Abstract
Within the European Human Biomonitoring (HBM) Initiative HBM4EU we derived HBM indicators that were designed to help answering key policy questions and support chemical policies. The result indicators convey information on chemicals exposure of different age groups, sexes, geographical regions and time points by comparing median exposure values. If differences are observed for one group or the other, policy measures or risk management options can be implemented. Impact indicators support health risk assessment by comparing exposure values with health-based guidance values, such as human biomonitoring guidance values (HBM-GVs). In general, the indicators should be designed to translate complex scientific information into short and clear messages and make it accessible to policy makers but also to a broader audience such as stakeholders (e.g. NGO's), other scientists and the general public. Based on harmonized data from the HBM4EU Aligned Studies (2014–2021), the usefulness of our indicators was demonstrated for the age group children (6–11 years), using two case examples: one phthalate (Diisobutyl phthalate: DiBP) and one non-phthalate substitute (Di-isononyl cyclohexane-1,2- dicarboxylate: DINCH). For the comparison of age groups, these were compared to data for teenagers (12–18 years), and time periods were compared using data from the DEMOCOPHES project (2011–2012). Our result indicators proved to be suitable for demonstrating the effectiveness of policy measures for DiBP and the need of continuous monitoring for DINCH. They showed similar exposure for boys and girls, indicating that there is no need for gender focused interventions and/or no indication of sex-specific exposure patterns. They created a basis for a targeted approach by highlighting relevant geographical differences in internal exposure. An adequate data basis is essential for revealing differences for all indicators. This was particularly evident in our studies on the indicators on age differences. The impact indicator revealed that health risks based on exposure to DiBP cannot be excluded. This is an indication or flag for risk managers and policy makers that exposure to DiBP still is a relevant health issue. HBM indicators derived within HBM4EU are a valuable and important complement to existing indicator lists in the context of environment and health. Their applicability, current shortcomings and solution strategies are outlined.
Keywords: indicator, Human biomonitoring (HBM), Phthalates, DINCH, Science-policy uptake, HBM4EU
Highlights
-
•
Pan-European HBM indicators are shown for one representative phthalate (DiBP) and the substitute DINCH.
-
•
They convey results of surveys and research in an easily accessible and interpretable way and allow to monitor progress.
-
•
Result indicators are a communication tool to show differences in exposure burden by comparing median exposure values.
-
•
Impact indicators assess health risks by comparing exposure with health-based guidance values.
-
•
An adequate database with comparable data is a prerequisite for deriving HBM indicators.
Abbreviations
- 3xG
Health – Municipalities – Births study (Belgium, BE)
- 5cx-MEPP
Mono(2-ethyl-5-carboxypentyl) phthalate
- 5OH-MEHP
Mono(2-ethyl-5-hydroxyhexyl) phthalate
- 5oxo-MEHP
Mono(2-ethyl-5-oxohexyl) phthalate
- BBzP
Butyl benzyl phthalate
- BE
Belgium
- BEA
Biomonitoring in Adolescents study (Spain, ES)
- BPA
Bisphenol A
- BPF
Bisphenol F
- BPS
Bisphenol S
- CELSPAC: TE
Central European Longitudinal Studies of Parents and Children: Teenagers (Czech Republic, CZ)
- CLP
Classification, Labelling and Packaging
- CROME
Cross-Mediterranean Environment and Health Network study (Greece, GR)
- CZ
Czech Republic
- cx-MiDP
Mono(2,7-methyl-7carboxy-heptyl) phthalate
- cx-MINCH
Cyclohexane-1,2- dicarboxylate-mono-(7- carboxylate-4- methyl)heptyl ester
- cx-MiNP
Mono(4-methyl-7-carboxyheptyl) phthalat
- DE
Germany
- DEHP
Diethylhexyl phthalate
- DEMOCOPHES
DEMOnstration of a study to COordinate and Perform Human biomonitoring on a European Scale project
- DEP
Diethyl phthalate
- DiBP
Diisobutyl phthalate
- DINCH
Di-isononyl cyclohexane-1,2- dicarboxylate
- DiNP
Diisononyl phthalate
- DK
Denmark
- DnBP
Di-n-butyl phthalate
- ED
Endocrine disruptor
- EE
Extent of exceedance
- EEA
European Environment Agency
- EFSA
European Food Safety Authority
- ES
Spain
- ESB
German Environmental Specimen Bank
- ESTEBAN
Health study on environment, biomonitoring, physical activity and nutrition study (France, FR)
- EU
European Union
- FLEHS IV
4th cycle of the Flemish Environment and Health Survey (Belgium, BE)
- FR
France
- GerES V-sub (unweighted)
5th cycle of the German Environmental Survey (subsample, unweighted data, DE)
- GM
Geometric mean
- GR
Greece
- HBM4EU
The European Human Biomonitoring Initiative
- HBM
Human Biomonitoring
- HBM-GV
Human Biomonitoring guidance values
- HI
Hazard index
- HU
Hungary
- InAirQ
Transnational Adaption Actions for Integrated Indoor Air Quality Management study (Hungary, HU)
- IT
Italy
- LoC
Level of confidence
- LOD
Limit of detection
- LOQ
Limit of quantification
- LU
Luxembourg
- MBzP
Mono-benzyl phthalate
- MEP
Mono-ethyl phthalate
- MiBP
Mono-isobutyl phthalate
- MnBP
Mono-n-butyl phthalate
- MRA
Mixture risk assessment
- NAC II
Northern Adriatic cohort II (Italy, IT)
- NEB II
Norwegian Environmental Biobank II (Norway, NO)
- n
Number of samples/participants exceeding the HBM-GV
- N
Total number of samples/participants
- NEP
N-Ethyl-2 pyrrolidone
- NGO
Non-Governmental Organisation
- NIPH
Norwegian Institute of Public Health
- NL
The Netherlands
- NMP
N-Methyl-2-pyrrolidone
- NO
Norway
- OCC
Odense child cohort (Denmark, DK)
- OH-MiDP
Mono-hydroxy-isodecyl phthalate
- OH-MINCH
Cyclohexane-1,2- dicarboxylate-mono-(7- hydroxy-4-methyl)octyl ester
- OH-MiNP
Mono(4-methyl-7-hydroxyoctyl) phthalate
- P50
50th percentile; median
- P95
95th percentile
- PCB
Polychlorinated biphenyls
- PCB cohort follow-up
Endocrine disruptors and health in children and teenagers in Slovakia study (follow-up study; Slovakia, SK)
- PE
Percentage exceedance
- PFAS
Per- and polyfluoroalkyl substances
- POLAES
Polish Aligned Environmental Study (Poland, PL)
- QA/QC
quality assurance/quality control
- RA
Risk assessment
- REACH
Registration, Evaluation, Authorisation and Restriction of Chemicals
- Riksmaten Adolescents
Riksmaten Adolescents 2016-17 (Sweden, SE)
- SE
Sweden
- SES
Socioeconomic status
- SIN-list
Substitute it now-list
- SK
Slovakia
- SL
Slovenia
- SLO CRP
Exposure of children and adolescents to selected chemicals through their habitat environment study (Slovenia, SL)
- SPECIMEn-NL
Survey on Pesticide Mixtures in Europe (The Netherlands, NL)
- SVHC
Substances of very high concern
- TWI
Tolerable weekly intake
- UBA
German Environment Agency
- US EPA
United States Environmental Protection Agency
- WHO
World Health Organization
1. Introduction
The „European Human Biomonitoring (HBM4EU) Initiative“ is a joint effort of 30 European countries, and the European Environment Agency (EEA), co-funded by the European Commission under the framework of Horizon 2020 aiming to improve and inform chemical safety. Using HBM methods the internal exposure of a chemical of interest is determined by measuring this substance in human samples such as urine, blood or hair. Since the internal exposure results from multiple sources, it represents the aggregated exposure from all routes (dermal, inhalation and oral). HBM has been identified as an important tool to support policy making (Ganzleben et al., 2017) but an improved science to policy transfer is urgently needed. Therefore, the focus of this publication is on how HBM indicators may answer policy-related questions and help identifying urgent needs for chemical regulation or management.
In the context of knowledge transfer and information processing, indicators are coming to the fore as they are known to be a valuable tool to illustrate rather complex scientific information in a concise and clear manner. Indicators can contribute to an effective science-policy translation for decision makers and direct communication to stakeholders, scientists and the general public (Buekers et al., 2018).
Indicators in the context of environment and health have been derived by the World Health Organization (WHO) (WHO, 1999; WHO, 2022) and the EEA (EEA, 2014; EEA, 2018). HBM indicators at the European level are scarce – to our knowledge only a few HBM indicators are publicly available in the WHO European Health Information Gateway (WHO, 2022). These include "Mean blood lead levels of children measured in areas without significant local sources of lead exposure” and “dioxin levels in human milk in selected countries” and some other persistent organic pollutants (POPs) in human milk. At the same time the progressive development and usage of chemicals demonstrate the need for implementing further European-wide HBM indicators. This is a relevant step towards prioritization of emerging chemicals and may highlight human health risks regarding chemical exposure. Including indicators for presenting HBM data in established indicator lists, such as the ones mentioned above, may provide further information on strategy and policy development in chemicals monitoring, while at the same time, checking progress towards policy targets that have already been set (Buekers et al., 2018).
For this purpose, within HBM4EU a concept has been elaborated to derive HBM indicators (Buekers et al., 2018). Based on Eurostat (2014), we decided to differentiate between two types of indicators, which we defined as follows:
A) result indicators (formerly described by Buekers et al., 2018 as ‘HBM indicator for internal exposure’), which compare internal exposure levels between selected population groups, sexes or between regions, and.
B) impact indicators (formerly described by Buekers et al., 2018 as ‘HBM indicator for health risk’), which compare exposure levels with health-based guidance values, such as the human biomonitoring guidance values (HBM-GVs) derived within HBM4EU (Apel et al., 2020a). These values allow a health risk assessment (RA) of available HBM data based on currently available scientific knowledge. They can be directly compared with measured internal values. „The HBM-GVs derived for the general population represent the concentration of a substance or its specific metabolite(s) in human biological media (e.g., urine, blood, hair) at and below which, according to current knowledge, there is no risk of health impairment anticipated, and consequently no need for action“ (Apel et al., 2020a).
To produce robust and scientifically sound answers to the policy questions, the indicators need to be based on harmonized and quality assured data (Buekers et al., 2018). Under HBM4EU, comparable HBM data with European wide exposure coverage from different countries have been aligned and collated under the HBM4EU Aligned Studies (Gilles et al., 2021 and Gilles et al., 2022). This harmonized data set (Esteban López et al., 2021; Mol et al., 2022) has been used for further development of the indicators. Depending on which information they should provide final visualization of the indicators can be either in a number format, or as an infographic (Buekers et al., 2018).
In this publication, we present the derived European HBM indicators for one selected phthalate Diisobutyl phthalate (DiBP) in children as a case study and the non-phthalate substitute Di-isononyl cyclohexane-1,2-dicarboxylate (DINCH). Phthalates are a group of industrial chemicals that are extensively used as plasticisers. They are used in a wide range of consumer products including vinyl flooring, food contact materials, personal care products and children's toys (German HBM Commission, 2011; Silano et al., 2019). In animal studies certain phthalates were found to affect fertility and reproduction of both sexes. Developmental effects in the offspring were also observed. The prenatal exposure to some phthalates during a critical time window (i.e. late 1st to early 2nd trimester in humans) induces adverse effects summarized as “phthalate syndrome” which comprises irreversible developmental and reproductive disorders mainly in male offspring (Main et al., 2009; German HBM Commission, 2011). Children are a sensitive group with regards to the adverse effects of phthalate exposure because of their development, their surface area per body weight and their specific behavior (like hand-to mouth behavior for younger children).
Phthalates are of great societal concern as revealed under the substance prioritization carried out by HBM4EU (Ougier et al., 2021). They are widely used, toxic and present in all humans. Greenpeace has highlighted the presence of phthalates in consumer products and the potential health effects of phthalate exposure due to their endocrine disrupting effects (Greenpeace International et al., 2006). All phthalates for which indicators have been derived are included in the SIN list (i.e. Substitute It Now1) for which they were nominated from an advisory committee of NGO's working in the fields of environment, health and consumer.
Of the phthalates considered for the derivation of indicators Butyl benzyl phthalate (BBzP), Di-n-butyl phthalate (DnBP), DiBP and Diethylhexyl phthalate (DEHP) are officially recognized in the EU as endocrine disrupting chemicals and as toxic to reproduction (ECHA, 2022a). Also, Diethyl phthalate (DEP) is under assessment as an endocrine disrupting chemical (ECHA, 2022b). HBM4EU has therefore developed the health-based guidance values HBM-GV for phthalates and DINCH (Lange et al., 2021).
DINCH was put on the market in 2002 as a substitute for high molecular weight phthalates such as DEHP (German HBM Commission, 2014). It has no toxic effects to reproduction and is not an endocrine disruptor, but nephrotoxic effects have been observed in rat studies at high doses (EFSA Panel on Food Additives, 2006; German HBM Commission, 2014). For the substitute DINCH, no hazards in CLP notifications have been classified.
