Abstract
Background and Aims
A better understanding of the underlying mechanism of acetaminophen (APAP)-induced liver injury (AILI) remains an important endeavor to develop new therapeutic approaches. Eosinophils have been detected in liver biopsies of patients with APAP overdose. We recently demonstrated a profound protective role of eosinophils against AILI; however, the molecular mechanism had not been elucidated.
Approach and Results
In agreement with our previous data from experiments using genetic deletion of eosinophils, we found that depletion of eosinophils in wild-type (WT) mice by an anti-IL-15 antibody resulted in exacerbated AILI. Moreover, adoptive transfer of eosinophils significantly reduced liver injury and mortality rate in WT mice. Mechanistic studies using eosinophil-specific IL-4/IL-13 knockout mice demonstrated that these cytokines, through inhibiting IFN-γ, mediated the hepato-protective function of eosinophils. Reverse phase protein array analyses and in vitro experiments using various inhibitors demonstrated that IL-33 stimulation of eosinophils activated p38MAPK, and in turn, cyclooxygenases (COX), which triggered NF-κB-mediated IL-4/IL-13 production. In vivo adoptive transfer experiments showed that in contrast to naive eosinophils, those pre-treated with cyclooxygenase inhibitors failed to attenuate AILI.
Conclusions
The current study revealed that eosinophil-derived IL-4/IL-13 accounted for the hepato-protective effect of eosinophils during AILI. The data demonstrated that the p38MAPK/COX/NF-κB signaling cascade played a critical role in inducing IL-4/IL-13 production by eosinophils in response to IL-33.
Keywords: eosinophils, acetaminophen, cyclooxygenase, IL-4, IL-13
Introduction
Acetaminophen (APAP) overdose is the single most important cause of acute liver failure in many developed countries(1). Although N-acetyl cysteine can reduce APAP-induced liver injury (AILI), its effectiveness is limited to the first few hours after APAP ingestion. Therefore, exploring the underlying pathogenesis of AILI in order to develop novel therapeutic approaches remains an important area of research.
In addition to APAP-induced direct hepatotoxicity, evidence supports that sterile inflammation contributes to AILI. Damage-associated molecular patterns (DAMPs) are released from damaged cells and activate innate immune cells to produce pro-inflammatory mediators (2). TNF-α and IL-1α are involved in the early phase of APAP-induced liver damage (3, 4). IFN-γ is shown to promote leukocyte infiltration, nitric oxide production, and the release of other inflammatory cytokines during AILI (5). The critical role of IFN-γ in AILI is further demonstrated by the reduced liver injury in both IFN-γ−/− and anti-IFN-γ antibody-treated mice (5, 6). Sterile inflammation is self-limiting owing to factors that counteract and restrain inflammation, and eventually restore tissue homeostasis. Similar to the initiation of inflammation, the resolution of inflammation is an active process and tightly controlled (7, 8). However, the pro-resolving or anti-inflammatory mechanisms in AILI are not completely understood.
Eosinophils are terminally differentiated and highly granulated myeloid-derived cells. They have been regarded as a cell type responding to parasitic infection and contributing to allergic diseases (9, 10). However, emerging evidence suggests that eosinophils play an important role in resolving inflammation. In a mouse model of IgG-mediated skin inflammation, eosinophils promote the resolution of inflammation via recruiting regulatory T cells (11). It is also reported that eosinophils are important in restraining inflammation and promoting tissue remodeling in allergic airway (12, 13). Although a small number of eosinophils are found in the liver in steady state (14), the number increases significantly under pathological conditions, such as viral hepatitis and acute liver injury (15, 16). Up to date, there are very few studies on the role of eosinophils in acute liver injury and they report controversial findings (17, 18). In a mouse model of halothane-induced acute liver injury, eosinophils have been associated with a pathological role (17), whereas in liver ischemia-reperfusion injury, eosinophil accumulation in the liver confers a profound hepato-protective function (18). Moreover, eosinophils have been detected in liver biopsies of patients with APAP overdose (19) and our recent study demonstrated a protective role of eosinophils in AILI (20).
The present study provide data supporting that eosinophil-derived IL-4/IL-13, through inhibition of IFN-γ, is an important mechanism of the hepato-protective function of eosinophils. The data also demonstrate that the IL-4/IL-13 production by eosinophils is mediated through IL-33-induced p38 MAPK phosphorylation, followed by cyclooxygenase and NF-κB activation.
Materials and Methods
Animal experiments
Breeders of Balb/c (cat#000651), ΔdblGata1 (cat#005653), and IL-4/GFP-enhanced transcript (4Get) reporter (cat#004190) mice were purchased from the Jackson laboratory. Breeders of IL-4/IL-13fl/fl×eoCre mice (backcrossed to C57BL/6J) (21) were obtained from Mayo Clinic Arizona. All mouse colonies were maintained at the UTHealth animal core facility. All experiments were performed according to the guidelines of the IACUC at UTHealth.
Female Balb/c mice (8–10 weeks old) were used in the majority of the experiments to avoid the effects of aggression among male mice. Male Balb/c and ΔdblGata1 mice were also used and the same phenotype was observed (data not shown). For APAP treatment, mice were fasted overnight (5:00pm – 9:00am) before injected intraperitoneally (i.p.) with APAP (Sigma-Aldrich) dissolved in warm phosphate-buffered saline (PBS) at the dose of 325 mg/kg (22). After APAP treatment, mice were euthanized to harvest blood and liver tissues for further analysis at 8h or 24h. For survival analyses, mice were treated with 600mg/kg of APAP.
To evaluate the extent of liver injury, serum concentrations of alanine transaminase (ALT) were measured by a diagnostic assay kit (Teco Diagnostics). Paraffin-embedded liver tissues were cut in 5μm sections and stained with hematoxylin and eosin for the examination of liver histopathology.
In the adoptive transfer experiments, 7.5 millions of bone marrow-derived eosinophils (bmEos) were injected intravenously (i.v.) into recipient mice immediately prior to APAP injection. For eosinophils depletion, WT mice were i.p. injected with anti-IL-5 antibody (200μg per mouse, clone: TRFK5, Bio X Cell) at 18h before APAP injection. To neutralize IL-4 and IL-13 in vivo, mice were i.v. injected with the combination of an anti-mouse IL-4 antibody (300μg per mouse, clone: 11B11, Bio X Cell) and an anti-mouse IL-13 antibody (200μg per mouse, clone: 38213, ThermoFisher) immediately prior to APAP treatment. Control mice were treated with the same amounts of rat IgG.
Flow Cytometric analyses
Liver mononuclear cells were isolated, stained with various antibodies and analyzed using a CytoFLEX flow cytometer (Beckman Coulter). Anti-CD16/CD32 antibody (2.4G2, BD Pharmingen) was used to block nonspecific binding. The fixable live/dead viability dye (ThermoFisher, cat#L34962) was used to define live cells. Anti-CCR3 (J073E5) was purchased from BioLegend. Anti-Siglec F (E50–2440), anti-CD3 (145–2C11), anti-CD11b (M1/70), and anti-CD45 (30-F11) were purchased from BD Pharmingen. For intracellular staining of IL-13, liver mononuclear cells obtained from APAP-treated mice were fixed and permeabilized by the kit (BD Cytofix/Cytoperm™ Fixation/Permeabilization Kit), and then stained with anti-IL-13 antibody (eBio13A, ThermoFisher) without further stimulation ex vivo. The data were analyzed with the FlowJo v10.7.1 software.
