Highlights:
-
•
TSC causes overactivation of the mTOR pathway, which is linked to neuroinflammation.
-
•
Most cortical tubers in TSC patients showed microglial activation.
-
•
Microglial activation was far greater in refractory seizure patients.
-
•
Microglial activation was reduced in patients treated with mTOR-inhibitor.
-
•
Microglial activation is correlated with epilepsy severity and cognitive dysfunction.
Keywords: Tuberous sclerosis complex, TSPO-PET, Neuroinflammation, Epilepsy, TAND
Abbreviations: TSC, Tuberous Sclerosis Complex; mTOR, mammalian target of rapamycin; PET, positron emission tomography; TSPO, translocator protein; SUVr, standardized uptake value ratio; AML, angiomyolipoma; ASD, autism spectrum disorder; ID, intellectual disability; WI, weighted images; FLAIR, fluid-attenuated inversion-recovery; DEE, developmental and epileptogenic encephalopathy; IQ, Intelligence Quotient; DQ, Developmental Quotient; LST, Lesion Segmentation Toolbox; ICV, intracranial volumes; IL, interleukin; SEN, subependymal nodules; SEGA, subependymal giant-cell astrocytoma; AED, anti-epileptic drug; GASE, Global Assessment of Severity of Epilepsy; EEG, electroencephalography; KSPD, Kyoto Scale of Psychological Development; VABS, Vineland Adaptive Behavior Scales
Abstract
Background and objectives
Neuroinflammation contributes to the severity of various neurological disorders, including epilepsy. Tuberous sclerosis complex (TSC) is a condition that results in the overactivation of the mammalian target of rapamycin (mTOR) pathway, which has been linked to the activation of microglia responsible for neuroinflammation. To clarify the involvement of neuroinflammation in the neuropathophysiology of TSC, we performed a positron emission tomography (PET) study using the translocator protein (TSPO) radioligand, [11C] DPA713, and investigated microglial activation in relation to neurological manifestations, especially epilepsy and cognitive function.
Methods
This cross-sectional study included 18 patients with TSC (6 in the no-seizure group, 6 in the refractory seizure group, and 6 in the mTOR-inhibitor [mTOR-i] group). All participants underwent [11C] DPA713-PET. PET results were superimposed with a 3D T2-weighted fluid-attenuated inversion-recovery (FLAIR) and T1-weighted image (T1WI) to evaluate the location of cortical tubers. Microglial activation was assessed using the standardized uptake value ratio (SUVr) of DPA713 binding. The volume ratio of the DPA713-positive area to the intracranial volume (volume ratio of DPA713/ICV) was calculated to evaluate the extent of microglial activation. A correlation analysis was performed to examine the relationship between volume ratio of DPA713/ICV and severity of epilepsy and cognitive function.
Results
Most cortical tubers with hyperintensity on FLAIR and hypo- or isointensity on T1WI showed microglial activation. The extent of microglial activation was significantly greater in the refractory seizure group than in the no-seizure or mTOR-i groups (p < 0.001). The extent of microglial activation in subjects without mTOR-i treatment correlated positively with epilepsy severity (r = 0.822, P = 0.001) and negatively with cognitive function (r = -0.846, p = 0.001), but these correlations were not present in the mTOR-i group (r = 0.232, P = 0.658, r = 0.371, P = 0.469, respectively).
Conclusion
Neuroinflammation is associated with the severity of epilepsy and cognitive dysfunction in brains with TSC. mTOR-i may suppress the extent of neuroinflammation in TSC. Investigating the spread of microglial activation using TSPO-PET in these patients may help to predict the progression of neuropathy by assessing the degree of neuroinflammation and therefore be useful for determining how aggressive the treatment should be and in assessing the effectiveness of such treatment in patients with TSC.
1. Introduction
Tuberous sclerosis complex (TSC) is a hereditary multiorgan disorder attributed to mutations in TSC1 (9q34, hamartin) or TSC2 (16p13, tuberin) (Dabora et al., 2001). TSC1 and TSC2 form complexes that inhibit the mammalian target of rapamycin (mTOR) cascade and Rheb-GTP. In normal cells, mTOR plays a central role in regulating cell growth and proliferation (Saxton and Sabatini, 2017), as well as innate immune homeostasis (Weichhart et al., 2008). TSC results in constitutive overactivation of the mTOR pathway (Crino et al., 2006).
Patients with TSC may develop hamartomas or malignant neoplastic tumors in multiple organs. Neuroimaging can detect structural alterations, such as subependymal nodules (SEN), cortical tubers, and subependymal giant-cell astrocytoma (SEGA) in >95 % of patients with TSC and contribute to early diagnosis (Roach et al., 1998). Although clinical manifestations and their severity are diverse, neurological symptoms, such as epilepsy and TSC-associated neuropsychiatric disorders (TANDs), including autism spectrum disorder (ASD), intellectual disability (ID), attention-deficit/hyperactivity disorder, anxiety disorder, and depressive disorder, have a significant impact on patients’ prognosis and quality of life (de Vries et al., 2015).
Some predictive factors of neurological manifestation have been reported, such as TSC2 mutation (Chu-Shore et al., 2010), larger cortical abnormalities on MRI (Jesmanas et al., 2018), and early onset refractory epilepsy (Chu-Shore et al., 2010). Cortical tubers typically appear hypointense on T1-weighted images (WI) and hyperintense on fluid-attenuated inversion-recovery (FLAIR) images (Grajkowska et al., 2010). Although many researchers have examined the relationship between cortical tubers and neurological deficits, it remains controversial; several studies have reported that the number of cortical nodules has no relationship to neurological severity (Chifari and Schiavella, 2020, Kaczorowska et al., 2011), while others have not (O'Callaghan et al., 2004).
Another major factor of neurological manifestation in patients is epilepsy, which manifests in over 80 % of patients with TSC (Nabbout et al., 2019). Most seizures (63.2 %) appear within the first year of life, including focal epilepsy and/or developmental and epileptogenic encephalopathy (DEE). Although most seizures originate from or near a cortical tuber (Major et al., 2009), not all cortical tubers are epileptogenic. In addition, as visual characteristics of cortical tubers are less obvious in the immature brain (Baskin, 2008, Grajkowska et al., 2010), it is difficult to detect the extension of cortical tubers before 2 years of age. Greater hypometabolism of the lesion on18F-fluoro-2-deoxyglucose (FDG)-PET compared to MRI was reported to detect epileptogenic cortical tubers (Chandra et al., 2006). However, epileptogenic lesions were also reported to be hypermetabolic on FDG-PET when the seizures are very frequent (Sakaguchi et al., 2018, Schur et al., 2018).
In recent studies, neuroinflammation was highlighted as the pathophysiological basis of epilepsy. Reactive microglia have especially been reported to be the main contributor to neuroinflammation in various epilepsy types, such as temporal lobe epilepsy with hippocampal sclerosis (Beach et al., 1995), focal cortical dysplasia (Boer et al., 2006), encephalomalacia, and Rasmussen’s encephalitis (Choi et al., 2009). In the TSC brain, microglial infiltrations within the surgical specimens of cortical tubers have been reported (Sun et al., 2016, Wirenfeldt et al., 2009).
The 18 kDa Translocator protein (TSPO), which accumulates in the outer mitochondrial membrane of activated microglia, has been described as a clinical biomarker of neuroinflammation. The radiotracers for TSPO positron emission tomography (TSPO-PET) are associated with the severity of clinical manifestations and are biomarkers for various neurological conditions, such as multiple sclerosis (Cosenza-Nashat et al., 2009, Herranz et al., 2016), chronic fatigue syndrome (Nakatomi et al., 2014), Alzheimer disease (Golla et al., 2015, Lyoo et al., 2015), and Huntington disease (Rocha et al., 2021).
Patients with temporal lobe epilepsy also showed significant uptake of TSPO in the ipsilateral hippocampus (Gershen et al., 2015, Hirvonen et al., 2012). In addition, we have previously reported that TSPO-PET detects the epileptogenic foci in children with various etiologies of epilepsy, including TSC, and microglial accumulation in surgical specimens correlated with the regions of uptake in TSPO-PET (Kagitani-Shimono et al., 2021).
The inhibition of the mTOR pathway is an adjunctive treatment for several manifestations of TSC (Franz, 2011). Among mTOR-inhibitors (mTOR-i), everolimus has been approved for the treatment of angiomyolipoma (AML), SEGA (Bissler et al., 2016, Franz et al., 2013), and epilepsy (Mizuguchi et al., 2019).
