Summary
Large-scale, site-directed mutagenesis enables rapid characterization of the biochemical and biological properties of proteins. Here, we present a cost-effective and adaptable cloning pipeline to generate arrayed gene libraries for a construct of interest. We detail steps to use an open access web app to automate the design of mutagenesis primers optimized for our cloning protocols in a 96-well plate format. The protocol allows most molecular biology labs to clone 96 mutants (from PCR to sequence ready plasmid) in 3 days.
Subject areas: Genetics, Sequencing, High Throughput Screening, Molecular Biology
Graphical abstract

Highlights
-
•
Web app for automated generation of arrayed mutagenesis primer libraries
-
•
Optimized high-throughput site-directed mutagenesis PCR protocol
-
•
Streamlined bacterial transformation in a 96-well plate format
-
•
Automated magnetic-bead-based plasmid purification from transformed E. coli colonies
Publisher’s note: Undertaking any experimental protocol requires adherence to local institutional guidelines for laboratory safety and ethics.
Large-scale, site-directed mutagenesis enables rapid characterization of the biochemical and biological properties of proteins. Here, we present a cost-effective and adaptable cloning pipeline to generate arrayed gene libraries for a construct of interest. We detail steps to use an open access web app to automate the design of mutagenesis primers optimized for our cloning protocols in a 96-well plate format. The protocol allows most molecular biology labs to clone 96 mutants (from PCR to sequence ready plasmid) in 3 days.
Before you begin
This protocol describes the steps for creating arrayed mutagenesis libraries for three categories of mutations: site-saturation, phospho-site specific, and custom scanning. We describe the timing for creating up to 96 mutations at a time in DNA constructs designed for protein expression in E. coli. Importantly, the pipeline is amenable to mutagenesis of constructs for bacterial and mammalian expression systems and the number of mutations can be scaled down or up as required to meet the needs of the individual researcher. We recommend before you start that you confirm desired expression levels of your protein (wild-type) from the vector you will use to create a gene library. Our protocol also provides instructions for performing a preliminary test of the cloning pipeline to test reagents and cloning efficiency prior to initiating production of a large-scale gene library.
Institutional permissions (if applicable)
Not applicable.
Design primers using our open-source mutagenesis primer program, Primutant
Timing: 5–10 min
-
1.Design PCR primers specific to your construct of interest.
-
a.Access our free mutagenesis primer design program (Primutant) at the following web address: https://moklab.biochem.ualberta.ca/Primutant/ (Figure 1A). Alternatively, you can download the original source code and essential packages at Github: https://github.com/sueannmok/Primutant.git. Fill in the input boxes on the homepage with the required information then hit the submit button.
-
i.Enter the gene name or other unique identifier for your job. This text will be appended to the beginning of every primer name and the file name of your primer output file.
-
ii.Select the codon usage table appropriate for your protein expression system.
-
iii.Enter the nucleotide sequence for the region of interest. Minimal sequence requirements are the coding sequence for the protein of interest (includes start and stop codons) plus the 30 nucleotides upstream and downstream of this coding sequence within the vector.
-
iv.Enter the position number within the inputted sequence that corresponds to the start codon. For example, if you entered the minimal sequence requirements then the position number = 31.
-
v.Input the nucleotide position numbers encompassing the sequence region that you wish to mutagenize. For example, the start site should correspond to the first nucleotide of the codon where mutagenesis will begin.
-
vi.Choose the type of mutagenesis you wish to perform: Site-saturation, custom scanning, phosphorylation site.
-
i.
-
b.The .csv output containing primer information will be automatically downloaded. Open the file in a compatible application (e.g., Excel, Numbers) and check that it contains the desired mutagenesis primer pair sequences. There are built-in features for each designed primer (see Figure 1B) that assist the user in verifying the desired mutagenesis output as follows:
-
i.the residue number of the desired mutation relative to the start site.
-
ii.the corresponding amino acid before and after mutation contained within the primer name.
-
iii.within each mutagenesis primer sequence, the codon to be mutated is written in lowercase.
-
iv.the sequence of the mutated codon before and after mutation (e.g., CCC to ACC).Note: The primer program designs overlapping mutagenesis primer pairs compatible with site-directed mutagenesis cloning methods. The primer design takes into consideration multiple parameters including melting temperature, GC content, potential secondary structures, number of nucleotide changes and codon usage. For codon usage, rare codons (less than 0.11 fraction of all codons for a given amino acid, source: https://www.genscript.com/tools/codon-frequency-table) are excluded from being selected as substituting mutations in primers. The program also attempts to minimize the length of the oligos to decrease synthesis costs. Codon usage tables and more information on primer design parameters can be found at: https://moklab.biochem.ualberta.ca/Primutant/about.html.
-
i.
-
a.
-
2.
Synthesize mutagenesis primers.
Figure 1.
Primer design using the mutagenesis primer program, Primutant
(A) Image of primer program submission page with example input text.
(B) Example of .csv output file generated by Primutant.
Use a commercial service that delivers each mutagenesis primer pair into an individual well of a 96-well plate format.
Note: Each oligo in the primer pair should be present in equal amounts with a minimum of 5 nmol per oligo, per well. Oligos are generally shipped dry and later resuspended to an appropriate working concentration.
Note: The column format of the output primer file should allow for easy cut and paste entry into commercial primer synthesis ordering forms. We typically use Integrated DNA Technologies, Inc for synthesis of arrayed primer pairs delivered in 96-well plates.
Prepare competent cells in a 96-well plate format
Timing: 2 days
Note: This is a procedure for preparing E. coli competent cells that has been adapted from Inoue and colleagues.1,2 This recipe makes enough competent cells for four 96-well plates and can be scaled as needed.
-
3.Inoculate a starter culture.
-
a.Add to a sterile 250 mL Erlenmeyer flask:
-
i.50 mL of LB.
-
ii.100 μL of commercial NEB 10-beta E. coli competent cells.Alternatives: Other commercial competent E. coli cells can be used in place of NEB 10-beta cells (e.g., DH5α, TOP10).
-
i.
-
b.Incubate culture for 6–8 h at 37°C, 250–300 rpm.
-
a.
-
4.Grow multiple main cultures.
-
a.Prepare three 2 L flasks with 500 mL of LB each. Add 20 mL, 10 mL or 8 mL of starter culture to the three flasks.Note: Using three cultures increases the chances that one of the cultures will achieve the correct OD600 after the incubation period.
