Abstract
BACKGROUND
Doppler studies of uteroplacental–fetal circulation have been proven useful in diagnosing fetal growth restriction, appropriately timing delivery, and improving perinatal morbidity and mortality. There has been an extensive search for the ideal means to identify fetuses between the compensatory and acidemic phase (ie, the “preacidemic phase”), and the aortic isthmus Doppler seems to show promise.
OBJECTIVE
This study aimed to investigate: (1) the prevalence of abnormal aortic isthmus Dopplers in a cohort of small-for-gestational-age fetuses and their correlation with other conventional Doppler abnormalities, and (2) the predictive ability of abnormal aortic isthmus Dopplers with regard to short-term adverse neonatal outcomes.
STUDY DESIGN
Fetuses diagnosed as small-for-gestational-age at ≥24 weeks’ gestation were included. Management was as per the standard protocol. Aortic isthmus Doppler was performed within a week of delivery with other conventional Dopplers. The adverse perinatal outcomes studied were: requirement of neonatal resuscitation at birth, Apgar score at 5 minutes <7, cord blood pH <7, presence of bronchopulmonary dysplasia, hypoxic-ischemic encephalopathy, grade III/IV intraventricular hemorrhage, necrotizing enterocolitis, sepsis, neonatal intensive care unit stay longer than 14 days, and stillbirth or neonatal death.
RESULTS
Among 121 small-for-gestational-age fetuses, 67 showed Doppler abnormalities in ≥1 vessels. The prevalence of abnormal aortic isthmus Doppler was 14.87%. Analysis was between group 1 with 103 normal aortic isthmus and group 2 with 18 abnormal aortic isthmus fetuses; 41 cases had some form of adverse perinatal outcome, the frequency of which was comparable between the groups. Abnormal aortic isthmus Doppler had a significant correlation with low cerebroplacental ratio, absent or reversed end-diastolic flow in the umbilical artery, and high pulsatility index in the ductus venosus. The positive likelihood ratio for predicting composite adverse perinatal outcome was 10.2 for absent or reversed end-diastolic flow in the umbilical artery, 9.6 for low cerebroplacental ratio, 2.28 for absent or retrograde flow in the aortic isthmus, and 2 for abnormal ductus venosus.
CONCLUSION
Predelivery abnormal aortic isthmus Dopplers performed worse than other conventional Dopplers in predicting abnormal perinatal outcomes.
Key words: aortic isthmus Doppler, clinical utility, fetal growth restriction, perinatal morbidity and mortality, small for gestational age
AJOG Global Reports at a Glance.
Why was this study conducted?
The study was conducted to evaluate the clinical utility of the aortic isthmus (AoI) in predicting perinatal outcome compared with other conventionally performed Doppler parameters. We also aimed to study the correlation of abnormal AoI Dopplers with other conventional Doppler abnormalities
Key findings
Predelivery abnormal AoI Dopplers performed worse than other conventional Dopplers in predicting abnormal perinatal outcomes.
What does this add to what is known?
Cerebral redistribution and absent or reversed end-diastolic flow in the umbilical artery are easily diagnosed, common, and have a good predictive value for adverse perinatal outcomes. Performing a technically challenging AoI Doppler may not add clinical benefit to the perinatal management of small-for-gestational-age fetuses or those with fetal growth restriction.
Introduction
A proportion of small-for-gestational-age (SGA) fetuses are categorized as cases of fetal growth restriction (FGR).1 Doppler studies of uteroplacental–fetal circulation have been proven useful in diagnosing FGR, appropriately timing the delivery, and improving perinatal morbidity and mortality.1 However, the prediction of perinatal outcomes in SGA/FGR fetuses is still far from ideal.
Since the 1990s, there has been an extensive search for the ideal means to identify fetuses between the compensatory and acidemic phase (the “preacidemic phase”). The aim is to balance the risks owing to extreme prematurity with the risks associated with severe asphyxia among preterm FGR fetuses, reduce morbidity and mortality, and improve perinatal outcomes.2, 3, 4, 5, 6, 7 In this regard, evaluation of aortic isthmus (AoI) Doppler has clearly shown some promise.8 However, decades of research have shown no additional clinical benefit of using AoI Doppler to predict perinatal morbidity or to time the delivery. Therefore, international recommendations have not mentioned AoI Dopplers as an essential part of fetal surveillance in SGA fetuses.1 Before incorporating any new Doppler parameter into surveillance pathways, it is important to establish how well it performs in predicting adverse perinatal outcomes relative to already existing, widely used Doppler parameters. Once this is established in observational studies, larger interventional studies would be needed to know when to use the new Doppler parameter as a threshold for delivery.
Objective
We performed AoI Doppler examinations of SGA fetuses close to delivery to compare clinical utility in predicting perinatal outcomes between AoI Doppler and other conventionally performed Doppler parameters. We also aimed to study the correlation of abnormal AoI Dopplers with other conventional Doppler abnormalities.
Materials and Methods
Study design and setting
This prospective observational cohort study was performed in a tertiary-care center from August 2017 to July 2019.
Participants
Individuals with singleton pregnancies were invited to participate at ≥24 weeks’ gestation when ultrasound-estimated fetal weight and/or abdominal circumference were <10th percentile for gestational age (GA), leading to the diagnosis of an SGA fetus. GA was determined using menstrual dating or first-trimester crown–rump length when menstrual dating was not appropriate. Multiple pregnancies and fetuses with known structural, chromosomal, or genetic abnormalities were excluded from the study.
The demographic profile and baseline pregnancy data were noted. The GE Voluson P8 and E8 ultrasound machines (GE Healthcare, Chicago, IL) with a curvilinear probe of 2 to 6 MHz were used for sonographic examination. All Doppler studies were performed by a single trained fetal medicine consultant (A.V.). When fetuses were diagnosed as SGA, amniotic fluid index, biophysical profile, and multivessel Doppler studies were performed. Doppler measurements were done in the absence of fetal movement or fetal breathing movement, with the mother's minimal respiratory effort. When the insonation angle was as close to 0 degrees as possible, the measurements were taken and 3 consecutive waveforms were used for analysis. The Doppler studies included the umbilical artery (UA) pulsatility index (PI) and end-diastolic flow, middle cerebral artery (MCA) PI, cerebroplacental ratio (CPR), ductus venosus (DV) PI/A-wave analysis, and the AoI Doppler waveforms. All the Doppler studies were stored for offline analysis.
