Abstract
Objectives
This study described and compared glycaemic changes with the use of the following Continuous Glucose Monitoring (CGM) metrics: time in range, time in hyperglycaemia and time in hypoglycaemia from retrospective CGM data among children and adolescents with Type 1 Diabetes Mellitus (T1DM), before and during Ramadan to better understand the impact of fasting during this season.
Methodology
This study was conducted in 2 tertiary centres: Hospital Putrajaya (HPJ) and Hospital Universiti Sains Malaysia (HUSM) from February to May 2020. Muslim T1DM patients between ages 8 to18 who intended to fast during Ramadan were given Ramadan-focused education. CGM iPro2® (Medtronic) was used before and during Ramadan, complemented by finger-prick glucose monitoring or self-monitoring of blood glucose (SMBG).
Results
Of the 32 patients, only 24 (12 female) were analysed. Mean age was 13.6 ± 3.1 years old, mean HbAlc was 9.6 ± 1.9% and mean duration of illness was 5.4 ± 3.4 years. Majority (91.7%) were on multiple dose injections (MDI) while only 8.3% were on continuous subcutaneous insulin infusion (CSII). All fasted in Ramadan without acute complications. Retrospective CGM analysis revealed similar results in time in range (TIR), time in hyperglycaemia and time in hypoglycaemia before and during Ramadan, indicating no increased hypoglycaemic or hyperglycaemic events related to fasting. Glycaemic variability before Ramadan as measured by the LBGI, HBGI and MAG, were similar to values during Ramadan.
Conclusion
Ramadan fasting among T1DM children and adolescents, by itself, is not associated with short-term glycaemic deterioration. T1DM youths can fast safely in Ramadan with the provision of focused education and regular SMBG.
Keywords: paediatric, T1DM, CGM, Ramadan fasting
INTRODUCTION
Fasting from dawn (Sahur) until sunset (Iftar) in Ramadan, the 9th month of the Islamic Calendar, is one of the five pillars of Islam and is obligatory for all healthy Muslim adults, adolescents, and children from the time of puberty.1 This one-month-long fasting is a period of spiritual contemplation and seeking nearness to God when the followers strictly refrain from eating or drinking during daylight and practice abstinence. For patients with T1DM, Ramadan fasting is even more demanding as their body’s glucose homeostasis is dependent on exogenous insulin and has been associated with higher risks of hypoglycaemia, hyperglycaemia, diabetic ketoacidosis (DKA), dehydration, and venous thrombosis.2–6
Despite exemption by religious authorities for medical concerns3–5 and alternatives like Fidya, which is a form of donation of food or money to the poor to compensate for the missed fasting days, most T1DM patients still insist on fasting during Ramadan.2–8 The Epidemiology of Diabetes and Ramadan study (EPIDIAR) in 2001 reported that 42.8% of T1DM patients fasted for at least 15 days during Ramadan.6 More recently, the DaR (Diabetes and Ramadan) Global Survey in 20208 by the International Diabetes Federation - Diabetes and Ramadan alliance (IDFDaR) looked at profiles of T1DM youths from 13 major Islamic countries, 75% of them fasted for a mean duration of 22 days, despite the COVID-19 pandemic. More than half (55.6%) had at least one daytime hypoglycaemia and only 71.2% performed regular SMBG.8 To better assess and manage risks associated with fasting, IDF-DaR in their updated guidelines 2021,5 introduced a risk calculator to determine the risk of a person with diabetes prior to fasting for Ramadan. The calculator, which included T1DM as one of the risks, provides a convenient way to determine risk but it is still advisable for diabetes care in Ramadan to be highly individualised. In addition, knowledge gaps regarding the true glycaemic impact of Ramadan fasting still remain.5
The International Society of Paediatric and Adolescent Diabetes (ISPAD) in their 2018 guidelines, recommended that Muslim T1DM youths may fast, provided they have reasonable glycaemic control, good hypoglycaemic awareness and willingness to frequently monitor blood glucose.2 Both the IDF and the ISPAD have emphasized the importance of glucose monitoring during Ramadan, either through SMBG or via advanced technology, such as intermittent flash CGM (iCGM) or real-time CGM (rt-CGM).
Before the era of CGM, most of the glycaemic data from previous Ramadan studies9-11 in T1DM children and adolescents were from SMBG records. Unlike CGM which provides continuous glucose data for the entire day, SMBG only provides single-point glucose readings and is unable to reflect the overall glycaemic picture. Following the increasing use of CGM in clinical and research settings, more Ramadan studies using CGM were conducted among paediatric T1DM in recent years.12–18 Instead of retrospective CGM, most of these authors12,14–18 utilized personal or real-time CGM (rt-CGM), which has the benefit of immediate glucose visualisation and intervention, as well as “communication,” or coupling with the insulin infusion pump, also referred to as the sensor-augmented pump (SAP). However, with retrospective or professional CGMs, patients wearing the devices are not aware of their glucose values until their care provider downloads and reviews data during an office visit. Despite being “blinded,” they provide important information to both patients and clinicians, especially in resource-limited countries. As opposed to rt-CGM, these “unaltered” glycaemic profiles from retrospective CGM could provide insights, as well as promote self-learning for Muslim T1DM patients who rely mostly on SMBG.
As one of the developing Islamic countries in Southeast Asia with 61.3% of its population comprising of Muslim population, Malaysia has a growing number of Muslim T1DM youths. Referring to the local diabetes registry or DICARE,19 70% of the diabetic population less than 18 years old were T1DM and the majority of them were Muslims. Despite the existing knowledge gap of Ramadan fasting, there is still a lack of local T1DM Ramadan studies specifically looking at CGM outcomes. Also, most previous Ramadan CGM fasting studies were conducted in either the Middle East or Central Asia countries, where fasting practices and local cultures may not be entirely similar to the Muslim countries in Southeast Asia.
For these reasons, we decided to investigate the CGM profiles of our Muslim T1DM youths during Ramadan fasting. Our study aimed to describe and compare the glycaemic changes among children and adolescents with T1DM before and during fasting in Ramadan month. These glycaemic changes were measured using CGM metrics, which include time in range, time in hypoglycaemia and time in hyperglycaemia. We hypothesized that Ramadan fasting is not associated with increased or worsened glycaemic risks. We also analyzed the impact of optimal HbA1c level and younger age on glycaemic changes.
We hope to support and empower Muslim T1DM youths for a safer Ramadan fasting experience though the conduct of this study.
METHODOLOGY
Patient and study design
This was a prospective study involving two tertiary centres in West and East Peninsular Malaysia, namely Hospital Putrajaya (HPJ) and Hospital Universiti Sains Malaysia (HUSM), respectively. It was conducted from February until May 2020 and included Ramadan 2020 (Hijri 1441) which was from 23rd April to 24th May 2020. Inclusion criteria were Muslim T1DM children and adolescents aged 8-18 years old, under follow-up care by paediatric endocrinologists and had expressed intentions to fast. Participants with all types of insulin delivery were included. Exclusion criteria were history of severe hypoglycaemia, recurrent hypoglycaemia episodes, hypoglycaemia unawareness, diabetic ketoacidosis (DKA) three months prior, intervening acute illnesses, pregnancy or chronic dialysis, in accordance to the Malaysia Paediatric T1DM Management Guideline.20 Stratified sampling proportionate to sample size was applied in recruitment from both centres.
