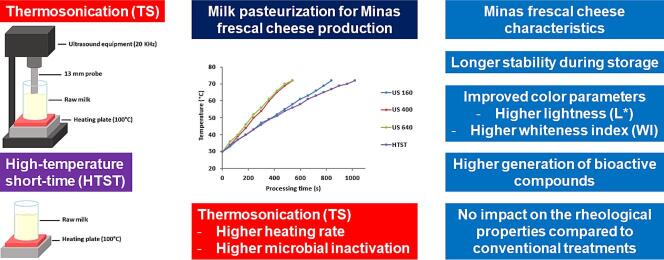
Graphical abstract
Keywords: Soft cheese, High-intensity ultrasound, Acoustic power, Nutritional aspects, Food processing, Dairy processing
Highlights
-
•
Thermosonication treatment showed a higher heating rate than conventional pasteurization.
-
•
Thermosonication using 20 kHz induced higher microbial inactivation.
-
•
Improved color parameters and higher storage stability were observed.
-
•
Thermosonication showed a higher generation of bioactive compounds in the cheese.
-
•
No alterations in the rheological aspects comparing the treatments.
Abstract
Minas frescal cheese is extremely popular in Brazil, with high perishability and acceptability. Among emerging technologies, ultrasound stands out for its satisfactory results regarding microbiological safety and technological and sensory aspects. The combined mild temperature application, called thermosonication, can generate even more promising results. In this study, a high-intensity ultrasound system combined with thermal heating (TS, thermosonication) was applied for the treatment of raw milk to produce Minas Frescal cheese. US energy was delivered to raw milk samples using a probe operating at a 20 kHz of frequency and nominal power of 160, 400, and 640 W. The TS system was compared with conventional pasteurization (HTST, high-temperature short-time pasteurization) at 72 to 75 °C and 15 s. Soft cheeses were prepared with different samples: (a) raw milk (control), b) conventionally pasteurized milk (HTST), and c) TS treat milk in different nominal power (TS160, TS400, and TS640). The produced cheeses were evaluated for microbiological behavior, rheology, color parameters, and bioactive compounds. TS treatment in milk resulted in higher microbial inactivation and stability during storage, improved color parameters (higher lightness (L*), and whiteness index (WI). TS treatment also showed a higher generation of bioactive compounds (higher antioxidant, and inhibitory activities of α-amylase, α-glucosidase, and angiotensin-converting enzymes) than HTST. The impact of TS on rheological properties was similar to HTST, resulting in more brittle and less firm products than the cheese produced with raw milk. The positive effects were more prominent using a nominal power of 400 W (TS400). Therefore, TS proved to be a promising process for processing milk for Minas Frescal cheese production.
1. Introduction
1.1. Industrial relevance
The processing of milk for Minas Frescal cheese production using thermosonication results in a higher microbial inactivation than conventional processing (HTST). It improves the product optical parameters without altering the rheological properties and can increase bioactive compounds' generation. In addition, the processing time is shorter (47 % for 400 W nominal power). No negative impacts were observed in the samples. In the industry, this time would be converted into income since more products could be produced per time. In this sense, this research's findings contribute to the dairy industry's use of emerging technologies.
Minas Frescal cheese is a high-moisture cheese very popular in Brazil, presenting soft texture, white appearance, fresh aroma, and slightly salty cheese. Due to its high moisture content and simple processing, it has an estimated 14 to 25-day shelf life. Furthermore, it has high consumer acceptability and low production cost, generally produced from pasteurized milk [1].
Conventional thermal treatments, such as pasteurization, can ensure the microbiological safety of processed foods, but their application can result in adverse effects such as nutrient degradation and sensory changes. Therefore, emerging technologies, particularly nonthermal treatments, have been increasingly studied and applied to process foods [2], [3], [4], [5], [6].
Ultrasound (US) is one of the most applied and versatile emerging technologies for process intensification in food processing, enabling greater microbial and enzymatic inactivation and nutritional preservation [7], [8]. The US comprehends mechanical waves above 20 kHz to 10 MHz, which can be classified as low frequency (20 to 100 kHz), intermediate frequency (100 kHz to 1 MHz), and high frequency (1 to 10 MHz)[9]. The physical and chemical effects observed in a US acoustic field occur due to acoustic cavitation. The cavitation phenomena are characterized by the formation, growth, and implosion of microbubbles of preexisting dissolved gas in the liquid media or gas sacs trapped on the surface of solids [10]. Under a US field, those bubbles are exposed to cycles of low pressure (rarefaction) and high pressure (compression). In a low-pressure cycle, the bubble expands due to the rectified diffusion of dissolved gases to the inner parts of the bubble cavity, in a higher pressure cycle, the bubble is compressed. The cycles of rarefaction and compression continue until the bubble reach a critical volume and collapses violently. The violent bubble collapse leads to mechanical effects such as shear forces, shock waves, and microjets [11].
The inactivation of bacteria under a US field may be due to the damage to the surface of bacteria cell wall caused by mechanical shear stress, with a pressure higher than 100 kPa, fast microjets with a speed of 100 m s−1, and shock waves leading to a pressure of 40–60 kbar at 20 kHz [12]. Bacteria inactivation can also occur due to the formation of highly reactive radical species caused by the sonolysis of water inside the cavitation bubbles. The species of radicals formed, mainly the hydroxyl radical, can damage the bacterial DNA and interfere with enzymatic activity [9].
The balance between ultrasound physical and chemical effects depends mainly on the frequency of the acoustic wave. At low frequencies (20 to 100 kHz) the physical effects are more prominent due to the higher size and lower number of bubbles. At higher frequencies, up to 500 kHz, chemical effects are more prominent due to the low size and higher number of bubbles [10]. The ultrasound effects depend also on other processing variables, such as treatment time, nominal power, and temperature [13], [14].
Dairy products have been the subject of numerous studies evaluating emerging technologies in which US technology stands out [15]. Raw milk, dairy beverages, fermented milk, and some types of cheese showed satisfactory results related to ultrasound processing [16]. Thermosonication (TS) is the utilization of low heat in addition to ultrasound waves. It can increase the shelf life of the product, making it safe for human consumption without altering its nutritional composition and sensory properties [11], [17]. TS has shown positive results in dairy food processing, such as whey drinks [18] and dairy desserts [19]. However, more investigations are needed to confirm its adequation, including studies with cheeses and the influence of nominal power as a process parameter.
Therefore, this study aimed to evaluate the influence of thermosonication, an emerging technology that has shown promise in food processing, in the processing of raw milk for the production of Minas Frescal cheese, a very popular soft cheese in Brazil. The products were evaluated for microbial behavior, bioactive compounds, and optical and rheological parameters.
2. Materials and methods
2.1. Thermosonication (TS) and HTST processing
The raw milk (about 3.4 % w/w) used to produce Minas Frescal cheese was obtained from Cooperativa de Produtores de Leite Barra Mansa, located in the city of Barra Mansa, Rio de Janeiro. It was kept under refrigeration (4 ± 2 °C) during the transport to the laboratory. For all the experiments of high-temperature short-time (HTST) pasteurization and thermosonication (TS), the initial milk temperature was established at 30 °C. The experiments were carried out in triplicate.
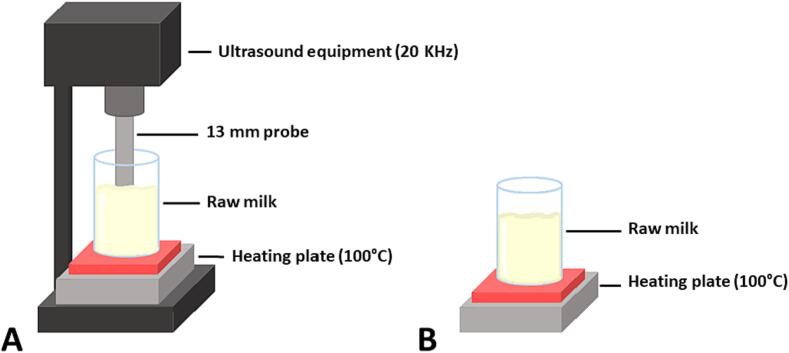
For HTST experiments a glass flask with 300 mL of raw milk was placed on a hot plate with magnetic agitation (SL-91, Biolab), as presented in Fig. 1A, until reaching the temperature of 72–75 °C, keeping in this range for 15 s, and immediately cooled in an ice bath to 35 °C.
Fig. 1.