-
b.Incubate all three flasks for 16–18 h at 18°C, 200 rpm.
 CRITICAL: Culturing temperatures >22°C reduces the resulting transformation efficiency of cells.
CRITICAL: Culturing temperatures >22°C reduces the resulting transformation efficiency of cells. -
c.After the initial incubation period, read all three cultures at OD600. The optimal OD600 = 0.55. Continue incubating and reading all cultures every 45 min until one culture is in the OD600 range of 0.55–0.6 but not higher. Chill the appropriate culture on an ice-water bath for 10 min. Discard the other two cultures.
-
d.Pellet the cells at 2,500 × g for 10 min at 4°C. Discard as much supernatant as possible. Use a vacuum aspirator if necessary to remove any remaining liquid drops from the vessel.
-
a.
-
5.Prepare and freeze the competent cells.
-
a.Resuspend the cells GENTLY in 160 mL of ice-cold Inoue transformation buffer. Swirling of the vessel is recommended over pipetting. Do not vortex the suspension.
 CRITICAL: Make sure the Inoue transformation buffer has been pre-chilled prior to use.
CRITICAL: Make sure the Inoue transformation buffer has been pre-chilled prior to use. -
b.Repeat steps 4d and 5a except resuspend cells in only 40 mL of ice-cold Inoue transformation buffer.
-
c.Add 3 mL of DMSO (3.75% vol/vol final) to the cell suspension. Mix gently by swirling then chill on ice for 10 min.
-
d.Working quickly dispense 100 μL per well in pre-chilled 96 deep well plates. We use a repeat pipettor to assist in accurate and rapid aliquoting. Cover the deep well plate with a sealing film (−80°C compatible). Immediately immerse the bottom 50% of the deep well plate into liquid nitrogen to snap freeze cells. Store frozen cells in well plates at −80°C until needed.Note: When aliquoting competent cells it is also recommended to aliquot 100 μL each into 3–4 chilled sterile microfuge tubes and snap freeze similar to the well plate procedure described above. These aliquots can be used to test for plasmid contamination and transformation efficiency as per standard lab protocols.
-
a.
Prepare 96 well plates to store master glycerol stocks
Timing: 1 h
-
6.
Pipette 50 μL of sterile 60% glycerol solution to each well of a 96 well PCR plate. Use wide mouth pipette tips for more accurate pipetting of the viscous solution.
-
7.
Cover the PCR plate with a sealing film (−80°C compatible).
-
8.
Store the plates at −20°C until needed.
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Bacterial and virus strains | ||
| 10-beta Competent E. coli | New England Biolabs Inc. | Cat#C3019I |
| Chemicals, peptides, and recombinant proteins | ||
| Manganese(II) chloride tetrahydrate | Sigma-Aldrich | Cat#M-3634 |
| Calcium chloride dihydrate | Sigma-Aldrich | Cat#223506 |
| Potassium chloride | Sigma-Aldrich | Cat#P9541 |
| Piperazine-N,N′-bis(2-ethanesulfonic acid), piperazine-1,4-bis(2-ethanesulfonic acid), 1,4-piperazinediethanesulfonic acid (PIPES) | Sigma-Aldrich | Cat#P-6757 |
| Dimethyl sulfoxide | Sigma-Aldrich | CAS 67-68-5 |
| Luria Broth Base (Miller’s LB Broth Base)™, powder | Thermo Fisher | Cat#12795027 |
| LB Agar, Miller | Fisher Scientific | Cat#BP9724-500 |
| HyClone™ Water, Molecular Biology Grade, Cytiva | Fisher Scientific | Cat#SH3053802 |
| Phusion Hot Start Flex DNA Polymerase - 500 units | New England Biolabs (NEB) | Cat#M0535S |
| Dpni Restriction Enzyme, 20,000 units/mL, 5,000 units, New England Biolabs (NEB) R0176L | New England Biolabs (NEB) | Cat#R0176L |
| dNTP Set (100 mM) | Thermo Fisher | Cat#10297018 |
| Glycerol | Sigma-Aldrich | CAS 56-81-5 |
| Q5 Hot Start DNA polymerase | New England Biolabs (NEB) | Cat#M0493L |
| Critical commercial assays | ||
| Magjet Plasmid DNA Kit | Thermo Fisher Scientific | Cat#K2791 |
| Oligonucleotides | ||
| Primers for site-directed mutagenesis | This paper | N/A |
| Primer: T7 Forward: TAA TAC GAC TCA CTA TAG GG |
IDT | Cat#51-01-20-01 |
| Primer: T7 Reverse: GCT AGT TAT TGC TCA GCG G |
IDT | Cat#51-01-20-02 |
| Recombinant DNA | ||
| pET28a-0N4R tau | Mok et al. and Thompson et al.3,4 | N/A |
| pMCSG7 His-TEV-DNAJA2 | Mok et al. and Rauch and Gestwicki3,5 | N/A |
| pUC19 | New England Biolabs (NEB) | Cat#N3041S |
| Software and algorithms | ||
| Primutant | This paper | https://moklab.biochem.ualberta.ca/Primutant/index.php |
| Python script for Primutant | This paper | GitHub: https://github.com/sueannmok/Primutant.git, Zenodo: https://zenodo.org/badge/latestdoi/529993406 |
| Benchling | Benchling | https://www.benchling.com/ |
| Other | ||
| Breathable Sealing Film | CELLTREAT | Cat#229130 |
| Axygen® 48-well Clear V-Bottom 5 mL Polypropylene Rectangular Well Deep Well Plate | Corning | Cat#P-5ML-48-C |
| PCR Plate, 96-well, non-skirted | Thermo Fisher Scientific | Cat#AB0600 |
| Barrier (Filter) Tips, 200 μL size | Thermo Fisher Scientific | Cat#AM12655 |
| Square Petri Dish w/ Grid | Thomas Scientific | Cat#1173B67 |
| Nunc™ 96-Well DeepWell Plates with Shared-Wall Technology, Thermo Scientific, Color=Natural, Material=Polypropylene, Well Volume=2 mL, Sterility=Nonsterile | Thermo Scientific | Cat#278752 |
| Sealing Film PCR Tubes and Plates | Fisher Scientific | Cat#08-408-240 |
| Aluminum PCR Plate Seal (for freezing competent cells) | Thomas Scientific | Cat#1165R30 |
| Kingfisher Duo Prime | Thermo Fisher Scientific | Cat#5400110 |
| Benchtop Refrigerated Centrifuge 5810R with S-4-104 rotor | Eppendorf | Cat#022628092 |
| Plate bucket adaptors for benchtop refrigerated centrifuge, aerosol-tight capable, incl. plate carrier, 2 pcs. | Eppendorf | Cat#895125000 |
| 96-well PCR Thermal Cycler, Mastercycler Nex Grad 115V | Eppendorf | Cat#6331000025 |
| Incubator Shaker, New Brunswick Innova 42R | Eppendorf | Cat#M1335-0010 |
| Microplate Rack for Incubator Shaker | Eppendorf | Cat#TTR-221 |
| -80 freezer | Fisher Scientific | Cat#09313870 |
Materials and equipment
-
•Bacteria shaking incubator capable of incubating at temperature ranges of at least 22°C–37°C.