Ramadan-focused education and regular clinic visits
Ramadan-focused education and single-day workshop were conducted for both patients and their caregivers before Ramadan, involving paediatric endocrinologists, diabetes educator nurses, and dieticians. A total of 4 clinic visits were performed throughout the study period: 2 clinic visits before and another 2 during Ramadan. Standardized diabetes assessment and Ramadan-focused education were provided during clinic visits. Individual insulin adjustment and dietary advice were provided according to published international guidelines.3–5
Retrospective CGM
Retrospective CGM data analysis was performed twice for all the participants, using the iPro2 device (Medtronic, 18000 Devonshire Street, Northridge, CA 91325, USA), before and during Ramadan fasting. The coin-sized glucose sensor was inserted into the participants’ abdominal subcutaneous tissue together with the iPro2 recorder for 6 to 7 days (according to manufacturer’s recommendation), this was then removed the subsequent week. Both the insertion and removal procedures were performed by skilled diabetes nurse educators (DNE). All participants were also given emergency contact information for support, in case of any medical or technical problems arising during the CGM periods.
Self-Monitoring of Blood Glucose (SMBG)
Standardised glucometers (Contour Plus One, Ascensia Diabetes Care, 600 North Bridge Road) with blue-tooth connectivity were provided to every participant together with an ample supply of glucose strips for use for SMBG throughout the study period, including the entire Ramadan month. Participants were advised to perform SMBG at least 4 times per day and more frequently during Ramadan fasting (pre-sahur, 2-hour post-sahur, pre-iftar, 2-hour post iftar, or when symptomatic). SMBG readings were recorded by participants into individual diabetes logbooks with other relevant details such as amount and type of food, physical activities, and insulin doses. During Ramadan, the participants could discontinue fasting, if they experience any symptoms of being unwell, hypoglycaemia, severe hyperglycaemia, or sudden change of decision for any personal reasons.
Ethical approval
This study received ethical approval from the Medical Research & Ethics Committee (MREC), Ministry of Health, Malaysia and the Human Research Ethics Committee of Universiti Sains Malaysia (USM). It had also received a research grant from the National Institute of Health (NIH) under the Ministry of Health, Malaysia.
Sample Size Estimation
For the specific objective of comparing the CGM glycaemic parameters (time in range, time in hyperglycemia and time in hypoglycemia) before and during fasting in Ramadan, sample size calculation was done using PS software (paired t-test). Alpha (α) was set at 0.05, power at 0.8.
With reference to earlier findings by Nader Lessan et al.,13 for time in range (TIR), the smallest difference that is of clinical significance was pre-determined at 4 hours. International consensus21,22 and experts have recommended targeting the TIR for 70% of the day (16.8 hours of the 24 hour-day), but no data have reported the smallest deviation that could result in unfavorable clinical outcomes, hence, we have set the smallest difference at 4 hours.
For hyperglycemia duration, the smallest difference that is of clinical significance was also predetermined at 4 hours based on clinical assumption.
For hypoglycemia duration, the smallest difference that is of clinical significance was set at the lowest, and for safety reasons, we predetermined the value to be 15 minutes or an equivalent of 0.25 hours. Prolonged hypoglycemia of more than 15 minutes may result in severe neuroglycopenic symptoms that would necessitate medical care. Table 1 summarizes sample size considerations for each of the prespecified CGM outcomes.
Table 1.
Summary of sample size considerations per CGM outcome
| α | β (power) | γ | σ | Sample size | |
|---|---|---|---|---|---|
| Time in range (hr) | 0.05 | 0.8 | 4 | 6.98 | 26 pairs |
| Hyperglycaemia duration (hr) | 0.05 | 0.8 | 4 | 7.49 | 30 pairs |
| Hypoglycemia duration (hr) | 0.05 | 0.8 | 0.25 | 0.38 | 20 pairs |
Taking into consideration a drop-out rate of 10%, the initial sample size required for this study is estimated to be 33 subjects. This sample size is however, was limited by the short duration of Ramadan month and the unexpected COVID-19 pandemic.
Statistical methods
SPSS version 22 statistical analysis software (SPSS Inc., Chicago, IL, USA) was used. CGM data were downloaded from Medtronic Care-link Pro to Microsoft Excel (2010) and transcribed into SPSS. Descriptive statistics were used to characterise demographics (Table 2). Normality was tested by Shapiro–Wilk test and graphical assessment of normality. Continuous variables were presented as mean (SD) or median (IQR) based on their normality distribution; categorical data were presented as frequency (percentage). Glycaemic variability (GV) were calculated using EasyGV Excel version 9.0.R2 (https://www.phc.ox.ac.uk/research/resources/easygv) that was developed by Nathan R Hill, (©University of Oxford 2010-2016).23 All CGM metrics were compared by paired t-test or Wilcoxon Signed Rank test, as appropriate, whereas Chi-Squared test and Fisher Exact test were used to assess for differences in the categorical variables, as appropriate. A value of P<0.05 was considered statistically significant.
Table 2.
Demographics
| Baseline characteristics | Centres | p-value | Total (n=24) | ||
|---|---|---|---|---|---|
| HPJ (n=10) | HUSM (n=14) | ||||
| Age* (years) | 12.8 ± 3.0 | 14.1 ± 3.1 | 0.30a | 13.6 ± 3.1 | |
| Duration of diabetes* (years) | 5.3 ± 3.6 | 5.4 ± 3.4 | 0.94a | 5.4 ± 3.4 | |
| Baseline HbA1c* (%) | 9.2 ± 1.5 | 10.0 ± 2.0 | 0.28a | 9.6 ± 1.9 | |
| Anthropometry* | Weight (kg) | 45.1 ± 14.9 | 42.8 ± 15.0 | 0.72a | 43.7 ± 14.7 |
| Weight SD | -0.1 ± 0.9 | -1.0 ± 1.4 | 0.09a | -0.6 ± 1.3 | |
| Height (m) | 1.5 ± 0.2 | 1.5 ± 0.1 | 0.74a | 1.5 ± 0.2 | |
| Height SD | -0.3 ± 1.0 | -1.3 ± 1.5 | 0.07a | -0.9 ± 1.4 | |
| BMI* (kg/m2) | 19.1± 2.7 | 18.6 ± 4.4 | 0.74a | 18.7 ± 3.7 | |
| BMI SD | 0.2 ± 0.8 | -0.5 ± 1.7 | 0.28a | -0.2 ± 1.4 | |
| Gender+ | Male | 5 (50) | 7 (50) | 1.00b | 12 (50) |
| Female | 5 (50) | 7 (50) | 4 (16.7) | ||
| Puberty+ | Tanner 1-2 | 1 (10) | 3 (21.4) | 0.39c | 20 (83.3) |
| Tanner 3-5 | 9 (90) | 11 (78.6) | 22 (91.7) | ||
| Insulin delivery+ | MDI | 9 (90) | 13 (92.9) | 1.00c | 2 (8.3) |
| CSII | 1 (10) | 1 (7.1) | 1.2 (0.2) | ||
| Daily insulin dose* (unit/kg/day) | Before Ramadan | 1.1 ± 0.13 | 1.25 (0.24) | 0.11a | 1.0 ± 0.2 |
| During Ramadan | 1.0 ± 0.14 | 1.01 (0.22) | 0.45a | 23 ± 95.8 | |
| Ramadan experience+ | Yes | 9 (90) | 14 (100) | 0.42c | 1 (4.2) |
| No | 1 (10) | 0 (0) | 12 (50) | ||
| Socio-economic+ | B40 | 1 (10) | 10 (71.4) | 0.003c | 11 (45.8) |
| M40 | 6 (60) | 4 (28.6) | 10 (41.7) | ||
| T20 | 3 (30) | 0 (0) | 3 (12.5) | ||
| HbA1c subgroups+ | HbA1c level <7.5% | 3 (30) | 1 (7.2) | 0.24c | 4 (16.6) |
| HbA1c level 7.5-9.0 % | 0 (0) | 3 (21.4) | 3 (12.5) | ||
| HbA1c level >9.0% | 7 (70) | 10 (71.4) | 17 (70.9) | ||
Numerical data, presented in means ± SD
Categorical data, presented in number (%)
Independent t-test
Chi-square test
Fisher-exact test
B40: Household income below RM 4850 per month
M40: Household income RM 4851 – RM 10,970 per month
T20: Household income above RM 10,971 per month
(Laporan Kaji Selidik Pendapatan dan Gaji 2019, Jabatan Statistik, Malaysia)38
Glossary of CGM outcomes
Time in Range (TIR): The percentage of time a person spends with their blood glucose levels in a target range which varies depending on the person, but as a general guideline, it is suggested to start with a range of 3.8 to 10 mmol/L.
