambios más comunes informados fueron menstruaciones irregulares (43%), aumento de síntomas premenstruales (34%), aumento de dolor menstrual o calambres (30%), y sangrado anormal abundante y prolongado (31%). Altos niveles de estrés se percibieron en las autoevaluaciones comparados con bajos niveles de estrés percibidos (OR, 2.22; 95% CI 1.12-4.37) y un mayor índice de masa corporal (OR, 1.04; 95% CI 1.00-1.07) fue asociado a mayor probabilidad de experimentar cambios en el ciclo menstrual después de la vacunación. Las participantes que tenían una historia de infección SARS-CoV-2 fueron menos propensas a informar cambios en su ciclo menstrual después de la vacunación comparadas con las participantes sin historia de infección SARS-CoV-2 (OR, 0.58; 95% CI 0.32-1.04).
Conclusión (es)
Entre las participantes vacunadas, aproximadamente el 25% informó predominantemente cambios temporales en el ciclo menstrual, sin embargo, somos incapaces de determinar si estos cambios se deben a la variabilidad normal del ciclo. Las vacunas COVID-19 son seguras y efectivas para todos, incluyendo gente embarazada y gente tratando de concebir; por tanto, estos hallazgos no deben desalentar la vacunación.
In December 2020, the United States Food and Drug Administration (FDA) authorized the emergency use of the Pfizer-BioNTech vaccine against SARS-CoV-2 infection and the associated disease state, COVID-19. After this approval along with Moderna and Janssen vaccines, the United States has undertaken the largest and most comprehensive vaccination campaign in recent history. While the information on traditional adverse reactions from vaccination is required in clinical trials by the FDA and has been systematically collected on a national level by the Centers for Disease Control and Prevention, standardized collection of information on menstrual cycle health changes has been neglected in the current surveillance.
There is increasing evidence to suggest that SARS-CoV-2 infection as well as societal changes associated with the COVID-19 pandemic, may influence menstrual cycle health (1, 2, 3, 4, 5, 6). Severe SARS-CoV-2 infection has been associated with adverse changes in ovarian function and menstruation, including decreased anti-mullerian hormone levels (1), as well as an increased prevalence of amenorrhea, menstrual volume changes, and dysmenorrhea among patients with COVID-19 (2). Other research focused on the societal impact of the COVID-19 pandemic and government-instituted lockdowns, observed that nearly half of the women surveyed reported changes to their menstrual cycle between March 2020 and September 2020 (3) which was attributable to stress. However, there has been limited published research on menstrual cycle health and COVID-19 vaccination (7), despite the biological plausibility of an association via immunomodulation of menstruation-associated hormones (8). The majority of the information on menstrual cycle changes after COVID-19 vaccination has come from media articles and anecdotal accounts having limited validity and may not be generalizable to all women considering vaccination (9). However, to date, there is a paucity of data regarding how long any potential changes to the menstrual cycle may persist after vaccination. Therefore, we utilized our ongoing Arizona COVID-19 Cohort Study (CoVHORT), to quantify the prevalence of menstrual changes after vaccination, the duration of change, and the associated risk factors among those vaccinated for COVID-19.
Materials and methods
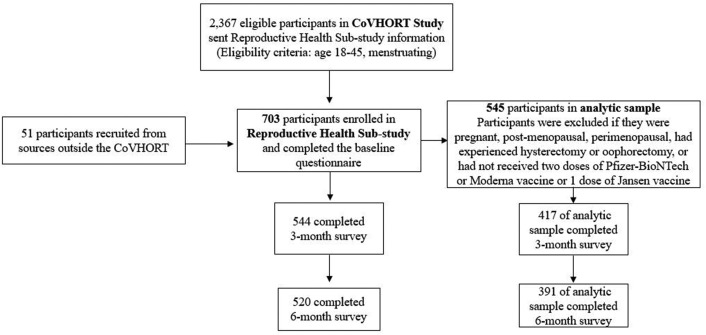
In May 2020, the Arizona CoVHORT began recruiting participants for a prospective cohort study (10). The primary objective of this cohort was to better understand all COVID-19 risk factors, as well as the short-and long-term sequelae associated with infection through enrolling a longitudinal cohort of individuals with and without a history of SARS-CoV-2 infection in Arizona state. In brief, SARS-CoV-2-positive individuals were recruited through case investigations and testing sites in collaboration with local health departments. Individuals without SARS-CoV-2 infection were recruited through a variety of sources including testing sites (those who tested negative), mailed postcards, flyers, and emails disseminated across the state. In May 2021, a reproductive health sub-study was launched which recruited CoVHORT study participants (n = 2,367) who were menstruating and aged 18–45 years. Enrollment in the reproductive sub-study was rolling and participants could enter the sub-cohort between May 2021 and December 2021 (n = 703). All participants were invited to complete a baseline (launched in May 2021), 3-month (launched in August 2021), and 6-month (launched in December 2021) questionnaire. While enrollment was active until December 2021, approximately 81% of our patients completed the baseline questionnaire in May 2021. For this analysis, we excluded participants who self-identified as pregnant, postmenopausal, perimenopausal, experienced hysterectomy or oophorectomy, or who did not receive 2 doses of the Pfizer-BioNTech or the Moderna vaccine or 1 dose of the Jansen vaccine, leaving a final sample of 545 participants (Fig. 1 ) who completed the baseline survey and were included in our analysis. The 3-and 6-month surveys were completed by 417 and 391 participants, respectively. We did not observe meaningful differences between the CoVHORT participants who were eligible, those enrolled in the reproductive sub-study, our analytic sample, and participants who completed the 3 and 6-month questionnaires (Supplemental Table 1, available online). The CoVHORT study and the reproductive sub-study were approved by the University of Arizona IRB (Protocol number: 2101406252).
