Abstract
Background and Aims
Aquatic carnivorous plants have typical rootless linear shoots bearing traps and exhibit steep physiological polarity with rapid apical growth. The aim was to analyse auxin and cytokinin metabolites in traps, leaves/shoots and shoot apices in several species of genera Aldrovanda and Utricularia to elucidate how the hormonal profiles reflect the specific organ functions and polarity.
Methods
The main auxin and cytokinin metabolites were analysed in miniature samples (>2 mg dry weight) of different organs of Aldrovanda vesiculosa and six Utricularia species using ultraperformance liquid chromatography coupled with triple quadrupole mass spectrometry.
Key Results
Total contents of biologically active forms (free bases, ribosides) of all four main endogenously occurring cytokinin types were consistently higher in traps than in leaves in four Utricularia species with monomorphic shoots and/or higher than in shoots in two Utricularia species with dimorphic shoots. In Aldrovanda traps, the total content of different cytokinin forms was similar to or lower than that in shoots. In U. australis leaves, feeding on prey increased all cytokinin forms, while no consistent differences occurred in Aldrovanda. In four aquatic Utricularia species with monomorphic shoots, the content of four auxin forms was usually higher in traps than in leaves. Zero IAA content was determined in U. australis leaves from a meso-eutrophic site or when prey-fed.
Conclusions
Different cytokinin and auxin profiles estimated in traps and leaves/shoots of aquatic carnivorous plants indicate an association with different dominant functions of these organs: nutrient uptake by traps versus photosynthetic function of traps. Interplay of cytokinins and auxins regulates apical dominance in these plants possessing strong polarity.
Keywords: Phytohormone profiles, Utricularia spp, Lentibulariaceae, Aldrovanda vesiculosa, Droseraceae, aquatic rootless plants, traps, leaves, apices, physiological polarity
INTRODUCTION
In plants, phytohormones, signalling molecules of endogenous origin, control plant responses to various endogenous and environmental factors (including crosstalk with other groups of phytohormones). Among them, auxins and cytokinins (CKs) are the most influential key regulators, having multiple roles in plants, which include their growth and development. Their interplay, which exerts a tight control on plant morphogenesis and differentiation, has been extensively studied during root and shoot development in land-based plants (Hussain et al., 2021; Tarkowská et al., 2014). However, their roles in the morphogenesis and physiology of aquatic carnivorous plants (ACPs) are largely unexplored (Šimura et al., 2016).
The ACPs are a special ecological subgroup of carnivorous plants comprising ~60 species from the genus Utricularia (Lentibulariaceae, Lamiales), which have suction traps, and the monotypic species Aldrovanda vesiculosa (Droseraceae, Nepenthales), with snapping traps (Juniper et al., 1989; Taylor, 1989; Cross, 2012; Adamec, 2018a, 2018b; Miranda et al., 2021). The ACPs usually grow in shallow, standing, barren humic waters and many of them can also grow as amphibious plants (Guisande et al., 2007; Adamec, 2018a, 2020). They are strictly rootless and most species have a linear, modular shoot structure comprising nodes with filamentous leaves bearing traps and fine tubular internodes. The shoots are poorly branched and are a means of vegetative propagation. Most Utricularia species with linear shoots, and Aldrovanda, have monomorphic, non-differentiated green shoots bearing traps, while several Utricularia species have dimorphic shoots differentiated into pale shoots bearing the majority of the traps, and green photosynthetic ones (Taylor, 1989; Cross, 2012; Adamec, 2018a). Nevertheless, the morphological and developmental relationships between Utricularia stems, branches, leaves and traps are not clear as all these organs, with great morphological and functional plasticity, might be considered homologous (‘fuzzy’ morphology; for a review see Miranda et al., 2021). However, in this paper we shall use the terms ‘stems’, ‘branches’, ‘leaves’ and ‘traps’ for these organs with the usual meaning (e.g. Taylor, 1989). Traps of both genera have quite different structure and function (Poppinga et al., 2018) and different regulation of the secretion of digestive enzymes. Jasmonate regulation of enzyme secretion has been confirmed only in Aldrovanda (Jakšová et al., 2021).
Aquatic carnivorous plants with linear shoots exhibit a marked physiological shoot polarity: they usually possess very rapid apical shoot growth (one to four leaf nodes per day) and high relative growth rates, while their basal shoot segments senesce and decompose at the same high rate so that the length of adult plants is approximately constant (Friday, 1989; Adamec, 2000, 2018a, 2018b). They possess adaptations associated with very rapid growth, including mineral nutrient uptake from carnivory, efficient N and P re-utilization (resorption) from senescent shoots, a record high net photosynthetic rate, a high affinity for mineral nutrient uptake from the ambient water and, also, the formation of commensal associations in Utricularia traps (Adamec, 2000, 2013, 2014, 2018a; Richards, 2001; Sirová et al., 2009). So far, over a dozen of species of ACPs (mostly A. vesiculosa and U. australis) have been used in various experimental ecophysiological studies (Adamec, 2018a). In both species, Šimura et al. (2016) analysed the profiles of CKs and auxins in shoot segments of different age in relation to steep shoot growth polarity. Only isoprenoid CKs were detected in both species. In Aldrovanda, cis- and trans-zeatin types prevailed over the N6-isopentenyladenine types along shoots, but the opposite occurred for U. australis. In both species, the content of biologically active forms (free bases + ribosides) and their biosynthetic precursors as well as the proportion of active to total CK content decreased basipetally from the shoot apices. In contrast, the IAA (indole-3-acetic acid) content was relatively stable along the shoots. The active CK:IAA content ratio thus markedly decreased basipetally in both species. These results suggest that phenomena of distinct physiological polarity and polar growth in both species are associated with a polar gradient of CKs.
In the above-mentioned study (Šimura et al., 2016), leaves/shoots and traps were not separated prior to the phytohormone analysis. However, recent improvements in instrumentation and sample purification have provided technology to detect phytohormones in milligram quantities of plant tissue and have greatly facilitated the investigation of some questions in developmental plant biology (Svačinová et al., 2012). Therefore, in the present study we determined the levels of CKs and auxins in miniature samples of traps and trap-free leaves or shoots of the ACPs A. vesiculosa and four Utricularia species with linear monomorphic shoots and also in both carnivorous and photosynthetic shoots in two species with dimorphic shoots. We also compared CK and auxin levels in trap-free leaves/shoots and shoot apices between main shoots and branches and in leaves of intact and decapitated plants to judge the strength of the apical dominance. To detect how the phytohormone profiles depend on ecological conditions, we determined phytohormone levels in the leaves of U. australis plants grown naturally at an oligotrophic and a meso-eutrophic site with different CO2 concentrations. Similarly, we determined the phytohormone profiles in leaves or shoots of two species grown in a greenhouse growth experiment with or without prey to learn how prey capture can influence CK and auxin levels. We interpret the levels of CKs and auxins in terms of their possible roles in facilitating special organ functions and maintaining physiological polarity and apical dominance in ACPs.
MATERIALS AND METHODS
Experiment A: comparison of phytohormone profiles between leaves or shoots and traps in Utricularia species and Aldrovanda
Phytohormone profiles were estimated in traps and leaves of four Utricularia species with monomorphic shoots: in U. reflexa, U. inflata, U. vulgaris and U. australis; in traps, pale carnivorous and green photosynthetic shoots of U. intermedia and U. stygia with dimorphic shoots, and in traps and shoots of Aldrovanda vesiculosa. For details of plant origin, cultivation type, plant length and organ position of sampled organs, see Table 1. Except for U. reflexa, growing indoors in a 3-L aquarium, all these species were grown outdoors in 300- to 1800-L laminate containers. Litter of robust Carex species was used as substrate. Water in the containers simulated natural conditions and was considered neutral or slightly acidic, oligo-mesotrophic and humic (for details see Sirová et al., 2003; Adamec, 2014). All plants were able to capture small zooplankton. All plant organs were sampled in the peak of the growing season between 8 June and 8 August within both seasons.
Table 1.