In the following the methodology for the HBM indicator derivation defined by Buekers et al., (2018) is summarized and the question as to whether policy–related questions can be addressed with these HBM indicators (for DiBP and DINCH) is examined.
2. Methods
During a HBM4EU workshop in Copenhagen in June 2017, a selection of substances was performed to identify substances that are relevant for the translation into HBM indicators. This was done by the application of criteria concerning relevance with regard to various aspects and data quality (see below). Consequently, the following substances were selected for indicator development: bisphenols A, F, S (BPA, BPF, BPS), some per- and polyfluoroalkyl substances (PFAS), cadmium, a number of pesticides, several phthalates and the non-phthalate substitute DINCH. Additionally, for showing the applicability of result indicators for investigating time trends data from the German Environment Specimen Bank (ESB) has been used from the HBM4EU dashboard for two aprotic solvents (N-Methyl-2-pyrrolidone; NMP and N-Ethyl-2-pyrrolidone; NEP). The above-mentioned selection criteria include a) relevance for EU policy, society and health and b) data quality, i. e. availability and comparability of data as described in the paper from Buekers et al. (2018).
Examples of EU policy relevance are the existence of a clear policy question or that the exposure to the selected substance may be a public health issue and there is a clear possibility for prevention and risk management options. Societal relevance was identified when public demand from newspapers or other lists, like the SIN list, was confirmed. Health relevance was confirmed for a substance when there was evidence of exposure for humans and an association with adverse health effects.
Concerning the criterium data quality, only substances were selected for which a) HBM data were available from European countries (at least 120 persons per study population), b) the comparability of the data was ensured due to harmonized procedures in, e.g., analytical methods. As an additional criterium it was decided to c) avoid overlaps to other existing indicator lists. Since all these criteria were met for the group of phthalates, we derived indicators for seven phthalates and a non-phthalate substitute. For the group of phthalates and DINCH, specific metabolites are measured in urine during human biomonitoring. For DiBP the measured metabolite is Mono-iso-butyl phthalate (MiBP), whereas for DINCH two metabolites were analysed, namely Cyclohexane-1,2- dicarboxylate-mono-(7-hydroxy-4-methyl)octyl ester (OH-MINCH) and Cyclohexane-1,2- dicarboxylate-mono-(7-carboxylate-4-methyl)heptyl ester (cx-MINCH). In Table 1, the two parent compounds are given together with their CAS numbers and their metabolites. For the other phthalates not presented in this publication, but for which indicators have been derived within HBM4EU, this information is given in the Supplementary Materials in Table S1.
Table 1.
Overview of the parent compounds for phthalates and DINCH and their respective metabolites investigated in this publication.
| Phthalate/DINCH compounds (acronym) | Full name | CAS-Number | Metabolite(s) investigated acronym | Full name |
|---|---|---|---|---|
| DiBP | Diisobutyl phthalate | 84-69-5 | MiBP | Mono-isobutyl phthalate |
| DINCH | Di-isononyl cyclohexane-1,2- dicarboxylate | 166412-78-8 | OH-MINCH | Cyclohexane-1,2- dicarboxylate-mono-(7-hydroxy-4-methyl)octyl ester |
| cx-MINCH | Cyclohexane-1,2- dicarboxylate-mono-(7-carboxylate-4- methyl)heptyl ester |
To show how suitable our indicators are for answering policy-related questions gathered within HBM4EU, two illustrative examples were selected. DiBP was selected to represent the indicators for those phthalates belonging to the highly regulated group. DINCH was selected to represent indicators for a non-phthalate substitute.
2.1. Available datasets for the derivation of HBM indicators
For the derivation of HBM indicators there are several requirements with regards to harmonized and quality approved data, and only a few studies have been conducted to date harmonizing HBM data at the European level.
The DEMOCOPHES project was a first HBM feasibility study in 17 European countries (Den Hond et al., 2015). DEMOCOPHES data comprises urine samples from children (6–11 years) in a selected study area (see Table S2), collected between 2011 and 2012 and are currently available in the HBM4EU data repository.2 This contains DEMOCOPHES data for DiBP from 8 countries and data for DINCH from 6 countries. DEMOCOPHES data for phthalates were quality controlled by a QA/QC scheme (Schindler et al., 2014). For the result indicators on “time patterns” (i.e. the comparison of data from two periods of time (DEMOCOPHES (2011–2012) versus HBM4EU Aligned Studies (2014–2021)) the median (50th percentile; P50) of metabolites of DiBP and DINCH were gathered from urine samples obtained from a sample of children (6–11 years) from the general population from different countries (see Table S3).
To get an overview of recent chemical exposure (sampling years 2014–2021) of European citizens, one aim under HBM4EU was to perform so called HBM4EU Aligned Studies (Gilles et al., 2021; Gilles et al., 2022). The ethics related to data and sample handling in studies included in HBM4EU was compliant with national and EU regulation as described in “Implementation and coordination of an ethics framework in HBM4EU – experiences and reflections” (Knudsen et al., 2022) (this issue). Both for DiBP and DINCH metabolites in children (6–11 years) data are available from 11 studies from the HBM4EU Aligned Studies (see Table S3). For teenagers (12–18 years) data are available from 9 studies both for DiBP and DINCH. These data were used for the comparison of age groups (see Table S4).
Chemical analysis of the biomarkers in the HBM4EU Aligned Studies was quality controlled (Esteban López et al., 2021; Mol et al., 2022) and was done for phthalates and DINCH by 7 different analytical laboratories (Esteban López et al., 2021).
Urine samples were either first morning urine samples or spot urine samples collected in the HBM4EU Aligned Studies. Urine samples taken in the frame of the DEMOCOPHES project in children were all first morning urine samples. All concentrations are given in μg/L and are not creatinine-adjusted. Since creatinine excretion is age dependent in children (German Human Biomonitoring German HBM Commission, 2005) we decided not to present the study results on a creatinine-adjusted basis. Data for specific gravity was only available for teenagers within the HBM4EU Aligned Studies (Gilles et al., 2022), and no adjustments were made to enable a better comparability of the data sets for children and teenagers. In addition, for the impact indicators a volume-based indication in μg/L of the results was necessary, since HBM-GVs are given in a volume basis in μg/L.
An overview of the corresponding studies used for the derivation of indicators including their study names, sampling years, number of participants, age range and matrix type (spot or first morning urine) for determination of the phthalates and DINCH is given in the Supplementary Materials Tables S2, S3 and S4.
2.2. Derivation of result indicators
Our result indicators compare internal exposure levels between selected population groups, sexes and regions. For illustrating exposure at the population level, the use of different percentiles is possible and we selected P50 (median) values for this comparison. Different types of result indicators have been derived for showing: 1) differences in exposure between age groups, 2) differences between geographical regions, 3) differences in exposure between boys and girls and 4) differences in exposure of different time points.
The policy-related questions to be answered by the result indicators are:
-
1.
What is the extent of the current exposure of the EU population, especially children, to DiBP and the non-phthalate substitute DINCH?
-
2.
Do the exposure levels differ between the studies from different countries?
-
3.
Is there a difference in internal exposure between boys and girls?
-
4.
Is there a difference in internal exposure between different age groups?
-
5.
Are there indications for an increase or decrease in internal exposure for DiBP and DINCH?
2.2.1. Calculation of result indicators 1) to 3) for geographical differences, sex differences and age differences
To show differences in internal exposure in the format of our result indicators, P50 values have been calculated from the data from the HBM4EU Aligned Studies (2014–2021). A difference in exposure has been defined when no overlap of the 95% confidence intervals between the P50 values of the different geographical regions/sexes/age groups was found. When an overlap was observed, no significance could be stated as further statistical tests should be performed. The P50 values and 95% confidence intervals for the calculated result indicators are given in the Supplementary Materials in Tables S6–S13. Meanwhile statistical analyses of the individual data of the HBM4EU Aligned Studies have been performed (Vogel et al., 2022a (this issue), Martinsone et al. (2022 in preparation)). Therefore, we decided to refer in the results sections to the outcomes from these statistical analyses.
For the indicator on sex differences, only 10 studies were available since for one study the number of participants was low, and no stratification could be made for sex (i.e., no P50 or P95 data for this study are available in the data repository).
2.2.2. Calculation of result indicators 4) for “time patterns”
For this indicator, P50 values of phthalate metabolites for children (6–11 years) from the HBM4EU Aligned Studies (2014–2021) were compared to P50 values from the previous conducted DEMOCOPHES project (2011–2012). A difference in exposure was defined when no overlap of the 95% confidence intervals between P50 internal exposure values of the different studies was seen. An analogous indicator was also derived for the non-phthalate substitute DINCH. To give an overview on P50 values for the two periods of time, all the available data from the HBM4EU repository are shown and this resulted in 8 studies with data for DiBP and 6 studies with data for DINCH. Since only data from countries with studies available for both time periods have been compared, the number of studies for this comparison was 4 for DiBP and 3 for DINCH.
2.3. Derivation of impact indicators
As impact indicators directly evaluate the internal exposure to a substance within a health risk context, the importance of having HBM-GVs is coming to the fore. HBM-GVs have been derived for selected substances under HBM4EU, and specifically for phthalates HBM-GVs have been derived for 5 phthalates and DINCH for the general population for the following two population groups: 1) children (6–13 years) and 2) adolescents, adults (from 14 years onwards) (Lange et al., 2021). The HBM-GVs for children for DiBP and DINCH, the sensitive endpoint on which the HBM-GV derivation was based and the level of confidence (LoC) evaluating the data that has been used for the derivation are presented in Table S5 in the Supplementary Materials. The P95 values (and 95% confidence intervals) for the impact indicator calculation are given in the Supplementary Materials for all graphs (Tables S14 and S15).
The policy-related questions to be answered by the impact indicators are:
-
1.
Is the exposure to phthalates, like DiBP and their substitutes of health-relevance for the general population and vulnerable groups e.g. children?
-
2.
In which countries does exposure exceed the HBM-GV for children?
2.3.1. Calculation of impact indicators
Two types of HBM impact indicators have been derived. The first one is the „percentage of sample (i.e., individuals) exceeding the HBM-GV (called PE), and the second is the „extent of exceedance“ on a sample level (called EE). As impact indicators evaluate exposure within a health risk context, it was decided to compare the HBM-GVs with the P95 values of the measured concentrations. A P95 can be interpreted as 95% of the study participants having an internal concentration equal or below this value. All values are given in μg/L. The P95 have been calculated with RStudio Team (2022).
-
1)
The percentage of population exceeding the HBM-GV (PE) can be calculated as follows:
| PE = n/N x 100% |
Where n is the number of samples/participants with HBM exposure levels above the HBM-GV (n > HBM-GV) and N is the total number of samples/participants participating in the study. Thus, the PE describes the percentage in a given population that exceeds the HBM-GV.
-
2)
The extent of exceedance (EE) describes the extent by which the HBM-GV is exceeded by the 95th percentile (P95). It is calculated by dividing the P95 percentile by the HBM-GV:
| EE = P95/HBM-GV |
A value > 1 means that the P95 exceeds the HBM-GV. Below 1 there is no exceedance by the P95. Thus, the EE describes the factor of exceedance or non-exceedance of the P95.
2.4. Visualization of indicators
For the visualization of the result and impact indicators, the data have been graphed using the R statistical analysis software package (R Core Team, 2018). These graphs show P50 and P95 as bars and the HBM-GVs as dotted lines.
For the result indicators, the indicators were stratified according to the selected parameters (e.g. sex and age). For the geographical comparison the studies from the respective countries have been assigned to one of the four regions according to the United Nations geoscheme in the HBM4EU Aligned Studies (United Nations, 1999,) (, i.e. North, South, East and West.
The graphs showing the impact indicator with the focus on illustrating the percentage of participants exceeding the HBM-GV have been plotted using Microsoft Excel (version 2019).
2.5. Workshop on policy uptake of HBM4EU results – a practical reality check
The workshop policy uptake of HBM4EU results was held in virtual format on 30–31 May 2022. Participants were experts from the HBM4EU project as well as policymakers from the European Commission. The purpose of this workshop was to present the highlights and main messages based on HBM4EU findings in response to policy questions and needs, in which the results for phthalates and DINCH were discussed. Topics that were discussed included: determination of main highlights, reflection of policy needs by results presented, addressees for further action in regulation and policy making, expectations towards the practices in risk assessment, management, regulation, policy, awareness raising and communication initiatives.
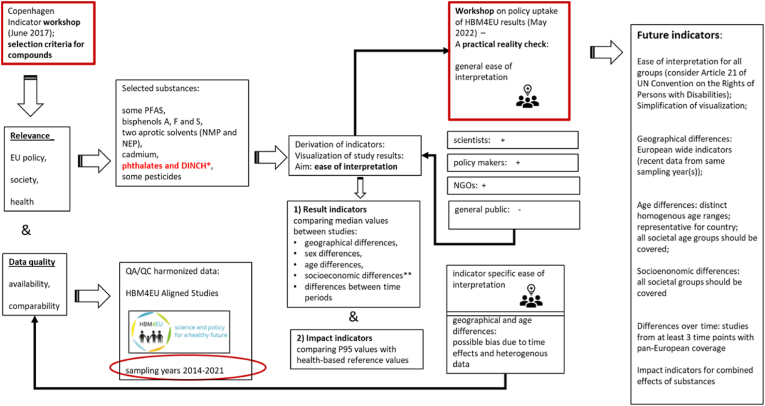
In Fig. 1, the process of indicator development within HBM4EU is presented.
