Abstract
Background
Due to the COVID-19 pandemic, the Substance Abuse and Mental Health Services Administration (SAMHSA) has relaxed restrictions on methadone treatment in the United States. There is concern that the relaxation may increase fatal overdose rates. This study examines opioid treatment program (OTP) changes to methadone treatment during COVID-19 and changes in fatal methadone-involved overdose rates in Connecticut.
Methods
From July 8th to August 18th, 2020, we conducted a comprehensive state-wide survey of all eight OTPs that dispense methadone in Connecticut to examine programmatic changes during COVID-19. We also analyzed state-level data on confirmed accidental opioid-involved deaths to assess if relaxation of take-home dosing restrictions and in-person attendance requirements correlated with increased methadone-involved fatal overdose rates.
Results
OTPs reported implementing multiple changes to methadone treatment in response to the COVID-19 pandemic. The percent of patients receiving 28-day take-home doses increased from 0.1% to 16.8%, 14-day take-home doses increased from 14.2% to 26.8%, and the percent receiving one or no take-home doses decreased from 37.5% to 9.6%. Monthly or more frequent drug testing decreased from 15% to 4.6% and 75.2% of individual counseling for methadone patients transitioned to telehealth. However, changes to methadone treatment varied considerably by program. OTP providers said restrictions on methadone should be relaxed and increases in take-home dosing as well as telehealth should be continued in non-pandemic situations. Methadone-involved fatalities relative to other opioid-involved fatalities did not increase in Connecticut following changes in OTP practices.
Conclusions
Connecticut OTPs relaxed methadone treatment requirements during COVID-19. Since relaxing restrictions on methadone treatment has not increased fatal overdoses, we recommend that the reductions in-person dosing and attendance requirements implemented during the COVID-19 pandemic should be continued and made permanent.
Keywords: Methadone, COVID-19, Opioid use disorder, Overdose, Telehealth, Medication-based treatment
1. Introduction
In the United States, approximately 1.6 million people live with opioid use disorder (OUD) (Substance Abuse and Mental Health Services Administration, 2020b). They suffer from high rates of overdose, suicide, and HIV and hepatitis C (HCV) infection that increase in lockstep with rising OUD rates (Hedegaard et al., 2020; Hodder et al., 2021; Olfson et al., 2019; Powell et al., 2019). Over 100,000 people have died from opioid overdoses during the past two years (National Institute on Drug Abuse, 2019). Overdose rates may be increasing due to the COVID-19 pandemic (Alter & Yeager, 2020; Mallet et al., 2020; Slavova et al., 2020).
More than 50 years of clinical evidence has shown that methadone is a safe and effective treatment for OUD that significantly reduces risk of overdose, illicit substance use, HIV and HCV infection risk, and overall morbidity and mortality (Dole & Nyswander, 1965; Kreek, 2000; Leshner & Dzau, 2019). Although decades of evidence show the substantial benefits of methadone for the treatment of OUD, it is heavily regulated in the United States (Jaffe & O'Keeffe, 2003). Only certified opioid treatment programs (OTPs) can dispense methadone, and patients typically receive methadone doses in-person while under direct supervision on a daily basis, with regular drug testing usually required (Bell, 2014; Stitzer & Vandrey, 2008). Daily supervised administration of methadone at OTPs was instituted for most patients because of concerns that take-home doses may lead to methadone diversion and subsequently to fatal overdose (Cicero & Inciardi, 2005; Fountain et al., 2000; H. Green et al., 2000; Seymour et al., 2003).
However, the COVID-19 pandemic raises concerns over these restrictions on methadone treatment, since in-person OTP visits for medication and required drug testing and counseling make social and physical distancing difficult, if not impossible. People with OUD may be at increased risk for COVID-19 due to the communal nature of drug use, high-rates of co-morbidities among people with OUD such as cardiovascular and respiratory diseases, and higher rates of housing instability, poverty, and unemployment (Mallet et al., 2020; Slaunwhite et al., 2020; van Dorn et al., 2020; Volkow, 2020). COVID-19-related loss of housing, jobs, and food security may increase the likelihood for relapse among people living with OUD (Alexander et al., 2020; Mallet et al., 2020).
In response to these issues, on March 16th, 2020, the Substance Abuse and Mental Health Services Administration (SAMHSA) released a guidance letter to OTPs on relaxing patient requirements in order to meet the urgent need to reduce the risk of COVID-19 infection through social distancing (Substance Abuse and Mental Health Services Administration, 2020a). The guidance contained a blanket exemption allowing increases in take-home dosing to 28 days for all stable patients and up to 14 days of take-home dosing for those who are considered less stable. It was further recommended that OTPs reduce or eliminate toxicology screens and in-person counseling sessions to further reduce potential exposure to COVID-19. The guidance also allowed OTPs to increase use of telemedicine and provide home delivery of medication, changes that others have also advocated for (Alexander et al., 2020; Heimer et al., 2020). Some public health scholars have raised concerns that relaxing OTP methadone patient requirements, particularly increased take-home doses, may increase risk of diversion and thus fatal overdose rates (Alexander et al., 2020; Becker & Fiellin, 2020; Dunlop et al., 2020; Volkow, 2020). However, little is known about if and how OTPs have been implementing changes that accord with the SAMHSA guidance and if methadone-involved overdose rates have increased as a result.