Mean absolute relative difference (MARD): Computed using temporally matched glucose data from CGM systems and comparison glucose measurements (most often obtained by capillary blood glucose (BG) measurements) of all subjects from a clinical study. For paediatric population the acceptable MARD is 12.2%.
Estimated A1C (eA1C): A measure converting the mean glucose derived from CGM or self-monitored blood glucose readings, using a formula obtained from glucose readings from a population into an estimate of a simultaneously measured laboratory A1C.
Standard Deviation (SD): A measure of the spread in glucose readings around the average – some call this the variation. If there are many highs and/or many lows on a given day, they will have a larger SD whereas a lower SD reflects a pretty stable glucose readings throughout a day.
Coefficient variant (CV): A term derived by dividing the SD by the mean glucose and multiplying by 100 to get a percentage. An acceptable CV is within or < 36%.
Glycaemic varialbility (GV): Refers to oscillations in blood glucose level or fluctuation of glucose over a given period of time.
Mean amplitude of glycemic excursion (MAGE): The mean of blood glucose values exceeding one SD from 24 hour mean blood glucose. This can be used to gauge the degree of glucose fluctuation or glycemic variability.
High blood glucose index (HBGI) and low blood glucose index (LBGI): Indexes designed based on symmetrisation of blood glucose ranges to summarize the number and extent of extreme blood glucose fluctuations into single number. LBGI accounts for hypoglycemic episodes and HBGI for hyperglycemic episodes.
Time in Hypoglycaemia:
Level 1: <54–70 mg/dL (3.0–3.9 mmol/l)
Level 2: <54 mg/dL (<3.0 mmol/l)
Time in Hyperglycaemia:
Level 1: >180 mg/dL (>10 mmol/l)
Level 2: >250 mg/dL (>13.9 mmol/l)
RESULTS
Clinical and demographic characteristics
A total of 32 participants were initially recruited (14 from HPJ and 18 from HUSM). Due to the escalating COVID-19 pandemic and implementation of Movement Control Order (MCO) by the Government of Malaysia (Phase 1; 16th March 2020),24 8 participants defaulted their follow-up visits in Ramadan. Only 24 participants (n=24), 10 from HPJ and 14 from HUSM, were eventually included for analysis. Their mean age was 13.6 ± 3.1 years old with 83.3% at either mid- or late-puberty and were of equal gender distribution. The baseline mean HbA1c was 9.6 ± 1.9% and the mean duration of diabetes was 5.4 ± 3.4 years. Anthropometry or BMI SDS was -0.2 ± 1.4. Insulin administration were though multiple daily injections (MDI) for 91.7% while 8.3% were through continuous subcutaneous insulin infusion (CSII). The mean daily insulin requirement was 1.2 ± 0.2 unit/kg/ day and 1.0 ± 0.2 unit/kg/day before and during Ramadan, respectively. Majority (95.2%) had past fasting experiences and 4.8% were attempting to fast for the first time. Most (87.5%) came from either the middle- or lower-income groups (M40 and B40, respectively), while 12.5% were from the upper-income group (T20). For the HbA1c subgroups, 16.6% had HbA1c <7.5%, 12.5% were within the HbA1c range of 7.5-9.0% and 70.9% had HbA1c >9%.
No significant differences were seen between participants from HPJ and HUSM, apart from socio-economic status. All participants fasted in Ramadan for at least 7 days while on CGM. None experienced complications of severe hyperglycaemia, DKA, or hypoglycaemia episodes requiring assistance or emergency visits.
Adherence of CGM data
As indicated in Table 3, total sensor glucose (SG) readings (per CGM-cycle) were 1739.8 (SD 366.9) and 1613.9 (SD 416.1) before and during Ramadan, respectively. This amounted to 41,755 and 38,734 SG readings before and during Ramadan, respectively. In addition, 23.7 ± 6.7 and 21.9 ± 8.2 valid calibrations (per CGM-cycle) were reported before and during Ramadan, respectively. The mean absolute relative difference (MARD) was 14.3 ± 7.7% and 15.0 ± 9.5% before and during Ramadan, respectively.
Table 3.
CGM Glycaemic outcome (before and during Ramadan)
| Before Ramadan (n=24) | During Ramadan (n=24) | p-value | ||
|---|---|---|---|---|
| Adherence and Sensor Accuracy | Sensor readings* (per CGM cycle) | 1739.8 ± 366.9 | 1613.9 ± 416.1 | 0.14a |
| MARD* (%) | 14.3 ± 7.7 | 15.0 ± 9.5 | 0.61a | |
| Calibrations* (per CGM cycle) | 23.7 ± 6.7 | 21.9 ± 8.2 | 0.29a | |
| Sensor Glucose (SG) data | Mean SG* (mmol/L) | 9.7 ± 2.2 | 10.6 ± 2.9 | 0.04a |
| Coefficient of variation*, CV (%) | 42.91 ± 8.1 | 40.76 ± 9.1 | 0.31a | |
| Estimated A1c* (%) | 7.7 ± 1.4 | 8.3 ± 1.8 | 0.03a | |
| Time in range_level 1* (%) | 51.1 ± 14.6 | 42.4 ± 20.9 | 0.05a | |
| Time in range_level 2* (%) | 34.6 ± 16.0 | 27.3 ± 17.4 | 0.02a | |
| Time in hyperglycemia_level 1* (%) | 21.2 ± 9.2 | 24.5 ± 12.0 | 0.12a | |
| Time in hyperglycemia_level 2* (%) | 19.5 ± 14.0 | 25.6 ± 18.6 | 0.10a | |
| Time in hypoglycemia_level 1* (%) | 4.0 ± 4.9 | 4.0 ± 6.2 | 0.96a | |
| Time in hypoglycemia_level 2* (%) | 4.2 ± 7.1 | 3.5 ± 5.3 | 0.54a | |
| Glycaemic Variability (GV) | MAGE* (mmol/L) | 8.2 ± 3.0 | 8.7 ± 2.3 | 0.48a |
| HBGI* | 14.3 ± 6.5 | 17.1 ± 8.0 | 0.07a | |
| LBGI* | 5.6 ± 4.2 | 6.2 ± 4.9 | 0.62a |
Numerical data, presented in means ± SD
Paired sample t-test
Time in range_level 1, SG readings between 3.9-10 mmo/L
Time in range_level 2, SG readings between 3.9-7.8 mmol/L
Time in hyperglycemia_level 1, SG readings 10-13.9 mmol/L
Time in hyperglycemia_level 2, SG readings >13.9 mmol/L
Time in hypoglycemia_level 1, SG readings 3.0-3.9 mmol/L
Time in hypoglycemia_level 2, SG readings <3.0 mmol/L
MAGE, mean amplitude glycaemic excursion
HBGI, high blood glucose index
LBGI, low blood glucose index
(Clinical targets for continuous glucose monitoring data interpretation: Recommendations from the international consensus on time in range)22
Outcomes from CGM data
As indicated in Table 4, the mean SG was 9.7 ± 2.2 mmol/L before Ramadan and increased to 10.6 ± 2.9 mmol/L during Ramadan (p=0.04). Estimated A1c similarly increased from 7.7 ± 1.4% before to 8.3 ± 1.8% during Ramadan (p=0.03). However, the coefficient of variation (CV) showed no difference with 42.9 ± 8.1% and 40.8 ± 9.1% before and during Ramadan, respectively (p=0.31).