Fig. 1.
Development of HBM indicators within HBM4EU. *phthalates and DINCH were used to exemplify the process of indicator development. **there were not sufficient data to derive this indicator for phthalates and DINCH.
3. Results
Within the HBM4EU Aligned Studies most of the phthalates including DiBP could be detected in the vast majority of samples with the percentage of values above the limit of quantification (LOQ) ranging from 90 up to 100% in children for the corresponding metabolites of the phthalates where indicators have been derived. This demonstrates the ubiquitous exposure of children in Europe to phthalates. Furthermore, the substitute DINCH could be detected in almost all samples (with the percentage of values for the corresponding metabolites that were above LOQ ranging from 96 up to 99% in children).
In the following sections, our HBM indicators are presented using DiBP as a case example for a regulated phthalate and DINCH as a non-phthalate substitute. The indicators will be further analysed in the Discussion.
Under HBM4EU indicators for other selected substances (including other phthalates) have been developed. They will be presented on the HBM4EU website.3
3.1. Result indicators
Result indicators illustrate differences in the internal exposure between geographical regions, sex, age, and time.
3.1.1. Result indicator for geographical differences
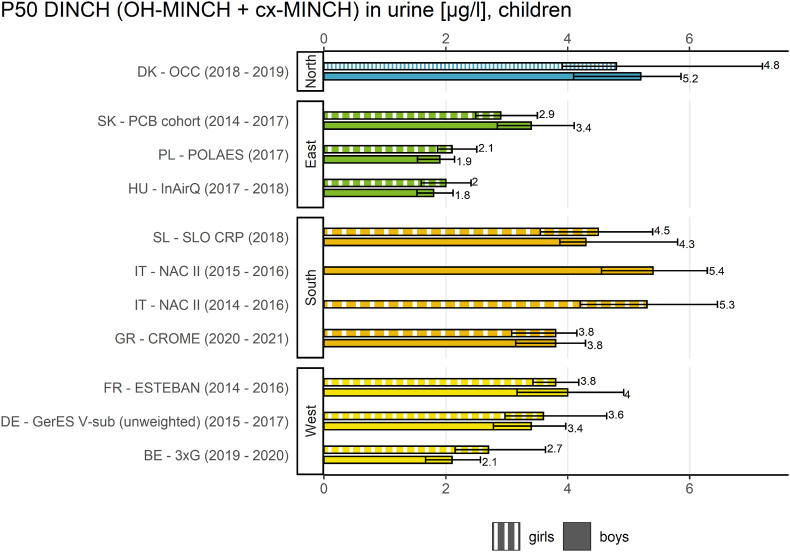
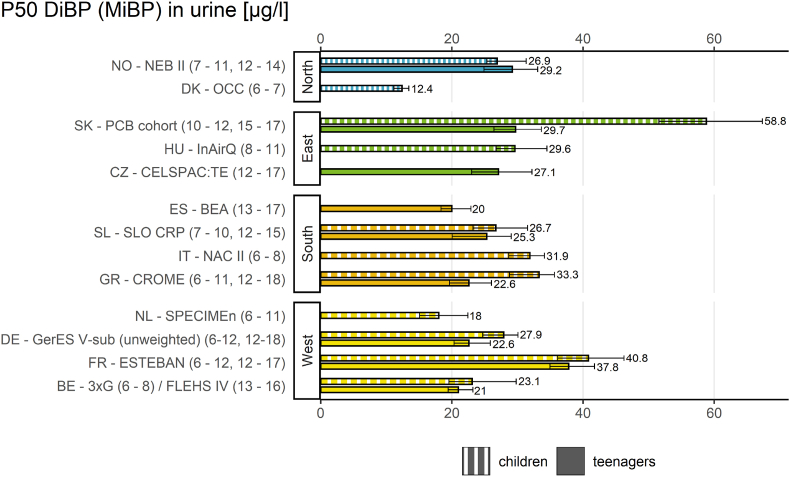
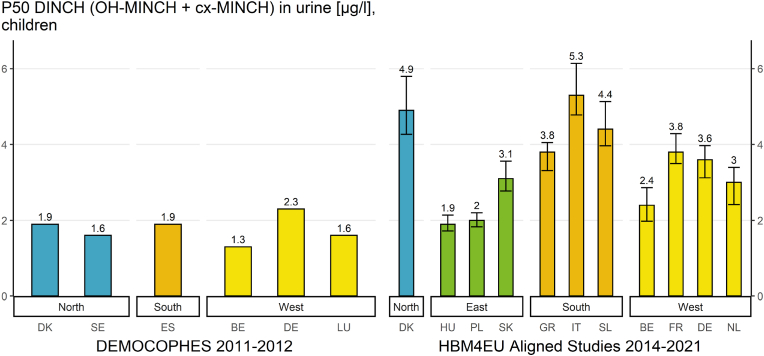
In Fig. 2 the result indicator for DiBP in children is shown for the different geographical regions. In Fig. 3 this indicator is shown for DINCH in children.
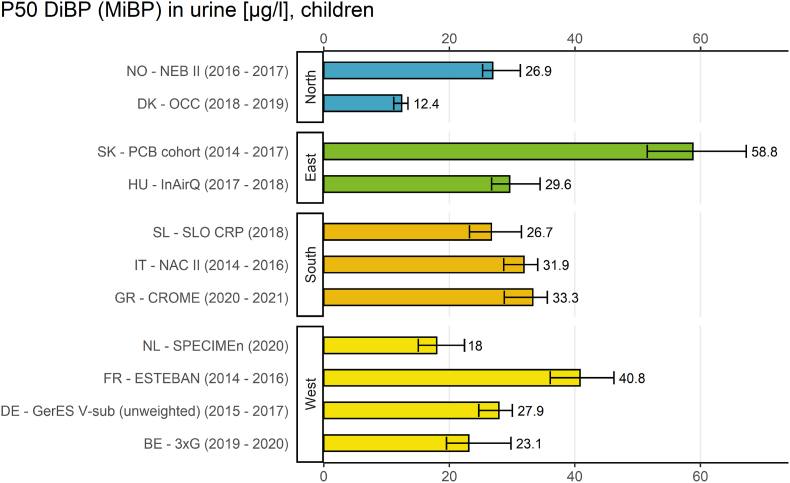
Fig. 2.
Result indicator for geographical differences of P50 values (and 95% confidence intervals) in DiBP exposure (MiBP in μg/L) in children (6–11 years) in the HBM4EU Aligned Studies. Country names, study names and sampling years (in brackets) are given. DiBP metabolite levels were either measured in first morning or random spot urine samples.
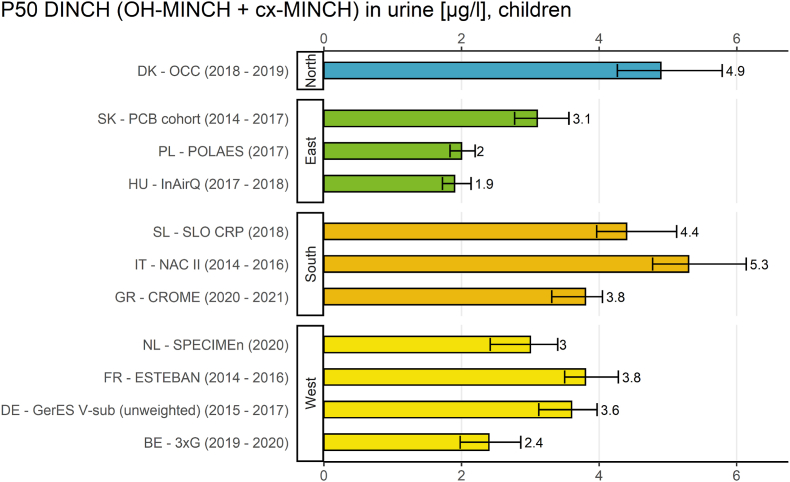
Fig. 3.
Result indicator for geographical differences of P50 values (and 95% confidence intervals) for DINCH exposure (∑(OH-MINCH + cx-MINCH) in μg/L) in children (6–11 years) in the HBM4EU Aligned Studies (collected in the years 2014–2021). Country names, study names and sampling years (in brackets) are given. DINCH metabolite levels were either measured in first morning or random spot urine samples.
The result indicator for geographical differences (Fig. 2) provides an overview of internal exposure to DiBP in European children (6–11 years) from studies in 11 European countries between 2014 and 2021 by plotting the P50 values of MiBP.
Regarding geographical differences, the exposure of children towards DiBP metabolites was highest in an eastern European study (Slovakia, PCB cohort study, 2014–2017) and lowest in a northern European study (Denmark, OCC study, 2018–2019). The P50 values differed more between the sampling sites than between regions. Also Vogel et al. (2022a) (this issue) found no differences between the regions in urinary metabolite concentrations of DiBP in multivariate analyses. The result indicator for geographical differences of DiBP metabolite concentrations in children showed that P50 values varied by a factor of almost 5 (4.7) between the sampling sites. It must be noted, that the MiBP measurements in PCB-cohort study from Slovakia were not quality assured within HBM4EU (Govarts et al., 2022) (this issue), therefore comparability cannot be guaranteed (see Esteban López et al., 2021).
The result indicator for geographical differences (Fig. 3) provides an overview of the internal exposure to DINCH of European children (6–11 years) from studies in 11 European countries between 2014 and 2021 by plotting the P50 values of the sum of OH-MINCH and cx-MINCH.
Regarding geographical differences, the exposure of children towards the sum of the two DINCH metabolites was highest in one southern European study (Italy, NAC II study, 2014–2016) and lowest in one eastern European study (Hungary, InAirQ study, 2017–2018). The P50 values for DINCH metabolites were also higher in the south compared to the east. The P50 values for DINCH metabolites were also higher in the northern region (but represented by only one country) than in the eastern region. This was statistically confirmed by Vogel et al. (2022a) (this issue) who also found further differences between the regions in multivariate analyses.
The P50 values varied by a factor >2 (2.76) between the sampling sites.
3.1.2. Result indicator regarding sex differences
Result indicators on sex differences for children are shown in Fig. 4 for DiBP and in Fig. 5 for DINCH.
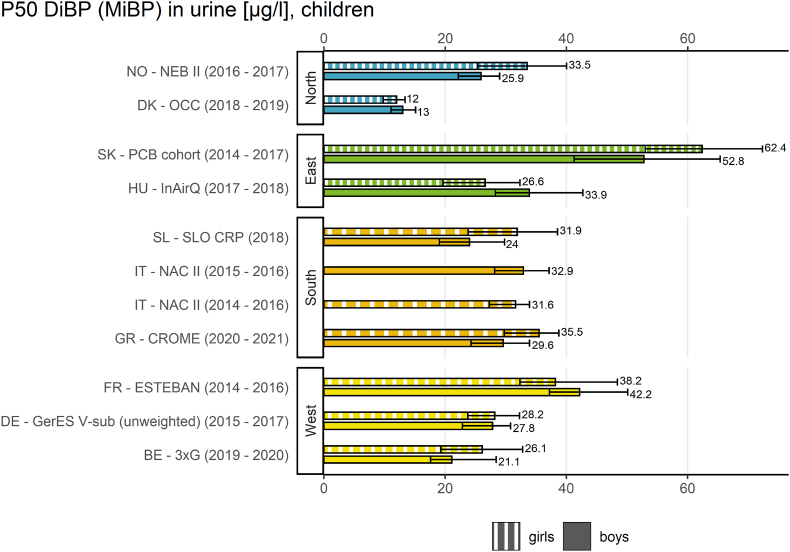
Fig. 4.
Result indicator regarding sex differences of P50 values (and 95% confidence intervals) of DiBP exposure (MiBP in μg/L) in children (6–11 years) in the HBM4EU Aligned Studies (collected in the years 2014–2021). Country names, study names and sampling years (in brackets) are given. DiBP metabolite (MiBP) levels were either measured in first morning or random spot urine samples.
Fig. 5.
Result indicator regarding sex differences of P50 values (and 95% confidence intervals) of DINCH exposure ((∑(OH-MINCH + cx-MINCH) in μg/L) in children (6–11 years) in the HBM4EU Aligned Studies (collected in the years 2014–2021). Country names, study names and sampling years (in brackets) are given. DINCH metabolite levels were either measured in first morning or random spot urine samples.
The result indicator regarding sex differences (Fig. 4) provides an overview of the internal exposure to DiBP of European boys and girls (6–11 years) from studies in 10 European countries between 2014 and 2021 by plotting the P50 values of MiBP.
The result indicator for sex differences showed that boys and girls were similarly exposed to DiBP. This was statistically confirmed by Martinsone et al. (2022, in preparation).