Standard protocols were followed to capture and analyze the umbilical, MCA, and DV waveforms.9 AoI Doppler was measured using the longitudinal aortic arch view or the 3-vessel trachea view by the standard technique described.2 The position of the fetus decided the view that could be most practically obtained in a given fetus.2 The ease of assessment was also determined on the basis of the time needed to obtain a satisfactory waveform in the chosen view. We noted multiple parameters such as peak systolic velocity, end-diastolic velocity, time-averaged maximum velocity, resistance index, and isthmic flow index in the AoI. However, during analysis we chose the PI and direction of flow in diastole because they were the most studied parameters in published literature, and the simplest for potential clinical use.10 AoI PI is a continuous variable known to have a positive linear correlation with GA.10, 11, 12 Complex Doppler indices other than PI may help identify subtle or earlier changes in hemodynamics, whereas absent or retrograde flow during diastole suggests the possibility of cerebral hypoxia in a more compromised fetus.7 We decided to include a high PI (>95th centile) in abnormal AoI Doppler to mark the onset of altered hemodynamics, which may help predict perinatal outcomes and optimal timing of delivery.13, 14, 15
Two Doppler recordings, one at diagnosis and the other a week before delivery, were noted to identify Doppler parameters’ progression. The Doppler parameters within a week before delivery were considered for analysis. Doppler waveforms were considered abnormal when UA PI was >95th centile or had an absent or reversed end-diastolic flow (A/REDF), MCA PI <5th centile, CPR <1, DV PI >95th centile, or an absent or reversed A-wave, and AoI PI >95th centile or an absent or retrograde flow if observed during diastole.
Patients were divided into 2 groups based on the last AoI Doppler: group 1 with normal AoI Doppler (PI <95th centile) and group 2 with abnormal AoI Doppler. Group 2 included: (1) cases with antegrade flow (AoI PI ≥95th centile) but positive end-diastolic flow, and (2) cases with absent end-diastolic flow or retrograde diastolic flow in AoI. A similar qualitative definition of abnormal AoI has been used earlier by researchers,16 to which we have included PI >95th centile. Although the obstetrician managing the pregnancy was informed if AoI waveform showed predominantly retrograde flow in diastole at ≥32 weeks, the decision for delivery was taken by the treating obstetrician as per the standard protocol because this was an observational study, considering the overall clinical picture and GA, maternal condition, Dopplers, and biophysical profile, and not AoI Dopplers.
Study size
This was a time-bound study, and cases with SGA fetuses from 2017 to 2019 were selected.
Outcome measures
Primary perinatal outcomes studied were the requirement of neonatal resuscitation at birth, Apgar score at 5 minutes <7, cord blood pH <7.0,17 presence of bronchopulmonary dysplasia, hypoxic-ischemic encephalopathy, grade III/IV intraventricular hemorrhage, necrotizing enterocolitis, sepsis, neonatal intensive care unit (NICU) stay longer than 14 days, and stillbirth or neonatal mortality. The presence of any of the above was considered as an adverse perinatal outcome. Mode of delivery and neonatal birthweight (along with their centiles plotted against local reference values for GA) were also obtained.
Statistical analyses
Doppler variables and perinatal outcome parameters in normal and abnormal AoI Doppler groups were analyzed using an independent t-test and a chi-square test. The role of AoI Doppler and other abnormal Doppler parameters in predicting adverse perinatal outcomes was evaluated by calculating sensitivity, specificity, positive and negative predictive values, and likelihood ratios (LRs). SPSS for Windows, Version 15.0 (SPSS Inc, Chicago, IL) was used for statistical analysis. Receiver operating characteristic (ROC) curve analysis was performed for the prediction of adverse perinatal outcomes using quantitative variables. Statistical significance was assumed as a P value <.05.
Ethical approval
Institutional ethical committee approval was obtained (IEC: 459/2017), and written informed consent was taken from the participants before enrollment.
Results
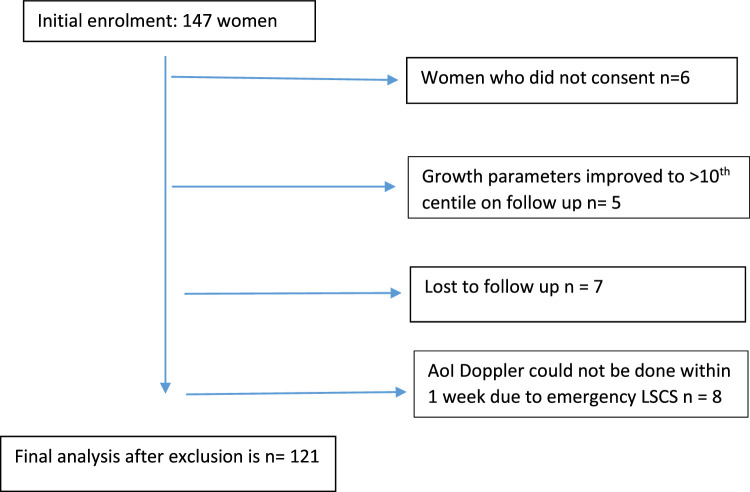
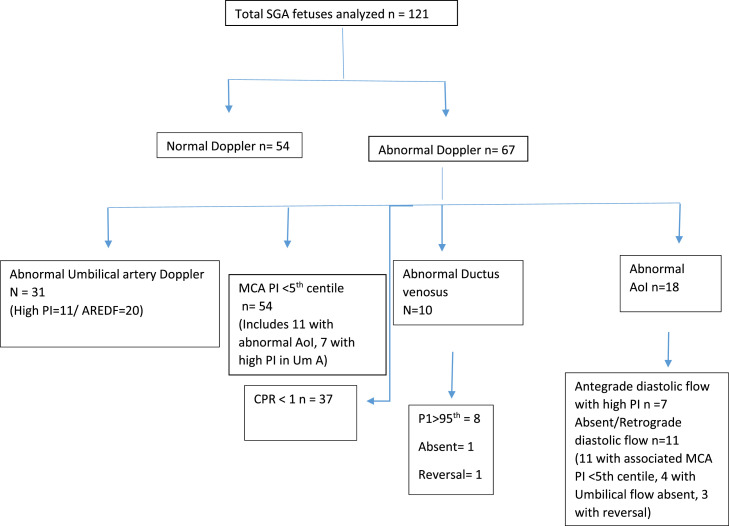
The recruitment of the study population is shown in Figure 1. Ultimately, a total of 121 SGA fetuses were recruited for AoI+ multivessel Doppler within a week before delivery and followed up until the perinatal period. Figure 2 describes the number of SGA fetuses showing various Doppler abnormalities. Among 121 SGA fetuses, 67 showed Doppler abnormalities, with many fetuses showing abnormal Dopplers in >1 vessel. Although this cohort of SGA fetuses were predominantly cases of early-onset SGA (mean GA at diagnosis of 30–31 weeks), cerebral redistribution (MCA PI <5th centile and/or CPR <1) was evident in 54 (44.6%) and UA Doppler abnormality in 31 (25.61%) fetuses. Hence, cerebral distribution in some form seems to be the most common Doppler abnormality, even among early-onset FGR fetuses.
Figure 1.