In focal ischemic stroke model rats, mTOR-i (rapamycin) significantly suppressed proinflammatory cytokines and chemokines, resulting in reduced lesion volume (Xie et al., 2014). However, there are no reports examining the extent to which mTOR-i suppresses neuroinflammation in TSC patients across the brain. We hypothesized that neuroinflammation is strongly associated with the severity of epilepsy and cognitive dysfunction and that mTOR-i could alter the epileptic seizure threshold by suppressing neuroinflammation.
Therefore, the purpose of this study was to evaluate neuroinflammation in TSC using TSPO-PET and determine whether TSPO-PET can be a biomarker for TSC pathology by identifying its association with neurological manifestations and treatment.
2. Methods
2.1. Standard protocol approvals, registrations, and patient consent
This cross-sectional study was approved by the Institutional Review Board of Osaka University Hospital (No.16092–6) and conducted according to the principles of the Declaration of Helsinki. All participants and/or their guardians provided written informed consent to participate in this study.
2.2. Participants
Patients who met the diagnostic criteria for TSC based on the International Tuberous Sclerosis Complex Diagnostic Criteria (Northrup et al., 2021) and were aged ≥ 1 year were recruited at the Department of Pediatrics, Osaka University Hospital, between May 2017 and December 2021. In total, a cohort of 20 patients with TSC was examined; however, two patients with a history of surgical intervention for refractory seizures were excluded from further analysis. A total of 18 TSC patients were analyzed: 6 without seizures for the 6 months preceding the study (No-seizure group); 6 with seizures within the previous 6 months (Refractory seizure group); 6 treated with mTOR-inhibitors (mTOR-i) (mTOR-i group). None of the patients, except for the 6 treated with mTOR-i, took any immunosuppressants, including steroids (e.g., adrenocorticotropic hormone), within the 3 months of the DPA713-PET study. Clinical information was obtained retrospectively from the participants’ medical records, including the type of epilepsy, age at epilepsy onset, type and frequency of seizures, seizure types, number of current antiepileptic drugs (AEDs), and information on mTOR-i treatment (indication, duration, and concentration). The severity of epilepsy was assessed by neurologists (SN, KT, and KKS) using the Global Assessment of Severity of Epilepsy (GASE) scale (Speechley et al., 2008), which consists of seven categories, including frequency of seizures, intensity of seizures, falls or injuries during seizure, duration/severity of the postictal period, total dose/number of AEDs, side effects of AEDs, and interference of epilepsy or drugs with daily life activities, using 7-point Likert scales for each of the 7 items (higher score indicates more severe).
Electroencephalography (EEG) (EEG1200; Nihon Kohden) was performed on all patients. To evaluate spike frequency, EEG data during sleep stage II were isolated, and automated spike detection was performed using Persyst 14 software (Persyst Development Corp., San Diego, CA). Then, an experienced pediatric neurologist (YI) examined all detected spikes, confirmed the precision of the analysis, and added spike markings if they were detected manually. In TSPO-PET, the affinity for ligands varies greatly among genetic polymorphisms (Owen et al., 2011), so all participants were checked for TSPO-binding polymorphisms by genotyping re6971. Cognitive scores used were the Intelligence Quotient (IQ) or the Developmental Quotient (DQ), calculated using a suitable test for age and cognitive function, such as the Wechsler Intelligence Scale for Children IV (WISC-IV) or the Kyoto Scale of Psychological Development 2001 (KSPD). For participants who could not complete the WISC-IV or KSPD due to severe ASD traits, the estimated IQ was calculated using the Japanese version of the Vineland Adaptive Behavior Scales (VABS)-Second Edition. The VABS is a standardized questionnaire that measures adaptive behavior through semi-structured interviews with guardians.
2.3. MRI acquisition
We performed 3-T MRI with a 24-channel-head coil (Discovery MR750W or SIGNA Architect; GE Healthcare, Milwaukee, WI, USA) using the following parameters: silent T1-weighted (repetition time (TR)/echo time (TE), 880/0.016 ms; field of view (FOV), 240 mm; matrix, 240 × 240; slice thickness, 1.0 mm; gap, 0.5 mm) and T2-weighted FLAIR (TR/TE, 6302/104.274 ms; inversion time, 1765 ms; matrix, 512 × 512; slice thickness, 1.0 mm; gap, 0.5 mm; flip angle, 90°). Children unable to keep still were sedated with either oral sedatives, such as risperidone and triclofos, or intravenous sedatives, such as pentobarbital, thiopental, pentazocine, levomepromazine, diazepam, midazolam, or a combination thereof.
2.4. MRI data analysis
To estimate the extent of cortical tubers, hyperintense lesions in FLAIR images were identified using the Lesion Segmentation Toolbox version 3.0.0 (LST) (Schmidt et al., 2019) in SPM12 (https://www.fil.ion.ucl.ac.uk/spm/software/spm12/) running in MATLAB R2017b (MathWorks Inc., Natick, MA). In addition, intracranial volumes (ICV, total volume with gray matter, and white matter) were calculated using LST in 3D-T1 images. The volume ratio of the FLAIR high-intensity area was calculated using the formula: high-intensity lesion (cm3)/ICV (cm3) × 100. Two patients (Pt 7 and Pt 9) could not be evaluated due to the absence of their 3D FLAIR images.
2.5. TSPO-PET imaging
[11C] DPA713 was prepared using a SUMITOMO gas-phase synthesizer C-GPS 100 system at the Radiopharmaceutical Laboratory of Osaka University Hospital. The mean molar activity at the end of synthesis was 141.0 ± 36.6 GBq/mmol (range, 53.5 – 192.1 GBq/mmol). [11C] DPA713, with a trace dose of approximately 7 MBq/kg, was administered intravenously within 30 s using an infusion pump. PET images were acquired for 20 min from 40 min to 60 min after administration of the tracer using an Eminence SOPHIA SET-3000 BCT/X (Shimazu Co.) in three-dimensional acquisition mode. For attenuation correction, transmission data were acquired using a rotating Cs-137-point source following the emission scan. Based on our previous protocol (Kagitani-Shimono et al., 2021), PET images acquired for 40 to 60 min were used for visual assessment.
2.6. PET data analysis
The standardized uptake values (SUVs), which were corrected for body weight and injected activity for each patient, were used for the static PET images and co-registered to the individuals' 3D T1-weighted (all patients) and 3D FLAIR images (all but Pt 7 and Pt 9) using the Image Registration and Fusion Tool in the PMOD 3.6 software package (PMOD Technologies ltd., Switzerland). The distribution of increased TSPO uptake was visually identified, then compared with the location of focal spikes in EEG. If the EEG spikes were in areas with increased TSPO uptake, it was defined as “concordant”.
The MRI images were examined for the absence of obvious cerebellar lesions, and DPA713 images were inspected for the absence of abnormal uptake in the cerebellum. To account for inter-participant differences, the standardized uptake value ratio (SUVr) was calculated using the cerebellum as a reference using PMOD 4.1 (PMOD Technologies ltd.) (Kagitani-Shimono et al., 2021, Lyoo et al., 2015). Then, the SUVr in the regions of interest in the cortical tubers was obtained. In addition, to estimate the extent of neuroinflammation, the areas where DPA713 lesions surpassed the threshold, which was set as the maximum SUVr in both cerebellar lobes, were measured. The volume ratio of the increased DPA713/ICV was also calculated.
2.7. Statistical analysis
The association between descriptive variables was compared using the chi-square (χ2) test. Since our small sample size made it difficult to estimate the data distribution, nonparametric tests were used for other analyses. Comparisons between the three groups were made using the Kruskal–Wallis test. In addition, post-hoc analysis (Dunn’s pairwise comparison test) was used to determine the significant differences among group pairs. Correlation analysis between the volume ratio of the hyperintensity areas on FLAIR and GASE or IQ/DQ was performed using Spearman’s rank-order correlation analysis. Correlation analysis between the volume ratio for increased uptake of [11C] DPA713 and spike frequency or the estimated IQ/DQ was performed using Spearman's rank-order correlation analysis. Statistical significance was set at P < 0.05. In addition, multiple linear regression was performed to evaluate the association between the volume ratio for increased DPA713/ICV and independent variables such as age, GASE, and use of mTOR-i. Statistical analyses were performed using STATA version 15.1 (Stata Corp LLC, College Station, Texas, USA).