We conducted a comprehensive state-wide survey of OTPs in Connecticut to examine programmatic changes to methadone maintenance treatment (MMT) during COVID-19. We then analyzed state-level data to assess if relaxation of OTP take-home dosing restrictions and in-person attendance requirements correlated with methadone-involved fatal overdose rates.
2. Methods
This analysis combines two sources of data. The first is a comprehensive state-wide survey of Connecticut OTP providers conducted from July 8th to August 18th, 2020 to examine programmatic changes during COVID-19. The second source is state-level data on autopsies conducted by the Office of the Chief Medical Examiner on confirmed accidental opioid-involved deaths. The Connecticut Department of Public Health provided data in the toxicology reports on the opioid(s) involved in each fatal event. With these data, we assessed whether the relaxation of take-home dosing restrictions and in-person attendance requirements correlated with changes in the rate of involvement of methadone in fatal overdoses. The Yale Human Research Protections Program exempted this study from review.
2.1. Surveying methadone providers in Connecticut
A survey was developed in collaboration with Robert Lambert, Chair, Opioid Treatment Programs of Connecticut. The survey was designed to collect data on the operations of all eight OTPs that operate in the state. The first section of the survey covered OTP characteristics including number and location of clinics, and number of patients receiving methadone and other forms of treatment for OUD. Subsequent sections covered OTP provision of methadone, counseling, drug testing, and other services before and after SAMHSA's COVID-19 guidance in March 2020. A final section covered OTPs' potential and observed problems associated with implementing the SAMHSA guidelines and solicited opinions on the suitability of continuing the relaxation in non-pandemic situations. The survey was uploaded to Qualtrics survey platform to allow on-line data collection. A letter with a link to the survey was sent to the directors of all eight OTPs in Connecticut, co-signed by Mr. Lambert and the study principal investigator. The letter was sent on July 2nd and responses were received between July 8th and August 18th.
2.2. Determining the involvement of methadone in opioid-involved accidental deaths in CT
The Office of the Chief Medical Examiner investigates deaths attributed to drug overdoses and the Connecticut Department of Public Health maintains summary data that include the drugs determined to be involved. Monthly summary data were obtained for all of 2015–19 and January through August of 2020. Data for 2020 are considered preliminary and are subject to change. Individuals receiving methadone through the OTPs in Connecticut are reported to the Connecticut Department of Mental Health and Addiction Services, which deduplicates records to produce an annual count of individuals who receive treatment in each calendar year.
2.3. Statistical analysis
We used descriptive statistics to describe trends in how OTPs changed their policies and practices in response to the COVID-19 pandemic, including changes in take-home dosing and drug testing practices before and after COVID-19. We used χ2 tests to compare opioid-involved overdose deaths and overdose deaths involving methadone specifically during the five-month period after lockdown (April–August 2020) to the same five-month period in the previous five years going back to 2015. We divided methadone-involved fatalities into methadone-only or in combination and determined the change in proportion of fatalities involving methadone.
3. Results
3.1. Study sample: patients dispensed methadone at Connecticut OTPs
The sample included all eight OTPs in the state of Connecticut. The eight programs operate 29 clinics in total, serving 24,261 MMT patients (Table 1 ). Each OTP operates four clinics on average (range 1 to 9 clinics) and serves an average of 837 patients at each clinic (range 74 to 5000 patients). Most OTP patients have insurance coverage through Medicare or Medicaid (78.4%), 10.4% hold private insurance, and 9.7% have no insurance. The OTPs adjusted services in response to COVID-19 between February 27th and March 27th (median: March 16th).
Table 1.
OTP Characteristics.
| Program |
||||||||
|---|---|---|---|---|---|---|---|---|
| #1 | #2 | #3 | #4 | #5 | #6 | #7 | #8 | |
| Number of clinics | 2 | 1 | 3 | 4 | 6 | 7 | 9 | 5 |
| Number of patients receiving methadone | 10,000 | 493 | 1690 | 775 | 817 | 4590 | 5528 | 368 |
| Insurance of patients | ||||||||
| Medicare/Medicaid | 87% | 85% | 85% | 94% | 73% | 65% | 75% | 92% |
| Private | 3% | 5% | 6% | 6% | 4% | 20% | 20% | 3% |
| None | 10% | 10% | 9% | 0% | 23% | 15% | 5% | 5% |
| Naloxone provision post COVID-19 | No | Request | All | No | No | All | All | Unstable patients |
| Change in the people seeking treatment | Decrease | None | Increase | None | None | Decrease | None | Decrease |
3.2. Patient-level changes in OTP treatment practices
OTP take-home dosing practices relaxed significantly during COVID-19 (Table 2 ). Prior to COVID-19, almost no MMT patients (0.1%) received 28-day take-home dosing. After COVID-19 changes were implemented, 16.8% of patients received 28-day take-home dosing. The number of patients receiving 14-day take-home dosing increased from 14.2% prior to COVID-19 to 26.8% during COVID-19. Furthermore, the number of patients receiving one or no take-home doses decreased from 37.5% to 9.6%. However, only 43.6% of patients were moved to the maximum 14- or 28-day take-home dosing schedule allowable by the SAMHSA guidance. Thus, 56.4% of MMT patients remained on lower take-home amounts than permitted by SAMHSA.