Recruitment of study population
AoI, aortic isthmus; LSCD, lower-segment cesarean delivery. Vasudeva. Clinical utility of aortic isthmus Doppler. Am J Obstet Gynecol Glob Rep 2022.
Figure 2.
Distribution of the study population
There was significant overlap of Doppler abnormalities among SGA fetuses, meaning that a single fetus showed >1 Doppler abnormality. AoI, aortic isthmus; A/REDF, absent or reversed end-diastolic flow; CPR, cerebroplacental ratio; MCA, middle cerebral artery; PI, pulsatility index; SGA, small-for-gestation-age; UA, umbilical artery. Vasudeva. Clinical utility of aortic isthmus Doppler. Am J Obstet Gynecol Glob Rep 2022.
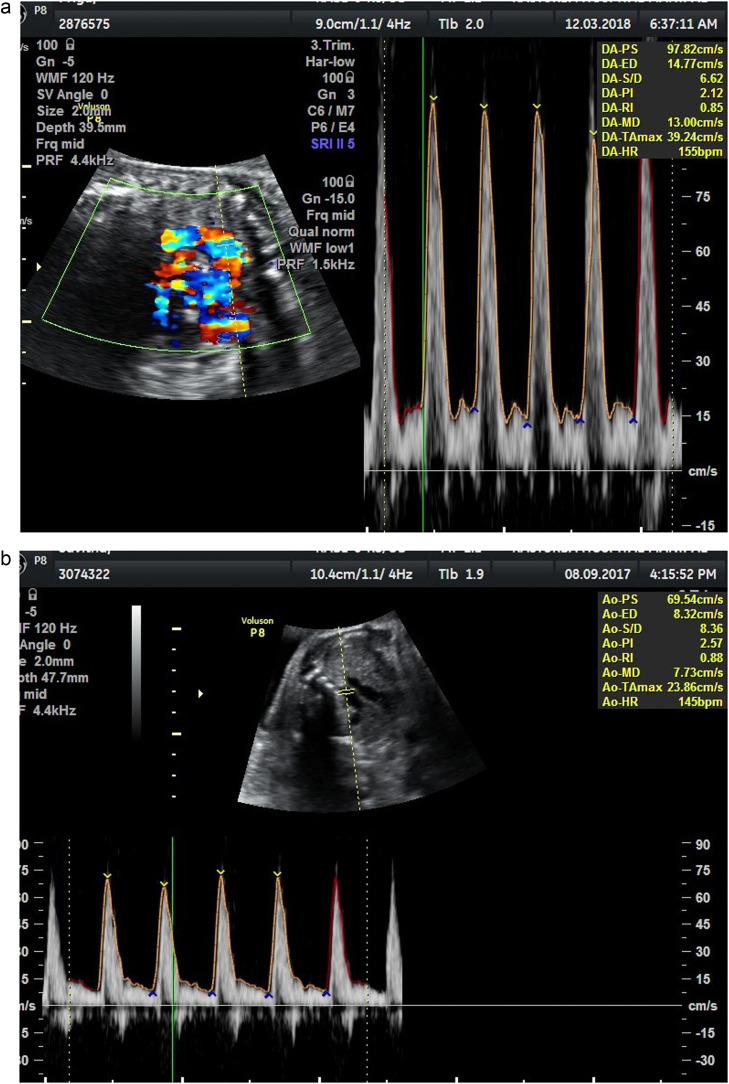
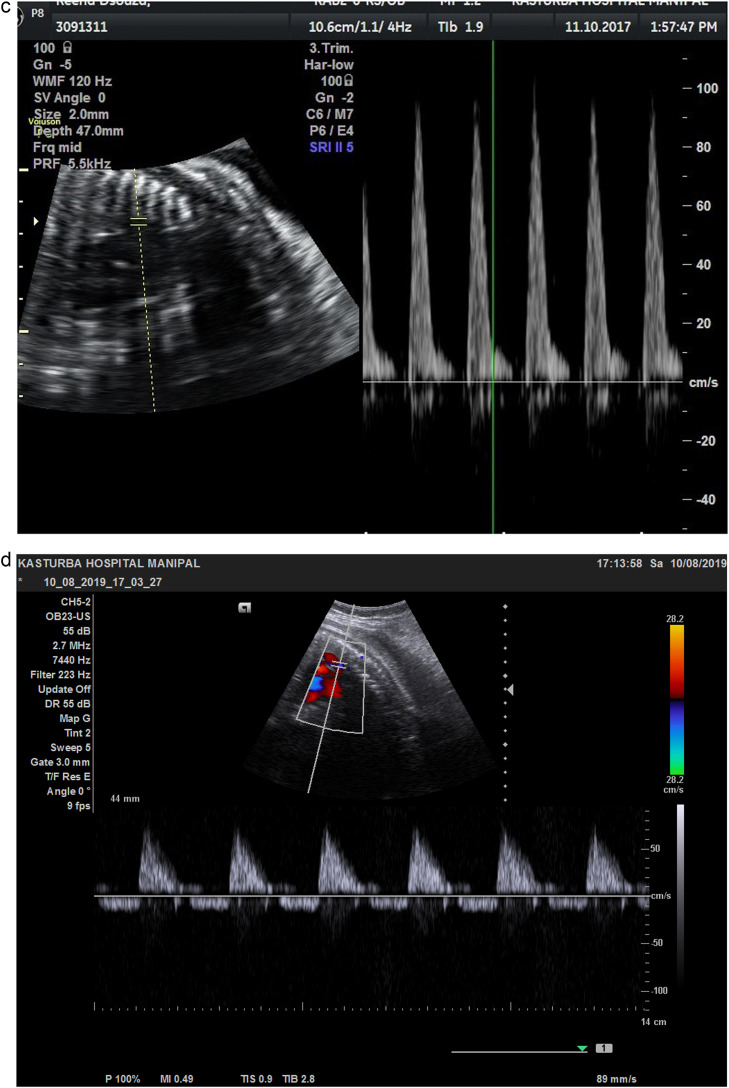
AoI waveforms were obtained in 3-vessel trachea view from 93 (76.85%) patients, taking an average of 1.17±0.92 minutes, and in longitudinal aortic arch view from 28 (23.14%) patients, taking an average of 1.54±1.06 minutes. Therefore, we found the 3-vessel trachea view easier to obtain and less time-consuming. Published literature suggests no significant differences between the reference ranges for these 2 sonographic views.15,18, 19, 20 We could obtain AoI Doppler waveforms in all fetuses. There were 103 patients with normal AoI Doppler (Figure 3, A), belonging to group 1, and 18 patients with abnormal AoI Doppler, belonging to group 2. In group 2, there were 7 fetuses with AoI PI ≥95th centile but antegrade flow during diastole (Figure 3, B), 3 fetuses with absent end-diastolic flow (Figure 3, C), and 8 fetuses with retrograde flow during diastole (Figure 3, D). Further analysis of our data follows these 2 groups because we aimed to know the significance of abnormal AoI Dopplers among SGA fetuses.