3. Results
3.1. Patients and clinical evaluation
A summary and each of the participants’ demographics is shown in Table. 1 and Table 2, respectively. There was no significant difference between the three groups in terms of age and sex (P = 0.36, P = 0.2, respectively). Most participants who had the TSPO genotype Ala147/Ala147 were classified as high-affinity binders. Only one participant (Pt 9) had Ala147/Thr147 (mixed affinity binder), while none had Thr147/Thr147 (low-affinity binders). Additionally, most of the participants, except two, had a history of epilepsy and DEE, and the number of AEDs did not show significant differences (p = 0.1, p = 0.4, p = 0.1, respectively). The severity of epilepsy, evaluated by GASE, was highest in the refractory seizure group (P = 0.001) compared to the no-seizure (P < 0.01) and mTOR-i groups (P < 0.05). Participants in the mTOR-i group had severe manifestations before treatment: GASE before the mTOR-i treatment was higher than after treatment (P = 0.02), with no difference compared to the refractory seizure group (P = 0.31). The average spike frequency in EEG was evaluated in all participants except one in the refractory seizure group, who could not achieve sleep during EEG. Although the average spike frequency was lower in the no-seizure group (12.1 ± 16.9) and mTOR-i group (10.9 ± 16.5) than in the refractory group (20.8 ± 16.5), there was no significant difference between the groups (P = 0.23). In addition, the volume ratios of FLAIR high-intensity area/ICV were 2.2 ± 1.4 for the no-seizure group, 3.0 ± 2.0 for the refractory seizure group, and 1.5 ± 1.2 for the mTOR-i group, which tended to be lower than those of the other groups, but not significantly (P = 0.34). The correlation analysis between the volume ratios of FLAIR high-intensity area/ICV and GASE showed no correlation (r = 0.26, p = 0.331). The estimated cognitive score (IQ/DQ) was highest (77.6 ± 40.4) in the no-seizure group, followed by the mTOR-i group (54.6 ± 15.3), and was significantly low (24.8 ± 19.9) in the refractory seizure group (p = 0.02). The correlation analysis between the volume ratios of FLAIR high-intensity area/ICV and the cognitive score showed a mild negative correlation (r = -0.549, p = 0.027). Comparison of pre- and post-treatment cognitive scores in the mTOR-i group showed maintenance (n = 2) or improvement (n = 2). One patient showed mild deterioration with age. As Pt 18 started treatment in the neonatal period, a comparison could not be made.
Table 1.
Summary of participant characteristics.
| No Seizure (N = 6) | Refractory Seizure (N = 6) | mTOR-I (N = 6) | P Value | |
|---|---|---|---|---|
| Age, y, (range) | 7.0 (4–11) | 9.5 (3–16) | 11.5 (1–22) | 0.36a |
| Sex distribution (M:F) | 5:1 | 3:3 | 2:4 | 0.2b |
| TSPO genotype, n (%) | ||||
| Thr147/Thr147 | 0 | 0 | 0 | |
| Ala147/Thr147 | 0 | 1 (16.6) | 0 | |
| Ala147/Ala147 | 6 (1 0 0) | 5 (83.3) | 6 (1 0 0) | |
| History of Epilepsy | 4 (66.6 %) | 6 (100 %) | 6 (100 %) | 0.1b |
| History of DEE | 2 (33.3 %) | 4 (66.6 %) | 4 (66.6 %) | 0.4b |
| Number of AEDs | 1.3 (0–2) | 2.8 (1–4) | 2.2 (1–4) | 0.1a |
| GASE | 9 (7–11) | 24.2 (21–30)** | pre mTOR-i, 18.8 (7–25) | 0.02d |
| On_mTOR-i, 14.5* (10–20) | 0.001c | |||
| EEG (spike frequency/min) | 12.1 ± 16.9 | 20.8 ± 16.5§ | 10.9 ± 16.5 | 0.23a |
| Volume ratio of FLAIR hyperintensity area/ICV | 2.2 ± 1.4 | 3.0 ± 2.0§ | 1.5 ± 1.2 | 0.34a |
| Estimate cognitive score | 77.6 (21–127) | 24.8 (5–57) | 54.6 (40–82) | 0.02a |
| SUVr in DPA713 hot area | 1.36 ± 0.07 | 1.36 ± 0.51 | 1.39 ± 0.14 | 0.367 a |
| Volume ratio of DPA713/ICV | 3.79 ± 1.74 | 11.62 ± 4.42 | 2.62 ± 2.58 | 0.007 a |
Abbreviations: mTOR-i, mTOR-inhibitor; DEE, developmental and epilepsy encephalopathy; AED, antiepileptic drug; GASE, Global Assessment of Severity of Epilepsy; FLAIR, fluid-attenuated inversion-recovery; ICV, intracranial volume.
a, Kruskal–Wallis test; b, Pearson χ2 test; c, Kruskal–Wallis followed by Dunn's Pairwise comparisons test: ** No seizure group vs Refractory seizure group, P < 0.01, *Refractory group vs mTOR-i group, P < 0.05; §, n = 4; d, Paired t-test (pre GASE > On_mTOR-i GASE).
Table 2.
Patient characteristics.
| Group | Pt. No | Age (y) | Sex | Type of Epilepsy | Age of Onset (y) | Sz Frequency (Sz-free period) | MRI (availability of 3D FLAIR) | EEG (spike/min) | mTOR-i (ng/mL) | GASE (pre mTOR-i) | Estimated IQ (pre mTOR-i) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| No Sz | 1 | 11 | M | FE | 1.6 | - (7.5y) | CT, SEN | 27.1 | – | 11 | 45 |
| 2 | 6 | F | FE | 2.4 | - (6y) | CT, SEN | 0.0 | – | 9 | 115 | |
| 3 | 5.7 | M | FE → DEE | 0.08 | - (4.7y) | CT, SEN | 39.1 | – | 9 | 74 | |
| 4 | 4 | M | FE | 0.5 | - (4y) | CT, SEN | 0.4 | – | 7 | 84 | |
| 5 | 7 | M | – | – | – | CT, SEN | 0.0 | – | 7 | 127 | |
| 6 | 8 | M | DEE | 0.4 | -(7y) | CT, SEN | 5.8 | – | 11 | 21 | |
| Ref Sz | 7 | 4 | M | DEE | 0.5 | daily | CT, SEN, NA | 43.9 | – | 30 | 10 |
| 8 | 3 | F | DEE | 0.3 | daily | CT, SEN | 10.2 | – | 29 | 15 | |
| 9 | 9 | M | DEE | 1 | daily | CT, SEN, NA | 8.2 | – | 22 | 22 | |
| 10 | 16 | F | FE | 4 | daily | CT, SEN | 8.8 | – | 21 | 57 | |
| 11 | 15 | M | FE | 1.6 | daily | CT, SEN | 32.8 | – | 21 | 5 | |
| 12 | 10 | F | DEE → FE | 5 | daily | CT, SEN | 0 (awake) | – | 22 | 40 | |
| mTOR-i | 13 | 22 | F | FE | 2.3 | monthly (1.5 m) | CT, SEN | 8.4 | 2.5 | (25) 20 | (46) 49 |
| 14 | 14 | F | FE | 5 | monthly (1 m) | CT, SEN | 2.6 | 2.5 | (23) 18 | (42) 61 | |
| 15 | 9 | M | DEE → FE | 0.7 | monthly (1 m) | CT, SEN | 0.0 | 3 | (21) 15 | (44) 43 | |
| 16 | 9 | F | FE → DEE → FE | 0.6 | - (10 m) | CT, SEN | 43.5 | 4.3 | (18) 10 | (82) 82 | |
| 17 | 14 | M | DEE → FE | 0.4 | monthly (1 m) | CT, SEN, SEGA | 1.2 | 5 | (19) 14 | (46) 40 | |
| 18 | 1 | F | DEE | 0.4 | - (9 m) | CT, SEN, SEGA | 9.4 | 2.5 | (7) 10 | (NA) 53 | |
Sz, seizure; mTOR-i, mammalian target of rapamycin inhibitor; FE, focal epilepsy; DEE, developmental and epileptic encephalopathy; EEG, electroencephalography; CT, Cortical tubers; SEN, subependymal nodule; SEGA, subependymal giant cell astrocytoma; AML, angiomyolipoma; NA, not available.