Figure 1.
Flow diagram of study participants.
On the baseline questionnaire of the reproductive sub-study, participants were asked detailed questions about their demographics, reproductive and overall health history, self-reported stress using the perceived stress scale (PSS-10) (4), COVID-19 vaccination status, and changes to the menstrual cycle after vaccination. Changes to the menstrual cycle after vaccination were also asked on the 3-month and 6-month surveys along with information on pre-and postvaccination menstrual cycle characteristics such as menstrual cycle length and heaviness of menstrual flow. Specifically, participants were asked, “Since receiving the COVID-19 vaccine, have you noticed a change in your menstrual cycle?” If participants indicated “yes,” they could choose one or more of the after responses: missed periods, infrequent menstruation (i.e. menstrual periods occurring at intervals >35 days), irregular menstruation (i.e. the number of days your menstrual period lasts or the time between each varies significantly), abnormal bleeding or spotting between the menstrual periods, abnormally light bleeding, abnormally heavy or prolonged bleeding (i.e. bleeding for longer than a week, need to use double sanitary protection to control the menstrual flow), increased menstrual pain or cramps, and increase in premenstrual symptoms (i.e. greater than usual mood swings, feelings of anxiety/depression, tiredness, trouble sleeping, bloating/stomach pain, breast tenderness, changes in appetite or sex drive). Participants were also asked when they first noticed menstrual changes (after dose 1, dose 2, and dose 3 (added to the 6-month questionnaire in December 2021), or unsure), whether the menstrual changes had resolved, and when they had resolved (approximately how many months after vaccination did these changes resolve). The survey item employed to address resolution was as follows: “Approximately how many months after vaccination did these changes resolve?”. Information on self-reported history of polymerase chain reaction and antigen-confirmed SARS-CoV-2 status and body mass index (BMI; kg/m2) were obtained through linkages with the parent CoVHORT study.
In this analysis, we quantified the prevalence of self-reported changes to the menstrual cycle among women who were vaccinated against SARS-CoV-2, the timing of onset and resolution, as well as type of menstrual changes. We also compared the demographic and reproductive characteristics of those participants who reported and did not report changes in their menstrual cycle after COVID-19 vaccination. We employed logistic regression analyses to calculate odds ratios and 95% confidence intervals of changes to the menstrual cycle for a variety of demographic and reproductive characteristics (Stata 17.0).
Results
Figure 1 presents the study flow diagram for the reproductive sub-cohort. Information regarding the study was sent to 2,367 eligible participants in the CoVHORT. A total of 703 participants completed baseline surveys and enrolled in the reproductive sub-cohort; whereas 545 participants were eligible for our analytic sample. Three- and six-month surveys were completed by 417 and 391 women, respectively in our analytic sample. Among participants (n = 545), the majority (64.2%) reported tracking their menstrual periods using a phone application, calendar, or other devices. We observed that 24.8% of patients reported a change in their menstrual cycle after vaccination (Table 1 ). Among participants who reported changes to their menstrual cycle (n = 135), the majority (56.3%) of them reported these differences after their second dose of vaccine as compared with their first (18%) and third (14%) doses. Among participants who reported changes in their menstrual cycles, 17.8% of patients reported resolution of changes, with the majority (58%) resolving within 2 months of vaccination. Of the specific changes to the menstrual cycle, irregular menstruation, defined as an affirmative response to the query of whether the number of days the menstrual period lasted or the time between each varied significantly, was the most commonly reported change (43.0%), followed by increased premenstrual symptoms (34.1%), increased menstrual pain/cramps (30.4%), and abnormally heavy or prolonged bleeding (31.1%).
Table 1.