-
○An incubator adaptor for holding well plates is required for transformation and culturing steps.
-
○
-
•
A table top centrifuge equipped with well plate adaptors capable of spinning to a minimum of 3,000 × g.
-
•
An automated processing instrument for magnetic beads (e.g., KingFisher Flex from Thermo Scientific) can reduce active work time for plasmid purification steps.
Alternatives: a 96-well magnetic plate separation rack plus thermomixer can be used in place of the above instrument.
Luria Broth (LB): Mix reagents in an autoclavable bottle and autoclave using a liquid cycle to sterilize.
| Reagent | Amount |
|---|---|
| Luria Broth powder | 25 g |
| Deionized water | Up to a total of 1 L |
Store capped at ∼21°C.
LB Agar plate: Mix reagents in an autoclavable bottle and autoclave using a liquid cycle to sterilize. Cool to between 37°C and 50°C. Add antibiotic (e.g., Kanamycin/Ampicillin) to appropriate final working concentration. Pour LB agar into sterile square (10 cm) petri dishes, 20 mL per plate. Allow LB Agar plates solidify at room temperature (∼ 21°C) before use.
| Reagent | Amount |
|---|---|
| Luria Broth powder | 25 g |
| Agar | 17 g |
| Deionized water | Up to a total of 1 L |
Store at 4°C for up to one month.
Inoue transformation buffer: Add reagents in table to a sterile vessel. Sterilize buffer by filtration with a 0.22 μm filter. Aliquot into sterile 50 mL falcon tubes.
| Reagent | Final concentration | Amount |
|---|---|---|
| MnCl2⋅4H2O | 55 mM | 10.88 g |
| CaCl2⋅2H2O | 15 mM | 2.20 g |
| KCl | 250 mM | 18.65 g |
| PIPES (0.5 M, pH 6.7) | 10 mM | 20 mL |
| ddH2O | N/A | Up to a total of 1 L |
Store at −20°C for up to 2 years.
Glycerol solution (60%): Mix reagents in an autoclavable bottle and autoclave using a liquid cycle to sterilize.
| Reagent | Final concentration | Amount |
|---|---|---|
| Glycerol (100%) | 60% | 60 mL |
| ddH2O | N/A | 40 mL |
Store capped at ∼21°C.
Step-by-step method details
The following step-by-step method describes the workflow for creating a 96-well arrayed gene library. We recommend that you start by running the method with the small subset of test mutagenesis primer pairs (8 total) indicated in your primer output file (see Figure 1B). These primer pairs were selected to examine efficiency of low, mid, and high GC content primers under our standard PCR conditions. The test primers can be used to identify issues and optimize conditions for your specific vector sequence (see troubleshooting section) prior to performing reactions in higher throughput. The step-by-step method can be readily scaled down for the test primer run.
Site directed mutagenesis PCR
Timing: 4 h
-
1.Prepare mutagenesis primer stocks.
-
a.Resuspend the primer pairs in the original well-plate to a final concentration of 100 μM with molecular biology grade nuclease-free water.Note: We use a multichannel pipette and pipette the solution up and down several times to resuspend.
-
b.Perform two 10-fold serial dilutions to generate a 1 μM working secondary stock.
-
i.In a PCR 96 well plate add 10 μL of primer stock to 90 μL of molecular biology grade nuclease-free water.
-
ii.Pipette up and down several times to mix.
-
iii.Repeat the process in a second PCR plate to generate the final 1 μM working primer stock.Note: Mutagenesis primer stocks should be kept on ice at all times and stored at −20°C when not in use. We recommend minimizing the freeze-thawing of working stocks (maximum of 5 times). Handling and freeze-thawing of primary stocks should be minimized.Note: We recommend using multichannel pipettes with sterile filter tips throughout the following step-by-step method details section.
-
i.
-
a.
-
2.
Prepare PCR master mix on ice in a sterile 15 mL falcon tube. Amounts are provided per well and per 96 reactions. Add all reagents to a sterile tube and vortex briefly to mix.
CRITICAL: Add reagents in the order listed within the table.
PCR master mix (Phusion Hot Start Flex DNA Polymerase)
| Reagent | Amount per reaction | Amount per 96 well plate (includes 10% excess volume) |
|---|---|---|
| Nuclease Free Water | 33 μL | 3.49 mL |
| 5× High Fidelity Buffer | 10 μL | 1.06 mL |
| 25 mM dNTPs | 0.5 μL | 52.8 μL |
| Purified plasmid template in nuclease-free water (20 ng/μL) | 0.5 μL | 52.8 μL |
| Phusion Hot Start Flex DNA Polymerase | 1 μL | 105.6 μL |
| TOTAL | 45 μL | 4.75 mL |
-
3.Assemble the PCR reactions at ∼21°C.
-
a.Transfer the PCR master mix to a reagent reservoir for pipetting.
-
b.Add 45 μL of PCR master mix to each well of a 96-well PCR plate using a multichannel pipette.
-
c.Transfer 5 μL of mutagenesis primer working stock to individual wells (1 primer pair per reaction).
-
d.Thoroughly mix the PCR master mix and primer stock by pipetting up and down several times (without introducing air bubbles) prior to transferring into the reaction plate.
-
e.Adhere a PCR compatible sealing film to the assembled plate.
-
a.
