Highlights
-
•
Empathic concern is an other-oriented emotional empathy that fosters compassion.
-
•
Amyloid-β positive healthy older adults had greater empathic concern gains.
-
•
Amyloid-β positivity related to greater salience network connectivity.
-
•
Empathic concern predicted salience network hyperconnectivity in the amyloid-β positive group.
Keywords: Preclinical, Alzheimer's disease, Social cognition, Compassion, Anterior cingulate cortex, Salience network
Abbreviations: AD, Alzheimer’s disease; MCI, mild cognitive impairment; APOE, apolipoprotein E; Aβ, amyloid-β; PET, positron emission tomography; MRI, magnetic resonance imaging; tf-fMRI, task-free functional magnetic resonance imaging; VBM, voxel-based morphometry; ACC, anterior cingulate cortex; PAG, periaqueductal gray; IRI, Interpersonal Reactivity Index
Abstract
Enhanced emotional empathy, the ability to share others’ affective experiences, can be a feature of Alzheimer’s disease (AD), but whether emotional empathy increases in the preclinical phase of the disease is unknown. We measured emotional empathy over time (range = 0 – 7.3 years, mean = 2.4 years) in 86 older adults during a period in which they were cognitively healthy, functionally normal, and free of dementia symptoms. For each participant, we computed longitudinal trajectories for empathic concern (i.e., an other-oriented form of emotional empathy that promotes prosocial actions) and emotional contagion (i.e., a self-focused form of emotional empathy often accompanied by feelings of distress) from informant ratings of participants’ empathy on the Interpersonal Reactivity Index. Amyloid-β (Aβ) positron emission tomography (PET) scans were used to classify participants as either Aβ positive (Aβ+, n = 23) or negative (Aβ-, n = 63) based on Aβ-PET cortical binding. Participants also underwent structural and task-free functional magnetic resonance imaging approximately two years on average after their last empathy assessment, at which time most participants remained cognitively healthy. Results indicated that empathic concern, but not emotional contagion, increased more over time in Aβ+ participants than in Aβ- participants despite no initial group difference at the first measurement. Higher connectivity between certain salience network node-pairs (i.e., pregenual anterior cingulate cortex and periaqueductal gray) predicted longitudinal increases in empathic concern in the Aβ+ group but not in the Aβ- group. The Aβ+ participants also had higher overall salience network connectivity than Aβ- participants despite no differences in gray matter volume. These results suggest gains in empathic concern may be a very early feature of AD pathophysiology that relates to hyperconnectivity in the salience network, a system that supports emotion generation and interoception. A better understanding of emotional empathy trajectories in the early stages of AD pathophysiology will broaden the lens on preclinical AD changes and help clinicians to identify older adults who should be screened for AD biomarkers.
1. Introduction
Emotional alterations are a poorly understood feature of Alzheimer’s disease (AD). While numerous prior studies of AD have investigated cognitive symptoms (McKhann et al., 1984), less is known about the changes in emotions and empathy that also emerge across the disease course (e.g., Fernandez-Duque et al., 2010, Lyketsos and Olin, 2002, Rankin et al., 2006). In the symptomatic phase of AD, progressive deterioration of the default mode network is associated with decline in episodic memory as well as other areas of cognitive functioning (Andrews-Hanna et al., 2010, Buckner et al., 2008, Greicius et al., 2003, Pini et al., 2016, Raichle, 2015, Seeley et al., 2009). The default mode network interacts with the salience network, a distributed brain system that supports emotion generation and interoception (Jilka et al., 2014, Menon and Uddin, 2010). With hubs in the anterior cingulate cortex (ACC) and ventral anterior insula and connections to the amygdala, hypothalamus, and periaqueductal gray (PAG), the salience network guides behavior by detecting and responding to personally relevant cues (Hermans et al., 2011, Seeley et al., 2007). Studies of healthy individuals have found that functional connectivity in the default mode and salience networks are inversely correlated, such that higher connectivity in one is often associated with lower connectivity in the other (Greicius et al., 2003, Seeley et al., 2007). The reciprocal relationship between these two networks suggests that decline in the default mode network may result in enhancement of the salience network, a pattern that has been found in the early clinical phase of AD (Balthazar et al., 2014, Zhou et al., 2010). In AD dementia, heightened salience network connectivity is associated with affective symptoms that might reflect elevated emotional responsivity including agitation, irritability, and disinhibition (Balthazar et al., 2014).
For people on an AD trajectory, salience network connectivity may begin to increase prior to the onset of dementia. Incipient AD pathology can be detected by measuring the abnormal accumulation of amyloid-β (Aβ), a hallmark feature of AD pathology (Braak and Braak, 1991, Fox et al., 1996, Hampel et al., 2008), in healthy older adults who lack cognitive symptoms (Insel et al., 2017, Jagust, 2016, Mormino, 2014, Mormino and Papp, 2018, Risacher and Saykin, 2013, Rowe et al., 2013, Sperling et al., 2011). Prior studies have shown that cognitively healthy older adults with elevated Aβ burden, as detected by molecular positron emission tomography (PET) imaging, have salience network hyperconnectivity (Brier et al., 2014, Fredericks et al., 2018). Similar salience network connectivity enhancements have also been reported in asymptomatic adults at genetic risk for AD (Machulda et al., 2011) and in individuals with mild cognitive impairment (MCI) (Bai et al., 2009), a clinical stage that often precedes AD dementia. In addition to lower scores on tests of episodic memory (Hedden et al., 2013), cognitively healthy older adults with elevated Aβ have greater increases in neuroticism (Binette et al., 2021, Fredericks et al., 2018, Snitz et al., 2015, Terracciano et al., 2021) as well as higher depression (Donovan et al., 2018, Yasuno et al., 2016) and loneliness (Donovan et al., 2016) than their peers, affective changes that may reflect enhanced connectivity in the salience network (Balthazar et al., 2014, Fredericks et al., 2018, Zhou et al., 2010).
Emotional empathy, the ability to share and experience others’ internal states via automatic physiological and motor mirroring mechanisms (Decety and Jackson, 2004), also depends on the salience network (Singer and Klimecki, 2014, Zaki and Ochsner, 2012) and may intensify over the course of AD. Emotional empathy can be further divided into two subtypes: emotional contagion and empathic concern (Davis, 1983, Decety and Jackson, 2004). Unlike emotional contagion, which is a self-focused form of emotional empathy that often leads to feelings of personal distress and overwhelm, empathic concern is an other-oriented form of emotional empathy that encourages compassionate, prosocial actions that prioritize the feelings and needs of other people (Batson et al., 1981, Hatfield et al., 1993). In our previous work, we found stepwise elevations in emotional contagion in MCI and AD dementia when compared to healthy older controls (Sturm et al., 2013b). Whether emotional empathy begins to increase prior to MCI, however, is unknown.
In the present study, we investigated emotional empathy trajectories in healthy older adults with elevated Aβ and their associations with salience network connectivity. Empathic concern is a more sophisticated form of emotional empathy than emotional contagion and requires individuals to downregulate their emotional reactions and respond to the needs of others (Roth-Hanania et al., 2011). Thus, we anticipated that empathic concern may begin to climb in the preclinical phase of AD, when people lack cognitive symptoms and can manage strong emotions despite the presence of early pathological changes (Jack et al., 2010, Sperling et al., 2011). We expected that participants with elevated Aβ burden would have elevated salience network connectivity and that those who exhibited the greatest increases in empathic concern would have higher connectivity between salience network node-pairs that generate emotions. A more complete understanding of the affective changes that arise in the early stages of Aβ aggregation will help to elucidate new domains, such as emotional empathy, in which changes herald an underlying AD process.
2. Materials and methods
2.1. Participants
Participants were 86 healthy older adults (aged 48 – 85 years at their first empathy assessment) recruited from the University of California, San Francisco (UCSF) Hillblom Healthy Aging Network, a longitudinal study of healthy aging (see Table 1). At each research visit, participants underwent multidisciplinary diagnostic evaluations that included a neurological examination, neuropsychological testing, neuroimaging, and an assessment of daily functioning. The neuropsychological assessment included tests of episodic memory, language, visuospatial processing, and executive functioning (Kramer et al., 2003). Mood was assessed with the Geriatric Depression Scale (GDS), a self-report measure that quantifies depressive symptoms over the past two weeks (Yesavage et al., 1982); GDS scores indicate the presence of mild (0 – 10 points), moderate (11 – 20 points), or severe (21 – 30 points) depressive symptoms. Participants also had available apolipoprotein E (APOE) genetic data, which was used to determine whether they were carriers of at least one copy of APOE*E4, an AD risk allele (Bertram and Tanzi, 2008).
Table 1.
Participant demographics and clinical data at the first empathy assessment.
| Measures | Aβ Burden |
Statistics | |
|---|---|---|---|
| Aβ- | Aβ+ | ||
| n | 63 | 23 | |
| Sex (% female) | 52.4% | 34.8% |
χ2(1, N = 86) = 2.092, p =.148 |
| Age: M (SD) | 70.0 (7.3) | 69.7 (5.2) |
t(55.109) = 0.212, p =.833 |
| Education years: M (SD) | 18.0 (2.0) | 17.3 (1.4) |
t(53.989) = 1.753, p =.085 |
| Handedness (left/right) | 6/55 | 1/21 |
χ2(1, N = 83) = 0.586, p =.444 |
| APOE*E4 carriers (% carriers) | 12.7%* | 39.1%* |
χ2(1, N = 86) = 7.422, p =.006 |
| APOE*E4 genotypes | 9 E2/E3 46 E3/E3 8 E3/E4 0 E4/E4 |
2 E2/E3 12 E3/E3 8 E3/E4 1 E4/E4 |
|
| Mini-Mental State Examination (/30): M (SD) | 29.5 (0.8) | 29.4 (0.7) |
t(46.172) = 0.504, p =.617 |
| Clinical Dementia Rating Scale Total Score (/3): M (SD) | 0 (0.0) | 0 (0.0) | |
| Geriatric Depression Scale (/30): M (SD) | 2.0 (2.4) | 2.3 (3.0) |
t(33.215) = -0.362, p =.720 |
| Modified Trails Number Lines Correct (/14): M (SD) | 14 (0.0) | 14 (0.0) | |
| Modified Trails Errors: M (SD) | 0.4 (0.8) | 0.2 (0.5) |
t(61.436) = 1.289, p =.202 |
| Phonemic Fluency: M (SD) | 15.1 (4.0) | 16.7 (4.3) |
t(36.233) = -1.480, p =.148 |
| Semantic Fluency: M (SD) | 23.6 (5.5) | 23.0 (4.6) |
t(47.547) = 0.546, p =.587 |
| Design Fluency Correct: M (SD) | 10.7 (3.4) | 11.6 (3.0) |
t(43.500) = -1.180, p =.244 |
| Digit Span Backward: M (SD) | 5.6 (1.3) | 5.7 (1.0) |
t(47.448) = -0.612, p =.544 |
| Benson Figure Copy 10-Minute Recall (/17): M (SD) | 12.3 (2.3) | 11.9 (2.3) |
t(31.782) = 0.585, p =.563 |
| Benson Figure Copy (/17): M (SD) | 15.5 (0.9) | 15.3 (0.8) |
t(35.485) = 1.023, p =.313 |
| Modified Boston Naming Test Correct (total score/15): M (SD) | 14.6 (0.7) | 14.5 (0.8) |
t(32.517) = 0.173, p =.864 |
* Indicates a significant difference between Aβ- and Aβ+ participants (p <.05, uncorrected). Analyses included Pearson’s Chi-squared tests and Welch's t-tests.
The multidisciplinary evaluations were used to confirm that all participants were cognitively normal and free of current neurological or psychiatric disorders at each of their empathy measurements. At the time of their first empathy assessment (within six months), all participants obtained a score of 26 or greater on the Mini-Mental State Examination (MMSE), a measure of overall mental status (Folstein et al., 1975), and had a score of 0 on the Clinical Dementia Rating scale (CDR), an informant-based measure of daily functioning (Morris, 1991).