This result indicator regarding sex differences (Fig. 5) provides an overview of the internal exposure to DINCH of European boys and girls (6–11 years) from studies in 10 European countries between 2014 and 2021 by plotting the P50 values of the sum of OH-MINCH and cx-MINCH.
The result indicator for sex differences showed that boys and girls were similarly exposed to DINCH. This was statistically confirmed by Martinsone et al., (2022 in preparation).
3.1.3. Result indicator regarding age differences
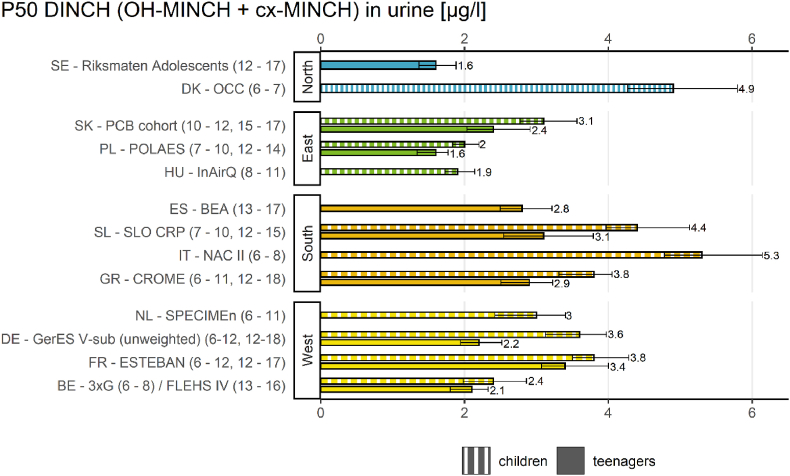
Result indicators on age differences in children are shown for DiBP in Fig. 6 and for DINCH in Fig. 7.
Fig. 6.
Result indicator regarding age differences of P50 values (and 95% confidence intervals) of DiBP exposure (MiBP in μg/L) in children (6–11 years) and teenagers (12–18 years) in the HBM4EU Aligned Studies (collected in the years 2014–2021) measured in 11 countries in children and in 9 countries in teenagers. Country names, study names and age range of participants (in brackets) are given. DiBP metabolite levels were either measured in first morning or random spot urine samples.
Fig. 7.
Result indicator regarding age differences of P50 values (and 95% confidence intervals) of DINCH exposure (∑(OH-MINCH + cx-MINCH) in μg/L) between children (6–11 years) and teenagers (12–18 years) in the HBM4EU Aligned Studies (collected in the years 2014–2021) measured in 11 countries in children and 9 countries in teenagers. Country names, study names and age range of participants (in brackets) are given. DINCH metabolite levels were either measured in first morning or random spot urine samples.
The result indicator on age differences (Fig. 6) provides an overview of the internal exposure to DiBP of European children (6–11 years) and teenagers (12–18 years). These were based on 11 studies in children and 9 studies in teenagers between 2014 and 2021 by plotting P50 values of MiBP.
Regarding the data from the HBM4EU Aligned Studies, the exposure towards DiBP metabolites was in a similar range in children (6–11 years) and teenagers (12–18 years) in most studies. This was statistically confirmed by Vogel et al. (2022a) (this issue). However, when the authors investigated the effect of age in years, decreasing levels of DiBP metabolites were found with increasing age.
Higher exposure in children was also observed compared to teenagers for studies from Slovakia and Greece, based on no overlap of 95% confidence intervals (Table S9). The highest exposure values for DiBP were found for children in the PCB cohort study from Slovakia. In Slovakia however, the samples in children were taken in 2014–2017 whereas samples in teenagers were taken in 2019–2020.
The result indicator regarding age differences (Fig. 7) provides an overview of the internal exposure to DINCH of European children (6–11 years) and teenagers (12–18 years) from 11 studies in children and 9 studies in teenagers between 2014 and 2021 by plotting P50 values of the sum of OH-MINCH and cx-MINCH.
The result indicator for age differences revealed that exposure towards ΣDINCH metabolites was higher in children than in teenagers in most studies, therefore an age difference can be confirmed, resulting in higher exposure for this sub-group of children. This age difference for DINCH exposure was statistically confirmed by Vogel et al. (2022a) (this issue).
3.1.4. Result indicator for “time pattern” regarding different periods of time
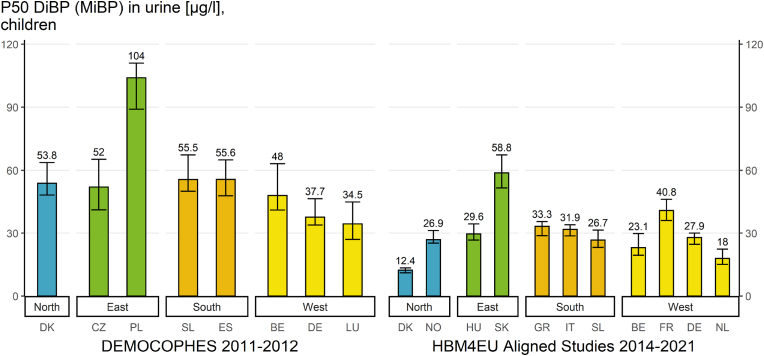
Result indicators on differences in phthalate and DINCH exposure for different periods of time have been developed (the so-called “time pattern” indicator). These indicators are shown in Fig. 8 for DiBP and in Fig. 9 for DINCH from children samples of the HBM4EU Aligned Studies.
Fig. 8.
Result indicator regarding different periods in time showing differences of P50 values of DiBP exposure (MiBP in μg/L). Direct comparison of DiBP exposure from two projects in different time periods in Europe: 1) in children (6–11 years) from 8 studies between 2011 and 2012 (DEMOCOPHES project) and 2) 11 studies in children between 2014 and 2021 (HBM4EU Aligned Studies). DiBP metabolite (MiBP) levels were either measured in first morning or random spot urine samples.
Fig. 9.
Result indicator regarding different periods in time in showing differences of P50 values of DINCH exposure (sum OH-MINCH + cx-MINCH in μg/L). Direct comparison of DINCH exposure from two projects in different time periods in Europe: 1) in children (6–11 years) from 6 studies between 2011 and 2012 (DEMOCOPHES project) and 2) 11 studies between 2014 and 2021 (HBM4EU Aligned Studies). DINCH metabolites (OH-MINCH and cx-MINCH) levels were either measured in first morning or random spot urine samples. 95% confidence intervals are only shown for the HBM4EU Aligned Studies as for DEMOCOPHES confidence intervals for the sum of the metabolites were not given for the aggregated data.
The result indicator regarding different periods in time (Fig. 8) provides an overview of the internal exposure to DiBP in European children (6–11 years) for two specific periods in time by plotting P50 MiBP values.
For the first time period, differences in exposure to DiBP metabolites in children from 8 countries based on data of the DEMOCOPHES project and collected in the years 2011–2012 are presented. For the second time period, the exposure of children from 11 European countries conducted under the HBM4EU Aligned Studies between 2014 and 2021 are shown.
Differences in exposure between the studies can be observed with P50 values ranging from 34.5 up to 104 μg/L in the DEMOCOPHES project (values varied by a factor of 3) and P50 values ranging from 12.4 up to 58.8 μg/L in the HBM4EU Aligned Studies (values varied by a factor of 4.7).
Data from 4 countries are available for both periods of time (i.e., DK, SL, BE and DE). The exposure of children to DiBP metabolites in these studies was lower in the more recent data from 2014 to 2021 compared to earlier data from 2011 to 2012, indicating a decrease in exposure over time These findings are based on visual comparison of an overlap or non-overlap of the confidence intervals (Table S11). Decreasing concentrations for DiBP in young adults were statistically confirmed by (Vogel et al., 2022b) (this issue) in a time trend study assessing data from Denmark and Germany.
The result indicator regarding different periods in times (Fig. 9) provides an overview of the internal exposure based on the sum of the DINCH metabolites OH-MINCH and cx-MINCH in children (6–11 years) for two specific periods in time periods by plotting P50 values. It compares the summed DINCH metabolites levels in children from 6 European countries collected under the DEMOCOPHES project (2011–2012) with data from children in 11 countries from the HBM4EU Aligned Studies (2014–2021).
For the sum of DINCH metabolites data for both periods of time are available from 3 countries (i.e., Denmark (DK), Belgium (BE) and Germany (DE)). The levels of DINCH metabolites in children were higher in samples from DK, BE and DE collected between 2014 and 2021 compared to samples collected in 2011–2012. These findings are based on visual comparison of an overlap or non-overlap of the confidence intervals (Table S12). Vogel et al., (2022b) (this issue) statistically confirmed increasing DINCH levels in young adults in a time trend study assessing data from Denmark and Germany.
3.2. Impact indicators
In the following section two types of impact indicators have been derived, as they are described in section 2.3.1.
-
1)
Percentage of population exceeding the HBM-GV (PE): comparison of P95 with the corresponding HBM-GV; 2) Extent of exceedance (EE): percentage participants exceeding the corresponding HBM-GV.
3.2.1. Impact indicator for DiBP exposure
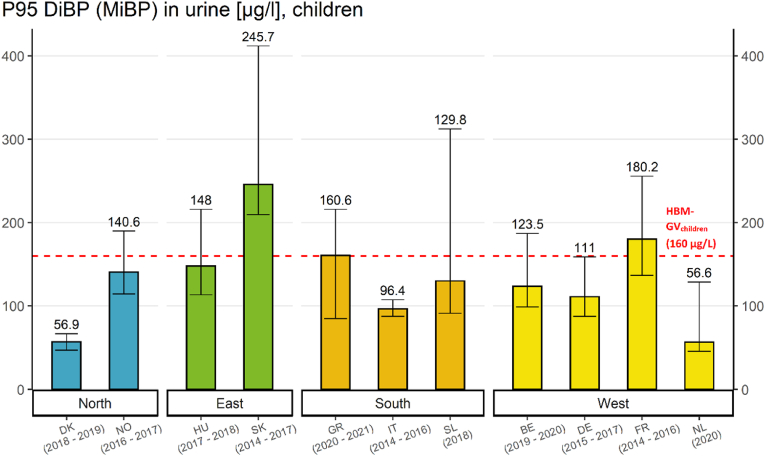
The impact indicator (Fig. 10) provides an overview of the P95 MiBP (biomarker for DiBP) levels in children (6–11 years) from the 11 European studies and the corresponding HBM-GV of 160 μg/L (Lange et al., 2021) for comparison.
Fig. 10.
Impact indicator for DiBP in children (P95 MiBP levels in μg/L with their corresponding 95% confidence intervals) in urine samples from children (age 6–11 years) from different European regions compared with the HBM-GV. The dotted line indicates the HBM-GVchildren of 160 μg/L. Studies are part of the HBM4EU Aligned Studies in children. Country names and sampling years (in brackets) are given. In three of these studies (i.e. Slovakia, Greece and France), P95 values exceed this guidance value, representing 5% of the most highly exposed children.
Since this indicator is intended to be used to estimate health risks, the complete set of results are presented in Table 2.
Table 2.
Impact indicators for DiBP in children (HBM4EU Aligned Study data).
| Study name, Country | N | P95 [μg/L] | Number of participants exceeding the HBM-GVchildren | Percentage of participants exceeding the HBM-GVchildren [%] | Extent of exceedance [P95/HBM-GVchildren] |
|---|---|---|---|---|---|
| OCC, Denmark | 300 | 56.9 | 0 | 0 | 0.36 |
| NEB II, Norway | 300 | 140.6 | 13 | 4.3 | 0.88 |
| InAirQ, Hungary | 262 | 148.0 | 11 | 4.2 | 0.92 |
| PCB cohort, Slovakia | 296 | 245.7 | 36 | 12.2 | 1.54 |
| CROME, Greece | 161 | 160.6 | 9 | 5.6 | 1.00 |
| NAC II, Italy | 299 | 96.4 | 5 | 1.7 | 0.60 |
| SLO CRP, Slovenia | 149 | 129.8 | 4 | 2.7 | 0.81 |
| 3xG, Belgium | 133 | 123.5 | 3 | 2.3 | 0.77 |
| ESTEBAN, France | 286 | 180.3 | 17 | 5.9 | 1.13 |
| GerES V, Germany | 300 | 111.1 | 8 | 2.7 | 0.69 |
| SPECIMEn-NL, The Netherlands | 89 | 56.6 | 1 | 1.1 | 0.35 |
N = number of participants.
In all but one study, at least some of the children were observed to have DiBP levels larger than the HBM-GVchildren of 160 μg/L (see Table 2). In these cases, a risk for adverse health effects cannot be excluded.
The extent of exceedance (EE) (i.e., P95/HBM-GV) in the different studies and locations ranges from 0.35 up to 1.54, meaning that the HBM-GV for DiBP was exceeded at the P95 of the population only in the case of Slovakia (54%) and France (13%). The studies with the largest extent of exceedance for DiBP exposure were the PCB cohort study from Slovakia (1.54) and the ESTEBAN study from France (1.13) (see Table S15). This shows that exposure to DiBP in France and Slovakia exceeded established health-based guidance values for DiBP. It has to be noted, that both studies were conducted prior to the other studies which were completed more recently. As regulation might have been effective over this time, this time difference might have influenced these results.
