Abstract
Background
Rebound pain is extreme pain that persists after the effects of regional anesthesia wear off. Rebound pain occurrence and intensity are influenced by patient, surgical, and anesthesia-related factors. The incidence and severity of rebound pain after peripheral nerve block resolution are both reduced by the use of perioperative multimodal strategy. The purpose of the current paper was to evaluate the frequency, seriousness, and risk factors for rebound pain following peripheral nerve block resolution.
Method
A cross-sectional study centred on 384 patients who had received peripheral nerve blocks was carried out from August 20, 2021, to June 30, 2022. A semi-structured questionnaire was used to gather information within 24 h following the block's performance. SPSS 25 was used to enter and analyze the data. The change from well-controlled pain while the block is operating to severe pain within 24 h of block performance is known as rebound pain. Both univariate and multivariable analyses were used to examine the relationship between various parameters (patient, surgical, and anesthetic-related factors) and rebound pain. In the multivariable analysis, a P-value of 0.05 or lower is regarded as statistically significant.
Results
The incidence of rebound pain after peripheral nerve block was resolved was 61.7% (95% CI: 56.5–66.7) with a mean rebound pain score of 4.19 ± 2. Of the total 237, 120(50.6%) had severe rebound pain after the peripheral nerve block was resolved. The use of preoperative intravenous dexamethasone (AOR: 2.6, 95%CI: 20.29–24.57), preoperative pain (AOR: 3.9, 95%CI: 41–57.4), type of surgery (AOR: 6.5, 95%CI: 1.45–11.7), post-operative NSAID (AOR: 2.2, 95%CI: 17.69–20.8), and opioid use (AOR: 2.2, 95%CI: 19.1–22.56) were independent risks associated with rebound pain.
Conclusions
and Recommendation: Rebound pain occurred in 61.7% of patients and had independent associations with preoperative pain, dexamethasone premedication, type of surgery, use of an adjuvant, use of postoperative opioids, and NSAIDs. Therefore, clinicians should continue to use preventative strategies, especially for patients at higher risk of experiencing rebound pain.
Keywords: Rebound pain, Regional anesthesia, Peripheral Nerve block
Highlights
-
•
Incidence of rebound pain was 61.7%.
-
•
Preoperative pain, premedication with dexamethasole, type of surgery, use of adjuvant, and postoperative analgesia were factors for rebound pain.
-
•
It is recommended to use preventative strategies.
1. Introduction
1.1. Statement of the problem
Regional anesthesia, specifically peripheral nerve blocks (PNBs), is routinely performed for perioperative analgesia and anesthesia in patients undergoing surgery [1]. It plays a great role in maximising post-operative pain control while minimising opioid consumption and allowing a fast hospital discharge [2,3].
Rebound pain (RP) is mechanical–surgical pain caused by unopposed nociceptive inputs that are brutally uncovered after PNB resolution [3] and characterised by sudden, significant pain following regional nerve blockade regression [4]. There is a quantifiable difference in pain scores when the block is working versus the increase in acute pain encountered during the first few hours after the effects of perineurally single-injection or continuous infusion local anaesthetics resolve [5]. It may reduce or even negate the overall benefits of a peripheral nerve block [2].
The incidence of rebound pain after peripheral nerve block (PNB) resolves could reach around 40% of patients and may be due to abnormal spontaneous C-fiber hyperactivity and nociceptor hyper-excitability without mechanical nerve lesion [3]. Even in a previous study in Canada overall incidence of rebound pain after PNB regression was 49.6% [6].
Patient-related factors such as severe pre-operative pain, age less than 60 years old, female sex, and psychosocial factors such as catastrophic pain perception and depression have all been found to have a significant impact on the occurrence and severity of rebound pain [4,[7], [8], [9]].
Surgical factors can produce an abnormal level of plasticity at the peripheral nociceptor level and in the central neurons involved in receiving and processing direct and indirect inputs [8]. Damage to the peripheral nociceptor provokes a continuous firing of pain signals, leading to either an exaggerated response to normally painful stimuli or a noxious response to normally non-painful stimulation [3].
Poorly managed postoperative pain can result in adverse consequences, including impaired quality of recovery, opioid dependence, PPSP, and increased medical costs [2]. Therefore it is important to examine if rebound pain may have a significant impact on other health-related outcomes [10].
Postoperative pain is one of the most feared surgical complications reported by patients, which is frequently followed by a painful recovery. Appropriate treatment of acute postoperative pain is associated with better clinical outcomes, while inadequate pain control may negatively impact patients' postoperative experience [4].
Some strategies used to prevent and manage rebound pain, like continuous PNB catheter techniques, using local anesthetic adjuvant, multimodal analgesics, and preoperative education and counselling regarding rebound pain were effective in preventing and managing rebound pain [2,11].
Due to the shortage of experimental and clinical studies, the incidence of the rebound pain phenomenon is still poorly documented. Nevertheless, its occurrence has been increasingly reported by researchers, and it could profoundly impact the patient's recovery experience [1].