2.2. Emotional empathy
Informants rated participants’ empathy using the Interpersonal Reactivity Index (IRI), a multidimensional empathy measure comprised of cognitive and emotional empathy subscales (Davis, 1983). For each item, informants evaluated participants’ current behavior on a scale of 1 (“does not describe well”) to 5 (“describes well”); scores on each IRI subscale range from 7 to 35, with higher scores reflecting greater empathy. Informant reports are a reliable approach to assessing personality and empathy in healthy individuals (Konrath, 2013, Saroglou et al., 2005) and in those with dementia (Rankin et al., 2006, Strauss et al., 1993). To build on our previous work (Sturm et al., 2013b), we focused on the emotional empathy IRI subscales: Empathic Concern and Personal Distress. The Empathic Concern subscale measures feelings of compassion and concern for others (e.g., “Would show tender, concerned feelings for people less fortunate than them” and, “They are often quite touched by things they see happen”). The Personal Distress subscale measures self-oriented feelings of distress in response to others’ suffering (e.g., “Being in a tense emotional situation scares them” and, “Sometimes would feel helpless when in the middle of a very emotional situation”) and was used as our measure of emotional contagion.
Empathy was assessed in participants for up to eight years (range = 0 – 7.3 years, mean = 2.4 years), and most participants (62.8%) had more than one empathy assessment (range = 1 – 7 IRI measurements, mean = 2.6 IRI measurements; Aβ+ mean = 2.5 IRI measurements over 2.5 years; Aβ- mean = 2.6 IRI measurements over 2.3 years). A total of 222 IRI measurements were collected from the 86 participants during this period. Informants who completed the IRI included participants’ spouses (68.5%), siblings (5.0%), children (8.6%), and friends (18.0%). Over the course of the study, although most participants had the same informant (89.5%) at each visit, some had different informants over time (10.4%).
2.3. Neuroimaging measures
2.3.1. Amyloid PET scan acquisition and processing
Participants underwent molecular Aβ-PET imaging. Aβ positivity was assessed with Florbetapir (18F-AV-45) or 11C-Pittsburgh compound B (PIB) PET scans. Standardized uptake value ratio (SUVR) values were calculated from data acquired between 50 and 70 minutes after tracer injection, with tracer-specific reference regions (whole cerebellum for Florbetapir, cerebellar cortex for PIB). Using previously established SUVR thresholds (Landau et al., 2013b, Villeneuve et al., 2015) and methodology (e.g., Asken et al., 2021), participants with either Florbetapir SUVR values greater than 1.11 or PIB SUVR values greater than 1.21 were categorized as Aβ positive (Aβ+). Participants that did not exceed these SUVR thresholds were categorized as Aβ negative (Aβ-). A total of 63 Aβ- participants and 23 Aβ+ participants were included in the current study. As the PET scan could have occurred at any time in relation to a participant’s disease course, we used the Aβ+ and Aβ- categories to distinguish between participants who, to the best of our knowledge, were and were not on an AD trajectory, respectively. All PET scans were conducted at the same time or after the last IRI measurement, except for one Aβ- participant who underwent PET imaging 2.6 years before their last IRI. The rest of the participants underwent PET imaging an average of 4.2 years after their last IRI (range = 0.5 years before last IRI to 11.9 years after last IRI; median = 4.0 years after last IRI). At the time of the PET scans, 91.3% of Aβ+ participants remained cognitively healthy, with only two Aβ+ participants (as well as three Aβ- participants) who had converted to MCI. See Supplemental Fig. 1 for more information about the timing of the PET scans in relation to the IRI measures in each participant.
2.3.2. Magnetic resonance imaging (MRI) acquisition
Structural MRI and task-free functional MRI (tf-fMRI) scans were completed during the same session on a Siemens 3.0 T Tim Trio scanner at the UCSF Neuroscience Imaging Center. Structural MRI scans were obtained using a whole-brain T1-weighted magnetization-prepared rapid gradient-echo (MPRAGE) imaging sequence (160 sagittal slices; slice thickness: 1.0 mm; field of view [FOV]: 256 × 240 mm; matrix: 256 × 240; voxel size: 1.0 × 1.0 × 1.0 mm; repetition time [TR]: 2,300 ms; echo time [TE]: 2.98 ms; flip angle: 9°) while tf-fMRI scans were collected using a whole-brain T2*-weighted echo-planar imaging sequence (axial interleaved acquisition with 36 slices; slice thickness: 3.0 mm; FOV = 230 × 230 mm; matrix: 92 × 92; voxel size: 2.5 × 2.5 × 3.0 mm; TR: 2,000 ms; TE: 27 ms; flip angle: 80°).
MRI and tf-fMRI scans were analyzed for a total of 72 participants (18 Aβ+ participants and 54 Aβ- participants). Gray matter volume and functional connectivity change slowly in preclinical AD (Jack et al., 2010; Twamley et al., 2006), and neural differences can be very subtle (Besson et al., 2015, Khan, 2018, Sheline and Raichle, 2013). To allow the longest period of time for structural and functional brain changes to develop in the Aβ+ participants, we used participants’ most recent scans that were acquired as a part of their longitudinal follow-up in the healthy aging study. On average, the MRI scans were acquired 1.9 years after participants’ last IRI (range = 0.9 years before last IRI to 9.4 years after last IRI, median = 1.6 years after last IRI). At the time of the MRI scans, 94.4% of Aβ+ participants remained cognitively healthy, with only one Aβ+ participant (as well as two Aβ- participants) who had converted to MCI.
2.3.3. Functional neuroimaging preprocessing
The tf-fMRI scans originally consisted of 240 functional volumes. The first five volumes were discarded to allow for magnetic field stabilization, and the remaining 235 volumes for each participant were analyzed. The structural T1-weighted MPRAGE scans acquired during the same scan session were used to aid preprocessing during the co-registration process.
FMRIPrep 1.2.5 (Esteban et al., 2018, Esteban and Cj, 2020) was employed to preprocess all tf-fMRI scans due to its transparency, integration of tools, and adeptness at reducing motion-related artifacts. The fMRIPrep 1.2.5 pipeline implemented conventional preprocessing procedures such as co-registration to the associated structural scan, slice time correction, motion correction, normalization to Montreal Neurological Institute (MNI) stereotactic space using the ICBM 152 Nonlinear Asymmetrical template v. 2009c (Fonov et al., 2009), and smoothing with a 6-mm full width at half maximum (FWHM) Gaussian kernel. Specifically, processing of the reference T1-weighted MPRAGE scans included: ANTs 2.2.0 procedures to correct the MPRAGE scans for bias field intensity non-uniformity (Tustison et al., 2010) and skull strip; FreeSurfer 6.0.1 (Dale et al., 1999) to reconstruct brain surfaces; custom processing with Mindboggle to enhance brain masks (Klein et al., 2017); ANTs 2.2.0′s antsRegistration (Avants et al., 2008) to perform spatial normalization to the ICBM 152 Nonlinear Asymmetrical template v. 2009c (Fonov et al., 2009) using nonlinear registration; and FSL 5.0.9′s FAST (Zhang et al., 2001) to segment tissue. Preprocessing of the blood-oxygen-level-dependent (BOLD) tf-fMRI scans with fMRIPrep 1.2.5 included: custom fMRIPrep creation of reference volumes; FreeSurfer’s bbregister to perform boundary-based coregistration (Greve and Fischl, 2009) of the reference volumes to the structural reference; FSL 5.0.9′s MCFLIRT (Jenkinson et al., 2002) to estimate head-motion parameters, slice time correction with AFNI 20160207 (Cox and Hyde, 1997); and spatial smoothing using a 6-mm FWHM Gaussian kernel.
Independent component analysis removal of motion artifacts was implemented through the fMRIPrep 1.2.5 preprocessing procedures using ICA-AROMA, a robust approach to diminishing motion-related signal artifacts without disrupting the functional data’s temporal characteristics (Pruim et al., 2015). Scans were bandpass filtered in the 0.0083 Hz to 0.15 Hz frequency range to minimize high frequency noise as well as low frequency drift (Lowe et al., 1998).
Stringent movement correction was applied after preprocessing by regressing out 24 nuisance variables including six movement parameters, six movement parameters derived from the prior time point, and the squares of the 12 corresponding terms (Friston et al., 1996) as well as the cerebrospinal fluid and white matter time series. Global signal regression was not utilized. Analyses incorporating higher-parameter nuisance variables have been shown to better account for motion artifacts relative to lower-parameter nuisance variables (Satterthwaite et al., 2013), and comparisons using this 24-parameter procedure demonstrate its ability to reduce motion-induced spikes (Yan et al., 2013). Although this set of denoising procedures reduces motion-related associations, excessive participant movement can produce spurious correlations (Power et al., 2012). To minimize artifacts from excessive head movement, participants were excluded if, relative to the estimated reference volume created by fMRIPrep 1.2.5, the functional scans included movement greater than 1 mm in more than 10% of the total 240 functional volumes, more than a maximum translational movement of 3 mm, or more than a maximum rotational movement of 3°. Prior studies have demonstrated that these exclusion criteria result in moderate to good test–retest reliability for the salience network node-pairs used in the present study (Guo et al., 2012). Only one Aβ+ participant was excluded based on these criteria. Thus, a total of 72 participants (18 Aβ+ and 54 Aβ-) were included in the tf-fMRI analyses.
2.3.4. Structural neuroimaging preprocessing
The T1-weighted MPRAGE structural scans were visually inspected prior to analysis to ensure adequate quality. Images were preprocessed with the Computational Anatomy Toolbox (CAT12; https://www.neuro.uni-jena.de/cat/) and Statistical Parametric Mapping 12 (SPM12; https://www.fil.ion.ucl.ac.uk/spm/software/spm12/) using standard structural imaging analysis procedures that included segmentation, normalization to MNI stereotactic space, modulation, and smoothing with an 8-mm FWHM Gaussian kernel. Normalization involved use of the Diffeomorphic Anatomic Registration Through Exponentiated Lie algebra (DARTEL; Ashburner, 2007) to warp participants’ images into MNI stereotactic space. No participants were initially excluded based on poor quality scans, but we excluded from the structural analyses the one Aβ+ participant whose tf-fMRI scan had been excluded. This resulted in a total of 72 participants (the same 18 Aβ+ and 54 Aβ- individuals as the tf-fMRI analyses) in the structural neuroimaging analyses.
2.4. Analyses
2.4.1. Emotional empathy analyses
All analyses of empathy were conducted in R v.4.0.3 (R Core Team, 2020). Participants were only included in the regression models for which they had available data, and all datapoints were analyzed. Fixed effects are reported for ease of interpretation.
We conducted multivariate mixed-model linear regressions, implemented through the nlme package (Pinheiro et al., 2018), to determine whether emotional empathy differed in the Aβ+ or Aβ- groups over time. To account for the variable amounts of time (in days) that elapsed between the collection of different measures within and across participants, we calculated a set of “interval” variables. First, we computed the “IRI – IRI interval” variable, which was the number of days between the participant’s first IRI (IRI1) and each subsequent IRI until their final IRI (IRIn). This variable (i.e., IRI1 – IRI2, IRI1 – IRI3 … IRI1 – IRIn) enabled us to control for the variable amount of time over which empathy was assessed. As IRI1 – IRIn reflected the total number of days over which empathy was measured in each participant, we refer to this interval variable as “time” in our analyses. Next, we computed the “IRI – PET interval” variable, which was the number of days between a participant’s PET scan and each of their IRI assessments. This variable (i.e., IRI1 – PET, IRI2 – PET … IRIn – PET) allowed us to account for the variable amount of time that passed between the determination of a participant’s Aβ status and their IRI measures.
In the regression models, the raw empathic concern or emotional contagion scores were entered as the dependent variables to compare participants’ emotional empathy slopes over time. Fixed effects in these models included Aβ status (Aβ+ = 1 and Aβ- = 0), sex, age, and APOE*E4 carrier status—covariates associated with AD, emotions, and empathy (Davis, 1980, Gard and Kring, 2007, Kring and Gordon, 1998)—as well as the IRI – IRI and IRI – PET interval variables. We also included a Aβ status by time (IRI1 – IRIn) interaction term as a fixed effect to examine changes in emotional empathy across time by group. Random effects in these models included slopes of time (IRI1 – IRIn) and intercepts for each participant to account for individual longitudinal trajectories. For participants who had only one IRI measure, estimates of the random effects component of their empathic concern and emotional contagion slopes were calculated as the deviation from the fixed effects of time (IRI1 – IRIn).