3.2. Increased microglial activation in the TSC brain
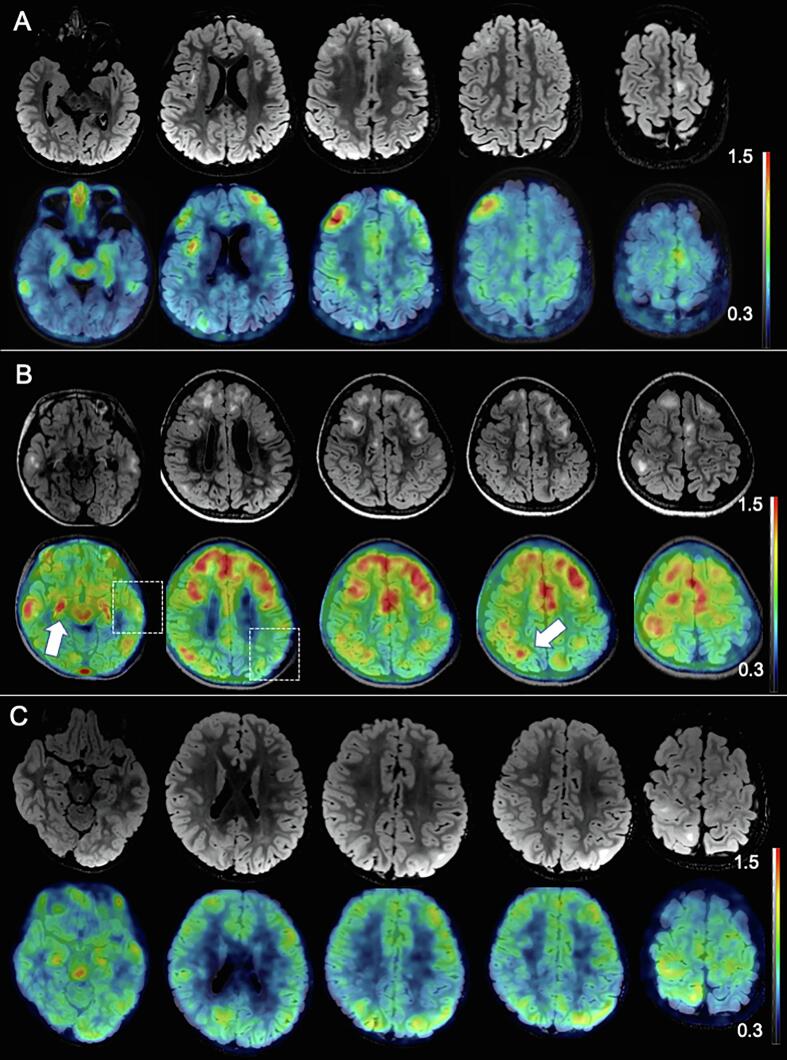
Fig. 1 shows that the representative TSC patient with refractory seizures (Fig. 1B) demonstrated high [11C] DPA713 SUVr in broad cortical lesions, which was consistent with the cortical tubers indicated by the hyperintensity lesions on FLAIR. However, a few lesions with hyperintensity on FLAIR did not show increased uptake of [11C] DPA713 (squares in Fig. 1B). In contrast, the propagated area, such as the ipsilateral hippocampus and area of isointensity, also demonstrated increased uptake of DPA713 (arrow in Fig. 1B). TSC patients without seizures also demonstrated high [11C] DPA713 SUVr lesions, which were strictly localized to a part of the cortical tubers (Fig. 1A). Patients with TSC treated with mTOR-i showed a mildly increased [11C] DPA713 SUVr lesion, which corresponded to the cortical tubers (Fig. 1C).
Fig. 1.
Association of cortical tubers and microglial activation. Representative cases from the three groups are shown. The upper rows represent FLAIR-MRI, while the lower rows represent [11C] DPA713-PET. [11C] DPA713-PET images of SUVr show massive microglial activation that mainly overlaps with cortical tubers. The severity of the increased microglial activation was higher in the refractory seizure group (B) than in the no-seizure (A) or mTOR-i groups (C). Some cortical tubers do not show microglial activation (dashed box), while increased microglial activation is shown in the FLAIR isointensity area (arrow).
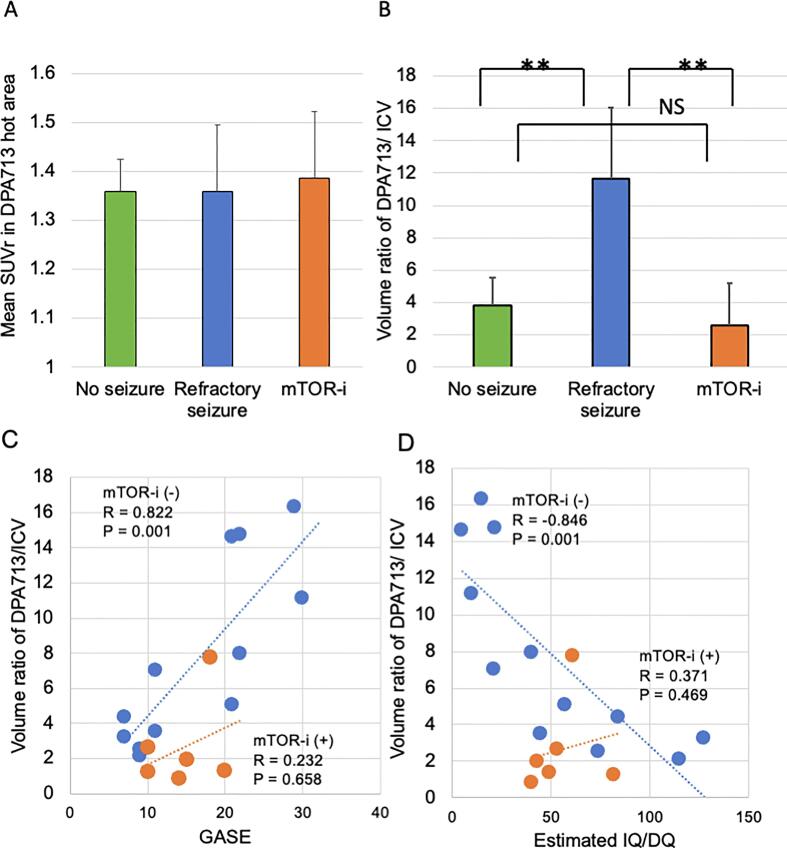
The average SUVr in the DPA713 increased area was not significantly different between the groups (P = 0.367, Fig. 2A). On comparing the locations of spikes on EEG and the DPA713-increased regions, all subjects except three without spikes were defined as concordant. The volume ratio of [11C] DPA713-PET/ICV was significantly higher in the refractory seizure group with respect to that in the no-seizure (P = 0.007) and mTOR-i groups (P = 0.007, Fig. 2B).
Fig. 2.
Extension of microglial activation is associated with epilepsy and cognitive deficit. The mean SUVr in the DPA713 hot area did not differ among the groups (p = 0.367, A). The volume ratio of DPA713/ICV was significantly higher in the refractory seizure group than in the no seizure (p = 0.007) and mTOR-i (p = 0.007) groups (B). The volume ratio of DPA713/ICV was associated with high GASE in subjects without mTOR-i (blue circle: mTOR-i(-)) (r = 0.822, p = 0.001), but not in the mTOR-i group (orange circle: r = 0.232, p = 0.658) (C). In contrast, the volume ratio of DPA713/ICV was negatively correlated with low estimated IQ/DQ in subjects without mTOR-i (blue circle: r = -0.843, p = 0.001) but not in the mTOR-i group (orange circle: r = 0.371, p = 0.469)(D). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.3. Correlation of microglial activation with neurological manifestation and mTOR-i treatment
The volume ratio of [11C] DPA713-PET/ICV in the no-seizure and refractory seizure groups (not treated with mTOR-i) was significantly correlated with the GASE score (r = 0.822, P = 0.001, Fig. 2C). However, the mTOR-i group did not show a correlation between the volume ratios of [11C] DPA713-PET/ICV and GASE (r = 0.232, P = 0.658). The spike frequency did not show a correlation with the volume ratios of [11C] DPA713-PET/ICV (r = 0.238, p = 0.357). In addition, the volume ratio of [11C] DPA713-PET/ICV in the no-seizure and refractory seizure groups was negatively correlated with the estimated IQ/DQ (r = -0.843, P = 0.001), but not in the mTOR-i group (r = 0.371, P = 0.469, Fig. 2D). Multiple linear regression showed that age had no effect on the volume ratio of DPA713/ICV (t = -0.62, P = 0.546). However, the volume ratio of DPA713/ICV was strongly affected by GASE (t = 4.52, P = 0.000) and mTOR-i (t = -2.33, P = 0.035) (adjusted R2 = 0.628).
4. Discussion
Using [11C] DPA713-PET, we first evaluated the microglial activation in the TSC whole brain and found that prominent microglial activation was mainly associated with cortical tubers, even without seizures. However, compared with the MRI findings, there were some inconsistencies, such as no microglial activation in some cortical tubers with hyperintensity on FLAIR and strong microglial activation even in the cortical tubers without hyperintensity on FLAIR. The extent of microglial activation was strongly associated with the severity of epilepsy and negatively associated with cognitive function. In addition, in the mTOR-i group, which showed refractory epilepsy before mTOR-i treatment, the extent of microglial activation was reduced compared with that in the refractory seizure group. These findings suggest that mTOR-i suppresses the extent of microglial activation.