Table 4.
Comparing the effect of HbA1c
| HbA1c <7.5% (n=4) | HbA1c ≥7.5% (n=20) | |||||
|---|---|---|---|---|---|---|
| Before Ramadan | During Ramadan | p-value | Before Ramadan | During Ramadan | p-value | |
| Mean SG* (mmol/L) | 8.6 ± 2.6 | 10.4 ± 3.2 | 0.03a | 9.9 ± 2.2 | 10.6 ± 2.9 | 0.15a |
| Estimated A1c* (%) | 7.1 ± 1.7 | 8.2 ± 2.0 | 0.03a | 7.8 ± 1.4 | 8.3 ± 1.8 | 0.12a |
| Time in range_level 1* (%) | 51.8 ± 7.9 | 39.3 ± 19.4 | 0.23a | 51.0 ± 15.7 | 43.0 ± 21.6 | 0.12a |
| Time in range_level 2* (%) | 38.3 ± 20.5 | 27.0 ± 18.9 | 0.04a | 33.9 ± 15.5 | 27.3 ± 17.6 | 0.06a |
| Time in hyper_level 1*(%) | 35.3 ± 23.0 | 52.3 ± 30.9 | 0.05a | 41.8 ± 18.3 | 49.8 ± 25.4 | 0.10a |
| Time in hyper_level 2* (%) | 10.8 ± 10.7 | 24.8 ± 18.5 | 0.08a | 21.2 ± 14.1 | 25.8 ± 19.0 | 0.28a |
| Time in hypo_level 1* (%) | 13.0 ± 19.5 | 8.5 ± 12.6 | 0.29a | 7.3 ± 8.4 | 7.2 ± 10.4 | 0.97a |
| Time in hypo_level 2* (%) | 6.5 ± 10.5 | 2.8 ± 2.6 | 0.46a | 3.8 ± 6.5 | 3.6 ± 5.8 | 0.90a |
| MAGE* (mmol/L) | 6.6 ± 1.3 | 8.1 ± 1.5 | 0.09a | 8.5 ± 3.2 | 8.8 ± 2.5 | 0.74a |
| HBGI* | 10.0 ± 5.4 | 15.4 ± 7.7 | 0.09a | 15.1 ± 6.5 | 17.4 ± 8.3 | 0.19a |
| LGBI* | 6.3 ± 4.7 | 7.0 ± 4.8 | 0.80a | 5.5 ± 4.2 | 6.1 ± 5.0 | 0.68a |
Numerical data presented in means (SD)
Paired sample t-test
Wilcoxon Sign Ranked test
Time in range_level 1, SG readings between 3.9-10 mmo/L
Time in range_level 2, SG readings between 3.9-7.8 mmol/L
Time in hyperglycemia_level 1, SG readings 10-13.9 mmol/L
Time in hyperglycemia_level 2, SG readings >13.9 mmol/L
Time in hypoglycemia_level 1, SG readings 3.0-3.9 mmol/L
Time in hypoglycemia_level 2, SG readings <3.0 mmol/L
MAGE, mean amplitude glycaemic excursion
HBGI, high blood glucose index
LBGI, low blood glucose index
(Clinical targets for continuous glucose monitoring data interpretation: Recommendations from the international consensus on time in range)22
Other important clinical CGM metrics, such as time in range level 1(SG 3.9-10 mmol/L), time in hypoglycaemia level (SG 3.0-3.9 mmol/L), time in hypoglycaemia level 2 (SG <3.0 mmol/L), time in hyperglycaemia level 1 (SG 10-13.9mmol/L) and time in hyperglycaemia level 2 (SG >13.9 mmol/L) showed no difference before and during Ramadan fasting. Only TIR level 2 (SG 3.9-7.8 mmol/L) demonstrated a difference but this was not applicable for paediatric patients.
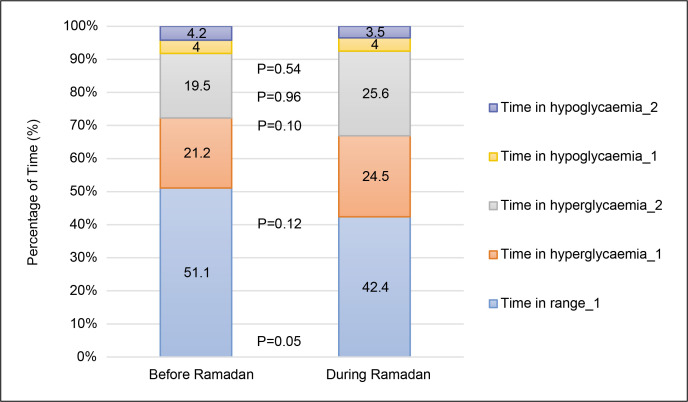
Breaking down each CGM metric for the periods before and during Ramadan, TIR level 1 (SG 3.9-10 mmol/L) was 51.1 ± 14.6% and 42.2 ± 20.9%, respectively (p=0.05); time in hypoglycaemia level 1 (SG 3.0-3.9 mmol/L) was 4.0 ± 4.9% and 4.0 ± 6.2%, respectively (p=0.96); time in hypoglycaemia level 2 (SG <3.0 mmol/L) was 4.2 ± 7.1% and 3.5 ± 5.3%, respectively (p=0.54); time in hyperglycaemia level 1 (SG 10-13.9 mmol/L) was 21.2 ± 9.2 and 24.5 ± 12.0%, respectively (p=0.12); time in hyperglycaemia level 2 (SG >13.9 mmol/L) was 19.5 ± 14.0% and 25.6 ± 18.6%, respectively (p=0.10). These are shown in Figure 1.
Figure 1.
CGM metrics before and during Ramadan.
For glycaemic variability (GV), represented by mean amplitude glycaemic excursion (MAGE), high blood glucose index (HBGI) and low blood glucose index (LBGI), there were no differences across both periods. Results before and during Ramadan were: MAGE 8.2 ± 3.0 mmol/L and 8.7 ± 2.3 mmol/L (p=0.48); HBGI 14.3 ± 4.5 and 17.1 ± 8.0 (p=0.07); LBGI 5.6 ± 4.2 and 6.2 ± 4.9 (p=0.62).
Comparing effect of HbA1c over Ramadan fasting
Analysis of CGM data for participants within optimal HbA1c group (HbA1c <7.5%; n =4), indicated higher mean SG (10.4 ± 3.2 mmol/L vs. 8.6 ± 2.6 mmol/L; p=0.03) and estimated A1c (8.2 ± 2.0% vs. 7.1 ± 1.7%; p=0.03) during Ramadan (Table 4). TIR level 2 (SG 3.9-7.8 mmol/L) was also reduced in Ramadan (27.0 ± 18.9% vs. 38.3 ± 20.5%; p=0.04).
Other important CGM metrics, such as TIR level 1 (SG 3.9-10 mmol/L), time in hypoglycaemia level 1 (SG 3.0-3.9 mmol/L), time in hypoglycaemia level 2 (SG <3.0 mmol/L), time in hyperglycaemia level 1 (SG 10-13.9 mmol/L) and time in hyperglycaemia level 2 (SG >13.9 mmol/L) showed no difference between the two periods. GV (MAGE, HBGI, and LBGI) also did not show a significant difference.
On the other hand, among participants with suboptimal HbA1c (HbA1c >7.5%; n =20), there was no difference across the two periods for all the above CGM metrics and for GV.
Comparing effect of age over Ramadan fasting
Sub-analysis of CGM data among the younger age group (10 years old and less, n=6) showed differences for TIR level 1 (SG 3.9-10mmol/L), TIR level 2 (SG 3.9-7.8 mmol/L), mean SG, and estimated A1c across both periods (Table 5).
Table 5.