Figure 3.
Aortic Isthmus Doppler waveforms: a) Good diastolic flow b) reduced diastolic flow c) absent diastolic low d) retrograde diastolic flow
Vasudeva. Clinical utility of aortic isthmus Doppler. Am J Obstet Gynecol Glob Rep 2022.
Table 1 shows the demographic data and obstetrical profiles of women in the 2 groups. Although GA at recruitment was similar between the 2 groups, women in group 2 delivered at an earlier GA with a lower mean birthweight compared with women in group 1, with the differences being statistically significant (P<.05). More women in group 2 had preeclampsia or gestational hypertension. A higher number of them required cesarean delivery, but these differences were statistically insignificant.
Table 1.
Demographic data and obstetrical profiles in normal and abnormal aortic isthmus Doppler groups (N=121)
| Parameters | Group 1aNormal AoI Doppler N =103 | Group 2aAbnormal AoI Doppler N=18 | P valueb |
|---|---|---|---|
| Age | 28.51±4.09 | 29.28±3.4 | .45 |
| BMI | 23.73±3.48 | 24.36±3.63 | .48 |
| Gestation at diagnosis | 31.19±3.4 | 30.4±3.7 | .40 |
| Gestation at delivery | 35.4±2.8 | 33.5±3.3 | .01 |
| Gestational hypertension | 13 (12.6) | 4 (22.2) | .27 |
| Preeclampsia | 06 (5.8) | 2 (11.1) | .40 |
| Apgar score <7 at 5 min | 0 | 1 (11.1) | — |
| Spontaneous vaginal delivery | 14 (13.6) | 0 | — |
| Induced vaginal delivery | 10 (9.7) | 2 (11.1) | .85 |
| Cesarean delivery | 79 (76.7) | 16 (88.9) | .24 |
| Birthweight | 1961.1±577.1c | 1608±715.1c | .02 |
Demographic data were compared using an independent t-test and obstetrical data using a chi-square test.
AoI, aortic isthmus; BMI, body mass index.
Values shown as mean±standard deviation and as numbers and percentages
P value <.05 was significant
Values shown as mean±standard deviation.
Vasudeva. Clinical utility of aortic isthmus Doppler. Am J Obstet Gynecol Glob Rep 2022.
Table 2 shows the adverse perinatal outcomes in the 2 groups. Among 121 deliveries, 41 cases had some form of adverse perinatal outcome. However, none of the adverse outcome parameters were found to be more common in group 2. These results remained the same among preacidotic fetuses after excluding the 10 cases with DV abnormality. Because there were only 8 fetuses with retrograde diastolic flow in AoI, it was not possible to analyze them separately to draw any meaningful conclusion on perinatal outcome. Hence, there was no significant association between abnormal AoI Doppler and adverse perinatal outcome in SGA fetuses in this study.
Table 2.
Adverse perinatal outcomes in normal and abnormal aortic isthmus Doppler groups (N=121)
| Perinatal outcome | Group 1a Normal AoI Doppler N=103 | Group 2a Abnormal AoI Doppler N=18 | P valueb |
|---|---|---|---|
| Stillbirth | 1 (1) | 1 (5.6) | .15 |
| Neonatal death | 2 (1.9) | 1 (5.6) | .36 |
| NICU stay >14 d | 31(25.6) | 6 (33.9) | .40 |
| Intraventricular hemorrhage | 1 (1) | 0 (0) | — |
| Bronchopulmonary dysplasia | 0 (0) | 0 (0) | — |
| Respiratory distress syndrome | 20 (19.4) | 4 (22.2) | .78 |
| Necrotizing enterocolitis | 2 (1.9) | 0 (0) | — |
| Sepsis | 9 (8.7) | 0 (0) | — |
| Need of resuscitation at birth | 18 (17.5) | 2 (11.1) | .50 |
| Cord blood pH <7 | 0 (0) | 1 (5.6) | — |
| Apgar score at 5 min <7 | 0 (0) | 1 (5.6) | — |
| Composite adverse outcome (any one of the above adverse outcomes) | 35 (34) | 6 (33.3) | — |
Perinatal outcome parameters compared using chi-square test.
AoI, aortic isthmus; NICU, neonatal intensive care unit.
Values shown as numbers and percentages
P value <.05 was significant.
Vasudeva. Clinical utility of aortic isthmus Doppler. Am J Obstet Gynecol Glob Rep 2022.
There were 20 SGA fetuses with only MCA Doppler abnormality, among whom 2 had abnormal AoI Doppler, and we observed that both of these fetuses had a normal perinatal outcome.
Table 3 shows a significant association between abnormal Doppler waveforms of AoI and other blood vessels of fetoplacental circulation. Specifically, in the abnormal AoI Doppler group, there was a significantly higher number of fetuses with A/REDF in the UA, CPR <1, and DV PI >95th centile.
Table 3.
Other Doppler parameters in normal and abnormal aortic isthmus Doppler groups (N=121)
| Parameters | Group 1aNormal AoI Doppler (N = 103) n (%) | Group 2aAbnormal AoI Doppler (N = 18) n (%) | P valueb |
|---|---|---|---|
| UA pulsatility index >95th centile | 8 (7.7) | 3 (16.6) | .22 |
| UA absent or reversed end-diastolic velocity | 13 (12.6) | 7 (38.9) | .005 |
| Middle cerebral artery pulsatility index <5th centile | 43 (41.7) | 11 (61.1) | .12 |
| Cerebroplacental ratio <1 | 27 (26.2) | 10 (55.5) | .01 |
| Ductus venosus pulsatility index >95th centile | 4 (3.8) | 4 (22.2) | .003 |
| Ductus venosus absent or reversed A-wave | 1 (0.97) | 1 (5.5) | 0.15 |
Doppler parameters compared using chi-square test.
AoI, aortic isthmus; UA, umbilical artery.
Values shown as numbers and percentages
P value <.05 was significant.
Vasudeva. Clinical utility of aortic isthmus Doppler. Am J Obstet Gynecol Glob Rep 2022.