Scheme of the thermosonication (A) and HTST processing (B).
For TS experiments a flask with 300 mL of raw milk was treated using an ultrasound probe device (QR750, Ultronique Disruptor/Sonicator, São Paulo, Brazil) with a 13-mm probe, operating at 20 kHz in different nominal power (160, 400, and 640 W), as presented in Fig. 1B. The ultrasound probe was dipped into the milk (10 mm of the milk surface) under magnetic agitation for the TS process. TS experiments were carried out until samples reached 72–75 °C. Then, the milk was kept at the target temperature for 15 s and immediately cooled in an ice bath to 35 °C. During processing, the temperature was not controlled, and the temperature increase was a response of acoustic cavitation in combination with heating by conduction. The temperature profile of the treatments was measured and presented in Fig. 2.
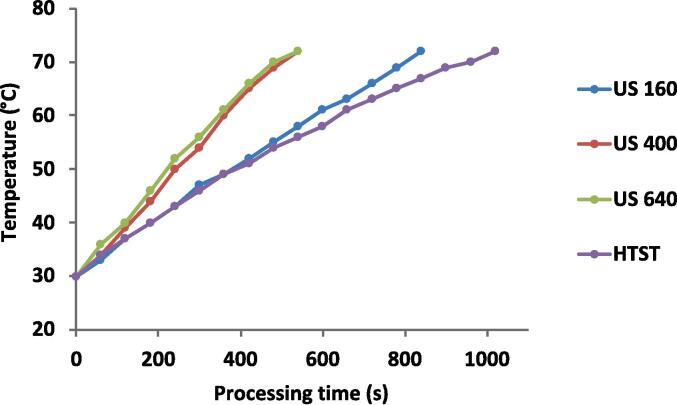
Fig. 2.
Temperature profiles of raw milk processing by Thermosonication (TS160, TS400, TS640) and conventional pasteurization (HTST).
The acoustic power of the process (AP) (Equation (1)), the acoustic intensity (AI) (Equation. (2)), the power density (PD) (Equation (3)), the specific energy (SE) (Equation (4)), and the energy density (ED) (Equation (5)) were calculated as suggested by Strieder et al.[20]. The specific heat capacity (Cp) was established according to Hu et al. [21], where the milk with 3.4 % fat has approximately 3.9 J g−1 °C−1.
| (1) |
where Cp is the specific heat capacity (J g−1 °C−1) measured at constant pressure, m is the mass (g) of the sample, and dT/dt is the heating rate in function of process time (°C s−1).
| (2) |
Where D is the probe diameter (cm).
| (3) |
| (4) |
where V is the sample volume (mL), t is the processing time (s), and m is the sample mass (g). The energy density (ED) was also calculated according to Equation (5).
| (5) |
where UP is the ultrasonic power (J s−1), t is the processing time (s), and V is the sample volume (cm3).
2.2. Minas Frescal cheese processing
Minas Frescal cheese processing was carried out according to traditional methodology, as described by Rocha et al.[1], with modifications. First, the treated milk samples of each treatment were mixed to obtain 900 mL of milk. Next, the milk was kept inside water baths at 35 °C. Then, the ingredients were added in the following concentrations considering 1 L of milk solution: calcium chloride (0.5 mL L-1), lactic acid (0.2 mL L-1), and liquid rennet (chymosin, HA LA, Chr Hansen, Valinhos, São Paulo, 0.8 mL L-1) and the mixture kept resting for 40 min until coagulation. Then, the obtained curd was cut into cubes of approximately 2 cm. Next, the curd was stirred as follows: 2 min slow stirring; 5 min rest; 5 min slow stirring; 5 min rest; then, the whey was partially drained, and the curd salted (NaCl, 1.5 % w/w of the initial volume of milk). A control sample with raw milk was also prepared. Subsequently, the curds were placed into polyethylene plastic cylindrical molds (10 cm diameter) and stored in a fridge (5 °C ± 2 °C) for 24 h. After that period, the cheeses were packed in sterile polyethylene plastic bags and kept under refrigeration for 24 days at 5 ± 2 °C.
2.3. Gross composition
The analysis was performed according to the traditional methods published elsewhere [22]. Moisture was determined gravimetrically after drying in an incubator at 105 °C (Micronal, São Paulo, Brazil) for 24 h. The total content of ash was also determined gravimetrically after heating 2 g of sample in a muffler furnace (Micronal) at 550 °C. Protein was calculated by determining total nitrogen by the Kjeldahl method using a conversion factor of 6.38. Finally, the fat content was determined using the Gerber method.
2.4. Microbiological analysis and predictive modeling
Initially, the samples were subjected to serial dilutions in buffered peptone water 0.1 % w/v. Plate count agar (Himedia®, India) was used to count total aerobic mesophilic bacteria (AMB) and aerobic psychotropic bacteria (APB), with incubation at 37 °C for 48 h and 7 °C for 10 days, respectively [23], [24]. Lactic acid bacteria (LAB) were enumerated using Man, Rogosa, and Sharp agar (MRS, Himedia®, India) supplemented with 100 mg L-1 of cycloheximide and incubation under anaerobic conditions (Anaerobac®, Probac, Brazil) at 37 °C for 48 h.
Microbiological analyzes were performed immediately after processing (day 0) to verify microbial inactivation and during storage every 4 days until day 24. In addition, the microbial count was evaluated in a sample produced with untreated milk (control). To assess microbial inactivation, the number of log reductions (γ) was calculated according to Equation (6) [23]:
| (6) |
where N0 is the number of viable microorganisms of the untreated sample (control), and Nf is the number of viable microorganisms after processing.
To evaluate microbial behavior along the storage time, predictive growth models were fitted by DMFit Excel add-in software, using the Baranyi model (Equations (7), (8)) [25]:
| (7) |
| (8) |
where y(t) is the cell concentration (CFU mL−1), at time t (d), ymax (k) is the maximum cell concentration (CFU mL−1), y0 is the initial cell concentration (CFU mL−1), μmax is the maximal specific growth rate (CFU mL−1.d-1), q0 is a measure of the physiological state of the cell, and m is the parameter related to curvature after exponential phase.
2.5. Color
The color of the samples was measured by a colorimeter (Colour Quest XE Hunter Lab, Northants, UK) and calibrated to illuminant D65. The parameters L*, a*, b*, C*, and h* of the CIE (Commission Internationale de l’Eclairage) system were obtained according to the procedure established by Balthazar et al. [26]. In addition, the whiteness index (WI, Equation (9)) was also calculated according to Balthazar et al. [27]:
| (9) |
2.6. Rheological analysis
The rheological behavior of the Minas Frescal cheese samples (control, HTST, TS160, TS400, and TS640) was evaluated in refrigerated storage at 10 °C. The rheological analysis was carried out using the TA-XT21 texture analyzer (Stable Micro Systems ltd., Surrey, UK), fitted with a 50 kg load cell. The texture analyzer had a fixed platform and an aluminum plate 35 mm in diameter. The plate and the platform were not lubricated since it was considered that the whey exuded during the test was sufficient to reduce the friction between the cheese surface and the plate/platform to insignificant levels. The samples were prepared by removing the cheese's cylindrical samples (20 mm diameter, 24 mm length). These samples were wrapped individually in PVC film, then in waterproof plastic bags, and maintained in a refrigerated bath at 10 °C for at least 90 min before testing. Four cylindrical replicates were measured for each sample.
A uniaxial compression test was performed by compressing the sample to 20 % of its initial length at a crosshead speed of 1 mm s−1 [1], [28]. Force-displacement data were converted to true stress () (Eq. (10)) and true strain () (Eq. (11)), as described by Gunasekaran & Ak [29]:
| (10) |
| (11) |
where is the applied force at any time, is the cross-sectional area at any time, is the initial cross-sectional area, is the current sample length, is the initial sample length, and is deformation.
The stress–strain curves were fitted by a fifth-order polynomial equation (Equation (12)) passing through the origin, as described by Ak and Gunasekaran [29]:
| (12) |
where denotes an index for the coefficients () and power for the true strain term (); and the coefficients () were determined using a curve-fitting procedure.