Table 2.
Patient Level Changes in Clinic Practices During COVID-19.
| Changes in clinic practices | Before COVID-19 | After COVID-19 | Change |
|---|---|---|---|
| Take-home Doses | |||
| 28-day take-home doses | 0.1% (25) | 16.8% (4076) | 16,700% |
| 14-day take-home doses | 14.2% (3445) | 26.8% (6499) | 89% |
| 4 to 6-day take-home doses | 13.3% (3236) | 15.3% (3710) | 15% |
| 3-day take-home doses | 16.8% (4069) | 20% (4853) | 19% |
| 2-day take-home doses | 18.1% (4383) | 11.5% (2789) | -36% |
| ≤1-day take-home doses | 37.5% (9103) | 9.6% (2333) | −74% |
| Drug testing frequencies | |||
| 8 times per year | 84.9% (20,608) | 92.2% (22,375) | 9% |
| Once or twice a month | 12.5% (3042) | 2.9% (692) | −77% |
| Weekly | 2.5% (612) | 1.7% (418) | −32% |
| Random | 0% | 1.2% (287) | – |
| No testing requirement | 0% | 2% (488) | – |
| Other clinic visits | |||
| In-person individual counseling | 57.5% (13,962) | 9.3% (2263) | −84% |
| In-person group counseling | 42.5% (10,299) | 8.2% (1996) | −81% |
| Telehealth individual counseling | 0% | 75.2% (18,244) | – |
| Telehealth group counseling | 0% | 7.2% (1758) | – |
| In-person case management | 46.3% (11,231) | 13.9% (3384) | −70% |
| Telehealth case management | 0% | 32.3% (7846) | – |
| No case management | 12.5% (3030) | 12.5% (3030) | 0% |
| In-person patient evaluations | 77.8% (18,883) | 55.6% (13,480) | −28% |
| Telehealth patient evaluations | 0% | 23.1% (5617) | – |
| In-person healthcare appointments | 17.7% (4286) | 7.9% (1921) | −55% |
| Telehealth healthcare appointments | 0% | 11.8% (2868) | – |
OTP drug testing frequency requirements decreased somewhat during COVID-19. The percentage of MMT patients tested once a month or more decreased from 15% to 4.6%. Further, after the COVID-19 pandemic began, 2% of patients were not required to be tested at all.
The majority of OTP in-person individual and group counseling transitioned to telehealth counseling during COVID-19. Before COVID-19, no MMT patients received telehealth counseling. During COVID-19, 75.2% of patients received telehealth individual counseling. Notably, the percentage of patients receiving group counseling— either in person or via telehealth— decreased from 42.5% to 15.4% while the percentage of patients receiving in-person or telehealth individual counseling increased from 57.5% to 84.5%. Some OTP case management (32.3%), patient evaluations (23.1%), and health care appointments (11.8%) also shifted to telehealth.
3.3. Program-level changes in OTP practices
Although Connecticut OTPs relaxed methadone treatment policies during COVID-19, not all programs instituted all SAMHSA-authorized changes. All OTPs made the following changes to clinic practices during COVID-19: increasing take-home doses, moving counseling services to telehealth, providing staff with personal protective equipment (PPE), providing curbside dosing, and mandating 6 ft of social distancing inside the clinic (Table 3 ). The majority of OTPs also made the following changes: posting information about COVID-19 on their websites, setting up handwashing stations, screening patients before entry, changing hours of operation, offering proxy pick-ups for sick patients, separating symptomatic patients, requiring and providing masks for patients, limiting entry to clinics, and mandating 6 ft of social distancing outside the clinic. Three of the OTPs provided naloxone to all patients during COVID-19 (Table 1). In summary, OTPs made a median number of 12 programmatic changes, with a range of 11 to 17 changes.
Table 3.