The occurrence of rebound pain may outweigh the benefits of PNBs and represent a clinically relevant problem [2].
Rebound pain is still a poorly understood concept, and few studies have evaluated its full impact on the use of regional anesthesia as a strategy to reduce long-term pain and opioid consumption. Rebound pain is a common, yet under-recognized, acute increase in pain and severity after a peripheral nerve block (PNB) has receded, typically manifesting within 24 h after the block was performed. Despite economic pressure and the well-known early benefits of PNBs, rebound pain unanswered questions are one more challenge in the area of perioperative management. Therefore, this study aimed to assess incidence, severity, and factors associated with rebound pain after peripheral nerve block is resolved.
2. Methodology
Ethical clearance was obtained from the institutional ethical review committee. The aim of the study was explained to each study participant, and informed consent was obtained. This study was registered with the UIN research registry (8161) and was reported in accordance with STROCSS criteria [12].
2.1. Study design, study setting, and population
An institution-based, longitudinal cross-sectional study was conducted from August 20, 2021, to June 30, 2022, at the PACU, recovery room, and wards. In this study, we included both elective and emergency procedures in patients who underwent an operation under peripheral nerve block alone or in combination with general anesthesia in the study period. However, patients who were lost to follow-up and Patients with a primary block failure were excluded from the study.
2.2. Operational definitions
Pain- Pain is defined as An unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage.
Rebound pain-defined as transient acute postoperative pain within 12–24hrs that ensues following resolution of sensory blocked [6].
Rebound pain score-the lowest pain score during the first 12 h before the PNB wears off is subtracted from the highest pain score during the first 12 h after the PNB wears off [13].
2.3. Sample size determination
To determine the sample size, the single population proportion formula was used. Since there was no previous study done similar to this topic, we took a proportion of 50% by assuming a 95%CI with a 5% margin of error, and finally, the sample size for the study was calculated as:
2.4. Sampling method
Study participants will be selected using a consecutive sampling technique.
2.5. Data collection process
Before data collection, training was given to data collectors. The data collection procedures included chart review, interview, and direct measurement of the pain score after peripheral nerve block resolved using NRS within 24 h of the block performed. The questionnaire was prepared in the English language. The questionnaire includes sociodemographic variables, anesthesia, and surgical-related risk factors. To ensure the quality of data, pretesting of the data collection tool was conducted on 20 patients, or 5% of the study sample size. Data collectors were provided adequate information regarding the questionnaires. The data collectors was closely monitored by the principal investigator throughout the study period. The collected data were checked for completeness, accuracy, and clarity on the day of data collection before being entered into the database by the principal investigator. A total of two BSC anaesthetists participated in the data collection process.
2.6. Data analysis and interpretation
The data was entered and analyzed with SPSS version 20. Descriptive statistics were used to explain to the study participants about study variables and were presented as mean and standard deviation. Rebound pain score was approximately normal in sample distribution and means (95% confidence interval [CI]) were used to report for each variable subgroup. Univariate comparisons were analyzed by logistic regression for dichotomous outcomes. The linearity of the continuous variables concerning the logit of the dependent variable rebound pain was assessed. Univariate linear regression and multivariable analysis were performed to analyze the association of variables with the RPS.
3. Results
3.1. Sociodemographic characteristics
A total of 384 patients were included, with a mean age distribution of 30.8 ± 5.8. From a total of 384 patients, 237 (61.7%) patients developed rebound pain after resolution of peripheral nerve block with a mean rebound pain score of 4.19 ± 2.1. Most of the participants (70.8) were male (Table 1).
Table 1.
Cross-tabulation of sociodemographic characteristics of the study participants.(N = 384).
| Variables | Rebound pain frequency% |
Mean RPS (95%CI) | |||
|---|---|---|---|---|---|
| Overall |
Yes (n = 237) |
No (n = 147) |
|||
| Frequency | 237(61.7%) | 147(38.7%) | |||
| Gender: | Male | 272 | 174(64%) | 98(36%) | 4.19 ± 2.1[3.94,4.5] |
| Female | 112 | 63(56.2%) | 48(42.8%) | 4.08[3.79,4.36] | |
| BMI: | 18.5–24.5 | 263 | 157(59.7%) | 106(40.3%) | 4.49[3.85,5.09] |
| 24.6–29.5 | 84 | 72(85.7%) | 12(14.3%) | 3.69[3.39,4.03] | |
| 29.6–35 | 37 | 8(21.6%) | 29(78.4%) | 5.1[4.65,5.63] 6[5,6.85] | |
4. Preoperative risk factors
As the distribution of preoperative risk factors showed, all patients were on ASA I and ASAII, 376 (97.9%), and 8 (2.1%) respectively. The majority of patients who had no history of coexisting disease were 352 (91.6%). Preoperative pain was experienced by the majority of patients 237 (61.7%), with 119 (30.9%) experiencing severe pain and 194 (50.5%) receiving preoperative analgesia. Of the total 384,259 patients, 67.4% were premedicated with dexamethasone(Table 2).