Comparing the effect of age
| Age ≤10 years old (n=6) | Age >10 years old (n=18) | |||||
|---|---|---|---|---|---|---|
| Before Ramadan | During Ramadan | p-value | Before Ramadan | During Ramadan | p-value | |
| Mean SG* (mmol/L) | 10.3 ± 1.1 | 12.4 ± 1.8 | 0.02 | 9.5 ± 2.5 | 10.0 ± 2.9 | 0.33 |
| Estimated A1c* (%) | 8.1 ± 0.7 | 9.4 ± 1.1 | 0.02 | 7.6 ± 1.6 | 7.9 ± 1.8 | 0.28 |
| Time in range_level 1* (%) | 48.5 ± 6.7 | 30.5 ± 12.5 | 0.00 | 51.9 ± 16.4 | 46.3 ± 21.9 | 0.32 |
| Time in range_level 2* (%) | 30.2 ± 5.5 | 16.2 ± 10.9 | 0.01 | 36.1 ± 18.1 | 30.9 ± 17.8 | 0.16 |
| Time in hyperglycaemia_level 1*(%) | 24.7 ± 9.0 | 30.7 ± 7.4 | 0.24 | 20.1 ± 9.2 | 22.5 ± 12.7 | 0.31 |
| Time in hyperglycaemia_level 2* (%) | 22.5 ± 5.5 | 37.5 ± 16.1 | 0.07 | 18.4 ± 15.8 | 21.7 ± 18.0 | 0.45 |
| Time in hypoglycaemia_level 1* (%) | 3.7 ± 4.3 | 0.8 ± 1.3 | 0.11 | 4.1 ± 5.1 | 5.0 ± 6.9 | 0.29 |
| Time in hypoglycaemia_level 2* (%) | 0.7 ± 1.2 | 0.5 ± 0.8 | 0.61 | 5.4 ± 7.9 | 4.4 ± 5.8 | 0.57 |
| MAGE* (mmol/L) | 9.2 ± 1.8 | 8.6 ± 1.5 | 0.52 | 7.9 ± 3.3 | 8.7 ± 2.6 | 0.34 |
| HBGI* | 15.3 ± 3.4 | 20.2 ± 5.5 | 0.08 | 13.9 ± 7.3 | 16.0 ± 8.6 | 0.25 |
| LGBI* | 3.9 ± 2.0 | 3.1 ± 2.9 | 0.47 | 6.2 ± 4.6 | 7.2 ± 5.1 | 0.50 |
Numerical data presented in means (SD)
Paired sample t-test
Time in range_level 1, SG readings between 3.9-10 mmo/L
Time in range_level 2, SG readings between 3.9-7.8 mmol/L
Time in hyperglycemia_level 1, SG readings 10-13.9 mmol/L
Time in hyperglycemia_level 2, SG readings >13.9 mmol/L
Time in hypoglycemia_level 1, SG readings 3.0-3.9 mmol/L
Time in hypoglycemia_level 2, SG readings <3.0 mmol/L
MAGE, mean amplitude glycaemic excursion
HBGI, high blood glucose index
LBGI, low blood glucose index
(Clinical targets for continuous glucose monitoring data interpretation: Recommendations from the international consensus on time in range)22
Values before and during Ramadan were: TIR level 1 was 48.5 ± 6.7% and 30.5 ± 12.5% (p=0.00); TIR level 2 was 30.2 ± 5.5% and 16.2 ± 10.9% (p=0.01); mean SG was 10.3 ± 1.1 mmol/L and 12.4 ± 1.8 mmol/L (p=0.02); and estimated A1c was 8.1 ± 0.7% and 9.4 ± 1.1%, (p=0.02). There were no differences demonstrated for time in hyperglycaemia level 1 (SG 10-13.9 mmol/), time in hyperglycaemia level 2 (SG >13.9 mmol/L), time in hypoglycaemia level 1 (SG 3.0-3.9 mmol/L) and time in hypoglycaemia level 2 (SG <3.0 mmol/L). There were also no differences in GV metrics (MAGE, HBGI, and LBGI) across both periods.
On the other hand, for those in the older age groups (more than 10 years old, n=18), all CGM metrics and GV show no difference across the two periods.
DISCUSSION
To the best of our knowledge, this is the only other study after Lessan et al.,13 that utilised retrospective CGM to investigate the glycaemic effect of Ramadan fasting among diabetes patients before and during Ramadan. Of the studies among T1DM children and adolescents, this is the first that selected retrospective CGM over rt-CGM, with its advantage of analysis of “unaltered” glycaemic data. This may be more reflective of the true glycemic profile of patients and is helpful for Muslim T1DM youths in this part of the world who have less access to rt-CGM technology.
Our demographic findings were generally similar with the global trend of Ramadan fasting among T1DM children and adolescents, evidenced by the younger age, shorter duration of diabetes, higher percentage of past experiences and the less optimal HbA1c. This is similar to the recent DaR Global Survey 2020,8 that had reported 75% of T1DM youths attempted Ramadan fasting with HbA1c of 9.5 ± 2.0%. Apart from religious inclination, this trend could be driven by our local socio-cultural background and preferences.4,5,25 In Malaysia, despite the reportedly growing prevalence of obesity,26 the prevalence of diagnosed T1DM youths in our cohort were not affected.
In terms of access to advanced diabetes technology, compared to the DaR Global Survey 2020 which had reported 93.8% of T1DM on MDI, 4.8% on CSII, and rest 1.5% on pre-mixed insulin,8 our cohort also showed lesser access to more advanced technology.
The Malaysia Health Technology Assessment Section (MaHTAS) in 2015 stated that the switch from MDI to CSII in T1DM would cost the public health care system an increased estimate of USD 1230 to USD 1900 per annum that would translate to an incremental cost-effectiveness ratio (ICER) of USD 12,930.27 At the time of writing of this report, CSII is yet to be included as part of public health care for T1DM children in Malaysia and is limited only to those who could afford it. That said, the existing public health care system that provides near-fully subsidised health care services to its citizens regardless of socio-economic class has effectively nearly eliminated the impact of financial gaps. T1DM youths from both West and East Peninsular Malaysia in our cohort were comparable, in terms of the diabetes care and insulin treatment they received, despite differences in socio-economic profiles. To further illustrate these socioeconomic differences, the Malaysia National Census 201928 reported that the Federal Territories of Putrajaya, Kuala Lumpur, and the state of Selangor (West peninsular Malaysia) had a higher median monthly income (USD 1,959 to RM 2,517), compared to the state of Kelantan (East peninsular Malaysia) with a reported median monthly income of USD 850.
The reported MARD was close to the data published by Medtronic®, which quoted 12.2% for paediatric populations.29 According to the latest CGM consensus guidelines by Advanced Technologies and Treatments for Diabetes (ATTD),21,22 recommended clinical targets for paediatric T1DM are as described: TIR level 1 (SG 3.9-10.0 mmol/L) of >60%; time in hyperglycaemia level 1 (SG 10-13.9 mmol/L) of <25%; time in hyperglycaemia level 2 (SG >13.9 mmol/L) of <5%; time in hypoglycaemia level 1 (SG 3.0-3.9 mmol/L) of <4%; and time in hypoglycaemia level 2 (SG <3.0 mmol/L) of <1%.
For ease of discussion, we used the term “rate” to refer to the percentage of time spent in a certain glycaemic range, e.g., mild hypoglycaemia rate as referring to the time in hypoglycaemia level 1 (SG 3.0-3.9 mmol/L), severe hypoglycaemia rate as referring to time in hypoglycaemia level 2 (SG <3.0 mmol/L), and as appropriate for the others.