Because the random effect variances differed between the Aβ+ and Aβ- groups, we next conducted multiple regressions in each group separately to capture participant-specific changes in emotional empathy. Here, the dependent variables were either the raw empathic concern or emotional contagion scores. Covariates included the same fixed and random effects as above (including the IRI – IRI and IRI – PET interval terms), with the omission of the Aβ status by time (IRI1 – IRIn) interaction term. From these models, we extracted the slopes of time (IRI1 – IRIn) for each participant (the coefficient combining the fixed and random effects of the IRI1 – IRIn time), which represented individualized emotional empathy trajectories, for use in the functional neuroimaging analyses (see next section 2.4.2).
2.4.2. Functional neuroimaging analyses
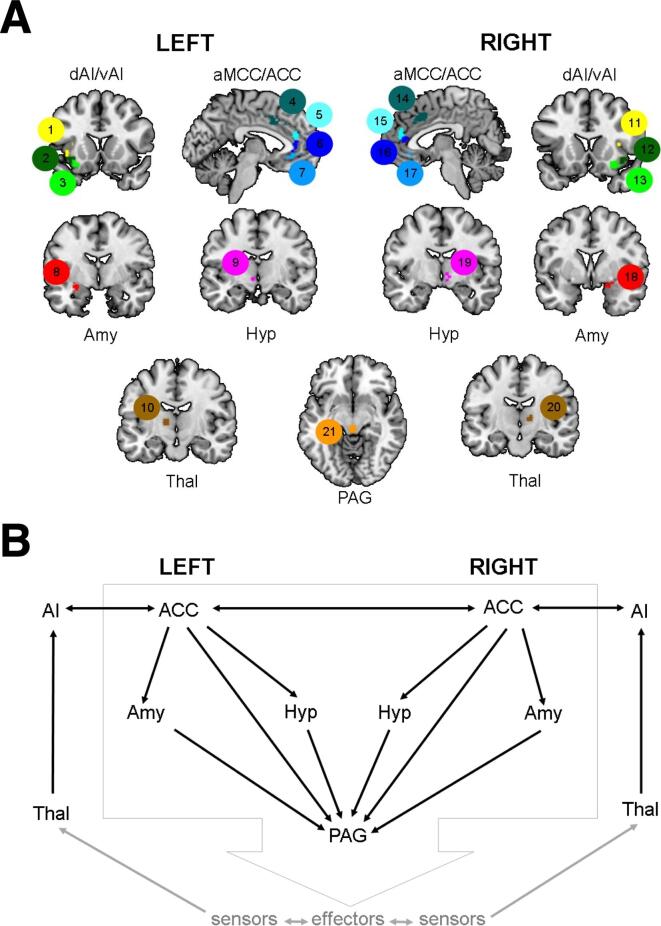
Salience network connectivity was examined using a priori nodes, a region of interest (ROI) approach that is preferable for matrix-based investigations (see Sturm et al. (2018)) (Fig. 1A). In brief, 21 ROIs corresponding to the salience network were created from tf-fMRI scans in an independent sample; see Guo et al. (2012) for more details. We used the CONN toolbox (Whitfield-Gabrieli and Nieto-Castanon, 2012) to calculate Fisher z-transformed bivariate correlation edge weights for the 74 node-pairs that corresponded to the connections depicted in Sturm et al. (2018) (Fig. 1B).
Fig. 1.
Salience network nodes and functional connections of interest. A) Functional connectivity in 21 cortical and subcortical salience network nodes were used in our analyses. The salience network nodes, and their corresponding ROI number, included: left dorsal anterior insula (ROI 1), right dorsal anterior insula (ROI 11), left ventral anterior insula (ROI 2), right ventral anterior insula (ROI 12), left ventral anterior insula (ROI 3), right ventral anterior insula (ROI 13), left anterior midcingulate cortex (ROI 4), right anterior midcingulate cortex (ROI 14), left pregenual anterior cingulate cortex (ROI 5), right pregenual anterior cingulate cortex (ROI 15), left pregenual anterior cingulate cortex (ROI 6), right pregenual anterior cingulate cortex (ROI 16), left subgenual anterior cingulate cortex (ROI 7), right subgenual anterior cingulate cortex (ROI 17), left amygdala (ROI 8), right amygdala (ROI 18), left hypothalamus (ROI 9), right hypothalamus (ROI 19), left thalamus (ROI 10), right thalamus (ROI 20), and PAG (ROI 21). ACC, anterior cingulate cortex; aMCC, anterior midcingulate cortex; Amy, amygdala; AI, anterior insula; dAI, dorsal anterior insula; vAI, ventral anterior insula; Hyp, hypothalamus; PAG, periaqueductal gray; and Thal, thalamus. B) Salience network node-pairs assessed in the current study. Both panels adapted with permission from Sturm et al. (2018).
First, we used hierarchical regressions to investigate whether salience network node-pair connectivity predicted emotional empathy trajectories in Aβ+ participants (Sturm et al., 2018, Sturm et al., 2013a). We first conducted Pearson’s bivariate correlational analyses to assess the correlation strength between Aβ+ participants’ emotional empathy trajectories and connectivity in each of the 74 salience network node-pairs; only node-pairs with a correlation coefficient of r ≥ 0.25 were included in subsequent analyses to avoid over-specification of the models. Forward-selection hierarchical regression models, which allow node-pairs to compete for entry in the final regression model (Akaike, 1998), were then conducted using the MASS package in R (Venables and Ripley, 2002) to identify the salience network node-pairs that predicted emotional empathy trajectories. To reduce the likelihood of collinearity between homologous salience network node-pairs in the left and right hemispheres, we ran three hierarchical regression models to examine ipsilateral left hemisphere connections, ipsilateral right hemisphere connections, and contralateral connections. As the PAG contained regions in both the left and right hemisphere, this ROI was included in both ipsilateral models but not the contralateral one. As an additional measure to avoid collinearity, only salience network node-pairs that were significant predictors of emotional empathy trajectories at p <.05 were allowed entry into the final regression model.
The regression models included fixed effects of sex, age at tf-fMRI scan, the interval in days between participants’ most recent IRI and their tf-fMRI scans (IRIn – tf-fMRI), and APOE*E4 carrier status as well as random intercepts for each participant. All histograms and P—P plots of standardized residuals demonstrated normally distributed error terms that indicated the assumptions of regression models were met. Variables included in the final models displayed very weak collinearity, with all variance inflation factors < 2.0.
Next, we employed a multivariate mixed-model linear regression to determine whether average overall connectivity across all 74 salience network node-pairs was higher in the Aβ+ than in the Aβ- group. To investigate whether specific node-pairs were driving potential group differences, we also conducted a series of regressions to compare the average connectivity in node-pairs in the left hemisphere (29 ipsilateral node-pairs), right hemisphere (29 ipsilateral node-pairs), and across hemispheres (16 contralateral node-pairs). Results were Bonferroni corrected (pBONFERRONI < 0.05) for the number of node-pairs within each set of analyses. These regression models included random intercepts for each participant as well as fixed effects of Aβ status (Aβ+ or Aβ-), sex, age at tf-fMRI scan, the interval in days between participants’ tf-fMRI and PET scans (tf-fMRI – PET), and APOE*E4 carrier status.
2.4.3. Structural neuroimaging analyses
We used CAT12 and SPM12 to conduct whole-brain voxel-based morphometry (VBM) analyses (Ashburner and Friston, 2000). We compared the Aβ+ and Aβ- groups to assess whether there were overall differences in gray matter volume. Nuisance covariates included sex, age at MRI scan, APOE*E4 status, and total intracranial volume, which is recommended for VBM analyses (Barnes et al., 2010) as it accounts for individual differences in head size that can vary with brain volume (Barnes et al., 2010, Good et al., 2001, Whitwell et al., 2001). We also included the time interval in days between each participant’s MRI and PET scan (MRI – PET) as an additional nuisance covariate to account for variability between the measurement of Aβ status and the structural MRI.
3. Results
3.1. Aβ+ and Aβ- participants had similar demographics and comparable cognitive functioning at their first empathy assessment
Aβ+ and Aβ- participants had similar demographic profiles, but there was a greater proportion of APOE*E4 carriers, χ2(1) = 7.422, p =.006, n = 86, in the Aβ+ group, as would be expected given the known associations between APOE*E4 and Aβ deposition (Caselli et al., 2010, Schmechel et al., 1993). At the time of the first empathy assessment, the groups had similar neuropsychological profiles and mood, with both groups performing well in all cognitive domains and reporting minimal depressive symptoms (see Table 1).
3.2. Empathic concern, but not emotional contagion, increased more over time in Aβ+ participants than in Aβ- participants despite similar initial empathy scores
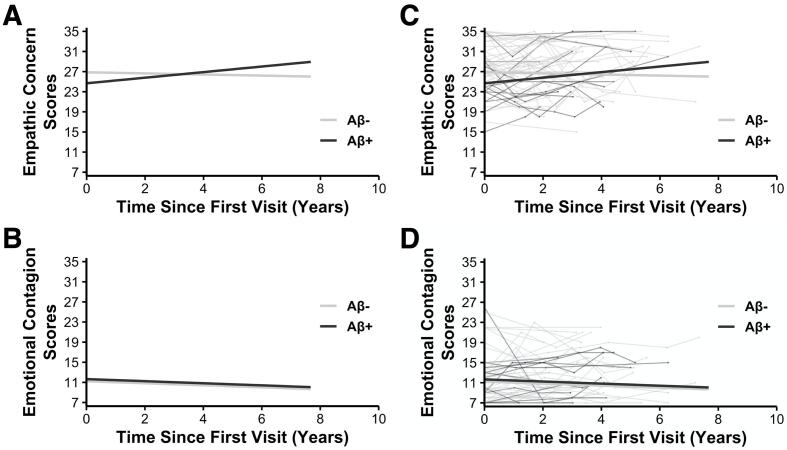
Analysis of the emotional empathy scores revealed an Aβ status by time interaction on empathic concern, b = 0.002, t(132) = 2.055, p =.042, n = 86 (Fig. 2A), but not on emotional contagion, b = -1.569 × 10-5, t(116) = -0.017, p =.986, n = 86 (Fig. 2B). These findings suggested that Aβ+ participants demonstrated greater preclinical increases in empathic concern, but not emotional contagion, than Aβ- participants (Table 2).The only other significant result in either model was a main effect of sex on empathic concern, b = 2.932, t(82) = 3.000, p =.004, n = 86, which indicated that women, on average, had greater empathic concern than men, consistent with prior studies (Davis, 1980, Davis, 1983).
Fig. 2.
Divergent empathic concern, but not emotional contagion, trajectories in Aβ+ and Aβ- participants. Multivariate mixed-model linear regression analyses revealed a significant interaction between A) Aβ status and time on empathic concern trajectories such that Aβ+ participants showed greater increases in empathic concern over time than Aβ- participants. There was no statistically significant interaction between Aβ status and time on participants’ B) emotional contagion trajectories in the respective multivariate mixed-model linear regression analysis. The depicted models controlled for additional fixed effects (Aβ status, time (IRI – IRI interval in days), age, sex, the interval between completion of participants’ emotional empathy measures and PET scans (IRI – PET), and APOE*E4 status), random intercepts for each participant, and random slopes of time (IRI – IRI interval). Panels C and D include participants’ individual trajectories for their respective emotional empathy measures.
Table 2.