Program Level COVID-19 Changes.
| Number of Clinics | Change in program operations |
|---|---|
| 8 | Increasing take-home doses, moving counseling to telehealth, providing PPE to staff, mandating social distancing within clinic, providing curbside dosing |
| 7 | Mandating social distancing outside clinic, providing home delivery, requiring PPE for patients, providing PPE for patients |
| 6 | Screening patients before entry, limiting site entry |
| 5 | Posting information on website, providing handwashing stations for patients, separating symptomatic patients |
| 4 | Changing hours of operation |
| 3 | Telehealth monitoring of take-home doses, distributing phone/email lists of staff, allowing proxy pick-ups for sick patients |
| 2 | Allowing support person for dose administration |
| 1 | Offering patients alternative treatment locations |
The degree of relaxation of in-person dosing requirements differed significantly by OTP. For instance, only 3 of the 8 OTPs provided 25% or more of their patients the maximum (28 day) take-home dosing allowable after COVID-19, and 3 of the 8 OTPs reduced the number of patients given one or no take-home doses per week to 15% or less of patients. During COVID-19, OTPs widely adopted telehealth for counseling and other services, but the degree of change differed between programs. For example, 5 of 8 OTPs provided telehealth individual counseling to at least 50% of patients and 3 of 8 OTPs reduced requirements for in-person individual and group counseling to less than 25% of patients.
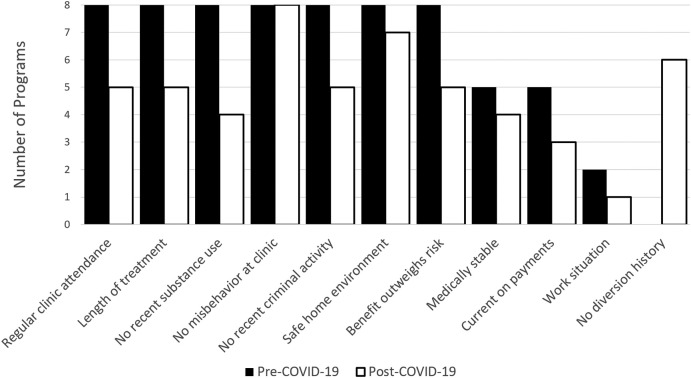
OTPs also differed in the changes they made to how they defined patient stability for take-home dose eligibility during COVID-19. Prior to COVID-19, all OTPs followed the SAHMSA 8 definition of stable, although some OTPs added additional criteria (Fig. 1 ). During COVID-19, all OTPs kept only two of the SAMHSA 8 criteria (absence of serious behavioral problems at the clinic and safe storage of take-home doses) and differed in their implementation of the other six criteria. Furthermore, some criteria increased in importance during COVID-19. For example, 6 of 8 OTPs added history or risk of diversion and 3 OTPs added regular attendance of counseling sessions to their take-home dose eligibility criteria.
Fig. 1.
Changes in criteria for determining eligibility for take-home doses. Prior to the SAMHSA guidance document, all eight programs used all seven previously established standards.
3.4. Consequences of COVID-19-related changes
OTPs reported some negative consequences of programmatic changes due to COVID-19 (Table 4 ). One OTP experienced liability issues as a consequence of programmatic changes and three OTPs reported diversion as a consequence of increased take-home doses. The extent of the problems encountered was generally less than what was anticipated. The consequences of OTP changes on congregation outside the clinic were mixed. Although two OTPs said patient congregation outside the clinic had increased due to programmatic changes that decreased patient density inside, six OTPs said congregation outside the clinic had decreased.
Table 4.
Program level concerns versus consequences.
| Concerns | Consequences | |
|---|---|---|
| Liability related issues because of increased take-home doses | 5 programs | 1 program |
| Patients taking more than the prescribed dose in one day | 5 programs | 3 programs |
| Diversion | 3 programs | 3 programs |
| Overdose of someone other than patient on take-home doses | 3 programs | 0 programs |
| Overdose of patients | 3 programs | 1 program |
| Patients having trouble paying for multiple doses at one time | 2 programs | 2 programs |
OTP directors said that patients were experiencing COVID-19-related difficulties including increased tensions within the clinic due to social distancing non-compliance, disputes over the use of personal protective equipment, and the effects of staff burnout. Regarding the transition to telehealth, OTPs said that it worked well for some but not all patients and it made building rapport difficult. OTPs reported many patients were experiencing multiple negative consequences from COVID-19 including mental health issues, grief, anxiety, depression, social isolation, unemployment, food insecurity, and housing difficulties.
OTPs said that in future epidemics take-home doses should be increased and expansion permitted sooner. OTPs also recommended giving patients tutorials on telehealth, expanding resources to create safe and reliable transportation, providing curbside and home dosing for immunocompromised patients, stockpiling reserves of personal protective equipment, and focusing on therapeutic relationships with patients. Further, they stated that more structured federal directives would help reduce variation in programmatic changes between clinics. Finally, OTPs said restrictions on MMT services in non-pandemic situations should be relaxed to increase take-home dosing and continue telehealth for patient care and counseling.
3.5. The changing environment of opioid overdose fatalities
Prior to COVID-19, the annual number for all opioid-involved accidental deaths in Connecticut increased by 71%, from 659 in 2015 to 1127 in 2019. In 2019, 82% of opioid-involved deaths included fentanyl (Connecticut Department of Health, 2021). Between 2015 and 2019 the number of methadone-involved fatalities, either as a single opioid or in combination with other opioids, increased by 27.8%. During this same five-year period, the number of people treated with methadone at the state's OTPs ranged from 19,203 patients in 2015 to 21,680 in 2019, averaging 20,929.8 ± 1012.8. However, the slight rise in patients was not significant.