When applying these recommended clinical targets, it is evident that the glycaemic profiles in our cohort were suboptimal even before Ramadan month, as seen in the lower TIR level 1, higher severe hyperglycaemia rate and higher severe hypoglycaemia rate. Both the mild hyperglycaemia rate and mild hypoglycaemia rate were within normal limits. During fasting, similar CGM profiles were observed with no further difference across both the periods for TIR level 1, mild hypoglycaemia rate, severe hypoglycaemia rate, mild hyperglycaemia rate, and severe hyperglycaemia rate. The mean SG and estimated A1c, referred to as glucose management indicator (GMI) to avoid confusion with the laboratory obtained HbA1c, even though different for before and during Ramadan, have limited clinical values reflecting glycaemic outcome by themselves.21,22
In contrast the CGM metric TIR correlates well with HbA1c.30,31 Specifically, TIR level 1 (SG 3.9-10 mmol/L) of >60%, is associated with a HbA1c level of <7.5%. Furthermore, Beck et al.,32 in 2019 who used the existing Diabetes Control and Complications Trial (DCCT) SMBG data set to compute TIR for validation, concluded that TIR is strongly associated with the risk of microvascular complications and should be used as another clinical outcome measure apart from HbA1c.32
The TIR level 2 (SG 3.9-7.8 mmol/L) despite being worse during Ramadan, carries no clinical implication for the paediatric T1DM population,21,22 as this CGM metric is specifically applied only for pregnant women with diabetes.
Similar to other authors,13,17 glycaemic variability (GV) in our cohort showed no difference before and during Ramadan, indicating no worsening of glucose variability during Ramadan. However, it should be noted that acceptable GV should ideally be within or less than 36% according to the ATTD consensus guidelines,21,22 and this threshold was exceeded by our cohort from the onset. The CGM findings from our cohort indicated that Ramadan fasting, did not negatively impact or worsen glycaemic control.
Considering the confounding effects of different insulin delivery systems and CGM technology (rt-CGM vs. retrospective CGM) over the glycaemic outcome, the comparison of our CGM data with previous authors12–18 should be adjusted to these differences.
Lessan et al.,13 who used retrospective CGM, reported similar outcomes of no difference for mean SG, hypoglycaemia rate, as well as MAGE, LBGI, HBGI, before and during fasting. Their study however, included 56 adult patients with unspecified diabetes and 7 of them were healthy volunteers, forming a heterogeneous cohort. The study of Kaplan et al.,17 with participants consisting of 12 T1DM adolescents with rt-CGM on CSII (85%) and 2 with rt-CGM on MDI (15%), reported no difference in the mean SG, hypoglycaemia rate, hyperglycaemia rate and TIR level 1 before and during Ramadan fasting. Their sub-analysis also reported that for HbA1c <8% (n=6), there was a lower hypoglycaemia percentage (6.2% vs. 9.6%) during Ramadan as opposed to before.17 This was not reproduced in our cohort, (Table 4) and will be discussed later.
Consistent with the baseline HbA1c of 9.6 ± 1.9%, the TIR level 1 in our cohort was 51.1 ± 14.6% and 42.2 ± 20.9% before and during Ramadan, respectively (p=0.05), which were worse than the study of Kaplan et al.,17 who had used more advanced diabetes technology. However, compared to the study of Alfadhli et al.,16 whose cohort characteristics were closer to ours, the reported TIR level 1 was 42% during Ramadan.
Nevertheless, it is noteworthy that despite advanced diabetes technology, most TIR during Ramadan fasting still fall short of the clinical targets, implying the importance of other aspects of diabetes care, such as nutritional interventions.
The similar CGM outcomes for before and during Ramadan reassures that fasting per se, is not associated with worse glycaemic outcomes during Ramadan. Instead, the differences in diabetes technology used seemed to be affect glycaemic outcomes more, and would be an interesting area of focus for future studies.
On the other hand, the CGM findings of high severe hypoglycaemia rates before and during Ramadan did indicate serious clinical concerns and reflected the extent of subclinical hypoglycaemia or unawareness that escapes clinical evaluation. The reported severe hypoglycaemia rate of 4% before Ramadan was equivalent to the duration of 1 hour per day, when blood glucose levels was below 3.0 mmol/L, far exceeding the clinical target of fewer than 15 minutes or 1%. This hypoglycaemia unawareness due to impaired counter-regulatory hormone response and defective adrenaline drive from hypoglycaemia-associated-autonomic failure (HAAF)33 could lead to detrimental and life-threatening complications, if left unrecognised. Hence, compliance to SMBG during fasting among T1DM youths cannot be over-emphasized. Most Ramadan guidelines,2,4,5 state that doing SMBG should not symptom-based alone, but should be done regularly for 7-points per day to include pre-suhur, morning, mid-day, mid-afternoon, pre-iftar, 2-hours post iftar, and at any time when symptomatic or unwell. This is also important because hypoglycaemia not only occurred during fasting hours but also post iftar, due to insulin carbohydrate mismatch or increased physical activites.12
On the other hand, the elevated severe hyperglycaemia rate in both periods raise a concern over the risk of DKA, and other long-term metabolic consequences. The severe hyperglycaemia rate of 25.6% during Ramadan in our cohort was equivalent to a duration of 6 hours per day when blood glucose was beyond 13.9 mmol/L. Most of this exposure happened at the post-iftar hours.12,16 Lessan et al.,13 described the mean CGM curve for diabetes patients during fasting, where the gradual fall of blood glucose during fasting hours was followed with an abrupt and sustained rise of blood glucose after the sunset meal, and this effect was exaggerated for the insulin-dependent. This hyperglycaemia phenomenon could be attributed to the large carbohydrate-rich food or drink with high fat and glycaemic index during iftar to compensate for daytime fasting. For the Muslim community in Malaysia, this could also be owing to the widespread culture of food Bazaars that commonly flourish during Ramadan, on top of our tradition of serving guests with sweet drinks (syrup), and baked goods (kuih) during family gatherings of breaking fast. Ramadan has been viewed as the most important time of the year for the food retail sector in Malaysia, with an increase in retail growth of baking ingredients and non-alcoholic beverages, compared to the non-Ramadan period.25
The suboptimal and at-risk CGM profiles among Muslim T1DM youths outside of and during Ramadan as seen in previous studies13,17 and our cohort, is indicative that work needs to be done to improve Ramadan glycemic outcome. Diabetes technology could potentially be part of the solution. In a meta-analysis and systematic review of 17 observational studies involving 1699 patients34, the use of CSII in Ramadan was associated with lower severe hyperglycaemia rate but higher mild hyperglycaemia rate when compared to MDI. Alamoudi et al.,35 similarly concluded that CSII use in Ramadan was associated with less glucose variability, while Khalil et al.,36 concluded that SAP in Ramadan was associated with more flexibility and reduced the severity and duration of hypoglycaemia.
However, as emphasized by the IDF-DaR guidelines,3–5 intensive Ramadan-focused education must be given to patients and families before the commencement of Ramadan, covering the aspects of risk quantification, blood glucose monitoring, fluids, and dietary advice, exercise and physical activity patterns, medication adjustments, when to stop fasting and the recognition of complications with self-management strategies. Diabetes-focused education and empowering both caregivers and patients is crucial, together with individualised care by clinicians who have an understanding of the patients’ lifestyles and cultures, to enable appropriate advice and suitable insulin titration.