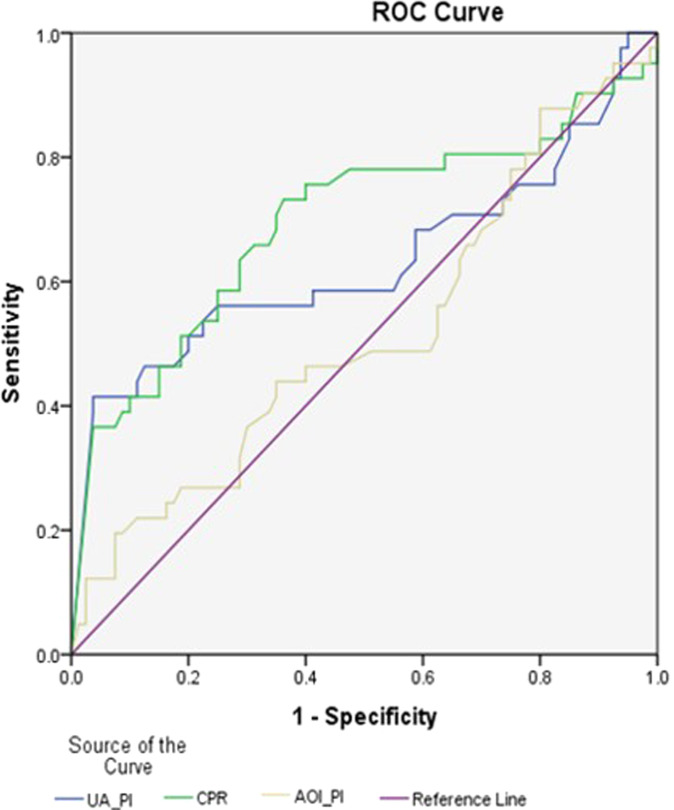
Table 4 describes how each of the Doppler abnormalities perform in predicting composite adverse perinatal outcomes. It was found that low CPR and A/REDF UA performed far better, with positive LRs of 9.6 and 10.26, respectively, as opposed to 1.56 for high PI in AoI, improving slightly to 2.28 for absent or retrograde diastolic flow in AoI. In our study, absent or retrograde flow in AoI performed nearly as well as absent or negative A-wave in DV in predicting adverse perinatal outcomes. Using ROC analysis (Figure 4), at a cutoff value of 0.5, we plotted ROC curves of abnormal AOI Doppler, abnormal UA Doppler, and low CPR to predict adverse perinatal outcomes. Sensitivity for predicting overall adverse perinatal outcome was 63.0% for abnormal UA, 59.5% for low CPR, and 51.6% for abnormal AoI Dopplers. This shows that performing AoI Doppler for SGA fetuses was not of additional benefit for predicting adverse perinatal outcomes in our study.
Table 4.
Prediction of composite adverse perinatal outcome using individual Doppler abnormality (n=41)
| Doppler parameters | Sensitivitya % | Specificitya % | Positive predictive valuea % | Negative predictive valuea % | Positive likelihood ratio % |
|---|---|---|---|---|---|
| AoI pulsatility index ≥95th centile | 4.8 | 93.75 | 28.5 | 65.7 | 0.048 |
| AoI absent end-diastolic flow or retrograde diastolic flow | 14.6 | 93.75 | 6.1 | 68.1 | 2.28 |
| Abnormal AoI (high PI or A/REDF) | 19.51 | 87.5 | 44.4 | 67.9 | 1.56 |
| UA pulsatility index >95th centile | 7.3 | 90 | 27.3 | 65.5 | 0.73 |
| UA absent or reversed end-diastolic velocity | 39 | 96.2 | 84.2 | 75.5 | 10.26 |
| Middle cerebral artery pulsatility index <5th centile | 56.1 | 61.3 | 42.6 | 73.1 | 1.44 |
| Ductus venosus pulsatility index >95th centile | 9.8 | 95 | 50 | 67.3 | 1.96 |
| Ductus venosus absent or reversed A-wave | 2.4 | 98.8 | 50 | 66.4 | 2.0 |
| Cerebroplacental ratio <1 | 36.6 | 96.2 | 83.3 | 74.8 | 9.6 |
Composite adverse perinatal outcome compared using chi-square test.
AoI, aortic isthmus; A/REDF, absent or reversed end-diastolic flow; PI, pulsatility index; UA, umbilical artery.
Values shown as percentages.
Vasudeva. Clinical utility of aortic isthmus Doppler. Am J Obstet Gynecol Glob Rep 2022.
Figure 4.
ROC curves of AoI PI, UA PI, and CPR for prediction of adverse perinatal outcomes. AoI, aortic isthmus; CPR, cerebroplacental ratio; PI, pulsatility index; ROC, receiver operating characteristic; UA, umbilical artery
Vasudeva. Clinical utility of aortic isthmus Doppler. Am J Obstet Gynecol Glob Rep 2022.
Table 5 shows that absent flow in the UA and CPR <5th centile had significant area under the curve with adverse perinatal outcome. Table 6 shows the logistic regression curve that reiterates that UA Dopplers performed better.
Table 5.
Area under the curve and the P value in the receiver operating characteristic curve for the different Doppler measurements
| Parameters | Area under the curve | P value |
|---|---|---|
| MCA PI <5th centile | 0.587 | .119 |
| High UA PI | 0.487 | .810 |
| Absent UA | 0.658 | .004 |
| Reversal UA | 0.530 | .586 |
| CPR <5th centile | 0.656 | .005 |
| Abnormal DV | 0.524 | .669 |
| Abnormal AoI | 0.535 | .529 |
AoI, aortic isthmus; CPR, cerebroplacental ratio; DV, ductus venosus; MCA, middle cerebral artery; PI, pulsatility index; UA, umbilical artery.
Vasudeva. Clinical utility of aortic isthmus Doppler. Am J Obstet Gynecol Glob Rep 2022.
Table 6.
Multiple logistic regression for the different Doppler measurements
| Parameters | B value adjusted odds ratio with 95% CI | P value |
|---|---|---|
| MCA PI <5th centile | −0.005 (−0.205 to 0.196) | .963 |
| High UA PI | 0.032 (−0.257 to 0.321) | .826 |
| Absent UA | 0.624 (0.327–0.920) | .000 |
| Reversal UA | 0.514 (0.015–1.012) | .043 |
| CPR <5th centile | 0.040 (−0.231 to 0.311) | .771 |
| Abnormal DV | −0.051 (−0.391 to 0.290) | .769 |
| Abnormal AoI | −0.027 (−0.266 to 0.212) | .824 |
AoI, aortic isthmus; CI, confidence interval; CPR, cerebroplacental ratio; DV, ductus venosus; MCA, middle cerebral artery; PI, pulsatility index; UA, umbilical artery.
Vasudeva. Clinical utility of aortic isthmus Doppler. Am J Obstet Gynecol Glob Rep 2022.
Discussion
This study attempted to investigate the clinical utility of AoI Doppler in a cohort of SGA fetuses, how well it correlates with other Doppler abnormalities, and how well it predicts poor perinatal outcomes.
AoI is a unique segment of the aorta just distal to the origin of the left subclavian artery that branches up to the area where the ductus arteriosus joins the descending part of the aorta. This area's uniqueness is that it reflects the resistance occurring in the cerebral, systemic, and placental vasculature.2,3 This watershed area is being explored to study hemodynamic alterations in fetal circulation.