Table 2.
Cross-tabulation of the preoperative factors for rebound pain after PNB resolved. (N = 384).
| Variables(n = 384) | Rebound pain frequency(%) |
Mean RPS (95%CI) |
|||
|---|---|---|---|---|---|
| Overall |
Yes n = 237 |
No = 102 |
|||
| Frequency | 237(61.7%) | 147(38.7%) | 4.19 ± 2.1[3.94,4.5] | ||
| Coexisting | Yes | 32 | 32(100%) | 0(0%) | 3.97[3.20,4.69] |
| No | 352 | 205(58.2%) | 147(41.8%) | 4.22[3.94,4.51] | |
| ASA | I | 376 | 229(60.9%) | 147(39.1%) | 4.17[3.89,4.44] |
| II | 8 | 8(100%) | 0(0%) | 4.62[3.33,6.0] | |
| Preoperative pain: | Yes | 237 | 213(89.9%) | 24(10.1%) | 4.43[4.15,4.71] |
| No | 147 | 69(46.9) | 78(53.1) | 2.0[1.69,2.33] | |
| Severity of preoperative pain: | Mild | 16 | 16(100%) | 0(0%) | 1.81[1.43,2.20] |
| Moderate | 147 | 117(79.6%) | 30(20.4%) | 4.86[4.53,5.21] | |
| Sever | 119 | 80(67.2%) | 39(32.8%) | 4.3[3.86,4.70] | |
| Preoperative analgesia: | Yes | 194 | 104(53.6%) | 90(46.4%) | 3.26[2.89,3.65] |
| No | 190 | 133(70%) | 57(30%) | 4.95[4.60,5.25] | |
| Dexamethasone premedication: | Yes | 259 | 120(46.3%) | 139(53.7%) | 3.15[2.78,3.45] |
| No | 125 | 117(93.6%) | 8(6.4%) | 5.35[5.04,5.66] | |
5. Intraoperative and postoperative risk factors
Among patients who underwent an operation, bone(orthopedics) surgeries were mostly procedures, 235 (61.2%) of those 149 (38.8%) operated under digital peripheral nerve block. Of the total 243 (63.3%) who took adjuvant during nerve block, of those, 160(41.7%) were lidocaine (Table 3).
Table 3.
Cross-tabulation of the intraoperative and postoperative factors and their association with rebound pain after PNB resolved. (N = 384).
| Variables | Rebound pain frequency(%) |
Mean RPS (95%CI) |
|||
|---|---|---|---|---|---|
| Overall |
Yes n = 237 |
No = 102 |
|||
| Frequency | 237(61.7%) | 147(38.7%) | 4.19 ± 2.1[3.94,4.5] | ||
| Type of surgery: | Soft tissue | 149 | 40(26.8%) | 109(73.2%) | 3.37[2.74,4.02] |
| Bone | 235 | 197(83.8%) | 38(16.2%) | 4.36[4.07.4.60] | |
| Surgical site: | Upper limb | 300 | 216(72%) | 84(28%) | 4.41[4.14,4.69] |
| Lower limb | 84 | 21(25%) | 63(75%) | 1.86[1.5,2.19] | |
| General Anesthesia: | Yes | 78 | 31(39.7%) | 47(60.3%) | 2.2[1.90,2.43] |
| No | 306 | 206(67.3%) | 100(32.7%) | 4.54[4.25,4.80] | |
| Type of PNB: | Interscalene | 61 | 61(100%) | 0(0%) | 5.24[4.72,5.72] |
| Supraclavicular | 90 | 90(100%) | 0(0%) | 4.39[4.0,4.77] | |
| Lumbar plexus | 42 | 5(11.9%) | 37(88.9%) | 1.6[0.5,2.5] | |
| Femoral compartment | 42 | 16(38.1%) | 26(61.9%) | 1.94[1.59,2.31] | |
| Digital peripheral NB | 149 | 65(43.6%) | 84(56.4%) | 3.71[3.24,4.23] | |
| Type of LA: Bupivacaine | 384 | 237(61.7%) | 147(38.3) | 4.19[3.92,4.45] | |
| Adjuvant used: | Yes | 243 | 108(44.4%) | 135(55.6%) | 2.64[2.39,2.90] |
| No | 141 | 129(91.5%) | 12(8.5%) | 5.55[5.27,5.84] | |
| Type of Adjuvant: | Opioid | 83 | 48(58.3%) | 35(42.2%) | 2.45[2.15,2.79] |
| Lidocaine | 160 | 60(37.5%) | 100(62.5%) | 2.8[2.4,3.21] | |
| Post-operative opioid: | Yes | 113 | 109(94.5%) | 4(5.5%) | 4.84[4.50,5.20] |
| No | 271 | 128(47.2%) | 143(52.8%) | 3.62[3.27,3.97] | |
| Post op NSAID: | Yes | 114 | 102(89.5%) | 12(10.5%) | 5.3[5,5.56] |
| No | 270 | 135(50%) | 135(50%) | 3.4[3.03,3.76] | |
| Post op paracetamol: | Yes | 8 | 8(100%) | 0(0%) | 5.63[4.86,6.4] |