Our study did not demonstrate the beneficial effect of optimal HbA1c, which was seen in the study of Kaplan et al.,17 which showed that patients below the threshold of HBA1C of 8% had less hypoglycaemia rates during fasting. For the purpose of standardization, we used a HbA1c threshold of <7.5%, in accordance to the latest IDF-DAR guideline 2021,4 which defines HbA1c risk score as “0” when <7.5%, “1” when HbA1c 7.5-9.0%, and “2” when HbA1c >9.0%. For the subgroup with optimal HbA1c (HbA1c <7.5%, n=4), there was no difference observed for all the CGM metrics across both periods. These findings suggest that among those with optimal HbA1c, Ramadan fasting did not adversely affect their glycaemic outcomes. We therefore surmised that the apparent benefit in Ramadan observed in the Kaplan study17 may be related to the effect of the diabetes technology used. Similarly, for the subgroup with poorer HbA1c (HbA1c >7.5%, n=20), no differences before and during Ramadan were demonstrated, implying no escalation of glycaemic risks during fasting. This was also been discussed by Zabeen et al.,37 based on SMBG readings, concluded that T1DM children and adolescents with poorly controlled HbA1 could fast safely in Ramadan, with no differences in glycaemic outcomes observed between the periods before and during Ramadan.
Since the appropriate age of fasting is controversial and could be further complicated by the trend of earlier puberty onset seen worldwide, we did a sub-analysis of our cohort by age, with 10 years old chosen arbitrarily as the cut-off. For those younger (10 years old or less, n=6), there was a seemingly worse glycaemic outcome during Ramadan fasting, evidenced by the reduction of both TIR level 1 and TIR level 2 during Ramadan compared to before.
However, this needs to be re-evaluated in future studies with a greater number of participants. The apparent glycaemic deterioration among younger children could be incidental and confounded by factors such as the “conservative approach” of insulin dosing by the clinicians or the caregivers that administer lower doses, due to concern for hypoglycaemia. Furthermore, reduction of physical activities, which usually occurs to a greater extent among younger children in Ramadan, could also be another reason. According to the IDF-DaR guidelines,5 there is no lower age limit or age-risk mentioned, except for those >70 years old. And when titrating the insulin dose, HbA1c levels rather than age were used to stratify the risk, in that when it exceeds 7.5%, no basal insulin reduction is required and prandial insulin could be given as per exchange for both suhur and iftar meals. Lastly, the relatively higher severe hypoglycaemia rate noticed in the older group is also likely to represent the effect of diabetes duration, which resulted in a higher likelihood of hypoglycaemic unawareness and implying the need for more frequent SMBG, even among older children.
Limitations
Sample size was limited primarily due to travel restrictions from the COVID-19 pandemic through the Movement Control Order (MCO) which made it difficult for some patients to participate in our study.
Future CGM studies with larger sample sizes should be conducted for better generalisability, and include different types of diabetes technology. If feasible, we also recommend that CGM be done for the entire duration of Ramadan for a more complete representation of glycaemic profiles.
CONCLUSION
Our study described and compared CGM outcomes of T1DM children and adolescents before and during Ramadan. We found that fasting by itself, is not associated with short-term glycaemic deterioration. This study had also confirms that with Ramadan-focused education and compliance to SMBG, fasting is feasible and safe.
With knowledge that fasting has a neutral effect on glycaemic profiles, Ramadan month may be viewed as an opportunity for T1DM youths to improve their glycaemic control, in conjunction with spiritual development.
Aside from pursuing advanced diabetes technology that could be helpful in better management of diabetes, efforts should also be focused on pre-Ramadan education and self-care empowerment.
Lastly, Ramadan diabetes care and insulin titration should be highly individualised and guided with an understanding of the individual’s background, lifestyle and culture.
Acknowledgments
The authors thank the following: the Director General and Deputy Director General (Research and Technical Support) of the Ministry of Health; Dr. E Puvanesvaran Ramachandran, the Medical Officer of Hospital Universiti Sains; Ms. Nurshazliza Zakaria and Ms. Syahidatun Mardhiah, dietitians from the Dietetics Department of Hospital Putrajaya; Ms. Mumtas Abu Gani and Ms. Ezzatulakma Mohd Nadzri, Diabetes Nurse Educators of the Diabetes Resource Center of Hospital Putrajaya and Dr. Lisa Mohamed Nor from the Clinical Research Centre of Hospital Putrajaya.
Statement of Authorship
All authors certified fulfilment of ICMJE authorship criteria.
Author Contribution Statement
STT, SH and JYHH conceived the study; developed the methodology; verified research outputs; reviewed and edited the manuscript and administered the research activity planning and execution. STT and SH synthesized the study data and provided the study materials. STT curated the data; prepared the original draft; prepared the data presentation and acquired financial support for the study. SH and JYHH supervised the research activity planning.
Author Disclosure
All authors declared no conflict of interest.
Funding Source
The study was funded by the National Institute of Health (NIH), Research and Technical Support Unit, Ministry of Health, Malaysia.
References
- 1.Holy Quran . Available from https://www.quran-pdf.com/en/
- 2.Deeb A, Elbarbary N, Smart CE, et al. ISPAD Clinical Practice Consensus Guidelines: Fasting during Ramadan by young people with diabetes. Pediatr Diabetes. 2020;21(1):5-17. PMID: . 10.1111/pedi.12920. [DOI] [PubMed] [Google Scholar]
- 3.Hassanein M, Al-Arouj M, Hamdy O, et al. Diabetes and Ramadan: Practical guidelines. Diabetes Res Clin Pract. 2017;126:303-16. PMID: . 10.1016/j.diabres.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 4.IDF and DAR Alliance . Diabetes and Ramadan Guidelines; 2016. Available from www.idf.org/guidelines/diabetes-in-ramadan. [Google Scholar]
- 5.Guidelines P. Diabetes and Ramadan Diabetes and Ramadan International Diabetes Federation (IDF), in Collaboration with the Diabetes and Ramadan (DAR) International Alliance; 2021. Available from www.idf.org/guidelines/diabetes-in-ramadan [Google Scholar]
- 6.Salti I, Bénard E, Detournay B, et al. A population-based study of diabetes and its characteristics during the fasting month of ramadan in 13 countries: Results of the epidemiology of diabetes and ramadan 1422/2001 (EPIDIAR) study. Diabetes Care. 2004;27(10):2306-11. PMID: . 10.2337/diacare.27.10.2306. [DOI] [PubMed] [Google Scholar]
- 7.Al-Arouj M, Bouguerra R, Buse J, et al. Recommendations for management of diabetes during Ramadan. Diabetes Care. 2005;28(9): 2305-11. PMID: . 10.2337/diacare.28.9.2305. [DOI] [PubMed] [Google Scholar]
- 8.Hassanein M, Alamoudi RM, Kallash MA, et al. Ramadan fasting in people with type 1 diabetes during COVID-19 pandemic: The DaR Global survey. Diabetes Res Clin Pract. 2021;172:108626. PMID: . PMCID: . 10.1016/j.diabres.2020.108626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.El-Hawary A, Salem N, Elsharkawy A, et al. Safety and metabolic impact of Ramadan fasting in children and adolescents with type 1 diabetes. J Pediatr Endocrinol Metab. 2016;29(5):533-41. PMID: . 10.1515/jpem-2015-0263. [DOI] [PubMed] [Google Scholar]