Isolation and ex vivo culturing of mouse bone marrow-derived eosinophils (bmEos)
Ex vivo culturing of mouse bmEos was performed as previously described (23). Briefly, bone marrow cells from mouse femurs and tibias were cultured in 10 ml of RPMI 1640 medium (Gibco) with 20% FBS and supplemented with FLT3-L (100ng/ml, PeproTech) and SCF (100ng/ml, PeproTech) for 4 days. On day 4, the medium was replaced with 10ml of fresh medium containing IL-5 (10ng/ml, PeproTech) only. On day 8, the medium was replaced with 20ml of fresh medium containing IL-5. On day 12, bmEos were collected and stimulated by IL-33 (20ng/ml) or the combination of IFN-γ and TNF-α (15ng/ml each) in presence of IL-5 (10ng/ml) for different times as indicated.
To inhibit the activity of COX-1, COX-2, p38, or NF-κB in vitro, the inhibitors of COX-1 (sc-560, 50μM), COX-2 (celecoxib, 50μM), COX-1/COX-2 (indomethacin, 50μM), p38 (SB203580, 10μM), or NF-κB (BAY 11–7821, 10μM) were used. The same amount of DMSO was used as control. All above inhibitors were purchased from MedChemExpress.
Quantitative real-time PCR, ELISA and IHC staining
Total RNA was extracted from bmEos using the RNeasy Mini Kit (QIAGEN) according to the manufacturer’s instruction. The cDNA synthesis kit (BIO-RAD, cat#1725035) and SyGreen Blue Mix (Genesee Scientific) were used to perform reverse transcription and real-time quantitative PCR. Results were normalized to 18s mRNA expression. PCR primers used are listed below:
IL-4: GGTCTCAACCCCCAGCTAGT (F), GCCGATGATCTCTCTCAAGTGAT (R);
IL-13: CCTGGCTCTTGCTTGCCTT (F), GGTCTTGTGTGATGTTGCTCA (R);
Ptgs2 (COX-2): TTCAACACACTCTATCACTGGC (F), AGAAGCGTTTGCGGTACTCAT (R);
18S: ACGGAAGGGCACCACCAGGA (F), CACCACCACCCACGGAATCG (R).
ELISA kits for the measurement of IL-4 and IFN-γ were purchased from BioLegend. ELISA kits for the measurement of IL-13 were purchased from R&D Systems. All measurements were performed according to the manufacturers’ instruction.
IHC staining was performed to detect eosinophils as previously described (18). Rat anti-mouse MBP antibody (MT2 14.7.3) was obtained from Mayo Clinic Arizona.
Western-blotting
The lysates of bmEos were electrophoresed on SDS-PAGE and transferred to a nitrocellulose membrane (Bio-Rad). COX-2, phospho-NF-κB p65 (Ser536), and NF-κB p65 were detected by using anti-COX-2 (cat#12282), anti-phospho-p65 (cat#3033), and anti-p65 (cat#8242) antibodies followed by anti-rabbit IgG-HRP antibody (cat#7074) as secondary antibody. All the above antibodies were purchased from Cell Signaling Technology. Anti-β-actin-HRP antibody was used as loading control (Sigma-Aldrich, cat#A3854).
Reverse Phase Protein Array (RPPA) analyses
BmEos were treated with PBS or IL-33 (20ng/ml) for 5min or 6h. Protein lysates were prepared and RPPA analyses were performed by the Antibody-based Proteomics Core Facility at The University of Texas MD Anderson Cancer Center.
Statistical analysis
All statistical analyses were conducted with GraphPad Prism version 8.0 (GraphPad Software Inc.). Results were presented as means ± SEM. To compare values obtained from three or more groups with one independent variable, a one-way analysis of variance (ANOVA) was used followed by Tukey’s test. To compare groups with two independent variables, a two-way ANOVA was used followed by Tukey’s test. Otherwise, a two-tailed unpaired Student’s t test was performed. The data were considered statistically significant when differences in values reached p < 0.05.
Results
Eosinophils protect against AILI by suppressing IFN-γ production through the release of IL-4/IL-13.
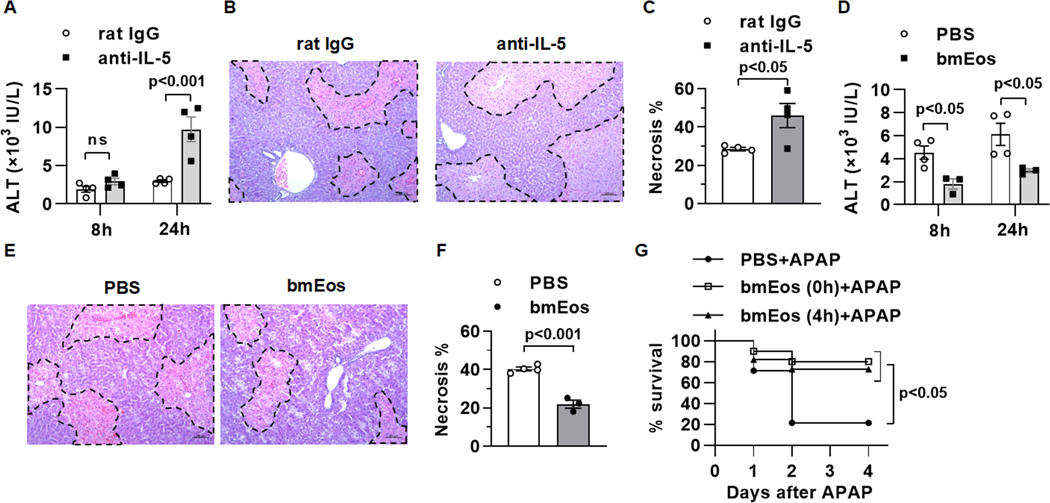
Our previous study using eosinophil-deficient ΔdblGATA1 mice showed that eosinophils play a protective role in AILI (Reference 20). As an alternative approach to genetic deletion of eosinophils, we treated WT mice with an anti-IL-5 antibody prior to APAP challenge (Supplemental Fig.1). Compared to IgG-treated controls, mice treated with anti-IL-5 developed much more severe liver injury, evident by an increased elevation of serum alanine aminotransferase (ALT) and more expansive areas of necrosis at 24h post-APAP (Fig. 1A–C). We previously showed that adoptive transfer of bone marrow-derived eosinophils (bmEos) to ΔdblGATA1 mice could normalize AILI toward the degrees observed in WT mice (Reference 20). An important next step toward probing the therapeutic potential of these cells is to investigate whether adoptive transfer of bmEos to WT mice can confer protection. The data showed that bmEos could markedly reduce ALT levels and liver necrosis in WT mice (Fig. 1D–F). More importantly, when we treated WT mice with a higher dose of APAP (600mg/kg) that caused around 80% mortality, we found that adoptive transfer of bmEos, either immediately or 4h after APAP treatment, could significantly reduce mortality rate (Fig. 1G).