| No | 368 | 221(60.1%) | 147(39.9%) | 4.3[3.98,4.52] | |
| Duration of surgery mean ± SD (2.87 ± 1.26): | ≤2.87 | 78 | 65(36.5%) | 113(63.5%) | 3.46[2.95,3.94] |
| >2.87 | 206 | 172(83.5%) | 34(16.5%) | 4.49[4.22,4.80] | |
| Duration of motor block mean ± SD (5.81 ± 1.54): | ≤5.81 | 66 | 136(81.9%) | 30(18.1%) | 3.9[3.53,4.29] |
| >5.81 | 218 | 101(46.3%) | 117(53.7%) | 4.58[4.21,4.98] | |
| Duration of sensory block mean ± SD (9.54 ± 2.9): | ≤9.54 | 08 | 167(80.3%) | 41(19.7%) | 4.13[3.84,4.44] |
| >9.54 | 176 | 70(39.8%) | 106(60.2%) | 4.34[3.84,4.84] | |
| The volume of LA mean ± SD (34.4 ± 7.8): | >34.4 | 44 | 69(47.9%) | 75(52.1%) | 2.92[2.46,3.37] |
| ≤34.4 | 240 | 168(70%) | 72(30%) | 4.74[4.45,5.05] | |
| Dose of LA mean ± SD (85.5 ± 19.7): | >85.5 | 161 | 86(53.4%) | 75(46.6%) | 2.94[2.57,3.32] |
| ≤85.5 | 223 | 151(67.7%) | 72(32.3%) | 4.92[2.46,3.37] | |
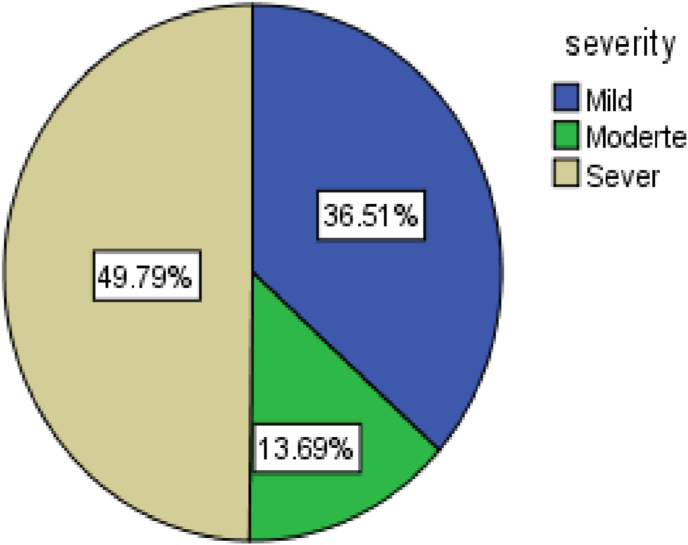
| The severity of Rebound pain | |||||
| Mild | 88(36.5%) | 2.03[1.87,2.26] | |||
| Moderate | 33(13.7%) | 4.21[3.82,4.62] | |||
| Sever | 120(49.8%) | 5.8[5.55,6.06] | |||
6. Magnitude of rebound pain after PNB resolved
The magnitude of rebound pain after resolution of peripheral nerve block was 237 (61.7%) (95%CI: 56.5–66.7)with a mean rebound pain score of 4.19 ± 2. Of the total 237, 120(50.6%) had severe rebound pain after PNB resolved(Fig. 1).
Fig. 1.
Severity of rebound pain after rebound pain resolved.(N = 384).
7. Factors associated with rebound pain after peripheral nerve block resolved
In the univariate logistic regression analysis, age and sex of the participants, having coexisting, having preoperative pain, premedication with dexamethasone, preoperative analgesia is given, type and site of surgery, having supraclavicular nerve block, use of an adjuvant, duration of surgery, the volume of local anaesthetics, and use of postoperative opioids and NSAIDs were significant at p-value <0.2. However, having preoperative pain, premedication with dexamethasone, type of surgery, use of an adjuvant, and use of postoperative opioids and NSAIDs were significantly associated with rebound pain in multivariable analysis at which p-value was <0.05. Participants who did not receive preoperative analgesia were 3.8 times (AOR: 3.8, 95%CI: 19.9–23.1), more likely to develop rebound pain when compared to those who had received preoperative analgesia. Similarly, those patients having preoperative pain were 3.9 times (AOR: 3.9, 95%CI: 41–57.4) more likely to develop rebound pain when compared to those who had no preoperative pain. Those patients premedicated with dexamethasone were 2.6 times(AOR: 2.6, 95%CI: 20.29–24.57), less likely to develop rebound pain compared to those not premeditated. Patients who underwent bone surgery 6.5times (AOR: 6.5, 95%CI: 1.45–11.7), were more likely to develop rebound pain compared to those who underwent soft tissue surgery. use of adjuvant for peripheral nerve block 0.4 times(AOR: 0.4, 95%CI: 18.37–19.9), less likely to develop rebound pain compared to nerve block without adjuvant. Patients who take postoperative opioids and NSAIDs were less likely to develop rebound pain with AOR: 2.2, 95%CI: 19.1–22.56 and AOR:2.2,95%CI, 17.69–20.8 compared to those not take postoperative analgesia respectively (Table 4, Table 5, Table 6).