Fixed and random effects of multivariate mixed-model linear regression analyses examining the impact of Aβ status on emotional empathy.
| Fixed Effects | Empathic Concern |
Emotional Contagion |
||||
|---|---|---|---|---|---|---|
| Estimates | Confidence Interval | p-value | Estimates | Confidence Interval | p-value | |
| Intercept | 19.089 | 6.880 – 31.298 | 0.002 | 11.403 | -0.922 – 23.729 | 0.069 |
| Aβ Status*Time | 0.002 | 6.795x10-5 – 0.004 | 0.042 | -1.569x10-5 | -0.002 – 0.002 | 0.986 |
| Aβ Status | -2.188 | -4.529 – 0.153 | 0.067 | 0.428 | -2.029 – 2.885 | 0.730 |
| Time | 3.016x10-4 | -0.002 – 0.001 | 0.644 | -0.001 | -0.002 – 0.001 | 0.392 |
| APOE*E4 Status | 0.107 | -2.341 – 2.556 | 0.931 | -0.182 | -2.644 – 2.281 | 0.884 |
| Sex | 2.932 | 0.988 – 4.877 | 0.004 | 1.356 | -0.602 – 3.314 | 0.172 |
| Age | 0.087 | -0.067 – 0.241 | 0.265 | 0.022 | -0.133 – 0.178 | 0.775 |
| IRI-PET Interval | -0.001 | -0.002 – 0.001 | 0.340 | 0.001 | -0.001 – 0.002 | 0.314 |
| Random Effects | Empathic Concern | Emotional Contagion |
| σ2 | 8.64 | 5.53 |
| τ Participant | 14.16 | 18.75 |
| ρ | 0.03 | -0.47 |
| ICC | 0.64 | 0.75 |
| Marginal R2 | 0.114 | 0.025 |
| Conditional R2 | 0.682 | 0.760 |
Follow-up regression analyses were conducted on participants’ first emotional empathy scores using the same covariates of non-interest (except the IRI – IRI time interval) to determine whether there were group differences at their initial assessment. Neither empathic concern, b = -2.050, t(80) = -1.734, p =.087, n = 86, nor emotional contagion, b = 0.560, t(80) = 0.426, p =.671, n = 86, differed between the Aβ+ and Aβ- participants at their first measurement. There was a significant main effect of sex such that women had higher empathic concern than men, b = 3.576, t(80) = 3.507, p =.001, n = 86. No participants were more than three standard deviations (SD) from the mean (M) for either of the emotional empathy subscales at their first assessment, suggesting that outliers did not have a significant impact on our results.
3.3. Increasing empathic concern over time related to higher salience network connectivity only in Aβ+ participants
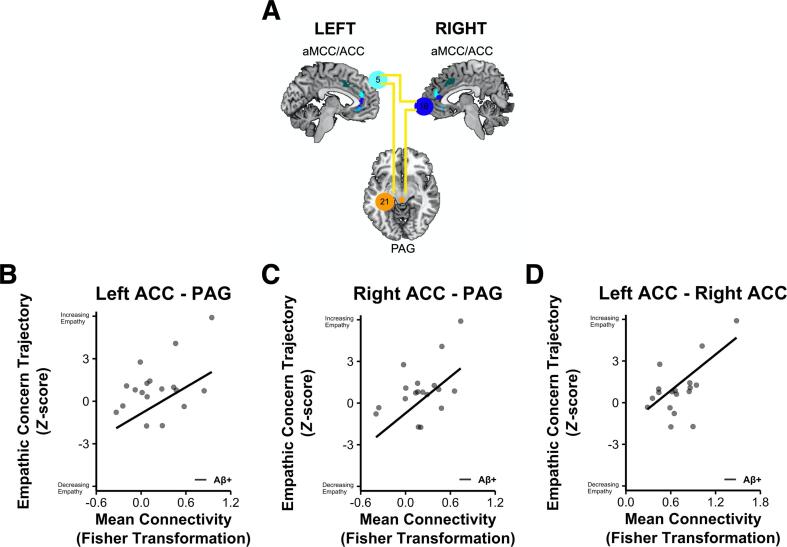
Given that empathic concern increased more over time in the Aβ+ participants than the Aβ- participants, we focused our functional neuroimaging analyses on this emotional empathy subtype. Forward-selection hierarchical regression models in the Aβ+ participants revealed that greater connectivity in three salience network node-pairs related to greater preclinical increases in empathic concern: (1) left pregenual ACC (ROI 5) and PAG (ROI 21) (final model b = 0.003, final model coefficient p =.034, model marginal R2 = 0.414, marginal R2 change from preliminary model = 0.292, n = 18); (2) right pregenual ACC (ROI 16) and PAG (ROI 21) (final model b = 0.004, final model coefficient p =.010, marginal R2 = 0.514, marginal R2 change from preliminary model = 0.392, n = 18); and (3) left pregenual ACC (ROI 5) and right pregenual ACC (ROI 16) (final model b = 0.004, final model coefficient p =.018, marginal R2 = 0.472, marginal R2 change from preliminary model = 0.350, n = 18) (Fig. 3).
Fig. 3.
Increasing empathic concern over time related to higher pregenual ACC-PAG and pregenual ACC-ACC connectivity in Aβ+ participants. A) Forward-selection hierarchical regression models in Aβ+ participants found greater connectivity between bilateral pregenual ACC and PAG as well as contralateral ACC predicted increasing empathic concern over time. Scatterplots are provided to illustrate the association that z-scored empathic concern trajectories have with mean connectivity between B) left pregenual ACC and PAG; C) right pregenual ACC and PAG; and D) left pregenual ACC and right pregenual ACC. These analyses controlled for fixed effects of age, sex, the interval between participants’ last IRI measure and tf-fMRI scans (IRI – tf-fMRI), and APOE*E4 status as well as random intercepts for each participant. ACC, anterior cingulate cortex; aMCC, anterior midcingulate cortex; and PAG, periaqueductal gray.
In the Aβ- participants, in contrast, no salience network node-pairs entered the forward-selection hierarchal regression models (all p >.05, n = 54), which indicated they did not explain a significant amount of variance in this group.
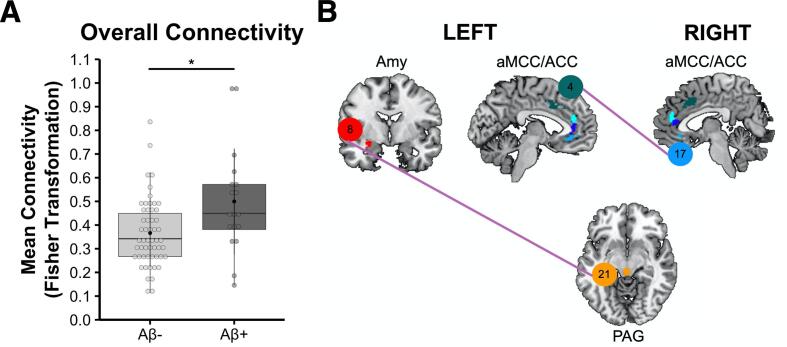
3.4. Aβ+ participants had elevated salience network connectivity despite normal gray matter volume
Multivariate mixed-model linear regressions showed that Aβ+ participants had higher overall mean salience network connectivity (averaged across node-pairs in the two hemispheres) than Aβ- participants, b = 0.117, t(66) = 2.460, p =.017, n = 72 (Fig. 4A). In analyses that compared mean connectivity in each of the salience network node-pairs, Aβ+ participants had higher connectivity than Aβ- participants in two node-pairs in particular (pBONFERRONI < 0.05): (1) left amygdala (ROI 8) and PAG (ROI 21), b = 0.280, t(66) = 3.760, p = 3.632 × 10-4, n = 72 and (2) left anterior midcingulate cortex (ROI 4) and right subgenual ACC (ROI 17), b = 0.318, t(66) = 3.338, p =.001, n = 72 (Fig. 4B).
Fig. 4.
Elevated salience network connectivity in Aβ+ participants. A) Relative to Aβ- participants, Aβ+ participants had greater mean overall connectivity across all 74 ipsilateral and contralateral salience network node-pairs. B) Aβ+ participants had higher connectivity than Aβ- participants (pBONFERRONI < 0.05) in two specific salience network node-pairs: (1) left amygdala (ROI 8) – PAG (ROI 21) and (2) left anterior midcingulate cortex (ROI 4) – right subgenual ACC (ROI 17). These analyses controlled for fixed effects of age, sex, the interval in days between participants’ tf-fMRI and PET scans (tf-fMRI – PET), and APOE*E4 status as well as random intercepts for each participant. At the time of the neuroimaging assessment, one Aβ+ participant (and two Aβ- participants) had converted to MCI. ACC, anterior cingulate cortex; aMCC, anterior midcingulate cortex; Amy, amygdala; and PAG, periaqueductal gray.
A whole-brain VBM analysis revealed no significant differences between the Aβ+ and Aβ- groups in gray matter volume (pFWE > 0.05, n = 72).
4. Discussion
The present study uncovered divergent empathic concern trajectories in cognitively healthy older adults with and without elevated cortical Aβ deposition. The Aβ+ participants demonstrated greater gains in empathic concern over time than the Aβ- participants, but the groups did not show a similar separation on emotional contagion. Functional connectivity neuroimaging analyses examined whether the Aβ+ participants with greater preclinical gains in empathic concern had higher connectivity in certain salience network hubs than those with less change in empathic concern. The neuroimaging measures were acquired at the same time or after participants’ last empathy assessment, at which time most participants remained cognitively healthy. We found greater increases in empathic concern in Aβ+ participants were associated with higher connectivity between bilateral pregenual ACC and PAG. On average, the Aβ+ participants also had higher salience network connectivity than the Aβ- participants but there were no differences in gray matter volume.
Empathic concern and emotional contagion are forms of emotional empathy that allow people to share and experience the emotions of others (Davis, 1983, Decety and Jackson, 2004). In a cohort of well-screened cognitively healthy older adults, we found Aβ+ participants demonstrated greater preclinical gains in empathic concern over time than those who were Aβ-. As the participants remained cognitively healthy and exhibited no cognitive symptoms during the period in which empathy was assessed, our findings suggest that empathic concern increases early in the context of Aβ aggregation. Just as the AD pathological cascade is a gradual process, with Aβ and tau aggregating over years or decades (Jack et al., 2010, Sperling et al., 2011), the changes in empathic concern we detected were also subtle, with levels climbing slowly over time and were present more in some individuals than others. As participants only underwent one PET scan, however, it is impossible to determine when an individual became Aβ+ in relation to the empathic concern changes. In the Aβ+ participants, however, the PET scans were acquired on average 4.0 years after their last IRI measurement (range = 0.5 years before last IRI to 7.2 years after last IRI), at which point only two of the Aβ+ participants had converted to MCI (but 91.3% of Aβ+ participants remained cognitively healthy), which suggests the gains in empathic concern that we captured in the years prior to these PET scans were preclinical features of an underlying AD process.
In cognitively healthy adults with elevated Aβ burden, changes in functional connectivity may precede brain atrophy, and early structural and functional brain changes are often subtle (Besson et al., 2015, Khan, 2018, Sheline and Raichle, 2013). To increase the likelihood of detecting associations between preclinical empathic concern changes and salience network connectivity, we used participants’ most recent MRI and tf-fMRI scans. These scans were obtained on average 1.9 years after the last IRI assessment, at which time most (94.4%) of the Aβ+ participants remained cognitively healthy, though one Aβ+ participant had converted to MCI. Within the Aβ+ group, participants who exhibited the greatest gains in empathic concern were those with higher connectivity between the PAG and bilateral pregenual ACC. Consistent with prior studies (Brier et al., 2014, Fredericks et al., 2018), Aβ+ participants also had higher average salience network connectivity than Aβ- participants at this time despite similar gray matter volume. These salience network elevations in the Aβ+ group were primarily driven by heightened connectivity between the left amygdala and PAG as well as the left anterior midcingulate cortex and right subgenual ACC. These tightly connected salience network structures are critical for producing the coordinated autonomic and motor changes that arise during emotions (Bandler et al., 1991, Devinsky et al., 1995, Gallagher and Chiba, 1996, Hillis, 2014, Kim et al., 2020, Singer and Klimecki, 2014, Zaki and Ochsner, 2012), and, thus, are critical for empathic concern.
The present research builds on our prior study that found greater emotional contagion in individuals with MCI and AD than in cognitively healthy individuals (Sturm et al., 2013b). Whereas emotional contagion is a self-focused form of emotional empathy that can be accompanied by feelings of personal distress (Hatfield et al., 1993), empathic concern is an other-oriented form of emotional empathy that promotes feelings of compassion and prosocial actions (Decety and Jackson, 2004, Goetz et al., 2010). Both forms of emotional empathy engage autonomic and motor mirroring systems that facilitate affect-sharing, but only empathic concern requires emotion downregulation, which prevents people from being overwhelmed by their own feelings and allows them to orient to the needs of others (Roth-Hanania et al., 2011). The present study shows that, in people on the AD continuum, empathic concern begins to climb even prior to MCI. We speculate that empathic concern may rise very early in the AD process when people become more sensitive to affective cues but can still regulate their emotions and focus on the needs of others. Gains in empathic concern may eventually subside, however, and give way to increases in emotional contagion in the symptomatic phase of the disease as autonomic, cognitive, or behavioral control systems begin to falter.