The fracture strain () and fracture stress () represent the highest strain and stress reached in the stress–strain curve before fracture, wherein fracture strain () was estimated by locating the peak strain at which the slope becomes zero and fracture stress () is the corresponding peak stress value. The modulus of toughness () is the amount of work per unit volume absorbed by a material until fracture, which was calculated (Equation (13)) as the area under the stress–strain curve up to the fracture point:
| (13) |
The linear region of the stress–strain curve was fitted by a linear equation passing through the origin. The modulus of elasticity () was calculated (Equation (14)) as the slope of the stress–strain curve in the linear-elastic region, as described by Gunasekaran & Ak [29]:
| (14) |
The modulus of resilience (), which is the amount of work per unit volume absorbed by a material in the elastic range, was calculated (Equation (15)) as the area under the stress–strain curve up to the elastic limit:
| (15) |
2.7. Bioactive compounds
The antioxidant activity was measured from the ability to reduce 2,2-diphenyl-1-picrylhydrazyl (DPPH), starting from the extract obtained by extraction in ethanolic solution followed by centrifugation and vacuum filtration [30]. From 150 μL of the extracts added with 2.85 mL of 0.006 mol L-1 DPPH solution, after standing in the absence of light for 60 min, the absorbance was measured at 517 nm [31].
The inhibitory activity of angiotensin-converting enzyme I (ACE) was determined from the addition of 20 μL of the ACE enzyme (0.1 unit mL−1) to the extracts obtained from the cheese samples, followed by a 30-min wait, the addition of 250 μL of 1 mol L-1 hydrochloric acid, drying and resuspension in deionized water and absorbance reading at 228 nm [32], [33]. In addition, the anti-diabetic activity, evaluated from the inhibition of α-glucosidase and α-amylase, was measured according to the methodology described by Lavelli et al. [34].
2.8. Statistical analysis
The experiment was designed in a completely randomized design, and analyzes were performed in triplicate. The data were submitted to the Analysis of variance (ANOVA) and Tukey test (significance level of p < 0.05) using XLSTAT software version 2020 (Adinsoft, Paris, France).
3. Results and discussion
3.1. TS and conventional processing performances
The heating rates for TS160, TS400, and TS600 were measured without the heating plate to measure the real heating caused by acoustic cavitation. The obtained heating rates were 0.003, 0.013, and 0.03 °C s−1 for TS160, TS400, and TS600, respectively. According to the equations, the ultrasound parameters applied to the raw milk were: acoustic power of 3.93, 15.72, and 39.3 W; an acoustic intensity of 8.5, 33.8, and 84.6 W cm−2; power density of 0.01, 0.05 and 0.13 W cm−3; and specific energy of 11, 28.3 and 70.7 J g−1, in treatments TS160, TS400 and TS600, respectively. The ED values were 0.45, 0.72 and 1.15 kJ cm−3 for TS160, TS400 and TS600, respectively.
Fig. 2 presents the heating performance of milk subjected to TS and HTST.
TS formulations took shorter times (849, 550, and 550 s for TS160, TS400, and TS640, respectively) to achieve the processing temperature (72 to 75 °C) than HTST (1050 s, p < 0.05). In addition, the increase in the nominal power from 160 to 400 W resulted in shorter processing times, in the same proportion that heat generation increased (0.003 and 0.013, respectively, p < 0.05). However, a further increase in nominal power (from 400 to 640 W) did not affect the processing times (p > 0.05). In practice, heat generation is related to cavitation bubble collapse, which leads to a local heating during the process [10], so an increase in the nominal power increases the heat generation. The results of the present study suggest that TS may be an alternative to conventional pasteurization leading to a processing time 47,5% shorter for TS400 and TS600, compared with HTST. Therefore, a nominal power of 400 W would be recommended.
3.2. Gross composition
Table 1 shows the gross composition of Minas Frescal cheese samples.
Table 1.
Gross composition of Minas Frescal cheese samples.
| Treatments | Moisture | Protein | Lipid |
|---|---|---|---|
| Control | 50.2b ± 1.23 | 15.7b ± 1.09 | 12.3b ± 0.45 |
| HTST | 52.3b ± 1.16 | 14.2b ± 1.21 | 12.9b ± 0.21 |
| TS160 | 47.6a ± 0.34 | 16.8a ± 0.76 | 14.1a ± 0.32 |
| TS400 | 46.9a ± 0.41 | 17.2a ± 0.98 | 14.9a ± 0.19 |
| TS640 | 46.1a ± 0.24 | 18.1a ± 1.12 | 15.5a ± 0.88 |
*Results are expressed as mean ± standard deviation. Different letters mean statistical difference among treatments according with the Fisher test (p < 0.05). Control, TS160, TS400, TS640, HTST = untreated, sonication at 160, 400 and 640 W, and conventional pasteurization. Moisture, Protein and Lipid are expressed in % w/w.
The moisture content ranged from 46.1 to 52.3 %. TS samples presented lower values and less variation between them (46.1 to 47.6 % w/w, p < 0.05) compared to the HTST sample. These findings can be explained by temperature as a function of the time profile of the treatments, as indicated in Fig. 2, where the TS treatments generated faster heating, which may cause a more significant loss of free water in the cheese.
Protein content also significantly differed between TS and HTST samples (values ranged from 16.8 to 18.1 against 14.2 % w/w, p < 0.05). The samples treated by TS generated Minas Frescal cheese with higher protein content, while the sample treated by HTST had the lowest content compared to the control sample (p < 0.05). The application of ultrasound affects the particle size of proteins and influences casein micelles [35]. The disruption of casein micelles can increase the effective concentration of casein [11], which, combined with lower observed moisture content, may explain the increased protein content in cheese samples treated by TS. In addition, TS can increase surface hydrophobicity and expose buried sulfhydryl groups (located inside the protein), thus improving gelling properties [36].
The same behavior was observed for the fat content, where the samples treated by TS had higher values when compared to HTST samples (values ranged from 14.1 to 15.5 against 12.9 % w/w). The application of ultrasound causes changes in the size of fat globules [35], but there is no record of changes in fat content in dairy products caused by the technology. Thus, the significant difference is attributed to the lower moisture value obtained in the TS-treated samples.
TS technology proved to be viable, regardless of the power applied, concerning meeting the composition parameters of Minas Frescal cheese required by Brazilian legislation, where it is classified as a high-moisture cheese (moisture between 46 and 54.9 %) and semi-fat (fat in the dry extract between 25 and 44.9 %) [37], [38].
3.3. Microbial inactivation and predictive modeling
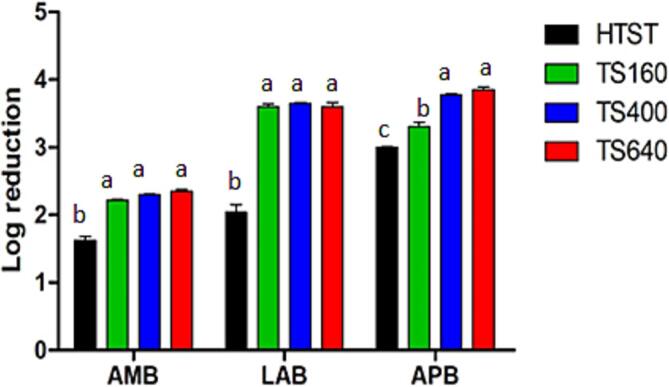
Fig. 3 shows the microbial inactivation observed for each microbial group evaluated in each treatment, represented by the reduction in log CFU g−1 (γ).
Fig. 3.
Microbial inactivation (γ) in Minas Frescal cheese samples compared to the control sample (manufactured from raw milk cheese). HTST, TS160, TS400, TS640, conventional pasteurization, Sonication at 160 W, 400 W, 640 W respectively. AMB, LAB, APB = Aerobic Mesophilic Bacteria, Lactic Acid Bacteria and Aerobic Psychotropic bacteria counts (log CFU g−1). Different letters for the same microbial group indicate differences among formulations (p < 0.05).
The HTST treatment generated less microbial inactivation for all microbial groups than TS treatments (p < 0.05). For example, AMB was reduced by 1.63 log CFU g−1 in the HTST treatment, while the TS treatments resulted in reductions between 2.22 and 2.35 log CFU g−1. Considering APB, the HTST treatment reduced 3.00 log CFUg−1, while the TS treatments reduced between 3.30 and 3.85 log CFU g−1. Regarding LAB, HTST treatment reduced 2.04 log CFU g−1, and the TS treatments reduced between 3.60 and 3.65 log CFU g−1. The nominal power significantly influenced APB, as the microbial inactivation was slightly higher with increasing operating power up to 400 W (p < 0.05). g−1
The higher inactivation of bacteria under a acoustic field of ultrasound can be explained by the cell disruption due to the physical forces generated in bubble collapse and chemical attack of cell constituents due the hydroxyl radical formation. Even though the cavitation phenomena cannot inactivate all the bacteria, the US chemical and physical forces can lead to a sub lethally damages that lead to a decrease of cell grow [39], [40], [41].