Table 4.
Univariate logistic regression analysis of patient characteristics and preoperative factors for association with incidence of rebound pain after PNB resolved. (N = 384).
| Variable | Reference group | OR | 95%CI | p-value |
|---|---|---|---|---|
| Age of participants | – | 0.28 | 0.74–0.183 | 0.001 |
| Sex of participants | Male | 0.85 | 0.197–1.01 | 0.18 |
| ASA physical status | One | 0.03 | −1.04–1.93 | 0.55 |
| Having co-existing disease | No | 3.7 | 2.22–5.21 | 0.002 |
| Having Preoperative pain | No | 6.86 | 5.91–7.815 | 0.01 |
| The severity of preoperative pain | ||||
| Mild | Mild | 0.01 | 2.3–6.1 | 0.52 |
| Moderate | 0.7 | 1.25–5.2 | 0.34 | |
| Sever | 2.339 | 0.96–3.7 | 0.01 | |
| Taking preoperative analgesia | Yes | 1.68 | 1.19–2.17 | 0.004 |
| Premedication with dexamethasone | Yes | 2.2 | 1.79–2.69 | 0.017 |
Table 5.
Univariate logistic regression analysis of patient characteristics and preoperative factors for association with incidence of rebound pain after PNB resolved. (N = 384).
| Variable | Reference group | OR | 95%CI | p-value |
|---|---|---|---|---|
| Type of surgery | Soft tissue | 0.17 | 0.29–1.69 | <0.001 |
| Site of surgery | Lower limb | 6.96 | 5.96–7.96 | 0.001 |
| General anesthesia | No | 0.4 | 1.69–3.06 | 0.74 |
| Type of PNB | Interscalene | 2.3 | 1.06–3.68 | 0.06 |
| Supraclavicular | 1.7 | 1.55–2 | <0.001 | |
| Lumbar plexus | 0.2 | 1.2–3.1 | 0.13 | |
| Femoral compartment | 1.2 | 0.25–1.2 | 0.62 | |
| Digital peripheral NB | 1.3 | 1.3–2.52 | 0.15 | |
| The dose of LA used | – | 0.42 | 0.038–0.67 | 0.27 |
| Adjuvant used | Yes | 0.69 | 2.52–3.29 | 0.01 |
| Type of adjuvant | Opioid | 0.12 | 0.17–0.86 | 0.28 |
| Duration of surgery | – | 0.13 | 0.017–0.45 | 0.035 |
| Duration of sensory block | – | 0.09 | −0.036–0.22 | 0.35 |
| Duration of motor block | – | 0.091 | −0.042–0.26 | 0.26 |
| Post-op opioid used | Yes | 1.22 | 0.71–1.73 | 0.002 |
| Post-operative NSAID used | No | −0.45 | −2.39- - 1.42 | 0.001 |
| Post-op PCM used | No | −0.122 | −2.88–0.68 | 0.61 |
| BMI | 18.5–24.5 | 0.2 | 0.31–1.33 | 0.002 |
| Dose category | Above mean | 0.45 | 0.128–1.79 | 0.024 |
| Volume category | Above mean | 0.399 | 1.289–2.35 | 0.022 |
| Duration of surgery | Below mean | 0.22 | 0.45–1.6 | <0.001 |
| Duration of sensory block | Above mean | 0.04 | 3.11–4.7 | 0.48 |
| Duration of motor block | Above mean | 0.27 | 0.14–1.2 | 0.34 |
Table 6.
Multivariable Logistic Regression with rebound pain after PNB resolved. (N = 384).
| Variables | Ref group | AOR | 95%CI | P-value |
|---|---|---|---|---|
| Having coexisting disease | No | 4.1 | 16.46–19.67 | 0.13 |
| Having preoperative pain | Yes | 3.9 | 41–57.4 | 0.01 |
| Premedication with dexamethasone | Yes | 2.6 | 20.29–24.57 | 0.008 |
| Pre-operative analgesia given | Yes | 3.8 | 19.9–23.1 | 0.01 |
| Type of surgery | Soft tissue | 6.5 | 1.45–11.7 | 0.04 |
| Adjuvant used | Yes | 0.4 | 18.37–19.9 | 0.01 |
| Duration of surgery | Below mean | 2.8 | 53.8–58.6 | 0.06 |
| Postoperative opioid used | Yes | 2.2 | 19.1–22.56 | 0.002 |
| Postoperative NSAID used | Yes | 2.2 | 17.69–20.8 | 0.001 |
8. Discussion
Rebound pain is a common, yet under-recognized, acute increase in pain severity after a peripheral nerve block (PNB) has receded, typically manifesting within 12–24 h after the block was performed and adversely affecting sleep quality [14]. The incidence of the rate of rebound pain could reach 40% of patients at PNB resolution [3].