Descriptive characteristics of participants who had received the COVID-19 vaccine and reported changes in their menstrual cycles (n = 545)
| n (%) | |
|---|---|
| Reported changes in the menstrual cycle after COVID-19 vaccination | 135 (24.8) |
| When they first noticed changes | |
| After 1st dose | 25 (18.5) |
| After 2nd dose | 76 (56.3) |
| After 3rd dose | 19 (14.1) |
| Unsure | 15 (11.1) |
| Menstrual changes resolveda | |
| Yes, I am no longer experiencing changes | 24 (17.8) |
| No, I am still experiencing changes | 71 (52.6) |
| I don’t know | 28 (20.7) |
| Time until changes resolved (mo)b | |
| 1 | 4 (16.7) |
| 2 | 10 (41.7) |
| 3 | 3 (12.5) |
| 4 | 3 (12.5) |
| 5 | 2 (8.3) |
| 6 | 2 (8.3) |
| Specific changes in the menstrual cycle–at the first reported change in the cycle | |
| One or more missed menstrual periods | 16 (11.9) |
| Infrequent menstruation (i.e., menstrual periods occurring at intervals >35 d) | 14 (10.3) |
| Irregular menstruation (i.e., the number of days your menstrual period lasts or the time between each varies significantly) | 58 (43.0) |
| Abnormal bleeding or spotting between normal menstrual periods | 32 (23.7) |
| Abnormally heavy or prolonged bleeding (i.e., bleeding for longer than a wk, needing to use double the sanitary protection to control your menstrual flow) | 42 (31.1) |
| Abnormally light bleeding | 22 (16.3) |
| Increase in menstrual pain or cramps | 41 (30.4) |
| Increase in premenstrual symptoms (i.e., greater than usual mood swings, feelings of anxiety/depression, tiredness, trouble sleeping, bloating/stomach pain, breast tenderness, changes in appetite or sex drive) | 46 (34.1) |
| Survey where participant reported their first vaccination | |
| Baseline | 135 (100) |
| 3 mo | 0 (0) |
| 6 mo | 0 (0) |
| Percent of participants that reported changes in their menstrual cycle by surveyc | |
| Baseline | 101 (74.8) |
| 3 mo | 22 (16.3) |
| 6 mo | 12 (8.9) |
| Mean (SD) Median [min-max] |
|
| Days from the first vaccine dose to the completion of the first survey where they reported a change in their menstrual cycle | 137 (79) 118.5 [29-380] |
| Days from the second vaccine dose to the completion of the first survey where they reported a change | 112 (79) 91 [6-359] |
| Days from the first vaccine dose to completion of the first survey where they reported resolution to menstrual changes | 166.8 (69.5) 152.5 [61-352] |
| Days from the second vaccine dose to completion of the first survey where they reported resolution to menstrual changes | 141.9 (69.8) 125.5 [39-331] |
Missing data for changes resolved (n = 12, 8.9%) at the last reported survey.
Among those who reported changes had resolved (n = 24); based on the write-in question: “Approximately how many months after vaccination did these changes resolve?”
545 participants completed the baseline questionnaire, 417 completed the 3-month questionnaire, 391 completed the 6-month questionnaire.
The majority of participants received the Pfizer-BioNTech vaccine (58%), were 26–35 years old (51%), non-Hispanic (84%), and White (88%) (Table 2 ). We observed no statistically significant differences in age, race/ethnicity, history of other chronic conditions, gravidity, subfertility, and age at first menses among vaccinated participants who did and did not report changes to their menstrual cycle after vaccination. A greater BMI was associated with greater odds of experiencing changes to the menstrual cycle (OR, 1.04; 95% CI, 1.00-1.07). Compared with the participants who reported low perceived stress at baseline, participants who reported high perceived stress had greater odds of reporting changes to their menstrual cycle (OR, 2.22; 95% CI, 1.12–4.37). Participants having a history of SARS-CoV-2 infection were less likely to report changes to their menstrual cycle after vaccination compared with the participants with no history of SARS-CoV-2 infection (OR, 0.58; 95% CI, 0.32–1.04). Participants who reported using any hormonal medication had 27% lower odds of reporting menstrual cycle changes (OR, 0.73; 95% CI, 0.49–1.08).
Table 2.