Physiologically, only forward flow is seen in the whole of the cardiac cycle. In case of FGR when there is very high resistance in the placental vasculature and low resistance in cerebral circulation, we can expect a gradual decrease and absent or reversed diastolic flow over time, which would also manifest as a gradual decrease in oxygen saturation of blood reaching the cerebral circulation.2,21 AoI PI is shown to be higher in FGR fetuses and SGA fetuses with normal UA Doppler near term.22,23
Principal findings
In this cohort of SGA fetuses, evidence of cerebral redistribution (low CPR and/or low MCA PI) was the most common Doppler abnormality observed among 54 (44%) SGA fetuses, followed by UA abnormality in 31 (25%), abnormal AoI in 18 (14.8%), and DV abnormality in 10 (8.2%) fetuses. Similar Doppler changes have been observed in other studies.23 A large multicenter trial showed that only 5% of SGA fetuses showed abnormal AoI Doppler, whereas 46% showed abnormal UA and 27% showed abnormal MCA Dopplers.24 The sequence of Doppler abnormality is known to start from the UA, followed by MCA, AoI, and finally DV.6,10,14,25, 26, 27 A similar progression of Doppler changes has been demonstrated by Figueras et al,14 thus proposing AoI PI as a useful parameter to time the delivery before severe decompensation. However, Doppler changes may be skipped, and unconventional progressions are observed very often.24 In this study, we analyzed the Dopplers before delivery, and thus we were unable to comment on the progression pattern in our sample. However, the prevalence of predelivery Doppler changes seems to indirectly reflect this sequential deterioration, except that cerebral redistribution was the most common Doppler change in our SGA cohort.
In our study, fetuses with abnormal AoI Doppler were significantly more likely to have A/REDF in the UA, low CPR, and high DV PI. This association is well-established in the published literature.6,28,29 Hemodynamic changes leading to high placental resistance and low-resistance flow in cerebral circulation are responsible for low CPR and abnormal AoI Doppler; hence, the association between the two is well-established.28,30, 31, 32 It is essential to understand that performing AoI Doppler requires a much higher level of training and expertise than performing UA or MCA Dopplers. Thus, establishing cerebral redistribution in fetuses is much easier and more practical in clinical management protocols compared with performing technically challenging AoI Doppler. Conversely, Mariola et al could not demonstrate a significant association between retrograde flow in AoI and abnormal flow in other vessels.4 Some researchers have suggested that changes in AoI are earlier and more pronounced than changes in UA.29,30 High PI in UA is shown to be reflected by some change in the AoI.33 These findings were not observed in our study cohort; in fact, abnormal AoI Doppler was much less common and not significantly associated with early Doppler changes such as high PI in UA and low PI in MCA.
Our study failed to show an association between abnormal AoI Doppler waveforms and short-term adverse perinatal outcomes in a cohort of SGA fetuses. Published literature is inconclusive on the association between abnormal AoI Doppler and short-term adverse perinatal outcome among SGA/FGR fetuses.4, 5, 6,27,34, 35, 36 In a large multicenter study (PORTO trial) involving >1000 FGR fetuses, UA+MCA Dopplers remained the most useful tools, and additional tools, including AoI Doppler, did not contribute significantly in predicting adverse perinatal outcomes.24 A large proportion of their sample included cases of late FGR, whereas the mean period of gestation at diagnosis in our cohort was 30 to 31 weeks.
Conversely, several prospective studies have shown AoI to be a good predictor of poor perinatal outcomes.6,27,34 The perinatal outcome depends on many factors, which are interdependent, thus making it difficult to assess the impact of an individual predictive parameter. The single best predictor of neonatal outcome has been the GA. Although GA at recruitment was comparable in our study, hemodynamic deterioration was faster in group 2, with abnormal AoI Dopplers leading to earlier GA at delivery and significantly lower birthweights. However, in the studies that showed that AoI Doppler significantly predicted poor perinatal outcome, either the sample size was small,18 sepsis was the only positively correlated outcome,18 or the GA at recruitment or delivery (along with respective birthweights) was vastly different (much lower) in the retrograde AoI groups, which might have acted as confounding factor responsible for poor perinatal outcome.6,27 In these studies, the most frequent adverse neonatal outcomes were mainly prolonged NICU stay and high perinatal mortality owing to extreme prematurity and very low birthweight.6 In one of the studies showing a positive correlation, there were no FGR fetuses with retrograde diastolic flow in AoI. The mean GA at recruitment of FGR fetuses was 36 weeks (wide range of 26–40 weeks), and all were delivered at >35 weeks, which makes it difficult to compare results between the studies.34 We used simple parameters in AoI Dopplers, whereas other researchers have used technically challenging semiquantitative methods such as isthmic flow index and several other grading systems.6,10
Although a positive association has been demonstrated between abnormal AoI Doppler and perinatal mortality, subsequent multivariate analyses37,38 have shown this association to be statistically insignificant when considered independently. Abnormal cardiac function and reversal of A-wave in DV have been shown to predict perinatal mortality reasonably well in very preterm FGR fetuses, and the addition of AoI Doppler was not found beneficial in this model.37 Our sample size was insufficient to comment on such association with perinatal mortality.
With regard to the role of individual Doppler abnormalities in predicting adverse outcomes, A/REDF in UA and low CPR showed the highest positive LR (close to 10) for the adverse perinatal outcome, and were found in 20 and 37 fetuses, respectively. The positive LR for absent or retrograde diastolic flow in AoI was 2.28, close to that of abnormal DV. Thus, in our cohort of SGA fetuses, low CPR and A/REDF in UA were more commonly observed, and were more predictive of adverse perinatal outcomes than abnormal AoI Doppler. Despite the lack of conclusive association between abnormal AoI Dopplers and poor immediate perinatal outcomes, retrograde AoI flow patterns are proven to be predictive of abnormal brain imaging in neonates and long-term neurodevelopmental issues in childhood.18,22 We are yet to follow up our neonates into their childhood.
Limitations
A small number of fetuses had abnormal AoI Doppler, specifically those with retrograde diastolic flow. Some of the critical adverse outcomes occurred rarely, which hindered comparison among the groups. We included all SGA fetuses with or without Doppler abnormality at recruitment. We did not study the long-term neurologic outcomes of these neonates. Finally, our observational study design did not incorporate AoI Dopplers into the decision-making protocols. The delivery decision was based on the clinician's discretion, taking into account the overall clinical picture and the multivessel Doppler parameters. Many fetuses with absent end-diastolic flow in the UA were delivered early (at or beyond 30–32 weeks), resulting in small numbers with retrograde flow in AoI Doppler. This observational design has been a limitation in most of the published literature with regard to AoI Dopplers.
Research implications and conclusion
Performing a technically challenging AoI Doppler examination does not add clinical benefit to the perinatal management of SGA and FGR fetuses. A large sample of fetuses with low CPR, A/REDF in UA, and normal DV Dopplers would be necessary to evaluate the clinical utility of AoI Dopplers in preacidotic fetuses. Importantly, it is essential to have uniform policies regarding GA at delivery to assess the clinical utility of AoI Dopplers.