The current paper was conducted to find out the magnitude, severity, and factors associated with rebound pain after resolution of peripheral nerve block. The overall incidence of rebound pain after peripheral nerve block was resolved was 61.7%(95% CI: 56.5–66.7) with a mean rebound pain score of 4.19 ± 2.1[95% CI: 3.94, 4.5].
A retrospective cohort study done in Canada showed that the incidence of rebound pain after PNB was resolved was 49.6%. This is relatively low when compared with current study. The possible explanation of this discrepancy could be the study design, sample size, and technique of peripheral nerve block [6]. In addition, a previous comparative study done in @New-York stated that a single injection had a higher risk of rebound pain compared to continuous peripheral nerve block [15]. However, in present work, all PNB done in a single injection could be the cause for higher incidence.
A prospective study carried out in Belgium found that the incidence of rebound pain after peripheral nerve block reached up to 40% [3]. Our finding is relatively higher than the above study. The possible explanation for the high magnitude of rebound pain in the current paper might be due to the small sample size and could be due to clinical set-up differences, and techniques of nerve block. In addition, in the present work all PNB was done under blind landmark and nerve stimulator technique. This might have caused mechanical and chemical nerve erosion/insult caused by PNB.
In the current paper, preoperative intravenous dexamethasone use was 2.6 times (AOR: 2.6, 95%CI: 20.29–24.57), less likely to develop rebound pain than those who had not taken intravenous dexamethasone. This present work is supported by previous studies in which using intravenous dexamethasone decreased the risk of rebound pain after PNB resolved or had a significant association with rebound pain [6]. Dexamethasone has been shown to prolong PNB duration when given perineurally compared with intravenously, although a recent systematic review showed that either route is equivalent in terms of duration of block analgesia, 24 h pain scores, and cumulative opioid consumption at 24 h postoperatively [16,17]. Dexamethasone at single doses greater than 0.1 mg/kg has been shown to reduce postoperative pain in a previous meta-analysis [18]. The reduction in rebound pain incidence and RPS found may be consistent with the known effect of iv dexamethasone on postoperative pain in general rather than any possible effect on PNB duration [6].
In the present study, those patients having preoperative pain were 3.9 times (AOR: 3.9, 95%CI: 41–57.4), more likely to develop rebound pain than those who had no preoperative pain. This might be supported by preoperative pain level was a significant predictor of severe postoperative pain in several studies across a variety of non-cardiac surgery [8,19]. Preoperative pain may be associated with rebound pain [[6], [7], [8],20]. This is also supported by the fact that patients with pre-existing joint pain who were more likely to report rebound pain following the use of PNB in total hip or knee arthroplasty [20].
In present work, patients who did not receive preoperative analgesia were 3.9 times (AOR: 3.9, 95%CI: 41–57.4) more likely to develop rebound pain when compared to those who had received preoperative analgesia. This might be explained by having preoperative analgesia as preemptive or preventive analgesia that decreases peripheral and central sensitization [21,22].
In the present work, patients who underwent bone surgery 6.5 times (AOR: 6.5, 95%CI: 1.45–11.7), were more likely to develop rebound pain compared to those who underwent soft tissue surgery. The current paper was supported by a previous study done in Canada that showed that patients having bone surgery had a significant association with rebound pain [6,8].
In the present work, the use of adjuvant for peripheral nerve block was 0.4 times(AOR: 0.4, 95%CI: 18.37 19.9), less likely to develop rebound pain compared to nerve block without adjuvant. This might be explained by a previous study [23] adding adjuvant on local anaesthetics in addition to prolonging the duration of analgesia, it helps to reduce overall dose requirements for local anaesthetics could decrease the incidence of rebound pain after PNB is resolved. In contradictory to a study done in Canada [24] in current paper, gender of the patients has no significant association with the occurrence of rebound pain. This finding supported by a previous study done in the Netherlands on predictors of postoperative pain [8].
In the present work, patients who received postoperative analgesia like opioids and NSAIDs were less likely to experience a rebound after PNB was resolved. This might be explained by using perioperative multimodal analgesia to decreases perioperative opioid use, which has an opioid sparing effect and also decrease the severity of postoperative pain [25,26].
9. Strength and limitation
The current paper's diversity of factors studied for association with rebound pain, potentially representing the largest single investigation on rebound pain, was considered the study's strength. Present work does not assess the specific time for which maximal rebound pain occurs, and the effect of continuous peripheral nerve block on rebound pain and severity is also not assessed.
10. Conclusion
The overall magnitude of rebound pain after PNB resolved was 61.7%, and patients having preoperative pain, premedication with dexamethasone, type of surgery, use of an adjuvant, use of postoperative opioids, and NSAIDs were independent factors associated with rebound pain.