Fig. 1. Eosinophils infiltrate the liver and exert a profound protection against AILI.
(A to C) WT mice were i.p. injected with anti-IL-5 antibody (200μg/mouse) to deplete eosinophils or rat IgG as control at 18h prior to APAP treatment (n=3–4/group). (A) Serum levels of ALT were measured at 8 and 24h after APAP treatment. (B and C) Liver necrosis (scale bars, 100μm) was evaluated and quantified at 24h after APAP treatment. (D to F) WT mice were adoptively transferred with PBS or WT bmEos immediately prior to APAP treatment (n=3–4/group). (D) Serum levels of ALT were measured at 8 and 24h after APAP treatment. (E and F) Liver necrosis (scale bars, 100μm) was examined and quantified at 24h after APAP treatment. (G) WT mice were treated with a lethal dose of APAP (600mg/kg). At 0h and 4h after APAP treatment, mice were i.v. injected with PBS or bmEos (7.5×106, n=10–14/group). The survival rates were recorded within 4 days after APAP treatment. Two-way ANOVA with Tukey post hoc test was performed in A and D. Two-tailed unpaired Student’s t test was performed in C and F. Long-rank (mantel-COX) test was performed in G.
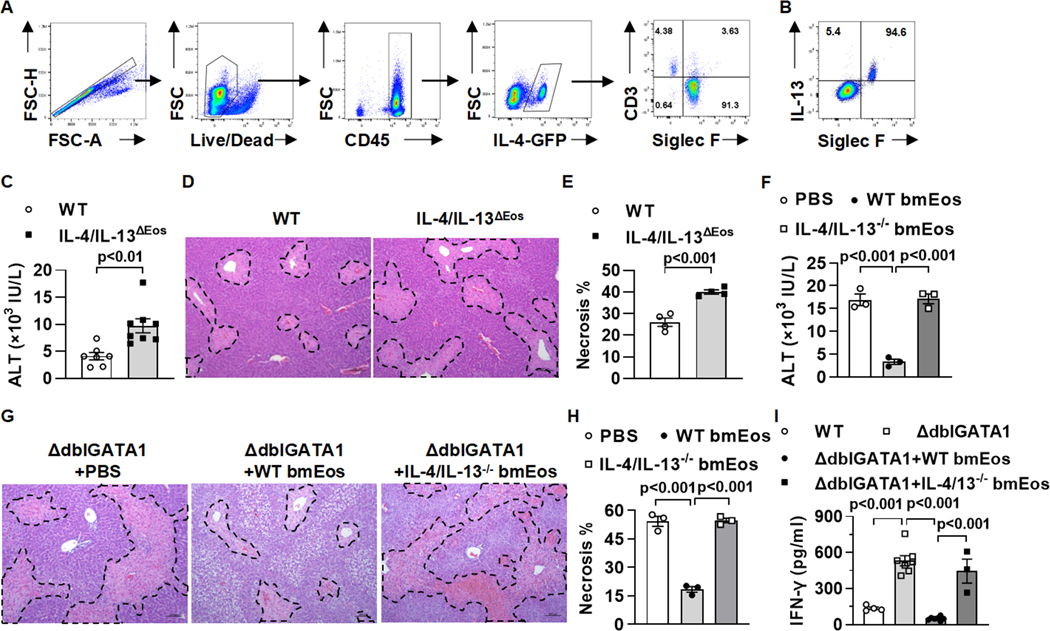
It is known that eosinophils represent a major cellular source of IL-4 and IL-13 (18, 23). To investigate if hepatic infiltrating eosinophils express IL-4 and/or IL-13 during AILI, we used IL-4/GFP-enhanced transcript (4Get) reporter mice to detect IL-4 and performed intracellular staining to detect IL-13 expression. The data showed that eosinophils accounted for more than 90% of IL-4-expressing cells and more than 90% of IL-13-expressing cells in the liver after APAP treatment (Fig. 2A and B). It has been reported that either IL-4- or IL-13-deficient mice develop exacerbated AILI compared with WT mice (24, 25), suggesting a protective role of IL-4 and IL-13. To confirm this finding, we treated mice with the combination of anti-IL-4 and anti-IL-13 neutralizing antibodies prior to APAP challenge and found a significant increase of serum ALT levels and liver necrosis compared to IgG-treated control mice (supplemental Fig. 2A–C).
Fig. 2. Eosinophil-derived IL-4 and IL-13 protect against AILI by inhibiting IFN-γ production.
(A and B) Liver mononuclear cells were isolated from 4Get reporter mice at 24h after APAP treatment (n=3/group). (A) IL-4-GFP–positive cells were gated, and shown are the proportions of eosinophils (Siglec F+) and T cells (CD3+) among all IL-4+ cells. (B) Intracellular staining for IL-13 was performed. Shown is the proportion of eosinophils (Siglec F+) that express IL-13 among total IL-13+ cells. (C to E) IL-4/IL-13ΔEOS mice and WT littermates were treated with APAP. Serum levels of ALT (C, n=6–8/group) and liver necrosis (D and E, n=4/group, scale bars, 100μm) were examined at 24h after APAP treatment. (F to H) ΔdblGATA1 mice were adoptively transferred with WT bmEos, IL-4/IL-13−/− bmEos or PBS as control prior to APAP treatment (n=3/group). Serum concentrations of ALT (F) and liver necrosis (G and H, scale bars, 100μm) were examined at 24h after APAP treatment. (I) WT, ΔdblGATA1, and ΔdblGATA1 mice adoptively transferred of WT bmEos or IL-4/IL-13−/− bmEos were challenged with APAP (n=4, 7, 7, and 3 respectively). Serum concentrations of IFN-γ were measured at 24h after APAP treatment by ELISA. One-way ANOVA with Tukey post hoc test was performed in F, H and I. Two-tailed unpaired Student’s t test was performed in C and E.
These findings led to our hypothesis that eosinophils protect against AILI through the release of IL-4 and IL-13. To examine this hypothesis, we used a mouse with eosinophil-specific deletion of IL-4 and IL-13 (IL-4/IL-13ΔEOS). Compared with their WT littermates, the IL-4/IL-13ΔEOS mice developed worsened liver injury after APAP treatment (Fig. 2C–E). Moreover, unlike WT bmEos, adoptive transfer of bmEos from IL-4/IL-13ΔEOS mice failed to reduce ALT levels nor liver necrosis in ΔdblGATA1 mice (Fig. 2F–H). These data strongly suggested that IL-4/IL-13 played a critical role in mediating the hepato-protective function of eosinophils during AILI.
IL-4 and IL-13 are Th2 cytokines, which could inhibit IFN-γ production and function. Given the pathological involvement of IFN-γ in AILI (5, 6), we hypothesized that eosinophil-derived IL-4/IL-13 played a protective role by inhibiting IFN-γ production. Indeed, serum levels of IFN-γ were much higher in ΔdblGATA1 mice than WT mice treated with APAP (Fig. 2I). Furthermore, adoptive transfer of WT bmEos, but not IL-4/IL-13ΔEOS bmEos, to ΔdblGATA1 mice reduced the level of IFN-γ to that of WT mice (Fig. 2I), indicating that eosinophil-derived IL-4/IL-13 suppressed IFN-γ production during AILI.