Footnotes
The authors report no conflict of interest.
The authors report no funding for this study.
Consent was obtained from all patients before recruitment.
Cite this article as: Vasudeva A, Padavagodu Shivananda R, Shree Belathur Shashidar D, et al. Clinical utility of aortic isthmus Doppler in the prediction of perinatal outcomes. Am J Obstet Gynecol Glob Rep 2022;2:100102.
References
- 1.Lees CC, Stampalija T, Baschat A, et al. ISUOG Practice Guidelines: diagnosis and management of small-for-gestational-age fetus and fetal growth restriction. Ultrasound Obstet Gynecol. 2020;56:298–312. doi: 10.1002/uog.22134. [DOI] [PubMed] [Google Scholar]
- 2.Acharya G. Technical aspects of aortic isthmus Doppler velocimetry in human fetuses. Ultrasound Obstet Gynecol. 2009;33:628–633. doi: 10.1002/uog.6406. [DOI] [PubMed] [Google Scholar]
- 3.Fouron JC. The unrecognized physiological and clinical significance of the fetal aortic isthmus. Ultrasound Obstet Gynecol. 2003;22:441–447. doi: 10.1002/uog.911. [DOI] [PubMed] [Google Scholar]
- 4.Ropacka-Lesiak M, Świder-Musielak J, Wójcicka M, Hamid A, Breborowicz GH. Retrograde diastolic blood flow in the aortic isthmus is not a simple marker of abnormal fetal outcome in pregnancy complicated by IUGR–a pilot study. Ginekol Pol. 2014;85:509–515. doi: 10.17772/gp/1762. [DOI] [PubMed] [Google Scholar]
- 5.Villalaín C, Herraiz I, Quezada MS, et al. Prognostic value of the aortic isthmus Doppler assessment on late onset fetal growth restriction. J Perinat Med. 2019;47:212–217. doi: 10.1515/jpm-2018-0185. [DOI] [PubMed] [Google Scholar]
- 6.Del Río M, Martínez JM, Figueras F, et al. Doppler assessment of the aortic isthmus and perinatal outcome in preterm fetuses with severe intrauterine growth restriction. Ultrasound Obstet Gynecol. 2008;31:41–47. doi: 10.1002/uog.5237. [DOI] [PubMed] [Google Scholar]
- 7.Kennelly MM, Farah N, Hogan J, Reilly A, Turner MJ, Stuart B. Longitudinal study of aortic isthmus Doppler in appropriately grown and small-for-gestational-age fetuses with normal and abnormal umbilical artery Doppler. Ultrasound Obstet Gynecol. 2012;39:414–420. doi: 10.1002/uog.9076. [DOI] [PubMed] [Google Scholar]
- 8.Eronen M, Kari A, Pesonen E, Kaaja R, Wallgren EI, Hallman M. Value of absent or retrograde end-diastolic flow in fetal aorta and umbilical artery as a predictor of perinatal outcome in pregnancy-induced hypertension. Acta Paediatr. 1993;82:919–924. doi: 10.1111/j.1651-2227.1993.tb12600.x. [DOI] [PubMed] [Google Scholar]
- 9.Nicolaides, K.H., & Rizzo, G. (2004). Placental and Fetal Doppler (1st ed.). CRC Press. 10.1201/9780367804329 [DOI]
- 10.Ruskamp J, Fouron JC, Gosselin J, Raboisson MJ, Infante-Rivard C, Proulx F. Reference values for an index of fetal aortic isthmus blood flow during the second half of pregnancy. Ultrasound Obstet Gynecol. 2003;21:441–444. doi: 10.1002/uog.105. [DOI] [PubMed] [Google Scholar]
- 11.Del Río M, Martínez JM, Figueras F, et al. Reference ranges for Doppler parameters of the fetal aortic isthmus during the second half of pregnancy. Ultrasound Obstet Gynecol. 2006;28:71–76. doi: 10.1002/uog.2827. [DOI] [PubMed] [Google Scholar]
- 12.Gámez F, Rodríguez MJ, Tenías JM, et al. Reference ranges for the pulsatility index of the fetal aortic isthmus in singleton and twin pregnancies. J Ultrasound Med. 2015;34:577–584. doi: 10.7863/ultra.34.4.577. [DOI] [PubMed] [Google Scholar]
- 13.Fouron JC, Gosselin J, Raboisson MJ, et al. The relationship between an aortic isthmus blood flow velocity index and the postnatal neurodevelopmental status of fetuses with placental circulatory insufficiency. Am J Obstet Gynecol. 2005;192:497–503. doi: 10.1016/j.ajog.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 14.Figueras F, Benavides A, Del Rio M, et al. Monitoring of fetuses with intrauterine growth restriction: longitudinal changes in ductus venosus and aortic isthmus flow. Ultrasound Obstet Gynecol. 2009;33:39–43. doi: 10.1002/uog.6278. [DOI] [PubMed] [Google Scholar]
- 15.Thanasuan S, Phithakwatchara N, Nawapan K. Reference values for fetal aortic isthmus blood flow parameters at 24 to 38weeks’ gestation. Prenat Diagn. 2014;34:241–245. doi: 10.1002/pd.4296. [DOI] [PubMed] [Google Scholar]
- 16.Rizzo G, Capponi A, Vendola M, Pietrolucci ME, Arduini D. Relationship between aortic isthmus and ductus venosus velocity waveforms in severe growth restricted fetuses. Prenat Diagn. 2008;28:1042–1047. doi: 10.1002/pd.2121. [DOI] [PubMed] [Google Scholar]
- 17.ACOG Committee on Obstetric Practice ACOG committee opinion no. 348, November 2006: umbilical cord blood gas and acid-base analysis. Obstet Gynecol. 2006;108:1319–1322. doi: 10.1097/00006250-200611000-00058. [DOI] [PubMed] [Google Scholar]
- 18.Cruz-Martinez R, Tenorio V, Padilla N, Crispi F, Figueras F, Gratacos E. Risk of ultrasound-detected neonatal brain abnormalities in intrauterine growth-restricted fetuses born between 28 and 34 weeks’ gestation: relationship with gestational age at birth and fetal Doppler parameters. Ultrasound Obstet Gynecol. 2015;46:452–459. doi: 10.1002/uog.14920. [DOI] [PubMed] [Google Scholar]
- 19.Rizzo G, Capponi A, Vendola M, Pietrolucci ME, Arduini D. Use of the 3-vessel view to record Doppler velocity waveforms from the aortic isthmus in normally grown and growth-restricted fetuses: comparison with the long aortic arch view. J Ultrasound Med. 2008;27:1617–1622. doi: 10.7863/jum.2008.27.11.1617. [DOI] [PubMed] [Google Scholar]