Recommendations
Future research should look into the specific time of occurrence of rebound pain after PNB has been resolved, as well as the effect of continuous PNB on rebound pain.
Ethics approval and consent to participate
Ethical clearance was obtained from the institutional ethical review committee. The aim of the study was explained to each study participant and informed consent was obtained. Anyone not volunteering for participation was informed that they had full right not to participate or stop at any time.
Funding source
University of Gondar.
Authors’ contributions
This work was carried out in collaboration among all authors. B.M Admassie contributed to the conception, the review, and interpreted the result. B. A Tegegne, W.M Alemu, A.B Getahun in commenting from conception till manuscript preparation.
Registration of research studies
-
1.
Name of the registry: research registry
-
2.
Unique Identifying number or registration ID: 8161
-
3.
Hyperlink to your specific registration (must be publicly accessible and will be checked): https://www.researchregistry.com/browse-the-registry#home/
Guarantor
The Guarantor is the one or more people who accept full responsibility for the work and/or the conduct of the study, had access to the data, and controlled the decision to publish. uogbelete@gmail.com Phone number:+251945567123 p. o.box:196.
Consent for publication
Not applicable.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Availability of data and materials
All data generated or analyzed during this study were included in this published article and available on request.
Declaration of competing interest
The authors declared that they have no competing interests.
Acknowledgments
We would like to express special gratitude to the University of Gondar for appropriate funding as required. We would like also to thank you for our colleagues for giving us the chance to conduct this study, for constructive comments regarding the statistical issues of the study, and the whole process of the study being completed. Also we would like to thank study participants for their volunteer and data collectors.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.amsu.2022.104915.
Contributor Information
Belete Muluadam Admassie, Email: uogbelete@gmail.com.
Biresaw Ayen Tegegne, Email: ayenbiresaw@gmail.com.
Wudie Mekonnen Alemu, Email: Getahunmekonnen45@gmail.com.
Amare Belete Getahun, Email: amarebelete2218@gmail.com.
Acronyms and Abbreviation
- EC
@Ethiopian Calendar
- GC
@Gregorian calendar
- ISB
@ Interscalene block
- MI
@Myocardial ischemia
- NRS
@Numerical rating scale
- PCA
@Patient controlled analgesia
- PNB
@peripheral nerve block
- RP
@Rebound pain
- RPS
@Rebound pain score
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Brenner D., Mahony S.O., Butt I., Switzer T., Brien N.O., Mcnamara B., et al. vol. 32. 2012. p. 2012. (Characterization of Rebound Pain Following a Peripheral Nerve Block). 3. [Google Scholar]
- 2.Dada O., Zacarias A.G., Ongaigui C. 2019. Does Rebound Pain after Peripheral Nerve Block for Orthopedic Surgery Impact Postoperative Analgesia and Opioid Consumption ? A Narrative Review Does Rebound Pain after Peripheral Nerve Block for Orthopedic Surgery Impact Postoperative Analgesia and Opioid? (September) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lavand P. 2018. Rebound Pain after Regional Anesthesia in the Ambulatory Patient; pp. 679–684. [DOI] [PubMed] [Google Scholar]
- 4.Thillainadesan T., Ortho M.S., Lee C., Mandaleson A., Hardinge A., Melb M., et al. Rebound pain after shoulder surgery with interscalene brachial Plexus blockade : how often. How bad ? 2019;12(2):5914. [Google Scholar]
- 5.Dien, et al. vol. 23. 2013. pp. 1–7. (基因的改变NIH Public Access. Bone. 2008). Sheean et al. 2013. 1. [Google Scholar]
- 6.Barry G.S., Bailey J.G., Sardinha J., Brousseau P., Uppal V. Factors associated with rebound pain after peripheral nerve block for ambulatory surgery. Br. J. Anaesth. 2020;(March:1–10. doi: 10.1016/j.bja.2020.10.035. [Internet] [DOI] [PubMed] [Google Scholar]
- 7.Ojer M., Martí A., Briones Z., Sariñena M., Canet J. Risk factors for moderate-severe postoperative pain. Eur. J. Anaesthesiol. 2008;25(Sup 44):205. [Google Scholar]
- 8.Gramke H.F., De Rijke J.M., Kleef M Van, Kessels A.G.H., Peters M.L., Sommer M., et al. Predictive factors of postoperative pain after day-case surgery. Clin. J. Pain. 2009;25(6):455–460. doi: 10.1097/AJP.0b013e31819a6e34. [DOI] [PubMed] [Google Scholar]