In the TSC brain, neuroinflammation is associated with the overactivation of the mTOR pathway and seizures. Especially, the overactivation of the mTOR pathway was suggested to be associated with cortical tubers. In the surgical tissues from the epileptogenic cortical tubers, the most prominent microglial activation was observed in giant-cell dysmorphic neurons with calcifications, followed by a high density of giant cells or dysmorphic neurons (Mühlebner et al., 2016). In gene expression analysis using human TSC brain tissues obtained via surgery or post-mortem, both cortical tubers and SEN/SEGA showed significant neuroinflammation, such as major histocompatibility class II (Martin et al., 2017) and toll-like receptors, which are involved in innate immunity and oxidative stress (Dombkowski et al., 2019). Meanwhile, CD47 and CD200, which inhibit proinflammatory cytokines, were downregulated in the epileptogenic foci of TSC (Sun et al., 2016).
Moreover, all types of tubers and peri-tuber tissue showed prominent mTOR activation. However, there was no association between the tuber types according to MRI characteristics (Gallagher et al., 2010) and the histological classification. In the present study, not only cortical tubers visualized with FLAIR hyperintensity or calcification but also those in the cortex without FLAIR high intensity and only with the blurring of the gray and white matter junction showed significant TSPO uptake. Even in the no-seizure group, the average SUVr of TSPO uptake in the cortical tubers was the same as that in the refractory seizure group. In TSC1-GFAP conditional knock-out mice, inflammatory markers were increased in astrocytes before the onset of epilepsy, and hence, not secondary to seizures (Zhang et al., 2015). These findings indicate that microglial activation is associated with proinflammatory features originally caused by mTOR pathway hyperactivation regardless of seizures.
In contrast, patients with refractory epilepsy showed broader microglial activation, affecting not only the cortical tubers but also the surrounding tissue. In fact, experimentally induced seizures in rodents demonstrate dynamic microglial activation (Eyo et al., 2014), which persists in the chronic phase (Amhaoul et al., 2015). In a human study, post-seizure TSPO-PET, compared with a TSPO scan during the seizure-free period, showed greater inflammation, suggesting induced microglial activation following a single seizure (Butler et al., 2016). Therefore, recurrent, persistent seizures could induce microglial activation in the surrounding and/or propagated areas of the refractory seizure group. Furthermore, proinflammatory mediators have been reported to induce neuronal excitation, reduce seizure threshold (Galic et al., 2012), and activate astrocytes, which have epileptogenic properties (He et al., 2013). These findings are consistent with our result of a strong correlation between the severity of epilepsy and microglia extension. Therefore, we hypothesize that the overactivation of the mTOR-pathway causes the initial neuroinflammation and triggers the onset of epilepsy, but that repeated epileptic seizures trigger further neuroinflammation.
Previous studies have reported close associations between neuroinflammation and cognition. Neuroinflammation using TSPO-PET was significantly correlated with cognitive dysfunction in Alzheimer’s disease (Yokokura et al., 2017) and multiple sclerosis (Herranz et al., 2016). In epilepsy, the levels of proinflammatory cytokines such as interleukin (IL)-6 and IL-1β increase after seizures (Lehtimäki et al., 2004, Lehtimäki et al., 2010), while inflammation can increase neuronal excitability, ultimately facilitating psychiatric comorbidities (Vezzani et al., 2013). Mazarati et al. (Mazarati et al., 2017) have suggested that the induction of inflammation in early life can precipitate both epilepsy and psychiatric comorbidities. In patients with TSC, refractory epilepsy, which develops in 62.5 % of cases, has been correlated with poor cognitive outcomes (Chu-Shore et al., 2010). Those findings are consistent with our result that the extent of microglial activation was significantly correlated with cognitive disability.
The effects of mTOR inhibitors in the TSC brain have been proposed based on animal studies. The First is an anti-inflammatory effect: mTOR inhibitors directly inhibited microglial accumulation in the lipopolysaccharide-induced neuroinflammation model (Yang et al., 2017), a focal ischemic stroke model (Guden et al., 2021), and a kainic acid-induced seizure model (Huang et al., 2021). The second is the effect on epileptogenicity: in the TSC1-knockout mouse model, an mTOR-inhibitor rescued the excitation/inhibition imbalance by changing the multiple ion channels in the hippocampus (Koene et al., 2021). Abs et al. (Abs et al., 2013) reported that mTOR overactivation by acute biallelic deletion of TSC1 in healthy adult mice facilitated the development of seizures without obvious cortical tubers within a few days and that rapamycin treatment completely stopped the seizures. The third is an effect on cognitive function by suppressing mTOR overactivity. In TSC1loxp/loxp mice, rapamycin treatment during the early postnatal days normalized aberrant arborization (Cox et al., 2018).Furthermore, cognitive deficits in TSC2+/- mice emerged by hyperactive hippocampal mTOR-signaling, even in the absence of neuropathology and seizures, and mTOR inhibitors rescued synaptic plasticity, behavioral deficits, and social behavior (Ehninger et al., 2008).
In a human clinical trial for TSC-associated refractory epilepsy, everolimus significantly reduced seizure frequency, with a high response rate and good seizure reduction of 57.7 % and 56.9 % at 2 years, respectively (Franz et al., 2018). However, evidence that mTOR-i suppresses microglial activation in vivo is lacking. Although TSPO-PET is a potential biomarker for anti-inflammatory therapeutics, it remains unclear whether the effectiveness of treatment can be determined (Scott et al., 2017). The results of this study indicate that mTOR-i suppresses microglial activation on tissues surrounding cortical tubers directly and/or indirectly by reducing seizures. These findings suggest that the effect of mTOR-i on epilepsy could be partially mediated by the suppression of microglial activation and the formation of epileptic networks by inhibiting abnormal neuronal connections.
In a human clinical trial, although mTOR-i showed a favorable trend of ASD symptoms (Mizuguchi et al., 2019), evidence for cognition and psychiatric comorbidity improvement with mTOR-i is lacking. Petrasek et al. (Petrasek et al., 2021) proposed a likely explanation that mTOR-i could improve the social deficit induced by TSC2 haploinsufficiency but not the deficits associated with developmental status epilepticus. In this study, although mTOR-i significantly reduced the severity of epilepsy and extension of microglial activation, the volume ratio DPA713/ICV did not correlate with GASE and IQ/DQ. This is because while mTOR-i treatment improved seizure frequency/severity and suppressed neuroinflammation, patients in the mTOR-i group maintained multidrug therapy. Furthermore, despite a mild increase or no decline in cognitive function, they already had irreversible cognitive dysfunction before mTOR-i, which might be a consequence of previous seizures and a genetic impact of TSC.
Even though the causality of neurological burden in TSC is complex and still unsolved, with multiple factors, TSPO-PET could be a potential biomarker that may help to evaluate the severity of neuroinflammation and provide an indication for mTOR-i therapy.
This study had several limitations. First, absolute radioligand values were not calculated. Since our participants were children, obtaining blood samples from arterial blood was difficult. We used the cerebellum as the reference tissue for comparisons among patients. Although the patients had no cerebellar lesions and semi-quantitative evaluation of the cerebellum has been proven reliable in previous studies (Lyoo et al., 2015), the cerebellum could be influenced by epileptic activity and overactivation of the mTOR pathway. Second, the sample size was insufficient to evaluate the pathophysiology of TSC. In addition, we used three types of cognitive scales because the ranges of the participants' intellectual ability and age were too large to be assessed using a single test. Since we recruited participants with TSC who were diagnosed during childhood and all participants had at least one cortical tuber, it is necessary to study adult patients without neurological symptoms in the future. Third, due to this being a cross-sectional study, the results for the mTOR-i group are for different treatment periods and without pre-treatment [11C] DPA713-PET. Therefore, we could not draw any conclusions regarding the causal relationships with mTOR-i treatment. A longitudinal study of the therapeutic effects of mTOR-i on neuroinflammation is required.
In conclusion, this study indicates that DPA713-PET can be used to evaluate microglial activation in patients with TSC. Microglial activation is strongly associated with epilepsy severity and has a negative impact on cognitive function in TSC patients who are not on mTOR-i. Treatment with mTOR-i may reduce microglial activation. We anticipate that the evaluation of microglial activation using DPA713-PET could be an important determinant in the clinical care of patients with TSC.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgments
The authors acknowledge and thank all participants in this study. The authors also thank the staff from the Radiology Center at Osaka University Hospital for their technical support.
Funding
This study was supported by JSPS KAKENHI (Grant Number, JP18K07843), AMED under Grant Number dm0307103h0003 and 22ym0126809j0001, Novartis Research Grants, Japan Epilepsy Research Foundation, and Eizai Scholarship. (All to KKS), and the Center of Innovation Program from the Japan Science and Technology Agency, JST, Japan (to MT).