Cyclooxygenases play a critical role in IL-4/IL-13 production by eosinophils
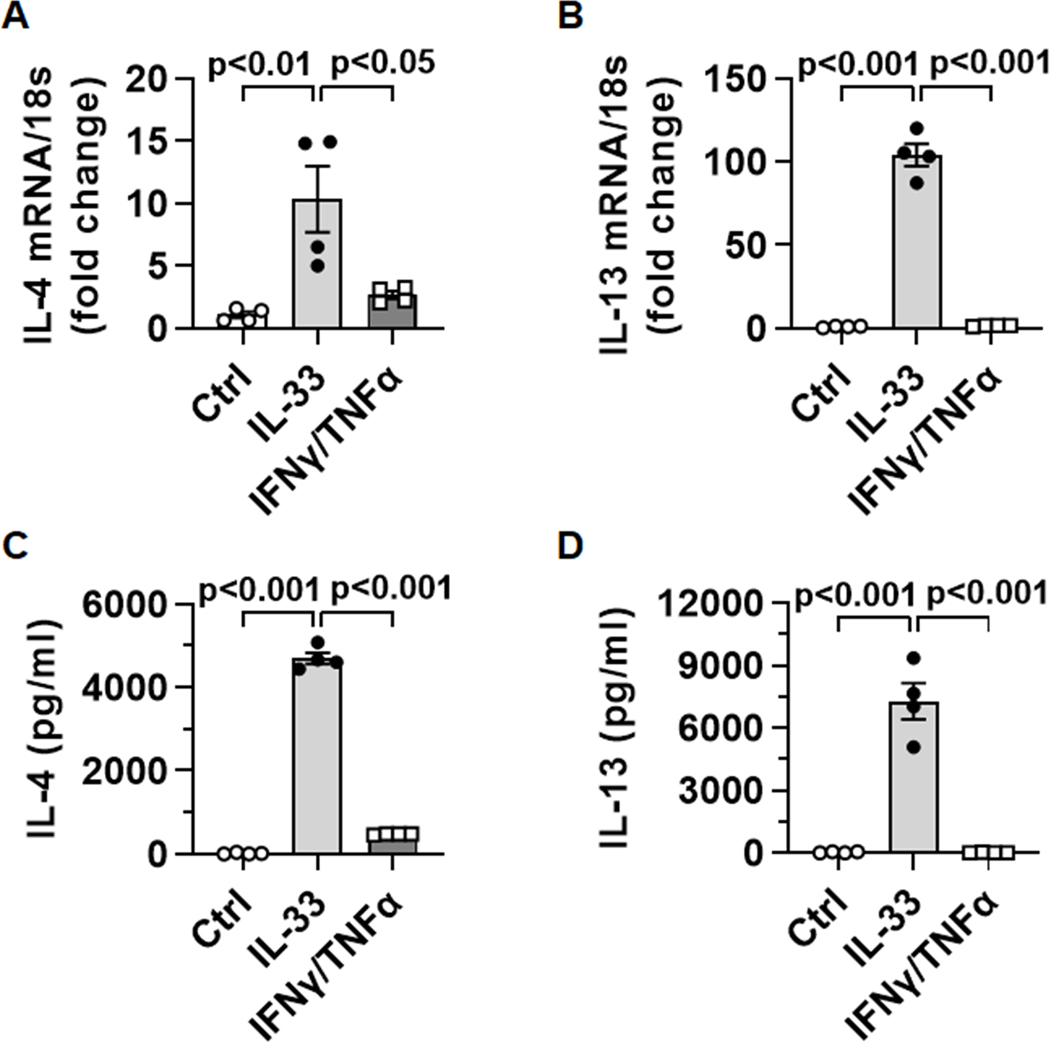
APAP-induced liver injury results in the release of a number of cytokines, some of which are known to activate eosinophils (e.g. IL-33, IFN-γ and TNF-α) (26, 27). We treated bmEos in vitro with IL-33 or the combination of IFN-γ and TNF-α. Although IFN-γ/TNF-α could induce a slight increase in IL-4 expression, IL-33-stimulated eosinophils produced much higher levels of both IL-4 and IL-13 (Fig. 3A–D).
Fig. 3. IL-4 and IL-13 expression in eosinophils in response to IL-33 or IFN-γ/TNF-α.
WT bmEos (n=4) were treated with nothing (Ctrl), IL-33 (20ng/ml), or the combination of IFN-γ and TNF-α (15 ng/ml each). (A and B) After 4h, mRNA levels of IL-4 and IL-13 were measured by qPCR. (C and D) The protein levels of IL-4 and IL-13 in culture supernatants were measured by ELISA after 24h. One-way ANOVA with Tukey post hoc test was performed in A to D.
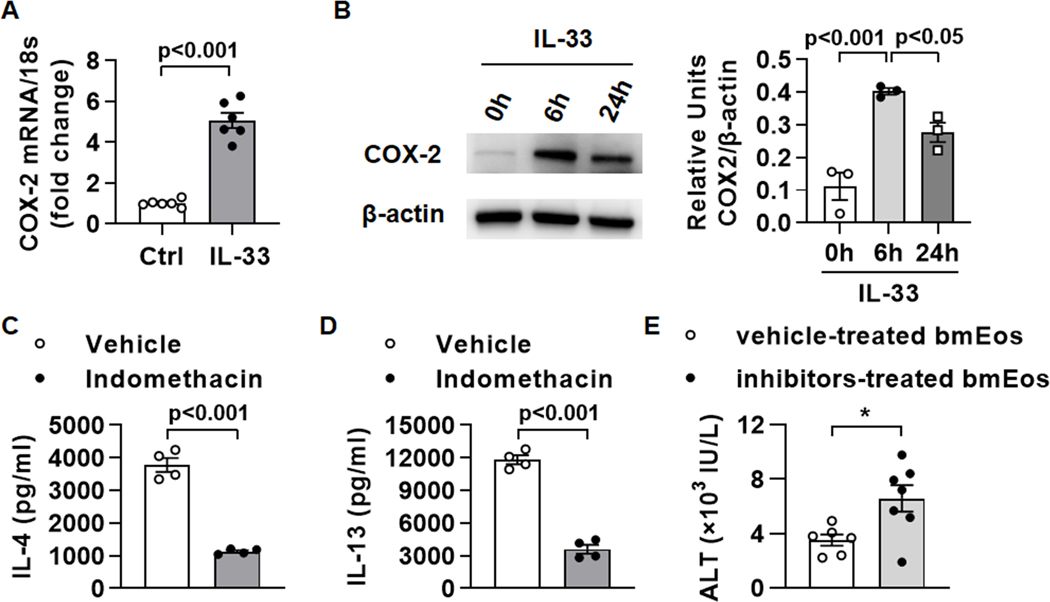
To explore the molecular signaling downstream of IL-33, we treated bmEos with IL-33 for 6h and then performed reverse phase protein array (RPPA) analyses. The data showed that cyclooxygenase (COX)-2 was the most significantly increased after IL-33 stimulation (supplemental Table. 1). This result was confirmed by qPCR and Western blot analyses of COX-2 in IL-33-stimulated bmEos (Fig. 4A and B). Next, we treated bmEos with IL-33 in presence of indomethacin and found that both IL-4 and IL-13 levels were decreased significantly (Fig. 4C and D). Since indomethacin inhibits both COX-1 and COX-2, we next used COX-1- or COX-2-specific inhibitors. Interestingly, inhibition of neither COX-1 (by sc-560) nor COX-2 (by celecoxib) alone could significantly reduce the levels of IL-4 (supplemental Fig. S3). However, the combination of sc-560 and celecoxib could dramatically inhibit IL-4 production. The results were similar for IL-13 (data not shown). These data indicate that both COX enzymes played a role in mediating the activation of eosinophils by IL-33. Moreover, we treated WT bmEos with sc-560 and celecoxib in vitro for 2h before adoptively transferring the cells to eosinophil-deficient ΔdblGATA1 mice. The data showed that COX-inhibited bmEos were not as efficient as vehicle-treated bmEos in attenuating liver injury in ΔdblGATA1 mice (Fig. 4E), suggesting that the COX-1/2 enzymes are important in the protective effect of eosinophils.