- 9.Sort R., Brorson S., Gögenur I., Nielsen J.K., Møller A.M. Rebound pain following peripheral nerve block anesthesia in acute ankle fracture surgery: an exploratory pilot study. Acta Anaesthesiol. Scand. 2019;63(3):396–402. doi: 10.1111/aas.13290. [DOI] [PubMed] [Google Scholar]
- 10.Tan M., Law L.S.C., Gan T.J. Optimizing pain management to facilitate Enhanced Recovery after Surgery pathways. Can. J. Anesth. 2015;62(2):203–218. doi: 10.1007/s12630-014-0275-x. [DOI] [PubMed] [Google Scholar]
- 11.Muñoz-leyva F. 2020. ABOUT ART I CLE CATEGORY BROWSE ART I CLES AUT H OR IN for M at I O N Managing Rebound Pain after Regional Anesthesia PDF Links PDF Links (Korean) PubReader E- M Ail Print Share : Editorial Board Best Practice Open Access Review Article Clinical Researc. [Google Scholar]
- 12.Mathew G., Agha R., Group S. 2021;72(October. STROCSS 2021 : Strengthening the Reporting of Cohort , Cross-Sectional and Case-Control Studies in Surgery. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lavand P. 2018. Rebound Pain after Regional Anesthesia in the Ambulatory Patient. [DOI] [PubMed] [Google Scholar]
- 14.Revisão A.D.E., Nobre L.V., Cunha G.P., César P., Branco C., Takeda A., et al. ARTICLE IN PRESS Bloqueio de nervos periféricos e dor rebote : revisão de literatura. Brazilian J Anesthesiol [Internet] 2019;(xx):1–7. doi: 10.1016/j.bjan.2019.05.001. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Continuous Peripheral Nerve Anesthesia Reduces Rebound Pain. 2021. p. 2021. [Google Scholar]
- 16.Heesen M., Klimek M., Imberger G., Hoeks S.E., Rossaint R., Straube S. Co-administration of dexamethasone with peripheral nerve block : intravenous vs perineural application : systematic review , meta-analysis , meta-regression and trial-sequential analysis. Br. J. Anaesth. 2017:16. doi: 10.1016/j.bja.2017.11.062. [Internet] [DOI] [PubMed] [Google Scholar]
- 17.Hussain N, Langenbergh T Van Den, Sermer C. Equivalent analgesic effectiveness between perineural and intravenous dexamethasone as adjuvants for peripheral nerve blockade : a systematic review and meta-analysis ' quivalence de l ’ efficacite ' analge ' sique entre la dexame ' thasone ' e par voie p. Can J Anesth Can d’anesthésie. [DOI] [PubMed]
- 18.De Oliveira G.S., Almeida M.D., Benzon H.T., McCarthy R.J. Perioperative single dose systemic dexamethasone for postoperative pain: a meta-analysis of randomized controlled trials. Anesthesiology. 2011;115(3):575–588. doi: 10.1097/ALN.0b013e31822a24c2. [DOI] [PubMed] [Google Scholar]
- 19.Kalkman C.J., Visser K., Moen J., Bonsel G.J., Grobbee D.E., Moons K.G.M. Preoperative prediction of severe postoperative pain. Pain. 2003;105(3):415–423. doi: 10.1016/S0304-3959(03)00252-5. [DOI] [PubMed] [Google Scholar]
- 20.Williams B.A., Ibinson J.W., Mangione M.P., Modrak R.T., Tonarelli E.J., Rakesh H., et al. Research priorities regarding multimodal peripheral nerve blocks for postoperative analgesia and anesthesia based on hospital quality data extracted from over 1,300 cases (2011-2014) Pain Med. 2015;16(1):7–12. doi: 10.1111/pme.12609. [DOI] [PubMed] [Google Scholar]
- 21.Kaye A.D., Urman R.D. 2014. Preventive Analgesia for Postoperative Pain Control : a Broader Concept; pp. 17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu J., Li H., Zheng C., Wang B., Shen P., Xie Z., et al. 2019. Arthroplasty the Efficacy of Pre-emptive Analgesia on Pain Management in Total Knee Arthroplasty : a Mini-Review; pp. 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bachir Cherif1 M., Temmar2 N., Demmene Debbih1 A.T., Bouafia 1 M.T. 1 clinic of internal medicine and cardiology blida Algeria 2 center of cardiology ghardaia Algeria U of B 1. Copyright © 2015 wolters kluwer health, inc. All rights reserved. 503. J. Hypertens. 2015;33(7) e435–e435. [Google Scholar]
- 24.Barry G.S., Bailey J.G., Sardinha J., Brousseau P., Uppal V. Factors associated with rebound pain after peripheral nerve block for ambulatory surgery. Br J Anaesth [Internet] 2021;126(4):862–871. doi: 10.1016/j.bja.2020.10.035. Available from: [DOI] [PubMed] [Google Scholar]
- 25.Hinther A., Nakoneshny S.C., Chandarana S.P., Matthews T.W., Hart R., Schrag C., et al. 2021. Efficacy of Multimodal Analgesia for Postoperative Pain Management in Head and Neck Cancer Patients; pp. 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Epidemic O. Postoperative multimodal analgesia pain management with nonopioid analgesics and techniques. Review. 2017;152(7) doi: 10.1001/jamasurg.2017.0898. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study were included in this published article and available on request.