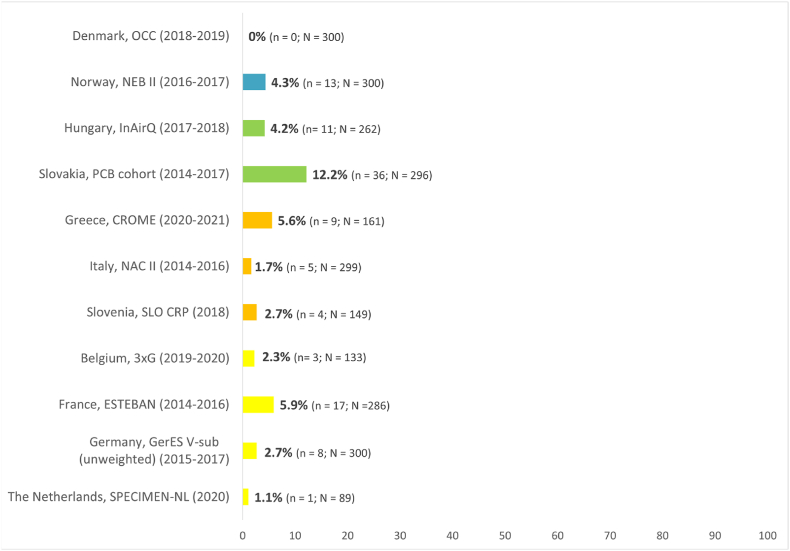
The “percentage of population exceeding the HBM-GV (PE)” is provided in Fig. 11.
Fig. 11.
Impact indicator for DiBP in children showing the percentage of children exceeding the HBM-GVchildren for DiBP of 160 μg/L. The sampling years are given in brackets after the study names. The different regions are highlighted by specific colours (north: blue, east: green, south: orange, west: yellow). n = number of samples/participants exceeding the HBM-GV, N = total number of samples/study participants. . (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
In cases where the HBM-GVs were exceeded, based on current knowledge a risk for adverse health effects cannot be excluded for part of the population.
3.2.2. Impact indicator for DINCH exposure
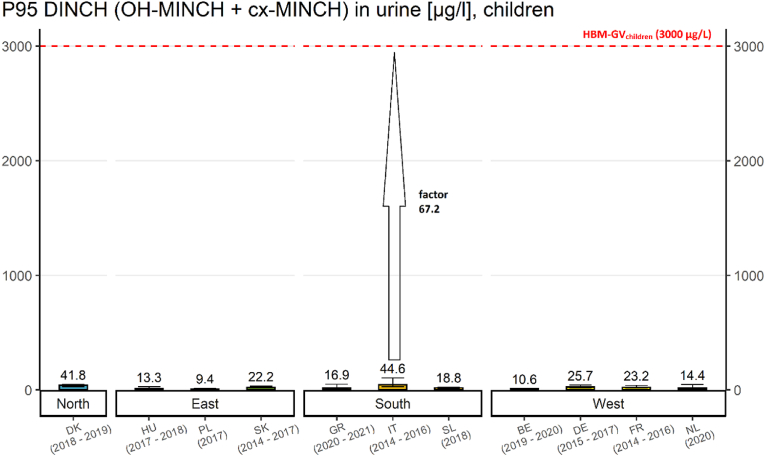
This impact indicator (Fig. 12) provides an overview of the P95 ΣDINCH metabolite levels in children (6–11 years) from 11 studies in Europe compared to the corresponding HBM-GV of 3000 μg/L (Lange et al., 2021).
Fig. 12.
Impact indicator for DINCH exposure in children (P95 ∑OH-MINCH + cx-MINCH in μg/L and their correspong 95% confidence intervals) in urine samples from children (age 6–11 years) from different European regions compared with HBM-GVchildren. The dotted line indicates the HBM-GVchildren of 3000 μg/L. Country names and sampling years (in brackets) are given.
The impact indicator for DINCH exposure in children showed P95 values that were considerably lower than the HBM-GVchildren of 3000 μg/L derived within HBM4EU in all considered countries. This indicates that there is no health concern regarding DINCH exposure for children based on current data.
The extent of exceedance (EE) in the different studies and locations ranges from 0.003 up to 0.015 (see Table 3).
Table 3.
Impact indicators for DINCH in children (HBM4EU Aligned Study data).
| Study name, Country | N | P95 [μg/L] | Number of participants exceeding the HBM-GV | Percentage participants exceeding the HBM-GV [%] | Extent of exceedance [P95/HBM-GVchildren] |
|---|---|---|---|---|---|
| OCC, Denmark | 300 | 41.8 | 0 | 0 | 0.014 |
| InAirQ, Hungary | 262 | 13.3 | 0 | 0 | 0.004 |
| POLAES, Poland | 300 | 9.4 | 0 | 0 | 0.003 |
| PCB cohort, Slovakia | 300 | 22.2 | 0 | 0 | 0.007 |
| CROME, Greece | 161 | 16.9 | 0 | 0 | 0.006 |
| NAC II, Italy | 300 | 44.6 | 0 | 0 | 0.015 |
| SLO CRP, Slovenia | 149 | 18.8 | 0 | 0 | 0.006 |
| 3xG, Belgium | 133 | 10.6 | 0 | 0 | 0.004 |
| ESTEBAN, France | 286 | 23.2 | 0 | 0 | 0.008 |
| GerES V, Germany | 299 | 25.7 | 0 | 0 | 0.009 |
| SPECIMEn-NL, The Netherlands | 89 | 14.4 | 0 | 0 | 0.005 |
N = number of participants.
The impact indicator for DINCH exposure in children showed no exceedance of the HBM-GV in all considered studies. Based on current knowledge, no health effects are expected for DINCH exposure in children from these findings.
3.3. Results from workshop on policy uptake of HBM4EU results
Within the framework of HBM4EU, a workshop on „Policy uptake of HBM4EU results“ took place on 30th and March 31, 2022. During this workshop it became clear that the indicators need to be further simplified to increase general understanding. In the current state, the indicators are well suited for answering policy-related questions, but further improvement and simplification is needed to make them accessible for the general population.
Some of the highlights mentioned at the workshop were related to availability of information on source contribution, such as exposure determinants which would be relevant for policymakers and risk assessors, and to a rapid response mechanism.
4. Discussion
This paper illustrates the usefulness of the result and impact indicators for human biomonitoring in answering policy-related questions regarding DiBP and DINCH exposure and outlines strategies for their future development. Due to the European Chemicals Regulation, decision makers need a transparent and easy interpretable way of setting new research needs or further actions for chemicals. Results from the HBM4EU Aligned Studies and the previously conducted DEMOCOPHES project provided information on the chemicals exposure burden at the European level. By preparing indicators, these study results can be used to provide direct answers to policy-related questions and are thus a valuable tool for decision making. Policy needs can already be identified by applying our indicators.
4.1. Result and impact indicators aspects
The result indicator on geographical differences for DiBP and DINCH exposure visualizes the extent of exposure for DiBP and DINCH of children in different European regions. For both DiBP and DINCH differences in exposure burdens were observed between the different studies. For DiBP, the P50 values differed a factor of five between the highest P50 concentrations compared to the lowest values. For ΣDINCH metabolites, the differences between the highest and lowest P50 values were somewhat smaller at a factor of 2.7. Regional differences were also found within DEMOCOPHES (Den Hond et al., 2015). In the HBM4EU Aligned Studies, the observed differences between the geographical regions are higher than those observed in the DEMOCOPHES project. As the HBM4EU Aligned Studies cover the period between 2014 and 2021, and several studies have shown a decreasing time trend for phthalates and an increasing one for DINCH (e.g., Lemke et al., 2021), the described exposure differences in the geographical regions might be biased by the factor of time. This effect could be confirmed by a recent study from the German Environmental Specimen Bank showing a continuous decrease in phthalate concentrations in the years 2014–2021 (Kasper-Sonnenberg et al., 2022 in preparation). Such a possible “time trend effect” within the Aligned Studies due to the large time span of the sampling years is also discussed by (Gilles et al., 2022) . The authors recommend a shortening of sampling periods to three-year sampling periods. For phthalates and DINCH, since they are short lived chemicals, samples for these compounds should preferably be taken within the same sampling year to increase the robustness of this indicator.
Nevertheless, the result indicator on geographical differences provides information that country or regional differences exist and highlights the need to find the sources of these differences. Reasons for different exposure patterns between countries may be related to country-specific behavioral patterns, differences in the regional presence of a naturally occurring substance or differences in product placement of regional markets. These exposure determinants may help in setting targeted actions for policy makers.
The result indicator on sex differences for DiBP and DINCH exposure in children visualizes the extent of exposure for DiBP and DINCH of European boys and girls. The indicator shows that internal exposure for DiBP and DINCH of boys and girls from the respective regions were in a similar concentration range. Since no major differences were observed for the sexes based on current data, no political measures are currently needed to lower the exposure of one sex or the other. Despite these results, our result indicator on sex differences may help in identifying the highly exposed sex for a respective substance. Having identified a sex difference in exposure, reasons for this should then be identified via exposure determinant studies (e.g. Martinsone et al., 2022 (in preparation)). If in other cases, differences between sexes are shown by the indicators, those differences might be related to variations in lifestyle behavior or physiological differences related to metabolism. If information is available it can help to prioritize further steps in chemical regulation or targeted education of product use for consumers.
The result indicator for age differences for DiBP showed that exposure to DiBP does not differ between children (6–11 years) and teenagers (12–18 years) in most studies. Vogel et al. (2022a) (this issue) analysed the individual data from HBM4EU and confirmed these findings for DiBP. However, when the authors used single years of age instead of the selected age groups of children and teenagers, they found lower levels for DiBP with increasing age. In contrast to our findings, differences in phthalate metabolite concentrations for different age groups have been reported in several studies, both when comparing children and adults but also for children and adolescents of different age groups (Bastiaensen et al., 2021; Den Hond et al., 2015; Schwedler et al., 2020a; Silva et al., 2004). In the DEMOCOPHES project, levels of phthalate metabolites were in general higher in children than in their mothers. Furthermore, younger children (5–8 years) had higher levels of phthalate metabolites compared to older children (9–11 years) (Den Hond et al., 2015). In a review paper from Choi et al. (2017), the authors found that children generally have higher body burdens of phthalate metabolites, with the exception of DEP. DEP is often present in cosmetics and a major part of the exposure to DEP is via personal care products (Wormuth et al., 2006), which might explain the higher exposure in the group of teenagers. One reason why an age dependency across all studies could not be shown for DiBP with the HBM4EU Aligned Studies might be due to the wide distribution of ages in the single studies (Gilles et al., 2022). In the HBM4EU children's group not all ages are represented equally. The same is the case for teenagers. Further the age range with children aged 6–11 years and teenagers aged 12–18 years also includes some “overlapping” of 12-year-old in the group of children (i.e. 3.9% of the children were 12 years old (Gilles et al., 2022). This had happened since some 11 year old children turned 12 in the course of the respective studies. Furthermore, very young children (<6 years) were not included. Since aggregated data were used for the derivation of the indicator, other age groups could not be constructed out of the available data. For infants and toddlers, different behavior patterns like crawling on the floor and hand-to-mouth behavior are well known. Also, the food intake in comparison to the body weight is much higher for the very young age groups (US EPA, 2011). Teenagers, on the other hand, are exposed via their usage of specific personal care products (Wormuth et al., 2006). Not all studies within the HBM4EU Aligned Studies provided data for both children and teenagers. Another influencing factor that may lead to an over- or underestimation is the wide range of sampling years (as has already been discussed above for the indicator on geographic differences). Here, time trend studies for both children and teenagers would be needed to adjust for possible time effects.
For DINCH, our result indicators on age differences revealed higher exposure of children compared to teenagers. An age dependency of DINCH exposure was also observed by Schwedler et al. (2020b), who reported HBM data from the German Environmental Survey of Children and Adolescents (GerES V, 2014–2017). The authors reported the highest geometric mean (GM) biomarker concentrations for the youngest children, while the concentrations decreased with increasing age. DINCH biomarker levels in children (3–5 years) were almost 3-fold higher than in teenagers (14–17 years) (Schwedler et al., 2020b). DINCH has been developed for sensitive applications, such as children's toys, as young children likelyhave the closest contact with these. This assumption of increased exposure to toys could be confirmed by our indicator in the group of children.
To summarize, the result indicator for age differences is suitable to highlight potential differences in exposure for different age groups. For DINCH and DiBP (in two studies) a higher exposure of children could be shown. The next step would be to determine the reasons for this higher exposure. Age differences may be related to differences in lifestyle behavior or differences in metabolism. Those reasons are relevant to know for taking further steps in chemical risk management.
Further, with our indicators on age differences, we could demonstrate the necessity of a valid database for the derivation of this indicator. Comparable data are a prerequisite for mapping existing differences.