Fig. 4. Cyclooxygenases are involved in IL-4/IL-13 production by IL-33-activated eosinophils.
(A) WT bmEos (5×106 cells/ml) were stimulated with IL-33 (20ng/ml) or nothing (Ctrl). After 2h, mRNA levels of COX-2 were measured by qPCR. (B) WT bmEos were treated with IL-33 (20ng/ml) for 0h, 6h, and 24h respectively, and then protein expression levels of COX-2 were measured by Western blotting. (C and D) WT bmEos (5×106 cells/ml) was treated with IL-33 (20ng/ml) in the presence or absence of indomethacin (50μM) for 24h. Protein levels of IL-4 (C) and IL-13 (D) in the supernatants were measured by ELISA. (E) WT bmEos were treated with sc-560 plus celecoxib (50μM each, COX inhibitors) or vehicle (10μl DMSO) in vitro for 2h, then the cells were washed 3 times and adoptively transferred to ΔdblGATA1 mice followed by APAP injection immediately. Serum levels of ALT were measured at 24h after APAP administration. Two-tailed unpaired Student’s t test was performed in A, and C to E. One-way ANOVA with Tukey post hoc test was performed in B.
The p38-COX-NF-κB signaling pathway is crucial for IL-4/IL-13 production by eosinophils
It has been reported that prostaglandin products of COX-1/2 can activate NF-κB (28, 29). We wondered if COX-1/2 play an important role in IL-33-induced NF-κB activation in eosinophils. We treated bmEos with IL-33 in the presence or absence of sc-560/celecoxib. The data showed that IL-33-induced p65 phosphorylation was abrogated by the combination of COX-1 and COX-2 inhibitors (Fig. 5A), suggesting that COX-1/2 mediate IL-33-induced NF-κB activation.
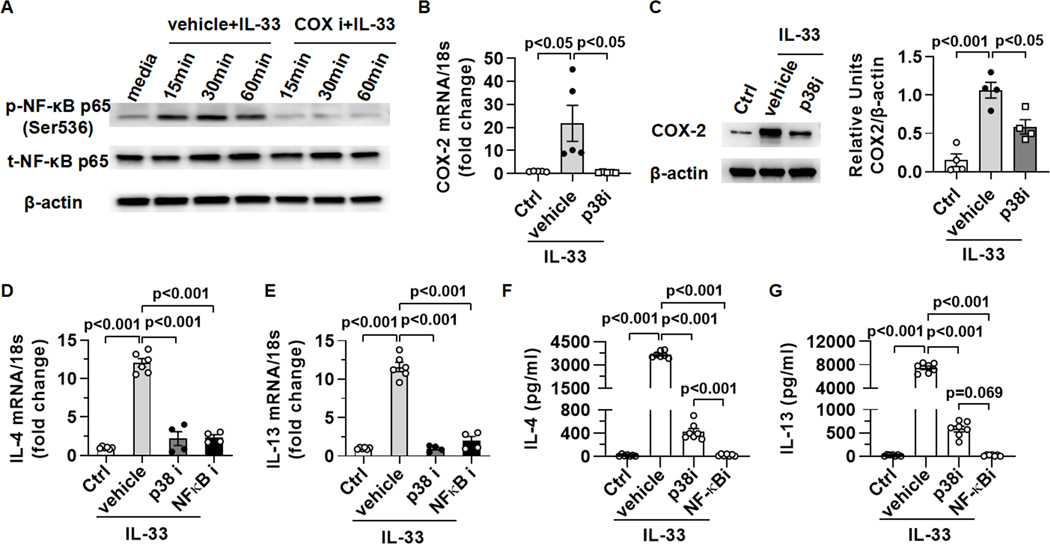
Fig. 5. The signal pathway of p38-COX-NF-κB is critical for IL-4/IL-13 production by eosinophils in response to IL-33.
(A) WT bmEos were treated with IL-33 (20ng/ml) in presence of sc-560 and celecoxib (50μM each, COXi ) or vehicle (10μl DMSO) in vitro for various times. Expression levels of phosphorylated-p65 and total-p65 were measured by Western blotting. (B and C) WT bmEos were treated with vehicle (1μl DMSO) or a p38 inhibitor (SB203580, 10μM) for 1h before stimulation by IL-33 (20ng/ml). WT bmEos without any treatment were regarded as control (Ctrl). (B) After 2h of IL-33 treatment, mRNA levels of COX-2 were measured by qPCR. (C) After 6h of IL-33 treatment, expression levels of COX-2 were measured by Western blotting. (D to G) WT bmEos were treated with vehicle (1μl DMSO), a p38 inhibitor (SB203580, 10μM), or a NF-κB inhibitor (BAY11–7821, 10μM) for 1h, then the cells were washed and stimulated with IL-33 (20ng/ml). WT bmEos without any treatment were regarded as control (Ctrl). (D and E) After 4h of IL-33 treatment, mRNA levels of IL-4 and IL-13 were measured by qPCR. (F and G) After 24h of IL-33 treatment, the concentrations of IL-4 and IL-13 in supernatants were measured by ELISA. One-way ANOVA with Tukey post hoc test was performed in B to G.
To identify upstream signals leading to COX-1/2 activation, we treated bmEos with IL-33 for 5min and performed a RPPA screen. The data showed that phospho-p38 mitogen-activated protein kinase (MAPK) was the most significantly increased by IL-33 treatment (supplemental Table. 2). Interestingly, treating bmEos with a p38MAPK inhibitor (SB203580) abrogated IL-33-induced upregulation of COX-2 mRNA and protein levels (Fig. 5B and C), placing p38 activation upstream of COX-2 upregulation by IL-33.