We compared all opioid-involved and methadone-involved overdose fatalities during the COVID-19 period (April–August 2020) to the same period in the preceding five years (Table 5 ). Chi-squared analysis revealed that neither methadone-only nor methadone-involved fatalities increased in the five-month period in 2020 compared to the same time period in earlier years, after controlling for the increase in overall fatal overdoses during this time period from 2015 to 2020.
Table 5.
Changes in Accidental Methadone-Involved Fatalities Recorded by Connecticut's Office of the Chief Medical Examiner. Comparison of fatalities involving methadone to fatalities involving opioids other than methadone.
| Period | All opioid fatalities |
Methadone-only fatalities |
All methadone-involved fatalities |
Opioid fatalities not involving methadone |
|||
|---|---|---|---|---|---|---|---|
| Number | Number | % | Number | % | Number | % | |
| April–August 2015 through 2019 | 1972 | 74 | 3.8% | 181 | 9.4% | 1791 | 90.6% |
| April–August 2020 | 539 | 22 | 4.1% | 59 | 10.9% | 480 | 89.1% |
| Χ2 value vs. other opioids | 1.803 | 1.539 | |||||
| p-value | 0.406 | 0.215 | |||||
4. Discussion
OTPs in Connecticut made substantial adjustments to methadone treatment practices in response to the COVID-19 pandemic. OTP changes included moving counseling to telehealth, taking measures to increase social distancing, increasing take-home doses and decreasing the percentage of patients with one or no take-home doses, in line with changes that others have called for (Tsai & Wilson, 2020; Wakeman et al., 2020). We see no evidence that increased take-home doses led to increased methadone-related fatal overdoses after taking into account changes in the number of patients receiving methadone for treatment of OUD (constant) and the number of opioid-involved fatal overdoses (increasing).
Transitioning to telemedicine and increasing take-home doses, as the OTPs in our study have done, can be an important part of efforts to decrease risk of COVID-19 infection and should be broadly implemented (Chen et al., 2020). Because in-person contact increases risk of COVID-19 infection for both patients and clinic staff, social distancing measures are primary prevention measures. Since methadone treatment reduces mortality for people with OUD (Sordo et al., 2017), and unsupervised take-home dosing is positively associated with treatment retention and improvement in quality of life and employment, and does not differ from supervised in-person dosing in illicit opioid use, diversion, or patient deaths (Gerra et al., 2011; King et al., 2006; Rhoades et al., 1998; Sarasvita et al., 2012; Shakira et al., 2017), methods other than daily dosing should be employed to ensure treatment compliance (Saulle et al., 2017). Telehealth to supervise at-home dosing appears to be a suitable alternative, at least in terms of preventing diversion that could increase methadone-involved fatalities. Further, OTPs should implement protections to limit respiratory disease spread similar to those used in other medical settings including PPE requirements for patients and staff alongside patient and staff screenings (Alexander et al., 2020). With their proven benefits and increasing availability, OTPs should offer COVID-19 vaccinations to staff and patients as a regular part of clinical practice and staff support.
Connecticut OTPs widely implemented telehealth to replace in-person counseling visits. Since counseling may increase treatment retention and decrease opioid use and HIV risk (Davstad et al., 2009; Dugosh et al., 2016; Kelly et al., 2011), switching counseling to telehealth so that patients may still receive its benefits while maintaining social distancing may be optimal for patient health. Further research might examine the impact of telehealth on patient retention and health.
This study shows that during COVID-19 different OTPs within one state instituted different changes in program practices. For example, only three of the eight OTPs distributed naloxone to all patients, although clinics are urged to distribute naloxone to patients because of the heightened overdose risks during COVID-19 (Alexander et al., 2020; Tsai & Wilson, 2020).
Survey responses from the OTPs endorsed the importance of relaxing in-person methadone treatment requirements during COVID-19, they also recommended expanding resources for patient tutorials on telehealth, creating safe and reliable transportation, providing curbside and home dosing for immunocompromised patients, and focusing on therapeutic relationships with patients. OTPs were consistent in holding that the range of changes in program services, including increased take-home dosing and expanded telehealth, should be implemented in non-pandemic situations.
4.1. Limitations
Although we received survey responses from all eight OTPs in Connecticut, we cannot generalize our findings to other states. This study is cross-sectional, so these findings represent OTP changes that were in place during the time of the survey; program changes may evolve over time. The data on fatal opioid-involved overdoses, including those involving methadone, are limited to decedents whose cases were reviewed by the Office of the Chief Medical Examiner. Nevertheless, it is clear that changes to methadone treatment are not contributing to the continued increase in overdose fatalities observed in Connecticut, at least during the initial five months of the COVID-19 pandemic. More research will be needed to determine if this situation is sustained.