Data on plant species, origin, cultivation type, shoot type, plant length and sampled organ position within Experiment A
| Species | Plant origin | Voucher | Cultivation | Shoot type | Plant length (cm) | Organ position | |
|---|---|---|---|---|---|---|---|
| Traps | Leaves or shoots | ||||||
| U. reflexa | Okavango, Botswana | BW 0 HBT 2017.04079 | Indoors, aquarium | 1 | 20–25 | 3–6 | 3–6, L |
| U. inflata | New Jersey, USA | US 0 HBT 2017.03538 | Outdoors, container | 1 | 45–65 | 3 | 3, L |
| U. vulgaris | Czechia | CZ 0 HBT 2017.03644 | Outdoors, container | 1 | 45–60 | 4–5 | 4–5, L |
| U. australis | Czechia | CZ 0 HBT 2017.04056 | Outdoors, container | 1 | 50–80 | 3–6 | 3–6, L |
| A. vesiculosa | E Poland | PL 0 HBT 2017.04079 |
Outdoors, container | 1 | 11–12 | 2–4 | 2–4, S |
| U. intermedia | Czechia | CZ 0 HBT 2017.04058 | Outdoors, container | 2 | 20–25 | Mature | 2–12, S |
| U. stygia | Czechia | CZ 0 HBT 2017.03767 | Outdoors, container | 2 | 25–30 | Mature | 2–12, S |
Shoot type: 1, monomorphic shoots; 2, dimorphic shoots. Sampled organ position shows the number of mature leaf nodes from the shoot apex where the organs were sampled: L, trap-free leaves; S, trap-free shoots. In dimorphic species, the number shows the sampled position in photosynthetic shoots.
In all species with monomorphic shoots, the position of sampled mature traps and leaves/shoots was selected in order to be identical or analogous to the ‘young’ shoot segments in the study by Šimura et al. (2016). As material of only 2 mg dry weight was sufficient for phytohormone analyses and steep polar gradients of phytohormones occur along the shoots, the sampled segments could be shortened as much as possible (Table 1). In the case of carnivorous shoots, both mature traps and the mature sections of shoots were sampled. Excised leaves or shoots with traps were usually pooled from two or three plant individuals and were thoroughly washed with tap water. Traps (23–95 pieces dependent on their size) and trap-free leaves or shoots were quickly separated from each other using a pair of tweezers and fine scissors, rinsed with distilled water, blotted dry using soft paper tissue, put in pre-weighed 2-mL plastic Eppendorf vials and placed in a freezer at ~−22 to −28 °C for several days or weeks before lyophilization. In all plant species, traps with visible prey items were discarded. After lyophilization, the Eppendorf vials were weighed again to estimate an approximate dry weight of the samples (it was 2–8 mg). Four parallel samples were prepared for each organ and species.
Experiment B: comparison of phytohormone profiles in plants from sites of different trophic level and/or growing with or without prey
We determined the levels of phytohormones in trap-free leaves (from the third to sixth mature leaf nodes; Table 1) in U. australis plants grown naturally at an oligotrophic and a meso-eutrophic site at different [CO2] and prey capture rates. The plants were collected from a softwater, oligotrophic shallow sand-pit near Cep, South Bohemia, Czechia (Adamec, 2009). The poorly branched plants were 25–45 cm long and their mature traps were <1.5 mm long, and only 10–20 % of them growing at the 12th node from the shoot apex contained visible prey items. The pH in the stand water was 5.79, electrical conductivity 2.79 mS m−1, total alkalinity 0.042 meq L−1, [CO2] 0.16 mm, NO3−-N below detection limit, NH4+-N 1.43 μm and PO4-P 0.12 μm. As a meso-eutrophic site, a small fishpond Namšal in the Lužnice village, South Bohemia, Czechia, was selected: the branched U. australis plants, 50–70 cm long, grew in a littoral zone in a loose common reed stand. Their mature traps were up to 3 mm long and ~70 % of them contained visible prey. The plants from both sites were transported to the laboratory in moist air and immediately processed. The pH in the stand water was 7.08, electrical conductivity 45.8 mS m−1, total alkalinity 2.00 meq L−1, [CO2] 0.38 mm, NO3−-N below detection limit, NH4+-N 2.96 μm and PO4-P 0.28 μm. For all water analyses, see Adamec (2009).
Similarly, we determined the phytohormone profiles in leaves or shoots of two species grown in a greenhouse growth experiment with or without prey to learn how prey capture can influence CK and auxin levels. Phytohormone profiles were determined in Aldrovanda and U. australis plants that had been pre-cultivated with or without prey in a growth experiment for 11 or 12 d, respectively (for several details see Adamec, 2016). Sub-adult stock U. australis plants and adult Aldrovanda plants were grown outdoors in laminate containers (see above and Table 1). On 21 June, 16 randomly selected, homogeneous non-branched U. australis plants were shortened to 25 mature apical leaf nodes (mean shoot length 18.4 cm). To estimate the apical shoot growth rate, the internodes between the third and fourth mature nodes were tagged with a fine thread (Adamec, 2009). Similarly, 24 randomly selected, homogeneous non-branched Aldrovanda plants were shortened to ten mature apical leaf nodes (mean shoot length 6.6 cm) and the internodes between the second and third mature nodes were tagged. These shortened shoots were transferred to grow in a 0.8-m2 (40 cm high) plastic container standing in a naturally lit greenhouse with open walls for cooling (Adamec, 2016). The container contained 300 L of tap water and 100 g (dry weight) of Carex litter as substrate on the bottom and the water chemistry resembled that in the stock cultures. A randomly selected half of the shortened shoots were placed in the container and could freely capture the added prey (variant +Prey), whereas three Aldrovanda and two U. australis shoots were transferred to each of four floating plastic frames (35 cm × 35 cm by ~7 cm depth) located in the same container. Mesh with a pore size of 150 µm created the bottom of the frames and excluded zooplankton capture but still allowed unimpeded water exchange (variant −Prey). The container was stirred by two aquarium water pumps to reduce concentration gradients between the frames and the free water. The pH inside and outside the floating frames never differed by more than 0.25 units. The water was considered oligo-mesotrophic and slightly humic: pH 7.28–7.30, electrical conductivity 33.1–34.4 mS m−1, total alkalinity 0.84 meq L−1, [CO2] 0.10 mm, NO3−-N below detection limit, NH4+-N 2.93 μm and PO4-P 0.092 μm. Every 2 d, the frames were thoroughly rinsed with tap water to exclude any zooplankton inside the frames. Small zooplankton (ostracods and daphnids, size 0.6–2 mm) was added regularly to the container to feed the control +Prey variant. A submersible temperature data logger (Minikin T, EMS, Brno, Czechia) monitored the water temperature at plant level. During the growth period, daily temperature maxima were within 20.0–27.5 °C and night minima within 17.6–24.0 °C with a total mean temperature of 23.1 °C; daily oscillations were 3–5 °C. The PAR irradiance at plant level 1 mm below the surface was ~20 % of that in the open, which is sufficient for both species (Adamec, 2016).
On 24 June, i.e. after 3 d of pre-cultivation as an adaptation period with or without prey, the position of the tag in all plants was estimated as a start of the apical shoot growth measurement. On 2 July, i.e. after the next 8 d, for U. australis and on 3 July, i.e. after the next 9 d, for Aldrovanda, the growth experiment with or without prey was terminated. The main shoot length, position of the tag and number of branches were measured in all plants to know the effect of prey feeding. Using a binocular loupe, the mean proportion of traps with a macroscopic prey was estimated to be ~10 % in the 12th leaf nodes in U. australis and ~56 % in the 8th leaf nodes in Aldrovanda in the +Prey variants, while all plants within the −Prey variants had zero prey capture. Trap-free leaves from third to sixth leaf nodes of two U. australis plants and trap-free shoots from the first to fourth leaf nodes of three Aldrovanda plants from one frame were pooled as one sample for phytohormone profiles. The samples were processed as above.
Experiment C: comparison of phytohormone profiles in organs on the main shoot and branches in U. australis
To consider the strength of apical dominance, phytohormone profiles were estimated in trap-free leaves and shoot apices of main shoots and branches of U. australis plants grown in an outdoor 300-L laminate container as above (pH 6.82, electrical conductivity 26.4 mS m–1, total alkalinity 0.80 meq L–1, [CO2] 0.28 mm, temperature ~20–25 °C, slightly shaded). On 3 June, mature leaf nodes were counted from the apex on the main shoot or branch to the point of branching in 26 adult plants 52–76 cm long. Linear regression analysis of these data determined the relationship of the apical growth rate between main shoots and branches as a measure of apical dominance (Adamec, 2011). Branches were removed from the plants and only one shorter branch close to the shoot apex remained on each plant. Between 8 and 13 June, when the branches had 10–12 mature leaf nodes, mature leaves from the third to sixth leaf nodes both from the main shoots and branches and also shoot apices from both organs (3–4 mm long) were sampled. Traps were removed and one sample was pooled from two or three plants. The sampled organs were processed as above.