To further confirm the roles of p38 and NF-κB activation in IL-33-induced IL-4/IL-13 production, we treated bmEos with p38 or NF-κB inhibitor for 1h prior to IL-33 stimulation. The data showed that IL-4 and IL-13 mRNA and protein levels were reduced significantly when p38 or NF-κB was inhibited (Fig. 5D–G). Interestingly, NF-κB inhibition, but not p38 inhibition, nearly completely abolished IL-4/IL-13 production. Since p38 inhibition does not affect COX-1, it is likely that COX-1-derived prostaglandin products could activate NF-κB and induce IL-4/IL-13 production, even when COX-2 was inhibited by the p38 inhibitor (Fig. 5F–G). Together, these data strongly support that in IL-33-stimulated eosinophils, the p38MAPK/COX/NF-κB signaling axis plays a critical role in inducing IL-4/IL-13 production.
Discussion
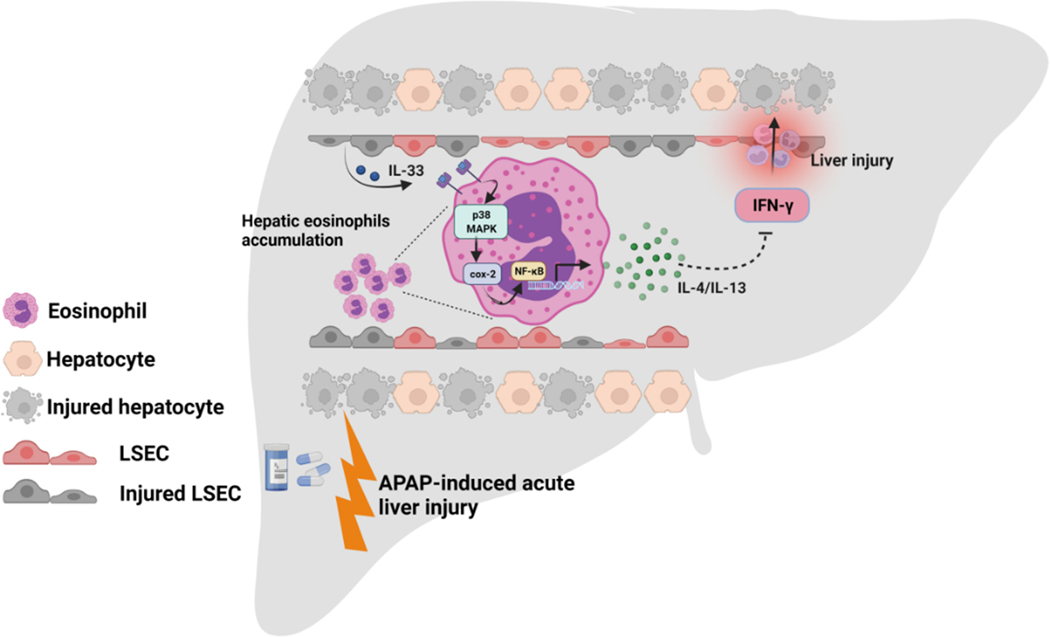
The current study elucidated the underlying mechanism of eosinophil-mediated hepato-protection against AILI. We provide evidence supporting that the protection is mediated by IL-4/IL-13 production through the p38 MAPK/COX/NF-κB signaling pathway (Fig. 6).
Fig. 6. Schematic summary of the main findings.
Eosinophil-derived IL-4/IL-13, through inhibition of IFN-γ, protect against APAP-induced liver injury. Eosinophils produce IL-4/IL-13 in response to IL-33 stimulation. IL-33 activated p38MAPK, resulting in cyclooxygenase activity, which triggers NF-κB-mediated IL-4/IL-13 production.
Accumulating evidence supports that eosinophils are recruited to injured/inflamed tissues and that they function to resolve inflammation and protect against tissue damage (11–13, 30, 31). For example, in staphylococcus aureus-induced acute lung injury, IL-33-induced eosinophilia plays a critical role in inhibiting neutrophilia and pulmonary edema, thereby promoting survival (32). In a mouse model of acute colitis, eosinophils exert a protective effect by attenuating neutrophil infiltration and reducing the levels of inflammatory cytokines through the production of anti-inflammatory lipid mediators (33). Eosinophil-derived anti-inflammatory lipid mediators are important in resolving inflammation (30). For example in zymosan-induced acute peritonitis, infiltrating eosinophils promote the resolution of inflammation by releasing protectin D1 (34). Our finding of a hepato-protective function of eosinophils in AILI is consistent with the clinical observation that hepatic eosinophil accumulation correlates with a better prognosis in patients with drug-induced liver injury (15, 35).
It has been reported that the serum levels of IL-4 and IL-13 are elevated after APAP administration (24, 25). Both cytokines play a protective role against AILI, as IL-4- or IL-13-deficient mice are more susceptible to AILI (24, 25). However, the cellular source of IL-4/IL-13 was not investigated in previous studies. Although multiple cell types, including CD4+ T cells, NKT cells, mast cells, eosinophils, and basophils, are capable of producing IL-4/IL-13 (36), our current study using 4Get mice and IL-13 intracellular staining unequivocally identified hepatic eosinophils as the main source of IL-4/IL-13 during AILI. Furthermore, the experiments using eosinophil-specific IL-4- and IL-13-deleted mice demonstrated that IL-4/IL-13 produced by eosinophils protected against AILI.
Eosinophils produce IL-4/IL-13 in response to IL-33 (18, 37), which is elevated during AILI (24, 25). It is known that IL-33 binds to its receptor, suppression of tumorigenicity 2 (ST2), and activates MAP kinases, including ERK, JNK and p38 MAPK (38, 39). It has also been shown that IL-33 activates MyD88/IRAK/TRAF6, culminating in NF-κB activation (38, 39). In addition, IL-33 can activate JAK2 which consequently induces NF-κB activation in murine embryonal fibroblasts (40). Experiments using inhibitors that block ERK, JNK, or JAK/STAT did not show any effect on IL-4 or IL-13 production (data not shown). In contrast, inhibition of p38 MAPK dramatically reduced IL-4/IL-13 production by bmEos. Further mechanistic study revealed that p38 MAPK was important in upregulating COX2, which in turn triggered NF-κB activation in eosinophils. Although this is the first time IL-33-induced COX-2 upregulation is observed in eosinophils, a similar finding has been reported in colorectal cancer cells (41). Interestingly, with regard to AILI, both COX-1−/− and COX-2−/− mice have been shown to develop worsened liver injury (42). These and the finding that pharmacological inhibition of COX-2 exacerbates AILI (42) suggest a protective role of COX-1 and COX-2 in AILI. The underlying mechanism of this protection is now unveiled by our data supporting a link between COX-1/2 and IL-4/IL-13 production by eosinophils.
In summary, the present study uncovers that hepatic infiltrating eosinophils protect against AILI through the release of IL-4/IL-13, in a COX-dependent manner. The findings raise a concern that nonsteroidal anti-inflammatory drugs, which are widely used to reduce pain and inflammation, may increase the risk of AILI by inhibiting COX-1/2 and reducing IL-4/IL-13 production by eosinophils. Our data also suggest that promoting hepatic eosinophil accumulation and/or adoptive transfer of eosinophils may be explored as strategies to treat AILI and conditions of acute liver injury.