Note: We recommend retaining the same well position for the primer working stock plate and the PCR plate to assist in identification. We have included an example .csv template (Data S1) for cataloguing the identity of each well position that can also be used throughout the procedure steps (transformation, glycerol stocks, plasmid purification).
-
4.
Place the PCR plate in the thermocycler and run under the following conditions:
PCR cycling conditions (Phusion Hot Start Flex DNA polymerase)
| Steps | Temperature | Time | Cycles |
|---|---|---|---|
| Initial Denaturation | 98°C | 30 s | 1 |
| Denaturation | 98°C | 30 s | 16 cycles |
| Annealing | 55°C | 60 s | |
| Extension | 72°C | 30 s per kb | |
| Final extension | 72°C | 5 min | 1 |
| Hold | 10°C | forever | |
Note: The standard annealing temperature (55°C) is optimized for compatibility with mutagenesis primers designed using our program (Primutant). The PCR cycling conditions are slightly modified from the recommended manufacturer protocols. We have found that longer annealing and extension times improve template amplification.
Alternatives: We have found Phusion Hot Start Flex polymerase yields positive results for the majority of cloning reactions we tested (see expected outcomes). However, for failed reactions we have found partial success with other high-fidelity polymerases such as Q5 DNA Polymerase (see troubleshooting 1). We recommend using a Hot Start polymerase to allow for assembly of reactions at ∼21°C. PCR reactions can be run in PCR tube strips if required.
-
5.Perform a DpnI digest to degrade the parent plasmid template.
-
a.Prepare a DpnI master mix:
Reagent Amount per reaction Amount per 96 well plate (includes 10% excess volume) 10× CutSmart Reaction Buffer 6 μL 633.6 μL DpnI (20 units/μL) 1 μL 105.6 μL -
b.Add 7 μL of DpnI master mix to each PCR reaction.
-
c.Incubate the PCR+DpnI reaction plate at 37°C for 90 min.
-
d.Heat inactivate the DpnI by incubating the PCR+DpnI reaction plate at 80°C for 20 min.
-
a.
-
6.Confirm successful PCR amplification.
-
a.Choose eight reactions from the PCR+DpnI reaction plate (e.g., 1 per row) and run 40 μL on a 1% agarose gel. One band corresponding to the size of the full-length vector should be observed in the presence of commercial DNA stains (Figure 2).
-
a.
Note: The expected yield of amplified PCR products in each reaction is low (<320 ng). Therefore, a sufficient volume of the PCR reaction must be loaded on the agarose gel to be observed by your DNA stain and detection system. If a PCR product band is faint/not observed, the PCR reaction may still yield successful clones at a reduced rate.
-
7.
Store the PCR+DpnI reaction plate at −20°C until ready to proceed to the Bacterial Transformation step.
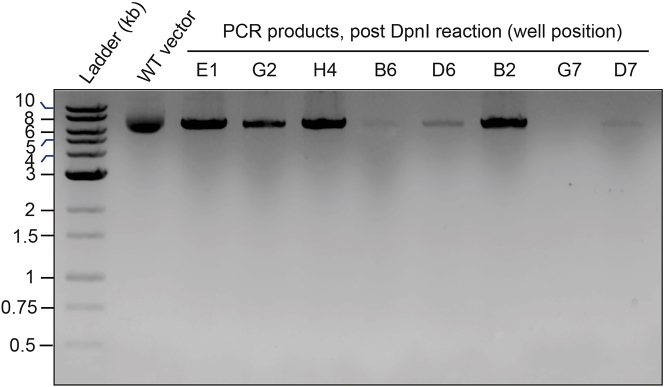
Figure 2.
Verification of amplified products following site-directed mutagenesis PCR and DpnI digestion
Samples of selected PCR+DpnI reactions using WT pET28a-0N4R tau vector template (6,493 bp) and mutagenesis primer pairs. A sample of WT pET28a-0N4R tau (WT vector) is run for reference. The intensity of the expected full-length PCR product can vary across the plate. Note: All presented reactions except for well G7 gave rise to clones with the desired mutation.
Bacterial transformation
Timing: 2 h
Amplified products from individual mutagenesis PCR reactions are transformed into competent E. coli cells in a 96-well plate format.
-
8.
Thaw a prepared 96 deep well plate containing E. coli competent cells on ice.
-
9.
Add 5 μL of each PCR+DpnI reaction to individual wells containing competent cells.
Note: We recommend retaining the same well positions for samples between the PCR+DpnI reaction plate and the competent cells plate to assist in identification.
Note: The PCR+DpnI reaction plate should be retained at −20°C in the event that the transformation step needs to be repeated.
-
10.
Incubate the competent cell plate on ice for 30 min.
-
11.
Heat shock competent cells by partially submerging the plate in a water bath at 42°C for 20 s.
Note: The length of the heat shock step will differ if using other strains of E. coli competent cells. Follow manufacturer protocols.
-
12.
Immediately return the plate on ice and incubate for 5 min.
-
13.
Add 700 μL of LB to each well.
-
14.
Cover the deep well plate with a sealing film and incubate in bacterial shaker at 37°C for 1 h, 250 rpm.
-
15.
Pellet the cells at 3,000 × g for 5 min at ∼21°C.
-
16.
Remove 600 μL of the supernatant and gently resuspend cells by pipetting solution up and down several times.
-
17.
Plate 8 μL spots of each cell suspension onto prepared LB agar plates containing the appropriate antibiotic.
Note: We recommend the following organization for placement of drops on each square LB agar plate: five drops of cell suspension per reaction, two rows of reaction per plate (Figure 3). You will need 6 LB agar plates total to plate all transformants from a full 96-well plate.
Note: We find that square LB agar plates are optimal for arraying transformant spots. However, standard round petri dishes can also be used (8 mutants per plate, 12 plates total).
-
18.
Incubate the LB agar plates containing transformants overnight (16–18 h) at 37°C. Representative results for a plate are shown in Figure 3B. The objective is to have spots containing multiple, single isolated colonies. If there are too many or no transformants see troubleshooting 2.
-
19.
Plates containing transformants can be stored at 4°C until ready to proceed to plasmid purification step or for up to 2 weeks.
Figure 3.