Experiment D: comparison of phytohormone profiles in leaves on shoots in intact and decapitated plants
The effect of shoot decapitation (removal of the shoot apex) on phytohormone profiles was investigated in outdoor-grown U. australis and Aldrovanda. On 25 June, eight adult plants of U. australis grown in an oligo-mesotrophic 1800-L laminate container (pH 7.67, electrical conductivity 30.7 mS m–1, total alkalinity 1.15 meq L–1, [CO2] 0.056 mm, unshaded) were slightly shortened to 60 mature leaf nodes (length 45–55 cm) and all visible branches were removed. In four randomly selected plants, their shoot apex ~2.2 cm long with immature leaf nodes was excised to simulate a common mechanical disturbance, e.g. by water birds. The remaining four intact plants were tagged by a fine thread on the main shoot for the estimation of apical shoot growth rate, and served as controls. After 4 d, the decapitated plants prolifically formed new axillary branches (mean 4.75, range 4–6, median 4.5, n = 4), while the intact controls did so only poorly (mean 1.0, range 0–2, median 1.0). The number of all branches and the position of tags were estimated and leaves from the third to sixth leaf nodes (new leaf nodes in the intact variant, old leaf nodes in the decapitated variant) sampled. Trap-free leaves from one plant represented one sample and were processed as above. Daily temperature maxima on the level of plants were within 25.2–30.6 °C and night minima within 17.4–21.5 °C with a total mean temperature of 23.2 °C; daily oscillations were ~8 °C.
Similarly on 26 June, 16 adult plants of A. vesiculosa grown in an oligotrophic 750-L laminate container (pH 7.62, electrical conductivity 19.1 mS m–1, total alkalinity 0.84 meq L–1, [CO2] 0.046 mm, unshaded) were slightly shortened to 14 mature leaf nodes (length 10–12 cm) and all visible branches were removed. In eight randomly selected plants, their shoot apex (~6 mm long) with unmatured leaf nodes was excised. The remaining eight intact plants were tagged by a fine thread on the main shoot for the estimation of apical shoot growth rate, and served as controls. After 3 d, the decapitated plants formed new axillary branches (mean 0.63, range 0–2, median 0.5, n = 8), while the intact controls did very poorly (mean 0.13, range 0–1, median 0). The number of all branches and the position of tags were estimated and trap-free shoots from the first to fourth leaf nodes (new leaf nodes in the intact variant, old leaf nodes in the decapitated variant) sampled. Samples always included two plants and were processed as above. Water temperature in the container was similar to that for U. australis above.
Quantitative determination of CK and auxin levels
Lyophilized plant material was divided into CK and auxin parts, each enabling three technical replicates with weights ~0.3 mg for CKs and 0.5 mg for auxins per sample. The CK samples were extracted in 0.5 mL of a modified Bieleski buffer (Bieleski, 1964; Hoyerová et al., 2006) and purified by in-tip µSPE with three layers of each different stationary phase – C18, SDB-RPS and Cation (AttractSPE Disks, Affinisep) – as described in Svačinová et al. (2012). To each sample, a mix of isotope-labelled CK internal standards (0.25 pmol per sample of B, R, 9G, 7G and 0.5 pmol per sample of OG, NT; see Supplementary Data File 1 for the list of abbreviations of phytohormone metabolites) was added to check the recovery of the purification step and to validate the determination. Eluates were evaporated to dryness and dissolved in 30 µL of 10 % methanol. Samples were analysed by ultraperformance liquid chromatography (Acquity UPLC® I-class system; Waters, Milford, MA, USA) coupled to a triple quadrupole mass spectrometer equipped with an electrospray interface (Xevo TQ-S, Waters, Manchester, UK) by a method described in Svačinová et al. (2012). Quantification was obtained by multiple reaction monitoring of [M + H]+ and the appropriate product ion. Optimal conditions, dwell time, cone voltage and collision energy in the collision cell were optimized for each CK metabolite (Novák et al., 2008; Svačinová et al., 2012). Quantification was performed by Masslynx software using a standard isotope dilution method. Auxin samples were extracted using 0.5 mL of 50 mm Na-phosphate buffer (pH 7.0; 4 °C) containing 0.1 % diethyldithiocarbamic acid salt. A mix of isotope-labelled auxin internal standards [13C6]IAAsp, [13C6]IAGlu, [13C6]IAA and [13C6]oxIAA was added to each sample (5 pmol per sample; Pěnčík et al., 2009). Extracted samples were acidified with 1 m HCl to pH 2.7 before purifying with HLB cartridges [Oasis®, HLB 1cc (30 mg), Waters]. Evaporated samples were then dissolved in 30 µL of 10 % MeOH, injected onto a reversed-phase column (Kinetex 1.7 μm C18 100A, 50 × 2.1 mm; Phenomenex) and analysed using an Acquity UPLC® I-class system (Waters, Milford, MA, USA) coupled to a Xevo™ TQ-S (Waters, Manchester, UK) equipped with an electrospray interface (ESI) by the method described in Pěnčík et al. (2009).
Evaluation and statistical treatment of data
Within each variant, sums of contents for the four CK types (cis-, trans-, dihydrozeatin and isopentenyladenine types) in nmol kg–1 dry weight as well as total CK contents and active forms (sum of free bases and their ribosides; Šimura et al., 2016) were estimated for each sample (Supplementary Data Files 2–6). Active forms of CKs as percentages of the total content were also estimated. The results are expressed as means ± s.e. interval for n = 4. As there was no indication that the distribution of the data was non-normal, log-transformation of the data was not performed. The statistical significance of differences in different CK or auxin forms between the variants was evaluated by one-way ANOVA and the Tukey HSD test was used for multiple comparisons. Within Experiment A, significant differences in CK contents between four Utricularia species and their organs (traps versus leaves) were evaluated by two-way ANOVA with Species and Organs as fixed factors, and the Tukey HSD test was used for multiple comparisons. The results of the growth experiment with or without prey (Experiment B) were evaluated by Student’s t-test for independent samples.
RESULTS
Cytokinin profiles
Analyses of CKs in traps and trap-free leaves in four aquatic Utricularia species with monomorphic shoots revealed a specific distribution of various types and forms of CKs in these organs (Table 2, Supplementary Data File 2). The total contents of each of the four CK types, trans-zeatin (tZ), cis-zeatin (cZ), dihydrozeatin (DHZ) and isopentenyladenine (iP) as well as biologically active forms (free bases + ribosides), except for the DHZ content in U. reflexa, were consistently slightly or statistically significantly higher in traps than in leaves. A greater predominance in traps over leaves was found in the contents of cZ, iP and total CKs. Nevertheless, due to the much higher total CK content in traps than in leaves, active forms as a proportion of total CK content (0.48–3.4 %) were distinctly lower in traps than in leaves (1.3–35 %) in all four species. In both organs in all Utricularia species, iP types were the most distributed CK form (Supplementary Data File 2). In Aldrovanda traps, however, the content of different forms was usually comparable to that in shoots or significantly lower and the proportion of active forms was 61–64 % of the total content (Table 2). Except for DHZ, all CK types had significantly lower contents in Aldrovanda traps than averaged values in Utricularia traps, but contents of CK bases in Aldrovanda were higher in traps than in its shoots (cZ, DHZ, iP) or similar in traps and shoots (tZ) . Two-way ANOVA confirmed that all CK types and total and active CKs differed significantly (P < 0.01) between the four Utricularia species and both organs. The same also applied (except for iP and total CKs) to the interaction of the two factors (Table 3). In U. stygia and U. intermedia with dimorphic shoots, all types of CKs and total and active CKs were usually significantly (P < 0.01) higher in traps than carnivorous shoots (Fig. 1, Supplementary Data Files 3 and 7) and their profiles in both organs were analogous to those in traps and leaves in monomorphic species (cf. Table 2 ). The content of all CK types in carnivorous and photosynthetic shoots was similar in both species.
Table 2.