Supplementary Material
WT mice were i.p. injected with anti-IL-5 antibody (200μg per mouse) to deplete eosinophils or the same amount of rat IgG as control at 18h prior to APAP treatment (n=3–4/group). The percentages and absolute numbers of eosinophils in liver were measured at 24h after APAP treatment by flow cytometry. One-way ANOVA was performed.
WT mice were treated with the combination of anti-IL-4 (300μg per mouse) and anti-IL-13 (200μg per mouse) neutralizing antibodies or the same amounts of rat IgG as control prior to APAP administration (n=3/group). (A) Serum level of ALT were measured at 8 and 24h after APAP treatment. (B, C) Liver necrosis (scale bars, 100μm) was evaluated and quantified at 24h after APAP treatment. Two-way ANOVA was performed in A. Two-tailed unpaired Student’s t test was performed in C.
WT bmEos was treated with IL-33 (20ng/ml) in the presence or absence of sc-560 (50μM), celecoxib (50μM), or sc-560 plus celecoxib (50μM each) for 24h. The concentrations of IL-4 ( n=3/group) in supernatant were measured by ELISA. One-way ANOVA was performed.
WT bmEos were treated with PBS or IL-33 (20ng/ml) for 6h. The cell lysates were prepared and used for RPPA screen. Proteins with more than 1.5-fold upregulation are shown.
WT bmEos were treated with PBS or IL-33 (20ng/ml) for 5min. The cell lysates were prepared and used for RPPA screen. Proteins with more than 1.5-fold upregulation are shown.
Financial support:
This work was supported by the NIH DK121330, DK122708, DK122796, DK109574 and the University of Texas System Translational STARs award to C.J.; the NSFC 81873570 and Talent Training Program 2022YPJH101 (School of Basic Medical Sciences, Anhui Medical University) to L.X.; the AST Research Network/CSL Behring Fellowship Basic Science Research grant to Y.Y..
Data Availability Statement:
The data that support the findings of this study are available on request from the corresponding author.
Footnotes
Competing interests: The authors declare that they have no competing interests.
References
- 1.Bunchorntavakul C, Reddy KR. Acetaminophen (APAP or N-Acetyl-p-Aminophenol) and Acute Liver Failure. Clin Liver Dis 2018;22:325–346. [DOI] [PubMed] [Google Scholar]
- 2.Bertheloot D, Latz E. HMGB1, IL-1alpha, IL-33 and S100 proteins: dual-function alarmins. Cell Mol Immunol 2017;14:43–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blazka ME, Wilmer JL, Holladay SD, Wilson RE, Luster MI. Role of proinflammatory cytokines in acetaminophen hepatotoxicity. Toxicol Appl Pharmacol 1995;133:43–52. [DOI] [PubMed] [Google Scholar]
- 4.Blazka ME, Elwell MR, Holladay SD, Wilson RE, Luster MI. Histopathology of acetaminophen-induced liver changes: role of interleukin 1 alpha and tumor necrosis factor alpha. Toxicol Pathol 1996;24:181–189. [DOI] [PubMed] [Google Scholar]
- 5.Ishida Y, Kondo T, Ohshima T, Fujiwara H, Iwakura Y, Mukaida N. A pivotal involvement of IFN-gamma in the pathogenesis of acetaminophen-induced acute liver injury. FASEB J 2002;16:1227–1236. [DOI] [PubMed] [Google Scholar]
- 6.Liu ZX, Govindarajan S, Kaplowitz N. Innate immune system plays a critical role in determining the progression and severity of acetaminophen hepatotoxicity. Gastroenterology 2004;127:1760–1774. [DOI] [PubMed] [Google Scholar]
- 7.Schett G, Neurath MF. Resolution of chronic inflammatory disease: universal and tissue-specific concepts. Nat Commun 2018;9:3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buckley CD, Gilroy DW, Serhan CN, Stockinger B, Tak PP. The resolution of inflammation. Nat Rev Immunol 2013;13:59–66. [DOI] [PubMed] [Google Scholar]
- 9.Klion AD, Nutman TB. The role of eosinophils in host defense against helminth parasites. J Allergy Clin Immunol 2004;113:30–37. [DOI] [PubMed] [Google Scholar]
- 10.Gleich GJ. Mechanisms of eosinophil-associated inflammation. J Allergy Clin Immunol 2000;105:651–663. [DOI] [PubMed] [Google Scholar]
- 11.Sezin T, Ferreiros N, Jennrich M, Ochirbold K, Seutter M, Attah C, Mousavi S, et al. 12/15-Lipoxygenase choreographs the resolution of IgG-mediated skin inflammation. J Autoimmun 2020;115:102528. [DOI] [PubMed] [Google Scholar]
- 12.Takeda K, Shiraishi Y, Ashino S, Han J, Jia Y, Wang M, Lee NA, et al. Eosinophils contribute to the resolution of lung-allergic responses following repeated allergen challenge. J Allergy Clin Immunol 2015;135:451–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Humbles AA, Lloyd CM, McMillan SJ, Friend DS, Xanthou G, McKenna EE, Ghiran S, et al. A critical role for eosinophils in allergic airways remodeling. Science 2004;305:1776–1779. [DOI] [PubMed] [Google Scholar]
- 14.Yu YR, O’Koren EG, Hotten DF, Kan MJ, Kopin D, Nelson ER, Que L, et al. A Protocol for the Comprehensive Flow Cytometric Analysis of Immune Cells in Normal and Inflamed Murine Non-Lymphoid Tissues. PLoS One 2016;11:e0150606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bjornsson E, Kalaitzakis E, Olsson R. The impact of eosinophilia and hepatic necrosis on prognosis in patients with drug-induced liver injury. Aliment Pharmacol Ther 2007;25:1411–1421. [DOI] [PubMed] [Google Scholar]
- 16.Tarantino G, Cabibi D, Camma C, Alessi N, Donatelli M, Petta S, Craxi A, et al. Liver eosinophilic infiltrate is a significant finding in patients with chronic hepatitis C. J Viral Hepat 2008;15:523–530. [DOI] [PubMed] [Google Scholar]
- 17.Proctor WR, Chakraborty M, Chea LS, Morrison JC, Berkson JD, Semple K, Bourdi M, et al. Eosinophils mediate the pathogenesis of halothane-induced liver injury in mice. Hepatology 2013;57:2026–2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Y, Yang Y, Wang M, Wang S, Jeong JM, Xu L, Wen Y, et al. Eosinophils attenuate hepatic ischemia-reperfusion injury in mice through ST2-dependent IL-13 production. Sci Transl Med 2021;13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pham BN, Bemuau J, Durand F, Sauvanet A, Degott C, Prin L, Janin A. Eotaxin expression and eosinophil infiltrate in the liver of patients with drug-induced liver disease. J Hepatol 2001;34:537–547. [DOI] [PubMed] [Google Scholar]