Across the AD spectrum, aggregation of Aβ and tau proteins is associated with cognitive as well as affective symptoms (e.g., Doraiswamy et al., 2012, Ellis et al., 2013, Lim et al., 2012, Ossenkoppele et al., 2016, Petersen et al., 2019). By altering salience network connectivity, early Aβ pathology may make older adults more sensitive to affective information. While increased emotional sensitivity may yield interpersonal benefits for some people as they become more attuned and responsive to the emotions of others, it may also make them more vulnerable to negative emotional experiences including depressed mood (Donovan et al., 2018, Yasuno et al., 2016), loneliness (Donovan et al., 2016), and neuroticism (Binette et al., 2021, Fredericks et al., 2018, Snitz et al., 2015, Terracciano et al., 2021). The Aβ+ participants in the present study did not exhibit significant depressive symptoms during the years in which empathy was assessed, however, which suggests our results were not accounted for by depressive symptoms or low mood. As there was variation across Aβ+ participants in the extent to which empathic concern increased over time, it is possible that certain salience network enhancements, such as heightened connectivity in node-pairs that support emotion generation, may predispose some individuals to greater changes in emotional empathy than others.
Prior research on healthy aging paints a mixed picture of the social and emotional lives of older adults, and our findings may help to explain some of the inconsistent results across studies. While some studies indicate healthy elders pay more attention to positive information, report greater experience of positive emotions, and invest more in emotion regulation goals (Carstensen, 1992) than their younger counterparts (Charles et al., 2003, Mather and Carstensen, 2005, Reed and Carstensen, 2012), others suggest more complexity in the emotional lives of older adults. Laboratory-based studies have found that, although healthy older adults are better than younger adults at using positive reappraisal strategies to manage their emotions, they are less able to distance themselves from certain negative affective cues (e.g., those that elicit sadness and distress) and, thus, are more reactive in some contexts (Katzorreck and Kunzmann, 2018, Kunzmann and Gruhn, 2005, Kunzmann and Richter, 2009, Lwi et al., 2019, Seider et al., 2011, Shiota and Levenson, 2009). Investigations of emotional empathy across the lifespan have also yielded variable conclusions. While some studies have found older and younger adults have similar levels of emotional empathy on questionnaire measures (Bailey et al., 2008, Phillips et al., 2002), others have found lower empathic concern in people of more advanced age (Chen et al., 2014, O'Brien et al., 2013). In laboratory-based studies, however, older adults tend to report and exhibit levels of empathic concern, sympathy, and prosocial behavior that are like—or even higher than—those of younger adults (Kunzmann and Gruhn, 2005, Kunzmann and Richter, 2009, Richter and Kunzmann, 2011, Sze et al., 2012, Wieck and Kunzmann, 2015). Some of these inconsistent results may be due, in part, to the presence of early, undetected AD pathology in some of the older participants who were included in healthy aging studies of emotional reactivity, emotion regulation, and emotional empathy (Brier et al., 2014). Additional research is needed, however, to determine whether emotional empathy changes can help to differentiate between individuals on typical aging and AD trajectories.
The present study has several limitations to consider. First, we used empathic concern slopes, which were calculated over time, to investigate subsequent salience network connectivity, but the interval of time between these measures differed across participants. Multimodal longitudinal studies that include time-locked measures of emotional empathy, salience network connectivity, and molecular pathology are needed to map the natural history of emotional empathy and its relation to AD pathophysiology. There is some evidence that salience network connectivity changes in AD are non-linear—with elevations in the mild to moderate clinical stages of disease progression (Balthazar et al., 2014, Zhou et al., 2010) that might wane as symptoms become more severe (Brier et al., 2012, Schultz et al., 2020)—and emotional empathy may also continue to change as the disease progresses. Given that empathic concern and emotional contagion rely on different salience network hubs (Christov-Moore and Iacoboni, 2016, Singer and Klimecki, 2014, Zaki and Ochsner, 2012), longitudinal imaging studies will also be needed to delineate how specific network-level changes relate to the trajectories of each type of emotional empathy. Additional studies that examine whether early AD structural or functional changes in brainstem nuclei, such as the locus coeruleus, (Braak and Braak, 1991, Fox et al., 1996, Hampel et al., 2008) that are associated with the salience network, emotional reactivity, and neuropsychiatric symptoms (Ehrenberg et al., 2018) will also be critical for illuminating the role of brainstem structures in affective symptoms across the AD spectrum.
Second, our measures of emotional empathy were derived from informant reports. Although informant reports are a reliable method of evaluating personality and empathy (Konrath, 2013, Saroglou et al., 2005), with substantial convergent validity between self and informant reports regarding personality assessments (Connolly et al., 2007, Kim et al., 2019, Mõttus et al., 2020, Olino and Klein, 2015), it is possible that informant reports may not be as accurate as self-reported measures of emotional empathy or that they emphasize observable behavioral reactions more than subjective feelings. Most informants in our study were spouses of participants, and it is possible that individual differences in the personality or emotional traits of the informants might have influenced their empathy ratings. Future studies of cognitively healthy older adults that include self-report measures would help detail whether participants’ perceptions of their own emotional empathy are more sensitive to underlying neuropathological changes.
Third, previous studies have established that different PET ligands offer convergent Aβ results (Chetelat et al., 2013, Johnson et al., 2013). Florbetapir and PIB, the ligands used in the present study, also provide measures of Aβ levels that are highly correlated (Landau et al., 2013a, Wolk et al., 2012) when established thresholds are used (Landau et al., 2013b, Villeneuve et al., 2015), making it possible to include participants that underwent either type of molecular imaging in the same study (e.g., Asken et al., 2021). Caution is still warranted when interpreting across scans that utilized different types of PET ligands, however. In addition, participants in the current study had only one PET scan, making it impossible to know when they became Aβ+ in relation to their emotional empathy changes. In cognitively healthy older adults, neuropathological changes emerge slowly (Chetelat et al., 2013), and aggregation of Aβ and tau may have different effects on salience network functioning over the disease course (Schultz et al., 2017). Although we focused on Aβ, future studies are needed to examine how changes in tau also influence salience network functioning in those with early AD pathology.
The present study contributes to a growing body of evidence that changes in emotions and empathy, as well as cognition, accompany incipient AD pathology. Our results suggest empathic concern increases in the very early stages of Aβ aggregation and relates to heightened functional connectivity in the salience network, a distributed system that guides behavior via its central role in emotions (Seeley et al., 2007, Singer et al., 2004). A better understanding of how emotions and empathy change in the context of AD pathophysiology will clarify the spectrum of alterations that arise early in the disease course and help clinicians to identify older adults who should be screened for AD biomarkers.
Funding
This study was supported by the Larry L. Hillblom Foundation, the John Douglas French Alzheimer’s Foundation, and grants from the National Institute on Aging (P30 AG062422, K23AG040127, K99AG065501, R01AG057204, and R01AG073244).
CRediT authorship contribution statement
Tiffany E. Chow: Investigation, Methodology, Formal analysis, Visualization, Writing – original draft, Writing – review & editing. Christina R. Veziris: Data curation, Visualization, Writing – original draft, Writing – review & editing. Renaud La Joie: Methodology, Writing – original draft, Writing – review & editing. Alex J. Lee: Methodology, Writing – original draft, Writing – review & editing. Jesse A. Brown: Methodology, Writing – original draft, Writing – review & editing. Jennifer S. Yokoyama: Data curation, Writing – original draft, Writing – review & editing. Katherine P. Rankin: Data curation, Writing – original draft, Writing – review & editing. Joel H. Kramer: Supervision, Writing – original draft, Writing – review & editing. Bruce L. Miller: Supervision, Writing – original draft, Writing – review & editing. Gil D. Rabinovici: Supervision, Writing – original draft, Writing – review & editing. William W. Seeley: Investigation, Methodology, Supervision, Writing – original draft, Writing – review & editing. Virginia E. Sturm: Funding acquisition, Investigation, Methodology, Supervision, Writing – original draft, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Dr. Rabinovici receives research support from Avid Radiopharmaceuticals, GE Healthcare, and Life Molecular Imaging; has received consulting fees or speaking honoraria from Axon Neurosciences, Avid Radiopharmaceuticals, GE Healthcare, Johnson & Johnson, Roche, Eisai, Genentech, and Merck; and is an associate editor of JAMA Neurology. Dr. Seeley received consulting fees or speaking honoraria from BridgeBio, Corcept Therapeutics, Biogen Idec, Bristol Myers-Squibb, Guidepoint Global, and GLG Council.
Acknowledgments
Acknowledgements
We would like to thank our participants and their families for their contributions to this work.
Data availability
UCSF Memory and Aging Center data are conditionally available upon request through the UCSF Memory and Aging Center Resource Request Form (https://memory.ucsf.edu/resources/data). Investigators must have Institutional Review Board approval from the UCSF Human Research Protection Program and be from academic, not-for-profit institutions.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2022.103282.
Contributor Information
Tiffany E. Chow, Email: Tiffany.Chow2@ucsf.edu.
Christina R. Veziris, Email: Christina.Veziris@ucsf.edu.
Renaud La Joie, Email: Renaud.LaJoie@ucsf.edu.
Alex J. Lee, Email: Alex.Lee@ucsf.edu.
Jesse A. Brown, Email: Jesse.Brown@ucsf.edu.
Jennifer S. Yokoyama, Email: Jennifer.Yokoyama@ucsf.edu.
Katherine P. Rankin, Email: Kate.Rankin@ucsf.edu.
Joel H. Kramer, Email: Joel.Kramer@ucsf.edu.
Bruce L. Miller, Email: Bruce.Miller@ucsf.edu.
Gil D. Rabinovici, Email: Gil.Rabinovici@ucsf.edu.
William W. Seeley, Email: Bill.Seeley@ucsf.edu.
Virginia E. Sturm, Email: Virginia.Sturm@ucsf.edu.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Akaike, H., 1998. Information theory and an extension of the maximum likelihood principle. Selected Papers of Hirotugu Akaike. Springer, New York, NY, pp. 199-213.
- Andrews-Hanna J.R., Reidler J.S., Sepulcre J., Poulin R., Buckner R.L. Functional-anatomic fractionation of the brain's default network. Neuron. 2010;65:550–562. doi: 10.1016/j.neuron.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38:95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Ashburner J., Friston K.J. Voxel-based morphometry–the methods. Neuroimage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Asken B.M., Mantyh W.G., La Joie R., Strom A., Casaletto K.B., Staffaroni A.M., Apple A.C., Lindbergh C.A., Iaccarino L., You M., Grant H., Fonseca C., Windon C., Younes K., Tanner J., Rabinovici G.D., Kramer J.H., Gardner R.C. Association of remote mild traumatic brain injury with cortical amyloid burden in clinically normal older adults. Brain Imaging Behav. 2021:1–9. doi: 10.1007/s11682-020-00440-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avants B.B., Epstein C.L., Grossman M., Gee J.C. Symmetric diffeomorphic image registration with cross-correlation: evaluating automated labeling of elderly and neurodegenerative brain. Med. Image Anal. 2008;12:26–41. doi: 10.1016/j.media.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai F., Watson D.R., Yu H., Shi Y., Yuan Y., Zhang Z. Abnormal resting-state functional connectivity of posterior cingulate cortex in amnestic type mild cognitive impairment. Brain Res. 2009;1302:167–174. doi: 10.1016/j.brainres.2009.09.028. [DOI] [PubMed] [Google Scholar]
- Bailey P.E., Henry J.D., Von Hippel W. Empathy and social functioning in late adulthood. Aging Ment Health. 2008;12:499–503. doi: 10.1080/13607860802224243. [DOI] [PubMed] [Google Scholar]
- Balthazar M.L., Pereira F.R., Lopes T.M., da Silva E.L., Coan A.C., Campos B.M., Duncan N.W., Stella F., Northoff G., Damasceno B.P., Cendes F. Neuropsychiatric symptoms in Alzheimer's disease are related to functional connectivity alterations in the salience network. Hum. Brain Mapp. 2014;35:1237–1246. doi: 10.1002/hbm.22248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandler R., Carrive P., Zhang S.P. Integration of somatic and autonomic reactions within the midbrain periaqueductal grey: viscerotopic, somatotopic and functional organization. Prog. Brain Res. 1991;87:269–305. doi: 10.1016/s0079-6123(08)63056-3. [DOI] [PubMed] [Google Scholar]
- Barnes J., Ridgway G.R., Bartlett J., Henley S.M., Lehmann M., Hobbs N., Clarkson M.J., MacManus D.G., Ourselin S., Fox N.C. Head size, age and gender adjustment in MRI studies: a necessary nuisance? Neuroimage. 2010;53:1244–1255. doi: 10.1016/j.neuroimage.2010.06.025. [DOI] [PubMed] [Google Scholar]
- Batson, C.D., Duncan, B.D., Ackerman, P., Buckley, T., Birch, K., 1981. Is empathic emotion a source of altruistic motivation? Journal of personality and social psychology 40.