Cytokinin contents (in nmol kgdw–1) of traps and leaves in four Utricularia species with monomorphic shoots and in Aldrovanda traps and shoots
| Cytokinin |
U. reflexa
traps |
U. reflexa leaves |
U. inflata
traps |
U. inflata
leaves |
U. vulgaris
traps |
U. vulgaris
leaves |
U. australis
traps |
U. aust.
leaves |
Aldrovanda
traps |
Aldrovanda shoots |
|---|---|---|---|---|---|---|---|---|---|---|
| ΣtZ | 425 | 290 | 248 | 157 | 809** | 137 | 952** | 96 | &25** | 68 |
| s.e. | 69 | 42 | 36 | 24 | 44 | 21 | 105 | 7.8 | 2.6 | 11 |
| ΣcZ | 516** | 231 | 477** | 95 | 385** | 80 | 242** | 25 | &&73 | 49 |
| s.e. | 55 | 25 | 38 | 2.9 | 16 | 8.6 | 6.7 | 1.7 | 27 | 17 |
| ΣDHZ | 84 | 168 | 123 | 74 | 294** | 61 | 90** | 20 | 35 | 40 |
| s.e. | 19 | 34 | 26 | 16 | 18 | 6.7 | 17 | 2.7 | 1.9 | 5.4 |
| ΣiP | 9533** | 813 | 5830** | 508 | 6515** | 87 | 6272** | 144 | &&55** | 110 |
| s.e. | 1719 | 164 | 196 | 35 | 333 | 15 | 63 | 5.6 | 5.2 | 12 |
| Σtotal | 10 558** | 1502 | 6677** | 834 | 8003** | 368 | 7557** | 285 | && 187 | 268 |
| s.e. | 1861 | 265 | 296 | 78 | 411 | 51 | 191 | 18 | 37 | 44 |
| Σactive | 59 | 38 | 32** | 11 | 273** | 129 | 69** | 25 | 120 | & 163 |
| s.e. | 8.2 | 13 | 3.4 | 1.5 | 31 | 17 | 6.1 | 3.0 | 9.6 | 17 |
| Active (%) | 0.56 | 2.5 | 0.48 | 1.3 | 3.4 | 35 | 0.91 | 8.8 | 64 | 61 |
Means ± s.e. intervals (in italics) of four parallel samples are shown for each species and organ for each of the four types of CK (see Supplementary Data File S1). Total contents and sum of active forms are shown in bold; the active:total ratio (%) is shown in the bottom line.
Significance of differences in different CK forms between the traps and leaves/shoots within each species is indicated by asterisks on the right side of the values (one-way ANOVA). The difference between averages for traps or leaves of four Utricularia species and values for Aldrovanda traps and shoots (one-way ANOVA) is indicated by ampersands on the left side of the values. && or **P < 0.01; & or *P < 0.05; no symbol, not significant, P > 0.05.
Table 3.
Significance of differences based on two-way ANOVA in the sums of four types of CK, total sums and sums of active forms between four Utricularia species (factor 1), two organs (traps versus leaves; factor 2) and their species × organ interaction
| Factor | d.f. Effect | d.f. Error | F | P |
|---|---|---|---|---|
| ΣtZ | ||||
| 1 | 3 | 24 | 14.94 | <0.0001 |
| 2 | 1 | 24 | 140.91 | <0.0001 |
| 1 × 2 | 3 | 24 | 26.94 | <0.0001 |
| ΣcZ | ||||
| 1 | 3 | 24 | 29.55 | <0.0001 |
| 2 | 1 | 24 | 258.74 | <0.0001 |
| 1 × 2 | 3 | 24 | 3.40 | <0.05 |
| ΣDHZ | ||||
| 1 | 3 | 24 | 13.64 | <0.0001 |
| 2 | 1 | 24 | 23.22 | <0.0001 |
| 1 × 2 | 3 | 24 | 21.90 | <0.0001 |
| ΣiP | ||||
| 1 | 3 | 24 | 4.85 | <0.01 |
| 2 | 1 | 24 | 225.59 | <0.0001 |
| 1 × 2 | 3 | 24 | 2.71 | n.s. |
| Σtotal | ||||
| 1 | 3 | 24 | 4.80 | <0.01 |
| 2 | 1 | 24 | 240.34 | <0.0001 |
| 1 × 2 | 3 | 24 | 1.89 | n.s. |
| Σactive | ||||
| 1 | 3 | 24 | 70.01 | <0.0001 |
| 2 | 1 | 24 | 34.72 | <0.0001 |
| 1 × 2 | 3 | 24 | 8.90 | <0.001 |
For details see Table 2 and Supplementary Data File S1. Summary of all effects evaluated by two-way ANOVA is shown; n.s., not significant, P > 0.05.
Fig. 1.
Cytokinin content (in nmol kgdw–1) in traps and carnivorous and photosynthetic shoots in U. stygia (solid bars) and U. intermedia (hatched bars) with dimorphic shoots. Means ± s.e. intervals of four parallel samples are shown for each species and organ for each of the four chemical types of CK (A–D). The total contents and the sum of active forms are shown in (E). Significance of differences in different CK forms between traps and carnivorous shoots and also between the two types of shoot within each species is indicated as follows (one-way ANOVA): **P < 0.01; no symbol, not significant, P > 0.05.
The comparison of CK profiles in U. australis leaves from the oligotrophic and meso-eutrophic sites showed a significantly (P < 0.01) greater content of cZ, iP, total and active CKs at the oligotrophic site, while that of tZ was the opposite (Fig. 2, Supplementary Data Files 4 and 8). The growth experiment (Experiment B) proved that both Aldrovanda and U. australis plants fed on zooplankton had significantly (P < 0.01) longer shoots and higher apical shoot growth rates than unfed plants (Table 4) and fed Aldrovanda plants were markedly branched. In U. australis, feeding on prey slightly or significantly increased all CK forms, while no consistent and significant differences in CK profiles occurred in Aldrovanda in the same experiment (Fig. 2, Supplementary Data Files 4 and 8). No significant differences in CK profiles were found between U. australis leaves on main shoots and branches (Experiment C, Fig. 3, Supplementary Data Files 5 and 9). The content of all CK types in shoot apices of main shoots and branches was usually around two to seven times greater than that in these leaves. The linear regression of the number of mature leaf nodes in main shoots and branches in U. australis in this experiment led to the following linear relationship between the growth of leaf nodes in both organs: nodes of branches = 0.812 nodes of main shoots –5.38 (n = 26, r = 0.973). Thus, the growth of branches starts between the fifth and sixth mature leaf node on the main shoot and the apical growth rate of branches is 81 % of that of the main shoots. CK profiles were also compared in young, trap-free leaves in intact and decapitated U. australis after 4 d and in shoots in intact and decapitated Aldrovanda (Experiment D) after 3 d of decapitation when the decapitated shoots of both species started forming new branches from axillary buds. In U. australis, only the contents of iP and total CKs were significantly higher in the decapitated plants, while the other forms were comparable (Fig. 4, Supplementary Data Files 6 and 10). However, in decapitated Aldrovanda shoots the content of all CK types (except for cZ) rose to around 10- to 48-fold that in intact shoots, probably mainly due to the effect of wounding, as previously suggested by Conrad and Kohn (1975), Crane and Ross (1986), Sano et al. (1994), Smigocki (1995) and many others.
Fig. 2.
Cytokinin contents (in nmol kgdw−1) in trap-free leaves in U. australis and in trap-free Aldrovanda shoots. Two experiments compared (A) U. australis leaves from an oligotrophic and a eutrophic site, and (C) CK content in plants of both species grown with or without prey in a greenhouse. Means ± s.e. intervals of four parallel samples are shown for each species and experiment for each of the four chemical types of CK. Total contents and the sum of active forms are shown in (B and/or D). Significance of differences in different CK forms between the two variants within each species is indicated as follows (one-way ANOVA): **P < 0.01; *P < 0.05; no symbol, not significant, P > 0.05. n.d., not detected.
Table 4.
Results of the growth experiment for phytohormone analyses on A. vesiculosa and U. australis fed or unfed on prey
| Species | Variant | Initial shoot length (cm) | Final shoot length (cm) | Apical shoot growth rate (nodes per day) | Final branches per plant |
|---|---|---|---|---|---|
| A. vesiculosa | –Prey | 6.82 ± 0.11 | 12.0 ± 1.0** | 0.94 ± 0.07** | 0.83 |
| A. vesiculosa | +Prey | 6.37 ± 0.18 | 17.7 ± 0.3 | 1.26 ± 0.08 | 2.10 |
| U. australis | –Prey | 18.6 ± 1.1 | 43.4 ± 1.9** | 2.53 ± 0.22** | 0.25 |
| U. australis | +Prey | 18.2 ± 0.5 | 59.1 ± 1.7 | 3.42 ± 0.22 | 0.38 |
The experiment on floating enclosures lasted 11 d for U. australis and 12 d for A. vesiculosa. Apical shoot growth rate (in new nodes per day) was estimated during the last 8 d for U. australis and 9 d for A. vesiculosa.