To inform on temporal trends, the HBM4EU Aligned Studies were compared to the earlier DEMOCOPHES project (2011–2012). This so-called “time pattern” indicator revealed lower levels of phthalate biomarkers and higher levels of DINCH biomarkers for children in the HBM4EU Aligned Studies. A decrease in DiBP metabolite levels (and metabolites of other regulated phthalates) in children and adolescents over time was also observed by Schwedler et al. (2020a). This decrease over time might largely be due to regulatory measures such as restrictions and regulations in consumer products being in place and being effective, as was also confirmed in time trends from the German Environmental Specimen Bank (Koch et al., 2017; Apel et al., 2020b) and studies from other European countries (e.g. Frederiksen et al., 2020; Bastiaensen et al., 2021).
DINCH data from the literature also reported an increasing time trend of DINCH biomarkers (e.g. Kasper-Sonnenberg et al. (2019), Lemke et al. (2021) and Schwedler et al. (2020b)). Lemke et al. (2021) reported that substitutes mimic the exposure behavior of REACH regulated phthalates. As a shift to non-regulated phthalates or substitutes (such as DINCH) takes place, an increase in measured concentrations for these substitutes can be followed. Vogel et al., 2022b (this issue) confirmed an increasing time trend for DINCH when analyzing HBM data from Denmark and Germany, including samples collected at different time points. The "time pattern” indicator is suitable for revealing differences in internal exposure over time. If lower concentrations are observed, this might be an indication that political measures were successful and effective, or that consumer use is decreasing or that maybe industry has changed their application. These changes in exposure need to be analysed to set priorities for further actions.
The impact indicators on health relevance of DiBP and DINCH exposure in children and teenagers confirmed their relevance for comparing exposure levels to existing guidance values and enable policy makers to set priorities for further actions. The impact indicators showed that even when single substances are assessed, the HBM-GV is exceeded for DiBP in some regions by a considerable number of participants even in the most recently collected data. This is a flag for risk managers and policy makers that exposure to DiBP still is a relevant health issue.
Despite regulations, bans and restrictions being in force for several phthalates, the impact indicator showed that there are still children with urinary phthalate levels exceeding the HBM-GV for DiBP. This is of particular concern since phthalates can act in a dose additive manner.
It should also be kept in mind that health-based guidance values, like the HBM-GVs may be revised when new scientific findings on dose-related health effects are available. Currently, the level of confidence (loC) for the derivation of HBM-GV for DiBP is low, whereas for DINCH the loC is medium. This is because the derivation of both is based solely on animal studies. A good example for this is the lowering of the tolerable weekly intake (TWI) for the combined exposure to some perfluoroalkyl substances (PFAS) due to new toxicological findings (Schrenk et al., 2020).
A continuous monitoring of phthalate exposure and their substitutes should be implemented to allow for a continuous monitoring of exposure levels of the population in Europe and to assess the effectiveness of new regulatory measures and to avoid regrettable substitution.
4.2. Limitations
Limitations regarding our indicators are mainly focused on data quality aspects and the availability of data. When using indicators for decision-making, the demand for data quality such as representativeness, reliability and comparability of data comes to the fore.
It has to be noted, that in a few exceptional cases, data were used for the derivation of indicators that were not quality assured within HBM4EU (Gilles et al., 2021;Govarts et al., 2022). Theses include 1) MiBP measurements for children in the PCB-cohort study (SK) and 2) MiBP measurements for teenagers from CELSPAC:TE (CZ) and the PCB cohort follow-up (SK). All data were included in the analysis of exposure determinants and risk assessment.
It was not possible to derive indicators for socioeconomic status (SES), as data on these were not available in a sufficiently large number of studies. However, many scientific studies (Den Hond et al., 2015; Bastiaensen et al., 2021; Schwedler et al., 2020a) have shown that socioeconomic status (indicated as educational level in some studies) can be a determinant of exposure to phthalates.
Another important aspect is the collection of urine samples for phthalate exposure. Phthalates are rapidly excreted via urine and do not circulate for a long time in the human body. After a single oral dose in experimental studies DEHP and DiNP (Diisononyl phthalate) metabolites indicated that around 50% were excreted in urine after 24 and 48 h, respectively (Koch et al., 2005, Koch and Angerer, 2007; ). Based on this relatively short half-life and rapid urinary excretion, it has to be kept in mind, that a single urine sample represents only the more recent exposure. Therefore, the assessment of spot and morning urine samples may lead to an over- or underestimation of internal exposure due to intra-individual variation over the day (Mok et al., 2022). Nevertheless, it has been shown for some phthalates that compared to the best case of a 24h-urine sample, the best comparability comes from morning urine samples (Frederiksen et al., 2013). Spot urine is less comparable as it can be collected at different time points during a day. However, sometimes the only available matrix in some studies is spot urine. In the HBM4EU Aligned Studies, urine samples were either first morning urine samples or spot urine samples. Urine samples taken in the frame of the DEMOCOPHES project in children were all first morning urine samples. Uncertainties regarding the determination of phthalate exposure of first morning versus spot urine samples needs to be considered.
Due to the wide use of phthalates, humans are exposed to a variety of these compounds simultaneously (Husøy et al., 2019). Mixtures of some of the selected phthalates can have direct combined effects (Howdeshell et al., 2017; Kortenkamp and Koch, 2020) as well as combined effects with other endocrine disrupting chemicals (Howdeshell et al., 2017; Kortenkamp 2020; Runkel et al., 2022). Therefore, mixture risk assessments (MRAs) have been performed for the cumulative risk of these compounds (e.g. Apel et al., 2020b). The importance of mixture risk assessment (MRA) is also addressed in several communication papers4 from the European Commission, and is also part of the European Chemicals Strategy for Sustainability5 (Bopp et al., 2018; Socianu et al., 2022). Within HBM4EU, chemical mixture risk assessment was also carried out for prioritized groups of chemicals, among them the phthalates. (Lange et al., 2022) (this issue) performed a mixture risk assessment of five selected phthalates (i.e., BBzP, DEHP, DiBP, DnBP and DiNP) and found that approximately one sixth of the European children and teenagers is at risk from adverse effects of combined exposure to these 5 phthalates. The mixture risk for the majority of children and teenagers would have gone unnoticed in single substance risk assessment. But in this study only single substance assessments for the derivation of indicators have been used and combination effects are not considered within our indicators at current state.
4.3. Future activities identified from workshop
During the workshop, two important topics were identified that are to be further processed within the future work on the indicators.
-
a)
Wish for simplification to increase general understanding
When using a simplified document targeted to the citizens, valuable information may be lost. Therefore, all information required for an explanation of the indicators has to be added in text boxes and adequate visuals have to be provided to summarize the statistical results. Further efforts are needed to convey a more informed message.
-
b)
Availability of information on source contribution influencing exposure
Information on relevant sources of phthalates is currently not integrated in our indicator concept. It is planned that also main sources of exposure to chemicals will be identified and shown in the form of indicators. This information may help to create targeted information materials that may help to minimize exposure.
5. General conclusions
The first set of pan-European HBM indicators for phthalates and DINCH provide a valuable tool to highlight differences in exposure between geographical regions, age groups, sexes and periods of time, but also to assess health impacts by comparing exposure values with HBM-GVs. The corresponding policy-related questions developed under HBM4EU could be answered by our indicators.
The HBM indicators can be compared with existing indicators based on environmental data, consumption data and food datasets. They are aimed at complementing other sets of indicators in the field of environment and health (EEA) and on human health (WHO).
With these indicators, a first step has been made for processing HBM findings in a clear and comprehensible manner. Further steps are needed to further simplify these messages, possibly in an interactive graphic design, as it has been done for the indicators which present blood lead levels of children by the U.S. EPA (US EPA, 2022).
Therefore, the HBM4EU indicators demonstrate that they are useful tools for a direct and simple interpretation of HBM data that can help policy makers to answer policy-related questions for the respective compounds and thus enable them to set priorities for further actions on their way to a toxic-free environment under the EU's chemicals strategy for sustainability (Ganzleben et al., 2017).
Lessons learned for deriving HBM indicators comprise aspects mainly related to data quality, namely:
-
a)
analytical methods should be quality assured and comparable between the studies/countries
-
b)
sampling should take place within a narrow time frame, especially for substances with a short half-life
-
c)
homogenous population groups should be compared to enable the mapping of existing differences for regions, sex or age groups
-
d)
samples should be representative for the parameter investigated: to map country differences a representativeness at national level should be the aim, to map differences between age groups these should be representative for the country
-
e)
exposure should be continuously monitored to enable time trend observations for the whole of Europe and thus create a database for deriving real “time trend indicators” at the European level
-
f)
the latest scientific findings and policy demands have to be considered, which also includes consideration of combination effects
Author contributions
Conceptualization: Jurgen Buekers, Jos Bessems, Ann Colles, Madlen David, Antje Gerofke, Joana Lobo Vicente, Greet Schoeters; Data Curation: Liese Gilles; Original Draft Preparation: Antje Gerofke, Madlen David; Visualization of indicators: Phillipp Schmidt; Commenting on draft version 19th May: all authors; finalization of manuscript: Antje Gerofke, Madlen David, Phillipp Schmidt; Providing data/study PIs: Michiel Bastiaensen, Adrian Covaci, Elly Den Hond, Gudrun Koppen, Michelle Laeremans, Veerle J Verheyen, Milena Černá, Jana Klánová, Andrea Krsková, Martin Zvonař, Lisbeth E. Knudsen, Holger M. Koch, Tina Kold Jensen, Loïc Rambaud, Margaux Riou, Nina Vogel, Catherine Gabriel, Spyros Karakitsios, Nafsika Papaioannou, Denis Sarigiannis, Réka Kakucs, Szilvia Középesy, Péter Rudnai, Tamás Szigeti, Fabio Barbone, Valentina Rosolen, Cedric Guignard, Arno C. Gutleb, Amrit Kaur Sakhi; Line Småstuen Haug, Beata Janasik, Danuta Ligocka, Milada Estokova, Lucia Fabelova, Branislav Kolena, Lubica Palkovicova Murinova, Ida Petrovicova, Denisa Richterova, Milena Horvat, Darja Mazej, Janja Snoj Tratnik, Agneta Annika Runkel, Argelia Castaño, Marta Esteban-López, Susana Pedraza-Díaz, Agneta Åkesson, Sanna Lignell, Jelle Vlaanderen, Jan-Paul Zock; Supervision: Joana Lobo Vicente, Greet Schoeters and Marike Kolossa-Gehring.
All authors have read and agreed to the published version of the manuscript.
Funding
The authors received funding from the EUHorizon 2020 framework (grant agreement No 733032). Additional financial support for the conduct of the studies is provided by each participating country.
The Norwegian Institute of Public Health (NIPH) has contributed to funding of the Norwegian Environmental Biobank (NEB). The laboratory measurements have partly been funded by the Research Council of Norway through research projects (275903 and 268465).
The funding of the German Ministry for the Environment, Nature Conservation, Nuclear Safety and Consumer Protection is gratefully acknowledged.
PCB cohort was supported by Ministry of Health of the Slovak Republic, grant no. 2012/47-SZU-11 and by Slovak Research and Development Agency, grant no. APVV-0571-12. PCB cohort follow-up received additional funding from the Ministry of Health of the Slovak Republic, program 07B0103.
Riksmaten Adolescents was performed by the Swedish Food Agency with financial support from the Swedish Environmental Protection Agency and the Swedish Civil Contingencies Agency.
The BEA study was co-funded by the Spanish Ministry of Agriculture, Fisheries and Food and the Instituto de Salud Carlos III (SEG 1321/15).
The CELSPAC study is supported by the MEYS (LM2018121, CZ.02.1.01/0.0/0.0/17_043/0009632 and CZ.02.1.01/0.0/0.0/15_003/0000469) and from the European Union’s Horizon 2020 research and innovation programme under grant agreement (857560). This publication reflects only the author's view and the European Commission is not responsible for any use that may be made of the information it contains.
NIRAS/ONDRAF (Belgian National Agency for Radioactive Waste and enriched Fissile Material), STORA (Study and Consultation Radioactive Waste Dessel) and MONA (Mols Overleg Nucleair Afval) financed the 3xG study.
The FLEHS IV study was conducted within the framework of the Flemish Center of Expertise on Environment and Health (FLEHS 2016–2020) and funded by the Flemish Government, Department of Environment & Spatial Development.
CROME study is co-funded by the European Commission research funds of Horizon 2020 and LIFE financial instrument of the European Community (LIFE12 ENV/GR/001040).
ESTEBAN is funded by Santé Publique France and the French ministries of Health and the Environment.
The Slovenian SLO-CRP study was co-financed by the Jožef Stefan Institute program P1- 0143, and a national project “Exposure of children and adolescents to selected chemicals through their habitat environment” (grant agreement No. C2715-16-634802).
The OCC studies were supported by Odense University Hospital, the Region of Southern Denmark, the Municipality of Odense, the Mental Health Service of the Region of Southern Denmark, Odense Patient data Exploratory Network (OPEN), Novo Nordisk Foundation [grant nr. NNF15OC00017734 and NNF19OC0058266], the Danish Council for Independent Research [4004-00352B_FSS].
POLAES study was co-financed by Ministry of Science and Education of Poland (contract no.3764/H2020/2017/2)
Declaration of competing interest
The authors declare no conflict of interest.