- 10.Zabeen B, Tayyeb S, Benarjee B, et al. Fasting during Ramadan in adolescents with diabetes. Indian J Endocrinol Metab. 2014;18(1): 44-7. PMID: . PMCID: . 10.4103/2230-8210.126530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Al-Khawari M, Al-Ruwayeh A, Al-Doub K, Allgrove J. Adolescents on basal-bolus insulin can fast during Ramadan. Pediatr Diabetes. 2010;11(2):96-100. PMID: . 10.1111/j.1399-5448.2009.00544.x. [DOI] [PubMed] [Google Scholar]
- 12.Al-Agha AE, Kafi SE, Aldeen AMZ, Khadwardi RH. Flash glucose monitoring system may benefit children and adolescents with type 1 diabetes during fasting at Ramadan. Saudi Med J. 2017;38(4): 366-71. PMID: . PMCID: . 10.15537/smj.2017.4.18750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lessan N, Hannoun Z, Hasan H, Barakat MT. Glucose excursions and glycaemic control during Ramadan fasting in diabetic patients: Insights from continuous glucose monitoring (CGM). Diabetes Metab. 2015;41(1):28-36. PMID: . PMCID: . 10.1016/j.diabet.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 14.Kaplan W, Afandi B. Blood glucose fluctuation during Ramadan fasting in adolescents with type 1 diabetes: Findings of continuous glucose monitoring. Diabetes Care. 2015;38(10):e162-3. PMID: . 10.2337/dc15-1108. [DOI] [PubMed] [Google Scholar]
- 15.Lessan N, Hasan H, Barakat MT. Ramadan fasting: A study of changes in glucose profiles among patients with diabetes using continuous glucose monitoring. Diabetes Care. 2012;35(5):2012. PMID: . PMCID: . 10.2337/dc11-2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alfadhli EM. Higher rate of hyperglycemia than hypoglycemia during Ramadan fasting in patients with uncontrolled type 1 diabetes: Insight from continuous glucose monitoring system. Saudi Pharm J. 2018;26(7):965-9. PMID: . PMCID: . 10.1016/j.jsps.2018.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaplan W, Afandi B, Al Hassani N, Hadi S, Zoubeidi T. Comparison of continuous glucose monitoring in adolescents with type 1 diabetes: Ramadan versus non-Ramadan. Diabetes Res Clin Pract. 2017;134: 178-82. PMID: . 10.1016/j.diabres.2017.10.010. [DOI] [PubMed] [Google Scholar]
- 18.Afandi B, Kaplan W, Majd L, Roubi S. Rate, Timing, and severity of hypoglycemia in adolescents with type 1 diabetes during Ramadan fasting: A study with freestyle libre flash glucose monitoring system. Ibnosina J Med Biomed Sci. 2018;10(1):9. 10.4103/ijmbs.ijmbs_73_17. [DOI] [Google Scholar]
- 19.Fuziah MZ, Hong JYH, Zanariah H, et al. A national database on children and adolescent with diabetes (e-DiCARE): Results from April 2006 to June 2007. Med J Malaysia. 2008;63(Suppl C):37-40. PMID: . [PubMed] [Google Scholar]
- 20.Ministry of Health Malaysia . Quick reference for healthcare providers: Diabetes Mellitus in manegment of nasopharyngeal carcinoma 2016. Putrajaya: MaHTAS Medical Development Ministry of Health Malaysia. https://www.moh.gov.my/moh/resources/Penerbitan/CPG/Kanser/QRNasopharyngealCarcinoma.pdf [Google Scholar]
- 21.Danne T, Nimri R, Battelino T, et al. International consensus on use of continuous glucose monitoring. Diabetes Care. 2017;40(12): 1631-40. PMID: . PMCID: . https://doi.org10.2337/dc17-1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Battelino T, Danne T, Bergenstal RM, et al. Clinical targets for continuous glucose monitoring data interpretation: Recommendations from the international consensus on time in range. Diabetes Care. 2019;42(8):1593-1603. PMID: . PMCID: . 10.2337/dci19-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hill NR, Oliver NS, Choudhary P, Levy JC, Hindmarsh P, Matthews DR. Normal reference range for mean tissue glucose and glycemic variability derived from continuous glucose monitoring for subjects without diabetes in different ethnic groups. Diabetes Technol Ther. 2011;13(9):921-8. PMID: . PMCID: . 10.1089/dia.2010.0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tang A. “Malaysia announces movement control order after spike in Covid-19 cases (updated).” The Star. March 16, 2020. https://www.thestar.com.my/news/nation/2020/03/16/malaysia-announces-restricted-movement-measure-after-spike-in-covid-19-cases. [Google Scholar]
- 25.Ramadan in Southeast Asia - statistics & facts. Statista; 2022. Available from https://www.statista.com/topics/6329/ramadan-in-southeast-asia/#topicHeader__wrapper. [Google Scholar]
- 26.NIH Ministry of Health Malaysia . National Health and Morbidity Survey 2019: NCDs - Non-Communicable Diseases: Risk factors and other health problems. Vol 1; 2019. Available from http://www.iku.gov.my/nhms-2019. [Google Scholar]
- 27.Insulin pump therapy for type 1 and type 2 diabetes. Putrajaya: MaHTAS Medical Development Ministry of Health Malaysia; 2015. Available from https://www.moh.gov.my/index.php/database_stores/attach_download/347/281. [Google Scholar]
- 28.Malaysia Statistical Handbook 2019. Department of Statistics Malaysia Official Portal; 2019. Available from https://www.dosm.gov.my/v1/index.php?r=column/cthemeByCat&cat=167&bul_id=OWZ0aFZIZmxVOTB3K1pFVjJrZFY2dz09&menu_id=WjJGK0Z5bTk1ZElVT09yUW1tRG41Zz09. [Google Scholar]
- 29.Weik MH. User guide. In: Computer science and communicationd dictionary. Springer, Boston, MA; 2000. 10.1007/1-4020-0613-6_20584. [DOI] [Google Scholar]
- 30.Vigersky RA, McMahon C. The Relationship of hemoglobin A1C to time-in-range in patients with diabetes. Diabetes Technol Ther. 2019;21(2):81-5. PMID: . 10.1089/dia.2018.0310. [DOI] [PubMed] [Google Scholar]
- 31.Beck RW, Bergenstal RM, Cheng P, et al. The relationships between time in range, hyperglycemia metrics and HbA1c. J Diabetes Sci Technol. 2019;13(4):614-26. PMID: . PMCID: . 10.1177/1932296818822496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beck RW, Bergenstal RM, Riddlesworth TD, et al. Validation of time in range as an outcome measure for diabetes clinical trials. Diabetes Care. 2019;42(3):400-5. PMID: . PMCID: . 10.2337/dc18-1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martín-Timón I, Del Cañizo-Gómez FJ. Mechanisms of hypoglycemia unawareness and implications in diabetic patients. World J Diabetes. 2015;6(7):912-26. PMID: . PMCID: . 10.4239/wjd.v6.i7.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Loh HH, Lim LL, Loh HS, Yee A. Safety of Ramadan fasting in young patients with type 1 diabetes: A systematic review and meta-analysis. J Diabetes Investig. 2019;10(6):1490-1501. PMID: . PMCID: . 10.1111/jdi.13054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alamoudi R, Alsubaiee M, Alqarni A, et al. Comparison of insulin pump therapy and multiple daily injections insulin regimen in patients with type 1 diabetes during ramadan fasting. Diabetes Technol Ther. 2017;19(6):349-54. PMID: . 10.1089/dia.2016.0418 [DOI] [PubMed] [Google Scholar]
- 36.Khalil AB, Beshyah SA, Abu Awad SM, et al. Ramadan fasting in diabetes patients on insulin pump therapy augmented by continuous glucose monitoring: An observational real-life study. Diabetes Technol Ther. 2012;14(9):813-8. PMID: . 10.1089/dia.2012.0061. [DOI] [PubMed] [Google Scholar]
- 37.Zabeen B, Nahar J, Ahmed B, Islam N, Azad K, Donaghue K. High HbA1c is not a reason not to fast during Ramadan in children, adolescents and young adults with type 1 diabetes – An observational study in Bangladesh. Diabetes Res Clin Pract. 2021;173:108673. PMID: . 10.1016/j.diabres.2021.108673. [DOI] [PubMed] [Google Scholar]
- 38.Department of Statistics Malaysia . House income basic amenities survey report 2019 [Laporan Survei Pendapatan Isi Rumah dan Kemudahan Asas 2019]; 2020. Available from https://www.dosm.gov.my/v1/index.php?r=column/cthemeByCat&cat=120&bul_id=TU00TmRhQ1N5TUxHVWN0T2VjbXJYZz09&menu_id=amVoWU54UTl0a21NWmdhMjFMMWcyZz09. [Google Scholar]