Within each species, statistical significance of difference between variants is indicated by asterisks (Student’s t-test: **P < 0.01; *P < 0.05). Means ± s.e. intervals are shown, n = 8–12.
Fig. 3.
Cytokinin contents (in nmol kgdw–1) in trap-free leaves and shoot apices in main shoots and shorter branches in U. australis grown in a container. Means ± s.e. intervals of four parallel samples are shown for each of the four chemical types of CK (A). Total contents and the sum of active forms are shown in (B). Significance of differences in different CK forms in the same organs between main shoots and branches is indicated as follows (one-way ANOVA): *P < 0.05; no symbol, not significant, P > 0.05.
Fig. 4.
Cytokinin contents (in nmol kgdw–1) in trap-free leaves of U. australis and trap-free Aldrovanda shoots in intact and decapitated plants. Means ± s.e. intervals of four parallel samples are shown for each variant for each of the four chemical types of CK (A). Total contents and the sum of active forms are shown in detail in (B). Significance of differences in different CK forms between intact and decapitated plants within each species is indicated as follows (one-way ANOVA): **P < 0.01; *P < 0.05; no symbol, not significant, P > 0.05.
Auxin profiles
In four aquatic Utricularia species with monomorphic shoots, the content of the four auxin forms IAA, 2-oxindole-3-acetic acid (oxIAA), indole-3-acetyl aspartic acid (IAAsp) and indole-3-acetyl-β-1-O-D-glucose (IAGlu) and their total content usually was markedly higher in traps than in leaves (Table 5). The main form was consistently IAAsp in traps in all species; IAGlu was found only in U. inflata. The only active form, IAA, made up 2–39 % of the total auxin content in traps, but 4.6–100 % in leaves. In Aldrovanda traps, however, only the IAA content was significantly higher than that in shoots and the content of the other auxin forms was significantly lower or the same in traps. The trap content of oxIAA and the foliar contents of oxIAA and total auxins in Aldrovanda were significantly higher than those in averaged values in Utricularia organs. Two-way ANOVA confirmed, for all auxin forms and the total content, a significant difference (P < 0.001) between the four Utricularia species and both organs as well as for the interaction of the two factors (Table 6). In U. stygia and U. intermedia with dimorphic shoots, the dominant auxin forms in all three organs were IAA and oxIAA except for a high IAAsp content in U. intermedia traps (Fig. 5, Supplementary Data File 11). In the former species, no significant differences in auxin profile were found between traps and carnivorous shoots or between the two shoot types. In the latter species, the IAAsp and total auxin contents in traps were significantly higher than those in carnivorous shoots, and the oxIAA and total auxin contents in photosynthetic shoots were significantly lower than those in carnivorous shoots. The proportion of IAA to total auxin content varied between 31 and 61 % in all organs.
Table 5.
Auxin contents (in nmol kgdw–1) in traps and leaves in four Utricularia species and in Aldrovanda traps and shoots
| Auxins |
U. reflexa
traps |
U. reflexa leaves |
U. inflata
traps |
U. inflata
leaves |
U. vulgaris
traps |
U. vulgaris
leaves |
U. australis
traps |
U. australis leaves |
Aldrovanda traps |
Aldrovanda shoots |
|---|---|---|---|---|---|---|---|---|---|---|
| IAA | 499** | 96 | 219** | 84 | 413** | 198 | 286** | 77 | 302** | 172 |
| s.e. | 56 | 2 | 16 | 13 | 24 | 25 | 46 | 4.0 | 2.5 | 8.1 |
| oxIAA | 268** | 0 | 0 | 0 | 138 | 174 | 327** | 137 | &&5210* | &&7859 |
| s.e. | 20 | 0 | 0 | 0 | 81 | 17 | 36 | 21 | 608 | 695 |
| IAAsp | 2374** | 0 | 9862** | 1520 | 558** | 0 | 1209** | 0 | 405 | 494 |
| s.e. | 163 | 0 | 596 | 183 | 76 | 0 | 89 | 0 | 82 | 65 |
| IAGlu | 0 | 0 | 706** | 212 | 0 | 0 | 0 | 0 | 0 | 0 |
| s.e. | 0 | 0 | 57 | 45 | 0 | 0 | 0 | 0 | 0 | 0 |
| Σtotal | 3141** | 96 | 10 787** | 1817 | 1109** | 373 | 1822** | 213 | 5918* | && 8525 |
| s.e. | 233 | 2 | 626 | 235 | 143 | 34 | 130 | 21 | 582 | 695 |
| IAA (%) | 16 | 100 | 2.0 | 4.6 | 39 | 53 | 16 | 36 | 5.1 | 2.0 |
Means ± s.e. intervals (in italics) of four parallel samples are shown for each species and organ for each of the four types of auxin measured. Total contents are shown in bold; the IAA:total content ratio (%) is shown in the bottom line.
Significance of differences in different types between the traps and leaves/shoots within each species is indicated by asterisks on the right side of the values (one-way ANOVA). The difference between averages for traps or leaves of four Utricularia species and values for Aldrovanda traps and shoots (one-way ANOVA) is indicated by ampersands on the left side of the values. && or **P < 0.01; & or *P < 0.05; no symbol, not significant, P > 0.05.
Table 6.
Results of two-way ANOVA to analyse significant differences in auxin type contents between four Utricularia species (factor 1) and organ type (leaves versus traps; factor 2)
| Factor | d.f. | d.f. Error |
F | P |
|---|---|---|---|---|
| IAA | ||||
| 1 | 3 | 24 | 14.39 | <0.0001 |
| 2 | 1 | 24 | 134.2 | <0.0001 |
| 1 × 2 | 3 | 24 | 7.575 | <0.001 |
| OxIAA | ||||
| 1 | 3 | 24 | 16.67 | <0.0001 |
| 2 | 1 | 24 | 19.94 | <0.0001 |
| 1 × 2 | 3 | 24 | 9.705 | <0.0001 |
| IAAsp | ||||
| 1 | 3 | 24 | 238.5 | <0.0001 |
| 2 | 1 | 24 | 363.3 | <0.0001 |
| 1 × 2 | 3 | 24 | 118.2 | <0.0001 |
| IAGlu | ||||
| 1 | 3 | 24 | 161.5 | <0.0001 |
| 2 | 1 | 24 | 46.65 | <0.0001 |
| 1 × 2 | 3 | 24 | 46.65 | <0.0001 |
| Σtotal | ||||
| 1 | 3 | 24 | 202.2 | <0.0001 |
| 2 | 1 | 24 | 381.4 | <0.0001 |
| 1 × 2 | 3 | 24 | 101.9 | <0.0001 |
For details see Table 9 and Supplementary Data File S1.
Summary of all effects is shown.
×, Interaction of factors.
Fig. 5.
Auxin contents (in nmol kgdw–1) in traps and carnivorous and photosynthetic shoots in U. stygia and U. intermedia with dimorphic shoots. Means ± s.e. intervals of four parallel samples are shown for each species and organ for each of the four types of auxin (IAGlu not detected). Significance of differences in different types between traps and carnivorous shoots and between the two types of shoot within each species is indicated as follows (one-way ANOVA): **P < 0.01; *, a, P < 0.05; no symbol, not significant, P > 0.05. n.d., not detected.