Data availability
Data will be made available on request.
References
- Abs E., Goorden S.M., Schreiber J., Overwater I.E., Hoogeveen-Westerveld M., Bruinsma C.F., Aganovic E., Borgesius N.Z., Nellist M., Elgersma Y. TORC1-dependent epilepsy caused by acute biallelic Tsc1 deletion in adult mice. Annals of Neurology. 2013;74:569–579. doi: 10.1002/ana.23943. [DOI] [PubMed] [Google Scholar]
- Amhaoul H., Hamaide J., Bertoglio D., Reichel S.N., Verhaeghe J., Geerts E., Van Dam D., De Deyn P.P., Kumar-Singh S., Katsifis A., Van Der Linden A., Staelens S., Dedeurwaerdere S. Brain inflammation in a chronic epilepsy model: Evolving pattern of the translocator protein during epileptogenesis. Neurobiology of Disease. 2015;82:526–539. doi: 10.1016/j.nbd.2015.09.004. [DOI] [PubMed] [Google Scholar]
- Baskin H.J., Jr. The pathogenesis and imaging of the tuberous sclerosis complex. Pediatric Radiology. 2008;38:936–952. doi: 10.1007/s00247-008-0832-y. [DOI] [PubMed] [Google Scholar]
- Beach T.G., Woodhurst W.B., MacDonald D.B., Jones M.W. Reactive microglia in hippocampal sclerosis associated with human temporal lobe epilepsy. Neuroscience Letters. 1995;191:27–30. doi: 10.1016/0304-3940(94)11548-1. [DOI] [PubMed] [Google Scholar]
- Bissler J.J., Kingswood J.C., Radzikowska E., Zonnenberg B.A., Frost M., Belousova E., Sauter M., Nonomura N., Brakemeier S., de Vries P.J., Berkowitz N., Miao S., Segal S., Peyrard S., Budde K. Everolimus for renal angiomyolipoma in patients with tuberous sclerosis complex or sporadic lymphangioleiomyomatosis: extension of a randomized controlled trial. Nephrology, Dialysis, Transplantation. 2016;31:111–119. doi: 10.1093/ndt/gfv249. [DOI] [PubMed] [Google Scholar]
- Boer K., Spliet W.G., van Rijen P.C., Redeker S., Troost D., Aronica E. Evidence of activated microglia in focal cortical dysplasia. Journal of Neuroimmunology. 2006;173:188–195. doi: 10.1016/j.jneuroim.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Butler T., Li Y., Tsui W., Friedman D., Maoz A., Wang X., Harvey P., Tanzi E., Morim S., Kang Y., Mosconi L., Talos D., Kuzniecky R., Vallhabjosula S., Thesen T., Glodzik L., Ichise M., Silbersweig D., Stern E., de Leon M.J., French J. Transient and chronic seizure-induced inflammation in human focal epilepsy. Epilepsia. 2016;57:e191. doi: 10.1111/epi.13457. e194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra P.S., Salamon N., Huang J., Wu J.Y., Koh S., Vinters H.V., Mathern G.W. FDG-PET/MRI coregistration and diffusion-tensor imaging distinguish epileptogenic tubers and cortex in patients with tuberous sclerosis complex: a preliminary report. Epilepsia. 2006;47:1543–1549. doi: 10.1111/j.1528-1167.2006.00627.x. [DOI] [PubMed] [Google Scholar]
- Chifari R., Schiavella M. Lack of Correlation between Tuber Number and Cognitive Level in Mild TSC Patients : a Clinical and Genetic Study. Cardiovascular Surgery International. 2020;1:20–24. [Google Scholar]
- Choi J., Nordli D.R., Jr., Alden T.D., DiPatri A., Jr., Laux L., Kelley K., Rosenow J., Schuele S.U., Rajaram V., Koh S. Cellular injury and neuroinflammation in children with chronic intractable epilepsy. Journal of Neuroinflammation. 2009;6:38. doi: 10.1186/1742-2094-6-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu-Shore C.J., Major P., Camposano S., Muzykewicz D., Thiele E.A. The natural history of epilepsy in tuberous sclerosis complex. Epilepsia. 2010;51:1236–1241. doi: 10.1111/j.1528-1167.2009.02474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosenza-Nashat M., Zhao M.L., Suh H.S., Morgan J., Natividad R., Morgello S., Lee S.C. Expression of the translocator protein of 18 kDa by microglia, macrophages and astrocytes based on immunohistochemical localization in abnormal human brain. Neuropathology and Applied Neurobiology. 2009;35:306–328. doi: 10.1111/j.1365-2990.2008.01006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R.L., Calderon de Anda F., Mangoubi T., Yoshii A. Multiple Critical Periods for Rapamycin Treatment to Correct Structural Defects in Tsc-1-Suppressed Brain. Frontiers in Molecular Neuroscience. 2018;11:409. doi: 10.3389/fnmol.2018.00409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crino P.B., Nathanson K.L., Henske E.P. The Tuberous Sclerosis Complex. New England Journal of Medicine. 2006;355:1345–1356. doi: 10.1056/NEJMra055323. [DOI] [PubMed] [Google Scholar]
- Dabora S.L., Jozwiak S., Franz D.N., Roberts P.S., Nieto A., Chung J., Choy Y.S., Reeve M.P., Thiele E., Egelhoff J.C., Kasprzyk-Obara J., Domanska-Pakiela D., Kwiatkowski D.J. Mutational analysis in a cohort of 224 tuberous sclerosis patients indicates increased severity of TSC2, compared with TSC1, disease in multiple organs. American Journal of Human Genetics. 2001;68:64–80. doi: 10.1086/316951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries P.J., Whittemore V.H., Leclezio L., Byars A.W., Dunn D., Ess K.C., Hook D., King B.H., Sahin M., Jansen A. Tuberous sclerosis associated neuropsychiatric disorders (TAND) and the TAND Checklist. Pediatric Neurology. 2015;52:25–35. doi: 10.1016/j.pediatrneurol.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dombkowski A.A., Cukovic D., Bagla S., Jones M., Caruso J.A., Chugani H.T., Chugani D.C. TLR7 activation in epilepsy of tuberous sclerosis complex. Inflammation Research. 2019;68:993–998. doi: 10.1007/s00011-019-01283-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehninger D., Han S., Shilyansky C., Zhou Y., Li W., Kwiatkowski D.J., Ramesh V., Silva A.J. Reversal of learning deficits in a Tsc2+/- mouse model of tuberous sclerosis. Nature Medicine. 2008;14:843–848. doi: 10.1038/nm1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyo U.B., Peng J., Swiatkowski P., Mukherjee A., Bispo A., Wu L.J. Neuronal hyperactivity recruits microglial processes via neuronal NMDA receptors and microglial P2Y12 receptors after status epilepticus. Journal of Neuroscience. 2014;34:10528–10540. doi: 10.1523/JNEUROSCI.0416-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franz D.N. Everolimus: an mTOR inhibitor for the treatment of tuberous sclerosis. Expert Review of Anticancer Therapy. 2011;11:1181–1192. doi: 10.1586/era.11.93. [DOI] [PubMed] [Google Scholar]
- Franz D.N., Belousova E., Sparagana S., Bebin E.M., Frost M., Kuperman R., Witt O., Kohrman M.H., Flamini J.R., Wu J.Y., Curatolo P., de Vries P.J., Whittemore V.H., Thiele E.A., Ford J.P., Shah G., Cauwel H., Lebwohl D., Sahmoud T., Jozwiak S. Efficacy and safety of everolimus for subependymal giant cell astrocytomas associated with tuberous sclerosis complex (EXIST-1): a multicentre, randomised, placebo-controlled phase 3 trial. Lancet. 2013;381:125–132. doi: 10.1016/S0140-6736(12)61134-9. [DOI] [PubMed] [Google Scholar]