Demographic and reproductive characteristics among participants who received the COVID-19 vaccine were stratified by whether they experienced changes to their menstrual cycle after vaccination
| Characteristics | People who reported changes in their menstrual cycle after vaccination (n = 135; 24.8%) | People who did not report a change to their menstrual cycle after vaccination (n = 410; 75.2%) | Odds of reporting menstrual changes after vaccination (95% CI) |
|---|---|---|---|
| Age (y), mean (SD) | 32.5 (6.4) | 32.3 (6.6) | 1.00 (0.97-1.03) |
| Age(y); n (%) | |||
| 18-25 | 21 (15.6) | 70 (17.0) | 1.00 (Referent) |
| 26-30 | 31 (23.0) | 94 (22.9) | 1.10 (0.58–2.07) |
| 31-35 | 40 (29.6) | 111 (27.1) | 1.20 (0.65–2.20) |
| 36-40 | 26 (19.3) | 83 (20.2) | 1.04 (0.54–2.01) |
| 41-45 | 17 (12.6) | 52 (12.7) | 1.09 (0.52–2.26) |
| Vaccine type, n (%) | |||
| Pfizer-BioNTech | 85 (63.0) | 229 (55.8) | 1.00 (Referent) |
| Moderna | 45 (33.3) | 171 (41.7) | 0.71 (0.47-1.07) |
| Janssen | 5 (3.7) | 10 (2.4) | 1.34 (0.45–4.05) |
| Race, n (%) | |||
| White | 111 (87.4) | 321 (87.5) | 1.00 (Referent) |
| Black or African American | 4 (3.2) | 6 (1.6) | 1.93 (0.53–6.96) |
| Asian | 3 (2.4) | 19 (5.2) | 0.46 (0.13–1.58) |
| Mixed Race | 9 (7.0) | 21 (5.7) | 1.24 (0.55–2.79) |
| Ethnicity, n (%) | |||
| Non-Hispanic | 108 (85.7) | 309 (83.7) | 1.00 (Referent) |
| Hispanic | 18 (14.3) | 60 (16.3) | 0.86 (0.49–1.52) |
| BMI (kg/m2), mean (SD) | 27.5 (7.3) | 26.0 (6.0) | 1.04 (1.00-1.07) |
| BMI (kg/m2), n (%) | |||
| <18.5 | 2 (1.6) | 7 (1.9) | 1.02 (0.21 -5.09) |
| 18.5-24.9 | 55 (43.3) | 198 (53.7) | 1.00 (Referent) |
| 25.0-29.9 | 37 (29.1) | 86 (23.2) | 1.55 (0.95–2.52) |
| 30.0-39.9 | 26 (20.5) | 64 (17.3) | 1.46 (0.85–2.52) |
| ≥40.0 | 7 (5.5) | 14 (3.8) | 1.80 (0.69–4.68) |
| History of SARS-CoV-2 infectiona, n (%) | 15 (11.1) | 73 (17.8) | 0.58 (0.32–1.04) |
| Perceived stress scoreb, mean, (SD) | 18.8 (7.2) | 17.3 (6.9) | 1.03 (1.00-1.06) |
| Low stress | 30 (22.2) | 133 (32.4) | 1.00 (Referent) |
| Moderate stress | 86 (63.7) | 239 (58.3) | 1.60 (1.00–2.54) |
| High stress | 19 (14.1) | 38 (9.3) | 2.22 (1.12–4.37) |
| History of other chronic conditions, n (%) | |||
| Endometriosis | 4 (3.0) | 21 (5.1) | 0.57 (0.19–1.68) |
| Polycystic ovary syndrome | 12 (8.9) | 31 (7.6) | 1.19 (0.59–2.39) |
| Uterine fibroids | 5 (3.7) | 9 (2.2) | 1.71 (0.56–5.20) |
| Diabetes (TI or TII) | 3 (2.2) | 3 (0.7) | 3.08 (0.61–15.46) |
| Hypertension | 10 (7.4) | 21 (5.1) | 1.48 (0.68–3.23) |
| Any chronic condition | 94 (69.6) | 280 (68.3) | 1.06 (0.70–1.62) |
| Reproductive history | |||
| Gravidity, mean (SD) | 0.9 (1.4) | 0.9 (1.3) | 1.00 (0.87-1.16) |
| History of subfertilityc, n (%) | 12 (8.9) | 47 (11.5) | 0.75 (0.39–1.46) |
| Specific infertility diagnosis, n (%) | |||
| tubal factor infertility | 0 (0) | 4 (1.0) | - |
| Polycystic ovary syndrome | 2 (1.5) | 8 (2.0) | - |
| Endometriosis | 1 (0.8) | 6 (1.5) | - |
| Uterine factor infertility | 0 (0) | 1 (0.2) | - |
| Male/Partner factor | 4 (3.0) | 5 (1.2) | - |
| Unknown | 4 (3.0) | 18 (4.4) | - |
| Other | 3 (0.7) | 3 (2.2) | - |
| Age at menses, y; n (%) | |||
| ≤10 | 7 (5.2) | 29 (7.2) | 0.69 (0.28–1.71) |
| 11 | 31 (23.1) | 85 (21.0) | 1.05 (0.61–1.81) |
| 12 | 41 (30.6) | 118 (29.2) | 1.00 (Referent) |
| 13 | 35 (26.1) | 96 (23.8) | 1.05 (0.62–1.77) |
| ≥14 | 20 (14.9) | 76 (18.8) | 0.76 (0.41–1.39) |
| Currently on any hormonal medication, n (%) | |||
| Any hormonal medication (OCP, Hormonal IUD, Implant, Shot, Other) | 57 (42.2) | 205 (50) | 0.73 (0.49-1.08) |
| Oral contraceptives–combination pill or progestin | 26 (19.3) | 96 (23.4) | 0.78 (0.48-1.27) |
| Hormonal IUD | 23 (17.0) | 96 (23.4) | 0.67 (0.41-1.11) |
| Hormonal implant | 7 (5.2) | 12 (2.9) | - |
| Hormonal shot | - | 1 (0.2) | - |
| Other hormonal medication | 1 (0.7) | 2 (0.5) | - |
| No hormonal medication use | 78 (57.8) | 205 (50.0) | 1.00 (Referent) |
IUD = intrauterine device. Prefer not to answer or missing: race (n = 45); ethnicity (n = 44); BMI (n = 49); history of subfertility (n = 1); age at first menstrual period (n = 7); missing first vaccination date (n = 32); missing second vaccination date (n = 33); time from last menstrual period to last change (n = 2); age at menses (n = 7);