The comparison of auxin profiles in U. australis leaves from an oligotrophic and a meso-eutrophic site showed significant differences in some auxin forms: a relatively high IAA content (456 ± 97 nmol kgdw−1, where dw is dry weight) at the oligotrophic site but zero content at the meso-eutrophic site, but significantly higher oxIAA, IAAsp and total auxin contents at the meso-eutrophic site (Fig. 6, Supplementary Data File 12). Thus, IAA as a proportion of total auxins content was 71 % at the oligotrophic site, but zero at the meso-eutrophic one. In the growth experiment on zooplankton feeding (Experiment B, Fig. 6, Supplementary Data File 12), the foliar IAA content in U. australis plants fed on prey was also zero and the difference between the two variants was non-significant. The other auxin forms were the same in both variants. Unlike the Aldrovanda shoots in this growth experiment, prey-fed plants had significantly higher contents of all auxin forms (except for IAGlu) than non-fed plants (Fig. 6, Supplementary Data File 12). The IAA content in U. australis leaves on main shoots (320 ± 34 nmol kgdw−1) differed significantly from that on branches, which was zero (Experiment C, Fig. 7, Supplementary Data File 13). Thus, IAA as a proportion of total auxin content in branches was zero. The contents of all auxin forms in apices of main shoots were similar to those in branches in U. australis, but all contents in apices were markedly higher than those in leaves, consistent with the CK profiles. The comparison of auxin profiles in young leaves in intact and decapitated shoots of U. australis and Aldrovanda (Experiment D) showed significantly lower contents of IAA, oxIAA and total auxins in decapitated U. australis plants and a similar pattern was also found in decapitated Aldrovanda shoots, where these contents were around 2-fold lower (Fig. 8, Supplementary Data File 13).
Fig. 6.
Auxin contents (in nmol kgdw−1) in trap-free leaves in U. australis and trap-free Aldrovanda shoots. Means ± s.e. intervals of four parallel samples are shown for each species and experiment for each of the four types of auxin (IAGlu not detected). Two experiments compared (A) U. australis leaves from oligotrophic and eutrophic sites, and (B) plants of both species grown with or without prey in a greenhouse. Note the logarithmic scale. Significance of differences in different types between the two variants within each species is indicated as follows (one-way ANOVA); **P < 0.01; *P < 0.05; no symbol, not significant, P > 0.05. n.d., not detected.
Fig. 7.
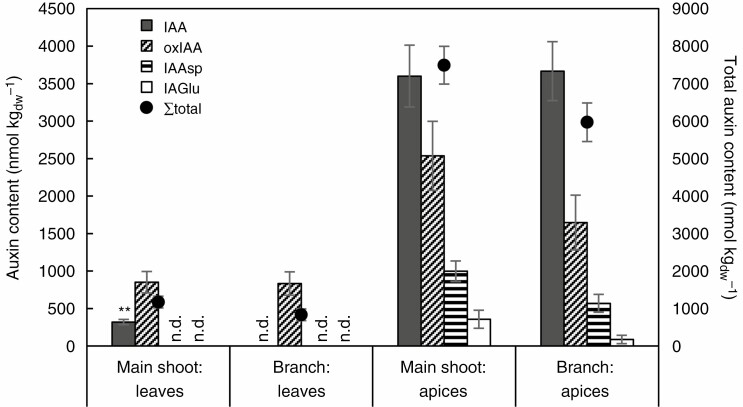
Auxin contents (in nmol kgdw−1) in trap-free leaves and shoot apices in main shoots and shorter branches in U. australis grown in a container. Means ± s.e. intervals of four parallel samples are shown for each variant for each of the four types of auxin. Total contents are shown by solid circles. Significance of differences in different components in the same organs between main shoots and branches is indicated as follows (one-way ANOVA): **P < 0.01; no symbol, not significant, P > 0.05. n.d. (not detected).
Fig. 8.
Auxin contents (in nmol kgdw–1) in trap-free leaves of U. australis and trap-free Aldrovanda shoots in intact or decapitated plants. Means ± s.e. intervals of four parallel samples are shown for each variant for each of the four chemical types of auxin (IAGlu not detected). Total contents are shown by solid circles. Significance of differences in different components between intact and decapitated plants within each species is indicated as follows (one-way ANOVA): **P < 0.01, *P < 0.05; no symbol, not significant, P > 0.05. n.d., not detected.
DISCUSSION
All aquatic carnivorous plants used in this study, six Utricularia species and Aldrovanda vesiculosa, are rootless and possess long and thin stems bearing dozens of leaf nodes or true leaf whorls in a regular, modular structure. Their linear shoots exhibit a marked physiological and growth polarity, associated with very rapid apical shoot growth, rapid gradual ageing, senescence and decay of basal shoot segments (Friday, 1989; Adamec, 2000, 2018a, 2018b). Moreover, their leaves bear one snapping trap (Aldrovanda) or dozens of suction traps of foliar origin (Poppinga et al., 2018). It is known that the traps of ACPs senesce similarly to leaves or stems or even more rapidly (Friday, 1989; Sirová et al., 2003; Adamec, 2014). It can therefore be assumed that the phenomena of the physiological and growth polarity of shoots and traps of ACPs are regulated by an interplay of phytohormones – mainly auxins and CKs. In Aldrovanda and U. australis, analyses of CKs and auxins in shoot segments of different ages revealed a relatively stable IAA content along this shoot gradient, but that of the active forms of CKs peaked in shoot apices and decreased basipetally, suggesting that the physiological polarity is regulated by the polarity of CKs (Šimura et al., 2016). Nevertheless, leaves/shoots and traps in ACPs have very different functions (photosynthetic versus prey capture and nutrient uptake) and could be regulated differently.
To the best of our knowledge, CKs and auxins were determined in traps of several ACP species for the first time (Tables 2 and 5, Supplementary Data Files 2–7 and 11). As soon as Utricularia traps mature and are able to capture prey, their growth generally ceases in 1–2 d (Friday, 1989) and then they only perform their demanding metabolic functions (water pumping, enzyme secretion, nutrient absorption). The same likely also occurs in Aldrovanda traps. Surprisingly, the total CK contents as well as biologically active forms in all Utricularia species were significantly higher in traps than in leaves and a great predominance of traps over leaves in the contents of cZ and iP was observed. One possible explanation of CK function in the traps can be their role in an efficient nutrient uptake mechanism (Kiba et al., 2010; Koeslin-Findeklee et al., 2015; Silva-Navas et al., 2019). It has been proposed that CKs modify sink/source nutrient mobilization (Werner et al., 2008). Plants use multiple signalling pathways that sense internal and external nitrogen status and some of these routes use CKs as a messenger (Gu et al., 2018). Recent data obtained also suggest that the cis- and trans-isomers of zeatin can play completely distinct roles in growth regulation by a complex crosstalk with IAA and ABA (Lacuesta et al., 2018). While tZ and iP regulate nitrate uptake, cZ regulates source and sink strength. Moreover, a specific role of cZ in plant responses to phosphate starvation has also been published recently (Silva-Navas et al., 2019). cZ-treated seedlings showed a longer root system as well as longer root hairs than tZ-treated ones, increasing the total absorbing surface. In ACPs, CKs could play a role in defining traps as nutrient uptake organs of these rootless plants. Also, analyses of U. stygia and U. intermedia dimorphic shoots and traps (Fig. 1, Supplementary Data File 7) showed a ten times higher total CK pool in the traps in comparison with both leaf types. Moreover, the comparison of CK profiles in U. australis leaves from an oligotrophic and a eutrophic site (Supplementary Data File 4) revealed significantly higher contents of cZ (and lower levels of tZ) in samples from the oligotrophic site, which is also in very good agreement with previous findings by Silva-Navas et al. (2019). All these data indirectly support our hypothesis about the important and distinct role of CKs in trap nutrient uptake in ACPs.
Nitrogen often limits plant growth and development (Hayat et al., 2009). In response to changes in its supply, plants adjust their growth and development at both physiological and morphological levels. Amongst phytohormones, auxins have also been considered signalling substances of such pathways (Kiba et al., 2010). Increasing evidence is revealing that N supply fluctuations have a significant impact on auxin distribution in plants. A decreased N supply commonly increases IAA accumulation (Hou et al., 2021). In our study, only U. australis leaves from an oligotrophic site contained active auxin, free IAA, whereas the entire auxin pool in the leaves from a eutrophic site was present in the inactive form of oxIAA and IAAsp (Fig. 6, Supplementary Data File 12). Also, IAA and the total auxin content in traps in all four Utricularia species studied were several times higher than in leaves (Tables 5 and 6). This can be associated with the fact that auxins can significantly improve N metabolism (Hayat et al., 2009) and therefore higher levels are needed in the traps as an alternative nutrient uptake organ. In Aldrovanda, the trap IAA content, but not the total auxin one, was also significantly higher due to the high content of inactive auxin metabolites in the shoots. Auxin analyses of U. stygia and U. intermedia dimorphic shoots and traps (Fig. 5, Supplementary Data File 11) did not show any clear trends, although the contents of active IAA (and also oxIAA in U. intermedia) were lower in photosynthetic than in carnivorous shoots.