- Franz D.N., Lawson J.A., Yapici Z., Ikeda H., Polster T., Nabbout R., Curatolo P., de Vries P.J., Dlugos D.J., Voi M., Fan J., Vaury A., Pelov D., French J.A. Everolimus for treatment-refractory seizures in TSC: Extension of a randomized controlled trial. Neurology, Clinical Practice. 2018;8:412–420. doi: 10.1212/CPJ.0000000000000514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galic M.A., Riazi K., Pittman Q.J. Cytokines and brain excitability. Frontiers in Neuroendocrinology. 2012;33:116–125. doi: 10.1016/j.yfrne.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher A., Grant E.P., Madan N., Jarrett D.Y., Lyczkowski D.A., Thiele E.A. MRI findings reveal three different types of tubers in patients with tuberous sclerosis complex. Journal of Neurology. 2010;257:1373–1381. doi: 10.1007/s00415-010-5535-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershen L.D., Zanotti-Fregonara P., Dustin I.H., Liow J.S., Hirvonen J., Kreisl W.C., Jenko K.J., Inati S.K., Fujita M., Morse C.L., Brouwer C., Hong J.S., Pike V.W., Zoghbi S.S., Innis R.B., Theodore W.H. Neuroinflammation in temporal lobe epilepsy measured using positron emission tomographic imaging of translocator protein. JAMA Neurology. 2015;72:882–888. doi: 10.1001/jamaneurol.2015.0941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golla S.S., Boellaard R., Oikonen V., Hoffmann A., van Berckel B.N., Windhorst A.D., Virta J., Haaparanta-Solin M., Luoto P., Savisto N., Solin O., Valencia R., Thiele A., Eriksson J., Schuit R.C., Lammertsma A.A., Rinne J.O. Quantification of [18F]DPA-714 binding in the human brain: initial studies in healthy controls and Alzheimer's disease patients. Journal of Cerebral Blood Flow and Metabolism. 2015;35:766–772. doi: 10.1038/jcbfm.2014.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grajkowska W., Kotulska K., Jurkiewicz E., Matyja E. Brain lesions in tuberous sclerosis complex. Review. Folia Neuropathologica. 2010;48:139–149. [PubMed] [Google Scholar]
- Guden D.S., Temiz-Resitoglu M., Senol S.P., Kibar D., Yilmaz S.N., Tunctan B., Malik K.U., Sahan-Firat S. mTOR inhibition as a possible pharmacological target in the management of systemic inflammatory response and associated neuroinflammation by lipopolysaccharide challenge in rats. Canadian Journal of Physiology and Pharmacology. 2021;99:921–934. doi: 10.1139/cjpp-2020-0487. [DOI] [PubMed] [Google Scholar]
- He J.J., Wu K.F., Li S., Shu H.F., Zhang C.Q., Liu S.Y., Yang M.H., Yin Q., Yang H. Expression of the interleukin 17 in cortical tubers of the tuberous sclerosis complex. Journal of Neuroimmunology. 2013;262:85–91. doi: 10.1016/j.jneuroim.2013.05.007. [DOI] [PubMed] [Google Scholar]
- Herranz E., Gianni C., Louapre C., Treaba C.A., Govindarajan S.T., Ouellette R., Loggia M.L., Sloane J.A., Madigan N., Izquierdo-Garcia D., Ward N., Mangeat G., Granberg T., Klawiter E.C., Catana C., Hooker J.M., Taylor N., Ionete C., Kinkel R.P., Mainero C. Neuroinflammatory component of gray matter pathology in multiple sclerosis. Annals of Neurology. 2016;80:776–790. doi: 10.1002/ana.24791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirvonen J., Kreisl W.C., Fujita M., Dustin I., Khan O., Appel S., Zhang Y., Morse C., Pike V.W., Innis R.B., Theodore W.H. Increased in vivo expression of an inflammatory marker in temporal lobe epilepsy. Journal of Nuclear Medicine. 2012;53:234–240. doi: 10.2967/jnumed.111.091694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X.Y., Hu Q.P., Shi H.Y., Zheng Y.Y., Hu R.R., Guo Q. Everolimus inhibits PI3K/Akt/mTOR and NF-kB/IL-6 signaling and protects seizure-induced brain injury in rats. Journal of Chemical Neuroanatomy. 2021;114 doi: 10.1016/j.jchemneu.2021.101960. [DOI] [PubMed] [Google Scholar]
- Jesmanas S., Norvainytė K., Gleizniene R., Simoliuniene R., Endziniene M. Different MRI-defined tuber types in tuberous sclerosis complex: Quantitative evaluation and association with disease manifestations. Brain and Development. 2018;40:196–204. doi: 10.1016/j.braindev.2017.11.010. [DOI] [PubMed] [Google Scholar]
- Kaczorowska M., Jurkiewicz E., Domańska-Pakiela D., Syczewska M., Lojszczyk B., Chmielewski D., Kotulska K., Kuczyński D., Kmieć T., Dunin-Wąsowicz D., Kasprzyk-Obara J., Jóźwiak S. Cerebral tuber count and its impact on mental outcome of patients with tuberous sclerosis complex. Epilepsia. 2011;52:22–27. doi: 10.1111/j.1528-1167.2010.02892.x. [DOI] [PubMed] [Google Scholar]
- Kagitani-Shimono K., Kato H., Kuwayama R., Tominaga K., Nabatame S., Kishima H., Hatazawa J., Taniike M. Clinical evaluation of neuroinflammation in child-onset focal epilepsy: a translocator protein PET study. Journal of Neuroinflammation. 2021;18:8. doi: 10.1186/s12974-020-02055-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koene L.M., Niggl E., Wallaard I., Proietti-Onori M., Rotaru D.C., Elgersma Y. Identifying the temporal electrophysiological and molecular changes that contribute to TSC-associated epileptogenesis. JCI Insight. 2021;6:e150120. doi: 10.1172/jci.insight.150120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehtimäki K.A., Keränen T., Huhtala H., Hurme M., Ollikainen J., Honkaniemi J., Palmio J., Peltola J. Regulation of IL-6 system in cerebrospinal fluid and serum compartments by seizures: the effect of seizure type and duration. Journal of Neuroimmunology. 2004;152:121–125. doi: 10.1016/j.jneuroim.2004.01.024. [DOI] [PubMed] [Google Scholar]
- Lehtimäki K.A., Keränen T., Palmio J., Peltola J. Levels of IL-1beta and IL-1ra in cerebrospinal fluid of human patients after single and prolonged seizures. Neuroimmunomodulation. 2010;17:19–22. doi: 10.1159/000243081. [DOI] [PubMed] [Google Scholar]
- Lyoo C.H., Ikawa M., Liow J.S., Zoghbi S.S., Morse C.L., Pike V.W., Fujita M., Innis R.B., Kreisl W.C. Cerebellum Can Serve As a Pseudo-Reference Region in Alzheimer Disease to Detect Neuroinflammation Measured with PET Radioligand Binding to Translocator Protein. Journal of Nuclear Medicine. 2015;56:701–706. doi: 10.2967/jnumed.114.146027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Major P., Rakowski S., Simon M., Cheng M., Eskandar E., Baron J., Leeman B.A., Frosch M., Thiele E. Are cortical tubers epileptogenic? Evidence from electrocorticography. Epilepsia. 2009;50:147–154. doi: 10.1111/j.1528-1167.2008.01814.x. [DOI] [PubMed] [Google Scholar]
- Martin K.R., Zhou W., Bowman M.J., Shih J., Au K.S., Dittenhafer-Reed K.E., Sisson K.A., Koeman J., Weisenberger D.J., Cottingham S.L., DeRoos S.T., Devinsky O., Winn M.E., Cherniack A.D., Shen H., Northrup H., Krueger D.A., MacKeigan J.P. The genomic landscape of tuberous sclerosis complex. Nature Communications. 2017;8:15816. doi: 10.1038/ncomms15816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazarati A.M., Lewis M.L., Pittman Q.J. Neurobehavioral comorbidities of epilepsy: Role of inflammation. Epilepsia. 2017;58:48–56. doi: 10.1111/epi.13786. [DOI] [PubMed] [Google Scholar]
- Mizuguchi M., Ikeda H., Kagitani-Shimono K., Yoshinaga H., Suzuki Y., Aoki M., Endo M., Yonemura M., Kubota M. Everolimus for epilepsy and autism spectrum disorder in tuberous sclerosis complex: EXIST-3 substudy in Japan. Brain and Development. 2019;41:1–10. doi: 10.1016/j.braindev.2018.07.003. [DOI] [PubMed] [Google Scholar]
- Mühlebner A., van Scheppingen J., Hulshof H.M., Scholl T., Iyer A.M., Anink J.J., van den Ouweland A.M., Nellist M.D., Jansen F.E., Spliet W.G., Krsek P., Benova B., Zamecnik J., Crino P.B., Prayer D., Czech T., Wöhrer A., Rahimi J., Höftberger R., Hainfellner J.A., Feucht M., Aronica E. Novel Histopathological Patterns in Cortical Tubers of Epilepsy Surgery Patients with Tuberous Sclerosis Complex. PloS One. 2016;11:e0157396. doi: 10.1371/journal.pone.0157396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabbout, R., Belousova, E., Benedik, M.P., Carter, T., Cottin, V., Curatolo, P., Dahlin, M., L, D.A., D Amato, L., d’Augères, G.B., de Vries, P.J., Ferreira, J.C., Feucht, M., Fladrowski, C., Hertzberg, C., Jozwiak, S., Lawson, J.A., Macaya, A., Marques, R., O'Callaghan, F., Qin, J., Sander, V., Sauter, M., Shah, S., Takahashi, Y., Touraine, R., Youroukos, S., Zonnenberg, B., Jansen, A., Kingswood, J.C., TOSCA Consortium and TOSCA Investigators, 2019. Epilepsy in tuberous sclerosis complex: Findings from the TOSCA Study. Epilepsia Open 4, 73-84.