Defined as polymerase chain reaction or antigen-confirmed positive COVID-19 test before second COVID-19 vaccination
Perceived stress was measured using the PSS-10
Subfertility was defined as a history of ≥6 months of trying to conceive without success
Among participants who reported ≥1 missed menstrual period on the baseline survey, 50% reported having missed 1 menstrual period, 14% reported missing 2, 14% missing 3, and 21% reported missing 4 menstrual periods on an average of 172 days (SD = 36) from the first vaccine. Among participants who reported infrequent or irregular menstruation after the vaccination, before the COVID-19 vaccine, they reported their cycles were an average of 27 days (SD = 6.1; median = 28); after the COVID-19 vaccine, when they noticed changes, their cycles were on average of 29 days (SD = 14; median = 31). Among participants who reported changes in bleeding heaviness (lighter or heavier), on average before vaccination within 4 hours, 11% reported experiencing spotting, 37% had experienced light bleeding, 41% had experienced moderate bleeding, and 11% had experienced heavy bleeding. After vaccination, on average within 4 hours, 15% reported experiencing spotting, 26% experienced light bleeding, 48% experienced moderate bleeding, and 11% experienced heavy bleeding. Among those who reported changes in premenstrual symptoms after vaccination, the most common symptoms reported to increase were tiredness (50%) and mood swings (43.5%).
Discussion
Overall, we observed that approximately 25% of the participants reported changes in the menstrual cycle after COVID-19 vaccination, with the most common changes related to menstrual timing, premenstrual symptoms, pain symptoms, and abnormally heavy or prolonged bleeding. Compared with those who did not report changes to their menstrual cycle after vaccination, those who did experience variation had higher stress levels and greater BMI at baseline and were also less likely to have had prior SARS-CoV-2 infection, suggesting that these variables may also be associated with postvaccination menstrual cycle changes, which requires further investigation.
There are several hypothesized mechanisms through which vaccinations may influence menstrual cycles. Changes in the menstrual cycle may be caused by several factors including an acute-phase immune response, inflammation, endogenous hormone levels, and/or immune cells in the endometrium. Moreover, prior research has suggested that some people may experience short-term changes to the menstrual cycle after other vaccinations (11, 12). Indeed, data collected from New York Presbyterian Hospital in 1911 reported anecdotal accounts of changes to the menstrual cycle after typhoid vaccination (12), and more recently, menstrual irregularities have been reported to the FDA’s Vaccine Adverse Events Reporting System after HPV vaccination (11). However, information on menstrual cycle health is not routinely collected in current vaccine trial documentation or surveillance. Previous research on menstrual cycle changes after COVID-19 vaccination among individuals using the digital application “Natural Cycles”(7), observed that compared with those who were unvaccinated, vaccinated individuals experienced a 0.6-day increase in their cycle after their first vaccine dose and a 0.8-day increase in their cycle after their second vaccine dose in adjusted models. Among individuals who received both vaccine doses within a single menstrual cycle, individuals experienced a 2.3-day increase in their menstrual cycle length compared with the unvaccinated individuals. Approximately, 10% of the participants experienced cycle length changes of ≥8 days, however this change attenuated after 2 postvaccine cycles. These findings are consistent with more recent findings among over 19,000 international users of the “Natural Cycles” application (13) and among nearly 4,000 participants in the Nurses’ Health Study 3 (14). In our study, we observed a two-day increase in menstrual cycle length among participants who reported infrequent or irregular menstruation after vaccination.