Another important developmental process, in which CKs and auxins are strongly involved, is plant morphogenesis and organ development. Apical dominance is a phenomenon in which a main shoot in an intact plant grows predominantly while suppressing the outgrowth of axillary buds (Shimizu-Sato et al., 2009). It is regulated by two plant hormones, auxin and CK. Interestingly, we found much higher levels of tZ CKs in apices of main shoots of U. australis in comparison with its shorter branches (Fig. 3, Supplementary Data Files 5 and 9). This is probably associated with more rapid growth of main shoots (1.23× greater than branches) of this species. Surprisingly, an opposite trend was found for cZ and iP types. An even more striking difference was found in auxin profiles (Fig. 7, Supplementary Data File 13): free IAA was present only in the main shoot leaves but not in the branch leaves. This finding is, however, in good agreement with the generally accepted inhibitory role of auxin in shoot branching (Shimizu-Sato et al., 2009).
Shoot decapitation breaks apical dominance (Shimizu-Sato et al., 2009). Significantly higher CK and decreased auxin contents measured after decapitation in young trap-free U. australis and Aldrovanda leaves (Supplementary Data Files 8 and 13), in comparison with leaves/shoots of intact plants, are also in good agreement with previously obtained data from land-based plants, e.g. Chrysanthemum (Sun et al., 2021), where decapitation leads to more flowers on lateral branches. Also in peas, CK content was shown to increase with decreasing IAA contents after decapitation and adenosine phosphate–isopentenyltransferase (IPT), a key enzyme in CK biosynthesis, was markedly induced in the nodal stem along with accumulation of CKs (Tanaka et al., 2006). These authors proposed a model in which, once the shoot apex is decapitated, the auxin level in the stem decreases, repression of IPT gene expression is released, CK contents increase and axillary buds sprout. After axillary buds sprout, de novo synthesized IAA derived from a new shoot apex flows to the stem and again suppresses IPT gene expression (Tanaka et al., 2006). In contrast, in Chrysanthemum (Sun et al., 2021), the expression levels of the IPT genes remained nearly unchanged, but expression of ARR12 was upregulated. They act as a molecular link between CK signalling and shoot meristem-specific genes.
It can be concluded that although the molecular mechanism of apical dominance still remains unresolved even in frequently studied rooted land species, our data (Figs 4 and 8, Supplementary Data Files 10 and 13) obtained on rootless, model aquatic carnivorous plants are in very good agreement with both previously proposed land plant models and can serve as a basis for future experiments. Yet it is possible to raise a general question about the extent to which the present CK and auxin profiles in rootless ACPs are representative of those in rapidly growing, rooted submerged aquatic plants with linear, modular shoots (e.g. Egeria densa, Myriophyllum spicatum), many of which are important aquatic weeds.
CONCLUSIONS
Levels of 33 CK and 4 auxin metabolites have been measured using ultraperformance liquid chromatography coupled with triple quadrupole mass spectrometry for the first time in traps, leaves/shoots and shoot apices in several species of genera Aldrovanda and Utricularia to elucidate how the hormonal profiles reflect the specific organ functions and polarity. Results show an association with different dominant functions of these organs: nutrient uptake by traps versus photosynthetic function of traps. Interplay of CKs and auxins regulates apical dominance in these plants possessing strong physiological and growth polarity.
SUPPLEMENTARY DATA
Supplementary data are available at Annals of Botany online (https://academic.oup.com/aob) and consist of the following. File 1: list of abbreviations. File 2: content of CK and auxin metabolites in traps and leaves in four Utricularia species with monomorphic shoots and in Aldrovanda traps and shoots. File 3: content of CK and auxin metabolites in traps and carnivorous and photosynthetic shoots in U. and U. intermedia with dimorphic shoots. File 4: content of CK and auxin metabolites in trap-free leaves in U. australis and trap-free Aldrovanda shoots. File 5: content of CK and auxin metabolites in trap-free leaves and shoot apices in main shoots and shorter branches in U. australis grown in a container. File 6: content of CK and auxin metabolites in trap-free leaves of U. australis and trap-free Aldrovanda shoots in intact and decapitated plants. File 7: CK content in traps and carnivorous and photosynthetic shoots in U. stygia and U. intermedia with dimorphic shoots. File 8: CK contents in trap-free leaves in U. australis and trap-free Aldrovanda shoots. File 9: CK contents in trap-free leaves and shoot apices in main shoots and shorter branches in U. australis grown in a container. File 10: CK contents in trap-free leaves of U. australis and in trap-free Aldrovanda shoots in intact and decapitated plants. File 11: auxin contents in traps and carnivorous and photosynthetic shoots in U. stygia and U. intermedia with dimorphic shoots. File 12: auxin contents in trap-free leaves in U. australis and trap-free Aldrovanda shoots. File 13: auxin contents in trap-free leaves and shoot apices in main shoots or shorter branches in U. australis grown in a container, in trap-free leaves of Utricularia australis and in trap-free Aldrovanda shoots in intact and decapitated plants.
ACKNOWLEDGEMENTS
We thank Dr Brian G. McMillan (Glasgow, UK) for English correction and Prof. Miroslav Strnad (Olomouc, Czechia) for critically reading the manuscript and valuable comments.
Contributor Information
Lubomír Adamec, Institute of Botany of the Czech Academy of Sciences, Dukelská 135, CZ-379 01 Třeboň, Czech Republic.
Lenka Plačková, Laboratory of Growth Regulators, Faculty of Science, Palacký University & Institute of Experimental Botany AS CR, Šlechtitelů 27, 78371 Olomouc, Czech Republic.
Karel Doležal, Laboratory of Growth Regulators, Faculty of Science, Palacký University & Institute of Experimental Botany AS CR, Šlechtitelů 27, 78371 Olomouc, Czech Republic; Department of Chemical Biology, Faculty of Science, Palacký University, Šlechtitelů 27, 78371 Olomouc, Czech Republic.
FUNDING
This work was supported by the long-term research developmental project (RVO 67985939) of the Czech Academy of Sciences (to L.A.) and by the ERDF project ‘Plants as a tool for sustainable global development’ (CZ.02.1.01/0.0/0.0/16_019/0000827).