4.2. Conclusion
The variability in programmatic changes by Connecticut OTPs suggests that more guidance should be provided on the state and federal level to OTPs for relaxing methadone treatment requirements as well as buprenorphine treatment requirements during COVID-19. OTPs should receive guidance for reducing drug testing and in-person group and individual counseling requirements, transitioning to telehealth for a spectrum of patient services, defining patient stability, establishing safety procedures, and stockpiling personal protective equipment.
The burdensome requirements for OTP patients and providers, the restrictions on methadone dispensation in primary care facilities or through pharmacies, and the stigma directed against agonist-based medications have created a treatment gap such that the majority of people with OUD are not receiving agonist medication-based treatment (Wu et al., 2016). Researchers have long called for expanding access to methadone treatment, reducing supervised in-person dosing, and decreasing regulations and the burden of compliance and attendance (Gerra et al., 2011; Radcliffe & Stevens, 2008; Zaller et al., 2009). During the COVID-19 pandemic such changes are more pressing (T.C. Green et al., 2020). Since methadone treatment serves as a protective factor against overdose, reducing barriers to methadone treatment is all the more urgent.
Since the relaxations in OTP policies in Connecticut did not lead to an increase in fatal methadone-involved overdoses, we recommend that the changes made to methadone treatment by the OTPs in this study should be expanded further, and many of the restrictions on OTP practices and policies should be permanently removed so that methadone and buprenorphine treatments for OUD are more accessible and less burdensome for patients.
Funding
No external funding was received for the conduct of this study.
CRediT authorship contribution statement
Sarah Brothers: Conceptualization, Investigation, Visualization, Methodology, Formal analysis, Writing – original draft. Adam Viera: Visualization, Methodology, Formal analysis, Writing – original draft. Robert Heimer: Conceptualization, Investigation, Visualization, Methodology, Formal analysis, Writing – original draft.
Declaration of competing interest
The authors have no conflicts to report.
Acknowledgements
We would like to thank Robert Lambert, Chair, Opioid Treatment Programs of Connecticut for assistance in developing the survey and Michele Sitler of Connecticut Counseling, Inc. for sending letters inviting the directors of the Connecticut's opioid treatment programs to participate.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jsat.2021.108449.
Appendix A. Supplementary data
Supplementary tables
References
- Alexander G.C., Stoller K.B., Haffajee R.L., Saloner B. An epidemic in the midst of a pandemic: Opioid use disorder and COVID-19. Annals of Internal Medicine. 2020:M20–1141. doi: 10.7326/M20-1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alter A., Yeager C. University of Baltimore. Baltimore; ODMAP: 2020. COVID-19 impact on US national overdose crisis.http://www.odmap.org/Content/docs/news/2020/ODMAP-Report-June-2020.pdf (Retrieved July 12, 2020) [Google Scholar]
- Becker W.C., Fiellin D.A. When epidemics collide: Coronavirus disease 2019 (COVID-19) and the opioid crisis. Annals of Internal Medicine. 2020:M20–1210. doi: 10.7326/M20-1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell J. Pharmacological maintenance treatments of opiate addiction. British Journal of Clinical Pharmacology. 2014;77(2):253–263. doi: 10.1111/bcp.12051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, K. L., Brozen, M., Rollman, J. E., Ward, T., Norris, K., Gregory, K. D., & Zimmerman, F. J. (2020). Transportation access to health care during the COVID-19 pandemic: Trends and implications for significant patient populations and health care needs. Los Angeles, CA: UCLA Institute of Transportation Studies. Report No.: UC-ITS-2021-11 | DOI:10.17610/T6RK5N. Accessed October 30, 2020.