- Nakatomi Y., Mizuno K., Ishii A., Wada Y., Tanaka M., Tazawa S., Onoe K., Fukuda S., Kawabe J., Takahashi K., Kataoka Y., Shiomi S., Yamaguti K., Inaba M., Kuratsune H., Watanabe Y. Neuroinflammation in Patients with Chronic Fatigue Syndrome/Myalgic Encephalomyelitis: An (11)C-(R)-PK11195 PET Study. Journal of Nuclear Medicine. 2014;55:945–950. doi: 10.2967/jnumed.113.131045. [DOI] [PubMed] [Google Scholar]
- Northrup, H., Aronow, M.E., Bebin, E.M., Bissler, J., Darling, T.N., de Vries, P.J., Frost, M.D., Fuchs, Z., Gosnell, E.S., Gupta, N., Jansen, A.C., Jozwiak, S., Kingswood, J.C., Knilans, T.K., McCormack, F.X., Pounders, A., Roberds, S.L., Rodriguez-Buritica, D.F., Roth, J., Sampson, J.R., Sparagana, S., Thiele, E.A., Weiner, H.L., Wheless, J.W., Towbin, A.J., Krueger, D.A., International Tuberous Sclerosis Complex Consensus, G., 2021. Updated International Tuberous Sclerosis Complex Diagnostic Criteria and Surveillance and Management Recommendations. Pediatric Neurology 123, 50-66. [DOI] [PubMed]
- O'Callaghan F.J., Harris T., Joinson C., Bolton P., Noakes M., Presdee D., Renowden S., Shiell A., Martyn C.N., Osborne J.P. The relation of infantile spasms, tubers, and intelligence in tuberous sclerosis complex. Archives of Disease in Childhood. 2004;89:530–533. doi: 10.1136/adc.2003.026815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen D.R., Gunn R.N., Rabiner E.A., Bennacef I., Fujita M., Kreisl W.C., Innis R.B., Pike V.W., Reynolds R., Matthews P.M., Parker C.A. Mixed-affinity binding in humans with 18-kDa translocator protein ligands. Journal of Nuclear Medicine. 2011;52:24–32. doi: 10.2967/jnumed.110.079459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrasek T., Vojtechova I., Klovrza O., Tuckova K., Vejmola C., Rak J., Sulakova A., Kaping D., Bernhardt N., de Vries P.J., Otahal J., Waltereit R. mTOR inhibitor improves autistic-like behaviors related to Tsc2 haploinsufficiency but not following developmental status epilepticus. Journal of Neurodevelopmental Disorders. 2021;13:14. doi: 10.1186/s11689-021-09357-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roach E.S., Gomez M.R., Northrup H. Tuberous sclerosis complex consensus conference: revised clinical diagnostic criteria. Journal of Child Neurology. 1998;13:624–628. doi: 10.1177/088307389801301206. [DOI] [PubMed] [Google Scholar]
- Rocha N.P., Charron O., Latham L.B., Colpo G.D., Zanotti-Fregonara P., Yu M., Freeman L., Furr Stimming E., Teixeira A.L. Microglia Activation in Basal Ganglia Is a Late Event in Huntington Disease Pathophysiology. Neurology, Neuroimmunology and Neuroinflammation. 2021;8:e984. doi: 10.1212/NXI.0000000000000984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi Y., Kidokoro H., Ogawa C., Okai Y., Ito Y., Yamamoto H., Ohno A., Nakata T., Tsuji T., Nakane T., Kawai H., Kato K., Naganawa S., Natsume J. Longitudinal Findings of MRI and PET in West Syndrome with Subtle Focal Cortical Dysplasia. AJNR. American Journal of Neuroradiology. 2018;39:1932–1937. doi: 10.3174/ajnr.A5772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxton R.A., Sabatini D.M. mTOR Signaling in Growth, Metabolism, and Disease. Cell. 2017;168:960–976. doi: 10.1016/j.cell.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt P., Pongratz V., Küster P., Meier D., Wuerfel J., Lukas C., Bellenberg B., Zipp F., Groppa S., Sämann P.G., Weber F., Gaser C., Franke T., Bussas M., Kirschke J., Zimmer C., Hemmer B., Mühlau M. Automated segmentation of changes in FLAIR-hyperintense white matter lesions in multiple sclerosis on serial magnetic resonance imaging. NeuroImage: Clinical. 2019;23 doi: 10.1016/j.nicl.2019.101849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schur S., Allen V., White A., Mirsky D., Stence N., O'Neill B., Handler M., Dudley R., Laoprasert P. Significance of FDG-PET Hypermetabolism in Children with Intractable Focal Epilepsy. Pediatric Neurosurgery. 2018;53:153–162. doi: 10.1159/000487088. [DOI] [PubMed] [Google Scholar]
- Scott G., Mahmud M., Owen D.R., Johnson M.R. Microglial positron emission tomography (PET) imaging in epilepsy: Applications, opportunities and pitfalls. Seizure. 2017;44:42–47. doi: 10.1016/j.seizure.2016.10.023. [DOI] [PubMed] [Google Scholar]
- Speechley K.N., Sang X., Levin S., Zou G.Y., Eliasziw M., Smith M.L., Camfield C., Wiebe S. Assessing severity of epilepsy in children: preliminary evidence of validity and reliability of a single-item scale. Epilepsy & Behavior. 2008;13:337–342. doi: 10.1016/j.yebeh.2008.05.001. [DOI] [PubMed] [Google Scholar]
- Sun F.J., Zhang C.Q., Chen X., Wei Y.J., Li S., Liu S.Y., Zang Z.L., He J.J., Guo W., Yang H. Downregulation of CD47 and CD200 in patients with focal cortical dysplasia type IIb and tuberous sclerosis complex. Journal of Neuroinflammation. 2016;13:85. doi: 10.1186/s12974-016-0546-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezzani A., Aronica E., Mazarati A., Pittman Q.J. Epilepsy and brain inflammation. Experimental Neurology. 2013;244:11–21. doi: 10.1016/j.expneurol.2011.09.033. [DOI] [PubMed] [Google Scholar]
- Weichhart T., Costantino G., Poglitsch M., Rosner M., Zeyda M., Stuhlmeier K.M., Kolbe T., Stulnig T.M., Hörl W.H., Hengstschl äger, M., Müller, M., Säemann, M.D., The TSC-mTOR signaling pathway regulates the innate inflammatory response. Immunity. 2008;29:565–577. doi: 10.1016/j.immuni.2008.08.012. [DOI] [PubMed] [Google Scholar]
- Wirenfeldt M., Clare R., Tung S., Bottini A., Mathern G.W., Vinters H.V. Increased activation of Iba1+ microglia in pediatric epilepsy patients with Rasmussen's encephalitis compared with cortical dysplasia and tuberous sclerosis complex. Neurobiology of Disease. 2009;34:432–440. doi: 10.1016/j.nbd.2009.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie L., Sun F., Wang J., Mao X., Xie L., Yang S.H., Su D.M., Simpkins J.W., Greenberg D.A., Jin K. mTOR signaling inhibition modulates macrophage/microglia-mediated neuroinflammation and secondary injury via regulatory T cells after focal ischemia. Journal of Immunology. 2014;192:6009–6019. doi: 10.4049/jimmunol.1303492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M.T., Lin Y.C., Ho W.H., Liu C.L., Lee W.T. Everolimus is better than rapamycin in attenuating neuroinflammation in kainic acid-induced seizures. Journal of Neuroinflammation. 2017;14:15. doi: 10.1186/s12974-017-0797-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokokura M., Terada T., Bunai T., Nakaizumi K., Takebayashi K., Iwata Y., Yoshikawa E., Futatsubashi M., Suzuki K., Mori N., Ouchi Y. Depiction of microglial activation in aging and dementia: Positron emission tomography with C-11 DPA713 versus C-11 (R)PK11195. Journal of Cerebral Blood Flow and Metabolism. 2017;37:877–889. doi: 10.1177/0271678X16646788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B., Zou J., Rensing N.R., Yang M., Wong M. Inflammatory mechanisms contribute to the neurological manifestations of tuberous sclerosis complex. Neurobiology of Disease. 2015;80:70–79. doi: 10.1016/j.nbd.2015.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.