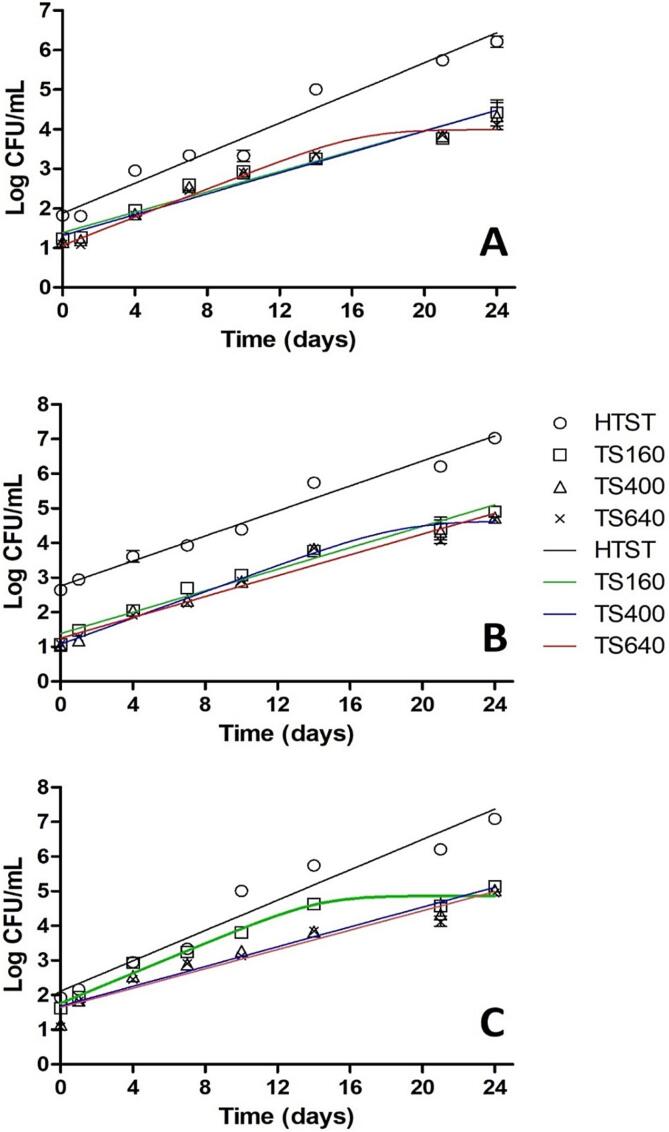
Fig. 4 shows the growth curves of the observed microbial groups, adjusted by the Baranyi predictive model. Experimental points are represented by symbols, while full curves indicate the model's fit.
Fig. 4.
Microbial behavior at the storage time of Minas Frescal cheese submitted to different treatments along the storage. Symbols with standard deviation bars represent experimental points and solid lines represent the fit of the growth curve by the Baranyi model. HTST, TS160, TS400, TS640 = conventional pasteurization, Sonication at 160 W, 400 W, 640 W respectively. A, B, C = Aerobic Mesophilic Bacteria, Lactic Acid Bacteria and Aerobic Psychotropic bacteria.
It is possible to observe that all microbial groups grew faster and reached a higher concentration at the end of storage in HTST-treated samples compared to TS-treated samples (p < 0.05). Among the TS treatments, slight differences in microbial behavior are observed, which can be more detailed based on the mathematical parameters obtained from the data adjustments to the Baranyi model, shown in Table 2.
Table 2.
Mathematical parameters obtained by fitting the data to the Baranyi predictive model.
| Aerobic Mesophilic Bacteria (AMB) | |||
|---|---|---|---|
| Treatment | R2 | yend | μ |
| HTST | 0.961359 | – | 0.190006 |
| TS160 | 0.962826 | – | 0.128742 |
| TS400 | 0.964003 | – | 0.131896 |
| TS640 | 0.986079 | 3.99 | 0.177909 |
| Lactic Acid Bacteria (LAB) | |||
| Treatment | R2 | yend | μ |
| HTST | 0.979936 | – | 0.180589 |
| TS160 | 0.976941 | – | 0.155093 |
| TS400 | 0.987198 | 4.65 | 0.190122 |
| TS640 | 0.963819 | – | 0.150204 |
| Aerobic Psychotropic Bacteria (APB) | |||
| Treatment | R2 | yend | μ |
| HTST | 0.951032 | – | 0.21899 |
| TS160 | 0.965305 | 4.86 | 0.216372 |
| TS400 | 0.948846 | – | 0.142906 |
| TS640 | 0.932284 | – | 0.139566 |
* yend: maximum population estimated by the model (log CFU g−1). μ: maximum growth rate (min−1). R2: Correlation coefficient. Control. TS160. TS400. TS640. HTST = untreated. sonication at 160. 400 and 640 W. conventional pasteurization. respectively.
Regarding the model's fit, correlation coefficient (R2) values above 0.93 were obtained in all curves, indicating a good fit of the Baranyi model to the experimental data. As seen in Fig. 4, none of the curves presented an adaptation phase (lag time); thus, this parameter is absent for this dataset. The yend values represent the maximum population estimated by the model in each curve. Only some curves associated with the TS treatment showed this value, indicating that it was possible to observe stability at the storage end. In the other curves, this was not possible, indicating that the growth would continue beyond the defined storage period.
Fig. 4A shows the curves related to AMB. While TS160 and TS400 exhibited practically equal curves, TS640 exhibited a stable behavior from day 16 onwards. When verifying the growth rate (Table 2), it is noted that the HTST treatment has the highest value (0.190 min−1) and that TS640 has a close value (0.178 min−1). Thus, the superiority of the treatments by TS compared to the HTST treatment concerning the control of AMB in Minas Frescal cheese is evidenced, presenting lower values of growth rate and stability of the population at the end of storage for the higher potency treatment (TS640).
Fig. 4B shows the curves related to the behavior of LAB during cheese storage. The three TS treatments showed similar curves, with TS400 exhibiting slight stability at the storage end. Although it has the highest growth rate, the existence of a maximum population value is an advantage over the HTST treatment, which has a similar growth rate value to LAB. The behavior of APB can be seen in Fig. 4C. In this case, TS160 caused a growth rate similar to that caused by the HTST treatment, but with population stability from day 16 onwards. The other TS treatments showed growth rates up to 35 % lower.
TS has been proven to generate positive effects when applied to foods regarding microbial inactivation. The cavitation bubble collapse generates a more significant microbial inactivation when compared to conventional heat treatments [10]. The turbulence generated by the collapse of the microbubbles can break the cell membrane of microorganisms, resulting in the leakage of cellular components. It can also interact with free radicals formed with cellular DNA, and hydrogen peroxide production in cavitation has bacteriostatic and bactericidal effects [16], [39], [42].
The results obtained in the present study corroborate the higher inactivation efficiency of ultrasound processing when applied to dairy products such as raw milk, dairy beverages, fermented milk, and cheeses [43], [44], [45]. In this way, this study confirms the applicability of TS for microbial inactivation of milk and further processing of Minas Frescal cheese.
3.4. Color
The science of color links the fundamental properties of light and matter to human perception. Therefore, the ability to capture and generate color is related to its importance in a wide range of manufacturing industries. Thus, this was divided into three branches: the color properties of the illumination (luminosity), the object's color properties, and the detector's or observer's color response [46]. The parameters related to luminosity/brightness (L*), chroma (C*), whiteness index (WI), and hue (h) in samples of Minas Frescal cheese processed by conventional pasteurization and TS are presented in Table 3.
Table 3.
Optical parameters of Minas Frescal cheese samples.
| Treatments | L* | a* | b* | C | h | WI |
|---|---|---|---|---|---|---|
| Control | 92.39d | −8.19a | 29.54a | 30.60a | 105.51d | 68.42d |
| HTST | 93.14c | −8.63d | 25.16b | 26.59b | 108.92c | 72.54c |
| TS160 | 94.41a | −8.32b | 22.61c | 24.09c | 110.21b | 75.27b |
| TS400 | 94.20a | −8.45c | 20.80d | 22.45d | 112.12a | 76.82a |
| TS640 | 93.63b | −8.38bc | 20.57d | 22.22d | 112.17a | 76.89a |
*Different letters mean significant difference between treatments (p < 0.05). L∗ = lightness. a∗ = from green (−) to red (+). b∗ = from blue (−) to yellow (+). WI = whiteness index. h = hue. C = chroma. Control. TS160. TS400. TS640. HTST = untreated. sonication at 160. 400 and 640 W. and the conventional pasteurization.