Representative results of plated colonies following bacterial transformation
(A) A schematic demonstrating organization system for plating transformations - deep well transformation plate with highlighted wells (left) and corresponding locations of positive transformants after plating on LB agar plates (right). Created with Biorender.com
(B) A representative example of plated transformants following overnight incubation (16–18 h). Note: the plated “transformation spots” contain separated single colonies suitable for plasmid purification.
Plasmid purification
Timing: 2 days
Individual colonies from transformant plates are isolated for high-throughput plasmid production and purification.
-
20.
Place 3 mL of LB and appropriate antibiotic into each well of two X 48-deep well plates.
-
21.
Use a sterile pipette tip to pick a single colony for each of the 16 different mutagenesis attempts per LB agar plate.
-
22.
Transfer each tip into one well of the 48-deep well plate.
Note: We recommend arranging the well positions of each colony so that they match the organization of the original PCR-DpnI reaction plate (Figure 4). This will also allow for easier transfer of bacteria to the 96 well format used during plasmid purification steps.
-
23.
Seal the 48- deep well plate with a breathable sealing film.
-
24.
Incubate the bacterial cell cultures at 37°C for up to 16 h, 300 rpm.
Note: The rpm conditions listed are for an incubator shaker with a 1-inch orbit diameter. Depending on your shaker parameters, the rpm may need to be adjusted to achieve aeration required for exponential bacterial growth. Changing the rpm could also lead to cross contamination if the liquid rises too high in the wells during shaking. Thus, the volume of the culture may need to be decreased at higher rpm settings.
-
25.Create a glycerol stock of each culture.
-
a.Thaw a glycerol master plate to room temperature (∼21°C).
-
b.Transfer 50 μL of each culture to individual wells of the glycerol master plate.
-
c.Freeze the glycerol master plate.
-
a.
-
26.
Pellet the remaining cells in the 48-deep well plates at 3,200 × g for 10 min at ∼21°C.
-
27.Lyse the bacterial cells and centrifuge debris.
-
a.Invert the plate to remove the supernatant without disturbing the cell pellet.
 Pause point: The plate containing cell pellets (no supernatant) can be covered and stored at −20°C.
Pause point: The plate containing cell pellets (no supernatant) can be covered and stored at −20°C. -
b.Add 200 μL of resuspension buffer to each well. Pipette up and down after addition to resuspend the cells.Note: Solutions for purifying plasmid DNA are provided in the MagJET Plasmid DNA Kit which is optimized for use with the KingFisher Flex/Duo instruments.
-
c.Add 200 μL of lysis solution and mix gently by shaking the 96 well plate on a flat surface for 4–6 times until the solution becomes viscous and slightly clear.
-
d.Let sit for 2 min at room temperature (∼21°C).
 CRITICAL: Do not shake the plate vigorously (shears chromosomal DNA) or allow the incubation to proceed past 2 min (denatures supercoil plasmid DNA).
CRITICAL: Do not shake the plate vigorously (shears chromosomal DNA) or allow the incubation to proceed past 2 min (denatures supercoil plasmid DNA). -
e.Add 200 μL neutralization solution and mix by shaking the 96 well plate on a flat surface for 4–6 times.
-
f.Add 50 μL of isopropanol per well and mix by shaking the 96 well plate on a flat surface for 4–6 times.
-
g.Once the entire plate is processed to step 27f, centrifuge at 3,200 × g for 10 min at room temperature (∼21°C).
 CRITICAL: Since it is difficult to achieve this timing with a 96 well plate, we recommend proceeding with steps 27b–27f for only 24 wells (3 plate columns) at a time then repeating the steps, 24 wells at a time, until the entire plate is processed (4 rounds total). For steps 27b–27f, add solutions to wells as quickly as possible and mix immediately.
CRITICAL: Since it is difficult to achieve this timing with a 96 well plate, we recommend proceeding with steps 27b–27f for only 24 wells (3 plate columns) at a time then repeating the steps, 24 wells at a time, until the entire plate is processed (4 rounds total). For steps 27b–27f, add solutions to wells as quickly as possible and mix immediately.
-
a.
-
28.While the plate is centrifuging, prepare deep well plates and elution strips for processing samples via KingFisher Duo/Flex instrument according to the MagJET Plasmid DNA kit User Guide.
-
a.Add wash 1, wash 2, and elution buffers to wells as directed.
-
b.Resuspend the MagJET Magnetic beads by vortexing and add 25 μL per well to the “Sample” plate. Add 250 μL of isopropanol to each well of the Sample plate.
-
a.
-
29.
Slowly transfer 500 μL of cleared lysate from each well into the Sample plate. Immediately seal the plate tightly and quickly shake up and down 4–6 times by hand.
CRITICAL: Avoid touching the pelleted debris during transfer of lysate to the Sample plate.
-
30.
Follow the MagJET Plasmid DNA kit User Guide for placing the required plates and elution strips into the KingFisher Flex/Duo instrument. Start the appropriate Plasmid DNA isolation program.
-
31.
Once the program is completed, transfer the eluate containing plasmid DNA to a sterile PCR plate. Cover the PCR plate with a sealing film and store at −20°C until ready to proceed with sequence verification of clones.
Note: We use a KingFisher Duo instrument for our magnetic bead-based purification of plasmid DNA. Other commercial instruments are capable of magnetic bead processing and may be substituted if they have a compatible plasmid DNA isolation kit. Plasmid purification may also be carried out manually using magnetic plate holders plus a thermomixer (see protocol in User Guide) but it will require more active work time to process the same number of samples.
Figure 4.
Reference well position system between 96 well plates and 48 well plates used for growth of bacterial cultures for plasmid purification
A schematic of a 96 well plate containing PCR+DpnI reactions (left) and the corresponding wells arranged in two 48 well plates, both rotated 90 degrees (right). Created with Biorender.com
Sequence verification of mutant clones
Timing: 2 days
Purified plasmid is prepared for Sanger sequencing and validated for desired mutation.
-
32.
Quantify DNA concentration of purified plasmids using a nanodrop or other low volume method. If plasmid yields are low, see troubleshooting 3.
Note: We have found that the plasmid concentrations for plasmids is fairly uniform across the plate when purified using the KingFisher Duo instrument. We typically spot check DNA concentrations for 10–12 samples chosen at random across the plate.
-
33.
Sequence the plasmids with primers that will provide sequence coverage across the region being mutated.
Note: There are commercial services for Sanger sequencing of DNA samples in high-throughput plate format. This is typically more economical than individual reaction rates and assists in sample organization.