LITERATURE CITED
- Adamec L. 2000. Rootless aquatic plant Aldrovanda vesiculosa: physiological polarity, mineral nutrition, and importance of carnivory. Biologia Plantarum 43: 113–119. doi: 10.1023/a:1026567300241. [DOI] [Google Scholar]
- Adamec L. 2009. Photosynthetic CO2 affinity of the aquatic carnivorous plant Utricularia australis (Lentibulariaceae) and its investment in carnivory. Ecological Research 24: 327–333. [Google Scholar]
- Adamec L. 2011. Shoot branching of the aquatic carnivorous plant Utricularia australis as the key process of plant growth. PHYTON Annales Rei Botanicae 51: 133–148. [Google Scholar]
- Adamec L. 2013. A comparison of photosynthetic and respiration rates in six aquatic carnivorous Utricularia species differing in morphology. Aquatic Botany 111: 89–94. doi: 10.1016/j.aquabot.2013.06.004. [DOI] [Google Scholar]
- Adamec L. 2014. Different reutilization of mineral nutrients in senescent leaves of aquatic and terrestrial carnivorous Utricularia species. Aquatic Botany 119: 1–6. doi: 10.1016/j.aquabot.2014.06.002. [DOI] [Google Scholar]
- Adamec L. 2016. Mineral nutrition in aquatic carnivorous plants: effect of carnivory, nutrient reutilization and K+ uptake. Fundamental and Applied Limnology 188: 41–49. doi: 10.1127/fal/2016/0780. [DOI] [Google Scholar]
- Adamec L. 2018a. Ecophysiology of aquatic carnivorous plants. In: Ellison AM, Adamec L, eds. Carnivorous plants: physiology, ecology, and evolution. Oxford: Oxford University Press, 256–269. [Google Scholar]
- Adamec L. 2018b. Biological flora of Central Europe: Aldrovanda vesiculosa L. Perspectives in Plant Ecology, Evolution and Systematics 35: 8–21. doi: 10.1016/j.ppees.2018.10.001. [DOI] [Google Scholar]
- Adamec L. 2020. Biological flora of Central Europe: Utricularia intermedia Hayne, U. ochroleuca R.W. Hartm., U. stygia Thor and U. bremii Heer ex Kölliker. Perspectives in Plant Ecology, Evolution and Systematics 44: 125520e125520. doi: 10.1016/j.ppees.2020.125520. [DOI] [Google Scholar]
- Bieleski RL. 1964. The problem of halting enzyme action when extracting plant tissues. Analytical Biochemistry 9: 431–442. doi: 10.1016/0003-2697(64)90204-0. [DOI] [PubMed] [Google Scholar]
- Conrad K, Kohn B.. 1975. Zunahme von Cytokinin und Auxin in verwundetem Speichergewebe von Solanum tuberosum. Phytochemistry 14: 325–328. doi: 10.1016/0031-9422(75)85083-7. [DOI] [Google Scholar]
- Crane KE, Ross CW.. 1986. Effects of wounding on cytokinin activity in cucumber cotyledons. Plant Physiology 82: 1151–1152. doi: 10.1104/pp.82.4.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross A. 2012. Aldrovanda. The waterwheel plant. Poole: Redfern Natural History Productions. [Google Scholar]
- Friday LE. 1989. Rapid turnover of traps in Utricularia vulgaris L. Oecologia 80: 272–277. doi: 10.1007/BF00380163. [DOI] [PubMed] [Google Scholar]
- Gu JF, Li ZK, Mao YQ, et al. 2018. Roles of nitrogen and cytokinin signals in root and shoot communications in maximizing of plant productivity and their agronomic applications. Plant Science 274: 320–331. [DOI] [PubMed] [Google Scholar]
- Guisande C, Granado-Lorencio C, Andrade-Sossa C, Duque SR.. 2007. Bladderworts. Functional Plant Science and Biotechnology 1: 58–68. [Google Scholar]
- Hayat Q, Hayat S, Ali B, Ahmad A.. 2009. Auxin analogues and nitrogen metabolism, photosynthesis, and yield of chickpea. Journal of Plant Nutrition 32: 1469–1485. doi: 10.1080/01904160903092671. [DOI] [Google Scholar]
- Hou MM, Wu DX, Li Y, Tao WQ, Chao L, Zhang YL.. 2021. The role of auxin in nitrogen-modulated shoot branching. Plant Signaling & Behavior 16: e1885888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyerová K, Gaudinová A, Malbeck J, et al. 2006. Efficiency of different methods of extraction and purification of cytokinins. Phytochemistry 67: 1151–1159. doi: 10.1016/j.phytochem.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Hussain S, Nanda S, Zhang JH, et al. 2021. Auxin and cytokinin interplay during leaf morphogenesis and phyllotaxy. Plants 10: e1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakšová J, Novák O, Adamec L, Pavlovič A.. 2021. Contrasting effect of prey capture on jasmonate signalling in two genera of aquatic carnivorous plants (Aldrovanda, Utricularia). Plant Physiology and Biochemistry 166: 459–565. [DOI] [PubMed] [Google Scholar]
- Juniper BE, Robins RJ, Joel DM.. 1989. The carnivorous plants. London: Academic Press. [Google Scholar]
- Kiba T, Kudo T, Kojima M, Sakakibara H.. 2010. Hormonal control of nitrogen acquisition: roles of auxin, abscisic acid, and cytokinin. Journal of Experimental Botany 62: 1399–1409. doi: 10.1093/jxb/erq410. [DOI] [PubMed] [Google Scholar]
- Koeslin-Findeklee F, Becker MA, van der Graaff E, Roitsch T, Horst WJ.. 2015. Differences between winter oilseed rape (Brassica napus L.) cultivars in nitrogen starvation-induced leaf senescence are governed by leaf-inherent rather than root-derived signals. Journal of Experimental Botany 66: 3669–3681. doi: 10.1093/jxb/erv170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacuesta M, Saiz-Fernandez I, Podlešáková K, et al. 2018. The trans and cis zeatin isomers play different roles in regulating growth inhibition induced by high nitrate concentrations in maize. Plant Growth Regulation 85: 199–209. doi: 10.1007/s10725-018-0383-7. [DOI] [Google Scholar]
- Miranda VFO, Silva SR, Reut MS, et al. 2021. A historical perspective of bladderworts (Utricularia): traps, carnivory and body architecture. Plants 10: 2656e2656. doi: 10.3390/plants10122656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novák O, Hauserová E, Amakorová P, Doležal K, Strnad M.. 2008. Cytokinin profiling in plant tissues using ultra-performance liquid chromatography-electrospray tandem mass spectrometry. Phytochemistry 69: 2214–2224. doi: 10.1016/j.phytochem.2008.04.022. [DOI] [PubMed] [Google Scholar]
- Pěnčík A, Rolčík J, Novák O, et al. 2009. Isolation of novel indole-3-acetic acid conjugates by immunoaffinity extraction. Talanta 80: 651–655. doi: 10.1016/j.talanta.2009.07.043. [DOI] [PubMed] [Google Scholar]
- Poppinga S, Bauer U, Speck T, Volkov AG.. 2018. Motile traps. In: Ellison AM, Adamec L, eds. Carnivorous plants: physiology, ecology, and evolution. Oxford: Oxford University Press, 180–193. [Google Scholar]
- Richards JH. 2001. Bladder function in Utricularia purpurea (Lentibulariaceae): is carnivory important? American Journal of Botany 88: 170–176. doi: 10.2307/2657137. [DOI] [PubMed] [Google Scholar]
- Sano H, Seo S, Orudgev E, et al. 1994. Expression of the gene for a small GTP binding protein in transgenic tobacco elevates endogenous cytokinin levels, abnormally induces salicylic acid in response to wounding, and increases resistance to tobacco mosaic virus infection. Proceedings of the National Academy of Sciences of the USA 91: 10556–10560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu-Sato S, Tanaka M, Mori H.. 2009. Auxin-cytokinin interactions in the control of shoot branching. Plant Molecular Biology 69: 429–435. [DOI] [PubMed] [Google Scholar]
- Silva-Navas J, Conesa CM, Saez A, et al. 2019. Role of cis-zeatin in root responses to phosphate starvation. New Phytologist 224: 242–257. [DOI] [PubMed] [Google Scholar]
- Šimura J, Spíchal L, Adamec L, et al. 2016. Cytokinin, auxin and physiological polarity in the aquatic carnivorous plants Aldrovanda vesiculosa and Utricularia australis. Annals of Botany 117: 1037–1044. doi: 10.1093/aob/mcw020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirová D, Adamec L, Vrba J.. 2003. Enzymatic activities in traps of four aquatic species of the carnivorous genus Utricularia. New Phytologist 159: 669–675. doi: 10.1046/j.1469-8137.2003.00834.x. [DOI] [PubMed] [Google Scholar]
- Sirová D, Borovec J, Černá B, Rejmánková E, Adamec L, Vrba J.. 2009. Microbial community development in the traps of aquatic Utricularia species. Aquatic Botany 90: 129–136. [Google Scholar]
- Smigocki AC. 1995. Expression of a wound-inducible cytokinin biosynthesis gene in transgenic tobacco: correlation of root expression with induction of cytokinin effects. Plant Science 109: 153–163. doi: 10.1016/0168-9452(94)04157-c. [DOI] [Google Scholar]
- Sun D, Zhang L, Yu Q, et al. 2021. Integrated signals of jasmonates, sugars, cytokinins and auxin influence the initial growth of the second buds of chrysanthemum after decapitation. Biology 10: 440e440. doi: 10.3390/biology10050440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svačinová J, Novák O, Plačková L, et al. 2012. A new approach for cytokinin isolation from Arabidopsis tissues using miniaturized purification: pipette tip solid-phase extraction. Plant Methods 8: e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M, Takei K, Kojima M, Sakakibara H, Mori H.. 2006. Auxin controls local cytokinin biosynthesis in the nodal stem in apical dominance. Plant Journal 45: 1028–1036. [DOI] [PubMed] [Google Scholar]
- Tarkowská D, Novák O, Floková K, et al. 2014. Quo vadis plant hormone analysis? Planta 240: 55–76. doi: 10.1007/s00425-014-2063-9. [DOI] [PubMed] [Google Scholar]
- Taylor P. 1989. The genus Utricularia: a taxonomic monograph. Kew Bulletin Additional Series XIV. London: HMSO. [Google Scholar]
- Werner T, Holst K, Pörs Y, et al. 2008. Cytokinin deficiency causes distinct changes of sink and source parameters in tobacco shoots and roots. Journal of Experimental Botany 59: 2659–2672. doi: 10.1093/jxb/ern134. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.