- Cicero T.J., Inciardi J.A. Diversion and abuse of methadone prescribed for pain management. JAMA. 2005;293(3):293–298. doi: 10.1001/jama.293.3.297. [DOI] [PubMed] [Google Scholar]
- Connecticut Department of Health . Connecticut Department of Public Health; Hartford: 2021. Drug overdose monthly report. Updated February 13, 2021. [Google Scholar]
- Davstad I., Stenbacka M., Leifman A., Romelsjö A. An 18-year follow-up of patients admitted to methadone treatment for the first time. Journal of Addictive Diseases. 2009;28(1):39–52. doi: 10.1080/10550880802544997. [DOI] [PubMed] [Google Scholar]
- Dole V.P., Nyswander M. A medical treatment for diacetylmorphine (heroin) addiction: A clinical trial with methadone hydrochloride. JAMA. 1965;193(8):646–650. doi: 10.1001/jama.1965.03090080008002. [DOI] [PubMed] [Google Scholar]
- Dugosh K., Abraham A., Seymour B., McLoyd K., Chalk M., Festinger D. A systematic review on the use of psychosocial interventions in conjunction with medications for the treatment of opioid addiction. Journal of Addiction Medicine. 2016;10(2):91. doi: 10.1097/ADM.0000000000000193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlop A., Lokuge B., Masters D., Sequeira M., Saul P., Dunlop G.…Lintzeris N. Challenges in maintaining treatment services for people who use drugs during the COVID-19 pandemic. Harm Reduction Journal. 2020;17:1–7. doi: 10.1186/s12954-020-00370-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fountain J., Strang J., Gossop M., Farrel M., Griffiths P. Diversion of prescribed drugs by drug users in treatment: Analysis of the UK market and new data from London. Addiction. 2000;95(3):393–406. doi: 10.1046/j.1360-0443.2000.95339310.x. [DOI] [PubMed] [Google Scholar]
- Gerra G., Saenz E., Busse A., Maremmani I., Ciccocioppo R., Zaimovic A.…Somaini L. Supervised daily consumption, contingent take-home incentive and non-contingent take-home in methadone maintenance. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2011;35(2):483–489. doi: 10.1016/j.pnpbp.2010.12.002. [DOI] [PubMed] [Google Scholar]
- Green H., James R.A., Gilbert J.D., Harpas P., Byard R.W. Methadone maintenance programs-a two-edged sword? The American Journal of Forensic Medicine and Pathology. 2000;21(4):359–361. doi: 10.1097/00000433-200012000-00012. [DOI] [PubMed] [Google Scholar]
- Green T.C., Bratberg J., Finnell D.S. Opioid use disorder and the COVID 19 pandemic: A call to sustain regulatory easements and further expand access to treatment. Substance Abuse. 2020;41(2):147–149. doi: 10.1080/08897077.2020.1752351. [DOI] [PubMed] [Google Scholar]
- Hedegaard H., Miniño A.M., Warner M. National Center for Health Statistics. NCHS Data Brief No; Atlanta, GA: 2020. Drug overdose deaths in the United States, 1999–2018; p. 356.https://www.cdc.gov/nchs/data/databriefs/db356-h.pdf (Last accessed October 30, 2020) [Google Scholar]
- Heimer R., McNeil R., Vlahov D. A community responds to the COVID-19 pandemic: A case study in protecting the health and human rights of people who use drugs. Journal of Urban Health. 2020;97(4):448–456. doi: 10.1007/s11524-020-00465-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodder S.L., Feinberg J., Strathdee S.A., Shoptaw S., Altice F.L., Ortenzio L., Beyrer C. The opioid crisis and HIV in the USA: Deadly synergies. The Lancet. 2021;397(10279):1139–1150. doi: 10.1016/S0140-6736(21)00391-3. [DOI] [PubMed] [Google Scholar]
- Jaffe J.H., O’Keeffe C. From morphine clinics to buprenorphine: Regulating opioid agonist treatment of addiction in the United States. Drug and Alcohol Dependence. 2003;70(2):S3–S11. doi: 10.1016/s0376-8716(03)00055-3. [DOI] [PubMed] [Google Scholar]
- Kelly S.M., O’Grady K.E., Mitchell S.G., Brown B.S., Schwartz R.P. Predictors of methadone treatment retention from a multi-site study: A survival analysis. Drug and Alcohol Dependence. 2011;117(2–3):170–175. doi: 10.1016/j.drugalcdep.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King V.L., Kidorf M.S., Stoller K.B., Schwartz R., Kolodner K., Brooner R.K. A 12-month controlled trial of methadone medical maintenance integrated into an adaptive treatment model. Journal of Substance Abuse Treatment. 2006;31(4):385–393. doi: 10.1016/j.jsat.2006.05.014. [DOI] [PubMed] [Google Scholar]
- Kreek M.J. In: Annals of the New York Academy of Sciences. Glick S.D., Maisonneuve I.M., editors. Vol. 909. New York Academy of Sciences; New York, NY: 2000. Methadone-related opioid agonist pharmacotherapy for heroin addiction: History, recent molecular and neurochemical research, and future in mainstream medicine; pp. 186–216. (New medications for drug abuse). [DOI] [PubMed] [Google Scholar]
- Leshner A.I., Dzau V.J. Medication-based treatment to address opioid use disorder. Jama. 2019;321(21):2071–2072. doi: 10.1001/jama.2019.5523. [DOI] [PubMed] [Google Scholar]
- Mallet J., Dubertret C., Le Strat Y. Addictions in the COVID-19 era: Current evidence, future perspectives a comprehensive review. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2020;110070 doi: 10.1016/j.pnpbp.2020.110070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIDA Overdose death rates. 2019. https://www.drugabuse.gov/relatedtopics/trends-statistics/overdose-death-rates
- Olfson M., Rossen L.M., Wall M.M., Houry D., Blanco C. Trends in intentional and unintentional opioid overdose deaths in the United States, 2000-2017. Jama. 2019;322(23):2340–2342. doi: 10.1001/jama.2019.16566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell D., Alpert A., Pacula R.L. A transitioning epidemic: How the opioid crisis is driving the rise in hepatitis C. Health Affairs. 2019;38(2):287–294. doi: 10.1377/hlthaff.2018.05232. [DOI] [PubMed] [Google Scholar]
- Radcliffe P., Stevens A. Are drug treatment services only for ‘thieving junkie scumbags’? Drug users and the management of stigmatised identities. Social Science & Medicine. 2008;67(7):1065–1073. doi: 10.1016/j.socscimed.2008.06.004. [DOI] [PubMed] [Google Scholar]
- Rhoades H.M., Creson D., Elk R., Schmitz J., Grabowski J. Retention, HIV risk, and illicit drug use during treatment: Methadone dose and visit frequency. American Journal of Public Health. 1998;88(1):34–39. doi: 10.2105/ajph.88.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAMHSA . Substance Abuse and Mental Health Services Administration; Rockville, MD: 2020. FAQs: Provision of methadone and buprenorphine for the treatment of opioid use disorder in the COVID-19 emergency.https://www.samhsa.gov/sites/default/files/faqs-for-oud-prescribing-and-dispensing.pdf (Last accessed October 28, 2020) [Google Scholar]
- SAMHSA Key substance use and mental health indicators in the United States: Results from the 2019 National Survey on Drug Use and Health (HHS Publication No. PEP20-07-01-001, NSDUH Series H-55). Rockville, MD: Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration. 2020. https://www.samhsa.gov/data/ Retrieved from.