Societal stress related to the COVID-19 pandemic, independent of infection and vaccination, has also been hypothesized to modify the menstrual cycle (3, 4, 15). Results of an anonymous digital survey of over 1,000 reproductive-aged women recruited through social media in September 2020 observed that 46% of respondents reported a change in their menstrual cycle during the COVID-19 pandemic with 53% reporting worsening menstrual symptoms, 18% reporting new menorrhagia, and 30% reporting new dysmenorrhea (3). However, the majority of study participants were White (97%) and from Ireland and the United Kingdom (98%) limiting generalizability. Additionally, given recruitment through social media, participants who enrolled in the study may have been more likely to participate because of having experienced adverse menstrual or reproductive outcomes, thus potentially oversampling individuals who had menstrual cycle changes.
In our data, we are unable to disentangle whether reported changes in the menstrual cycle after COVID-19 vaccination can be attributed to other endogenous or exogenous factors. We observed that vaccinated participants who reported changes to their menstrual cycle had higher perceived stress at the study baseline compared with participants who did not report changes. However, while stress is known to influence menstrual cycle regularity (16, 17), both groups had, on average, moderate stress. The instrument used to measure stress in our study was the PSS-10 which queries about stress in the previous 30 days. Hence, our measure of stress may be prone to reverse causation because participants completed the PSS-10 at baseline, on average 137 days after their first vaccine dose, and therefore, may not represent stress levels at the time of menstrual changes. We also observed that individuals with greater BMI may be more likely to experience menstrual cycle changes. Body size is associated with endogenous hormone levels and conditions that influence menstrual cycle regularity such as polycystic ovary syndrome (18, 19) and endometriosis (20). However, we did not observe clinically meaningful differences in polycystic ovary syndrome or endometriosis diagnoses between individuals who did and did not report menstrual changes. In addition, our data suggest that individuals who experienced changes in their menstrual cycle after vaccination were less likely to have had a history of SARS-CoV-2 infection (11%) compared with the individuals without any change in their menstrual cycle (18%). Prior research from our team has shown that acute infection may influence the menstrual cycle, as ∼16% of individuals with SARS-CoV-2 infection reported changes to their menstrual cycles after their illness (2), with individuals who had more severe acute illness presentation and larger body size more likely to experience menstrual cycle changes. It is possible that because some individuals experienced changes after infection, they may underreport menstrual changes after vaccination.
Lastly, while the menstrual cycle is traditionally thought to be relatively stable among women 20–40 years old (21), it is normal for many women to experience variability in cycle length and symptoms (22, 23, 24). A recent analysis of >4 million menstrual cycles observed a median cycle length difference of 4.15 days (SD = 4.94) across cycles, with approximately 8% of women consistently experiencing highly variable cycle lengths (>9 days) (23). Further, another study has demonstrated that 46% of individuals report a menstrual cycle range of ≥7 days, with 20% reporting a variation of ≥14 days (24). Therefore, random fluctuations in the menstrual cycle cannot be completely ruled out.
While this study has several strengths including being one of the first studies to quantify changes in the menstrual cycle after vaccination, we also recognize its limitations. The analysis utilizes self-reported, cross-sectional data, and some associations, such as with stress, may be due to reverse causation. Recruitment for the study came from the parent CoVHORT study and did not emphasize vaccination or menstrual cycle changes, however, women who have a history of reproductive health issues may be more likely to participate in a sub-study advertised as being focused on reproductive health. This study did not ask for menstrual cycle information among the unvaccinated and therefore we are unable to determine whether these changes would be expected among the unvaccinated. Participants who indicated resolution of changes to their menstrual cycle were asked to write “Approximately how many months after vaccination did these changes resolve?” Unfortunately, this question wording is not granular enough to allow for a detailed statistical analysis of time-to-symptom resolution, which should be incorporated into future research. Additionally, while our study could not account for effect modification by time, there may be differences observed by the calendar time of data collection given changes in vaccination rates and population-level awareness about menstrual changes after vaccination (25). Moreover, the phrasing of our survey about menstrual cycle changes was directly linked to vaccination status (“Since receiving the COVID-19 vaccine, have you noticed a change in your menstrual cycle?”) which may have caused participants to over-estimate the association between menstrual changes and vaccination status. Finally, bias with recall may have played a role in the findings of this study, as those who have been vaccinated may potentially be more likely to have noticed and reported changes to their menstrual cycle after media reporting before the survey and given the wording of the survey and as such, these results must be confirmed in larger studies.
Some individuals may notice changes to their menstrual cycle after COVID-19 vaccination. These findings should inform patient counseling regarding what to expect after COVID-19 vaccination. Menstrual changes occurred in 25% of our vaccinated patients. However, whether the COVID-19 vaccine directly caused these menstrual changes for all participants or whether these changes were part of normal fluctuations or caused by other factors warrants further investigation. The COVID-19 vaccines are safe and effective for everyone including pregnant people (26, 27) and people trying to conceive (28, 29). Future, prospective research is needed to replicate these findings. Moreover, additional research is needed to understand the potential mechanism of association and the duration of these changes.