The samples processed by TS presented higher L* and consequently WI (Table 3, p < 0.05). Although the TS160 sample (L*: 94.412) was significantly brighter than the TS640 sample (L*: 93.632), the latter (WI: 76.889) was significantly whiter than the former (WI: 75.272) (p < 0.05). In contrast, raw milk samples (control, L*: 92.387, WI: 68.415) and HTST (L*: 93.142, WI: 72.536) were considered less bright and white (p < 0.05), respectively.
In opposition to the whiteness result, it was expected that the control sample (C*: 30.598) had higher chroma than the other samples, followed by the pasteurized sample (C*: 26.592) and the samples processed by TS (p < 0.05) when the tonality values (hab) showed the same trend of chroma values.
Chromance is considered a quantitative attribute of color and determines the degree of difference between a hue concerning a gray color with the same luminosity. A sample with higher chroma is related to the greater intensity of color perceived by the eyes [47]. The presence of carotenoids in bovine milk is responsible for the yellowish pigmentation and less whiteness as occurs in milk from other sources, such as sheep [27]. However, as verified in the present study, this component is susceptible to heating and degradation at temperatures above 50 °C [48].
The color results presented in this work showed that the increase in temperature, even if the processing is nonthermal such as ultrasound, influences the degradation of carotenoids responsible for the less white coloration in bovine milk, verified by the color parameters, mainly L* and WI. Compared to the HTST treatment, the samples treated by TS had higher indices related to white color. This can be explained by the fact that the lower temperature applied in the TS treatment does not trigger the Maillard reaction, which promotes the formation of dark pigments, even in low amounts [49]. White color is desired for Minas Frescal cheese, therefore, the higher L* and consequently WI values of TS-treated cheeses would be interesting from the consumer point of view.
3.5. Rheological properties
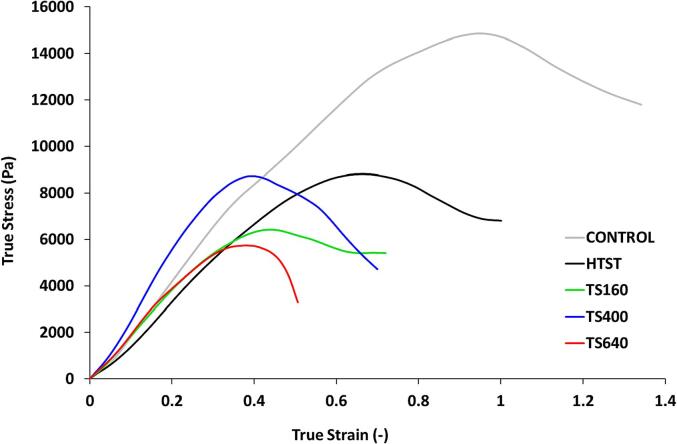
Fig. 5 exhibits the true stress-true strain curves of the Minas Frescal cheese samples, and Table 4 shows the values of the rheological parameters: modulus of elasticity (), fracture strain (), fracture stress (), modulus of resilience (), and modulus of toughness ().
Fig. 5.
True stress ()-true strain () curves of the cheese samples. Control, TS160, TS400, TS640, HTST = untreated, Sonication at 160 W, 400 W and 640 W and conventional pasteurization, respectively.
Table 4.
Rheological parameters obtained from uniaxial compression test.
| Treatments | E (kPa) | ef (-) | sf (kPa) | We (kJ/m3) | Wf (kJ/m3) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | 18.3 | ± | 3.5a | 1.055 | ± | 0.176a | 13.8 | ± | 1.1a | 2.2 | ± | 0.5a | 8.9 | ± | 1.0a |
| HTST | 16.6 | ± | 0.5a | 0.651 | ± | 0.008b | 8.8 | ± | 0.3b | 1.6 | ± | 0.1a | 3.3 | ± | 0.1b |
| TS160 | 18.2 | ± | 1.2a | 0.458 | ± | 0.060bc | 6.6 | ± | 0.4b | 1.2 | ± | 0.2b | 1.8 | ± | 0.3c |
| TS400 | 21.1 | ± | 1.8a | 0.404 | ± | 0.017c | 8.7 | ± | 2.1b | 1.6 | ± | 0.6a | 2.1 | ± | 0.6bc |
| TS640 | 18.7 | ± | 2.8a | 0.373 | ± | 0.002c | 5.8 | ± | 1.0b | 0.9 | ± | 0.1b | 1.3 | ± | 0.2c |
*Different letters mean significant difference between treatments (p < 0.05). E: modulus of elasticity; ef: fracture strain; sf: fracture stress; We: modulus of resilience; Wf: modulus of toughness. Control. TS160. TS400. TS640. HTST = untreated. sonication at 160. 400 and 640 W. and the conventional pasteurization.
The modulus of elasticity () is a measure of a material’s resistance to axial deformation, representing the slope of the linear portion of the stress–strain curve obtained by linear regression (0.9942 ≤ R2 ≤ 0.9995). It also expresses the material's stiffness to an applied load, where a higher value of modulus of elasticity corresponds to a higher material stiffness. No significant impact of HTST or TS was observed for the modulus of elasticity (p > 0.05).
The fracture strain () and fracture stress () are respective measures of the strain and stress required to bring about complete fracture of the sample. Generally, the fracture strain can be inversely correlated to the brittleness of the samples, while the fracture stress can be directly correlated to the firmness [28]. The control sample has shown significantly higher fracture strain (1.055, p = 0.002) and fracture stress (13.8 kPa, p = 0.006) than the other samples. The fracture strain has presented a gradual decrease across HTST (0.651), TS160 (0.458), TS400 (0.404) and TS640 (0.373) samples. In this way, TS-treated samples showed higher brittleness than the other samples. Likewise, no significant differences among HTST or TS-treated samples were observed for the fracture stress () (p > 0.05), suggesting that both treatments resulted in firmer products than the control.
The modulus of resilience () is the amount of work per unit volume absorbed by a material until the elastic limit, where the material can sustain stress without any permanent strain remaining upon release of the stress. Thus, the material will return to its original shape and size when the stress is removed [50]. The control sample has shown the highest resilience value (2.2 kJ m−3), twofold higher than the TS640 sample (0.9 kJ m−3), indicating a decrease in resilience caused by this TS treatment. However, at nominal power of 400 W, no significant difference was observed (p > 0.05).
The modulus of toughness () is the amount of work per unit volume absorbed by a material until the fracture point. The control sample showed the highest toughness (8.9 kJ m−3), followed by HTST (3.3 kJ m−3), and then the TS samples presented values ranging from 1.3 to 2.1 kJ m−3 and suggesting a pronounced decrease in toughness caused by heat and sonication. It has been reported the US can improve the renneting processing of the milk [51], producing firmer gels. However, the use of heat can contribute to a decreased performance of the structural aspects of the cheese.
Overall, the rheological parameters , , and found for untreated cheese (control) and conventionally pasteurized cheese (HTST) were in a range wherein they were lower than some studies reported in the literature [52], [53] but higher than others [28], [32]. The variation of rheological parameters indicated that conventional pasteurization and TS processing promoted a similar weakening effect on the intermolecular bonds of the protein network, making the Minas Frescal cheese more brittle and less firm than the untreated cheese, which are characteristics desired for Minas Frescal cheeses by consumers [1].
3.6. Bioactive compounds
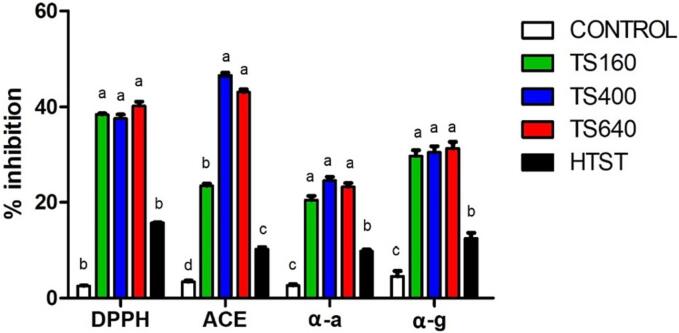
Fig. 6 illustrates the results related to the quantification of bioactive compounds in the cheese samples treated by HTST and TS and the control sample made with raw milk.