Acknowledgements
We would like to thank all data owners for providing us with the data to realise this publication. Further we would like to thank everyone who contributed to the HBM4EU Aligned Studies and the DEMOCOPHES project.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijheh.2022.114073.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Apel P., Kortenkamp A., Koch H.M., Vogel N., Rüther M., Kasper-Sonnenberg M., Conrad A., Brüning T., Kolossa-Gehring M. Time course of phthalate cumulative risks to male developmental health over a 27-year period: biomonitoring samples of the German Environmental Specimen Bank. Environ. Int. 2020;137 doi: 10.1016/j.envint.2020.105467. Epub 2020 Feb 6. PMID: 32036120. [DOI] [PubMed] [Google Scholar]
- Apel P., Rousselle C., Lange R., Sissoko F., Kolossa-Gehring M., Ougier E. Human biomonitoring initiative (HBM4EU) - strategy to derive human biomonitoring guidance values (HBM-GVs) for health risk assessment. Int. J. Hyg Environ. Health. 2020;230 doi: 10.1016/j.ijheh.2020.113622. Epub 2020 Oct 9. PMID: 33045523. [DOI] [PubMed] [Google Scholar]
- Bastiaensen M., Gys C., Colles A., Malarvannan G., Verheyen V., Koppen G., Govarts E., Bruckers L., Morrens B., Franken C., Den Hond E., Schoeters G., Covaci A. Biomarkers of phthalates and alternative plasticizers in the Flemish Environment and Health Study (FLEHS IV): time trends and exposure assessment. Environ. Pollut. 2021;276 doi: 10.1016/j.envpol.2021.116724. Epub 2021 Feb 11. PMID: 33631684. [DOI] [PubMed] [Google Scholar]
- Bopp S.K., Barouki R., Brack W., Dalla Costa S., Dorne J.C.M., Drakvik P.E., Faust M., Karjalainen T.K., Kephalopoulos S., van Klaveren J., Kolossa-Gehring M., Kortenkamp A., Lebret E., Lettieri T., Nørager S., Rüegg J., Tarazona J.V., Trier X., van de Water B., van Gils J., Bergman Å. Current EU research activities on combined exposure to multiple chemicals. Environ. Int. 2018;120:544–562. doi: 10.1016/j.envint.2018.07.037. Epub 2018 Aug 28. PMID: 30170309; PMCID: PMC6192826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buekers J., David M., Koppen G., Bessems J., Scheringer M., Lebret E., Sarigiannis D., Kolossa-Gehring M., Berglund M., Schoeters G., Trier X. Development of policy relevant human biomonitoring indicators for chemical exposure in the European population. Int. J. Environ. Res. Publ. Health. 2018;15(10):2085. doi: 10.3390/ijerph15102085. PMID: 30248963; PMCID: PMC6209865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J., Knudsen L.E., Mizrak S., Joas A. Identification of exposure to environmental chemicals in children and older adults using human biomonitoring data sorted by age: results from a literature review. Int. J. Hyg Environ. Health. 2017;220(2 Pt A):282–298. doi: 10.1016/j.ijheh.2016.12.006. Epub 2016 Dec 21. PMID: 28159478. [DOI] [PubMed] [Google Scholar]
- Den Hond E., Govarts E., Willems H., Smolders R., Casteleyn L., Kolossa-Gehring M., Schwedler G., Seiwert M., Fiddicke U., Castaño A., Esteban M., Angerer J., Koch H.M., Schindler B.K., Sepai O., Exley K., Bloemen L., Horvat M., Knudsen L.E., Joas A., Joas R., Biot P., Aerts D., Koppen G., Katsonouri A., Hadjipanayis A., Krskova A., Maly M., Mørck T.A., Rudnai P., Kozepesy S., Mulcahy M., Mannion R., Gutleb A.C., Fischer M.E., Ligocka D., Jakubowski M., Reis M.F., Namorado S., Gurzau A.E., Lupsa I.R., Halzlova K., Jajcaj M., Mazej D., Tratnik J.S., López A., Lopez E., Berglund M., Larsson K., Lehmann A., Crettaz P., Schoeters G. First steps toward harmonized human biomonitoring in Europe: demonstration project to perform human biomonitoring on a European scale. Environ. Health Perspect. 2015;123(3):255–263. doi: 10.1289/ehp.1408616. Epub 2014 Dec 11. PMID: 25493439; PMCID: PMC4348748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECHA 2022. https://www.echa.europa.eu/candidate-list-table Candidate List of substances of very high concern for Authorisation. Available online:
- ECHA Endocrine disruptor assessment list. 2022. https://echa.europa.eu/de/ed-assessment Available online:
- EEA . 2014. Digest of EEA Indicators.https://www.eea.europa.eu/publications/digest-of-eea-indicators-2014 Available online: [Google Scholar]
- EEA Environmental Indicator Report 2018. https://www.eea.europa.eu//publications/environmental-indicator-report-2018
- EFSA Panel on Food Additives Flavourings, Processing Aids and Materials in Contact with Food, 2006. Opinion of the Scientific Panel on food additives, flavourings, processing aids and materials in contact with food (AFC) related to the 12th list of substances for food contact materials. EFSA J. 2006;4(10):395. doi: 10.2903/j.efsa.2006.395. https://efsa.onlinelibrary.wiley.com/doi/epdf/10.2903/j.efsa.2006.395 21. [DOI] [Google Scholar]
- EFSA CEP Panel (EFSA Panel on Food Contact Materials, Enzymes and Processing Aids) Silano V., Barat Baviera J.M., Bolognesi C., Chesson A., Cocconcelli P.S., Crebelli R., Gott D.M., Grob K., Lampi E., Mortensen A., Riviere G., Steffensen I.-L., Tlustos C., Van Loveren H., Vernis L., Zorn H., Cravedi J.-P., Fortes C., Tavares Pocas M.F., Waalkens-Berendsen I., Wölfle D., Arcella D., Cascio C., Castoldi A.F., Volk K., Castle L. Scientific Opinion on the update of the risk assessment of di-butylphthalate (DBP), butyl-benzyl-phthalate (BBP), bis(2-ethylhexyl)phthalate (DEHP), di-isononylphthalate (DINP) and di-isodecylphthalate (DIDP) for use in food contact materials. EFSA J. 2019;17(12):5838. doi: 10.2903/j.efsa.2019.5838. 2019. 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteban López M., Göen T., Mol H., Nübler S., Haji-Abbas-Zarrabi K., Koch H.M., Kasper-Sonnenberg M., Dvorakova D., Hajslova J., Antignac J.P., Vaccher V., Elbers I., Thomsen C., Vorkamp K., Pedraza-Díaz S., Kolossa-Gehring M., Castaño A. The European human biomonitoring platform - design and implementation of a laboratory quality assurance/quality control (QA/QC) programme for selected priority chemicals. Int. J. Hyg Environ. Health. 2021;234 doi: 10.1016/j.ijheh.2021.113740. Epub 2021 Mar 26. PMID: 33774419. [DOI] [PubMed] [Google Scholar]
- Frederiksen H., Kranich S.K., Jørgensen N., Taboureau O., Petersen J.H., Andersson A.M. Temporal variability in urinary phthalate metabolite excretion based on spot, morning, and 24-h urine samples: considerations for epidemiological studies. Environ. Sci. Technol. 2013;47(2):958–967. doi: 10.1021/es303640b.Epub.2012.Dec.24. PMID: 23234290. [DOI] [PubMed] [Google Scholar]
- Frederiksen H., Nielsen O., Koch H.M., Skakkebaek N.E., Juul A., Jørgensen N., Andersson A.M. Changes in urinary excretion of phthalates, phthalate substitutes, bisphenols and other polychlorinated and phenolic substances in young Danish men; 2009-2017. Int. J. Hyg Environ. Health. 2020;223(1):93–105. doi: 10.1016/j.ijheh.2019.10.002. Epub 2019 Oct 25. PMID: 31669154. [DOI] [PubMed] [Google Scholar]
- Ganzleben C., Antignac J.P., Barouki R., Castaño A., Fiddicke U., Klánová J., Lebret E., Olea N., Sarigiannis D., Schoeters G.R., Sepai O., Tolonen H., Kolossa-Gehring M. Human biomonitoring as a tool to support chemicals regulation in the European Union. Int. J. Hyg Environ. Health. 2017;220(2 Pt A):94–97. doi: 10.1016/j.ijheh.2017.01.007. Epub 2017 Feb 22. PMID: 28284775. [DOI] [PubMed] [Google Scholar]
- German HBM Commission Normierung von Stoffgehalten im Urin – kreatinin Stellungnahme der Kommission „Human-Biomonitoring“ des Umweltbundesamtes. Bundesgesundheitsblatt. 2005;48:616–618. [Google Scholar]
- German HBM Commission Stoffmonographie für Phthalate – neue und aktualisierte Referenzwerte für Monoester und oxidierte Metabolite im Urin von Kindern und Erwachsenen. Bundesgesundheitsblatt. 2011;54:770–785. doi: 10.1007/s00103-011-1278-1. [DOI] [PubMed] [Google Scholar]
- German HBM Commission Stoffmonographie für 1,2-Cyclohexandicarbonsäuredi-isononylester (Hexamoll® DINCH®) – HBM-Werte für die Summe der Metabolite Cyclohexan-1,2-dicarbonsäuremono-hydroxyisononylester (OH-MINCH) und Cyclohexan-1,2- dicarbonsäure-mono-carboxyisooctylester (cx-MINCH) im Urin von Erwachsenen und Kindern Stellungnahme der Kommission „HumanBiomonitoring“ des Umweltbundesamtes. Bundesgesundheitsblatt. 2014;57:1451–1461. doi: 10.1007/s00103-014-2069-2. [DOI] [PubMed] [Google Scholar]
- Gilles L., Govarts E., Rambaud L., Vogel N., Castaño A., Esteban López M., Rodriguez Martin L., Koppen G., Remy S., Vrijheid M., Montazeri P., Birks L., Sepai O., Stewart L., Fiddicke U., Loots I., Knudsen L.E., Kolossa-Gehring M., Schoeters G. HBM4EU combines and harmonises human biomonitoring data across the EU, building on existing capacity - the HBM4EU survey. Int. J. Hyg Environ. Health. 2021;237 doi: 10.1016/j.ijheh.2021.113809. Epub 2021 Aug 26. PMID: 34455198; PMCID: PMC8504197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilles L, Govarts E, Rodriguez Martin L, Andersson AM, Appenzeller BMR, Barbone F, Castaño A, Coertjens D, Den Hond E, Dzhedzheia V, Eržen I, Esteban López M ME, Fábelová L, Fillol C, Franken C, Frederiksen H, Gabriel C, Haug LS, Horvat M, Halldórsson TI, Janasik B, Holcer NJ, Kakucs R, Karakitsios S, Katsonouri A, Klánová J, Kold-Jensen T, Kolossa-Gehring M, Konstantinou C, Koponen J, Lignell S, Lindroos AK, Makris KC, Mazej D, Morrens B, Murínová ĽP, Namorado S, Pedraza-Diaz S, Peisker J, Probst-Hensch N, Rambaud L, Rosolen V, Rucic E, Rüther M, Sarigiannis D, Tratnik JS, Standaert A, Stewart L, Szigeti T, Thomsen C, Tolonen H, Eiríksdóttir Á, Van Nieuwenhuyse A, Verheyen VJ, Vlaanderen J, Vogel N, Wasowicz W, Weber T, Zock JP, Sepai O, Schoeters G. Harmonization of Human Biomonitoring Studies in Europe: Characteristics of the HBM4EU-Aligned Studies Participants. Int J Environ Res Public Health. 2022;19(11):6787. doi: 10.3390/ijerph19116787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govarts Eva, et al. Harmonized human biomonitoring in European children, teenagers and adults: EU-wide exposure data of 11 chemical substance groups from the HBM4EU Aligned Studies (2014-2021) International Journal of Hygiene and Environmental Health. 2022;(this issue) doi: 10.1016/j.ijheh.2023.114119. [DOI] [PubMed] [Google Scholar]
- Greenpeace International . In: Our Reproductive Health and Chemical Exposure: a Review of the Evidence for Links between Declines in Human Reproductive Health and Our Exposure to Hazardous Chemicals. Allsopp M., Santillo D., Kallee U., Hojsík M., editors. 2006. p. 28. [Google Scholar]
- Howdeshell K.L., Hotchkiss A.K., Gray L.E., Jr. Cumulative effects of antiandrogenic chemical mixtures and their relevance to human health risk assessment. Int. J. Hyg Environ. Health. 2017;220(2 Pt A):179–188. doi: 10.1016/j.ijheh.2016.11.007. Epub 2016 Nov 19. PMID: 27923611; PMCID: PMC5401775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husøy T., Andreassen M., Hjertholm H., Carlsen M.H., Norberg N., Sprong C., Papadopoulou E., Sakhi A.K., Sabaredzovic A., Dirven H.A.A.M. The Norwegian biomonitoring study from the EU project EuroMix: levels of phenols and phthalates in 24-hour urine samples and exposure sources from food and personal care products. Environ. Int. 2019;132 doi: 10.1016/j.envint.2019.105103. Epub 2019 Aug 27. PMID: 31470218. [DOI] [PubMed] [Google Scholar]
- Kasper-Sonnenberg M., Koch H.M., Apel P., Rüther M., Pälmke C., Brüning T., Kolossa-Gehring M. Time trend of exposure to the phthalate plasticizer substitute DINCH in Germany from 1999 to 2017: biomonitoring data on young adults from the Environmental Specimen Bank (ESB) Int. J. Hyg Environ. Health. 2019;222(8):1084–1092. doi: 10.1016/j.ijheh.2019.07.011. Epub 2019 Aug 1. PMID: 31378638. [DOI] [PubMed] [Google Scholar]
- Kasper-Sonnenberg et al., (2022 in preparation).