- Sarasvita R., Tonkin A., Utomo B., Ali R. Predictive factors for treatment retention in methadone programs in Indonesia. Journal of Substance Abuse Treatment. 2012;42(3):239–246. doi: 10.1016/j.jsat.2011.07.009. [DOI] [PubMed] [Google Scholar]
- Saulle, R., Vecchi, S., & Gowing, L. (2017). Supervised dosing with a long-acting opioid medication in the management of opioid dependence. Cochrane Database of Systematic Reviews, (4), Article CD011983. [DOI] [PMC free article] [PubMed]
- Seymour A., Black M., Jay J., Cooper G., Weir C., Oliver J. The role of methadone in drug-related deaths in the west of Scotland. Addiction. 2003;98(7):995–1002. doi: 10.1046/j.1360-0443.2003.00425.x. [DOI] [PubMed] [Google Scholar]
- Shakira R.H., .W., Sarimah A., Norsa’adah B. Factor predictive of 1-year retention on methadone maintenance therapy program: A survival analysis study. Addictive Disorders & Their Treatment. 2017;16(2):64–69. [Google Scholar]
- Slaunwhite A.K., Gan W.Q., Xavier C., Zhao B., Buxton J.A., Desai R. Overdose and risk factors for severe acute respiratory syndrome. Drug and Alcohol Dependence. 2020;212:108047. doi: 10.1016/j.drugalcdep.2020.108047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavova S., Rock P., Bush H.M., Quesinberry D., Walsh S.L. Signal of increased opioid overdose during COVID-19 from emergency medical services data. Drug and Alcohol Dependence. 2020;214:108176. doi: 10.1016/j.drugalcdep.2020.108176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sordo L., Barrio G., Bravo M.J., Indave B.I., Degenhardt L., Wiessing L.…Pastor-Barriuso R. Mortality risk during and after opioid substitution treatment: Systematic review and meta-analysis of cohort studies. BMJ. 2017;357:j1550. doi: 10.1136/bmj.j1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitzer M.L., Vandrey R. Contingency management: Utility in the treatment of drug abuse disorders. Clinical Pharmacology & Therapeutics. 2008;83(4):644–647. doi: 10.1038/sj.clpt.6100508. [DOI] [PubMed] [Google Scholar]
- Tsai J., Wilson M. COVID-19: A potential public health problem for homeless populations. The Lancet Public Health. 2020;5(4):e186–e187. doi: 10.1016/S2468-2667(20)30053-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dorn A., Cooney R.E., Sabin M.L. COVID-19 exacerbating inequalities in the US. Lancet. 2020;395(10232):1243. doi: 10.1016/S0140-6736(20)30893-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow N.D. Collision of the COVID-19 and addiction epidemics. Annals of Internal Medicine. 2020;173(1):61–62. doi: 10.7326/M20-1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakeman S.E., Green T.C., Rich J. An overdose surge will compound the COVID-19 pandemic if urgent action is not taken. Nature Medicine. 2020;26:819–820. doi: 10.1038/s41591-020-0898-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L.T., Zhu H., Swartz M.S. Treatment utilization among persons with opioid use disorder in the United States. Drug and Alcohol Dependence. 2016;169:117–127. doi: 10.1016/j.drugalcdep.2016.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaller N.D., Bazazi A.R., Velazquez L., Rich J.D. Attitudes toward methadone among out-of-treatment minority injection drug users: Implications for health disparities. International Journal of Environmental Research and Public Health. 2009;6(2):787–797. doi: 10.3390/ijerph6020787. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary tables