Fig. 6.
Functional activities of Minas Frescal cheese samples. Different letters mean significant difference between treatments (p < 0.05). DPPH: 2,2-diphenyl-1-picrylhydrazyl. ACE: Angiotensin converting enzyme. α-a: α-amylase. α-g: α-glucosidase. Control, TS160, TS400, TS640, HTST = untreated, Sonication at 160 W, 400 W and 640 W and conventional pasteurization, respectively.
It is possible to observe that all analyzed parameters, exhibited the lowest levels of the referred compounds, and the samples treated by HTST exhibited lower values concerning the samples treated by TS, presenting a statistically significant difference (p < 0.05). The higher levels of bioactive compounds in the treated samples, in general, can be explained by the release of some bioactive peptides [54].
Among the TS samples, only the ACE values showed a statistically significant difference between them, where TS160 presented a significantly lower value (23.5 %, compared to 46.5 and 43 % of TS400 and TS640, respectively, p < 0.05). The DPPH values were higher in the samples treated by TS by about 2.5 times concerning the HTST treatment, while α-glucosidase and α-amylase presented values more than double.
The formation of bioactive compounds is strongly related to the technology used. The antioxidant activity, measured through the DPPH radical, is related to the release of phenolic compounds and peptides as a function of the treatment applied. In TS treatment, this release is favored by the disruption of cells caused due the physical effects of cavitation bubble collapse [18]. Inhibition of ACE, α-glucosidase, and α-amylase is also related to the release of bioactive peptide fragments, originating from the hydrolysis of cheese proteins, which end up exerting an inhibitory or shielding function on enzymes [30], [55].
Reinforcing the results observed in this work, other studies also show the impact of ultrasound application in dairy products with positive effects on the content of bioactive compounds [18], [19], [44], [56].
4. Conclusions
Thermosonication of raw milk is an effective option for Minas Frescal cheese processing. Simultaneous use of heat and acoustic cavitation proportionated higher microbial reductions than conventional thermal treatments (HTST) and improved the product optical parameters without significant impact on the rheological parameters. In addition, TS increased bioactive compound generation, which is relevant for the functional potential. In addition, for the TS system the processing time is 47 % when compared to HTST processing.
Therefore, TS must be considered by the cheese industry as an interesting alternative to manufacturing fresh cheese, such as Minas Frescal cheese, offering advantages to the product in relation to traditional processing, without presenting negative impacts. The nominal power of 400 W stands out as a processing parameter that offers the best benefits to the treated food.
CRediT authorship contribution statement
Hugo Scudino: Writing – original draft. Jonas T. Guimarães: Writing – original draft. Rafaella Silva Moura: Writing – original draft. Gustavo Luis P. A. Ramos: Writing – original draft. Tatiana C. Pimentel: Methodology, Validation, Formal analysis. Rodrigo N. Cavalcanti: Writing – original draft. Louise A. Sobral: Writing – original draft. Marcia Cristina Silva: Methodology, Validation, Formal analysis. Eliane T. Mársico: Methodology, Validation, Formal analysis. Erick A. Esmerino: Methodology, Validation, Formal analysis. Monica Q. Freitas: Methodology, Validation, Formal analysis. Thiago C. Pereira: Conceptualization, Writing – review & editing. Erico M. M. Flores: Conceptualization, Writing – review & editing. Adriano G. Cruz: Conceptualization, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
A.G. Cruz, M. C. Freitas, and T.C. Pimentel are grateful for the productivity grants (CNPq, CAPES and FAPERJ). J. T. Guimarães thanks to Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for the postdoctoral scholarship (finance code 001). T. C. Pereira thanks CNPq for the doctoral scholarship (grant 141044/2021-7). E. M. M. Flores acknowledges Instituto Nacional de Ciência e Tecnologia de Bioanalítica (INCTBio, Process Number 88887.137487/2017-00); Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Grant Nr. 313786/2019-4, 409548/2021-9, 312271/2017-4); and Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul (FAPERGS, Grant Nr. 17/2551-0000960-6, 22/2551-0000389-3, 21/2551-0002091-1) for the support.
References
- 1.Rocha R.S., Silva R., Guimarães J.T., Balthazar C.F., Pimentel T.C., Neto R.P.C., Tavares M.I.B., Esmerino E.A., Freitas M.Q., Cappato L.P., Calvacanti R.N., Rodrigues F.N., Raices R.S.L., Silva M.C., Cruz A.G. Possibilities for using ohmic heating in Minas Frescal cheese production. Food Res. Int. 2020;131 doi: 10.1016/j.foodres.2020.109027. [DOI] [PubMed] [Google Scholar]
- 2.Neoκleous I., Tarapata J., Papademas P. Non-thermal Processing Technologies for Dairy Products: Their Effect on Safety and Quality Characteristics. Front. Sustain. Food Syst. 2022;6 doi: 10.3389/fsufs.2022.856199. [DOI] [Google Scholar]
- 3.Shabbir M.A., Ahmed H., Maan A.A., Rehman A., Afraz M.T., Iqbal M.W., Khan I.M., Amir R.M., Ashraf W., Khan M.R., Aadil R.M. Effect of non-thermal processing techniques on pathogenic and spoilage microorganisms of milk and milk products. Food Sci. Technol. 2020;41:279–294. doi: 10.1590/fst.05820. [DOI] [Google Scholar]
- 4.Mukhtar K., Nabi B.G., Arshad R.N., Roobab U., Yaseen B., Ranjha M.M.A.N., Ibrahim S.A., Aadil R.M. Potential Impact of Ultrasound, Pulsed Electric Field, High-Pressure Processing, Microfludization Against Thermal Treatments Preservation Regarding Sugarcane Juice (Saccharum officinarum) Ultrason. Sonochem. 2022;90 doi: 10.1016/j.ultsonch.2022.106194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yıldız G., Khan M.R., Aadil R.M. High-intensity ultrasound treatment to produce and preserve the quality of fresh-cut kiwifruit. J. Food Process. Preserv. 2022:e16542. [Google Scholar]
- 6.Ahmad T., Butt M.Z., Aadil R.M., Inam-ur-Raheem M., Bekhit A.E.D., Guimarães J.T., Balthazar C.F., Rocha R.S., Esmerino E.A., Freitas M.Q., Silva M.C., Sameen A., Cruz A.G. Impact of nonthermal processing on different milk enzymes. Int. J. Dairy Technol. 2019;72:481–495. doi: 10.1111/1471-0307.12622. [DOI] [Google Scholar]
- 7.Chávez-Martínez A., Reyes-Villagrana R.A., Rentería-Monterrubio A.L., Sánchez-Vega R., Tirado-Gallegos J.M., Bolivar-Jacobo N.A. Low and High-Intensity Ultrasound in Dairy Products: Applications and Effects on Physicochemical and Microbiological Quality. Foods. 2020;9:1688. doi: 10.3390/foods9111688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aadil R.M., Zeng X.A., Han Z., Sun D.W. Effects of ultrasound treatments on quality of grapefruit juice. Food Chem. 2013;141:3201–3206. doi: 10.1016/j.foodchem.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 9.Gao S., Hemar Y., Lewis G.D., Ashokkumar M. Inactivation of Enterobacter aerogenes in reconstituted skim milk by high- and low-frequency ultrasound. Ultrason. Sonochem. 2014;21:2099–2106. doi: 10.1016/j.ultsonch.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 10.Meroni D., Djellabi R., Ashokkumar M., Bianchi C.L., Boffito D.C. Sonoprocessing: From Concepts to Large-Scale Reactors. Chem Rev. 2022;122:3219–3258. doi: 10.1021/acs.chemrev.1c00438. [DOI] [PubMed] [Google Scholar]
- 11.Abrahamsen R.K., Narvhus J.A. Can ultrasound treatment replace conventional high temperature short time pasteurization of milk? A critical review. Int. Dairy J. 2022;131 doi: 10.1016/j.idairyj.2022.105375. [DOI] [Google Scholar]