- Knudsen, et al. 2022. Implementation and Coordination of an Ethics Framework in HBM4EU – Experiences and Reflections. (also in this special issue) [DOI] [PubMed] [Google Scholar]
- Koch HM, Angerer J. Di-iso-nonylphthalate (DINP) metabolites in human urine after a single oral dose of deuterium-labelled DINP. Int J Hyg Environ Health. 2007;210(I):9–19. doi: 10.1016/j.ijheh.2006.11.008. [DOI] [PubMed] [Google Scholar]
- Koch HM, Bolt HM, Preuss R, Angerer J. New metabolites of di(2-ethylhexyl)phthalate (DEHP) in human urine and serum after single oral doses of deuterium-labelled DEHP. Arch Toxicol. 2005;79:367–376. doi: 10.1007/s00204-004-0642-4. [DOI] [PubMed] [Google Scholar]
- Koch H.M., Rüther M., Schütze A., Conrad A., Pälmke C., Apel P., Brüning T., Kolossa-Gehring M. Phthalate metabolites in 24-h urine samples of the German Environmental Specimen Bank (ESB) from 1988 to 2015 and a comparison with US NHANES data from 1999 to 2012. Int. J. Hyg Environ. Health. 2017;220(2 Pt A):130–141. doi: 10.1016/j.ijheh.2016.11.003. Epub 2016 Nov 9. PMID: 27863804. [DOI] [PubMed] [Google Scholar]
- Kortenkamp A. Which chemicals should be grouped together for mixture risk assessments of male reproductive disorders? Mol. Cell. Endocrinol. 2020;499 doi: 10.1016/j.mce.2019.110581. Epub 2019 Sep 13. PMID: 31525431. [DOI] [PubMed] [Google Scholar]
- Kortenkamp A., Koch H.M. Refined reference doses and new procedures for phthalate mixture risk assessment focused on male developmental toxicity. Int. J. Hyg Environ. Health. 2020;224 doi: 10.1016/j.ijheh.2019.113428. Epub 2019 Dec 23. PMID: 31978726. [DOI] [PubMed] [Google Scholar]
- Lange R., Apel P., Rousselle C., Charles S., Sissoko F., Kolossa-Gehring M., Ougier E. The European Human Biomonitoring Initiative (HBM4EU): human biomonitoring guidance values for selected phthalates and a substitute plasticizer. Int. J. Hyg Environ. Health. 2021;234 doi: 10.1016/j.ijheh.2021.113722. Epub 2021 Mar 9. PMID: 33711757. [DOI] [PubMed] [Google Scholar]
- Lange R, Vogel N, Schmidt P, Gerofke A, Luijten M, Bil W, Santonen T, Schoeters G, Gilles L, Sakhi AK, Haug LS, Jensen TK, Frederiksen H, Koch HM, Szigeti T, Szabados M, Tratnik JS, Mazej D, Gabriel C, Sarigiannis D, Dzhedzheia V, Karakitsios S, Rambaud L, Riou M, Koppen G, Covaci A, Zvonař M, Piler P, Klánová J, Fábelová L, Richterová D, Kosjek T, Runkel A, Pedraza-Díaz S, Verheyen V, Bastiaensen M, Esteban-López M, Castaño A, Kolossa-Gehring M. Cumulative risk assessment of five phthalates in European children and adolescents. International Journal of Hygiene and Environmental Health. 2022;(this issue) doi: 10.1016/j.ijheh.2022.114052. Epub 2022 Oct 30. PMID: 36323174. [DOI] [PubMed] [Google Scholar]
- Lemke N., Murawski A., Lange R., Weber T., Apel P., Dębiak M., Koch H.M., Kolossa-Gehring M. Substitutes mimic the exposure behaviour of REACH regulated phthalates - a review of the German HBM system on the example of plasticizers. Int. J. Hyg Environ. Health. 2021;236 doi: 10.1016/j.ijheh.2021.113780. Epub 2021 Jun 11. PMID: 34126298. [DOI] [PubMed] [Google Scholar]
- Main K.M., Skakkebaek N.E., Toppari J. Cryptorchidism as part of the testicular dysgenesis syndrome: the environmental connection. Endocr. Dev. 2009;14:167–173. doi: 10.1159/000207485. Epub 2009 Feb 27. PMID: 19293583. [DOI] [PubMed] [Google Scholar]
- Martinsone, et al. 2022. Determinants of Phthalates Exposure and Their Substitutes Among Children, Teenagers and Adults in Europe. (in preparation) [Google Scholar]
- Mok S., Lim J.E., Lee A., Kim S., Kim S., Lee I., Kho Y., Park J., Kim S., Choi K., Moon H.B. Within- and between-person variability of urinary phthalate metabolites and bisphenol analogues over seven days: considerations of biomonitoring study design. Environ. Res. 2022;209 doi: 10.1016/j.envres.2022.112885. Epub 2022 Feb 4. PMID: 35131323. [DOI] [PubMed] [Google Scholar]
- Mol H.G.J., Elbers I., Pälmke C., Bury D., Göen T., López M.E., Nübler S., Vaccher V., Antignac J.P., Dvořáková D., Hajšlová J., Sakhi A.K., Thomsen C., Vorkamp K., Castaño A., Koch H.M. Proficiency and interlaboratory variability in the determination of phthalate and DINCH biomarkers in human urine: results from the HBM4EU project. Toxics. 2022;10(2):57. doi: 10.3390/toxics10020057. PMID: 35202244; PMCID: PMC8878211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ougier E., Ganzleben C., Lecoq P., Bessems J., David M., Schoeters G., Lange R., Meslin M., Uhl M., Kolossa-Gehring M., Rousselle C., Vicente J.L. Chemical prioritisation strategy in the European human biomonitoring initiative (HBM4EU) - development and results. Int. J. Hyg Environ. Health. 2021;236 doi: 10.1016/j.ijheh.2021.113778. Epub 2021 Jun 2. PMID: 34089975. [DOI] [PubMed] [Google Scholar]
- R Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2018. R: A Language and Environment for Statistical Computing. [Google Scholar]
- Runkel A.A., Mazej D., Snoj Tratnik J., Tkalec Ž., Kosjek T., Horvat M. Exposure of men and lactating women to environmental phenols, phthalates, and DINCH. Chemosphere. 2022;286(Pt 3) doi: 10.1016/j.chemosphere.2021.131858. Epub 2021 Aug 10. PMID: 34399256. [DOI] [PubMed] [Google Scholar]
- Schindler B.K., Esteban M., Koch H.M., Castano A., Koslitz S., Cañas A., Casteleyn L., Kolossa-Gehring M., Schwedler G., Schoeters G., Hond E.D., Sepai O., Exley K., Bloemen L., Horvat M., Knudsen L.E., Joas A., Joas R., Biot P., Aerts D., Lopez A., Huetos O., Katsonouri A., Maurer-Chronakis K., Kasparova L., Vrbík K., Rudnai P., Naray M., Guignard C., Fischer M.E., Ligocka D., Janasik B., Reis M.F., Namorado S., Pop C., Dumitrascu I., Halzlova K., Fabianova E., Mazej D., Tratnik J.S., Berglund M., Jönsson B., Lehmann A., Crettaz P., Frederiksen H., Nielsen F., McGrath H., Nesbitt I., De Cremer K., Vanermen G., Koppen G., Wilhelm M., Becker K., Angerer J. The European COPHES/DEMOCOPHES project: towards transnational comparability and reliability of human biomonitoring results. Int. J. Hyg Environ. Health. 2014;217(6):653–661. doi: 10.1016/j.ijheh.2013.12.002. Epub 2013 Dec 15. PMID: 24405937. [DOI] [PubMed] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain) Schrenk D., Bignami M., Bodin L., Chipman J.K., del Mazo J., Grasl-Kraupp B., Hogstrand C., Hoogenboom L.R., Leblanc J.-C., Nebbia C.S., Nielsen E., Ntzani E., Petersen A., Sand S., Vleminckx C., Wallace H., Barregard L., Ceccatelli S., Cravedi J.-P., Halldorsson T.I., Haug L.S., Johansson N., Knutsen H.K., Rose M., Roudot A.-C., Van Loveren H., Vollmer G., Mackay K., Riolo F., Schwerdtle T. Scientific Opinion on the risk to human health related to the presence of perfluoroalkyl substances in food. EFSA J. 2020;18(9):391. doi: 10.2903/j.efsa.2020.622. 2020. 6223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwedler G., Rucic E., Lange R., Conrad A., Koch H.M., Pälmke C., Brüning T., Schulz C., Schmied-Tobies M.I.H., Daniels A., Kolossa-Gehring M. Phthalate metabolites in urine of children and adolescents in Germany. Human biomonitoring results of the German Environmental Survey GerES V, 2014-2017. Int. J. Hyg Environ. Health. 2020;225 doi: 10.1016/j.ijheh.2019.113444. Epub 2020 Feb 12. PMID: 32058939. [DOI] [PubMed] [Google Scholar]
- Schwedler G., Conrad A., Rucic E., Koch H.M., Leng G., Schulz C., Schmied-Tobies M.I.H., Kolossa-Gehring M. Hexamoll® DINCH and DPHP metabolites in urine of children and adolescents in Germany. Human biomonitoring results of the German Environmental Survey GerES V, 2014-2017. Int. J. Hyg Environ. Health. 2020;229 doi: 10.1016/j.ijheh.2019.09.004. Epub 2019 Oct 1. PMID: 31585790. [DOI] [PubMed] [Google Scholar]
- Silva M.J., Barr D.B., Reidy J.A., Malek N.A., Hodge C.C., Caudill S.P., Brock J.W., Needham L.L., Calafat A.M. Urinary levels of seven phthalate metabolites in the U.S. Population from the national health and nutrition examination survey (NHANES) 1999-2000. Environ. Health Perspect. 2004;112(3):331–338. doi: 10.1289/ehp.6723. Erratum in: Environ Health Perspect. 2004 Apr;112(5):A270. PMID: 14998749; PMCID: PMC1241863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Socianu S., Bopp S.K., Govarts E., Gilles L., Buekers J., Kolossa-Gehring M., Backhaus T., Franco A. Chemical mixtures in the EU population: composition and potential risks. Int. J. Environ. Res. Publ. Health. 2022;19:6121. doi: 10.3390/ijerph19106121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United Nations S.D Standard country or area codes for statistical use (M49) 1999. https://unstats.un.org/unsd/methodology/m49/
- US EPA . 2022. Indicators on Blood Lead Levels in Children.https://www.epa.gov/americaschildrenenvironment/biomonitoring-lead [Google Scholar]
- US EPA Exposure Factors Handbook: 2011 Edition. 2011. https://www.epa.gov/sites/default/files/2015-09/documents/efh-frontmatter.pdf
- Vogel, et al. 2022. Current Exposure to Phthalates and DINCH in European Children and Adolescents - Results from the HBM4EU Aligned Studies 2014 to 2021 (Also in This Special Issue) [DOI] [PubMed] [Google Scholar]
- Vogel, et al. 2022. Publication on Time Trends of Phthalate Exposure (Also in This Special Issue) [Google Scholar]
- WHO . World Health Organization; 2022. European Health Information Gateway.https://gateway.euro.who.int/en/indicators/enhis_45-mean-blood-lead-levels-of-childrenmeasured-in-areas-without-significant-local-sources-of-lead-exposure/https://gateway.euro.who.int/en/indicators/enhis_41-dioxin-levels-in-human-milk-in-selected-countries/ Available online: [Google Scholar]
- World Health Organization (WHO) 1999. Environmental Health Indicators: Framework and Methodologies; p. 47pp. Genva. [Google Scholar]
- Wormuth M., Scheringer M., Vollenweider M., Hungerbühler K. What are the sources of exposure to eight frequently used phthalic acid esters in Europeans? Risk Anal. 2006;26(3):803–824. doi: 10.1111/j.1539-6924.2006.00770.x.PMID:16834635. [DOI] [PubMed] [Google Scholar]
- RStudie, 2022. Integrated Development for R. RStudio, PBC, Boston, MA URL http://www.rstudio.com/.
- Eurostat, 2014. Towards a harmonised methodology for statistical indicators — Part 1: Indicator typologies and terminologies - 2014 edition - Products Manuals and Guidelines - Eurostat (europa.eu);available online: https://ec.europa.eu/eurostat/web/products-manuals-and-guidelines/-/KS-GQ-14-011 (Accessed 21 June 2022).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.