-
34.Validate sequencing in batch using Benchling web-based software.
-
a.Import your plasmid sequence into Benchling using an appropriate file format (e.g., Genbank, FASTA).
-
b.Annotate the sequence using the annotations tab if needed. For example, you can indicate the coding sequence, reading frame of translated protein, and the sub-regions you are mutagenizing to assist in verifying mutations.
-
c.Use the alignments tab to align your Sanger sequence files with the plasmid sequence. We prefer uploading the .ab1 file format so that we can easily visualize chromatogram quality within the alignment. Multiple sequence files can be uploaded at the same time and analyzed in batch format.
-
d.Check sequence alignments.
-
i.Use the alignment window at the bottom of the page to easily find mismatches that potentially correspond to the mutagenized codon.
-
ii.Zoom in on individual sequence regions to verify the correct mutation has been created. Verify that the quality of the sequencing chromatogram supports the base calling. See troubleshooting 4 and 5 if the correct mutation cannot be verified.
-
i.
-
a.
Note: We use Benchling to align sequencing results because the alignment tools are free for academic users and it is user friendly with respect to creating and analyzing batch alignments. However, any alignment program can be used.
Note: If the sequencing result does not confirm the desired mutation, more colonies can be picked from the transformant plate for plasmid purification and sequencing.
Expected outcomes
Here we have provided a standardized cloning pipeline for the creation of arrayed, single missense mutation libraries for your expression construct of interest. The procedures are designed to be applied to any vector template of interest with minimal optimization required. Our primer program provides options for three categories of mutagenesis: phospho-site, site saturation, and custom scanning. We have tested outputs from each of these categories using our cloning pipeline. Primer output files are found in Data S2. Sequence files for target plasmids are provided in Data S3 and S4.
We performed a site saturation mutagenesis on four separate residue sites in pET28a 0N4R tau, a bacterial expression vector for the human tau protein isoform, 0N4R. A total of four residues in the tau coding sequence were chosen to test the effects of GC content variation in the primer pair sequences (N279=39%, P301=49%, S352= 52%, G272= 61%). For each residue being tested, 19 mutagenesis primer pairs were generated to mutate the residue of interest to the other 19 amino acid possibilities in separate reactions. We ran all the primer pairs through the cloning pipeline and reported the results for the first attempt (Figure 5A). We observed a high success rate, with 83% (63/74 reactions) producing a clone with the desired mutated construct. For the remaining reactions, there were multiple sources of failure: insertion of primer sequence concatemers into the coding sequence (2 clones), retention of the WT codon (5 clones) or failed sequencing reactions (4 clones). Only 2 out of 74 reactions did not yield any colonies following transformation. To validate more positive clones, we followed our standard Troubleshooting procedures. First, we picked a second colony when available (14.5%, 11 reactions). For 9 out of 11 reactions the second colony contained the desired mutation, increasing the success rate for the experiment to 95% overall (Figure 5B). For the remaining failed reactions, we performed a second run, repeating the entire cloning procedure. This yielded only one additional validated clone upon sequencing. We carried out further analysis of our dataset to try and detect other factors that could influence success rates of mutagenesis. We did not observe any relationship between the mutated amino acid identity and success rates (Figure 5C). In Figure 5D, we graphed the success rate of generating a positive mutant clone according to the number of mismatched nucleotide bases between the primer and the template (1, 2, or 3 nucleotides). We found high cloning efficiency for mutagenesis of 1 base (100%) and 2 bases (97.4%). The cloning efficiency for 3 mismatched nucleotides in the primer was slightly lower at 81.3%. When we mapped the cloning results for each mutagenesis reaction according to GC content, we did not find that high or low GC content of the primers greatly altered the cloning efficiency (Figure 5E).
Figure 5.
Compiled results for generation of an arrayed site-saturation mutagenesis library
Mutagenesis targets were four residues (G272, N279, P301, S352) in the 0N4R tau coding sequence of pET28a-0N4R tau.
(A) The results for all targeted mutant reactions after the first run-through of the cloning procedure.
(B–E) Results indicating the stage within the cloning pipeline when a positive clone was isolated for a targeted mutant within the target set (n=76) but partitioned according to sequence features. (B) Overall results, no partitioning. (C) Results arranged according to WT residue (left to right) and mutation cloned. White, hatched boxes indicate a match with the WT residue in which case no primer pair was generated. (D) By number of nucleotide mismatches in the primer sequence. (E) By GC content (%) of the primer.
We performed phospho-site mutagenesis in the same pET28a 0N4R tau vector. All 66 Serine and Threonine codons located within the 0N4R tau coding sequence were targeted to generate mutagenesis primer pairs for conversion to glutamate codons. Note that the targeted serine and threonine sites spanned throughout the coding sequence region (Figure 6A). We ran all the primer pairs though the cloning pipeline and sequenced purified plasmids from the first colony picked. We achieved an overall success rate of 62.1% (41/66) for generating the desired mutation within the first clone tested (Figure 6B). The 25 unsuccessful mutagenesis reactions could partially be attributed to errors in the clone sequence such as incorporated primer concatemers (3%, 2 clones), unwanted mutations (3%), or retention of the WT codon (4.5%). For the remaining reactions, the cloning procedure failed at an intermediate stage i.e., sequencing reaction failed (9%) or the transformation reaction failed to produce colonies (18%). To validate more positive clones, we again followed the strategies outlined in the troubleshooting section. First, we picked a second colony if available (19.5%, 13 clones). We were able to validate successful mutagenesis for 10/13 of these clones (Figure 6C) bringing the overall mutagenesis success rate up to 77.3% (51/66). For the remaining failed reactions, we found that repeating a second run or reordering the primer pairs led to success for an additional 12 out of 15 reactions. Thus, we were able to obtain a final tally of 63 out of 66 desired clones (95.5%). The effect of reordering primers on producing validated clones points to potential issues that can arise due to compromised synthesis, resuspension or integrity of primer stocks. Similar to our site saturation experiments, we performed an analysis to identify primer sequence parameters regulating cloning efficiency. We graphed the stage at which a positive clone was validated based on whether the phosphosite was a serine or threonine (Figure 6D), the number of nucleotide mismatches in the primer sequence (Figure 6E), the location of the mutation site (Figure 6F) and the GC content of the mutagenesis primer (Figure 6G). The only trend we detected was that initial success rates were lower when the primer sequence contained three nucleotide mismatches, similar to our site saturation experiment.