- 12.Gao S., Lewis G.D., Ashokkumar M., Hemar Y. Inactivation of microorganisms by low-frequency high-power ultrasound: 2. A simple model for the inactivation mechanism. Ultrason. Sonochem. 2014;21:454–460. doi: 10.1016/j.ultsonch.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 13.Bhargava N., Mor R.S., Kumar K., Sharanagat V.S. Advances in application of ultrasound in food processing: A review. Ultrason. Sonochem. 2021;70 doi: 10.1016/j.ultsonch.2020.105293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pagnossa J.P., Rocchetti G., Ribeiro A.C., Piccoli R.H., Lucini L. Ultrasound: beneficial biotechnological aspects on microorganisms-mediated processes. Curr. Opin. Food Sci. 2020;31:24–30. doi: 10.1016/j.cofs.2019.10.006. [DOI] [Google Scholar]
- 15.Ribeiro N.G., Xavier-Santos D., Campelo P.H., Guimarães J.T., Pimentel T.C., Duarte M.C.K.H., Freitas M.Q., Esmerino E.A., Silva M.C., Cruz A.G. Dairy foods and novel thermal and non-thermal processing: A bibliometric analysis. Innov. Food Sci. Emerg. Technol. 2022;76 doi: 10.1016/j.ifset.2022.102934. [DOI] [Google Scholar]
- 16.Guimarães J.T., Scudino H., Ramos G.L., Oliveira G.AR., Margalho L.P., Costa L.EO., Freitas M.Q., Duarte M.C.KH., Sant'Ana A.S., Cruz A.G. Current applications of high-intensity ultrasound with microbial inactivation or stimulation purposes in dairy products. Curr. Opin. Food Sci. 2021;42:140–147. [Google Scholar]
- 17.Aadil R.M., Zeng X.A., Zhang Z.H., Wang M.S., Han Z., Jing H., Jabbar S. Thermosonication: A potential technique that influences the quality of grapefruit juice. Int. J. Food Sci. Technol. 2015;50(2015):1275–1282. doi: 10.1111/ijfs.12766. [DOI] [Google Scholar]
- 18.Oliveira G.A.R., Guimarães J.T., Ramos G.L.P.A., Esmerino E.A., Pimentel T.C., Neto R.P.C., Tavares M.I.B., Sobral L.A., Souto F., Freitas M.Q., Costa L.E.O., Cruz A.G. Benefits of thermosonication in orange juice whey drink processing. Innov. Food Sci. Emerg. Technol. 2022;75 doi: 10.1016/j.ifset.2021.102876. [DOI] [Google Scholar]
- 19.Lino D.L., Guimarães J.T., Ramos G.L.P.A., Sobral L.A., Souto F., Neto R.P.C., Tavares M.I.B., Celso Sant’Anna, Esmerino E.A., Mársico E.T., Freitas M.Q., Flores E.M.M., Raices R.S.L., Campelo P.H., Pimentel T.C., Cristina Silva M., Cruz A.G. Positive effects of thermosonication in Jamun fruit dairy dessert processing. Ultrason. Sonochem. 2022;86 doi: 10.1016/j.ultsonch.2022.106040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Strieder M.M., Silva E.K., Meireles M.A.A. Advances and innovations associated with the use of acoustic energy in food processing: An updated review. Innov. Food Sci. Emerg. Technol. 2021;74 doi: 10.1016/j.ifset.2021.102863. [DOI] [Google Scholar]
- 21.Hu J., Sari O., Eicher S., Rakotozanakajy A.R. Determination of specific heat of milk at different fat content between 1°C and 59°C using micro DSC. J. Food Eng. 2009;90:395–399. doi: 10.1016/j.jfoodeng.2008.07.009. [DOI] [Google Scholar]
- 22.Brazil, Normative instruction No 68 from 12/12/2006 – Official Analytical and Physical-chemical methods for milk and dairy control. Union Official Diary (2006). Avaliable in: https://pesquisa.in.gov.br/imprensa/jsp/visualiza/index.jsp?data=14/12/2006&jornal=1&pagina=8&totalArquivos=124 (accessed May 25, 2022).
- 23.H.M. Wehr, J.F. Frank, Standard Methods for the Examination of Dairy Products, 6th ed., American Public Health Association, New York, USA, 2003. 10.2105/9780875530024.
- 24.Alcántara-Zavala A.E., de J., Figueroa-Cárdenas D., Pérez-Robles J.F., Arámbula-Villa G., Miranda-Castilleja D.E. Thermosonication as an alternative method for processing, extending the shelf life, and conserving the quality of pulque: A non-dairy Mexican fermented beverage. Ultrason. Sonochem. 2021;70 doi: 10.1016/j.ultsonch.2020.105290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baranyi J., Roberts T.A. Mathematics of predictive food microbiology. Int. J. Food Microbiol. 1995;26:199–218. doi: 10.1016/0168-1605(94)00121-L. [DOI] [PubMed] [Google Scholar]
- 26.Balthazar C.F., Silva H.L.A., Celeguini R.M.S., Santos R., Pastore G.M., Junior C.A.C., Freitas M.Q., Nogueira L.C., Silva M.C., Cruz A.G. Effect of galactooligosaccharide addition on the physical, optical, and sensory acceptance of vanilla ice cream. J. Dairy Sci. 2015;98:4266–4272. doi: 10.3168/jds.2014-9018. [DOI] [PubMed] [Google Scholar]
- 27.Balthazar C.F., Silva H.L.A., Vieira A.H., Neto R.P.C., Cappato L.P., Coimbra P.T., Moraes J., Andrade M.M., Calado V.M.A., Granato D., Freitas M.Q., Tavares M.I.B., Raices R.S.L., Silva M.C., Cruz A.G. Assessing the effects of different prebiotic dietary oligosaccharides in sheep milk ice cream. Food Res. Int. 2017;91:38–46. doi: 10.1016/j.foodres.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 28.Dantas A.B., Jesus V.F., Silva R., Almada C.N., Esmerino E.A., Cappato L.P., Silva M.C., Raices R.S.L., Cavalcanti R.N., Carvalho C.C., Sant’Ana A.S., Bolini H.M.A., Freitas M.Q., Cruz A.G. Manufacture of probiotic Minas Frescal cheese with Lactobacillus casei Zhang. J. Dairy Sci. 2016;99(1):18–30. doi: 10.3168/jds.2015-9880. [DOI] [PubMed] [Google Scholar]
- 29.Ak M.M., Gunasekaran S. Stress-Strain Curve Analysis of Cheddar Cheese under Uniaxial Compression. J Food Sci. 1992;57:1078–1081. doi: 10.1111/j.1365-2621.1992.tb11268.x. [DOI] [Google Scholar]
- 30.Cappato L.P., Ferreira M.V.S., Moraes J., Pires R.P.S., Rocha R.S., Silva R., Neto R.P.C., Tavares M.I.B., Freitas M.Q., Rodrigues F.N., Calado V.M.A., Raices R.S.L., Silva M.C., Cruz A.G. Whey acerola-flavoured drink submitted Ohmic Heating: Bioactive compounds, antioxidant capacity, thermal behavior, water mobility, fatty acid profile and volatile compounds. Food Chem. 2018;263:81–88. doi: 10.1016/j.foodchem.2018.04.115. [DOI] [PubMed] [Google Scholar]
- 31.Amaral G.v., Silva E.K., Cavalcanti R.N., Martins C.P.C., Andrade L.G.Z.S., Moraes J., Alvarenga V.O., Guimarães J.T., Esmerino E.A., Freitas M.Q., Silva M.C., Raices R.S.L., Sant’ Ana A.S., Meireles M.A.A., Cruz A.G. Whey-grape juice drink processed by supercritical carbon dioxide technology: Physicochemical characteristics, bioactive compounds and volatile profile. Food Chem. 2018;239:697–703. doi: 10.1016/j.foodchem.2017.07.003. [DOI] [PubMed] [Google Scholar]
- 32.Ferreira M.V.S., Cappato L.P., Silva R., Rocha R.S., Guimarães J.T., Balthazar C.F., Esmerino E.A., Freitas M.Q., Rodrigues F.N., Granato D., Neto R.P.C., Tavares M.I.B., Silva P.H.F., Raices R.S.L., Silva M.C., Cruz A.G. Ohmic heating for processing of whey-raspberry flavored beverage. Food Chem. 2019;297 doi: 10.1016/j.foodchem.2019.125018. [DOI] [PubMed] [Google Scholar]
- 33.Konrad B., Anna D., Marek S., Marta P., Aleksandra Z., Józefa C. The Evaluation of Dipeptidyl Peptidase (DPP)-IV, α-Glucosidase and Angiotensin Converting Enzyme (ACE) Inhibitory Activities of Whey Proteins Hydrolyzed with Serine Protease Isolated from Asian Pumpkin (Cucurbita ficifolia) Int. J. Pept. Res. Ther. 2014;20:483–491. doi: 10.1007/s10989-014-9413-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lavelli V., Sri Harsha P.S.C., Ferranti P., Scarafoni A., Iametti S. Grape skin phenolics as inhibitors of mammalian α-glucosidase and α-amylase – effect of food matrix and processing on efficacy. Food Funct. 2016;7:1655–1663. doi: 10.1039/C6FO00073H. [DOI] [PubMed] [Google Scholar]
- 35.Carrillo-Lopez L.M., Garcia-Galicia I.A., Tirado-Gallegos J.M., Sanchez-Vega R., Huerta-Jimenez M., Ashokkumar M., Alarcon-Rojo A.D. Recent advances in the application of ultrasound in dairy products: Effect on functional, physical, chemical, microbiological and sensory properties. Ultrason Sonochem. 2021;73 doi: 10.1016/j.ultsonch.2021.105467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shokri S., Javanmardi F., Mohammadi M., Mousavi Khaneghah A. Effects of ultrasound on the techno-functional properties of milk proteins: A systematic review. Ultrason. Sonochem. 2022;83 doi: 10.1016/j.ultsonch.2022.105938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brazil, Resolution MERCOSUL/GMC/RES. No 145/96 . MERCOSUL Technical resolution for the identity and quality of Minas Frescal cheese. Avaliable in: http://www.sice.oas.org/trade/mrcsrs/resolutions/res4498p.asp (accessed May 17, 2022).