- 20.Hecher K, Snijders R, Campbell S, Nicolaides K. Fetal venous, arterial, and intracardiac blood flows in red blood cell isoimmunization. Obstet Gynecol. 1995;85:122–128. doi: 10.1016/0029-7844(94)00331-7. [DOI] [PubMed] [Google Scholar]
- 21.Mäkikallio K. Is it time to add aortic isthmus evaluation to the repertoire of Doppler investigations for placental insufficiency? Ultrasound Obstet Gynecol. 2008;31:6–9. doi: 10.1002/uog.5239. [DOI] [PubMed] [Google Scholar]
- 22.Ferraz MM, Araújo FDV, Carvalho PRN, Sá RAM. Aortic Isthmus Doppler Velocimetry in Fetuses with Intrauterine Growth Restriction: A Literature Review. Rev Bras Ginecol Obstet 2020;42:289–96. English. doi: 10.1055/s-0040-1710301. Epub 2020 May 29. Erratum in: Rev Bras Ginecol Obstet. 2020 Aug 26; PMID: 32483809. [DOI] [PMC free article] [PubMed]
- 23.Cruz-Martinez R, Figueras F, Benavides-Serralde A, Crispi F, Hernandez-Andrade E, Gratacos E. Sequence of changes in myocardial performance index in relation to aortic isthmus and ductus venosus Doppler in fetuses with early-onset intrauterine growth restriction. Ultrasound Obstet Gynecol. 2011;38:179–184. doi: 10.1002/uog.8903. [DOI] [PubMed] [Google Scholar]
- 24.Unterscheider J, Daly S, Geary MP, et al. Predictable progressive Doppler deterioration in IUGR: does it really exist? Am J Obstet Gynecol. 2013;209(539) doi: 10.1016/j.ajog.2013.08.039. :e1–7. [DOI] [PubMed] [Google Scholar]
- 25.Esercan A, Karakuş R, Seval A, Özgü Erdİnç AS. Aortic Isthmus Doppler For Fetal Assessment. Gynecol Obstet Reprod Med [Internet]. 2013 Dec. 30 [cited 2022Oct.3];19(3):197-202. Available from: https://gorm.com.tr/index.php/GORM/article/view/220.
- 26.Cruz-Martinez R, Figueras F, Hernandez-Andrade E, Oros D, Gratacos E. Changes in myocardial performance index and aortic isthmus and ductus venosus Doppler in term, small-for-gestational age fetuses with normal umbilical artery pulsatility index. Ultrasound Obstet Gynecol. 2011;38:400–405. doi: 10.1002/uog.8976. [DOI] [PubMed] [Google Scholar]
- 27.Abdelrazzaq K, Yeniel AÖ, Ergenoglu AM, Yildirim N, Akercan F, Karadadaş N. Fetal aortic isthmus Doppler measurements for prediction of perinatal morbidity and mortality associated with fetal growth restriction. Acta Obstet Gynecol Scand. 2013;92:656–661. doi: 10.1111/aogs.12070. [DOI] [PubMed] [Google Scholar]
- 28.Fouron JC, Skoll A, Sonesson SE, Pfizenmaier M, Jaeggi E, Lessard M. Relationship between flow through the fetal aortic isthmus and cerebral oxygenation during acute placental circulatory insufficiency in ovine fetuses. Am J Obstet Gynecol. 1999;181:1102–1107. doi: 10.1016/s0002-9378(99)70089-x. [DOI] [PubMed] [Google Scholar]
- 29.Benavides-Serralde A, Scheier M, Cruz-Martinez R, et al. Changes in central and peripheral circulation in intrauterine growth-restricted fetuses at different stages of umbilical artery flow deterioration: new fetal cardiac and brain parameters. Gynecol Obstet Investig. 2011;71:274–280. doi: 10.1159/000323548. [DOI] [PubMed] [Google Scholar]
- 30.Sonesson SE, Fouron JC. Doppler velocimetry of the aortic isthmus in human fetuses with abnormal velocity waveforms in the umbilical artery. Ultrasound Obstet Gynecol. 1997;10:107–111. doi: 10.1046/j.1469-0705.1997.10020107.x. [DOI] [PubMed] [Google Scholar]
- 31.Garcia-Canadilla P, Rudenick PA, Crispi F, et al. A computational model of the fetal circulation to quantify blood redistribution in intrauterine growth restriction. PLoS Comput Biol. 2014;10 doi: 10.1371/journal.pcbi.1003667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chabaneix J, Fouron JC, Sosa-Olavarria A, et al. Profiling left and right ventricular proportional output during fetal life with a novel systolic index in the aortic isthmus. Ultrasound Obstet Gynecol. 2014;44:176–181. doi: 10.1002/uog.13345. [DOI] [PubMed] [Google Scholar]
- 33.Bonnin P, Fouron JC, Teyssier G, Sonesson SE, Skoll A. Quantitative assessment of circulatory changes in the fetal aortic isthmus during progressive increase of resistance to umbilical blood flow. Circulation. 1993;88:216–222. doi: 10.1161/01.cir.88.1.216. [DOI] [PubMed] [Google Scholar]
- 34.Karakus R, Ozgu-Erdinc AS, Esercan A, Dogan MM. Doppler assessment of the aortic isthmus in intrauterine growth-restricted fetuses. Ultrasound Q. 2015;31:170–174. doi: 10.1097/RUQ.0000000000000126. [DOI] [PubMed] [Google Scholar]
- 35.Hidar S, Zaafouri R, Bouguizane S, et al. Prognostic value of fetal aortic isthmus Doppler waveform in intrauterine growth retardation: prospective longitudinal study. J Gynecol Obstet Biol Reprod (Paris) 2004;33:745–752. doi: 10.1016/s0368-2315(04)96637-9. [DOI] [PubMed] [Google Scholar]
- 36.Raboisson MJ, Huissoud C, Lapointe A, et al. Assessment of uterine artery and aortic isthmus Doppler recordings as predictors of necrotizing enterocolitis. Am J Obstet Gynecol. 2012;206(232) doi: 10.1016/j.ajog.2011.11.005. e1–6. [DOI] [PubMed] [Google Scholar]
- 37.Hernandez-Andrade E, Crispi F, Benavides-Serralde JA, et al. Contribution of the myocardial performance index and aortic isthmus blood flow index to predicting mortality in preterm growth-restricted fetuses. Ultrasound Obstet Gynecol. 2009;34:430–436. doi: 10.1002/uog.7347. [DOI] [PubMed] [Google Scholar]
- 38.Cruz-Lemini M, Crispi F, Van Mieghem T, et al. Risk of perinatal death in early-onset intrauterine growth restriction according to gestational age and cardiovascular doppler indices: a multicenter study. Fetal Diagn Ther. 2012;32:116–122. doi: 10.1159/000333001. [DOI] [PubMed] [Google Scholar]