Figure 6.
Compiled results for generation of an arrayed phosphomimetic mutagenesis library
Targeted mutation of serine and threonine residues in the human 0N4R tau coding sequence to glutamate (vector = pET28a-0N4R tau).
(A) Schematic showing the relative location of serine and threonine residues in the human 0N4R tau coding sequence. Proline-rich and repeat domains (R1-R4) associated with the microtubule-binding region are indicated. Numbering of the 0N4R tau sequence is based on standard convention using the longest tau isoform, 2N4R. The grey hatched box indicates the residues present in the 2N4R tau isoform sequence but lacking in 0N4R tau.
(B) The results for all targeted mutant reactions after the first run-through of the cloning procedure.
(C–G) Results indicating the stage within the cloning pipeline when a positive clone was isolated for a targeted mutant within the group (n=66) but partitioned according to sequence features. (C) Overall results, no partitioning. (D) By identity of the WT residue, serine (S) or threonine (T). (E) By number of nucleotide mismatches in the primer sequence. (F) By location of the targeted residue within the 0N4R sequence. The grey hatched segment indicates residues present in the 2N4R tau isoform sequence but lacking in 0N4R. (G) By GC content (%) of the primer.
Finally, we tested our scanning mutagenesis option on a bacterial expression vector for the molecular chaperone, human DNAJA2 (pMCSG7-His-TEV-DNAJA2). We chose to mutate all residues in the region spanning 78–168 of the DNAJA2 coding sequence to alanine (82 reactions total). We achieved success rates of 78% for the first attempt. Similar to the site saturation experiment, reactions failed for typical reasons: primer concatemers (10 clones), unwanted secondary mutations (3 clones) or failed sequencing reactions (5 clones) (Figure 7A). Testing a second clone increased the success rate to 93%, leaving only 6 out of 82 reactions with no clone verified (Figure 7B). We did not find any clear factors (GC content, 1 versus 2 nucleotide mismatches) tied to mutagenesis reaction success (Figures 7C and 7D).
Figure 7.
Compiled results for generation of an arrayed custom scanning mutagenesis library
Residues 78–169 of the human DNAJA2 coding sequence were targeted for mutation to alanine (vector = pMCSG7-His-TEV-DNAJA2).
(A) The results for all targeted mutant reactions after the first run-through of the cloning procedure.
(B–E) Results indicating the stage within the cloning pipeline when a positive clone was isolated for a targeted mutant within the group (n=82) but partitioned according to sequence features. (B) Overall results, no partitioning. (C) By number of nucleotide mismatches in the primer sequence. (D) By location of the targeted residue within the human DNAJA2 coding sequence. (E) By GC content (%) of the primer.
In summary, we found 90%–100% success rates in generating each category of arrayed mutant libraries using our cloning pipeline. We believe this procedure will be accessible to most standard molecular biology labs. Moreover, the cloning pipeline can be readily adapted to create combinations of mutations within the same coding sequence or in different expression constructs. In many cases, the same primer pairs from the original library can be reused leading to significant cost savings.
Limitations
We have had success with our mutagenesis cloning pipeline in the context of multiple vectors and sequences. However, variation in the sequence composition of a vector template (e.g., DNA secondary structure potential, GC-rich regions, vector length) could affect mutagenesis cloning efficiency when using our protocol. For this reason, we recommend first running the entire procedure with the test primer pairs indicated in the primer output file and optimizing key conditions if necessary (See troubleshooting section). The source code for our primer program is also available if the end user wishes to modify the original primer design parameters for their project.
Troubleshooting
Problem 1
Step 6: No PCR product is observed when run on a DNA agarose gel.
Potential solution
If no DNA band corresponding to the full-length product is observed it does not always mean that the reaction will not yield transformants. However, the probability of success is lower than if a band is detected. First, check that a sufficient amount of DNA sample is loaded which can be detected by your stain and detector system. Quantified DNA ladder markers can be used to estimate your limit of DNA detection. Detection of as little as 50 ng of a 1 kb DNA fragment is preferable. If loading is not the source of error, we have found that the three major sources of failed PCR are issues with 1) human error, 2) primer quality, and 3) DNA polymerase selection. We recommend repeating the cloning procedure as pipetting errors can lead to failed cloning. If the desired mutation is not achieved after a second attempt, we recommend reordering the primers and repeating the cloning pipeline again. If the PCR still does not produce the desired amplified product, consider performing the PCR reaction with another high-fidelity polymerase such as Q5 DNA polymerase. We have provided example PCR conditions for use with Q5 DNA polymerase below. If amplified products are now detected, proceed with the rest of the method as written. If switching the DNA polymerase still fails to produce correct mutant clones, reorder the corresponding primer pairs and repeat the entire cloning procedure.
PCR master mix (Q5 Hot Start DNA polymerase)
| Reagent | Amount per reaction | Amount per 96 well plate (includes 10% excess volume) |
|---|---|---|
| 5× Q5 reaction Buffer | 10 μL | 1.06 mL |
| 5× GC Enhancer | 10 μL | 1.06 mL |
| 25 mM dNTPs | 0.5 μL | 52.8 μL |
| Q5 Hot Start DNA Polymerase | 1 μL | 105.6 μL |
| Purified plasmid template in ddH2O (20 ng/μL) | 0.5 μL | 52.8 μL |
| ddH2O | 23 μL | 2.43 mL |
| TOTAL | 45 μL | 4.75 mL |
∗Aliquot 45 μL of master mix into each reaction. Add 5 μL of primer pair working stock (1 μM) to each reaction before proceeding to PCR cycling.
PCR cycling conditions (Q5 Hot Start DNA polymerase)
| Steps | Temperature | Time | Cycles |
|---|---|---|---|
| Initial Denaturation | 98°C | 30 s | 1 |
| Denaturation | 98°C | 30 s | 16 cycles |
| Annealing | 64°C | 60 s | |
| Extension | 72°C | 30 s per kb | |
| Final extension | 72°C | 5 min | 1 |
| Hold | 10°C | forever | |
Problem 2
Step 18: No transformed colonies or densely transformed areas lacking single isolated colonies.