- 38.Brazil, Directive No 146 from 07/03/1996. Technical resolution for the identity and quality of cheese. Avaliable in: https://www.defesa.agricultura.sp.gov.br/legislacoes/portaria-mapa-146-de-07-03-1996,669.html (accessed May 17, 2022).
- 39.Cichoski A.J., Rampelotto C., Silva M.S., de Moura H.C., Terra N.N., Wagner R., de Menezes C.R., Flores E.M.M., Barin J.S. Ultrasound-assisted post-packaging pasteurization of sausages. Innov. Food Sci. Emerg. Tech. 2015;30:132–137. doi: 10.1016/j.ifset.2015.04.011. [DOI] [Google Scholar]
- 40.Halpin R.M., Duffy L., Cregenzán-Alberti O., Lyng J.G., Noci F. The effect of non-thermal processing technologies on microbial inactivation: An investigation into sub-lethal injury of Escherichia coli and Pseudomonas fluorescens. Food Control. 2014;41:106–115. doi: 10.1016/j.foodcont.2014.01.011. [DOI] [Google Scholar]
- 41.Noci F., Walkling-Ribeiro M., Cronin D.A., Morgan D.J., Lyng J.G. Effect of thermosonication, pulsed electric field and their combination on inactivation of Listeria innocua in milk. Int. Dairy J. 2009;19:30–35. doi: 10.1016/j.idairyj.2008.07.002. [DOI] [Google Scholar]
- 42.Evelyn, Silva F.V.M. Silva, Ultrasound assisted thermal inactivation of spores in foods: Pathogenic and spoilage bacteria, molds and yeasts. Trends Food Sci. Technol. 2020;105:402–415. [Google Scholar]
- 43.Scudino H., Silva E.K., Gomes A., Guimarães J.T., Cunha R.L., Sant’Ana A.S., Meireles M.A.A., Cruz A.G. Ultrasound stabilization of raw milk: Microbial and enzymatic inactivation, physicochemical properties and kinetic stability. Ultrason. Sonochem. 2020;67 doi: 10.1016/j.ultsonch.2020.105185. [DOI] [PubMed] [Google Scholar]
- 44.Monteiro S.H.M.C., Silva E.K., Alvarenga V.O., Moraes J., Freitas M.Q., Silva M.C., Raices R.S.L., Sant'Ana A.S., Meireles M.A.A., Cruz A.G. Effects of ultrasound energy density on the non-thermal pasteurization of chocolate milk beverage. Ultrason. Sonochem. 2018;42:1–10. doi: 10.1016/j.ultsonch.2017.11.015. [DOI] [PubMed] [Google Scholar]
- 45.Jalilzadeh A., Hesari J., Peighambardoust S.H., Javidipour I. The effect of ultrasound treatment on microbial and physicochemical properties of Iranian ultrafiltered feta-type cheese. J Dairy Sci. 2018;101:5809–5820. doi: 10.3168/jds.2017-14352. [DOI] [PubMed] [Google Scholar]
- 46.Joiner A., Hopkinson I., Deng Y., Westland S. A review of tooth colour and whiteness. J. Dent. 2008;36:2–7. doi: 10.1016/j.jdent.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 47.Pathare P.B., Opara U.L., Al-Said F.-A.-J. Colour Measurement and Analysis in Fresh and Processed Foods: A Review. Food Bioproc. Tech. 2013;6:36–60. doi: 10.1007/s11947-012-0867-9. [DOI] [Google Scholar]
- 48.Fratianni A., Niro S., Messia M.C., Cinquanta L., Panfili G., Albanese D., di Matteo M. Kinetics of carotenoids degradation and furosine formation in dried apricots (Prunus armeniaca L.) Food Res. Int. 2017;99:862–867. doi: 10.1016/j.foodres.2016.12.009. [DOI] [PubMed] [Google Scholar]
- 49.Kutlu N., Pandiselvam R., Kamiloglu A., Saka I., Sruthi N.U., Kothakota A., Socol C.T., Maerescu C.M. Impact of ultrasonication applications on color profile of foods. Ultrason. Sonochem. 2022;89 doi: 10.1016/j.ultsonch.2022.106109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gunasekaran S., Ak M.M. CRC Press; 2002. Cheese Rheology and Texture. Doi: 10.1201/9781420031942. [Google Scholar]
- 51.Liu Z., Juliano P., Williams R.P.W., Niere J., Augustin M.A. Ultrasound improves the renneting properties of milk. Ultrason. Sonochem. 2014;21:2131–2137. doi: 10.1016/j.ultsonch.2014.03.034. [DOI] [PubMed] [Google Scholar]
- 52.Silva H.L.A., Balthazar C.F., Esmerino E.A., Vieira A.H., Cappato L.P., Neto R.P.C., Verruck S., Cavalcanti R.N., Portela J.B., Andrade M.M., Moraes J., Franco R.M., Tavares M.I.B., Prudencio E.S., Freitas M.Q., Nascimento J.S., Silva M.C., Raices R.S.L., Cruz A.G. Effect of sodium reduction and flavor enhancer addition on probiotic Prato cheese processing. Food Res. Int. 2017;99:247–255. doi: 10.1016/j.foodres.2017.05.018. [DOI] [PubMed] [Google Scholar]
- 53.Silva S.F., Rocha R.S., Esmerino E.A., Pimentel T.C., Gomes da Cruz A., C.a. Rodrigues Anjos, Impact of different modified atmosphere packaging on quality parameters and probiotic survival during storage of Minas Frescal cheese. Food Biosci. 2021;43 doi: 10.1016/j.fbio.2021.101338. [DOI] [Google Scholar]
- 54.Silveira M.R., Coutinho N.M., Esmerino E.A., Moraes J., Fernandes L.M., Pimentel T.C., Freitas M.Q., Silva M.C., Raices R.S.L., Senaka Ranadheera C., Borges F.O., Neto R.P.C., Tavares M.I.B., Fernandes F.A.N., Fonteles T.V., Nazzaro F., Rodrigues S., Cruz A.G. Guava-flavored whey beverage processed by cold plasma technology: Bioactive compounds, fatty acid profile and volatile compounds. Food Chem. 2019;279:120–127. doi: 10.1016/j.foodchem.2018.11.128. [DOI] [PubMed] [Google Scholar]
- 55.Martinez-Gonzalez A.I., Díaz-Sánchez Á.G., de la Rosa L.A., Bustos-Jaimes I., Alvarez-Parrilla E. Inhibition of α-amylase by flavonoids: Structure activity relationship (SAR) Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019;206:437–447. doi: 10.1016/j.saa.2018.08.057. [DOI] [PubMed] [Google Scholar]
- 56.Gholamhosseinpour A., Hashemi S.M.B., Raoufi Jahromi L., Sourki A.H. Conventional heating, ultrasound and microwave treatments of milk: Fermentation efficiency and biological activities. Int. Dairy J. 2020;110 doi: 10.1016/j.idairyj.2020.104809. [DOI] [Google Scholar]