- 20.Xu L, Yang Y, Wen Y, Jeong JM, Emontzpohl C, Atkins CL, Sun Z, et al. Hepatic recruitment of eosinophils and their protective function during acute liver injury. J Hepatol 2022.DOI: 10.1010/j.jhep.2022.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Doyle AD, Mukherjee M, LeSuer WE, Bittner TB, Pasha SM, Frere JJ, Neely JL, et al. Eosinophil-derived IL-13 promotes emphysema. Eur Respir J 2019;53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gao RY, Wang M, Liu Q, Feng D, Wen Y, Xia Y, Colgan SP, et al. Hypoxia-Inducible Factor-2alpha Reprograms Liver Macrophages to Protect Against Acute Liver Injury Through the Production of Interleukin-6. Hepatology 2020;71:2105–2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dyer KD, Moser JM, Czapiga M, Siegel SJ, Percopo CM, Rosenberg HF. Functionally competent eosinophils differentiated ex vivo in high purity from normal mouse bone marrow. J Immunol 2008;181:4004–4009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yee SB, Bourdi M, Masson MJ, Pohl LR. Hepatoprotective role of endogenous interleukin-13 in a murine model of acetaminophen-induced liver disease. Chem Res Toxicol 2007;20:734–744. [DOI] [PubMed] [Google Scholar]
- 25.Ryan PM, Bourdi M, Korrapati MC, Proctor WR, Vasquez RA, Yee SB, Quinn TD, et al. Endogenous interleukin-4 regulates glutathione synthesis following acetaminophen-induced liver injury in mice. Chem Res Toxicol 2012;25:83–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Antunes MM, Araujo AM, Diniz AB, Pereira RVS, Alvarenga DM, David BA, Rocha RM, et al. IL-33 signalling in liver immune cells enhances drug-induced liver injury and inflammation. Inflamm Res 2018;67:77–88. [DOI] [PubMed] [Google Scholar]
- 27.Krenkel O, Mossanen JC, Tacke F. Immune mechanisms in acetaminophen-induced acute liver failure. Hepatobiliary Surg Nutr 2014;3:331–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang D, Fu L, Sun H, Guo L, DuBois RN. Prostaglandin E2 Promotes Colorectal Cancer Stem Cell Expansion and Metastasis in Mice. Gastroenterology 2015;149:1884–1895 e1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang X, Yan K, Deng L, Liang J, Liang H, Feng D, Ling B. Cyclooxygenase 2 Promotes Proliferation and Invasion in Ovarian Cancer Cells via the PGE2/NF-kappaB Pathway. Cell Transplant 2019;28:1S–13S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Isobe Y, Kato T, Arita M. Emerging roles of eosinophils and eosinophil-derived lipid mediators in the resolution of inflammation. Front Immunol 2012;3:270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee JJ, Jacobsen EA, McGarry MP, Schleimer RP, Lee NA. Eosinophils in health and disease: the LIAR hypothesis. Clin Exp Allergy 2010;40:563–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krishack PA, Hollinger MK, Kuzel TG, Decker TS, Louviere TJ, Hrusch CL, Sperling AI, et al. IL-33-mediated Eosinophilia Protects against Acute Lung Injury. Am J Respir Cell Mol Biol 2021;64:569–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Masterson JC, McNamee EN, Fillon SA, Hosford L, Harris R, Fernando SD, Jedlicka P, et al. Eosinophil-mediated signalling attenuates inflammatory responses in experimental colitis. Gut 2015;64:1236–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yamada T, Tani Y, Nakanishi H, Taguchi R, Arita M, Arai H. Eosinophils promote resolution of acute peritonitis by producing proresolving mediators in mice. FASEB J 2011;25:561–568. [DOI] [PubMed] [Google Scholar]
- 35.Kleiner DE, Chalasani NP, Lee WM, Fontana RJ, Bonkovsky HL, Watkins PB, Hayashi PH, et al. Hepatic histological findings in suspected drug-induced liver injury: systematic evaluation and clinical associations. Hepatology 2014;59:661–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Junttila IS. Tuning the Cytokine Responses: An Update on Interleukin (IL)-4 and IL-13 Receptor Complexes. Front Immunol 2018;9:888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Qiu Y, Nguyen KD, Odegaard JI, Cui X, Tian X, Locksley RM, Palmiter RD, et al. Eosinophils and type 2 cytokine signaling in macrophages orchestrate development of functional beige fat. Cell 2014;157:1292–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pinto SM, Subbannayya Y, Rex DAB, Raju R, Chatterjee O, Advani J, Radhakrishnan A, et al. A network map of IL-33 signaling pathway. J Cell Commun Signal 2018;12:615–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Funakoshi-Tago M, Tago K, Hayakawa M, Tominaga S, Ohshio T, Sonoda Y, Kasahara T. TRAF6 is a critical signal transducer in IL-33 signaling pathway. Cell Signal 2008;20:1679–1686. [DOI] [PubMed] [Google Scholar]
- 40.Funakoshi-Tago M, Tago K, Sato Y, Tominaga S, Kasahara T. JAK2 is an important signal transducer in IL-33-induced NF-kappaB activation. Cell Signal 2011;23:363–370. [DOI] [PubMed] [Google Scholar]
- 41.Li Y, Shi J, Qi S, Zhang J, Peng D, Chen Z, Wang G, et al. IL-33 facilitates proliferation of colorectal cancer dependent on COX2/PGE2. J Exp Clin Cancer Res 2018;37:196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reilly TP, Brady JN, Marchick MR, Bourdi M, George JW, Radonovich MF, Pise-Masison CA, et al. A protective role for cyclooxygenase-2 in drug-induced liver injury in mice. Chem Res Toxicol 2001;14:1620–1628. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
WT mice were i.p. injected with anti-IL-5 antibody (200μg per mouse) to deplete eosinophils or the same amount of rat IgG as control at 18h prior to APAP treatment (n=3–4/group). The percentages and absolute numbers of eosinophils in liver were measured at 24h after APAP treatment by flow cytometry. One-way ANOVA was performed.
WT mice were treated with the combination of anti-IL-4 (300μg per mouse) and anti-IL-13 (200μg per mouse) neutralizing antibodies or the same amounts of rat IgG as control prior to APAP administration (n=3/group). (A) Serum level of ALT were measured at 8 and 24h after APAP treatment. (B, C) Liver necrosis (scale bars, 100μm) was evaluated and quantified at 24h after APAP treatment. Two-way ANOVA was performed in A. Two-tailed unpaired Student’s t test was performed in C.
WT bmEos was treated with IL-33 (20ng/ml) in the presence or absence of sc-560 (50μM), celecoxib (50μM), or sc-560 plus celecoxib (50μM each) for 24h. The concentrations of IL-4 ( n=3/group) in supernatant were measured by ELISA. One-way ANOVA was performed.
WT bmEos were treated with PBS or IL-33 (20ng/ml) for 6h. The cell lysates were prepared and used for RPPA screen. Proteins with more than 1.5-fold upregulation are shown.
WT bmEos were treated with PBS or IL-33 (20ng/ml) for 5min. The cell lysates were prepared and used for RPPA screen. Proteins with more than 1.5-fold upregulation are shown.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author.