- Bertram L., Tanzi R.E. Thirty years of Alzheimer's disease genetics: the implications of systematic meta-analyses. Nat. Rev. Neurosci. 2008;9:768–778. doi: 10.1038/nrn2494. [DOI] [PubMed] [Google Scholar]
- Besson F.L., La Joie R., Doeuvre L., Gaubert M., Mezenge F., Egret S., Landeau B., Barre L., Abbas A., Ibazizene M., de La Sayette V., Desgranges B., Eustache F., Chetelat G. Cognitive and brain profiles associated with current neuroimaging biomarkers of preclinical Alzheimer's disease. J Neurosci. 2015;35:10402–10411. doi: 10.1523/JNEUROSCI.0150-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binette A.P., Vachon-Presseau E., Morris J., Bateman R., Benzinger T., Collins D.L., Poirier J., Breitner J.C.S., Villeneuve S., Net D.I.A., Grp P.-A.-R. Amyloid and tau pathology associations with personality traits, neuropsychiatric symptoms, and cognitive lifestyle in the preclinical phases of sporadic and autosomal dominant Alzheimer's disease. Biol. Psychiatry. 2021;89:776–785. doi: 10.1016/j.biopsych.2020.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H., Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Brier M.R., Thomas J.B., Snyder A.Z., Benzinger T.L., Zhang D., Raichle M.E., Holtzman D.M., Morris J.C., Ances B.M. Loss of intranetwork and internetwork resting state functional connections with Alzheimer's disease progression. J. Neurosci. 2012;32:8890–8899. doi: 10.1523/JNEUROSCI.5698-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brier M.R., Thomas J.B., Snyder A.Z., Wang L., Fagan A.M., Benzinger T., Morris J.C., Ances B.M. Unrecognized preclinical Alzheimer disease confounds rs-fcMRI studies of normal aging. Neurology. 2014;83:1613–1619. doi: 10.1212/WNL.0000000000000939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner R.L., Andrews-Hanna J.R., Schacter D.L. The brain's default network: anatomy, function, and relevance to disease. Ann. N. Y. Acad. Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Carstensen L.L. Social and emotional patterns in adulthood: support for socioemotional selectivity theory. Psychol. Aging. 1992;7:331–338. doi: 10.1037//0882-7974.7.3.331. [DOI] [PubMed] [Google Scholar]
- Caselli R.J., Walker D., Sue L., Sabbagh M., Beach T. Amyloid load in nondemented brains correlates with APOE e4. Neurosci. Lett. 2010;473:168–171. doi: 10.1016/j.neulet.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles S.T., Mather M., Carstensen L.L. Aging and emotional memory: the forgettable nature of negative images for older adults. J. Exp. Psychol. Gen. 2003;132:310–324. doi: 10.1037/0096-3445.132.2.310. [DOI] [PubMed] [Google Scholar]
- Chen Y.-C., Chen C.-C., Decety J., Cheng Y. Aging is associated with changes in the neural circuits underlying empathy. Neurobiol. Aging. 2014;35:827–836. doi: 10.1016/j.neurobiolaging.2013.10.080. [DOI] [PubMed] [Google Scholar]
- Chetelat G., La Joie R., Villain N., Perrotin A., de La Sayette V., Eustache F., Vandenberghe R. Amyloid imaging in cognitively normal individuals, at-risk populations and preclinical Alzheimer's disease. Neuroimage-Clinical. 2013;2:356–365. doi: 10.1016/j.nicl.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christov-Moore L., Iacoboni M. Self-other resonance, its control and prosocial inclinations: Brain–behavior relationships. Hum. Brain Mapp. 2016;37:1544–1558. doi: 10.1002/hbm.23119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly J.J., Kavanagh E.J., Viswesvaran C. The convergent validity between self and observer ratings of personality: A meta-analytic review. Int. J. Sel. Assess. 2007;15:110–117. [Google Scholar]
- Cox, R.W., Hyde, J.S., 1997. Software tools for analysis and visualization of fMRI data. NMR in Biomedicine: An International Journal Devoted to the Development and Application of Magnetic Resonance In Vivo 10, 171-178. [DOI] [PubMed]
- Dale A.M., Fischl B., Sereno M.I. Cortical surface-based analysis: I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Davis M.H. Measuring individual differences in empathy: evidence for a multidimensional approach. J. Pers. Soc. Psychol. 1983;44:113. [Google Scholar]
- Davis, M.H., 1980. A multidimensional approach to individual differences in empathy.
- Decety J., Jackson P.L. The functional architecture of human empathy. Behav. Cogn. Neurosci. Rev. 2004;3:71–100. doi: 10.1177/1534582304267187. [DOI] [PubMed] [Google Scholar]
- Devinsky O., Morrell M.J., Vogt B.A. Contributions of anterior cingulate cortex to behaviour. Brain. 1995;118:279–306. doi: 10.1093/brain/118.1.279. [DOI] [PubMed] [Google Scholar]
- Donovan N.J., Okereke O.I., Vannini P., Amariglio R.E., Rentz D.M., Marshall G.A., Johnson K.A., Sperling R.A. Association of higher cortical amyloid burden with loneliness in cognitively normal older adults. JAMA Psychiat. 2016;73:1230–1237. doi: 10.1001/jamapsychiatry.2016.2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan N.J., Locascio J.J., Marshall G.A., Gatchel J., Hanseeuw B.J., Rentz D.M., Johnson K.A., Sperling R.A., Harvard Aging Brain Study Longitudinal association of amyloid beta and anxious-depressive symptoms in cognitively normal older adults. Am. J. Psychiatry. 2018;175:530–537. doi: 10.1176/appi.ajp.2017.17040442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doraiswamy P.M., Sperling R.A., Coleman R.E., Johnson K.A., Reiman E.M., Davis M.D., Grundman M., Sabbagh M.N., Sadowsky C.H., Fleisher A.S., Carpenter A., Clark C.M., Joshi A.D., Mintun M.A., Skovronsky D.M., Pontecorvo M.J., AV45-A11 Study Group Amyloid-β assessed by florbetapir F 18 PET and 18-month cognitive decline: a multicenter study. Neurology. 2012;79:1636–1644. doi: 10.1212/WNL.0b013e3182661f74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrenberg A.J., Suemoto C.K., Franca Resende E.P., Petersen C., Leite R.E.P., Rodriguez R.D., Ferretti-Rebustini R.E.L., You M., Oh J., Nitrini R., Pasqualucci C.A., Jacob-Filho W., Kramer J.H., Gatchel J.R., Grinberg L.T. Neuropathologic correlates of psychiatric symptoms in Alzheimer’s disease. J. Alzheimers Dis. 2018;66:115–126. doi: 10.3233/JAD-180688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis K.A., Lim Y.Y., Harrington K., Ames D., Bush A.I., Darby D., Martins R.N., Masters C.L., Rowe C.C., Savage G., Szoeke C., Villemagne V.L., Maruff P., For the AIBL Research Group Decline in cognitive function over 18 months in healthy older adults with high amyloid-β. J. Alzheimers Dis. 2013;34:861–871. doi: 10.3233/JAD-122170. [DOI] [PubMed] [Google Scholar]
- Esteban, O.M., CJ., 2020. fMRIPrep: a robust preprocessing pipeline for functional MRI. doi: 10.5281/zenodo.4252786. [DOI] [PMC free article] [PubMed]
- Esteban, O., Markiewicz, C.J., Blair, R.W., Moodie, C.A., Isik, A.I., Erramuzpe, A., Kent, J.D., Goncalves, M., DuPre, E., Snyder, M., Oya, H., Ghosh, S.S., Wright, J., Durnez, J., Poldrack, R.A., Gorgolewski, K.J., 2018. FMRIPrep: a robust preprocessing pipeline for functional MRI. bioRxiv, 306951. [DOI] [PMC free article] [PubMed]
- Fernandez-Duque D., Hodges S.D., Baird J.A., Black S.E. Empathy in frontotemporal dementia and Alzheimer's disease. J. Clin. Exp. Neuropsychol. 2010;32:289–298. doi: 10.1080/13803390903002191. [DOI] [PubMed] [Google Scholar]
- Folstein M.F., Folstein S.E., McHugh P.R. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Fonov V.S., Evans A.C., McKinstry R.C., Almli C., Collins D. Unbiased nonlinear average age-appropriate brain templates from birth to adulthood. Neuroimage. 2009;S102 [Google Scholar]
- Fox N., Warrington E., Freeborough P., Hartikainen P., Kennedy A., Stevens J., Rossor M.N. Presymptomatic hippocampal atrophy in Alzheimer's disease: a longitudinal MRI study. Brain. 1996;119:2001–2007. doi: 10.1093/brain/119.6.2001. [DOI] [PubMed] [Google Scholar]
- Fredericks C.A., Sturm V.E., Brown J.A., Hua A.Y., Bilgel M., Wong D.F., Resnick S.M., Seeley W.W. Early affective changes and increased connectivity in preclinical Alzheimer's disease. Alzheimer's & Dementia: Diagnosis, Assessment & Disease Monitoring. 2018;10:471–479. doi: 10.1016/j.dadm.2018.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston K.J., Williams S., Howard R., Frackowiak R.S., Turner R. Movement-related effects in fMRI time-series. Magn. Reson. Med. 1996;35:346–355. doi: 10.1002/mrm.1910350312. [DOI] [PubMed] [Google Scholar]
- Gallagher M., Chiba A.A. The amygdala and emotion. Curr. Opin. Neurobiol. 1996;6:221–227. doi: 10.1016/s0959-4388(96)80076-6. [DOI] [PubMed] [Google Scholar]
- Gard M.G., Kring A.M. Sex differences in the time course of emotion. Emotion. 2007;7:429. doi: 10.1037/1528-3542.7.2.429. [DOI] [PubMed] [Google Scholar]
- Goetz J.L., Keltner D., Simon-Thomas E. Compassion: an evolutionary analysis and empirical review. Psychol. Bull. 2010;136:351. doi: 10.1037/a0018807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good C.D., Johnsrude I., Ashburner J., Henson R.N., Friston K.J., Frackowiak R.S. Cerebral asymmetry and the effects of sex and handedness on brain structure: a voxel-based morphometric analysis of 465 normal adult human brains. Neuroimage. 2001;14:685–700. doi: 10.1006/nimg.2001.0857. [DOI] [PubMed] [Google Scholar]
- Greicius, M.D., Krasnow, B., Reiss, A.L., Menon, V., 2003. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proceedings of the National Academy of Sciences 100, 253-258. [DOI] [PMC free article] [PubMed]
- Greve D.N., Fischl B. Accurate and robust brain image alignment using boundary-based registration. Neuroimage. 2009;48:63–72. doi: 10.1016/j.neuroimage.2009.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo C.C., Kurth F., Zhou J., Mayer E.A., Eickhoff S.B., Kramer J.H., Seeley W.W. One-year test–retest reliability of intrinsic connectivity network fMRI in older adults. Neuroimage. 2012;61:1471–1483. doi: 10.1016/j.neuroimage.2012.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampel H., Bürger K., Teipel S.J., Bokde A.L., Zetterberg H., Blennow K. Core candidate neurochemical and imaging biomarkers of Alzheimer’s disease. Alzheimers Dement. 2008;4:38–48. doi: 10.1016/j.jalz.2007.08.006. [DOI] [PubMed] [Google Scholar]
- Hatfield E., Cacioppo J.T., Rapson R.L. Emotional contagion. Curr. Dir. Psychol. Sci. 1993;2:96–100. [Google Scholar]