In summary, among participants vaccinated for COVID-19, approximately 1 in 4 participants reported changes in their menstrual cycle after vaccination, similar to reported changes after COVID-19 disease (2, 6), and may be due to normal menstrual cycle variability observed in the general population. These findings should not deter individuals from receiving the COVID-19 vaccine, as the vaccine is safe and effective for pregnant people (26, 27) and people trying to conceive (28, 29, 30). Additionally, vaccine safety and surveillance data should systematically collect information on the menstrual cycle and reproductive health. While we observed that COVID-19 vaccination was associated with menstrual cycle changes among some participants, these appear to be temporary. Moreover, we could not disentangle the influence of outside factors or normal variability. The risk of long-term health consequences after COVID-19 disease is substantial and may also include changes to the menstrual cycle; therefore, menstrual side effects should not prevent individuals from getting vaccinated.
Footnotes
L.V.F. has nothing to disclose. S.M. K. has nothing to disclose. A.S. has nothing to disclose. K.M.H. has nothing to disclose. P.I. has nothing to disclose. A.M.A. has nothing to disclose. M.M.H-K. has nothing to disclose. N.D.M. has nothing to disclose. K.P-B. has nothing to disclose. K.C.E. has nothing to disclose. E.T.J. has nothing to disclose.
Supported by a challenge grant from the University of Arizona and by the BIO-5 Institute at the University of Arizona.
Supplementary data
References
- 1.Ding T., Wang T., Zhang J., Cui P., Chen Z., Zhou S., et al. Analysis of ovarian injury associated with COVID-19 disease in reproductive-aged women in Wuhan, China: an observational study. Front Med (Lausanne) 2021;8:1–11. doi: 10.3389/fmed.2021.635255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khan S.M., Shilen A., Heslin K.M., Ishimwe P., Allen A.M., Jacobs E.T., et al. SARS-CoV-2 infection and subsequent changes in the menstrual cycle among participants in the Arizona CoVHORT study. Am J Obstet Gynecol. 2022;226:270–273. doi: 10.1016/j.ajog.2021.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Phelan N., Behan L.A., Owens L. The impact of the COVID-19 pandemic on women's reproductive health. Front Endocrinol. 2021;12:1–8. doi: 10.3389/fendo.2021.642755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bruinvels G., Goldsmith E., Blagrove R.C., Martin D., Shaw L., Piasecki J. How lifestyle changes within the COVID-19 global pandemic have affected the pattern and symptoms of the menstrual cycle. Int J Environ Res Public Health. 2022;19:1–19. doi: 10.3390/ijerph192013622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carfì A., Bernabei R., Landi F. Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324:603–605. doi: 10.1001/jama.2020.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li K., Chen G., Hou H., Liao Q., Chen J., Bai H., et al. Analysis of sex hormones and menstruation in COVID-19 women of child-bearing age. Reprod Biomed Online. 2021;42:260–267. doi: 10.1016/j.rbmo.2020.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Edelman A., Boniface E.R., Benhar E., Han L., Matteson K.A., Favaro C., et al. Association between menstrual cycle length and coronavirus disease 2019 (COVID-19) vaccination: a U.S. cohort. Obstet Gynecol. 2022;139:940–941. doi: 10.1097/AOG.0000000000004695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Male V. Menstrual changes after Covid-19 vaccination. BMJ. 2021;374:n2211. doi: 10.1136/bmj.n2211. [DOI] [PubMed] [Google Scholar]
- 9.Alice Lu-Culligan R.H.E. 2021. Can Covid shots affect periods? In:New York Times. New York; p. 25. [Google Scholar]
- 10.Catalfamo C.J., Heslin K.M., Shilen A., Khan S.M., Hunsaker J.R., Austhof E., et al. Design of the Arizona CoVHORT: a population-based COVID-19 cohort. Front Public health. 2021;9:1–11. doi: 10.3389/fpubh.2021.620060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gong L., Ji H.H., Tang X.W., Pan L.Y., Chen X., Jia Y.T. Human papillomavirus vaccine-associated premature ovarian insufficiency and related adverse events: data mining of vaccine adverse event reporting system. Sci Rep. 2020;10:1–8. doi: 10.1038/s41598-020-67668-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lamb A.R. Experiences with prophylactic typhoid vaccination: its effect on menstruation. Archives of Internal Medicine. 1913;XII:565–577. [Google Scholar]