Potential solution
Individual preparations of competent cells may have varying transformation efficiencies. In our quality control tests, we aim to have a transformation efficiency of 1 × 106 colony forming units for every 1 ng of pUC19 plasmid transformed into 50 μL of competent cells. If your transformation efficiency is a much higher or lower value, it may be necessary to adjust the concentration of the competent cell suspension to be plated to achieve the correct transformant density. This can be readily done via modification of step 16 of the protocol, by adjusting the amount of supernatant removed from the plate before resuspending cells. Another solution is to perform several 2× serial dilutions of your cell suspension and spotting 8 μL of each diluted sample onto your transformant plate. You may also check if the lack of colonies is due to failure upstream at the PCR reaction step (see troubleshooting 1).
Problem 3
Step 20–32: Low plasmid yields after purification.
Potential solution
One common source of error for this problem is poor health of the bacterial culture. Insufficient aeration of the culture or prolonged growth of the culture can lead to slow growth or cell death and lysis. An indicator of a healthy culture in our hands is an OD600 between 2.0-3.0 following a 16 h incubation. Picking colonies from old transformant plates can also lead to poor plasmid yields. In this case, redo the transformations for the corresponding PCR+DpnI reactions. If the culturing conditions appear correct, there are additional sources of error during the plasmid purification procedure that can lead to low plasmid yields. Consult the MagJET Plasmid DNA kit User Guide for other troubleshooting solutions.
Problem 4
Step 34: Clone contains the wild-type sequence at targeted residue site.
Potential solution
Pick another colony from the transformant plate for plasmid purification and sequence verification. If the second clone is also wild-type, check the activity of your DpnI enzyme. Incubate 500 ng of your template plasmid with 20 units of DpnI in a total reaction volume of 60 μL. Also perform a control reaction with no DpnI enzyme. Follow digestion conditions described in steps 5c and 5d of the step-by-step method. Run 40 μL of the digest and control reactions on a 1% agarose gel as described in step 6 of the step-by step method. In the lane containing the control reaction sample, you should observe bands corresponding to migration of the supercoiled/nicked plasmid. You should only observe smaller or no fragments in the lane containing the DpnI digested reaction. If your DpnI appears active, repeat the PCR reaction with the corresponding primer pair and test new transformants. If you still obtain the wild-type sequence, check for other solution in section troubleshooting 1.
Problem 5
Step 34: Clone sequence contains extra mutations, insertions or deletions.
Potential solution
Pick another colony from the transformant plate for plasmid purification and sequence verification. If the second clone also has undesired mutations, try repeating the entire cloning procedure. If the desired mutation is still not achieved, repeat the cloning procedure using a different high-fidelity polymerase such as Q5 DNA polymerase (see troubleshooting 1). On some occasions, we have observed insertion of concatenated primer sequences at the targeted residue site. Although we still do not understand the mechanism by which this error occurs, we have found some success in eliminating this issue by varying our PCR conditions. Firstly, lowering the primer concentration or adjusting the annealing temperature can resolve this issue. We recommend trying a gradient of annealing temperatures ranging from 54°C to 68°C and lowering the primer content to half of the initial concentration. If the issue is still not resolved, using a high-fidelity DNA polymerase such as Q5 may resolve the issue.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Sue-Ann Mok (sueann@ualberta.ca).
Materials availability
This study did not generate new unique reagents.
Acknowledgments
We thank Hallie Ng and Akhil Patel for their technical support on the project. We thank Allan Yarahmady and the other members of the Mok Lab for helpful discussions. This work was supported by the following funding sources: Alberta Prion Research Institute, Alzheimer Society of Alberta and Northwest Territories, Canada Foundation for Innovation, and donors of the ADR, a program of the BrightFocus Foundation (Award #: A2022044S).
Author contributions
Conceptualization, S.A.M.; Investigation and testing, K.T.S., T.S.P., J.K., H.S.H.T., G.E.-S., H.S., D.S., and S.A.M.; Writing – Original draft, K.T.S., T.S.P., and S.A.M.; Writing – Review and editing, K.T.S., T.S.P., and S.A.M.
Declaration of interests
The authors declare no competing interests.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.xpro.2022.101930.
Contributor Information
Kerry T. Sun, Email: ksun4@ualberta.ca.
S.A. Mok, Email: sueann@ualberta.ca.
Supplemental information
3
Data and code availability
The code generated for our primer program, Primutant, is available and deposited in GitHub: https://github.com/sueannmok/Primutant.git. The code is also archived at Zenodo: https://zenodo.org/badge/latestdoi/529993406. The plasmid sequences and primer output files used in this study are available in Data S2, S3, and S4.
References
- 1.Inoue H., Nojima H., Okayama H. High efficiency transformation of Escherichia coli with plasmids. Gene. 1990;96:23–28. doi: 10.1016/0378-1119(90)90336-. [DOI] [PubMed] [Google Scholar]
- 2.Sambrook J., Russell D.W. 3rd ed. Cold Spring Harbor Laboratory Press; 2001. Molecular Cloning: A Laboratory Manual. [Google Scholar]
- 3.Mok S.-A., Condello C., Freilich R., Gillies A., Arhar T., Oroz J., Kadavath H., Julien O., Assimon V.A., Rauch J.N., et al. Mapping interactions with the chaperone network reveals factors that protect against tau aggregation. Nat. Struct. Mol. Biol. 2018;25:384–393. doi: 10.1038/s41594-018-0057-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thompson A.D., Scaglione K.M., Prensner J., Gillies A.T., Chinnaiyan A., Paulson H.L., Jinwal U.K., Dickey C.A., Gestwicki J.E. Analysis of the tau-associated proteome reveals that exchange of Hsp70 for Hsp90 is involved in tau degradation. ACS Chem. Biol. 2012;7:1677–1686. doi: 10.1021/cb3002599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rauch J.N., Gestwicki J.E. Binding of human nucleotide exchange factors to heat shock protein 70 (Hsp70) generates functionally distinct complexes in vitro. J. Biol. Chem. 2014;289:1402–1414. doi: 10.1074/jbc.M113.521997. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
3
Data Availability Statement
The code generated for our primer program, Primutant, is available and deposited in GitHub: https://github.com/sueannmok/Primutant.git. The code is also archived at Zenodo: https://zenodo.org/badge/latestdoi/529993406. The plasmid sequences and primer output files used in this study are available in Data S2, S3, and S4.