- Hedden T., Oh H., Younger A.P., Patel T.A. Meta-analysis of amyloid-cognition relations in cognitively normal older adults. Neurology. 2013;80:1341–1348. doi: 10.1212/WNL.0b013e31828ab35d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermans E.J., van Marle H.J., Ossewaarde L., Henckens M.J., Qin S., van Kesteren M.T., Schoots V.C., Cousijn H., Rijpkema M., Oostenveld R., Fernandez G. Stress-related noradrenergic activity prompts large-scale neural network reconfiguration. Science. 2011;334:1151–1153. doi: 10.1126/science.1209603. [DOI] [PubMed] [Google Scholar]
- Hillis A.E. Inability to empathize: brain lesions that disrupt sharing and understanding another’s emotions. Brain. 2014;137:981–997. doi: 10.1093/brain/awt317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel P.S., Ossenkoppele R., Gessert D., Jagust W., Landau S., Hansson O., Weiner M.W., Mattsson N., Alzheimer's Disease Neuroimaging, I., Time to amyloid positivity and preclinical changes in brain metabolism, atrophy, and cognition: evidence for emerging amyloid pathology in Alzheimer's disease. Front. Neurosci. 2017;11:281. doi: 10.3389/fnins.2017.00281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack C.R., Jr., Knopman D.S., Jagust W.J., Shaw L.M., Aisen P.S., Weiner M.W., Petersen R.C., Trojanowski J.Q. Hypothetical model of dynamic biomarkers of the Alzheimer's pathological cascade. Lancet Neurol. 2010;9:119–128. doi: 10.1016/S1474-4422(09)70299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagust W. Is amyloid-beta harmful to the brain? Insights from human imaging studies. Brain. 2016;139:23–30. doi: 10.1093/brain/awv326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M., Bannister P., Brady M., Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Jilka S.R., Scott G., Ham T., Pickering A., Bonnelle V., Braga R.M., Leech R., Sharp D.J. Damage to the salience network and interactions with the default mode network. J. Neurosci. 2014;34:10798–10807. doi: 10.1523/JNEUROSCI.0518-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K.A., Minoshima S., Bohnen N.I., Donohoe K.J., Foster N.L., Herscovitch P., Karlawish J.H., Rowe C.C., Carrillo M.C., Hartley D.M., Hedrick S., Pappas V., Thies W.H. Appropriate use criteria for amyloid PET: A report of the Amyloid Imaging Task Force, the Society of Nuclear Medicine and Molecular Imaging, and the Alzheimer's Association. Alzheimers & Dementia. 2013;9:E1–E16. doi: 10.1016/j.jalz.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzorreck M., Kunzmann U. Greater empathic accuracy and emotional reactivity in old age: The sample case of death and dying. Psychol Aging. 2018;33:1202–1214. doi: 10.1037/pag0000313. [DOI] [PubMed] [Google Scholar]
- Khan, T.K., 2018. An algorithm for preclinical diagnosis of Alzheimer's disease. Front. Neurosci. 12, 275. [DOI] [PMC free article] [PubMed]
- Kim J.J., Cunnington R., Kirby J.N. The neurophysiological basis of compassion: An fMRI meta-analysis of compassion and its related neural processes. Neurosci. Biobehav. Rev. 2020;108:112–123. doi: 10.1016/j.neubiorev.2019.10.023. [DOI] [PubMed] [Google Scholar]
- Kim H., Di Domenico S.I., Connelly B.S. Self-Other Agreement in Personality Reports: A Meta-Analytic Comparison of Self- and Informant-Report Means. Psychol. Sci. 2019;30:129–138. doi: 10.1177/0956797618810000. [DOI] [PubMed] [Google Scholar]
- Klein A., Ghosh S.S., Bao F.S., Giard J., Hame Y., Stavsky E., Lee N., Rossa B., Reuter M., Neto E.C., Keshavan A. Mindboggling morphometry of human brains. PLoS Comput. Biol. 2017;13:e1005350. doi: 10.1371/journal.pcbi.1005350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konrath, S., 2013. A critical analysis of the Interpersonal Reactivity Index. MedEdPORTAL Directory and Repository of Educational Assessment Measures (DREAM).
- Kramer J.H., Jurik J., Sharon J.S., Rankin K.P., Rosen H.J., Johnson J.K., Miller B.L. Distinctive neuropsychological patterns in frontotemporal dementia, semantic dementia, and Alzheimer disease. Cogn. Behav. Neurol. 2003;16:211–218. doi: 10.1097/00146965-200312000-00002. [DOI] [PubMed] [Google Scholar]
- Kring A.M., Gordon A.H. Sex differences in emotion: expression, experience, and physiology. J. Pers. Soc. Psychol. 1998;74:686. doi: 10.1037//0022-3514.74.3.686. [DOI] [PubMed] [Google Scholar]
- Kunzmann U., Gruhn D. Age differences in emotional reactivity: the sample case of sadness. Psychol Aging. 2005;20:47–59. doi: 10.1037/0882-7974.20.1.47. [DOI] [PubMed] [Google Scholar]
- Kunzmann U., Richter D. Emotional reactivity across the adult life span: the cognitive pragmatics make a difference. Psychol. Aging. 2009;24:879–889. doi: 10.1037/a0017347. [DOI] [PubMed] [Google Scholar]
- Landau, S.M., Breault, C., Joshi, A.D., Pontecorvo, M., Mathis, C.A., Jagust, W.J., Mintun, M.A., Neuroimaging, A.s.D., 2013a. Amyloid-beta Imaging with Pittsburgh Compound B and Florbetapir: Comparing Radiotracers and Quantification Methods. Journal of Nuclear Medicine 54, 70-77. [DOI] [PMC free article] [PubMed]
- Landau S.M., Lu M., Joshi A.D., Pontecorvo M., Mintun M.A., Trojanowski J.Q., Shaw L.M., Jagust W.J., Initiative A.D.N. Comparing positron emission tomography imaging and cerebrospinal fluid measurements of β-amyloid. Ann. Neurol. 2013;74:826–836. doi: 10.1002/ana.23908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim Y.Y., Ellis K.A., Pietrzak R.H., Ames D., Darby D., Harrington K., Martins R.N., Masters C.L., Rowe C., Savage G., Szoeke C., Villemagne V.L., Maruff P., For the AIBL Research Group Stronger effect of amyloid load than APOE genotype on cognitive decline in healthy older adults. Neurology. 2012;79:1645–1652. doi: 10.1212/WNL.0b013e31826e9ae6. [DOI] [PubMed] [Google Scholar]
- Lowe M., Mock B., Sorenson J. Functional connectivity in single and multislice echoplanar imaging using resting-state fluctuations. Neuroimage. 1998;7:119–132. doi: 10.1006/nimg.1997.0315. [DOI] [PubMed] [Google Scholar]
- Lwi S.J., Haase C.M., Shiota M.N., Newton S.L., Levenson R.W. Responding to the emotions of others: Age differences in facial expressions and age-specific associations with relational connectedness. Emotion. 2019;19:1437–1449. doi: 10.1037/emo0000534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyketsos C.G., Olin J. Depression in Alzheimer’s disease: overview and treatment. Biol. Psychiatry. 2002;52:243–252. doi: 10.1016/s0006-3223(02)01348-3. [DOI] [PubMed] [Google Scholar]
- Machulda M.M., Jones D.T., Vemuri P., McDade E., Avula R., Przybelski S., Boeve B.F., Knopman D.S., Petersen R.C., Jack C.R. Effect of APOE ε4 status on intrinsic network connectivity in cognitively normal elderly subjects. Arch. Neurol. 2011;68:1131–1136. doi: 10.1001/archneurol.2011.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather M., Carstensen L.L. Aging and motivated cognition: the positivity effect in attention and memory. Trends Cogn. Sci. 2005;9:496–502. doi: 10.1016/j.tics.2005.08.005. [DOI] [PubMed] [Google Scholar]
- McKhann, G., Drachman, D., Folstein, M., Katzman, R., Price, D., Stadlan, E.M., 1984. Clinical diagnosis of Alzheimer's disease: Report of the NINCDS‐ADRDA Work Group* under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology 34, 939-939. [DOI] [PubMed]
- Menon V., Uddin L.Q. Saliency, switching, attention and control: a network model of insula function. Brain Struct. Funct. 2010;214:655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mormino E.C. The relevance of beta-amyloid on markers of Alzheimer's disease in clinically normal individuals and factors that influence these associations. Neuropsychol. Rev. 2014;24:300–312. doi: 10.1007/s11065-014-9267-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mormino, E.C., Papp, K.V., 2018. Amyloid accumulation and cognitive decline in clinically normal older individuals: implications for aging and early Alzheimer's disease. J. Alzheimers Dis. 64, S633-S646. [DOI] [PMC free article] [PubMed]
- Morris J.C. The Clinical Dementia Rating (CDR): current version and. Young. 1991;41:1588–1592. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- Mõttus R., Allik J., Realo A. Do self-reports and informant-ratings measure the same personality constructs? Eur. J. Psychol. Assess. 2020;36:289–295. [Google Scholar]
- O'Brien E., Konrath S.H., Gruhn D., Hagen A.L. Empathic concern and perspective taking: linear and quadratic effects of age across the adult life span. J Gerontol B Psychol Sci Soc Sci. 2013;68:168–175. doi: 10.1093/geronb/gbs055. [DOI] [PubMed] [Google Scholar]
- Olino T.M., Klein D.N. Psychometric comparison of self- and informant-reports of personality. Assessment. 2015;22:655–664. doi: 10.1177/1073191114567942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossenkoppele R., Schonhaut D.R., Scholl M., Lockhart S.N., Ayakta N., Baker S.L., O'Neil J.P., Janabi M., Lazaris A., Cantwell A., Vogel J., Santos M., Miller Z.A., Bettcher B.M., Vossel K.A., Kramer J.H., Gorno-Tempini M.L., Miller B.L., Jagust W.J., Rabinovici G.D. Tau PET patterns mirror clinical and neuroanatomical variability in Alzheimer’s disease. Brain. 2016;139:1551–1567. doi: 10.1093/brain/aww027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen, C., Nolan AL, de Paula França Resende E, Miller Z, Ehrenberg AJ, Gorno-Tempini ML, Rosen HJ, Kramer JH, Spina S, Rabinovici GD, Miller BL, Seeley WW, Heinsen H, Grinberg LT 2019. Alzheimer’s disease clinical variants show distinct regional patterns of neurofibrillary tangle accumulation. Acta neuropathologica 138, 597-612. [DOI] [PMC free article] [PubMed]
- Phillips, L.H., MacLean, R.D., Allen, R., 2002. Age and the understanding of emotions: neuropsychological and sociocognitive perspectives. J Gerontol B Psychol Sci Soc Sci 57, P526-530. [DOI] [PubMed]
- Pinheiro, J., Bates, D., DebRoy, S., Sarkar, D., R Core Team, 2018. nlme: Linear and Nonlinear Mixed Effects Models. R package version 3.1-137, URL https://CRAN.R-project.org/package=nlme.
- Pini L., Pievani M., Bocchetta M., Altomare D., Bosco P., Cavedo E., Galluzzi S., Marizzoni M., Frisoni G.B. Brain atrophy in Alzheimer’s disease and aging. Ageing Res. Rev. 2016;30:25–48. doi: 10.1016/j.arr.2016.01.002. [DOI] [PubMed] [Google Scholar]
- Power J.D., Barnes K.A., Snyder A.Z., Schlaggar B.L., Petersen S.E. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59:2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruim R.H., Mennes M., van Rooij D., Llera A., Buitelaar J.K., Beckmann C.F. ICA-AROMA: A robust ICA-based strategy for removing motion artifacts from fMRI data. Neuroimage. 2015;112:267–277. doi: 10.1016/j.neuroimage.2015.02.064. [DOI] [PubMed] [Google Scholar]
- R Core Team, 2020. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/.