- 13.Edelman A., Boniface E.R., Male V., Cameron S.T., Benhar E., Han L., et al. Association between menstrual cycle length and Covid-19 vaccination: global, retrospective cohort study of prospectively collected data. BMJ Medicine. 2022;1:1–21. doi: 10.1136/bmjmed-2022-000297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang S., Mortazavi J., Hart J.E., Hankins J.A., Katuska L.M., Farland L.V., et al. A prospective study of the association between SARS-CoV-2 infection and COVID-19 vaccination with changes in usual menstrual cycle characteristics. Am J Obstet Gynecol. 2022;227:739. doi: 10.1016/j.ajog.2022.07.003. e1–e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Demir O., Sal H., Comba C. Triangle of COVID, anxiety and menstrual cycle. J Obstet Gynaecol. 2021;41:1257–1261. doi: 10.1080/01443615.2021.1907562. [DOI] [PubMed] [Google Scholar]
- 16.Critchley H.O.D., Babayev E., Bulun S.E., Clark S., Garcia-Grau I., Gregersen P.K., et al. Menstruation: science and society. Am J Obstet Gynecol. 2020;223:624–664. doi: 10.1016/j.ajog.2020.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nagma S., Kapoor G., Bharti R., Batra A., Batra A., Aggarwal A., et al. To evaluate the effect of perceived stress on menstrual function. J Clin Diagn Res. 2015;9 doi: 10.7860/JCDR/2015/6906.5611. Qc01–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Legro R.S., Arslanian S.A., Ehrmann D.A., Hoeger K.M., Murad M.H., Pasquali R., et al. Diagnosis and treatment of polycystic ovary syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2013;98:4565–4592. doi: 10.1210/jc.2013-2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goodarzi M.O., Dumesic D.A., Chazenbalk G., Azziz R. Polycystic ovary syndrome: etiology, pathogenesis and diagnosis. Nat Rev Endocrinol. 2011;7:219–231. doi: 10.1038/nrendo.2010.217. [DOI] [PubMed] [Google Scholar]
- 20.Farland L.V., Harris H.R. Long-term health consequences of endometriosis—pathways and mediation by treatment. Curr Obstet Gynecol Rep. 2020;9:79–88. doi: 10.1007/s13669-020-00287-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Treloar A.E., Boynton R.E., Behn B.G., Brown B.W. Variation of the human menstrual cycle through reproductive life. International journal of fertility. 1967;12:77–126. [PubMed] [Google Scholar]
- 22.Fehring R.J., Schneider M., Raviele K. Variability in the phases of the menstrual cycle. J Obstet Gynecol Neonatal Nurs. 2006;35:376–384. doi: 10.1111/j.1552-6909.2006.00051.x. [DOI] [PubMed] [Google Scholar]
- 23.Li K., Urteaga I., Wiggins C.H., Druet A., Shea A., Vitzthum V.J., et al. Characterizing physiological and symptomatic variation in menstrual cycles using self-tracked mobile-health data. NPJ Digit Med. 2020;3:1–13. doi: 10.1038/s41746-020-0269-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Creinin M.D., Keverline S., Meyn L.A. How regular is regular? An analysis of menstrual cycle regularity. Contraception. 2004;70:289–292. doi: 10.1016/j.contraception.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 25.Fell D.B., Dimitris M.C., Hutcheon J.A., Ortiz J.R., Platt R.W., Regan A.K., et al. Guidance for design and analysis of observational studies of fetal and newborn outcomes following COVID-19 vaccination during pregnancy. Vaccine. 2021;39:1882–1886. doi: 10.1016/j.vaccine.2021.02.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Razzaghi H., Meghani M., Pingali C., Crane B., Naleway A., Weintraub E., et al. COVID-19 vaccination coverage among pregnant women during pregnancy-eight integrated health care organizations, United States, December 14, 2020-May 8, 2021. MMWR Morb Mortal Wkly Rep. 2021;70:895–899. doi: 10.15585/mmwr.mm7024e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Joseph N.T., Metz T.D. Coronavirus disease 2019 (COVID-19) and pregnancy outcomes: state of the science. Obstet Gynecol. 2021;138:539–541. doi: 10.1097/AOG.0000000000004548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morris R.S. SARS-CoV-2 spike protein seropositivity from vaccination or infection does not cause sterility. F S Rep. 2021;2:253–255. doi: 10.1016/j.xfre.2021.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Orvieto R., Noach-Hirsh M., Segev-Zahav A., Haas J., Nahum R., Aizer A. Does mRNA SARS-CoV-2 vaccine influence patients' performance during IVF-ET cycle? Reprod Biol Endocrinol. 2021;19:69. doi: 10.1186/s12958-021-00757-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wesselink A.K., Hatch E.E., Rothman K.J., Wang T.R., Willis M.D., Yland J., et al. A prospective cohort study of COVID-19 vaccination, SARS-CoV-2 infection, and fertility. Am J Epidemiol. 2022;191:1383–1395. doi: 10.1093/aje/kwac011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.