- Raichle M.E. The brain's default mode network. Annu. Rev. Neurosci. 2015;38:433–447. doi: 10.1146/annurev-neuro-071013-014030. [DOI] [PubMed] [Google Scholar]
- Rankin K.P., Gorno-Tempini M.L., Allison S.C., Stanley C.M., Glenn S., Weiner M.W., Miller B.L. Structural anatomy of empathy in neurodegenerative disease. Brain. 2006;129:2945–2956. doi: 10.1093/brain/awl254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed, A.E., Carstensen, L.L., 2012. The theory behind the age-related positivity effect. Frontiers in Psychology 3. [DOI] [PMC free article] [PubMed]
- Richter D., Kunzmann U. Age differences in three facets of empathy: performance-based evidence. Psychol Aging. 2011;26:60–70. doi: 10.1037/a0021138. [DOI] [PubMed] [Google Scholar]
- Risacher S.L., Saykin A.J. Neuroimaging and other biomarkers for Alzheimer's disease: the changing landscape of early detection. Annu. Rev. Clin. Psychol. 2013;9:621–648. doi: 10.1146/annurev-clinpsy-050212-185535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth-Hanania R., Davidov M., Zahn-Waxler C. Empathy development from 8 to 16 months: Early signs of concern for others. Infant Behav. Dev. 2011;34:447–458. doi: 10.1016/j.infbeh.2011.04.007. [DOI] [PubMed] [Google Scholar]
- Rowe C.C., Bourgeat P., Ellis K.A., Brown B., Lim Y.Y., Mulligan R., Jones G., Maruff P., Woodward M., Price R., Robins P., Tochon-Danguy H., O'Keefe G., Pike K.E., Yates P., Szoeke C., Salvado O., Macaulay S.L., O'Meara T., Head R., Cobiac L., Savage G., Martins R., Masters C.L., Ames D., Villemagne V.L. Predicting Alzheimer disease with beta-amyloid imaging: results from the Australian imaging, biomarkers, and lifestyle study of ageing. Ann. Neurol. 2013;74:905–913. doi: 10.1002/ana.24040. [DOI] [PubMed] [Google Scholar]
- Saroglou V., Pichon I., Trompette L., Verschueren M., Dernelle R. Prosocial behavior and religion: New evidence based on projective measures and peer ratings. J. Sci. Study Relig. 2005;44:323–348. [Google Scholar]
- Satterthwaite T.D., Elliott M.A., Gerraty R.T., Ruparel K., Loughead J., Calkins M.E., Eickhoff S.B., Hakonarson H., Gur R.C., Gur R.E., Wolf D.H. An improved framework for confound regression and filtering for control of motion artifact in the preprocessing of resting-state functional connectivity data. Neuroimage. 2013;64:240–256. doi: 10.1016/j.neuroimage.2012.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmechel D., Saunders A., Strittmatter W., Crain B.J., Hulette C., Joo S., Pericak-Vance M., Goldgaber D., Roses A. Increased amyloid beta-peptide deposition in cerebral cortex as a consequence of apolipoprotein E genotype in late-onset Alzheimer disease. Proc. Natl. Acad. Sci. 1993;90:9649–9653. doi: 10.1073/pnas.90.20.9649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz A.P., Chhatwal J.P., Hedden T., Mormino E.C., Hanseeuw B.J., Sepulcre J., Huijbers W., LaPoint M., Buckley R.F., Johnson K.A., Sperling R.A. Phases of hyperconnectivity and hypoconnectivity in the default mode and salience networks track with amyloid and tau in clinically normal individuals. J. Neurosci. 2017;37:4323–4331. doi: 10.1523/JNEUROSCI.3263-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz A.P., Buckley R.F., Hampton O.L., Scott M.R., Properzi M.J., Pena-Gomez C., Pruzin J.J., Yang H.S., Johnson K.A., Sperling R.A., Chhatwal J.P. Longitudinal degradation of the default/salience network axis in symptomatic individuals with elevated amyloid burden. NeuroImage: Clin. 2020;26 doi: 10.1016/j.nicl.2019.102052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley W.W., Menon V., Schatzberg A.F., Keller J., Glover G.H., Kenna H., Reiss A.L., Greicius M.D. Dissociable intrinsic connectivity networks for salience processing and executive control. J. Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley W.W., Crawford R.K., Zhou J., Miller B.L., Greicius M.D. Neurodegenerative diseases target large-scale human brain networks. Neuron. 2009;62:42–52. doi: 10.1016/j.neuron.2009.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seider B.H., Shiota M.N., Whalen P., Levenson R.W. Greater sadness reactivity in late life. Soc Cogn Affect Neurosci. 2011;6:186–194. doi: 10.1093/scan/nsq069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline Y.I., Raichle M.E. Resting state functional connectivity in preclinical Alzheimer’s disease. Biol. Psychiatry. 2013;74:340–347. doi: 10.1016/j.biopsych.2012.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiota M.N., Levenson R.W. Effects of aging on experimentally instructed detached reappraisal, positive reappraisal, and emotional behavior suppression. Psychol Aging. 2009;24:890–900. doi: 10.1037/a0017896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer, T., Klimecki, O.M., 2014. Empathy and compassion. Current Biology 24, R875-R878. [DOI] [PubMed]
- Singer, T., Seymour, B., O'doherty, J., Kaube, H., Dolan, R.J., Frith, C.D., 2004. Empathy for pain involves the affective but not sensory components of pain. Science 303, 1157-1162. [DOI] [PubMed]
- Snitz B.E., Weissfeld L.A., Cohen A.D., Lopez O.L., Nebes R.D., Aizenstein H.J., McDade E., Price J.C., Mathis C.A., Klunk W.E. Subjective cognitive complaints, personality and brain amyloid-beta in cognitively normal older adults. Am. J. Geriatr. Psychiatr. 2015;23:985–993. doi: 10.1016/j.jagp.2015.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling R.A., Aisen P.S., Beckett L.A., Bennett D.A., Craft S., Fagan A.M., Iwatsubo T., Jack C.R., Jr., Kaye J., Montine T.J., Park D.C., Reiman E.M., Rowe C.C., Siemers E., Stern Y., Yaffe K., Carrillo M.C., Thies B., Morrison-Bogorad M., Wagster M.V., Phelps C.H. Toward defining the preclinical stages of Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss M.E., Pasupathi M., Chatterjee A. Concordance between observers in descriptions of personality change in Alzheimer's disease. Psychol. Aging. 1993;8:475. doi: 10.1037//0882-7974.8.4.475. [DOI] [PubMed] [Google Scholar]
- Sturm V.E., Sollberger M., Seeley W.W., Rankin K.P., Ascher E.A., Rosen H.J., Miller B.L., Levenson R.W. Role of right pregenual anterior cingulate cortex in self-conscious emotional reactivity. Soc. Cogn. Affect. Neurosci. 2013;8:468–474. doi: 10.1093/scan/nss023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturm V.E., Yokoyama J.S., Seeley W.W., Kramer J.H., Miller B.L., Rankin K.P. Heightened emotional contagion in mild cognitive impairment and Alzheimer’s disease is associated with temporal lobe degeneration. Proc. Natl. Acad. Sci. 2013;110:9944–9949. doi: 10.1073/pnas.1301119110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturm V.E., Brown J.A., Hua A.Y., Lwi S.J., Zhou J., Kurth F., Eickhoff S.B., Rosen H.J., Kramer J.H., Miller B.L., Levenson R.W., Seeley W.W. Network architecture underlying basal autonomic outflow: Evidence from frontotemporal dementia. J. Neurosci. 2018;38:8943–8955. doi: 10.1523/JNEUROSCI.0347-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sze J.A., Gyurak A., Goodkind M.S., Levenson R.W. Greater emotional empathy and prosocial behavior in late life. Emotion. 2012;12:1129–1140. doi: 10.1037/a0025011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terracciano A., Bilgel M., Aschwanden D., Luchetti M., Stephan Y., Moghekar A.R., Wong D.F., Ferrucci L., Sutin A.R., Resnick S.M. Personality associations with amyloid and tau: Results from the Baltimore Longitudinal Study of Aging and meta-analysis. Biol. Psychiatry. 2021 doi: 10.1016/j.biopsych.2021.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tustison N.J., Avants B.B., Cook P.A., Zheng Y., Egan A., Yushkevich P.A., Gee J.C. N4ITK: improved N3 bias correction. IEEE Trans. Med. Imaging. 2010;29:1310–1320. doi: 10.1109/TMI.2010.2046908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twamley E.W., Ropacki S.A., Bondi M.W. Neuropsychological and neuroimaging changes in preclinical Alzheimer's disease. J Int Neuropsychol Soc. 2006;12:707–735. doi: 10.1017/S1355617706060863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venables W.N., Ripley B.D. fourth ed. Springer; New York: 2002. Modern Applied Statistics with S. [Google Scholar]
- Villeneuve S., Rabinovici G.D., Cohn-Sheehy B.I., Madison C., Ayakta N., Ghosh P.M., La Joie R., Arthur-Bentil S.K., Vogel J.W., Marks S.M., Lehmann M., Rosen H.J., Reed B., Olichney J., Boxer A.L., Miller B.L., Borys E., Jin L.W., Huang E.J., Grinberg L.T., DeCarli C., Seeley W.W., Jagust W. Existing Pittsburgh Compound-B positron emission tomography thresholds are too high: statistical and pathological evaluation. Brain. 2015;138:2020–2033. doi: 10.1093/brain/awv112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S., Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012;2:125–141. doi: 10.1089/brain.2012.0073. [DOI] [PubMed] [Google Scholar]
- Whitwell J.L., Crum W.R., Watt H.C., Fox N.C. Normalization of cerebral volumes by use of intracranial volume: implications for longitudinal quantitative MR imaging. Am. J. Neuroradiol. 2001;22:1483–1489. [PMC free article] [PubMed] [Google Scholar]
- Wieck C., Kunzmann U. Age differences in empathy: Multidirectional and context-dependent. Psychol Aging. 2015;30:407–419. doi: 10.1037/a0039001. [DOI] [PubMed] [Google Scholar]
- Wolk D.A., Zhang Z., Boudhar S., Clark C.M., Pontecorvo M.J., Arnold S.E. Amyloid imaging in Alzheimer's disease: comparison of florbetapir and Pittsburgh compound-B positron emission tomography. J. Neurol. Neurosurg. Psychiatry. 2012;83:923–926. doi: 10.1136/jnnp-2012-302548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan C.-G., Cheung B., Kelly C., Colcombe S., Craddock R.C., Di Martino A., Li Q., Zuo X.-N., Castellanos F.X., Milham M.P. A comprehensive assessment of regional variation in the impact of head micromovements on functional connectomics. Neuroimage. 2013;76:183–201. doi: 10.1016/j.neuroimage.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuno F., Kazui H., Morita N., Kajimoto K., Ihara M., Taguchi A., Yamamoto A., Matsuoka K., Kosaka J., Kudo T. High amyloid-β deposition related to depressive symptoms in older individuals with normal cognition: a pilot study. Int. J. Geriatr. Psychiatry. 2016;31:920–928. doi: 10.1002/gps.4409. [DOI] [PubMed] [Google Scholar]
- Yesavage J.A., Brink T.L., Rose T.L., Lum O., Huang V., Adey M., Leirer V.O. Development and validation of a geriatric depression screening scale: a preliminary report. J. Psychiatr. Res. 1982;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- Zaki J., Ochsner K.N. The neuroscience of empathy: progress, pitfalls and promise. Nat. Neurosci. 2012;15:675–680. doi: 10.1038/nn.3085. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Brady M., Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans. Med. Imaging. 2001;20:45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]
- Zhou J., Greicius M.D., Gennatas E.D., Growdon M.E., Jang J.Y., Rabinovici G.D., Kramer J.H., Weiner M., Miller B.L., Seeley W.W. Divergent network connectivity changes in behavioural variant frontotemporal dementia and Alzheimer’s disease. Brain. 2010;133:1352–1367. doi: 10.1093/brain/awq075. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
UCSF Memory and Aging Center data are conditionally available upon request through the UCSF Memory and Aging Center Resource Request Form (https://memory.ucsf.edu/resources/data). Investigators must have Institutional Review Board approval from the UCSF Human Research Protection Program and be from academic, not-for-profit institutions.